1. Introduction
Wet oxidation (WO) is a well-known process for treating liquid wastes with a high organic matter content. This process has shown high efficiency and cost-effective behavior compared to those technologies applied to wastes too diluted to be incinerated but too concentrated or poorly biodegradable to be treated by the usual biological or chemical treatments [
1,
2,
3]. These wastes include sewage sludge from municipal or industrial wastewater treatment plants (WWTPs), without dewatering and/or drying the sludge, with a solids content up to 5% and mainly organic. WO process has been studied in different operating conditions, mainly related to temperature (100-330 ºC) and pressure (40-230 bar). In this sense, using pure oxygen, and maintaining the liquid phase, is the way to break C-C bonds of a large amount of toxic and hazardous organic compounds present in the sewage sludge [
4,
5,
6].
The end-products of WO are mainly CO
2 and H
2O, but the generation of short-chain organic acids, such as volatile fatty acids (VFA), as reaction intermediate compounds is common. All of them are easily biodegradable but refractory to oxidation, being acetic acid the most important among them. Also, liquid effluent is free of pathogens, while in the case of nutrients, nitrogen in the form of ammonium and phosphorus in soluble form, as orthophosphates, were detected. The solid fraction is stable and mostly inorganic, with heavy metals and insoluble phosphorus content, while the gaseous effluent is free of the toxic compounds usually produced in the incineration process. For all these reasons, wet oxidation has been installed in about 200 industrial-scale plants worldwide to treat municipal and industrial sewage sludge [
7,
8,
9]. Due to its exothermic nature, self-sustained from a certain organic matter content, energy recovery is one of the main technology's characteristics [
10,
11].
On the other hand, nutrients play an essential role in WWTPs from both a sustainability and legislative point of view. To avoid eutrophication and pollution caused by an excessive nutrient content in the aqueous medium, the discharge legislation in WWTPs regarding nutrients is becoming more and more restrictive, especially concerning to phosphorus discharge.
Phosphorus is an indispensable compound for life, necessary for the formation of genetic chains (DNA and RNA) to constitute the cell membrane of organisms. Thus, it is a crucial compound to the energy metabolism (ATP). Widely used as an agricultural fertilizer, phosphorus is a non-renewable resource obtained from phosphate rock mines. Multiple authors and agencies confirm its depletion in the short-medium term (30-200 years) [
12,
13,
14]. Furthermore, the high geographic concentration of phosphate rock reserves (up to 72%) in Morocco and Western Sahara is a challenge to environmental issues but also of political point of view [
15,
16,
17].
All these aspects have driven the need of phosphorus recovery around the circular economy concept, making sewage sludge a fascinating source for this purpose. After the discovery of struvite deposits formed by spontaneous precipitation in the pipes of several WWTPs, this compound began to receive significant interest. Struvite is a white mineral with a crystalline and pyramidal orthorhombic structure. Also, generally, it is easy to recover and valuable as a slow-release fertilizer [
18,
19,
20]. Current ways of recovering phosphorus in the form of struvite are mainly carried out in the liquid fraction resulting from anaerobic sludge digestion since it concentrates large amounts of P-PO
43- and N-NH
4+ generated by the microorganisms involved in the biological process.
The usual treatments in WWTPs are designed to remove phosphorus from the water line to ensure discharges with a phosphorus concentration below the legislation limits. The addition of metallic coagulants such as FeCl
3, Ca(OH)
2 or Al
2(SO
4)
3 in the primary treatment and settling after secondary treatment means that more than 90% of the phosphorus at the inlet of WWTPs ends up in the sludge line [
21,
22,
23]. Moreover, according to our characterizations, analyzing a mixed sludge from an urban WWTP, it has been found that the solid in the sludge concentrated almost 20 times more phosphorus than that accompanying liquid fraction. This is why recovering phosphorus from the sewage sludge and not only from the liquid fraction is fascinating, as it is currently mainly accomplished.
In this way, several industrial-scale applications have been developed to recover phosphorus in the form of struvite using sewage sludge as precursor. Thus, the AirPrex® process, currently operated at several WWTPs in Germany and the Netherlands, uses the effluent after anaerobic digestion of the sludge without being dewatered. In contrast, the PHOSHPAQ process, implemented in a WWTP in the Netherlands, treats the liquid fraction of the dewatered sludge. Both treatments require the addition of Mg and, through aeration, achieve CO
2 stripping to reach a slightly basic pH (8-8.5) which causes the spontaneous precipitation of struvite in the presence of ammonium [
24,
25,
26]. At the laboratory scale, phosphorus recovery as struvite was studied in a liquid effluent after WO treatment of municipal sewage sludge [
27,
28], process similar to the applied in this work.
Generally, struvite is obtained following Equation 1, using equimolar relations between the three main components in aqueous media. The presence of other compounds, mainly Ca, can compete in the phosphate precipitation process at fundamental pH values, forming different compounds such as hydroxyapatite or monocalcium phosphate [
29,
30,
31,
32].
Struvite precipitation occurs spontaneously at basic pH, the main operative parameter that governs the process, with the excess of Mg, which implies the supersaturation of the medium. The stirring speed and time or temperature parameters also affect the process, but they are kept constant in this work. Furthermore, to consider obtaining struvite on an industrial scale, other parameters specific to the formation and growth of crystalline structures must be regarded to get specific particle sizes [
18,
20]. However, these factors have yet to be considered in this work since it has been intended to demonstrate the technical feasibility of phosphorus recovery in the form of struvite.
In addition, after WO treatment, the stabilized and mostly inorganic solid effluent contains amounts of inorganic phosphorus that can be valorized. Direct agricultural application is not possible due to the toxicity attributed to the high presence of heavy metals in this fraction. Therefore, several studies have focused on extracting the phosphorus from the solid matrix into an aqueous one as soluble orthophosphates, similar to the recovery of incinerated sewage sludge ash (ISSA) and comparable in composition to the solid after the WO process [
33,
34,
35].
Previous studies about the chemical extraction of phosphorus have reported promising results, using ISSA to solid after WO [
36,
38]. Phosphorus leaching was studied in an initial approximation to determine the P-extractable in the solid, aiming to maximize its recovery.
Thus, this work aims to evaluate an integral valorization of phosphorus from the liquid and solid fractions generated after a wet oxidation treatment of sewage sludge. Liquid effluent containing dissolved orthophosphates was used to obtain optimal conditions for the struvite precipitation. Also, solid effluent was treated with acid solutions to maximize the phosphorus leaching.
2. Materials and Methods
2.1. Wet Oxidation Process
WO process was carried out in a pilot plant, owned by ECOLOTUM, Energía Recuperable S.L., and operating in a Spanish wastewater treatment plant (WWTP).
The sewage sludge was obtained by collecting and mixing the sludge generated after the primary and secondary treatments of the WWTP, as mentioned above. Experiments were accomplished over two weeks, taking different samples of the initial sludge and the final effluent throughout the reaction time. As the conditions were kept constant, these samples were mixed to obtain only one input and one output samples, which were characterized and used for the different valorization experiments.
The reactor operates at high pressure and temperature, at subcritical water conditions, but close to it, in a continuous volumetric flowrate of 50 L h-1 and using pure oxygen in excess respect to the stoichiometric amount.
The effluent obtained after the WO process, a mixture of liquid and solid fractions, was collected, conveniently transported, and stored at 5 ºC to carry out the phosphorus valorization experiments further. It was separated by vacuum filtration on filter paper, and the solid collected was dried in an oven at 105 ºC for 12 h before being ground and sieved from 355 to 500 µm.
The inlet sewage sludge and the outlet stream from the WO process were analyzed to evaluate the organic matter and solids content using Standard Methods [
39]. The measured parameters were chemical oxygen demand (COD) by spectrophotometry following the colorimetric method using acid-dichromate commercial vials. Total solids (TS), total volatile solids (TVS) and total fixed solids (TFS) contents were analyzed by gravimetric method. The solid after WO was analyzed by X-ray fluorescence (XRF) and micro-elemental analysis to determine heavy metals and other inorganic compounds, as well as the organic compounds percentage, such as C and N content.
2.2. Struvite Precipitation Experiments
Struvite precipitation experiments were carried out using a Jar Test equipment that allow accomplish several experiments simultaneously. 500 mL of liquid effluent were placed in 600 mL borosilicate beakers, and then, the required amount of the NaOH, 1M solution and the solid MgCl2∙6H2O were dosed. The struvite obtained was separated from the liquid by vacuum filtration on filter paper (0.45 μm), and after washing with ultrapure water, it was dried at room temperature for 24 h before be characterized.
