Submitted:
26 April 2023
Posted:
27 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
Multispecies biofilm formation
3. Results
Statistical analysis
Results
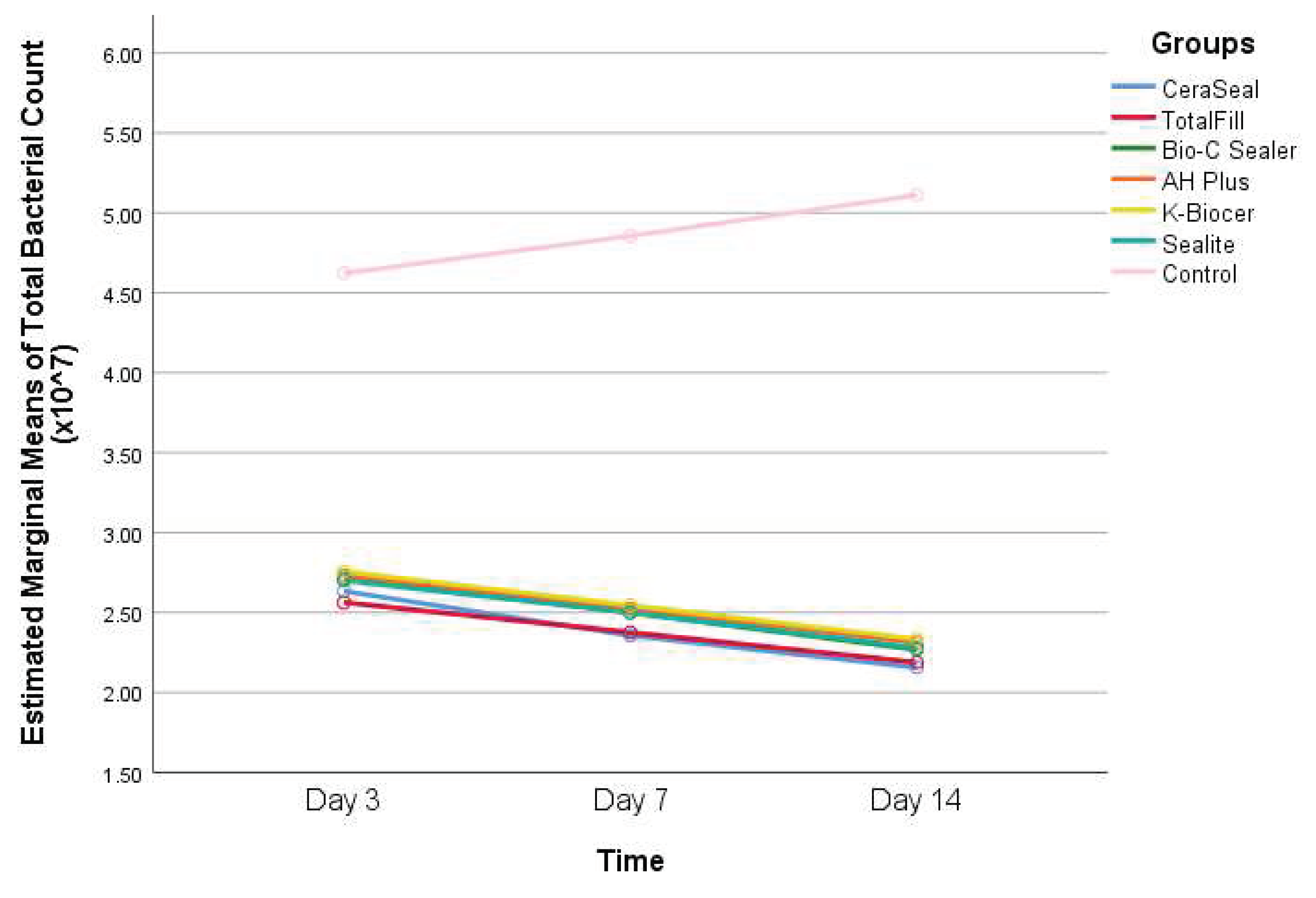
- Descriptive statistics of total bacterial count according to the seven different groups and the three different time points, and results of the comparisons between values of every sealer and the control group within each timepoint, and between the timepoints within each group are shown in Table 1.
