Submitted:
27 April 2023
Posted:
27 April 2023
You are already at the latest version
Abstract
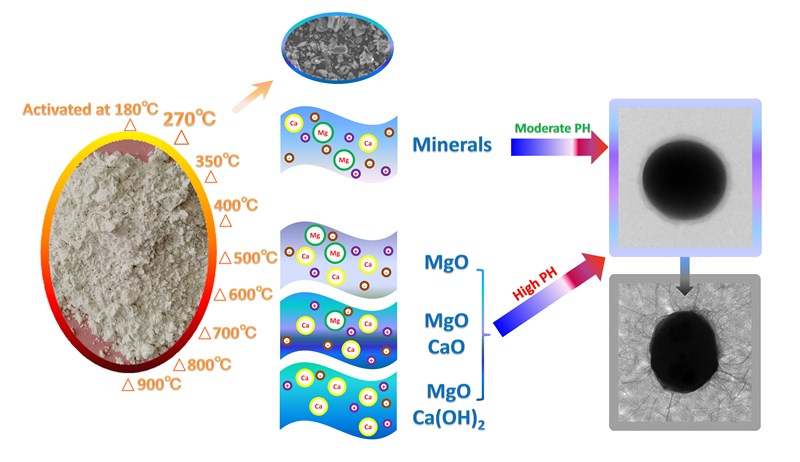
Keywords:
1. Introduction
2. Experimental
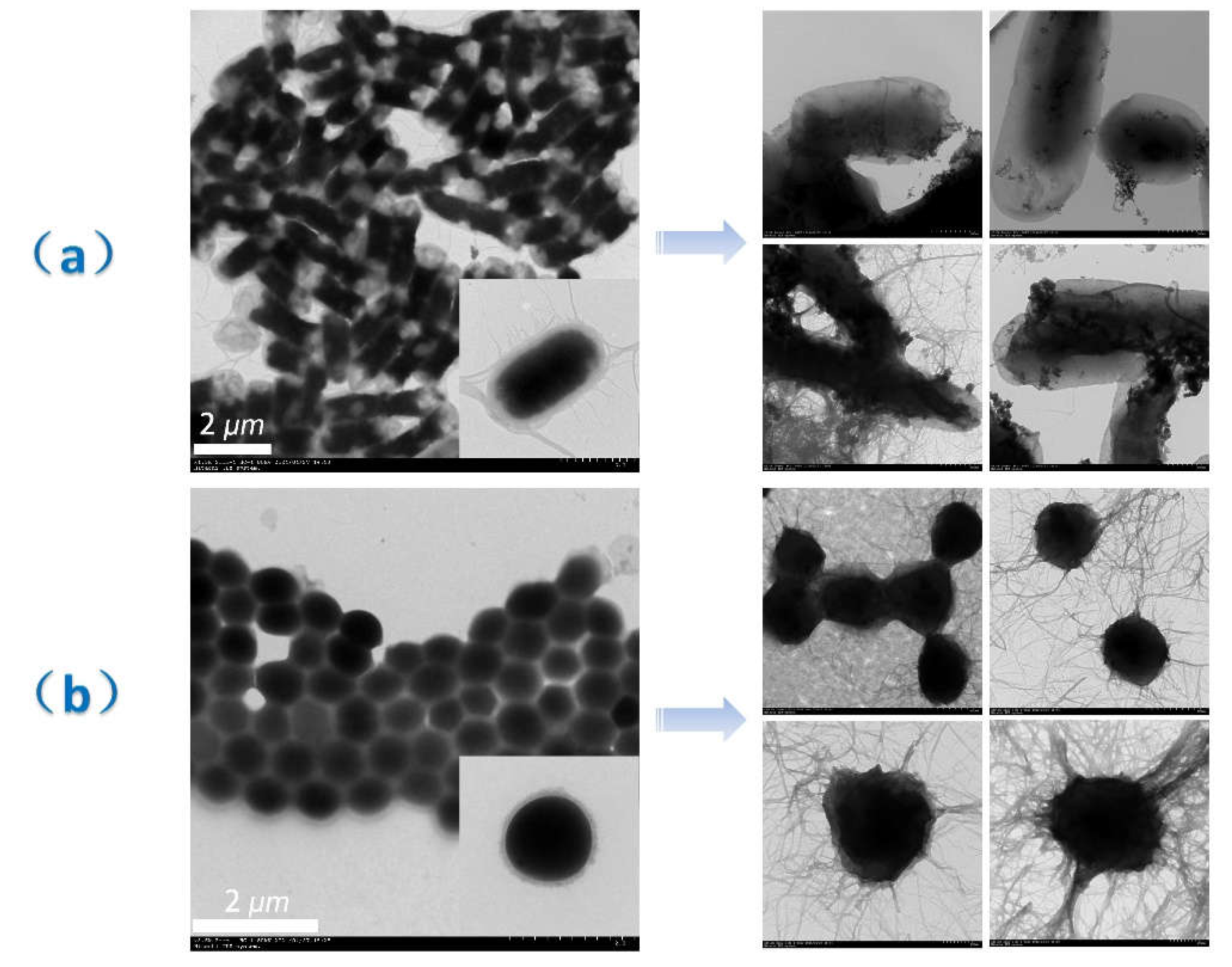
2.1. Materials and Methodology
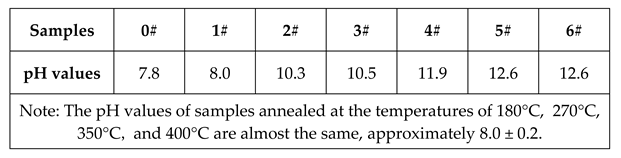
2.2 Metal leaching test and pH value
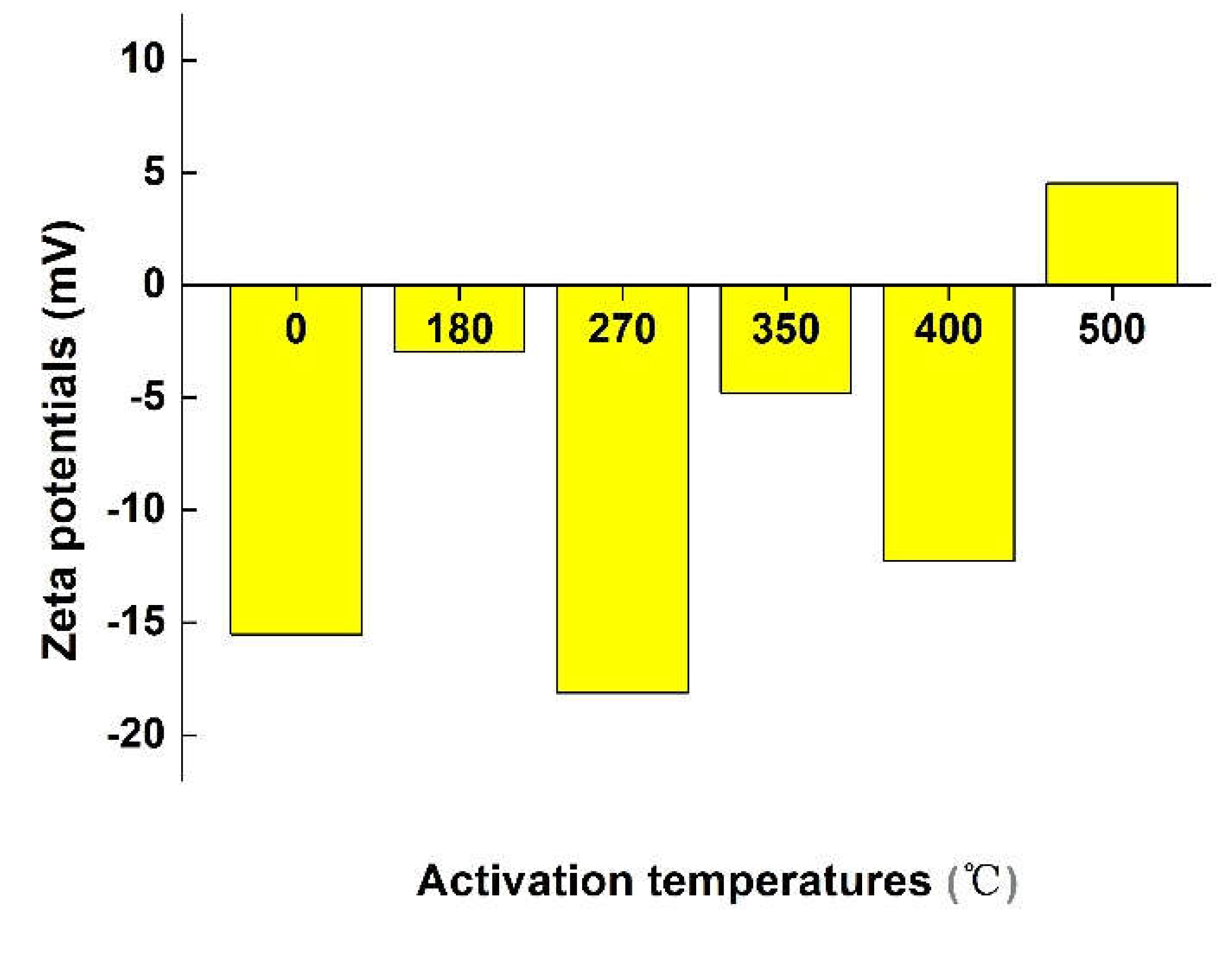
2.3 Zeta Potential
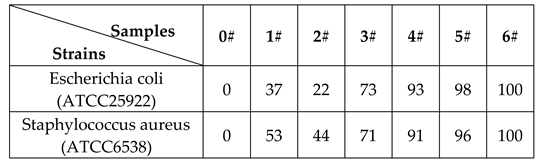
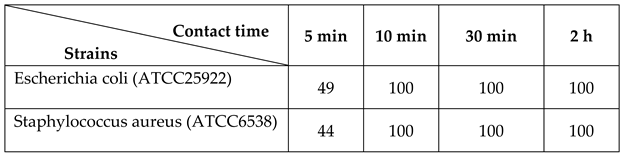
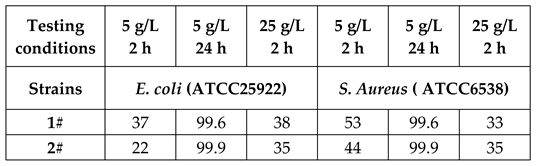
2.4 Bactericidal Test
3. Results and Discussion
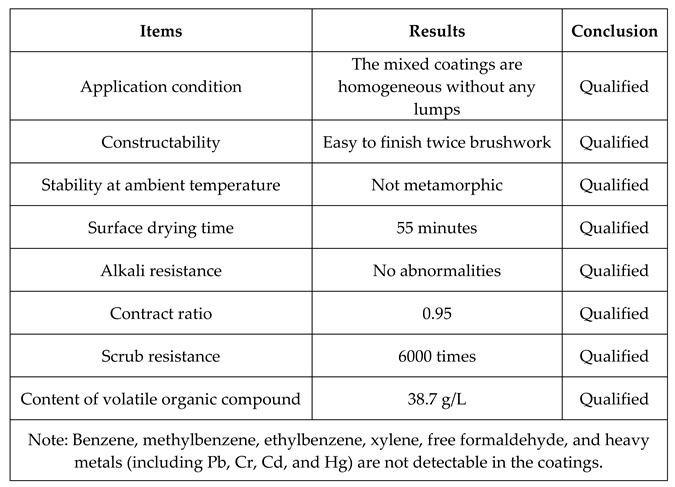
3.1 Application in Antibacterial Coatings
3.2 Outstanding Antibacterial Performance for Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.J.; Guo, X.D.; Zhang, H.M.; Liu, Y.X.; Wang Y., X.; Liu, K.; Liang, H.F.; Ming, W.Y. Recent advances in superhydrophobic and antibacterial coatings for biomedical materials. Coatins 2022, 12, 1469. [Google Scholar] [CrossRef]
- Gawlik-Maj, M.; Babczyńska, A.; Gerber, H.; Kotuła, J.; Sobieszczańska, B.; Sarul, M. Cytotoxicity of Silver-Containing Coatings Used in Dentistry, a Systematic Review. Coatings 2022, 12, 1338. [Google Scholar] [CrossRef]
- Ren, Y.; Fan, T.; Wang, X.; Guan, Y.; Zhou, L.; Cui, L.; Li, M.; Zhang, G. In Situ Reduction of Silver Nanoparticles on the Plasma-Induced Chitosan Grafted Polylactic Acid Nonwoven Fabrics for Improvement of Antibacterial Activity. Coatings 2021, 11, 1517. [Google Scholar] [CrossRef]
