Submitted:
25 April 2023
Posted:
26 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Thickness test
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
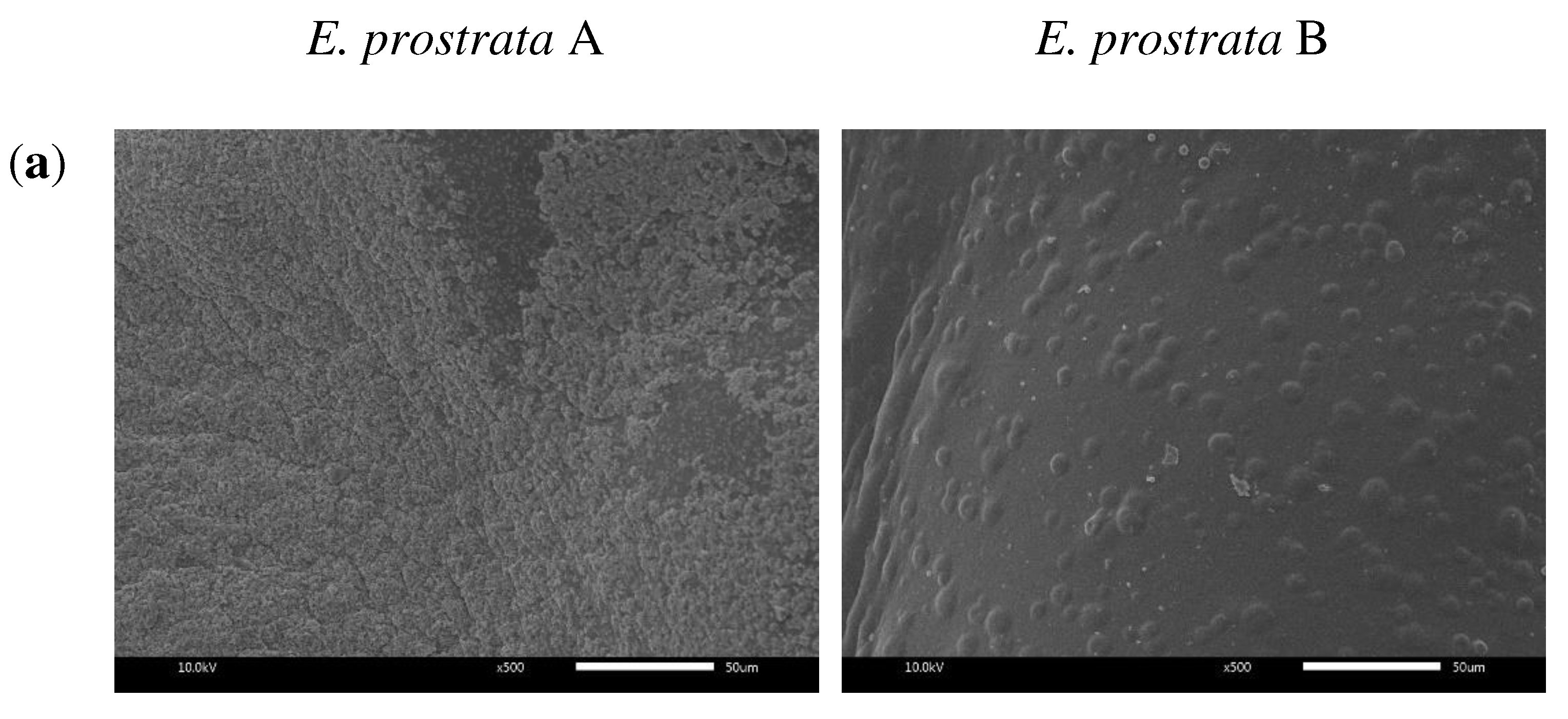
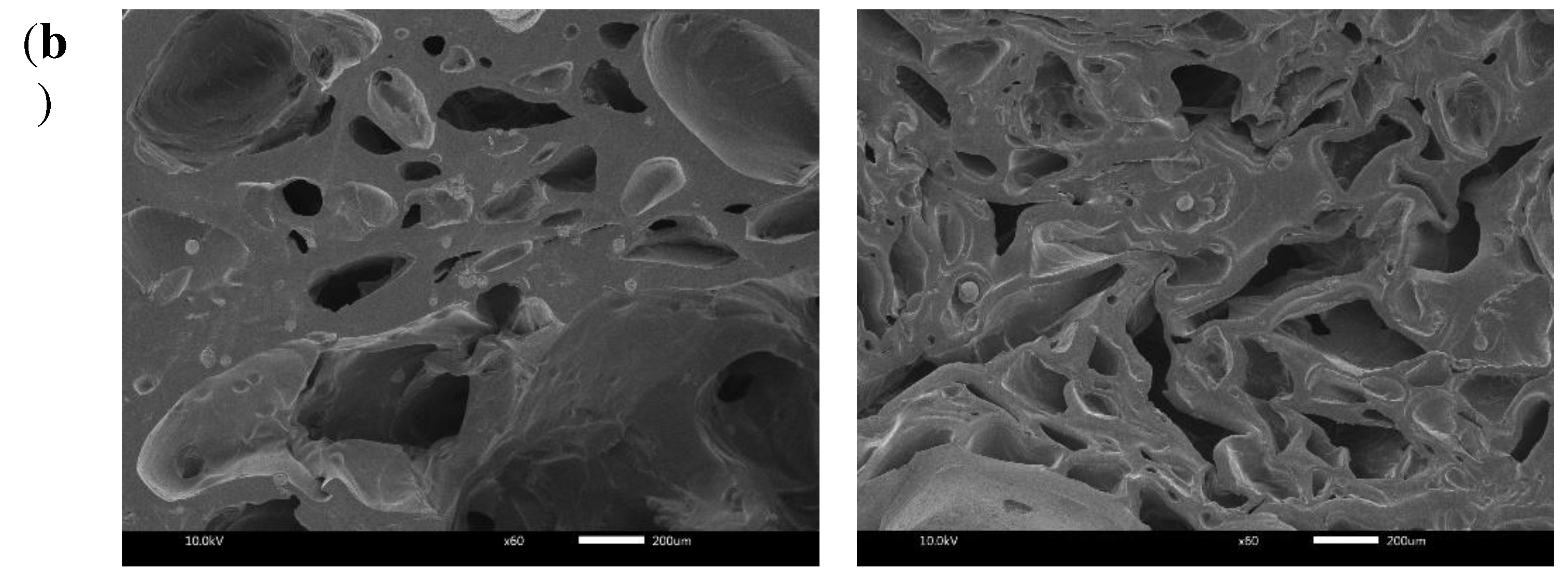
2.3. Morphological Properties
2.4. Absorption Properties
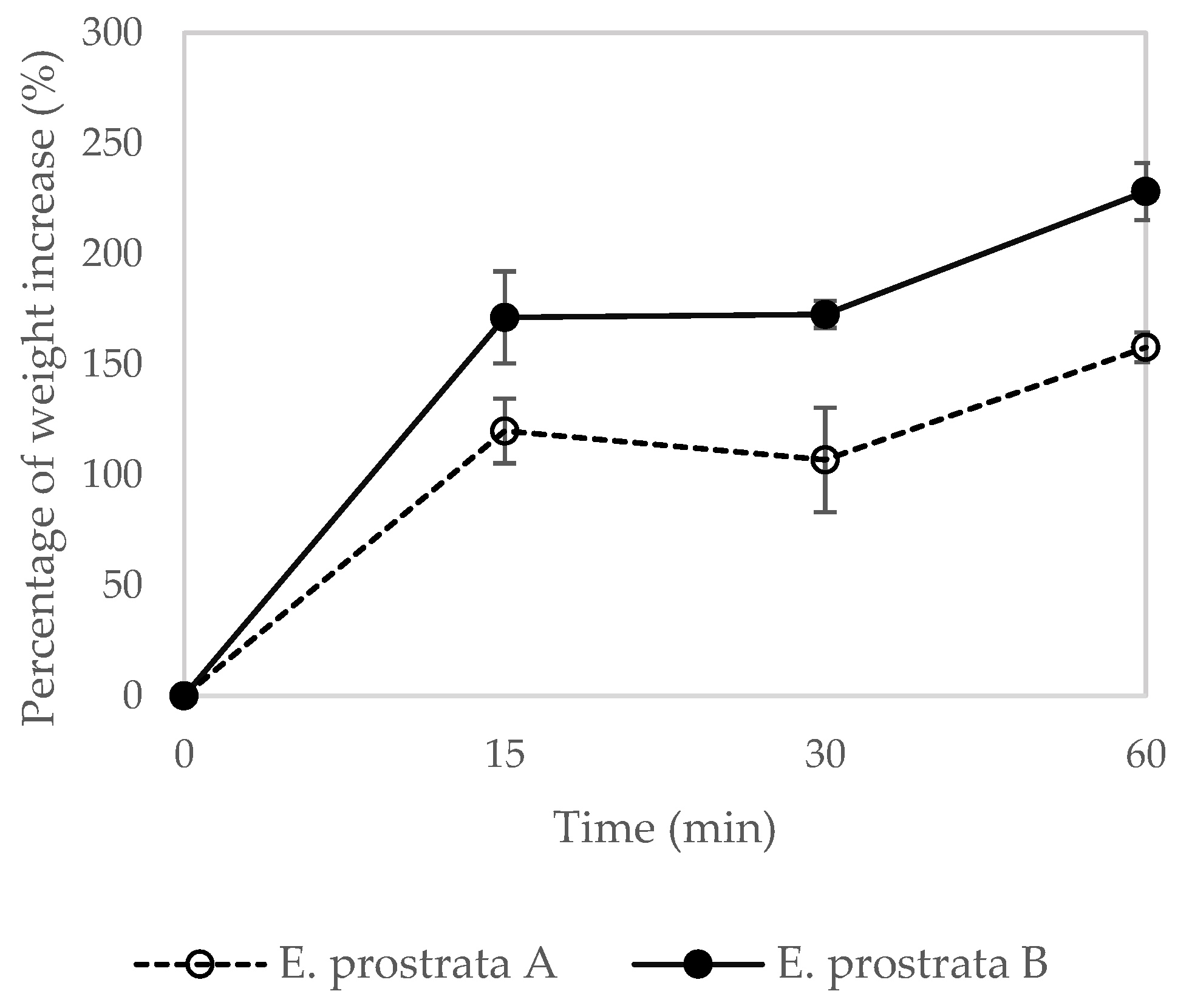
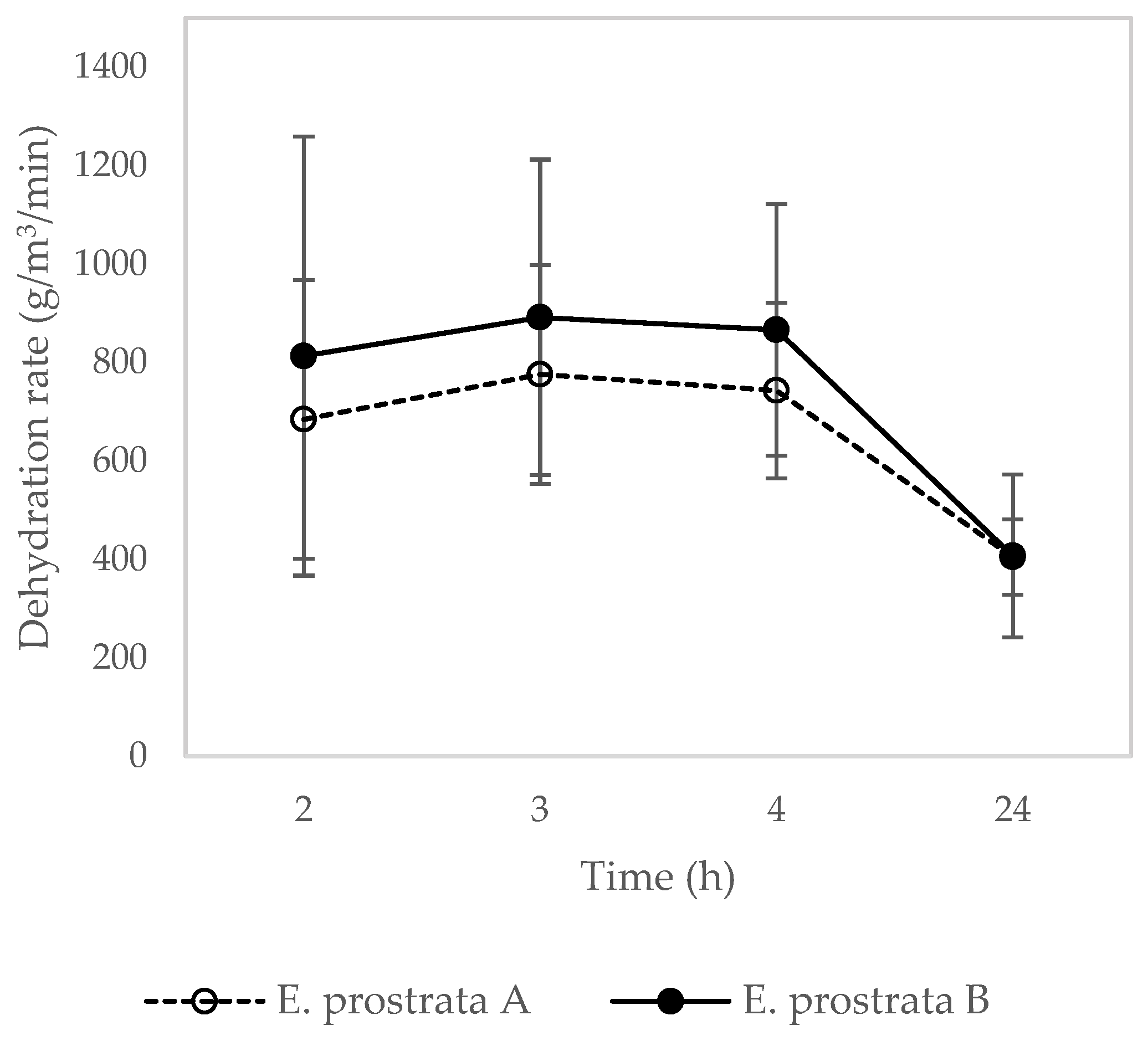
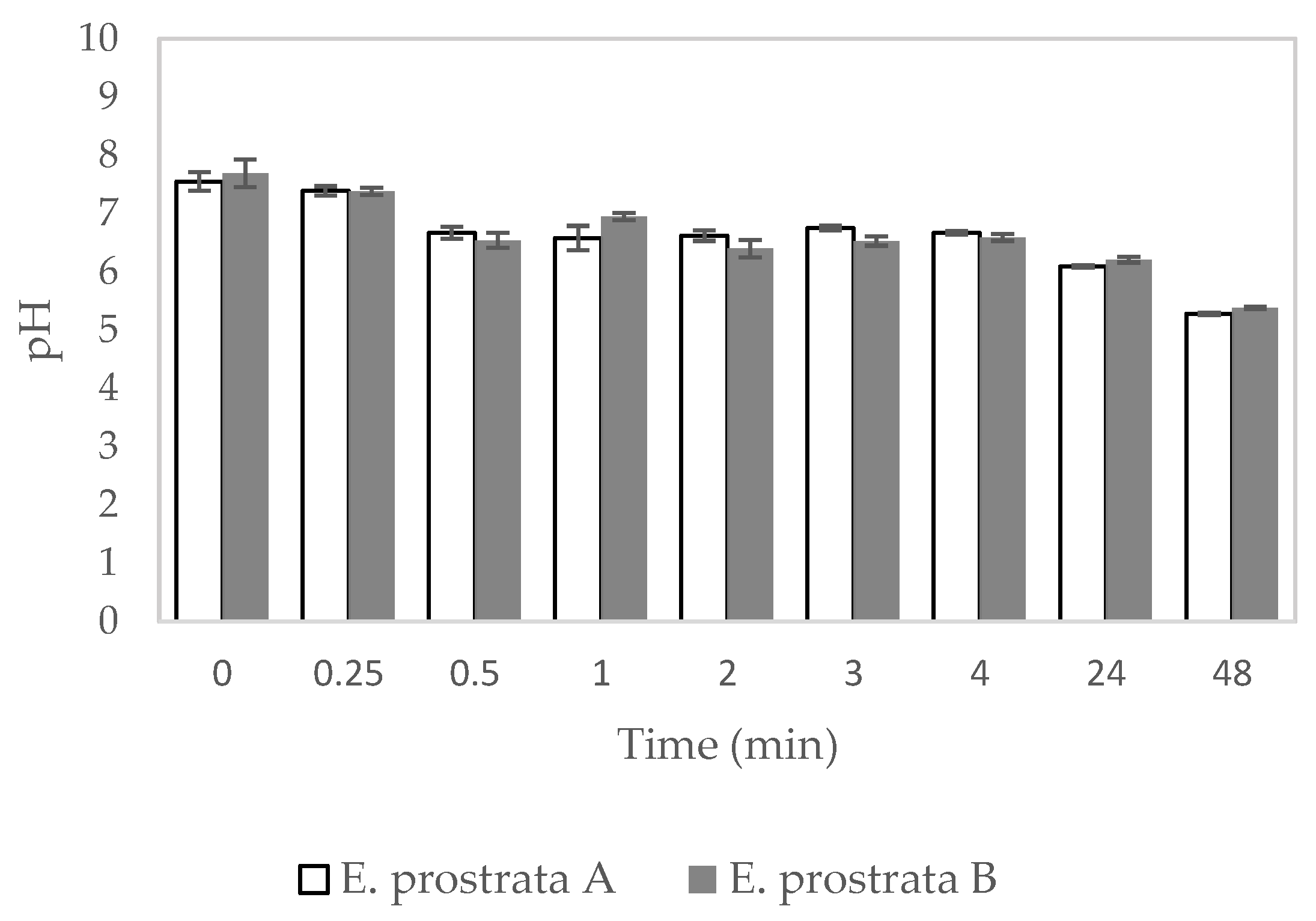
2.5. Dehydration Properties
2.7. Dispersion Characteristics
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of foam dressing containing E. prostrata extract and gelatin
4.3. Thickness Test
4.4. Fourier Transform Infrared Spectroscopy (FTIR)
4.5. Morphological Properties
4.6. Absorption Properties
4.7. Dehydration Properties
4.9. Dispersion Characteristics