| Groups | Time | |||
|---|---|---|---|---|
| Day 3 | Day 7 | Day 14 | p-value | |
|
Control (n=9) Mean ± SD Median (Q1 – Q3) |
4.62 ± 0.73C 4.6 (3.95 – 5.30)C |
4.86 ± 0.72B 4.8 (4.2 – 5.55)B |
5.11 ± 0.71A 5.1 (4.45 – 5.75)A |
<0.001* |
|
CeraSeal (n=9) Mean ± SD Median (Q1 – Q3) |
2.63 ± 0.23A 2.6 (2.4 – 2.75)A |
2.36 ± 0.22B 2.3 (2.2 – 2.5)B |
2.16 ± 0.17C 2.1 (2.05 – 2.5)C |
<0.001* |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
TotalFill (n=8) Mean ± SD Median (Q1 – Q3) |
2.56 ± 0.17A 2.5 (2.42 – 2.67)A |
2.37 ± 0.18B 2.3 (2.22 – 2.55)B |
2.19 ± 0.17C 2.1 (2.1 – 2.35)C |
<0.001* |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
Bio-C Sealer (n=9) Mean ± SD Median (Q1 – Q3) |
2.71 ± 0.23A 2.7 (2.55 – 2.9)A |
2.50 ± 0.21B 2.5 (2.35 – 2.7)B |
2.27 ± 0.19C 2.3 (2.1 – 2.4)C |
<0.001* |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
AH Plus (n=9) Mean ± SD Median (Q1 – Q3) |
2.73 ± 0.24A 2.8 (2.45 – 2.95)A |
2.52 ± 0.26B 2.6 (2.25 – 2.75)B |
2.31 ± 0.26C 2.3 (2.05 – 2.55)C |
<0.001* |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
K-Biocer (n=9) Mean ± SD Median (Q1 – Q3) |
2.76 ± 0.25A 2.8 (2.5 – 2.95)A |
2.54 ± 0.25B 2.6 (2.3 – 2.75)B |
2.33 ± 0.25C 2.4 (2.1 – 2.55)C |
<0.001* |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
Sealite (n=9) Mean ± SD Median (Q1 – Q3) |
2.70 ± 0.22A 2.7 (2.5 – 2.9)A |
2.50 ± 0.22B 2.5 (2.3 – 2.7)B |
2.28 ± 0.22C 2.3 (2.1 – 2.5)C |
<0.001* |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
-
Interpretation of Table 1: - Within the control group, the total bacterial count has significantly increased between day 3 and day 14 (P<0.05).
- –
- Within all the other groups (CeraSeal, TotalFill, Bio-C Sealer, AH Plus, K-Biocer, and Sealite), the total bacterial count has significantly decreased between day 3 and day 14 (P<0.05).
- –
- At days 3, 7, and 14, the total bacterial count was significantly greater in the control group than that of all the other groups (P<0.05).
- As for the total bacterial count, no statistically significant differences were found among the different sealers’ groups at all timepoints (P>0.05).
- Descriptive statistics of Candida Albicans count according to the seven different groups and the three different timepoints, and results of the comparisons between values of every sealer and the control group within each timepoint, and between the timepoints within each group are shown in Table 2.
| Groups | Time | |||
|---|---|---|---|---|
| Day 3 | Day 7 | Day 14 | p-value | |
|
Control (n=9) Mean ± SD Median (Q1 – Q3) |
12.78 ± 2.91 13 (10.5 – 15) |
13.56 ± 2.96 14 (11 – 15) |
14.22 ± 3.38 13 (11 – 17.5) |
0.308 |
|
CeraSeal (n=9) Mean ± SD Median (Q1 – Q3) |
6.89 ± 1.62 7 (5 – 8.5) |
5.67 ± 1.50 6 (4 – 7) |
5.78 ± 1.30 5 (5 – 7) |
0.072 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
TotalFill (n=8) Mean ± SD Median (Q1 – Q3) |
7.00 ± 1.60 7 (5.25 – 8.75) |
7.12 ± 1.25 7 (6.25 – 8) |
6.00 ± 1.07 6 (5.25 – 7) |
0.252 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
Bio-C Sealer (n=9) Mean ± SD Median (Q1 – Q3) |
7.22 ± 1.30 8 (6 – 8) |
6.00 ± 1.50 6 (4.5 – 7.5) |
6.00 ± 0.87 6 (5 – 7) |
0.106 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
AH Plus (n=9) Mean ± SD Median (Q1 – Q3) |
7.33 ± 1.00 7 (6.5 – 8) |
6.22 ± 0.97 6 (5.5 – 7) |
6.56 ± 1.33 7 (5.5 – 7.5) |
0.070 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
K-Biocer (n=9) Mean ± SD Median (Q1 – Q3) |
7.56 ± 1.24 7 (6.5 – 9) |
6.56 ± 0.88 7 (6 – 7) |
6.78 ± 1.20 7 (5.5 – 8) |
0.148 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
Sealite (n=9) Mean ± SD Median (Q1 – Q3) |
6.78 ± 1.56A 6 (5.5 – 8.5)A |
6.44 ± 1.01A 7 (5.5 – 7)A |
4.67 ± 0.87B 4 (4 – 5.5)B |
0.001* |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
-
Interpretation of Table 2: - The Candida Albicans count has increased in the control group between days 3 and 14, but this increase was not statistically significant (P>0.05).