- Xia, Y.J.; Jiang, X.Y.; Zhang, J.; Lin, M.; Tang, X.S.; Zhang, J.; Liu, H.J. Synthesis and characterization of antimicrobial nanosilver/diatomite nanocomposites and its water treatment application. Appl. Surf. Sci. 2017, 396, 1760–1764. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Kozlowski, H.; Janicka-Klos, A.; Brasun, J.; Gaggelli, E.; Valensin, D.; Valensin, G. Copper, iron, and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation). Co-ord. Chem. Rev. 2009, 253, 2665–2685. [Google Scholar] [CrossRef]
- Behrangi, S.; Sedláček, I.; Štěrba, J.; Suková, G.; Czigány, Z.; Buršíková, V.; Souček, P.; Sochora, V.; Balázsi, K.; Vašina, P. An Assessment of the Bactericidal and Virucidal Properties of ZrN-Cu Nanostructured Coatings Deposited by an Industrial PVD System. Coatings 2022, 12, 1330. [Google Scholar] [CrossRef]
- Wang, F.; Yao, J.; Si, Y.; Chen, H.L.; Russel, M.; Chen, K.; Qian, Y.G.; Zaray, G.; Bramanti, E. Short-time effect of heavy metals upon microbial community activity. J. Hazard. Mater. 2010, 173, 510–516. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal oxide nanoparticles as antimicrobial agents: a promise for the future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Tsikourkitoudi, V.; Henriques-Normark, B.; A Sotiriou, G. Inorganic nanoparticle engineering against bacterial infections. Curr. Opin. Chem. Eng. 2022, 38. [Google Scholar] [CrossRef]
- Verdier, T.; Bertron, A.; Erable, B.; Roques, C. Bacterial Biofilm Characterization and Microscopic Evaluation of the Antibacterial Properties of a Photocatalytic Coating Protecting Building Material. Coatings 2018, 8, 93. [Google Scholar] [CrossRef]