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kepekçi RA, Yener İlçe B, Demir Kanmazalp S. Plant-derived biomaterials for wound healing. In: Studies in Natural Products Chemistry. Elsevier; 2021. p. 227–64.
- Martin C, Low WL, Amin MCIM, Radecka I, Raj P, Kenward K. Current trends in the development of wound dressings, biomaterials and devices. Pharm Pat Anal. 2013 May;2(3):341–59. [CrossRef]
- Yazarlu O, Iranshahi M, Kashani HRK, Reshadat S, Habtemariam S, Iranshahy M, et al. Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacol Res. 2021 Dec 1;174:1–37. [CrossRef]
- Timalsina D, Devkota HP. Eclipta prostrata (L.) l. (asteraceae): Ethnomedicinal uses, chemical constituents, and biological activities. Biomolecules. 2021 Nov 1;11(11):1–18.
- Feng L, Zhai YY, Xu J, Yao WF, Cao YD, Cheng FF, et al. A review on traditional uses, phytochemistry and pharmacology of Eclipta prostrata (L.) L. J Ethnopharmacol. 2019 Dec 5;245:1–14. [CrossRef]
- Gurrapu S, Mamidala E, Mamidala E, Mamidala E. In vitro Antibacterial Activity of Alkaloids Isolated from Leaves of Eclipta alba Against Human Pathogenic Bacteria. Pharmacognosy Journal. 2017 Jul 1;9(4):573–7. [CrossRef]
- Nahid A, Neelabh C, Navneet K, Kumar Navneet C. Evaluation of antioxidant and antimicrobial potentials of Eclipta prostrata collected from the Nepal region. The Pharma Innovation Journal. 2017;6(11):4–7.
- Singh L, Antil R, Kumar D, Dahiya P. Phytochemical analysis and In-vitro assays for antimicrobial and antioxidant activity of Bhringraj herb Eclipta prostrata (L.). J Pharmacogn Phytochem. 2019;8(3):4527–33.
- Arunachalam G, Subramanian N, Pazhani GP, Ravichandran V. Anti-inflammatory activity of methanolic extract of Eclipta prostrata L. (Astearaceae). Afr J Pharm Pharmacol. 2009;3(3):97–100.
- Kang YM, Kim HM, Lee H, Lee DS, An HJ. Anti-inflammatory effects of Eclipta prostrata Linné on house dust mite-induced atopic dermatitis in vivo and in vitro. J Ethnopharmacol. 2022 Jun 28;292:1–11. [CrossRef]
- Raoul A, CyrJonas M, MatokoChristevyRommelle S, ItouDeGardeRomaric E, Martin D, AngeAntoine A. Antidiabetic and Wounds Healing Activities of Eclipta prostrata (Asteraceae) Leaves. Int J Adv Res (Indore). 2018 Nov 30;6(12):393–8. [CrossRef]
- Babu IS, Bhramaramba R, Tejaswini S Satya Naga. Formulation and Evaluation of Herbal Gel containing Eclipta alba Linn., leaves extract. IJAPBC. 2015;4(2):496–500.