- –
- In the Sealite group, Candida Albicans count has significantly decreased between day 3 and day 14 (P<0.05), and day 7 and 14.
- –
- In the rest of the groups, the count of Candida Albicans has decreased between days 3 and 14 but not significantly (P>0.05).
- –
- Candida Albicans count was significantly greater in the control group at all timepoints, compared to all the other groups (P<0.05).
-
Regarding the Candida Albicans count, a statistically significant difference was observed at day 7 between CeraSeal and TotalFill (P=0.047).
- –
- A statistically significant difference was observed at day 14 between TotalFill and Sealite (P=0.021).
- –
- A statistically significant difference was observed at day 14 between Bio-C Sealer and Sealite (P=0.011).
- –
- A statistically significant difference was observed at day 14 between AH Plus and Sealite (P=0.006).
- –
- A statistically significant difference was observed at day 14 between K-Biocer and Sealite (P=0.002).

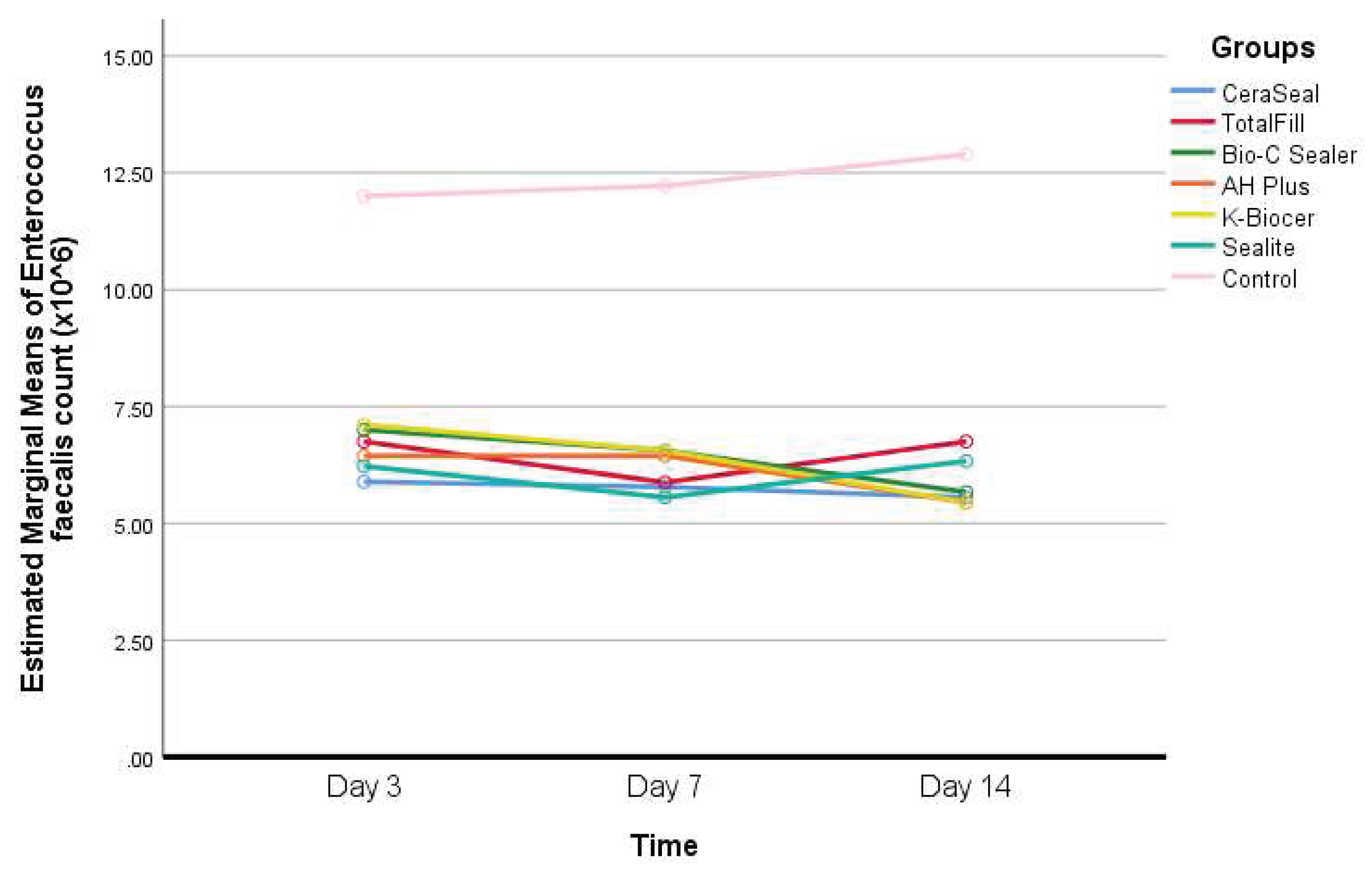
- Descriptive statistics of Enterococcus faecalis count according to the seven different groups and the three different timepoints, and results of the comparisons between values of every sealer and the control group within each timepoint, and between the timepoints within each group are shown in Table 3.