- Demirci, S.; Yildirim, B.K.; Tünçay, M.M.; Kaya, N.; Güllüoğlu, A.N. Synthesis, characterization, thermal, and antibacterial activity studies on MgO powders. J. Sol-Gel Sci. Technol. 2021, 99, 576–588. [Google Scholar] [CrossRef]
- Sawai, J.; Yoshikawa, T. Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J. Appl. Microbiol. 2004, 96, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, O.; Ohira, T.; Alvarez, K.; Fukuda, M. Antibacterial characteristics of CaCO3–MgO composites. Mater. Sci. Eng. B 2010, 173, 208–212. [Google Scholar] [CrossRef]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2014, 5, 12293–12299. [Google Scholar] [CrossRef]
- Pan, X.H.; Wang, Y.H.; Chen, Z.; Pan, D.M.; Cheng, Y.J.; Liu, Z.J.; Lin, Z.; Guan, X. Investigation of Antibacterial Activity and Related Mechanism of a Series of Nano-Mg(OH)2. ACS Appl. Mater. Interfaces 2013, 5, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Dey, A.; Neogi, S. An insight into the mechanism of antibacterial activity by magnesium oxide nanoparticles. J. Mater. Chem. B 2021, 9, 5329–5339. [Google Scholar] [CrossRef]
- Chaturvedi, U.C.; Shrivastava, R. Interaction of viral proteins with metal ions: role in maintaining the structure and functions of viruses. FEMS Immunol. Med Microbiol. 2005, 43, 105–114. [Google Scholar] [CrossRef]
