1. Introduction
The prevalence of peanut allergy in the developed world has increased significantly over the last few decades and is now estimated at 1% to 3% of children. Peanuts are therefore one of the most common food allergens in children and allergic reactions to peanuts are often more severe compared to other common food allergens. In addition, peanut allergy is often lifelong and represents a major daily burden that negatively affects quality of life [
1,
2]. Compared to other food allergens, peanut allergy is associated with a higher rate of accidental exposure and anaphylaxis, the latter occurring in up to 50% of all allergic reactions to peanuts in children [
3].
The etiopathogenesis of peanut allergy is not fully understood, although genetic factors play an important role and peanut allergy is much more common in children with a close family member who is allergic to peanuts [
4,
5]. Other risk factors for the development of peanut allergy are hypersensitivity to several other food and/or airborne allergens, chicken egg allergy, vitamin D deficiency, impaired skin barrier in patients with atopic dermatitis and late introduction of peanuts [
6,
7,
8,
9,
10].
Tolerance is more common than peanut allergy in peanut-sensitized patients. However, the prevalence of sensitization, defined by a positive skin prick test (SPT) or detection of specific immunoglobulin class E (sIgE) to whole peanut extract in the general population of children, is estimated to be approximately 10%. The prevalence of sensitization to peanut is even higher in patients with atopic dermatitis or allergic respiratory disease, mainly due to sensitization to non-specific and cross-reactive peanut proteins, such as Ara h8, resulting in mild symptoms such as oral allergy syndrome (OAS) or even tolerance to peanut [
11,
12]. Therefore, patient history is very important in the diagnosis of peanut allergy [
1].
The spectrum of allergic reactions to peanuts ranges from mild local reactions (such as OAS) to generalized skin reactions (e.g., urticaria), angioedema and life-threatening anaphylaxis [
13].
The potential risk factors for severe allergic reactions in peanut allergic individuals are not fully elucidated. In the adult population, an association between severe reactions to peanut and female sex, atopic dermatitis, family history of atopy or peanut allergy, and allergy to house dust mite or birch pollen has been observed [
14].
When an oral food challenge (OFC) was performed to diagnose food allergy in children, the severity of peanut allergy was significantly associated with the amount of peanuts ingested and levels of sIgE to peanut. A weak association was also found for allergic rhinoconjunctivitis and maternal history of asthma. In the same study, older age was reported as a risk factor for the severity of an accidental allergic reaction to peanut [
15]. Asthma has also been reported as an independent risk factor for anaphylaxis in food-allergic children [
16].
In the evaluation of a child with suspected peanut allergy skin prick tests (SPT) and peanut sIgE determination are usually performed as first-line investigations. However, both tests have low specificity (while being highly sensitive) and do not correlate well with the severity of the allergic reaction [
17].
Component resolved diagnostics determine serum IgE antibody levels against specific allergenic peanut protein components. In particular, sIgE against the Ara h2 peanut protein has been shown to be a predictive factor for allergy, and its serum level correlates with the likelihood of clinically relevant allergy and a positive OFC [
4,
18].
Recently, the basophil activation test (BAT) has been shown to improve the specificity of peanut allergy testing. BAT can predict the dose-response relationship when eating peanuts and thus potentially reduce the need for OFC [
19,
20].
The aim of our study was to identify epidemiological, clinical and laboratory characteristics of children that may predict the severity of allergic reactions in patients with peanut allergy.
We hypothesized that some risk factors for the development of peanut allergy and biomarkers for its diagnosis are also associated with the severity of peanut allergy. Therefore, we expected that more severe allergic reactions to peanut would occur in patients with a family history of food allergy or asthma, late introduction of peanut and older age at first reaction, hypersensitivity to other foods or airborne allergens, comorbidities such as atopic dermatitis or allergic respiratory diseases.