An experimental design was carried out to optimize the operating conditions evaluated; i.e., pH and Mg/P molar ratio, in order to maximize phosphorus recovery in the liquid after precipitation. The operational requirements selected ranged between pH = 7.5 to 9.5 and 1 to 4 Mg/P molar ratio, following previous research studies [
18,
27].
The orthophosphate content was analyzed in the liquid, both before and after precipitation. Total phosphorous was determined to verify that the presence of orthophosphates is the primary form of soluble phosphorous. Both measurements were accomplished by UV-Vis spectrophotometry in a pF11 Macherey-Nagel spectrophotometer, using the molybdate/ascorbic acid blue method. The pH was monitored using a micropH meter 2002 (Crison Instruments).
The chemicals used were NaOH (quality analysis, provided by Merck), and MgCl2∙6H2O (99% purity, provided by Panreac).
Overall, the solid obtained was characterized by micro-elemental analysis, and inductively coupled plasma optical emission spectrometry (ICP-OES) after acid digestion in 1% v/v of nitric acid and heating up to 100 ºC. In addition, some XRF analyses were performed to discard the co-precipitation of other ions.
2.3. Solid Leaching Experiments
In this research, the solid effluent was dried, grounded and sieved. For each experiment, the solid fraction and ultrapure water were placed in a 250 mL Erlenmeyer flask with constant magnetic stirring at 450 rpm, room temperature conditions and measuring pH in continuous mode.
A sequential extraction was carried out to characterize the solid after WO to determine the fractions of phosphorus bound to other compounds in the solid, using 1 g of each solid. Between each extraction, the solid was washed with 20 mL KCl, 1M solution and filtered again. The procedure was carried out following the next steps according to EN-12457:2002 standard [
37,
38]:
- i)
NaHCO3 fraction. 20mL of NaHCO3, 0.5M, to determine the P fraction weakly bound.
- ii)
NaOH fraction. 20mL of NaOH, 0.1M to determine Al and Fe bound P.
- iii)
HCl diluted fraction. 20mL of HCl, 1M to define Ca bound P.
- iv)
HCl concentrated and hot fraction. 12mL of HCl, 10M in a water bath at 80ºC to extract stable P. This fraction needs a large amount of energy to be recovered.
Preliminary tests were accomplished using HCl solution to evaluate P leaching by varying contact time, acid concentration and liquid/solid ratio, using 1 g of solid. After that, an experimental design was performed using four different acids with varying dosages to determine the optimal parameters of phosphorus extraction. Some leachates were also analyzed in order to identify the presence of heavy metals or toxic compounds.
The chemicals used were: KCl (chemically pure crystal, provided by Probys), NaHCO3 (chemically pure, provided by Solvay), HCl (fuming ≥37% quality analysis, supplied by Honeywell Fluka), HNO3 (purity 65-67%, provided by Sigma Aldrich) and citric acid (solid purity >99%, obtained by ProBys).
The solid used as raw material was characterized by X-ray fluorescence (XRF) and micro-elemental analysis techniques, while total phosphorus content was measured after digestion in aqua regia (1:3 HNO3:HCl v/v) following the UNE-EN 13657:2003 standard, using the ascorbic acid blue method. The phosphorus content as orthophosphate was measured in the liquid before and after leaching experiments. In some cases, total phosphorus after leaching was analyzed to compare how much phosphorus was extracted in the orthophosphate form.
3. Results and Discussion
3.1. Wet Oxidation Process
Several wet oxidation (WO) sewage sludge experiments were accomplished operating in a Spanish WWTP. The characterization results of both initial sludge and effluent after the oxidation process have been collected in
Table 1.
After that, a mixed effluent was separated, obtaining liquid and solid fractions used as raw material for both valorization processes. The characterization parameters of the effluent liquid fraction are shown in
Table 2, as well as the results related to the solid fraction have been collected in
Table 3.
As can be observed, in the wet oxidation sewage sludge treatment, conversion values of up to 83% in chemical oxygen demand (COD) and up to 75% in total solids (TS), of which up to 95% were total volatile solids (TVS), associated to organic matter content, could be achieved. Thus, the fixed total solids (TFS) are related to inorganic compounds, making phosphorus the most valuable.
The organic matter in the liquid effluent after the oxidation process is mainly composed of short-chain fatty acids, which are the majority of acetic acid. Therefore, the liquid effluent contains highly soluble and biodegradable organic matter to ensure possible future valorization in a biological process.
Solid effluent is mainly inorganic, being a total carbon content lower than 1%. The main compounds are micronutrients, such as Si, Fe, and Al oxides, but a high phosphorus content was also found.
This phosphorus content, both in the liquid and solid effluents, motivated the valorization studies as an integral mechanism to improve the oxidation process from a sustainable point of view. Thus, WO can be understood as a process of sludge pretreatment for subsequent valorization or recovery in an easier way; in this case, by transforming phosphorus into soluble species in the aqueous media that can be quickly recovered.
3.2. Struvite Precipitation Experiments
The struvite chemical precipitation was the operation selected to recover phosphorus as orthophosphates in aqueous media. The liquid effluent from the oxidation process (characterization parameters described in
Table 2) is the raw material used for struvite precipitation tests.
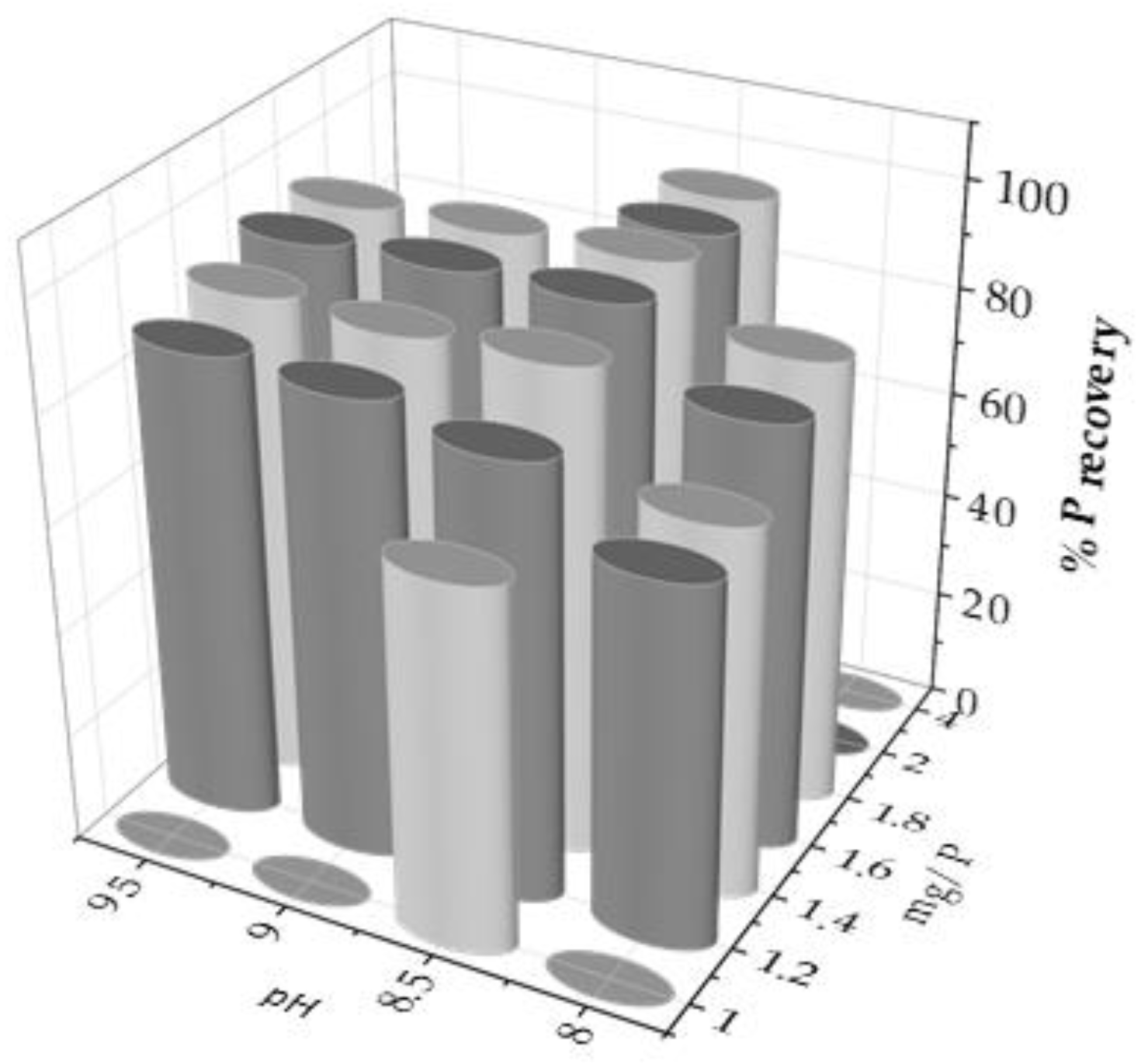
The main objective is to maximize phosphorus recovery, measured in the liquid before and after precipitation. For this purpose, several experiments were performed by varying the two main operating parameters; i.e., pH and Mg/P molar ratio. The experimental design results of the selected operating conditions are shown in
Figure 1.