- Sun, S.S.; Zhang, Y.M. Preparation of Transparent Glass-ceramics Based on Xiuyan Jade Waste. J. Shenyang Univ. Chem. Techn. 2010, 24(1), 44–47. [Google Scholar]
- Hou, M.Y. Preparation and Properties of Alkali Activated Slag-based Foam Concrete. Master Thesis, Shandong University of Science and Technology, China, Jun 3, 2020. [Google Scholar]
- Zhang, N. Effect of Content of Calcium and Magnesium on the Properties and Structure of Simiulated-slag Powders and Their Alkaliactivated Products. Master Thesis, Guangxi University, China, Jul 11, 2020. [Google Scholar]
- Xu, F.; Zou, H.Z. Techniques and Applications of Building Coatings., 1st ed.; China Building Industry Press: Beijing, China, 2009; pp. 88–97. [Google Scholar]
- Huang, Q.; Wei, K.; Xia, H.D. A novel perspective of dolomite decomposition: Elementary reactions analysis by thermogravimetric mass spectrometry. Thermochim. Acta 2019, 676, 47–51. [Google Scholar]
- Fang, Q.; Zhang, H.; Guo, Y. Thermal Decomposition of Dolomite. Adv. Mater. Res. 2010, 177, 617–619. [Google Scholar] [CrossRef]
- Wang, X.L. Study on antibacterial activity and mechanism of metal ions. Master Thesis, South China University of Technology, China, Apr 20, 2015. [Google Scholar]
- Wyness, A.J.; Paterson, D.M.; Defew, E.C.; Stutter, M.I.; Avery, L.M. The role of zeta potential in the adhesion of E. coli to suspended intertidal sediments. Water Res. 2018, 142, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bundeleva, I.A.; Shirokova, L.S.; Pascale Bénézeth, P.; Pokrovsky, O.S.; Kompantseva, E.I.; Balor, S.