2. Materials and Methods
2.1. Participants
We performed a cross-sectional study and included all patients with peanut allergy aged 3 months to 18 years referred to the pediatric allergy clinic of our pediatric department from 1 January 2020 to 31 December 2022. Diagnosis was based on a history of an allergic reaction within 2 hours of peanut consumption and a positive allergy test. In patients with a positive history and a negative allergy test, we performed an open OFC with peanut and if it was negative, we did not include the patient in the study [
21,
22]. We also did not include patients with positive allergy tests and no reaction to peanuts, or those who had never eaten peanuts. When there was no history of anaphylaxis and the sIgE on Ara h2 was < 2 IU/ml, we also performed OFC if the children and their guardians (in children aged < 15 years) agreed to it (22). OFC was performed in 44 (46.%) of the children included in the study.
Epidemiological data such as age, sex, family history of allergy (in parents or siblings), breastfeeding, age at first peanut introduction and age at first allergic reaction to peanut were recorded. Comorbidities (atopic dermatitis, viral-induced wheeze, allergic airway disease) and sensitisation to other foods or airborne allergens were also recorded. The amount of peanuts that triggered the allergic reaction has also been recorded, with the assumption that one peanut kernel weighs around 0.5 g [
22].
Allergic reaction to peanuts was assessed according to the Ring and Messmer scale [
23]. When a patient had more than one allergic reaction to peanut in his/her lifetime, the most severe, including the reaction during OFC, was recorded for further statistical analysis. Patients were classified into three groups for statistical analysis. In the first group, patients with a mild reaction to peanuts, such as OAS or worsening of atopic dermatitis within 2 hours after peanut ingestion, were classified. Patients with urticaria and/or angioedema (grade 1 according to the Ring and Messmer scale) were classified in the second group and patients with anaphylaxis to peanuts (grade 2 or more according to the Ring and Messmer scale) were classified in the third group.
2.2. Methods
Skin prick tests were performed on the volar side of the forearm using a positive control (histamine dihydrochloride 10 mg/ml), a negative control (diluent solution) and a potential allergen (Lofarma SpA, Milan, Italy). Results were recorded after 15 min and the test was positive if the maximum diameter of the wheal was at least 3 mm larger than the negative control. Serum sIgE was measured using the Pharmacia CAP method (Uppsala, Sweden) and a cut-off value of 0,35 IU/ml was used to confirm sensitization.
Open OFC with peanut was performed when appropriate (see above under Participants), and always in a hospital setting. We started with 0.25 g of 100 % peanut butter (or half of one peanut kernel) and doubled the dose every 30 min until signs of a type I hypersensitivity reaction appeared or the final dose was reached (4 g of peanuts in children < 5 years and 8 g in children > 5 years) [
22,
24].
2.3. Ethical approval
The study was approved by the Ethics Committee of the University Clinical Centre Maribor (UKC-MB-KME-25/20) and was conducted in accordance with the Helsinki Declaration of 1975, as revised in Edinburgh in 2000. All participants or their legal guardians (for children under 16 years of age) signed an informed consent form, and additional separate consent was obtained prior to the performance of OFC.
2.4. Statistical analysis
Statistical analysis was performed using IBM SPSS 24.0 software (IBM Inc.). The association of categorical variables (e.g., gender, family history of allergy, presence of comorbidities, sensitization to other foods or airborne allergens) with the severity of the allergic reaction (considered as an ordinal variable) to peanuts was analyzed by Mann-Whitney-U test and chi-square or Fisher exact test for association with anaphylaxis (as opposed to less severe reaction). The association of continuous variables (e.g., duration of breastfeeding, age at first introduction of peanut, age at first allergic reaction to peanut, amount of peanuts triggering the reaction, number of allergens to which the patient is sensitized, size of the wheal at SPT with peanut, blood levels of sIgE to whole peanut and its Ara h2 protein) with the severity of the allergic reaction was analyzed by ordinal regression after to the Kolmogorov-Smirnov normality test. Receiver-operating characteristic (ROC) curve analysis was used to estimate optimal cut-off values to discriminate between anaphylaxis and less severe allergic reaction to peanut for those variables found to be significantly associated with reaction severity. The α level for all tests was set at 0.05 and p values are presented for two-sided tests.