| Groups | Time | |||
|---|---|---|---|---|
| Day 3 | Day 7 | Day 14 | p-value | |
|
Control (n=9) Mean ± SD Median (Q1 – Q3) |
12.00 ± 2.78 13 (9.5 – 15) |
12.22 ± 2.44 13 (10 – 13) |
12.89 ± 2.42 14 (10.5 – 15) |
0.284 |
|
CeraSeal (n=9) Mean ± SD Median (Q1 – Q3) |
5.89 ± 1.54 6 (4.5 – 7.5) |
5.78 ± 1.64 5 (4.5 – 7) |
5.56 ± 1.67 5 (4 – 6.5) |
0.892 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
TotalFill (n=8) Mean ± SD Median (Q1 – Q3) |
6.75 ± 1.03 7 (6 – 7.75) |
5.87 ± 2.10 5.5 (4 – 8.25) |
6.75 ± 1.28 7 (5.5 – 7) |
0.261 |
| p-value (difference with the control group) | <0.001* | <0.001* | 0.018* | |
|
Bio-C Sealer (n=9) Mean ± SD Median (Q1 – Q3) |
7.00 ± 0.87 7 (6 – 8) |
6.56 ± 1.01 6 (6 – 7.5) |
5.67 ± 1.50 6 (4 – 7) |
0.067 |
| p-value (difference with the control group) | 0.003* | <0.001* | <0.001* | |
|
AH Plus (n=9) Mean ± SD Median (Q1 – Q3) |
6.44 ± 1.59 6 (5 – 8) |
6.44 ± 1.24 6 (5.5 – 7) |
5.44 ± 1.67 5 (4 – 6.5) |
0.233 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
K-Biocer (n=9) Mean ± SD Median (Q1 – Q3) |
7.11 ± 0.93 7 (6.5 – 7.5) |
6.56 ± 1.42 7 (5.5 – 8) |
5.44 ± 1.01 5 (5 – 6.5) |
0.053 |
| p-value (difference with the control group) | 0.001* | <0.001* | <0.001* | |
|
Sealite (n=9) Mean ± SD Median (Q1 – Q3) |
6.22 ± 1.56 7 (4.5 – 7.5) |
5.56 ± 0.73 5 (5 – 6) |
6.33 ± 0.71 6 (6 – 7) |
0.217 |
| p-value (difference with the control group) | <0.001* | <0.001* | 0.004* | |
-
Interpretation of Table 3: - In the control group, Enterococcus faecalis count has increased with time, but this increase was not statistically significant (P>0.05).
- –
- In the K-Biocer group, there was a decrease in Enterococcus faecalis with a borderline significance unlike the rest of the groups (P≈0.05).
- –
- At days 3, 7, and 14, the Enterococcus faecalis count was significantly greater in the control group, compared to all the other groups (P<0.05).
- At day 7, a statistically significant difference was observed in Enterococcus faecalis count between Bio-C Sealer and Sealite (P=0.040).