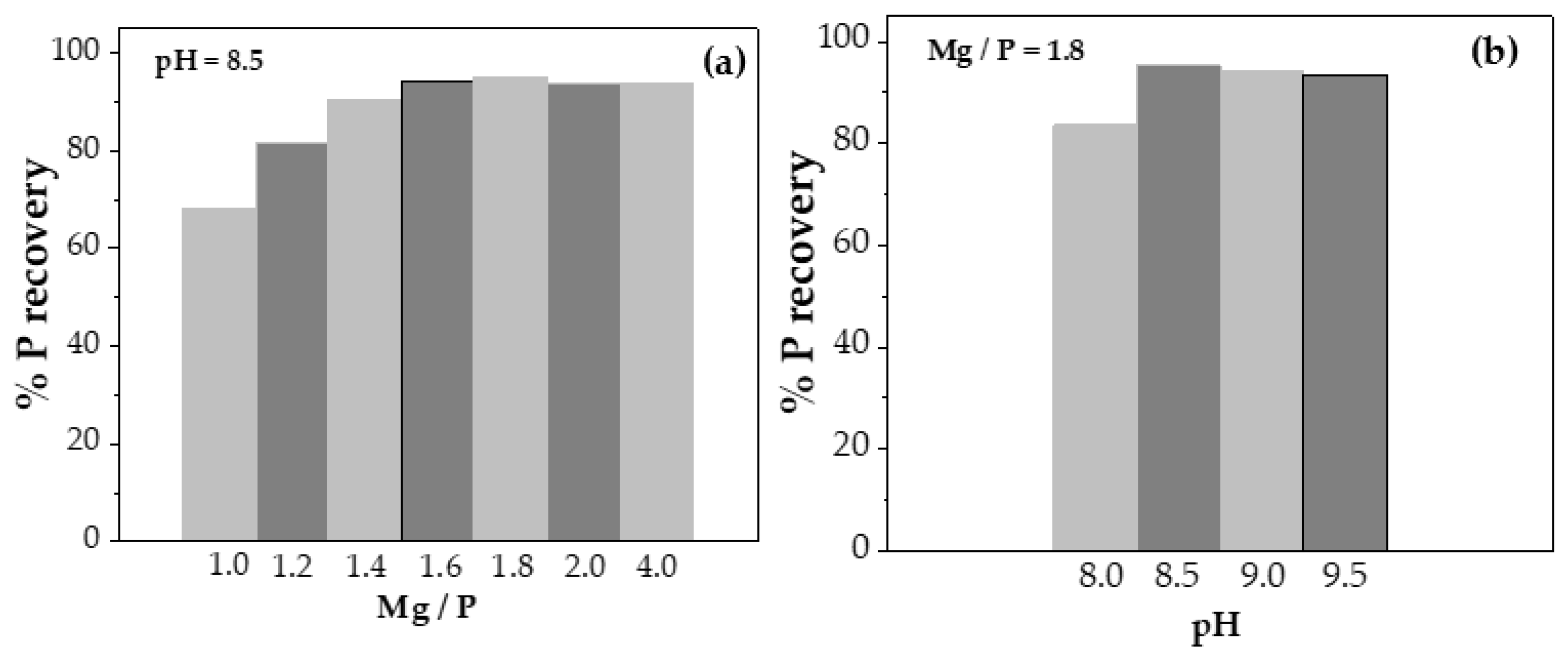
From these experiments, it could be stated that pH is the limiting parameter of the process, considering that at pH 7.5, and lower, no precipitate was obtained. In addition, phosphorus recovery increased up to the pH value of 8.5, while when it was exceeded, P recovery did not increase and even slightly decreased. Therefore, pH 8.5 was the optimum value for P recovery (
Figure 2b).
On the other hand, the Mg/P molar ratio seemed to behave more linearly, always favouring P recovery when the excess of Mg
2+ increased. Therefore, the P recovery percentage was compared as a function of the Mg/P molar ratio at pH 8.5, as shown in
Figure 2(a). Thus, the recovery reached a maximum when the Mg/P molar ratio was 1.8 (%P recovery slightly greater than 95%), increasing clearly from 1.0, then decreasing slightly at higher ratio values, corresponding to 2.0 and 4.0. Consequently, the variation pH experiments were carried out at a Mg/P molar ratio of 1.8.
From this moment on, the struvite obtained under the operating conditions of pH 8.5 and Mg/P molar ratio of 1.8 will be referred to as optimal struvite. Still, it is emphasized that it has been defined only in the range studied and taking into account only phosphorus recovery. An additional economical study will be necessary to determine the parameters that, in addition to maximizing the recovery, it is necessary to minimize the costs [
40].
Then, after precipitation and filtration, the solid obtained was characterized. The three main components (P, N and Mg) concentration values were determined by ICP-OES technique and compared to the literature reference values that define the struvite precipitated purity. At the same time, organic matter content was analyzed by elemental analysis. In addition, ICP-OES analysis of the optimum struvite was carried out and included the presence of other co-precipitated compounds. The results are shown in
Table 4.
As the pH increased, the concentration of both phosphorus and nitrogen decreased in the struvite, slightly reducing the purity values of the precipitate. The reduction could be considered almost negligible, only 5% in both compounds, being the experiments at pH 8.0, the ones that provided the precipitate with the highest content in P and N. Thus, the purity values of both compounds were very high, greater than 90% under optimum conditions. The magnesium content followed a similar trend but with a much lower richness than pure struvite, reaching up to 74%.
In the range of Mg/P molar ratio between 1.2-1.8, the composition of any of the three compounds (P, N, Mg) in the precipitate did not follow any trend. Similar contents were obtained in all the experiments carried out. In the experiments at pH 8.5, where a more significant excess of magnesium was used, it was observed that the content of the three compounds that formed part of the precipitate was reduced as the Mg/P molar ratio increased, being more pronounced at a molar ratio of 4.
On the other hand, the organic matter content behaved oppositely. It was minimal at pH 8 and increased when the pH was raised. However, the percentage of carbon in struvite did not exceed 0.35%. This aspect is essential for the possible application of struvite as a fertilizer.
Regarding to the compounds found in the optimum struvite, which can be considered impurities, the sum of all of them resulted in 2.2%, with Si being the main compound, reaching 1.93%. In addition, micronutrients such as Fe, Cu, Zn or Co were detected. However, small amounts of toxic or dangerous agricultural compounds, such as Cr, As or Hg, were also present [
28,
41].
According to recent European legislation, struvite is no longer considered waste but a potential fertilizer product [
42], offering a new alternative towards the future of the circular economy about the valorization of phosphorus by this way.