- Edwards H, Gibb M, Finlayson K, Jensen R. Wound Dressing Guide. Institute of health and Biomedical Innovation; 2013. 1–49 p.
- Dabiri G, Damstetter E, Phillips T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv Wound Care (New Rochelle). 2016;5(1):32–41. [CrossRef]
- Vivcharenko V, Przekora A. Modifications of wound dressings with bioactive agents to achieve improved pro-healing properties. Applied Sciences . 2021 May 1;11(9):1–16. [CrossRef]
- Broussard KC, Powers JG. Wound dressings: Selecting the most appropriate type. Am J Clin Dermatol. 2013 Dec;14(6):449–59. [CrossRef]
- Cutting KF, White RJ. Maceration of the skin and wound bed. 1: Its nature and causes. J Wound Care. 2002;11(7):275–8. [CrossRef]
- Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna S. Wound dressings: Current advances and future directions. J Appl Polym Sci. 2019 Jul 15;136(27):1–12.
- Gardner S. Managing high exudate wounds. Wound essential. 2012;7(1):1–3.
- Hasatsri S, Pitiratanaworanat A, Swangwit S, Boochakul C, Tragoonsupachai C. Comparison of the Morphological and Physical Properties of Different Absorbent Wound Dressings. Dermatol Res Pract. 2018;1–6. [CrossRef]
- Dhivya S, Padma VV, Santhini E. Wound dressings - A review. BioMedicine (Netherlands). 2015 Dec 1;5(4):24–8.
- Butcher M. Moist wound healing, exudate and management of the wound bed. J Wound Care. 2013 Sep 29;19(5 SUPPL.):10–3. [CrossRef]
- Nuutila K, Eriksson E. Moist Wound Healing with Commonly Available Dressings. Adv Wound Care (New Rochelle). 2021 Dec 1;10(12):685–98. [CrossRef]
- Sharman D. Moist Wound Healing: A Review of Evidence, Application and Outcome. The Diabetic Foot. 2003;6(3):112–20.
- Sim P, Strudwick XL, Song YM, Cowin AJ, Garg S. Influence of Acidic pH on Wound Healing In Vivo: A Novel Perspective for Wound Treatment. Int J Mol Sci. 2022 Nov 1;23(21):1–15. [CrossRef]
- Aly R, Shirley C, Cunico B, Maibach HI. Effect of prolonged occlusion on the microbial flora, pH, carbon dioxide and transepidermal water loss on human skin. Journal of Investigative Dermatology. 1978;71(6):378–81. [CrossRef]
- Toker-Bayraktar M, Erenay B, Altun B, Odabaş S, Garipcan B. Plant-derived biomaterials and scaffolds. Cellulose 2023. 2023 Feb 3;1–21. [CrossRef]
- Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–91. [CrossRef]
- Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010 Mar;89(3):219–29. [CrossRef]
- Evren Okur M, Karantas ID, enyi S, Üstünda N, Siafaka PI. Recent trends on wound management: New therapeutic choices based on polymer ic carr iers. Asian J Pharm Sci. 2020;15:661–84.
- Sultana N, Hassan MI, Ridzuan N, Ibrahim Z, Soon CF. Fabrication of gelatin scaffolds using thermally induced phase separation technique. International Journal of Engineering, Transactions B: Applications. 2018 Aug 1;31(8):1302–7.
- Annabi N, Nichol JW, Zhong X, Ji C, Koshy S, Khademhosseini A, et al. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. 2010;16(4):371–83. [CrossRef]
- Ndlovu SP, Ngece K, Alven S, Aderibigbe BA. Gelatin-based hybrid scaffolds: Promising wound dressings. Polymers (Basel). 2021 Sep 1;13(17):1–31. [CrossRef]
- Naomi R, Bahari H, Ridzuan PM, Othman F. Natural-based biomaterial for skin wound healing (Gelatin vs. collagen): Expert review. Polymers (Basel). 2021 Jul 2;13(14):1–20. [CrossRef]
- Cebi N, Durak MZ, Toker OS, Sagdic O, Arici M. An evaluation of Fourier transforms infrared spectroscopy method for the classification and discrimination of bovine, porcine and fish gelatins. Food Chem. 2016 Jun 30;190:1109–15. [CrossRef]
- Mahmoud AA, Osman O, Eid K, al Ashkar E, Okasha A, Atta D, et al. FTIR Spectroscopy of Natural Bio-Polymers Blends. Middle East Journal of Applied Sciences. 2014;4(4):816–24.
- Thomas M, Hamdan M, Hailes S, Walker M. An investigation into the conformability of wound dressings. Wounds UK. 2011;7(3):14–24.
- Said NS, Sarbon NM. Physical and Mechanical Characteristics of Gelatin-Based Films as a Potential Food Packaging Material: A Review. Membranes (Basel). 2022 May 1;12(5):1–26. [CrossRef]
- Sadat A, Joye IJ. Peak fitting applied to fourier transform infrared and raman spectroscopic analysis of proteins. Applied Sciences (Switzerland). 2020 Sep 1;10(17):1–16. [CrossRef]
- Chaudhari AA, Vig K, Baganizi DR, Sahu R, Dixit S, Dennis V, et al. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int J Mol Sci. 2016 Dec 1;17(12):1–31. [CrossRef]
- Murphy CM, Haugh MG, O’Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010 Jan;31(3):461–6.