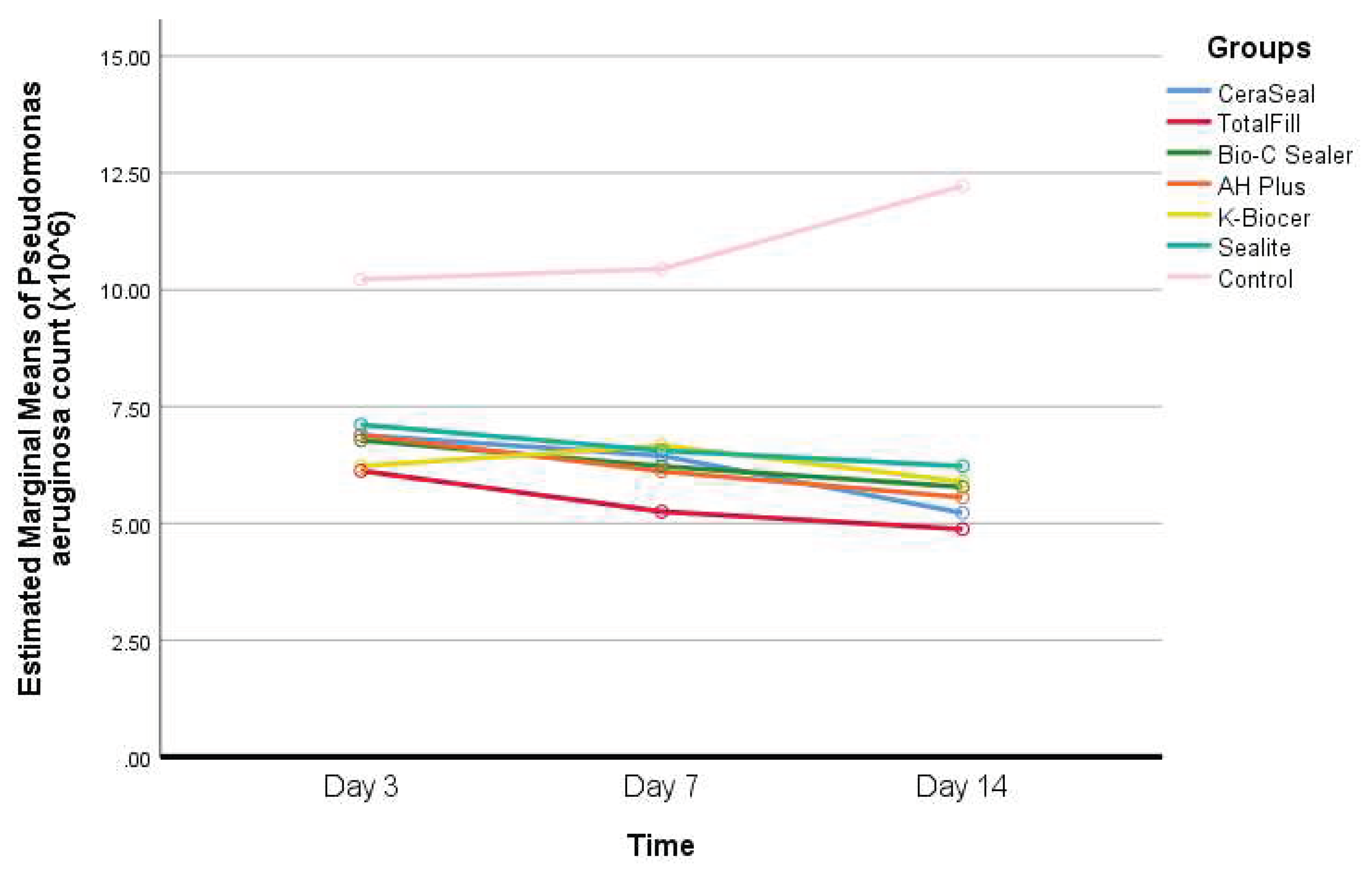
- Descriptive statistics of Pseudomonas aeruginosa count according to the seven different groups and the three different timepoints, and results of the comparisons between values of every sealer and the control group within each timepoint, and between the timepoints within each group are shown in Table 4.
| Groups | Time | |||
|---|---|---|---|---|
| Day 3 | Day 7 | Day 14 | p-value | |
|
Control (n=9) Mean ± SD Median (Q1 – Q3) |
10.22 ± 1.92B 9 (9 – 11.5)B |
10.44 ± 2.24B 12 (8 – 12)B |
12.22 ± 2.82A 12 (10 – 14.5)A |
0.012* |
|
CeraSeal (n=9) Mean ± SD Median (Q1 – Q3) |
6.89 ± 1.69A 7 (5.5 – 8.5)A |
6.44 ± 1.59AB 6 (5.5 – 8)AB |
5.22 ± 1.39B 5 (4 – 7)B |
0.040* |
| p-value (difference with the control group) | 0.001* | <0.001* | <0.001* | |
|
TotalFill (n=8) Mean ± SD Median (Q1 – Q3) |
6.12 ± 1.73 5.5 (5 – 7.75) |
5.25 ± 1.03 5 (4.25 – 6) |
4.87 ± 0.83 5 (4 – 5.75) |
0.199 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
Bio-C Sealer (n=9) Mean ± SD Median (Q1 – Q3) |
6.78 ± 1.39 7 (5.5 – 8) |
6.22 ± 1.56 7 (5 – 7) |
5.78 ± 1.39 6 (4.5 – 7) |
0.356 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
AH Plus (n=9) Mean ± SD Median (Q1 – Q3) |
6.89 ± 1.17 7 (6 – 7.5) |
6.11 ± 1.62 6 (5 – 7.5) |
5.56 ± 1.42 5 (4 – 7) |
0.347 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
K-Biocer (n=9) Mean ± SD Median (Q1 – Q3) |
6.22 ± 1.39 6 (5 – 7.5) |
6.67 ± 1.22 6 (6 – 7.5) |
5.89 ± 1.90 6 (4 – 8) |
0.462 |
| p-value (difference with the control group) | 0.001* | 0.001* | 0.001* | |
|
Sealite (n=9) Mean ± SD Median (Q1 – Q3) |
7.11 ± 1.54 7 (5.5 – 8.5) |
6.56 ± 1.74 7 (5 – 8) |
6.22 ± 1.39 6 (5 – 8) |
0.244 |
| p-value (difference with the control group) | 0.002* | 0.001* | 0.005* | |
-
Interpretation of Table 4: - The increase in Pseudomonas aeruginosa count in the control group was statistically significant between day 3 and day 14 (P<0.05).