3. Results
3.1. Epidemiological, clinical and laboratory characteristics and the severity of allergic reaction
We enrolled 94 peanut-allergic children, 30 (31.9%) of whom were female. Mild reactions to peanuts (e.g., OAS or exacerbation of atopic dermatitis) occurred in 31 (33.0%) patients, moderate reactions (urticaria and/or angioedema) in 30 (31.9%) patients and anaphylaxis in 33 (35.1%) patients. The epidemiological, clinical and laboratory characteristics of the patients are presented in
Table 1.
A comparison of the epidemiological, clinical and laboratory characteristics of patients with mild allergy, urticaria and/or angioedema and anaphylaxis after peanut consumption is presented in the
Table 2.
When children with mild and moderate reactions to peanuts were combined into one group, the median sIgE level on Ara h2 in this group was 1.51 IU/ml. We also analyzed the association or correlation of all the characteristics presented in
Table 2 with the degree of anaphylaxis, but no significant results were obtained.
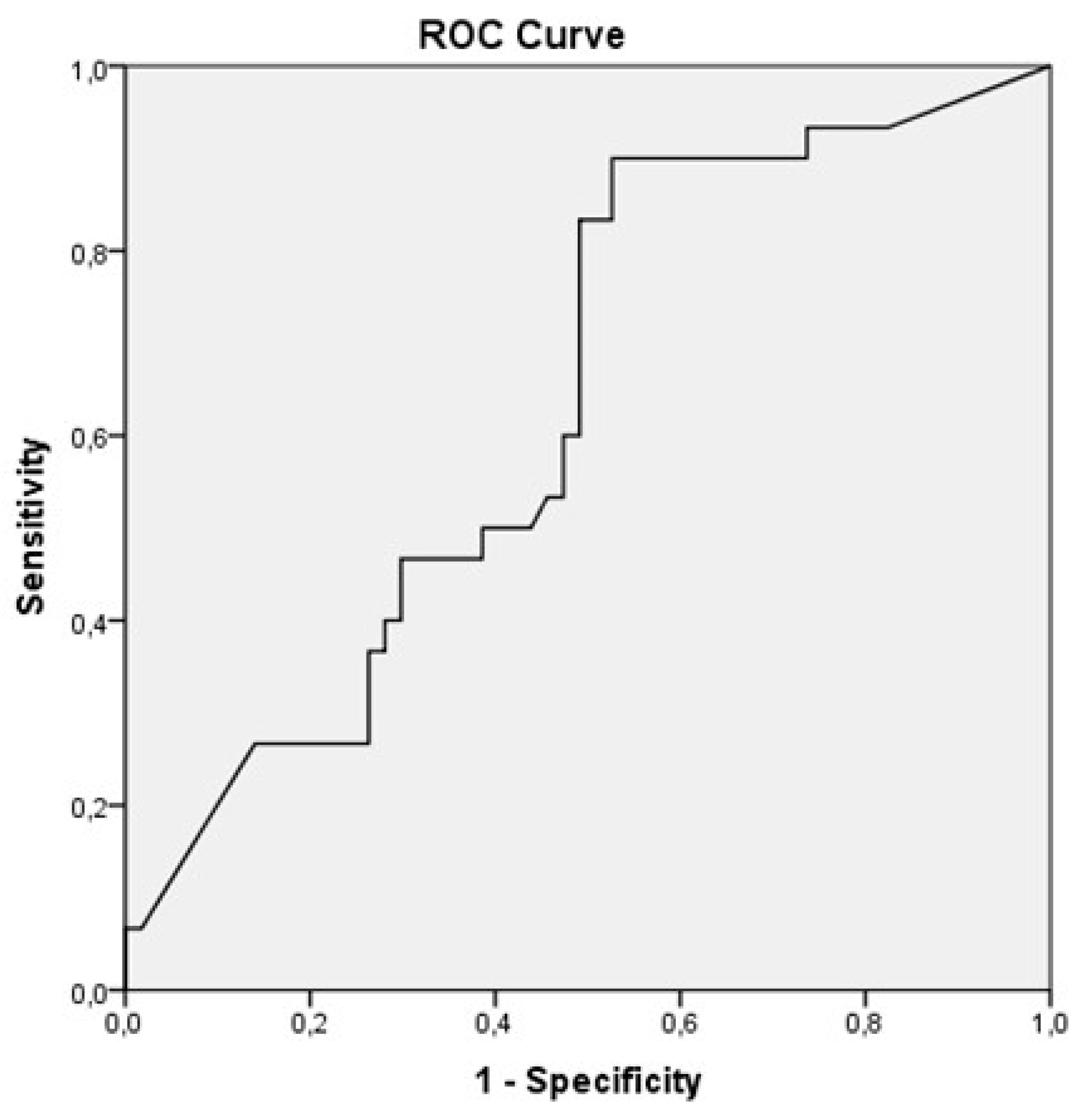
The ROC curve analysis (
Figure 1) showed that the optimal cut-off to discriminate between anaphylaxis and a less severe allergic reaction to peanut was an sIgE level to Ara h2 of 0.92 IU/ml, with a sensitivity of 90% and a specificity of 47.5% for the prediction of anaphylaxis. The area under the ROC curve was 0.63 (p=0.04; 95% CI 0.52-0.75).
