1. Introduction
Asthma is a chronic respiratory disease that affects around 334 million people globally, and it is attributed to 250,000 deaths yearly. It is estimated that the prevalence of asthma will increase by another 100 million by 2025 as asthma incidence continues to grow [
1,
2]. Asthma is characterized by airway inflammation, airway hyperresponsiveness, and airflow limitation, which can lead to respiratory symptoms such as coughing, wheezing, and shortness of breath [
3]. The pathogenesis of asthma is complex and involves various genetic, environmental, and immunological factors. One of the potential environmental factors that has been studied in relation to asthma is vitamin D [
4].
Vitamin D is a fat-soluble vitamin that is synthesized in the skin upon exposure to sunlight. It is also found in certain foods such as fatty fish, egg yolks, and fortified dairy products [
5,
6]. Vitamin D has been shown to have immunomodulatory effects and is involved in the regulation of various immune cells, including T cells and dendritic cells.
Over the past twenty years, numerous research groups have explored the connection between vitamin D and asthma pathogenesis. Their findings indicate that asthma patients with insufficient levels of vitamin D tend to have more severe symptoms and poorer lung function [
7,
8]. Various epidemiological and meta-analysis studies have indicated that children with low levels of vitamin D in their blood are more likely to develop asthma and experience more severe symptoms, exacerbations, and reduced lung function if they already have asthma [
9]. Additionally, there appears to be a correlation between low maternal vitamin D intake and levels during pregnancy and an increased likelihood of wheezing in children [
9,
10]. The evidence suggests that vitamin D affects both innate and adaptive immune system cells and structural cells in the airways, and that deficiency can promote inflammation while supplementation can alleviate these effects [
8,
11,
12].
Several clinical trials have investigated the effect of vitamin D supplementation on asthma outcomes, including airway remodeling. Current scientific knowledge confirms that structural cells, such as epithelial and ASM cells, play a role in the development of asthma through complex interactions with inflammatory lymphocytes. The degradation of airway epithelial integrity and the process of epithelial-mesenchymal transition (EMT) during airway remodeling are substantial factors in the pathogenesis of asthma [
13].A systematic review of these studies can provide a comprehensive evaluation of the current evidence regarding the relationship between vitamin D and asthma airway remodeling. Therefore , a systematic review of experimental studies and randomized controlled trials (RCTs) was carried out to examine the impact of vitamin D supplementation on airway remodeling in asthma.
2. Materials and Methods
2.1. Protocol and registration
The systematic review was conducted following the guidelines set forth by the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews (PRISMA) statement. We registered our study in the PROSPERO database
https://www.crd.york.ac.uk/PROSPERO/ and were assigned the registration ID ( CRD42023413798).
2.2. Search strategy and study selection
The review search strategy was begun by conducting systematic search of the PubMed, Embase, Clinical trails.gov and CINAHL databases using MeSH (Medical Subject Headings) using the terms “vitamin D” OR “25-hydroxyvitamin D” OR “vit d” and asthma and airway remodeling. Additionally, manual searches were performed followed by cross-referencing relevant articles to ensure a comprehensive review of the literature. The selected studies were analyzed through Review Manger 5.3 software developed by the Cochrane Library and the level of significance was considered as 5% (p < 0.05). The study’s inclusion criteria were limited to randomized controlled human trials or experimental studies published in the English language over the past decade (January 1, 1990, to February 28, 2023). Exclusion criteria included studies involving children or maternal populations. Articles such as review and editorial pieces, letters to editors, brief communications, short communications, personal opinions, and commentaries were excluded from consideration for this study.
2.3. Quality assurance and Data extraction
Two independent reviewers (L.S, B.M) were objectively reviewed and a consensus was reached by ensuring that they matched the inclusion criteria and MeSH terms. The data were extracted and organized based on the following criteria: first author’s name and year of publication, study design, country of origin, cell types used , vitamin D intervention details, outcome measurements of airway remodeling and methods used to assess airway remodeling .we attempted to identify randomized controlled trials and experimental studies investigating the effects of interventions on airway remodeling. All studies that measured airway remodeling were experimental studies rather than randomized controlled trials.