- –
- Decreases were observed in Pseudomonas aeruginosa count in the sealers’ groups between days 3 and 14, but this decrease was statistically significant in the CeraSeal group only (P<0.05).
- –
- At days 3, 7, and 14, the Pseudomonas aeruginosa count was significantly greater in the control group, compared to all the other groups (P<0.05).
- At day 7, a statistically significant difference in Pseudomonas aeruginosa counts was observed between TotalFill and K-Biocer (P=0.022).
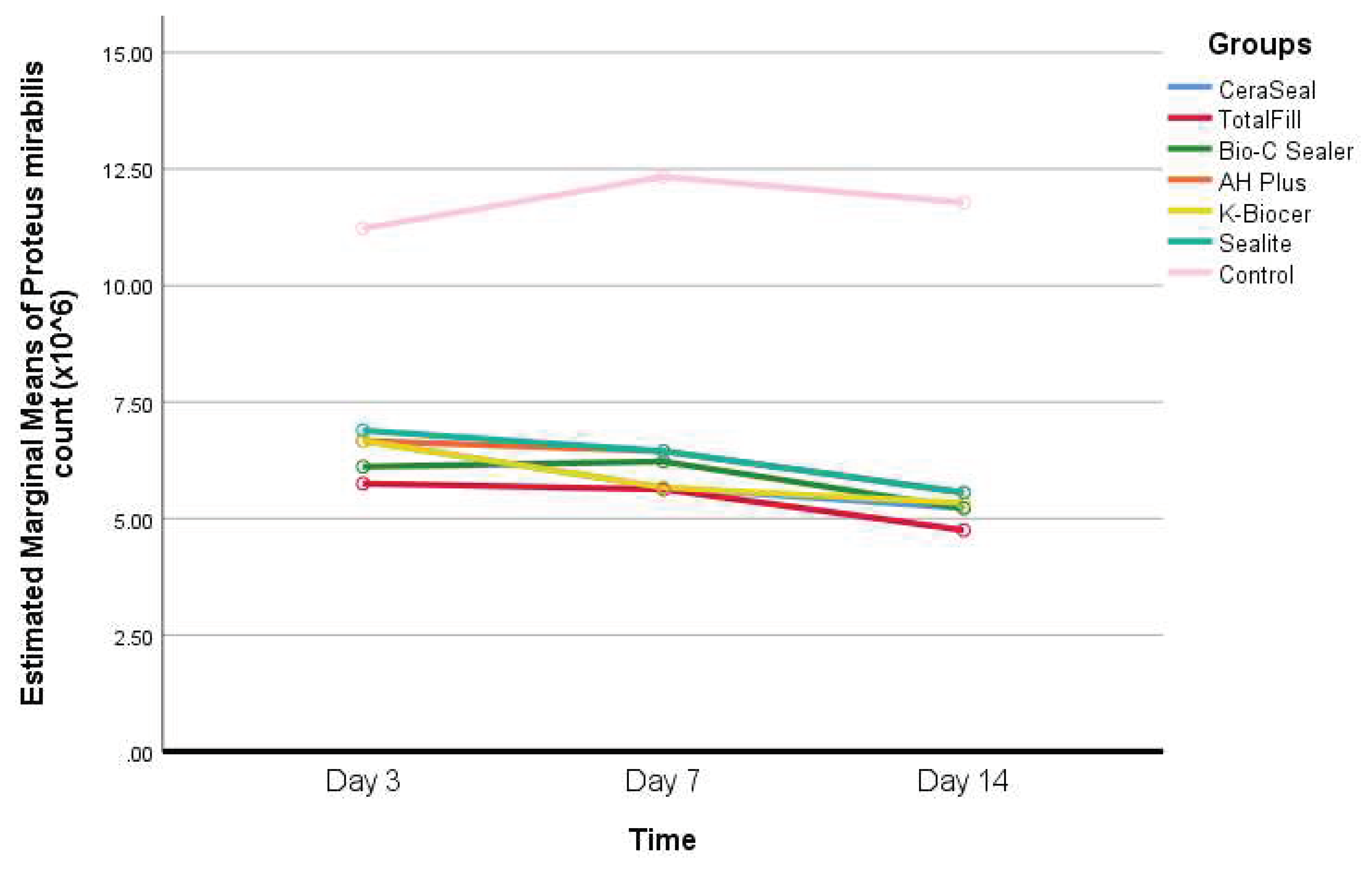
- Descriptive statistics of Proteus Mirabilis count according to the seven different groups and the three different timepoints, and results of the comparisons between values of every sealer and the control group within each timepoint, and between the timepoints within each group are shown in Table 5.