Figure 1 Receiver Operating Characteristic (ROC) curve analysis of specific IgE levels to Ara h2 discriminating between anaphylaxis and less severe peanut allergy.
4. Discussion
In our study, we found that the epidemiological and clinical characteristics of children with peanut allergy are not related to the severity of the reaction and cannot predict anaphylaxis. As regards the results of standard peanut allergy testing, our study found that only the level of specific IgE to the Ara h2 component of peanuts was weakly associated with anaphylaxis.
We found that 35% of the children who reacted to peanut had anaphylaxis. This percentage is higher compared with a systematic review by Bassegio Conrado et al. who reported that approximately 17% of allergic reactions to peanut in European children occurred as anaphylaxis [
25]. The percentage of anaphylaxis in our patients would have been even higher if we had not included mild reactions (such as OAS or exacerbation of atopic dermatitis) to peanut, which are often reported only by parents. However, the higher percentage of anaphylaxis observed is probably due to the design of our study, as in patients with multiple allergic reactions to peanuts, only the most severe one was considered for statistical analysis.
Risk factors for peanut allergy, such as family history of peanut allergy, atopic dermatitis, allergic airway disease, allergy to other foods or airborne allergens, late introduction of peanuts [
4,
5,
6,
7,
8,
9], were not associated with the severity of peanut allergy in our study.
We also did not confirm the findings of Datema et al. who reported several epidemiological risk factors (female sex, early onset of peanut allergy, atopic dermatitis, familial atopy, and sensitization to house dust mite) associated with a more severe allergic reaction to peanut in peanut-allergic adults [
14]. However, we confirmed their finding that the level of sIgE to the peanut protein Ara h2 is an independent risk factor for anaphylaxis in peanut allergic adults, although in our study we did not find significant differences in the level of sIgE to Ara h2 between the groups with moderate and severe allergic reactions. The mean level of sIgE to Ara h2 was even higher (although not significantly) in our group with a moderate reaction to peanuts compared to patients with anaphylaxis. The optimal cut-off value of sIgE to Ara h2 to discriminate between anaphylaxis and less severe reactions to peanut is therefore not easy to determine, and the value of 0.92 IU/ml is sensitive (90%), but at the expense of less than 50% specificity.
This threshold is higher than the 0.35 IU/ml suggested by Keet et al. and Nicolaeu et al. although they reported similar sensitivity and slightly higher specificity. The higher cut-off value in our study may be explained by the different study designs, as Keet et al. and Nicolaeu et al. were determining a cut-off value that would distinguish between clinically significant allergy and harmless sensitization [
22,
26], whereas we were trying to determine a cut-off value that would distinguish between anaphylaxis and milder forms of peanut allergy. A similar study was performed by Martinet et al. who reported that sIgE levels to Ara h2 below 0.44 IU/ml were associated with a low risk of anaphylaxis in children, whereas levels above 14 IU/ml were associated with a high risk of anaphylaxis [
27]. Our results are in agreement with those of Santos et al. who established an optimal cutoff level of sIgE to Ara h2 of 1.4 IU/ml to discriminate between severe and less severe allergic reactions to peanuts during OFC. In contrast to our results, they reported that SPT and sIgE to whole peanut also correlated with the severity and lower threshold for the occurrence of an allergic reaction to peanuts during OFC. Santos et al. also found that the BAT test was the best predictor of the severity and threshold of allergic reaction to peanuts during OFC [
20].