- potential of anoxygenic phototrophic bacteria and Ca adsorption at the cell surface: Possible implications for cell protection from CaCO3 precipitation in alkaline solutions. J. Colloid Interf. Sci. 2011, 360(1), 100–109. [CrossRef] [PubMed]
- Tang, H.M.; Liu, Z.S.; Hu, B.L.; Zhu, L.Z. Effects of iron mineral adhesion on bacterial conjugation: Interfering the transmission of antibiotic resistance genes through an interfacial process. J. Haz. Mater. 2022, 435(5), 128889. [Google Scholar] [CrossRef]
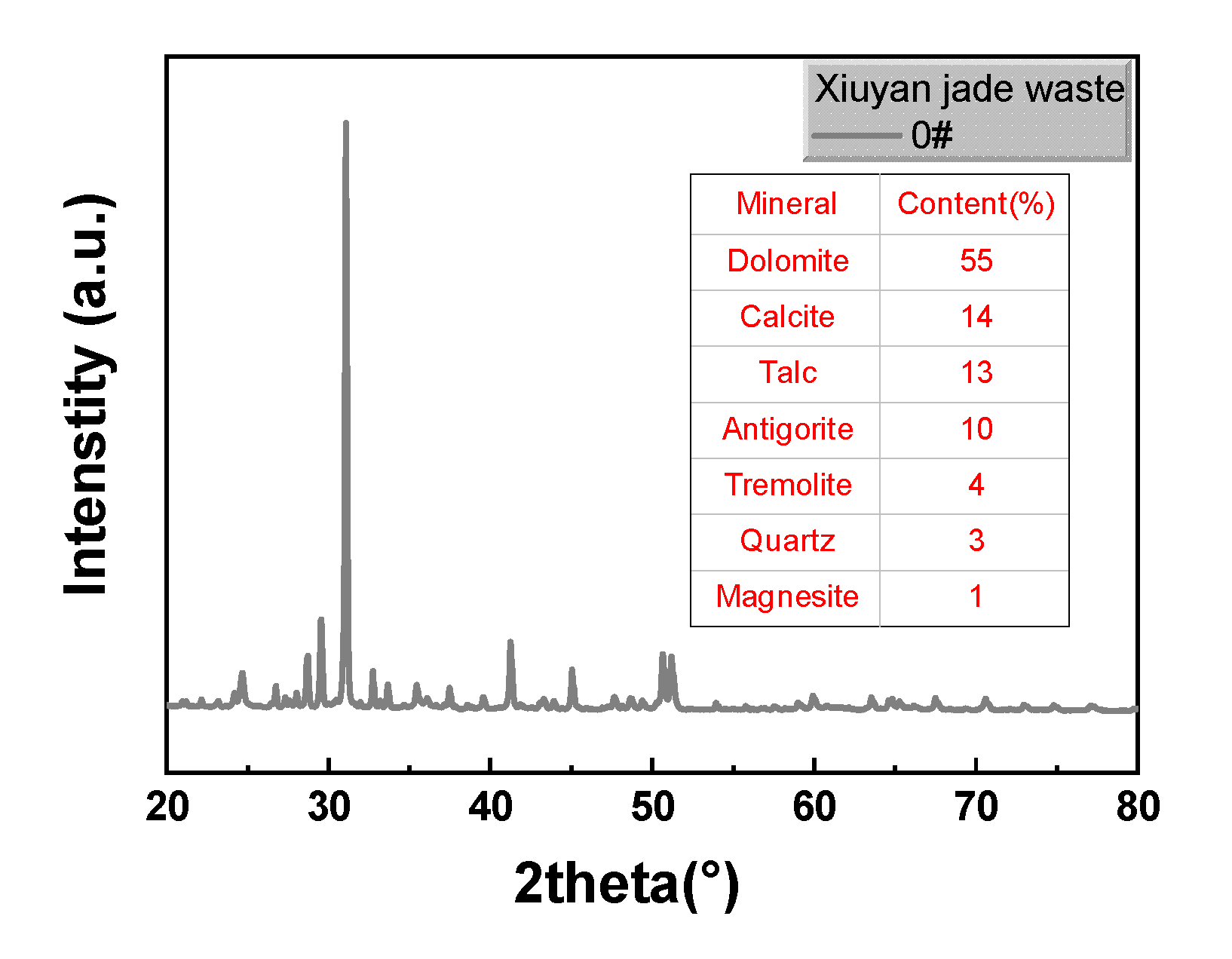
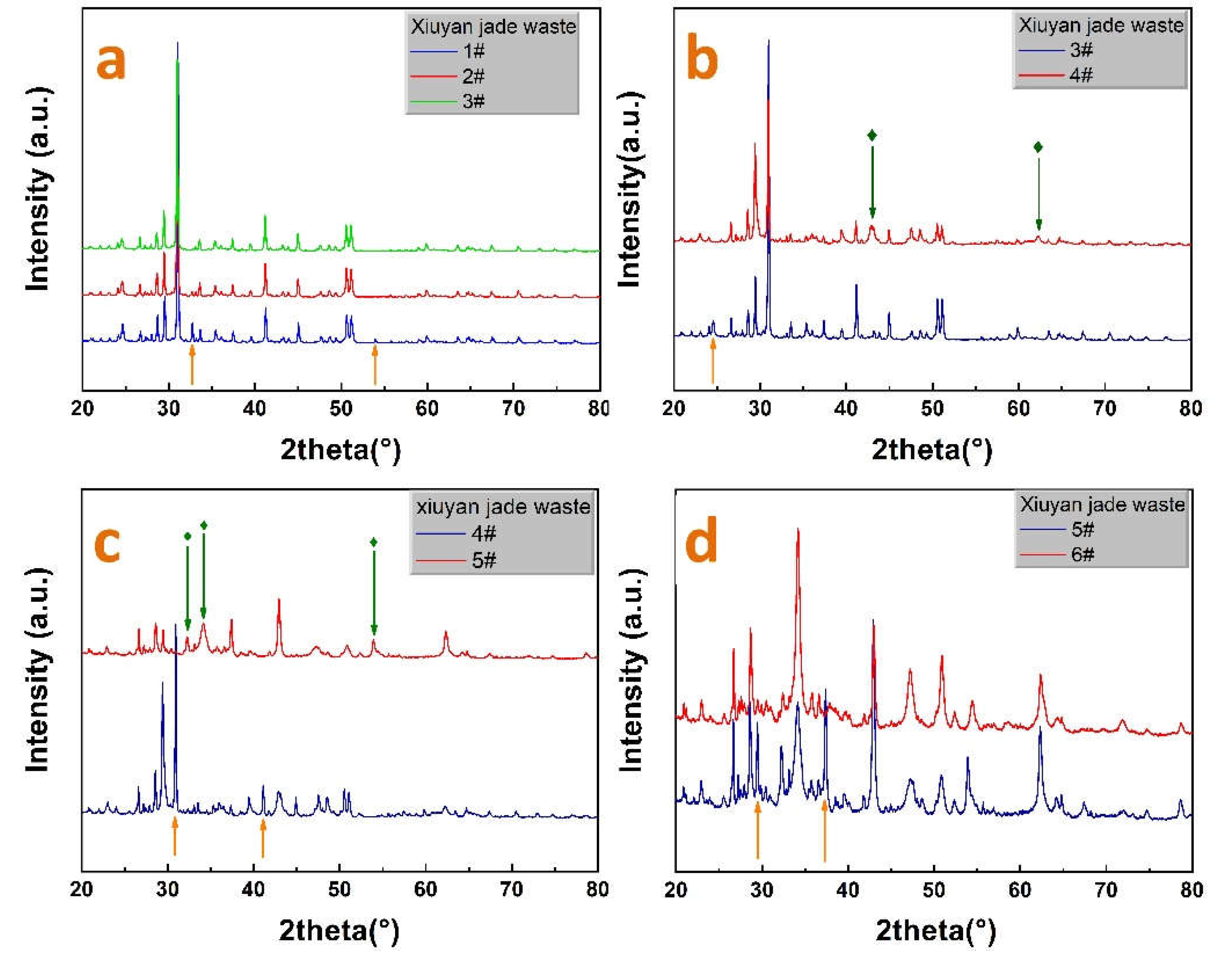
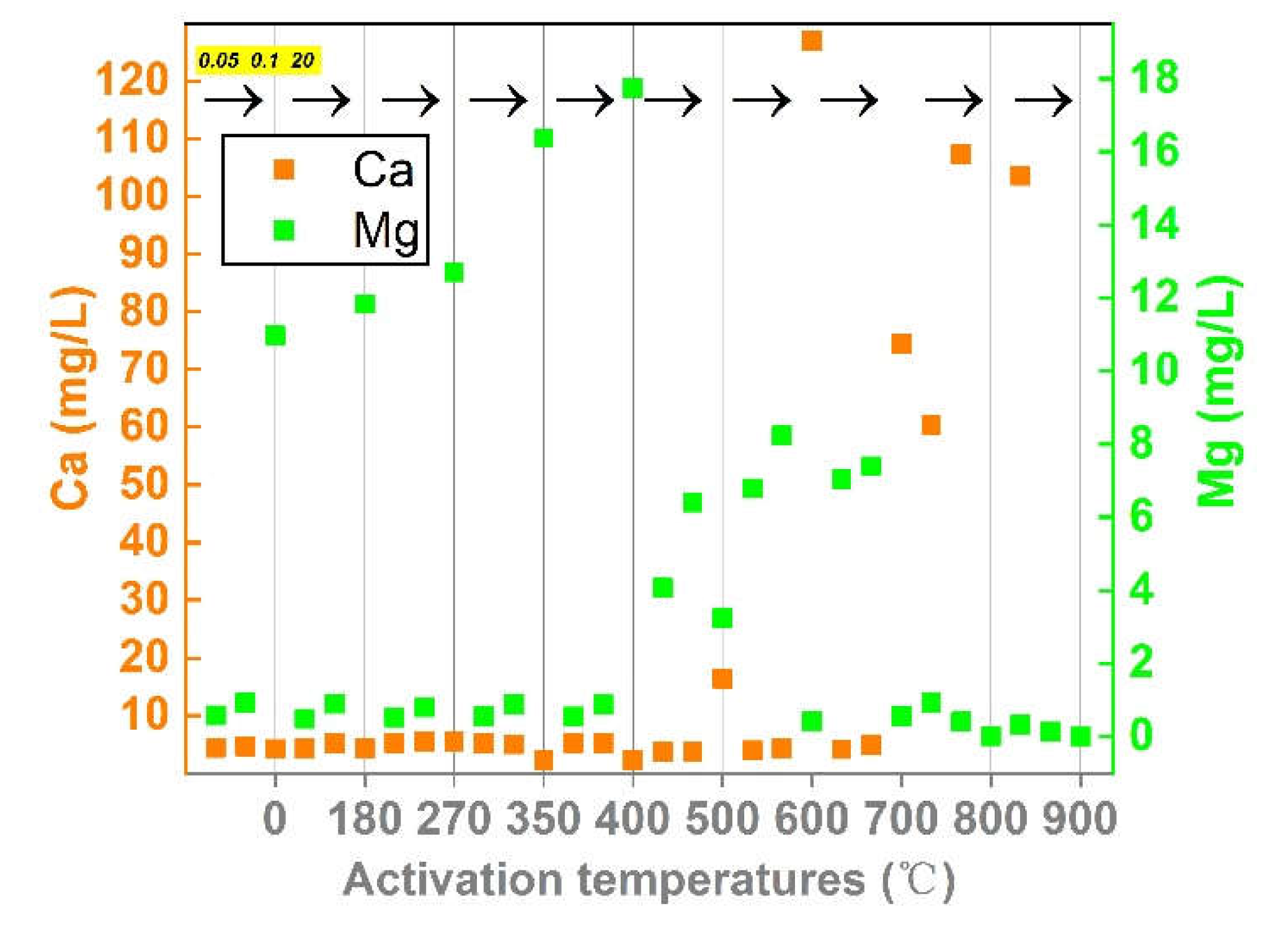
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