3.3. Solid Phosphorous Extraction: Preliminary Experiments
The solid fraction characterization has been shown in
Table 3, and preliminary solid leaching tests were performed following the sequential extraction described above. The average results of the duplicate extraction tests have been presented in
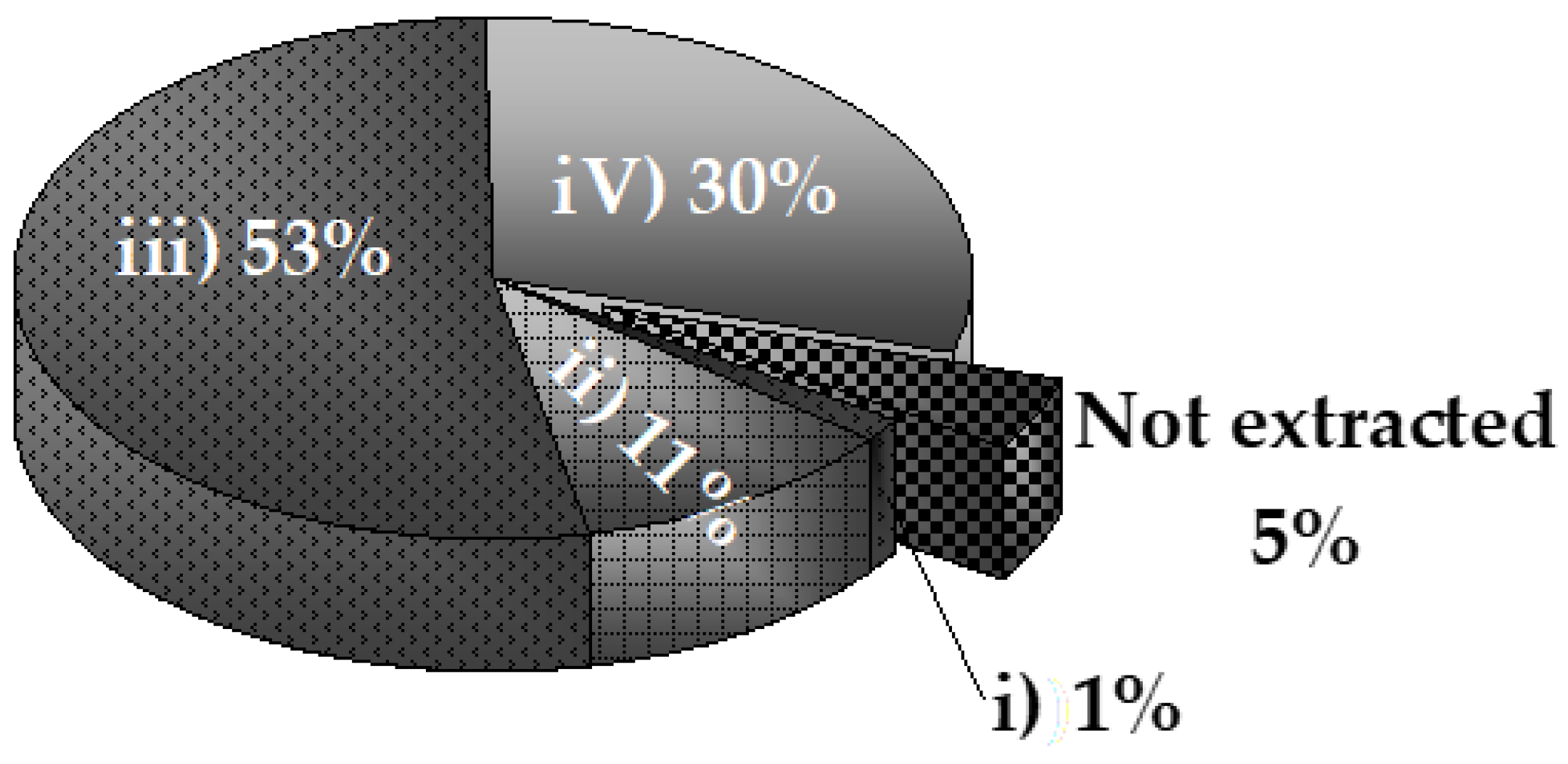
Figure 3.
As can be seen in
Figure 3, most phosphorus in the solid corresponded to that extracted with dilute acid (HCl solution) (53%), related to Ca-bound phosphorus. Then, 30% was stable phosphorus extracted with concentrated acid at hot conditions. Thus, more than 80% of the phosphorus was removed using acid solutions, while bases account for only 12%, of which 11% corresponded to the strong base NaOH, and 1% to the weak base NaHCO
3 solution. Only 5% fraction was not extracted.
Considering this, leaching tests will be carried out using dilute HCl solution. Some operating parameters, such as the contact time between phases, the acid concentration, and the liquid/solid ratio used, were modified in order to determine their effect on phosphorus leaching. In addition, both total phosphorus and phosphorus in the form of orthophosphates will be analyzed in the liquid effluent to determine how much was extracted in this form.
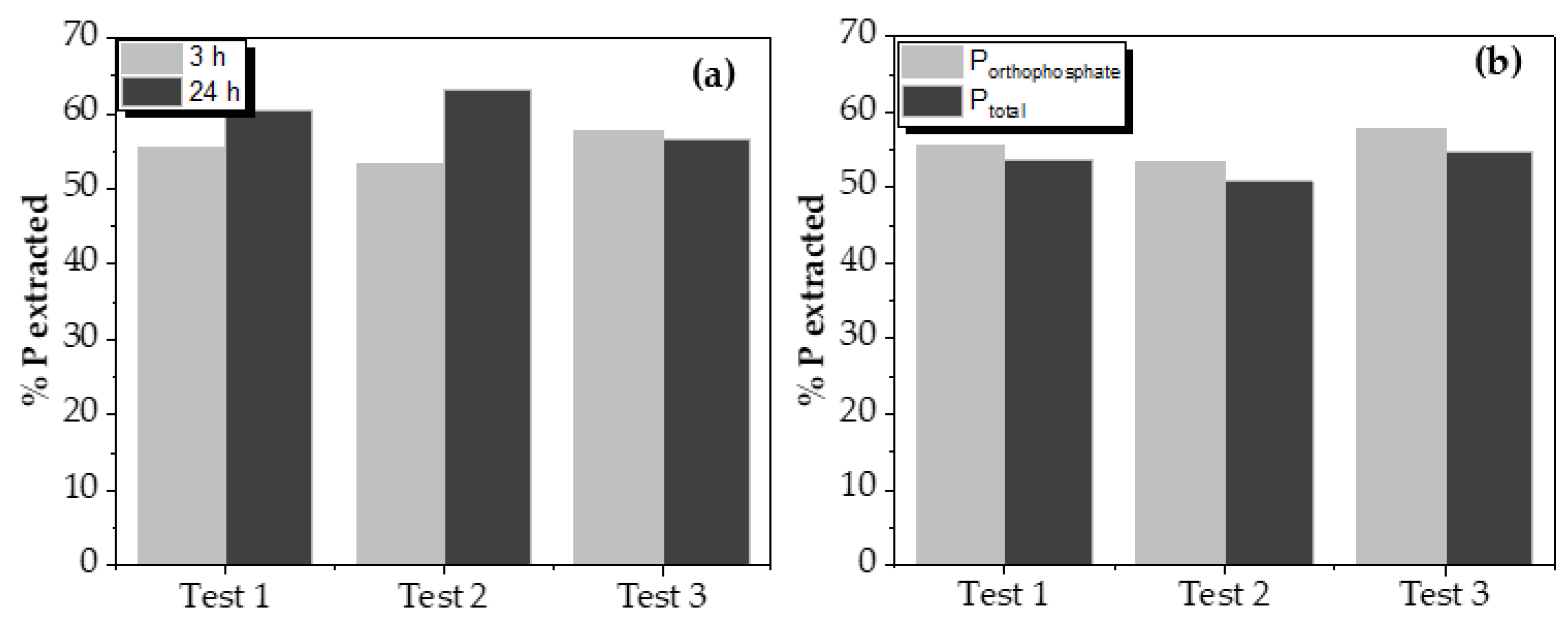
Leaching test results varying contact time and comparing total phosphorus and orthophosphate (P-PO
43-) are shown in
Figure 4. The influence of acid concentration and liquid/solid ratio on phosphorus recovery by leaching can be seen in
Figure 5. The main factor defining the efficiency of each experiment is the amount of phosphorus leached from the solid to the liquid phase.
Increasing the contact time between the two phases from 3 to 24 h did not significantly increase the phosphorus leaching, although, in one of the tests, it reached 10%. Despite this, increasing contact time did not imply a considerable increase in the leached phosphorus quantity. So, it could be assumed that this increase is not such as to justify such a significant difference.
Orthophosphates are the form in which phosphorus was obtained after the leaching process of the solid from the oxidation process. This is important since this leached phosphorus will be recovered as struvite. Measurements even indicated a higher presence of orthophosphates than total phosphorus, which can be assumed as a standard deviation of measurements.