| Groups | Time | |||
|---|---|---|---|---|
| Day 3 | Day 7 | Day 14 | p-value | |
|
Control (n=9) Mean ± SD Median (Q1 – Q3) |
11.22 ± 1.92 11 (10 – 13) |
12.33 ± 1.93 12 (10.5 – 14.5) |
11.78 ± 1.30 11 (11 – 13) |
0.328 |
|
CeraSeal (n=9) Mean ± SD Median (Q1 – Q3) |
6.67 ± 1.41 7 (5.5 – 8) |
5.67 ± 1.58 5 (4 – 7) |
5.22 ± 0.83 5 (4.5 – 6) |
0.177 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
TotalFill (n=8) Mean ± SD Median (Q1 – Q3) |
5.75 ± 2.05 5 (4 – 7.75) |
5.62 ± 1.30 5.5 (4.25 – 7) |
4.75 ± 0.89 4.5 (4 – 5.75) |
0.341 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
Bio-C Sealer (n=9) Mean ± SD Median (Q1 – Q3) |
6.11 ± 1.69AB 6 (4.5 – 7.5)AB |
6.22 ± 1.20A 6 (5.5 – 7)A |
5.22 ± 1.39B 5 (4 – 6)B |
0.040* |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
AH Plus (n=9) Mean ± SD Median (Q1 – Q3) |
6.67 ± 0.87 6 (6 – 7.5) |
6.44 ± 1.67 6 (5 – 8) |
5.56 ± 1.13 6 (4.5 – 6.5) |
0.261 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
K-Biocer (n=9) Mean ± SD Median (Q1 – Q3) |
6.67 ± 0.87 6 (6 – 7.5) |
5.67 ± 1.58 5 (4 – 7) |
5.33 ± 0.87 6 (4.5 – 6) |
0.085 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
|
Sealite (n=9) Mean ± SD Median (Q1 – Q3) |
6.89 ± 1.05 7 (6 – 8) |
6.44 ± 1.94 6 (4.5 – 8.5) |
5.56 ± 1.01 5 (5 – 6.5) |
0.234 |
| p-value (difference with the control group) | <0.001* | <0.001* | <0.001* | |
-
Interpretation of Table 5: - Only in the Bio-C Sealer group, a statistically significant decrease in Proteus Mirabilis count was observed between day 7 and day 14 (P<0.05).
- –
- The differences between timepoints in all the other groups were not statistically significant (P>0.05).
- –
- At days 3, 7, and 14, the Proteus Mirabilis count was significantly greater in the control group, compared to all the other groups (P<0.05).
- As for the Proteus Mirabilis count, no statistically significant differences were found among the different sealers’ groups at all timepoints (P>0.05).
4. Discussion
5. Conclusion
References
- Byström A., Sundqvist G. Bacteriologic evaluation of the effect of 0.5 percent sodium hypochlorite in endodontic therapy. Oral Surg. Oral Med. Oral Pathol. 1983;55:307–312. [CrossRef]
- Waltimo T., Trope M., Haapasalo M., Ørstavik D. Clinical efficacy of treatment procedures in endodontic infection control and one year follow-up of periapical healing. J. Endod. 2005;31:863–866. [CrossRef]
- Duggan J.M., Sedgley C.M. Biofilm formation of oral and endodontic Enterococcus faecalis. J. Endod. 2007;33:815–818. [CrossRef]
- Swimberghe RCD, Coenye TRJ, De Moor G, et al. Biofilm model systems for root canal disinfection: a literature review. Int Endod J. 2019;52(5):604–628. [CrossRef]
- Camilleri J. Will Bioceramics be the Future Root Canal Filling Materials? Curr. Oral Health Rep. 2017;4:228–238. [CrossRef]
- Camps J., Jeanneau C., El Ayachi I., Laurent P., About I. Bioactivity of a Calcium Silicate–based Endodontic Cement (BioRoot RCS): Interactions with Human Periodontal Ligament Cells in Vitro. J. Endod. 2015;41:1469–1473. [CrossRef]
- Silva Almeida L.H., Moraes R.R., Morgental R.D., Pappen F.G. Are Premixed Calcium Silicate–based Endodontic Sealers Comparable to Conventional Materials? A Systematic Review of In Vitro Studies. J. Endod. 2017;43:527–535.. [CrossRef]
- Lim M., Jung C., Shin D.-H., Cho Y.-B., Song M. Calcium silicate-based root canal sealers: A literature review. Restor. Dent. Endod. 2020;45:e35. [CrossRef]
- Zordan-Bronzel C.L., Tanomaru-Filho M., Rodrigues E.M., Chávez-Andrade G.M., Faria G., Guerreiro-Tanomaru J.M. Cytocompatibility, bioactive potential and antimicrobial activity of an experimental calcium silicate-based endodontic sealer. Int. Endod. J. 2019;52:979–986. [CrossRef]