We also found an association between the amount of peanuts consumed and the severity of the allergic reaction, and an association between a higher number of previous allergic reactions and anaphylaxis. These findings suggest that allergic reactions of different severity may occur in the same patient, depending also on the amount of peanuts consumed.
Our study has several limitations. We had to rely on the history presented by the caregivers regarding the severity of the allergic reaction in a significant proportion of the patients. Secondly, the amount of peanuts and the severity of the allergic reaction depend on the circumstances and may differ between an allergic reaction triggered during OFC and treated appropriately and an accidental and uncontrolled peanut ingestion at home. We sought to address these issues by performing OFC in all patients with mild and moderate reactions that were not documented in the medical records or when the history of anaphylaxis presented by caregivers and the results of allergy testing were inconclusive.
5. Conclusions
In conclusion, epidemiological, clinical and laboratory characteristics as risk factors for anaphylaxis are of limited value in peanut-allergic children, and reactions of different severity may occur in the same patient. Therefore, more accurate diagnostic tools that can also predict dose-response relationships (e.g., BAT) may be useful in clinical practice in selected patients. Children with a clinically manifest peanut allergy still need to be treated with extreme caution and prescribed with an epinephrine autoinjector.
Author Contributions
Conceptualization, T.P., M.L., B.Kr., P.K. and V.B.; methodology, T.P., P.K. and V.B.; software, T.P., M.L., B.Kr. and V.B.; validation, T.P., M.L., B.Kr. and V.B.; formal analysis, V.B.; investigation, resources and data curation, T.P., M.L., B.Kr., M.H., M.K., P.K., B.Ko., M.T., T.H. and V.B.; writing—original draft preparation, T.P. and V.B.; writing—review and editing, T.P., M.L., B.Kr., and V.B.; visualization, V.XB; supervision, V.B.; project administration, T.P. and V.B.; funding acquisition, T.P. and V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University Medical Centre Maribor, internal research grant number IRP-2021/02-12.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the University Clinical Centre Maribor (UKC-MB-KME-25/20).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data used in this study are openly available in Kaagle at doi 10.34740/kaggle/dsv/5528447.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Al-Muhsen, S.; Clarke, A.E.; Kagan, R.S. Peanut allergy: an overview. CMAJ 2003, 168, 1279–1285. [Google Scholar] [PubMed]
- Lieberman, J.A.; Gupta, R.S.; Knibb, R.C.; Haselkorn, T.; Tilles, S.; Mack, D.P. , et al. The global burden of illness of peanut allergy: a comprehensive literature review. Allergy 2021, 76, 1367–1384. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Chan, E.S.; Sicherer, S. Peanut allergy: new advances and ongoing controversies. Pediatrics 2020, 145, e20192102. [Google Scholar] [CrossRef] [PubMed]
- Bégin, P.; Graham, F.; Killer, K.; Paradis, J.; Paradis, L.; Des Roches, A. Introduction of peanuts in younger siblings of children with peanut allergy: a prospective, double-blinded assessment of risk, of diagnostic tests, and an analysis of patient preferences. Allergy 2016, 71, 1762–1771. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.; Koplin, J.J.; Ponsonby, A.L.; Gurrin, L.C.; Wake, M.; Vuillermin, P. , et al. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. J Allergy Clin Immunol 2013, 131. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Sicherer, S. Cutaneous sensitization to peanut in children with atopic dermatitis: a window to prevention of peanut allergy. JAMA Dermatology 2019, 155, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S. , et al. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr Allergy Immunol 2021, 32, 843–858. [Google Scholar] [CrossRef]