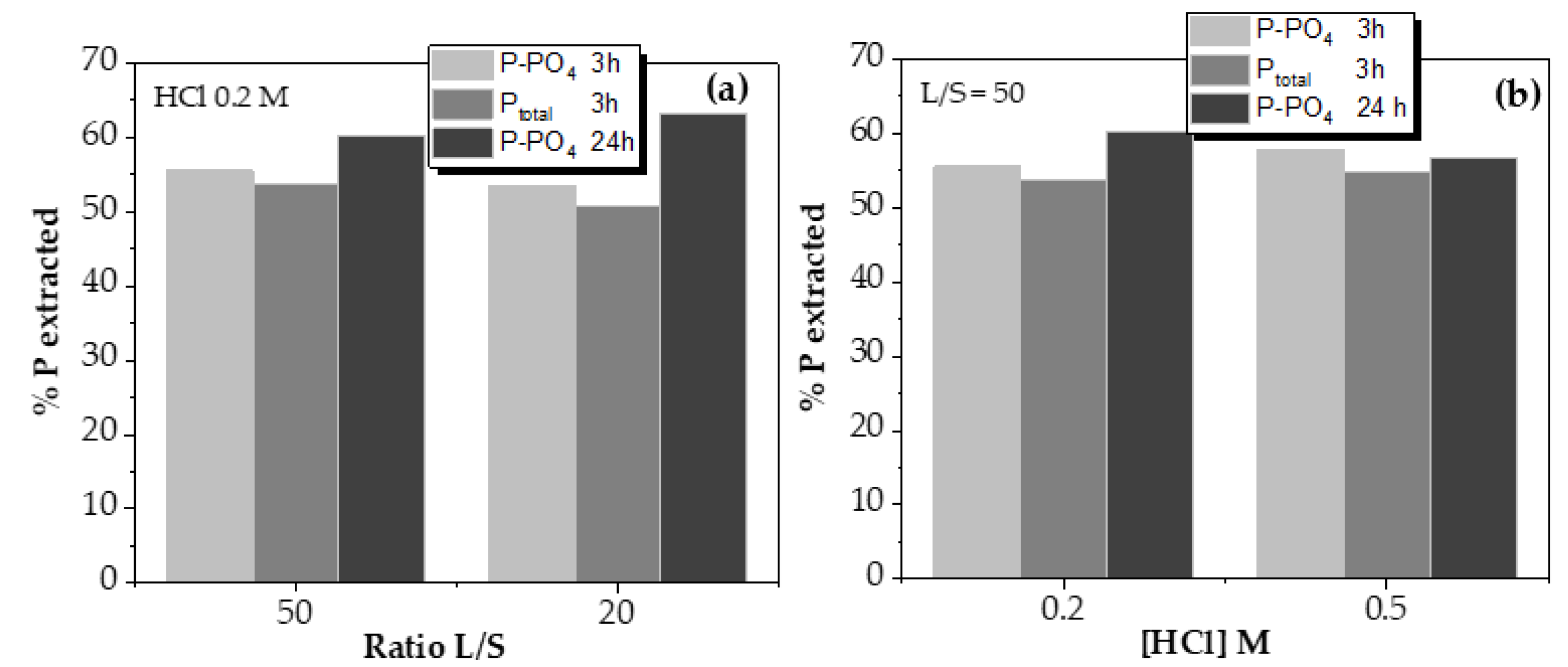
As can be observed, reducing the liquid-to-solid ratio (L/S) from 50 to 20 only reduced the phosphorus leaching over 2% on average, so it could be considered that the amount of liquid is, in both cases, in enough excess not to be a limiting factor in the process. Thus, phosphorus extraction is not affected by the ratio liquid/solid value at the range studied.
The objective of varying HCl solution concentration is to reduce the amount of acid used, evaluating whether phosphorus recovery is seen decreased. Phosphorus leaching tests using 0.5 and 0.2 M HCl solutions reported similar values, with deviations of 2%, which can be considered as negligible. This indicated that the minimum acid dose to maximize the phosphorus leaching recovery might be even lower than 0.2 M HCl, so it is experimented below that value by monitoring the pH, as shown in
Figure 6.
As conclusion, the maximum phosphorus extracted value using HCl 0.2 M solution reached up to 63%, as shown in
Figure 5(a), with a contact time of 24 h. Reducing to 3 h this parameter, the maximum recovery was of 60%, using the same operational conditions. However, the acid dosage is the most critical parameter related to phosphorus extraction, both from a technical and economic point of view. Hence, its reduction is essential for the efficiency of the process.
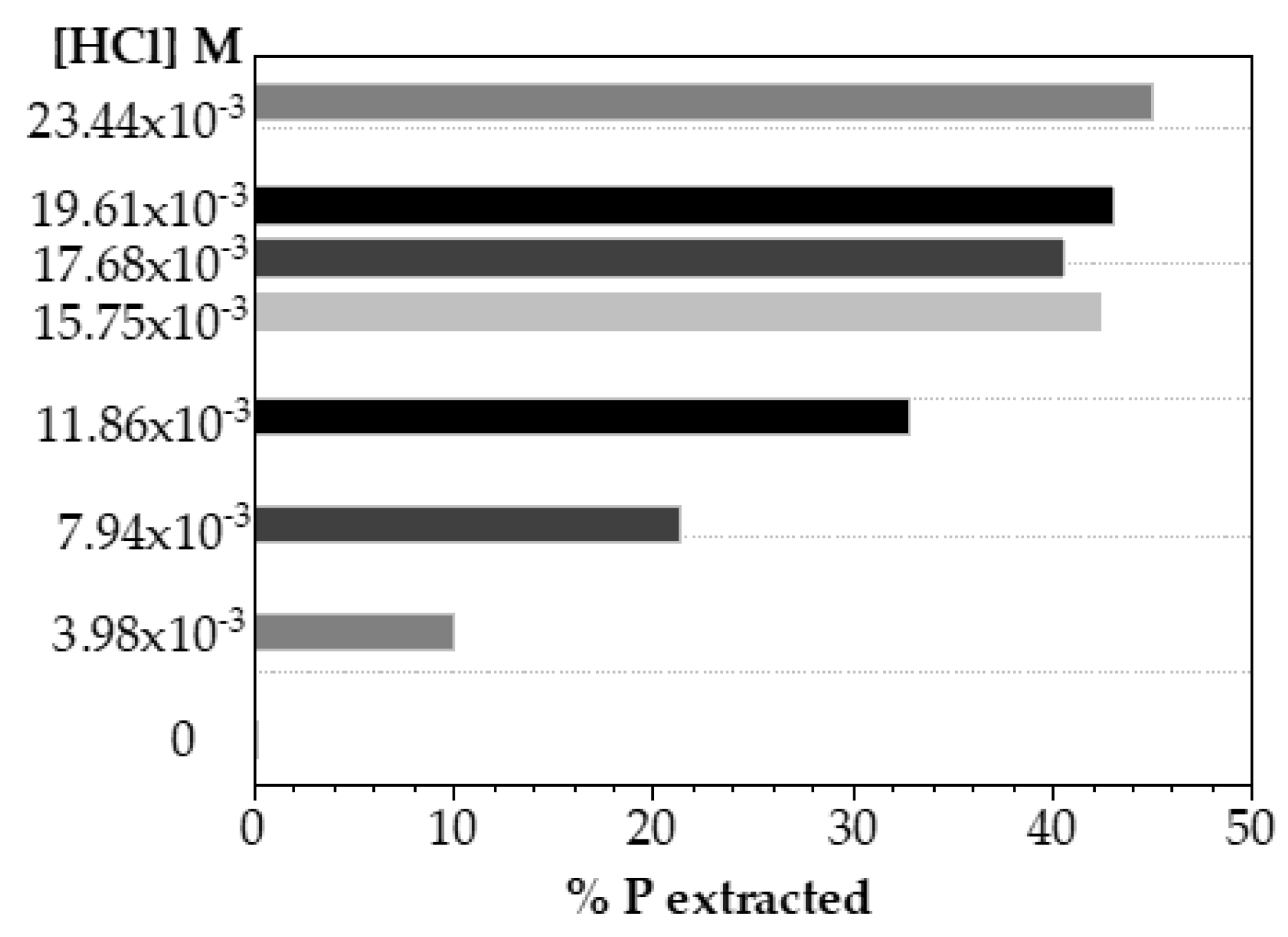
In order to discuss the decreasing of acid concentration for phosphorus extraction, several experiments were carried out using HCl solutions at concentrations considerably lower than those already studied, as shown in
Figure 6. It can be seen that reducing almost 10 times the acid concentration (0.0234 M), it could be reached up to 45% of phosphorus recovery.