- Shi C, Wang C, Liu H, Li Q, Li R, Zhang Y, et al. Selection of Appropriate Wound Dressing for Various Wounds. Front Bioeng Biotechnol. 2020 Mar 19;8:1–17. [CrossRef]
- World Union of Wound Healing Societies. World Union of Wound Healing Societies (WUWHS) Consensus Document. Wound exudate: effective assessment and management. Wounds International. 2019;1–34.
- Ennis WJ, Hill D. Wound Healing: A Comprehensive Wound Assessment and Treatment Approach. In: Skin Tissue Engineering and Regenerative Medicine. Elsevier Inc.; 2016. p. 239–63.
- Rippon M. Tissue Viability. British Journal of Nursing. 2016;25(20):1–8.
- Negut I, Dorcioman G, Grumezescu V. Scaffolds for Wound Healing Applications. Polymers (Basel). 2020 Sep 3;12(9):1–19. [CrossRef]
- Braun-Falco O, Korting H. [Normal pH value of human skin]. Hautarzt. 1986 Mar 1;37(3):126–9.
- Wallace LA, Gwynne L, Jenkins T. Challenges and opportunities of pH in chronic wounds. Ther Deliv. 2019;10(11):719–35. [CrossRef]
- Ekawati ER, Darmanto W, Wahyuningsih SPA. Detection of Staphylococcus aureus in wound infection on the skin surface. In: IOP Conference Series: Earth and Environmental Science. Institute of Physics Publishing; 2020. [CrossRef]
- Iyer V, Raut J, Dasgupta A. Impact of pH on growth of Staphylococcus epidermidis and Staphylococcus aureus in vitro. J Med Microbiol. 2021;70(9). [CrossRef]
- Romanelli M, Schipani E, Piaggesi A, Barachini P. Evaluation of Surface pH on Venous Leg Ulcers Under Allevyn Dressings. In: International congress and symposium series- Royal Society Of Medicine. Evidence-based wound care; 1997. p. 57–60.
- Tsukada K, Tokunaga K, Iwama T, Mishima Y. The pH changes of pressure ulcers related to the healing process of wounds. Wounds. 1992;2(4):16–20.
- Wilson I, Henry M, Quill R, Byrne P. The pH of varicose ulcer surfaces and its relationship to healing. Vasa. 1979;8:339–42.
- Gethin G. The significance of surface pH in chronic wounds. Wounds. 2007;3(3):52–6.
- McCarty SM, Percival SL. Proteases and Delayed Wound Healing. Adv Wound Care (New Rochelle). 2013 Oct;2(8):438–47. [CrossRef]
- Leveen HH, Falk G, Borek B, Diaz C, Lynfield Y, Wynkoop BJ, et al. Chemical Acidification of Wounds An Adjuvant to Healing and the Unfavorable Action of Alkalinity and Ammonia. Ann Surg. 1973;178(6):745–53.
- Lengheden A, Jansson L. pH effects on experimental wound healing of human fibroblasts in vitro. Eur J Oral Sci. 1995;103(3):148–55. [CrossRef]
- Sim P, Song Y, Yang GN, Cowin AJ, Garg S. In Vitro Wound Healing Properties of Novel Acidic Treatment Regimen in Enhancing Metabolic Activity and Migration of Skin Cells. Int J Mol Sci. 2022 Jul 1;23(13):1–15. [CrossRef]
- Welz MM, Ofner CM. Examination of self-crosslinked gelatin as a hydrogel for controlled release. J Pharm Sci. 1992 Jan 1;81(1):85–90. [CrossRef]
- Liu S, Zhang H, Ahlfeld T, Kilian D, Liu Y, Gelinsky M, et al. Evaluation of different crosslinking methods in altering the properties of extrusion-printed chitosan-based multi-material hydrogel composites. 2023;6:150–73. [CrossRef]
- British Standards Institution. Part 1; Aspects of absorbency. Section 3.2- Free Swell Absorptive capacities. In: Test methods for primary wound dressings. BS EN 13726-1; 2002.
- Parsons D, Bowler PG, Myles V, Jones S. Silver antimicrobial dressings in wound management: A comparison of antibacterial, physical and chemical characteristics. Wounds. 2005;17(8):222–32.
- British Standards Institution. Part 1; Aspects of absorbency. Section 3.6 - Dispersion characteristics. In: Test methods for primary wound dressings. BS EN 13726-1; 2002.