- Urban K., Neuhaus J., Donnermeyer D., Schäfer E., Dammaschke T. Solubility and pH Value of 3 Different Root Canal Sealers: A Long-term Investigation. J. Endod. 2018;44:1736–1740.. [CrossRef]
- Candeiro G.T.d.M., Correia F.C., Duarte M.A.H., Ribeiro-Siqueira D.C., Gavini G. Evaluation of Radiopacity, pH, Release of Calcium Ions, and Flow of a Bioceramic Root Canal Sealer. J. Endod. 2012;38:842–845. [CrossRef]
- Hage, W.; De Moor, R.J.G.; Hajj, D.; Sfeir, G.; Sarkis, D.K.; Zogheib, C. Impact of Different Irrigant Agitation Methods on Bacterial Elimination from Infected Root Canals. Dent. J. 2019, 7, 64. [CrossRef]
- Kapralos V., Koutroulis A., Ørstavik D., Sunde P.T., Rukke H.V. Antibacterial Activity of Endodontic Sealers against Planktonic Bacteria and Bacteria in Biofilms. J. Endod. 2018;44:149–154. [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. Present status and future directions: Microbiology of endodontic infections. Int. Endod. J. 2021. Int Endod J. 2022; 55: 512-530. [CrossRef]
- Garg A, Mala K, Kamath PM. Biofilm models in endodontics-A narrative review. J Conserv Dent. 2021 Jan-Feb;24(1):2-9. Epub 2021 Jul 5. PMID: 34475672; PMCID: PMC8378488. [CrossRef]
- Jhajharia K, Parolia A, Shetty KV, Mehta LK. Biofilm in endodontics: A review. J Int Soc Prev Community Dent. 2015;5:1–2. [CrossRef]
- Alghamdi F, Shakir M. The influence of Enterococcus faecalis as a dental root canal pathogen on endodontic treatment: A systematic review. Cureus. 2020 Mar 13;12(3). [CrossRef]
- Colaco AS. Extreme resistance of Enterococcus faecalis and its role in endodontic treatment failure. Prog Med Sci. 2018;2(1):9-13. [CrossRef]
- Sfeir G, Zogheib C, Patel S, Giraud T, Nagendrababu V, Bukiet F. Calcium silicate-based root canal sealers: A narrative review and clinical perspectives. Materials. 2021 Jul 15;14(14):3965.. [CrossRef]
- Bukhari S., Karabucak B. The Antimicrobial Effect of Bioceramic Sealer on an 8-week Matured Enterococcus faecalis Biofilm Attached to Root Canal Dentinal Surface. J. Endod. 2019;45:1047–1052. [CrossRef]
- Šimundić Munitić M, Poklepović Peričić T, Utrobičić A, Bago I, Puljak L. Antimicrobial efficacy of commercially available endodontic bioceramic root canal sealers: A systematic review. PLoS One. 2019;14(10):e0223575. [CrossRef]
- Šimundić Munitić M, Budimir A, Jakovljević S, Anić I, Bago I. Short-Term Antibacterial Efficacy of Three Bioceramic Root Canal Sealers Against Enterococcus Faecalis Biofilms. Acta Stomatol Croat. 2020 Mar;54(1):3-9. PMID: 32523152; PMCID: PMC7233124. [CrossRef]
- Wang Z, Shen Y, Haapasalo M. Antimicrobial and Antibiofilm Properties of Bioceramic Materials in Endodontics. Materials (Basel). 2021 Dec 10;14(24):7594. PMID: 34947188; PMCID: PMC8706218. [CrossRef]
- Zhang H, Shen Y, Ruse ND. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod. 2009. July;35(7):1051–5. [CrossRef]
- de Souza LC, Neves GS, Kirkpatrick T, Letra A, Silva R. Physicochemical and Biological Properties of AH Plus Bioceramic. Journal of Endodontics. 2023 Jan 1;49(1):69-76. [CrossRef]
- Kharouf N, Arntz Y, Eid A, Zghal J, Sauro S, Haikel Y, Mancino D. Physicochemical and antibacterial properties of novel, premixed calcium silicate-based sealer compared to powder–liquid bioceramic sealer. Journal of Clinical Medicine. 2020 Sep 25;9(10):3096. [CrossRef]
- Harini Priya M, Bhat SS, Sandeep Hegde K. Comparative evaluation of bactericidal potential of four root canal filling material against microflora of infected non-vital primary teeth. J Clin Pediatr Dent. 2010;35(1):23–29. [CrossRef]
- Saha S, Samadi F, Jaiswal JN, et al. Antimicrobial activity of different endodontic sealers: an in vitro evaluation. J Indian Soc Pedod Prev Dent. 2010;28(4):251–257. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