To compare phosphorus extracted as a function of the amount of acid solution used, the ratio mL HCl/gP extracted was evaluated. In a quick mass balance, the phosphorus recovery using HCl 0.0234 M solution needed 3.13 mL HCL/gPrecovered, while the use of HCl 0.2 M implied 19.28 mL HCl/gPrecovered.
An economic study not included in this work is necessary to determine which option involved a more cost-effective process. However, based on these data, decreasing the acid dose by 10 times while losing 15% recovery will be more profitable for the process. Therefore, the experimental design is focused on analysing phosphorus recovery at these low doses, using the above mentioned ratio and molar concentrations of different acids for comparison.
3.4. Solid Phosphorous Extraction: Experimental Design
Taking into account the previous conclusions, an experimental design was carried out comparing four different acids (hydrochloric, nitric, sulfuric and citric acid) varying concentrations (in a low range, 0.00794 to 0.0234 M) and monitoring pH while contact time and liquid/solid ratio were kept constant (24 h, and L/S ratio of 50). The obtained results are shown in
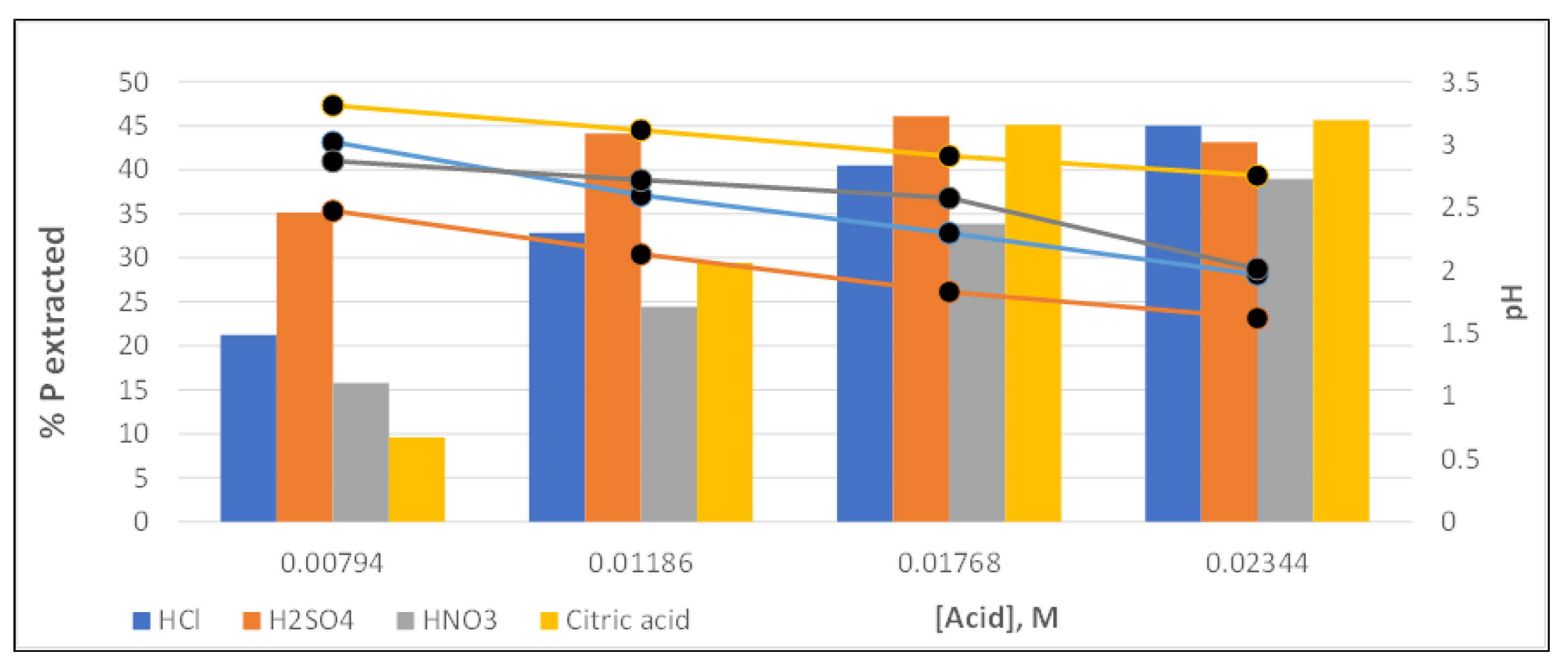
Figure 7.
As can be seen, sulfuric acid is the one that achieved the highest phosphorus recoveries, with more significant differences with the other acids, at low concentration values. This is because this acid is that most fastly reduces the pH solution.
On the other hand, nitric acid is the one that provided the lowest recovery values at all the concentrations studied. Concerning hydrochloric and citric acid, the first achieved higher recoveries than the second at low concentrations, while the trend seemed to be reversed in the range of higher acid doses (especifically, for 0.01768 M,
Figure 7). Citric acid reached the highest phosphorus recovery value (45.66%) at the highest dose (0.0234 M), although the differences found were small, less than 3% among the other three acids tested (hydrochloric, sulfuric and citric).
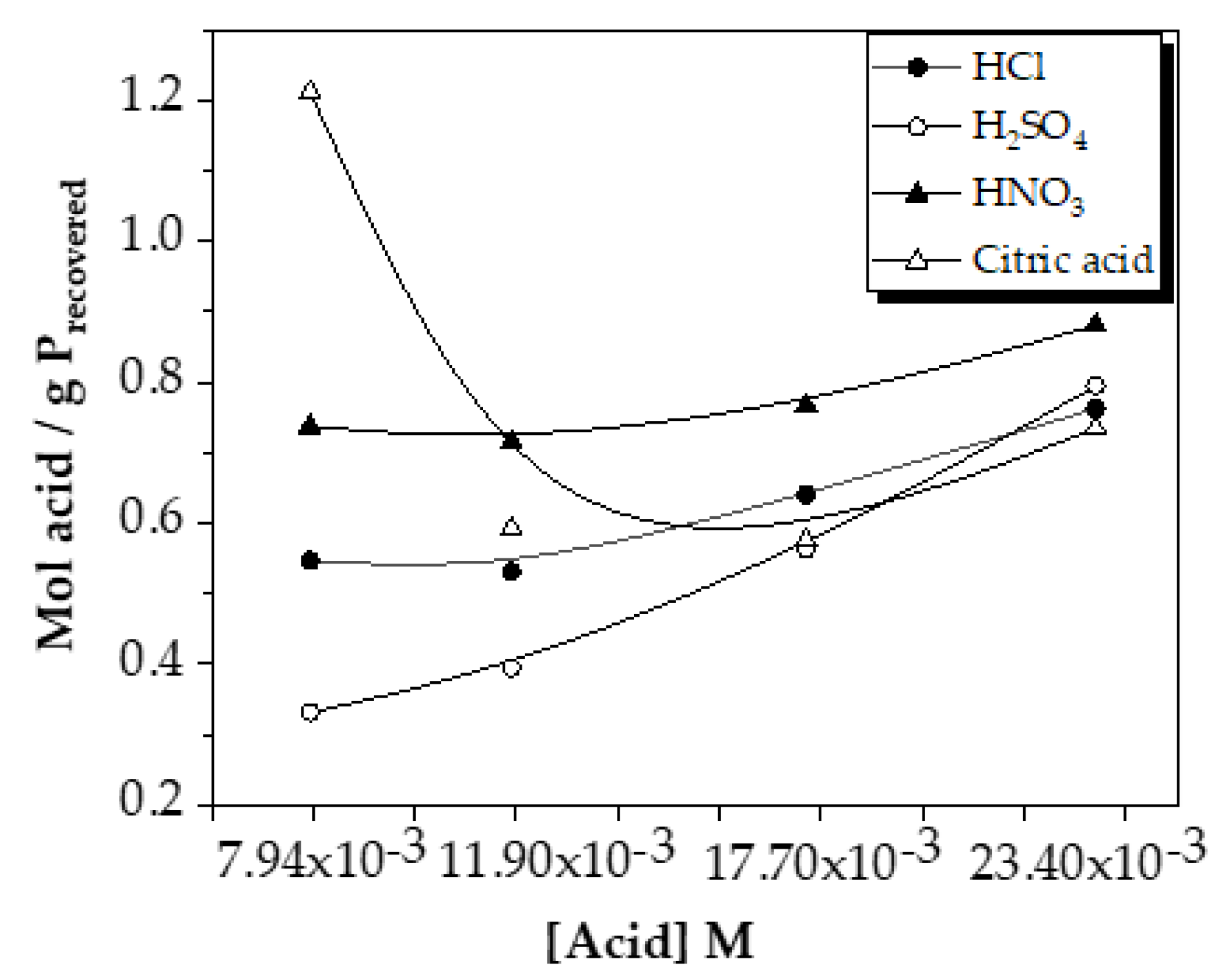
The comparison of mol acid used/g P recovered is shown in
Figure 8. This ratio tries to indicate the amount of acid necessary to extract phosphorus according to the results shown in
Figure 7; the results are expressed in molar values, allowing the comparison between citric acid (solid), versus the rest of the acids (liquid).