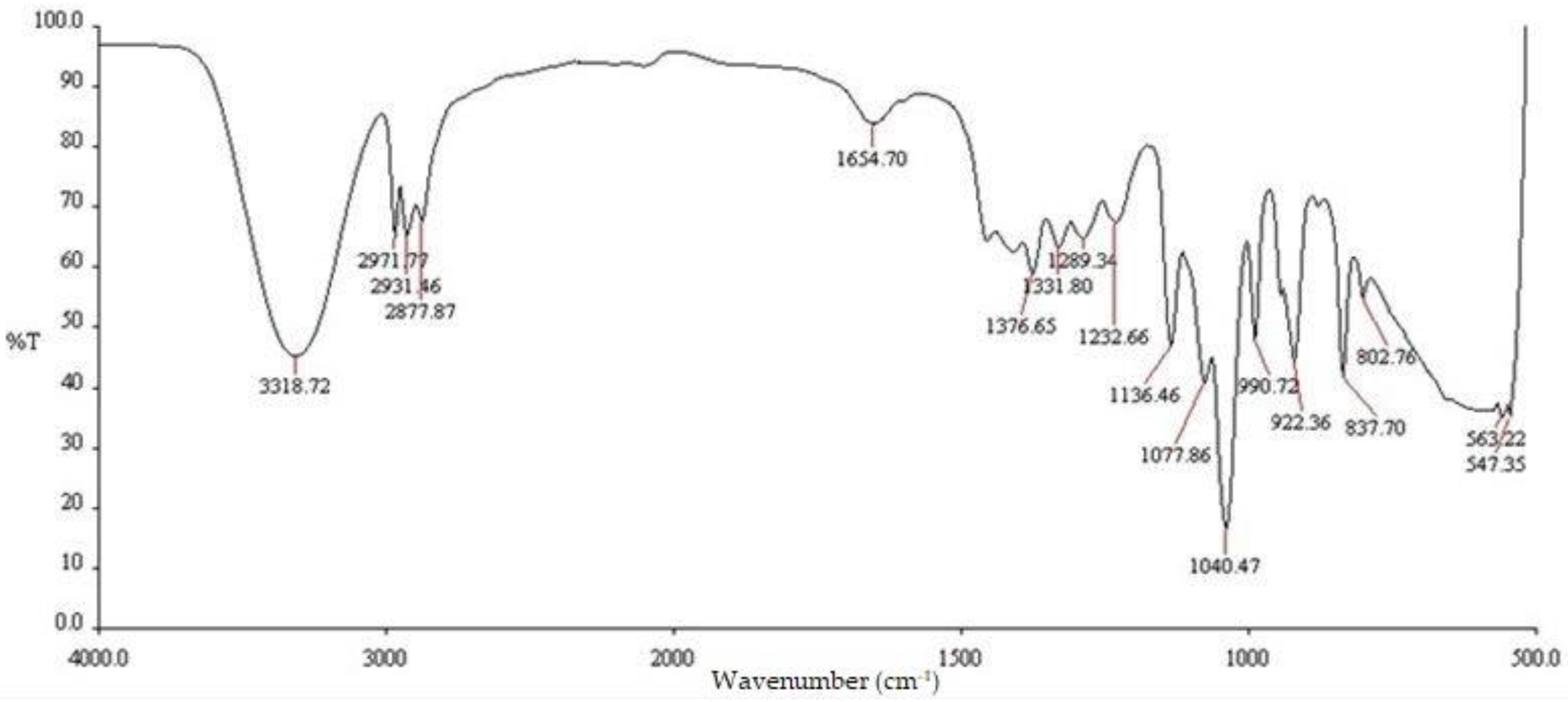
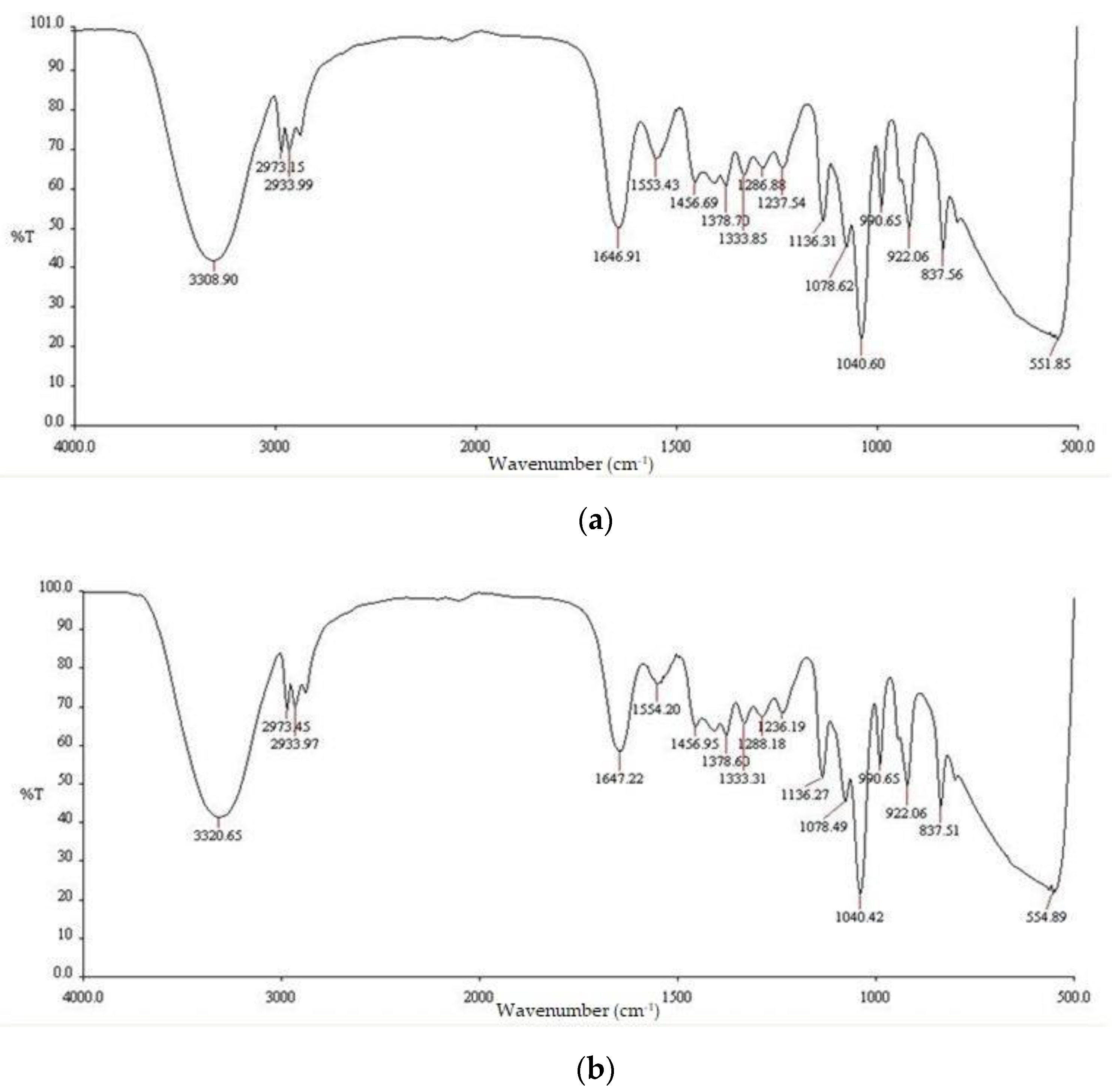

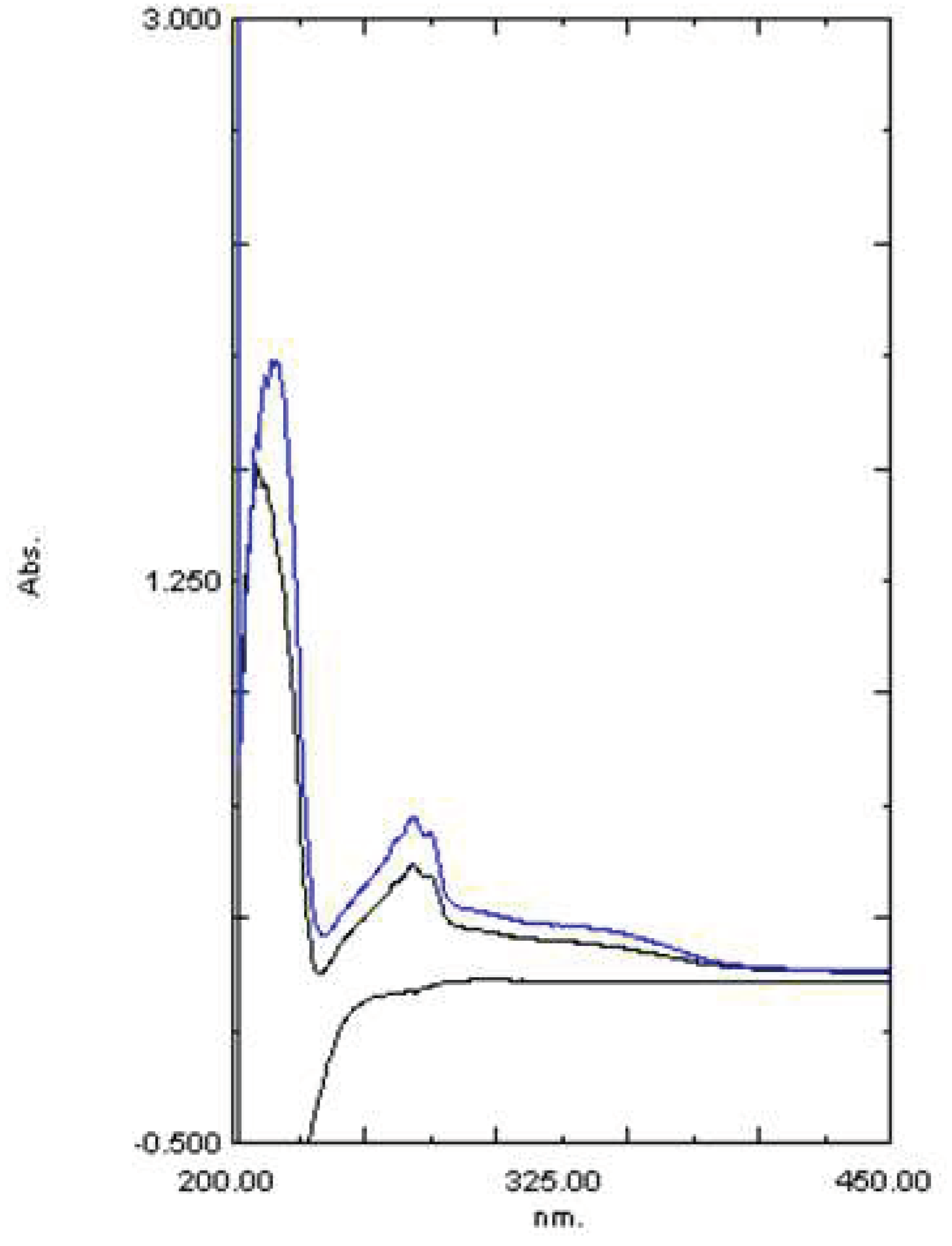
| Functional groups | Peak values | ||
| E. prostrata leaf extract | E. prostrata A dressings. | E. prostrata B dressings. | |
| Alkane | 1376.65 2877.87 2931.46 2971.77 |
1378.70 2933.99 2973.15 |
1378.60 2933.97 2973.45 |
| Alkene | 1654.70 | 1646.91 | 1647.22 |
| Halo compound |
802.76 837.70 |
837.56 | 837.51 |
| E. prostrata | E. prostrata leaf extract : Gelatin (v/v) |
| A | 3:7 |
| B | 2:3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