- Kotsapas, C.; Nicolaou, N.; Haider, S.; Kerry, G.; Turner, P.J.; Murray, C.S. , et al. Early-life predictors and risk factors of peanut allergy, and its association with asthma in later-life: Population-based birth cohort study. Clin Exp Allergy 2022, 52, 646–657. [Google Scholar] [CrossRef]
- Sikić Pogačar, M.; Mičetić-Turk, D. Vitamin D in human health. Acta medico-biotechnica 2017, 10, 12–24. [Google Scholar] [CrossRef]
- Johnson, J.; Malinovschi, A.; Lidholm, J.; Petersson, C.J.; Nordvall, L.; Janson, C. , et al. Sensitization to storage proteins in peanut and hazelnut is associated with higher levels of inflammatory markers in asthma. Clin Mol Allergy 2020, 18, 11. [Google Scholar] [CrossRef]
- Čelakovská, J.; Bukač, J.; Vaňková, R.; Krejsek, J.; Andrýs, C. Peanuts allergy in atopic dermatitis patients, analysis of sensitization to molecular components. Agric Immunol 2021, 32, 221–36. [Google Scholar] [CrossRef]
- Sampson, H.A. Clinical practice. Peanut allergy. N Engl J Med 2002, 346, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Datema, M.R.; Lyons, S.A.; Fernández-Rivas, M.; Ballmer-Weber, B.; Knulst, A.C.; Asero, R. , et al. Estimating the risk of severe peanut allergy using clinical background and IgE sensitization profiles. Front Allergy 2021, 2, 670789. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.E.; Koppelman, G.H.; Flokstra-de Blok, B.M.J.; Kollen, B.J.; Dubois, A.E.J. Prediction of the severity of allergic reactions to foods. Allergy 2018, 73, 1532. [Google Scholar] [CrossRef]
- Foong, R.X.; du Toit, G.; Fox, A.T. Asthma, food allergy, and how they relate to each other. Front Pediatr 2017, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Sicherer, S.H. Diagnosis and management of food allergy. CMAJ 2016, 188, 1087–1093. [Google Scholar] [CrossRef]
- Koplin, J.J.; Perrett, K.P.; Sampson, H.A. Diagnosing peanut allergy with fewer oral food challenges. J Allergy Clin Immunol Pract 2019, 7, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Ruinemans-Koerts, J.; Brouwer, M.L.; Schmidt-Hieltjes, Y.; Stevens, P.; Merkus, P.J.F.M.; Doggen, C.M.J. , et al. The indirect basophil activation test is a safe, reliable, and accessible tool to diagnose a peanut allergy in children. J Allergy Clin Immunol Pract 2022, 10, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Du Toit, G.; O’Rourke, C.; Becares, N.; Couto-Francisco, N.; Radulovic, S. , et al. Biomarkers of severity and threshold of allergic reactions during oral peanut challenges. J Allergy Clin Immunol 2020, 146, 344. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F. , et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Keet, C.A.; Johnson, K.; Savage, J.H.; Hamilton, R.G.; Wood, R.A. Evaluation of Ara h2 IgE thresholds in the diagnosis of peanut allergy in a clinical population. J Allergy Clin Immunol Pract 2013, 1, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Messmer, K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet 1977, 309, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.; Lack, G. Diagnosing peanut allergy with skin prick and specific IgE testing. J Allergy Clin Immunol 2005, 115, 1291–1296. [Google Scholar] [CrossRef]
- Baseggio Conrado, A.; Patel, N.; Turner, P.J. Global patterns in anaphylaxis due to specific foods: A systematic review. J Allergy Clin Immunol 2021, 148, 1515. [Google Scholar] [CrossRef]
- Nicolaou, N.; Custovic, A. Molecular diagnosis of peanut and legume allergy. Curr Opin Allergy Clin Immunol 2011, 11, 222–228. [Google Scholar] [CrossRef]
- Martinet, J.; Couderc, L.; Renosi, F.; Bobée, V.; Marguet, C.; Boyer, O. Diagnostic value of antigen-specific immunoglobulin E immunoassays against Ara h 2 and Ara h 8 peanut components in child food allergy. Int Arch Allergy Immunol 2016, 169, 216–222. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).