As the dosage of each acid increased, the ratio of acid used/phosphorus recovered increased slowly in the case of sulfuric, hydrochloric and nitric acids. This indicated that as phosphorus was extracted, more and more acid was needed, making the extraction less efficient from the point of view of the acid dosage used.
Moreover, the difference between them decreased as the dose increased, reaching similar final values among the four acids (~0.7-0.8 mol acid/g P
recovered). This fact, which had already been observed in
Figure 7, indicated that the recovery, as the dose was increased, did not depend on the type of acid used.
At low doses, sulfuric acid is the most profitable in terms of the ratio of acid used and the amount of phosphorus recovered. In contrast, as the dose increased, the results were very similar.
To determine the most profitable dose in a possible scale-up, an economic balance is required that, in addition to the acid concentration, could evaluate its cost. This aspect will be considered in further future work.
4. Conclusions
The liquid and solid effluents generated in a wet oxidation process contained quantities of phosphorus that could be recovered. In the liquid effluent, 86.11 mg P L-1 was found as orthophosphates, easily recoverable by struvite precipitation. At the same time, in the solid fraction, 68.2 mg P gsolid-1 was found, that can be also extracted as orthophosphates by leaching using acid.
Phosphorus recovery from the liquid effluent by struvite (MgNH4PO4∙6H2O) precipitation reached up to 95.24% at pH 8.5, and an optimum Mg/P molar ratio of 1.8. Increasing the excess of Mg above this value did not increase the phosphorus recovery, as with pH. An economic study would be necessary to define the optimal parameters, also considering the economic costs.
The purity of the struvite related to its nutrients content (P, N) was greater than 90% in the experiments carried out at pH 8.0 and 8.5, decreasing slightly as the operating pH increased. Magnesium presence in precipitated also followed the same trend, but its content concerning the reference mineral was lower, up to 74%. As the Mg/P molar ratio increased above 2.0, the purity of struvite decreased, while below 2.0, no significant differences were appreciated.
The organic matter content in struvite is minimal at lower pH within the range studied, always below 0.35%, valid for agricultural use. Co-precipitated impurities in optimal struvite mainly comprise harmless inorganic compounds such as Si, representing 89% of them. Also, there are micronutrients such as Fe and Cu and small amounts of toxic heavy metals such as Cr, As or Hg.
The initial characterization of the solid effluent was performed in a sequential extraction in which 53% of the phosphate present was leached using a dilute acid solution (HCl, 1M) related to Ca-bound phosphorus. Preliminary experiments were conducted by varying the contact time, acid concentration and liquid/solid (L/S) volumetric ratio.
Increasing the leaching contact time from 3 to 24 h did not significantly increase phosphorus recovery. As for the liquid/solid (L/S) ratio, experiments using 50 and 20 values reported similar results on leached phosphorus, so it could be considered to have enough excess liquid. Experiments using 0.5 and 0.2 M solutions led to similar values of phosphorous extracted, so it could be assumed that its dosage can still be further reduced. Spot measurements of both total phosphorus and orthophosphates were carried out, confirming that it was found in this latter form in its totality.
Comparing experiments using HCl concentrations of 0.2 and 0.0234 M, phosphorus recovery results of 63 and 45%, respectively, were achieved. It means that 19.28 mL HCl/gPrecovered was necessary when 0.2 M solution was used, while 3.13 mL HCl/gPrecovered was used with a 0.0234 M solution, which suggested that low acid solution doses are more profitable.
Among the studied acids, sulfuric acid achieved the highest phosphorus recoveries at the lowest doses, with significant differences for the other acids. Since at higher doses, the values were more similar for all the acids tested (less than 3%, except for nitric acid, which is lower), with the highest recovery using citric acid (45.66%).
Evaluating the ratio of mol acid/gP extracted, sulfuric acid is the most profitable acid at lower doses; then, the recovery values were very similar as the acid concentration increased.
Future experimentation would be accomplished in order to optimize these operating parameters, applying economic criteria.
Author Contributions
Conceptualization, J.G., S.A., J.C. and B.H.; methodology, J.C., S.A.; software, J.C..; validation, J.C..; formal analysis, J.G., S.A., J.C. and B.H.; investigation, J.C.; writing—original draft preparation, J.C., S.A., J.C..; writing—review and editing, J.G., S.A., J.C. and B.H.; visualization, J.G., S.A., J.C. and B.H.; supervision, J.G., S.A. and B.H.; project administration, J.G and B.H.; funding acquisition, J.G and B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by MICINN, through the CATAD3.0 project PID2020-116478RB-I00. In addition, the authors acknowledge funding by the Comunidad de Madrid (Spain) through the Industrial PhD projects (IND2017/AMB-7720 and IND2019/AMB-17114), as well as the REMTAVARES Network (S2018/EMT-4341), the European Social Fund and the Spanish Center for Technological and Industrial Development (CDTI). JCJ is grateful to the CAM for granting his Industrial Doctorate.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Foussard, J.-N.; Debellefontaine, H.; Besomes-Vailhe, J. Efficient elimination of organic liquid wastes: Wet Air Oxidation. J. Environ. Eng. 1989, 115, 367–385. [Google Scholar] [CrossRef]
- Wurm, J.; Poschmann, R.; Thielert, H.; von Morstein, O.; Repke, J.U. Kinetics of ammonium thiosulfate wet air oxidation under high-temperature conditions. Chem. Eng. Technol. 2022, 45, 1588–1597. [Google Scholar] [CrossRef]
- Benitez, F.J.; García, J.; Acero, J.L.; Real, F.J.; Roldan, G. Non-catalytic and catalytic wet air oxidation of pharmaceuticals in ultra-pure and natural waters. Process Saf. Environ. Prot. 2011, 89, 334–341. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, P.; Álvarez-Torrellas, S.; Larriba, M.; Gil, M.V.; Garrido-Zoido, J.M.; García, J. Efficient removal of antibiotic ciprofloxacin by catalytic wet air oxidation using sewage sludge-based catalysts. Degradation mechanism by DFT studies. J. Environ. Chem. Eng. 2023, 11, 109344. [Google Scholar] [CrossRef]
- Hii, K.; Baroutian, S.; Parthasarathy, R.; Gapes, D.J.; Eshtiaghi, N. A review of wet air oxidation and Thermal Hydrolysis technologies in sludge treatment. Bioresour. Technol. 2014, 155, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Vallet, A.; Ovejero, G.; Rodríguez, A.; Peres, J.A.; García, J. Ni/MgAlO regeneration for catalytic wet air oxidation of an azo-dye in trickle-bed reaction. J. Hazard. Mater. 2013, 244, 46–53. [Google Scholar] [CrossRef]
- Slavik, E.; Galessi, R.; Rapisardi, A.; Salvetti, R.; Bonzagni, P.; Bertanza, G.; Menoni, L.; Orhon, D.; Sözen, S. Wet oxidation as an advanced and sustainable technology for sludge treatment and management: Results from research activities and industrial-scale experiences. Dry Technol. 2015, 33, 1309–1317. [Google Scholar] [CrossRef]
- Urrea, J.L.; Collado, S.; Oulego, P.; Diaz, M. Wet oxidation of the structural sludge fractions. J. Clean Prod. 2017, 168, 1163–1170. [Google Scholar] [CrossRef]
- Besagni, G. The effect of operating and design parameters on bubble column performance: The LOPROX case study. Chin. J. Chem. Eng. 2022, 40, 48–52. [Google Scholar] [CrossRef]
- Liu, X.M.; Wan, J.; Zhu, C.B.; Zhou, Z.; Zhang, F.; Zhang, Z.B. Energy optimisation of wet air oxidation reactors with sub-millimetre bubble intensification. Chem. Eng. Sci. 2022, 260, 117864. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, H.; Li, D.B. Maximise methane recovery from sludge anaerobic digestion by combining an optimal wet air oxidation process. Renew. Energy 2021, 179, 359–369. [Google Scholar] [CrossRef]
- De Boer, M.A.; Wolzak, L.; Slootweg, J.C. Phosphorus: Reserves, production, and applications; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Blackwell, M.; Darch, T.; Haslam, R. Phosphorus use efficiency and fertilizers: Future improvement opportunities. Front. Agric. Sci. Eng. 2019, 6, 332–340. [Google Scholar] [CrossRef]
- Panagos, P.; Koningner, J.; Ballabio, C.; Liakos, L.; Muntwyler, A.; Borrelli, P.; Lugato, E. Improving the phosphorus budget of European agricultural soils. Sci. Total Environ. 2022, 853, 158706. [Google Scholar] [CrossRef]
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The future distribution and production of global phosphate rock reserves. Resour. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- El Bamiki, R.; Raji, O.; Ouabid, M.; Elghali, A.; Yazami, O.K.; Bodinier, J.L. Phosphate rocks: A review of sedimentary and igneous occurrences in Morocco. Minerals 2021, 11, 1137. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; El Boukhari, M.E.; Mphatso, C.; Zeroual, Y.; Lyamlouli, K. Conversion of waste into organo-mineral fertilizers: Current technological trends and prospects. Rev. Environ. Sci. Biotechnol. 2022, 21, 425–446. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus recovery from wastewater by struvite crystallization: A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Zhang, T.; He, X.Y.; Deng, Y.X.; Tsang, D.C.W.; Jiang, R.F.; Becker, G.C.; Kruse, A. Phosphorus recovered from digestate by hydrothermal processes with struvite crystallization and its potential as a fertilizer. Sci. Total Environ. 2019, 698, 134240. [Google Scholar] [CrossRef]
- Brye, K.R.; Omidire, N.S.; English, L.; Parajuli, R.; Kekedy-Nagy, L.; Sultana, R.; Popp, J.; Thoma, G.; Roberts, T.L.; Greenlee, L.F. Assessment of struvite as an alternative sources of fertilizer-phosphorus for flood-irrigated rice. Sustainability 2022, 14, 9621. [Google Scholar] [CrossRef]
- Butusov, M.; Jernelöv, A. Phosphorus: An Element that could have been called Lucifer. Springer: Berlin/Heidelberg, Germany, 2013; p. 101. [CrossRef]
- Cañas, J.; García, J.; Hermana, B.; Águeda, V.I.; Álvarez-Torrellas, S. Revision of the most harmful organic compounds present in sewage and sludge. Núñez-Delgado, A., Arias-Estévez, M., Eds.; In Emerging pollutants in sewage sludge and soils. The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Saoudi, M.A.; Dabert, P.; Vedrenne, F.; Daumer, M.L. Mechanisms governing the dissolution of phosphorus and iron in sewage sludge by the bioacidification process and its correlation with iron phosphate speciation. Chemosphere 2022, 307, 135704. [Google Scholar] [CrossRef]
- Schaum, C. Phosphorus: Polluter and resource of the future - Removal and recovery from wastewater. 2018. 17. [CrossRef]
- Korchef, A.; Naffouti, S.; Souid, I. Recovery of high concentrations of phosphorus and ammonium through struvite crystallization by CO2 repelling. Cryst. Res. Technol. 2022, 57, 2200123. [Google Scholar] [CrossRef]
- Zin, M.M.T.; Kim, D.J. Simultaneous phosphorus and nitrogen recovery from sewage sludge ash and food wastewater as struvite by Mg-biochar. J. Hazard. Mater. 2021, 403, 123704. [Google Scholar] [CrossRef]
- Munir, M.T.; Li, B.; Mardon, I.; Young, B.R.; Baroutian, S. Integrating wet oxidation and struvite precipitation for sewage sludge treatment and phosphorus recovery. J. Clean. Prod. 2019, 232, 1043–1052. [Google Scholar] [CrossRef]
- Ovsyannikova, E.; Kruse, A.; Becker, G.C. Valorization of byproducts from hydrothermal liquefaction of sewage sludge and manure: The development of a struvite-producing unit for nutrient recovery. Energy Fuels 2021, 35, 9408–9423. [Google Scholar] [CrossRef]
- Hao, X.D.; Wang, C.C.; Lan, L.; Van Loosdrecht, M.C.M. Struvite formation, analytical methods and effects of pH and Ca2+. Water Sci. Technol. 2008, 58, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Impact of calcium on struvite crystal size, shape and purity. J. Cryst. Growth 2005, 283, 514–522. [Google Scholar] [CrossRef]
- Daneshgar, S.; Cecconet, D.; Capsoni, D.; Capodaglio, A.G. Side-stream phosphorus recovery in activated sludge processes. Water 2022, 14, 1861. [Google Scholar] [CrossRef]
- Wang, Y.C.; Kuntke, P.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N.; Lei, Y. Electrochemically mediated precipitation of phosphate minerals for phosphorus removal and recovery: Progress and perspective. Water Res. 2022, 209, 117891. [Google Scholar] [CrossRef]
- Cieślik, B.; Konieczka, P. A review of phosphorus recovery methods at various wastewater treatment and sewage sludge management steps. The concept of no solid waste generation and analytical methods. J. Clean. Prod. 2017, 142, 1728–1740. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhai, Y.B.; Li, S.H.; Liu, X.M.; Wang, B.; Liu, X.P.; Fan, Y.W.; Shi, H.R.; Li, C.T.; Zhu, Y. Thermal treatment of sewage sludge: A comparative review of the conversion principle, recovery methods and bioavailability-predicting of phosphorus. Chemosphere 2022, 291, 133053. [Google Scholar] [CrossRef]
- Husek, M.; Mosko, J.; Pohorely, M. Sewage sludge treatment methods and P-recovery possibilities: Current state-of-the-art. J. Environ. Manag. 2022, 315, 115090. [Google Scholar] [CrossRef]
- Donatello, S.; Cheeseman, C.R. Recycling and recovery routes for incinerated sewage sludge ash (ISSA): A review. Waste Manage. 2013, 33, 2328–2340. [Google Scholar] [CrossRef] [PubMed]
- Barca, C.; Martino, M.; Hennebert, P.M.; Roche, N. Kinetics and capacity of phosphorus extraction from solid residues obtained from wet air oxidation of sewage sludge. Waste Manag. 2019, 89, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Chen, Y.; Liu, M.; Xu, L. Phosphorus extraction from sludge incinerated bottom ash with hydrochloric acid at low liquid-solid ratio. Environ. Eng. Sci. 2021, 39. [Google Scholar] [CrossRef]
- American Public Health Association. Standard methods for the examination of water and waste water, 21st ed. Washington, D.C. USA, 2005.
- Fernandez-Delgado, M.; Del Amo-Mateos, E.; Garcia-Cubero, M.T.; Coca, M.; Lucas, S. Phosphorus recovery from organic waste for its agronomic valorization: Technical and economic evaluation. J. Chem. Technol. Biotechnol. 2021, 97, 167–178. [Google Scholar] [CrossRef]
- Abeysiriwardana-Arachchige, I.S.A.; Samarasingha, N.; Rosalez, R.; Munasinghe-Arachchige, S.P.; Delanka-Pedige, H.M.K.; Brewer, C.E.; Nirmalakhandan, N. Maximizing phosphorus recovery as biofertilizer in an algal wastewater treatment system. Resour. Conserv. Recycl. 2021, 170, 105552. [Google Scholar] [CrossRef]
- European Parliament. Regulation (EU) 2019/1009 of the European Parliament and of the Council of laying down rules on the making available on the market of EU fertilizing products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation. 2019. 5 June.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).