3. Results
3.1. Study selection
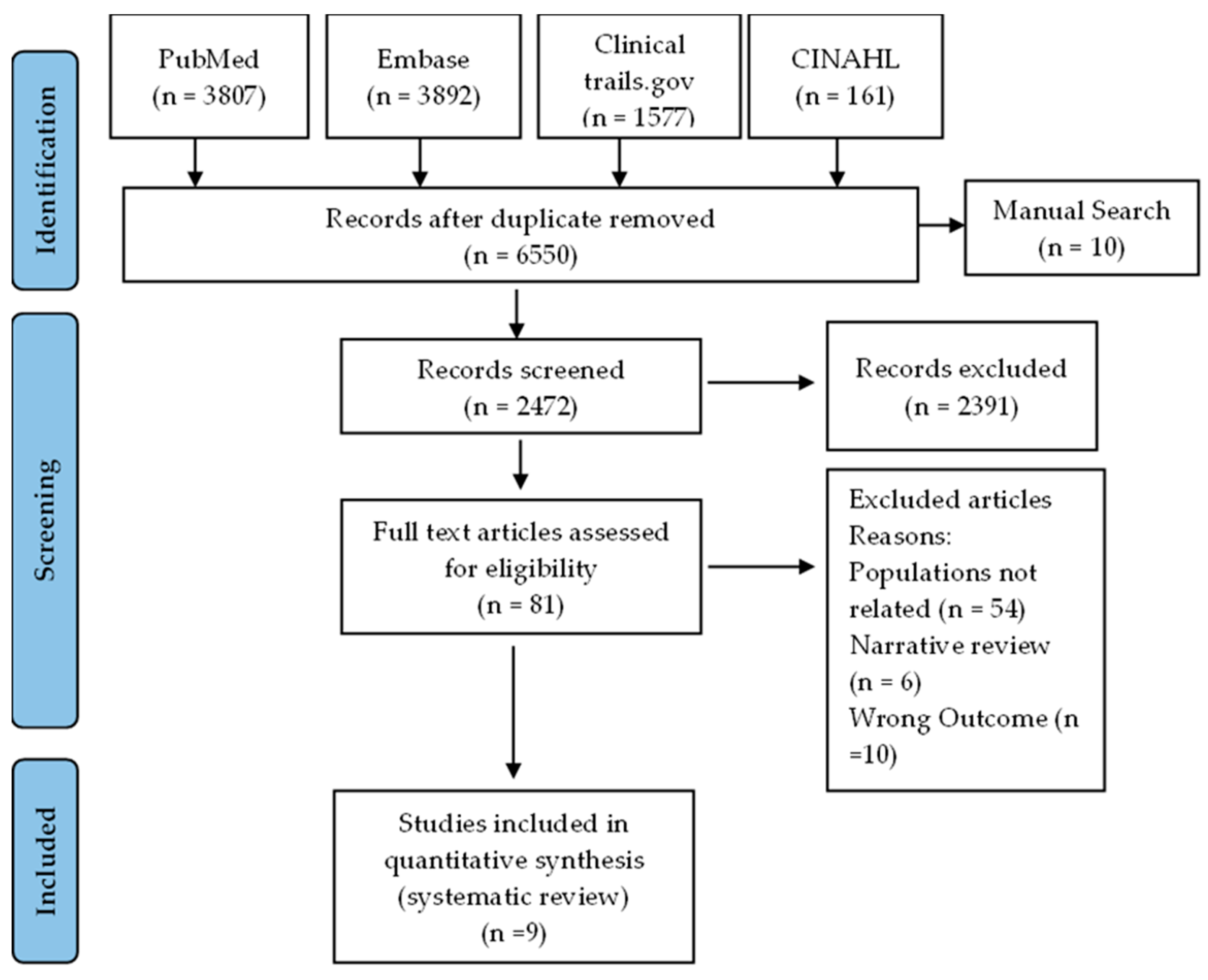
A comprehensive literature search was conducted using PubMed, Embase, Clinical trails.gov, and CINAHL , as well as a manual search, which retrieved a total of 9447 studies related to vitamin D, 25-hydroxyvitamin D, vit d, asthma, and airway remodeling. After removing 6650 duplicate studies, the titles and abstracts of the remaining studies were screened, resulting in the exclusion of 425 and 2391 studies, respectively. The full text of the remaining 81 articles was reviewed, and 72 were excluded based on pre-specified criteria (
Figure 1). The excluded studies were Populations not related ( 54 articles), Narrative review (6 articles), wrong outcome (10 articles), Duplicate (2 articles). Of the remaining articles, 9 articles included for narrative systematic review.
3.2. Study characteristics
Out of 9447 studies identified during the initial search, 9 studies (0.1%) met the inclusion criteria and were included in the systematic review. All the included studies were invitro experimental studies and conducted between 2007 to 2021.
Four of the included studies were from China [
14,
15,
16,
17], two from USA [
18,
19], two from Canada [
20,
21] and one study from Pennsylvania [
22]. Five studies used Human airway smooth muscle cells [
15,
16,
19,
21,
22] while the other three studies used bronchial fibroblast [
14,
18,
20]. Two studies measured airway remodeling directly [
16,
19] while the other six studies didn’t measured remodeling directly .Instead, they investigated the underling mechanisms impairing airway epithelial or fibroblast cells which will lead to airway remodeling [
14,
15,
18,
20,
21,
22]
Table 1.
3.3. Vitamin D inhibits airway smooth muscle cell contraction and remodeling.
Of the nine reviewed studies , five studies suggested that vitamin D may have a protective effect against airway smooth muscle cell contraction and remodeling in asthma [
15,
17,
19,
21,
22]. Bossé et al. 2007 found that treatment with 1,25-dihydroxyvitamin D3 (the active form of vitamin D) induced autocrine (self-stimulating), contractility, and remodeling processes in bronchial smooth muscle cells. Specifically, the authors found that vitamin D increased the expression of genes involved in muscle contraction, such as smooth muscle alpha-actin, and in remodeling, such as matrix metalloproteinase-9. While Song et al. 2007 examined the effects of 1,25-dihydroxyvitamin D3 on passively sensitized human airway smooth muscle cells (cells that have been exposed to allergens). They found that vitamin D reduced the contractile response of these cells to acetylcholine, a neurotransmitter that promotes muscle contraction. The authors suggested that this effect may be mediated by decreased intracellular calcium levels, which are necessary for muscle contraction. In the other hands, Damera et al. 2009 investigated the mechanisms by which vitamin D inhibits the growth of human airway smooth muscle cells. They found that vitamin D reduced the expression of genes involved in cell cycle progression, such as cyclin D1, and induced the phosphorylation of retinoblastoma protein and checkpoint kinase 1, which are key regulators of the cell cycle. These effects ultimately led to decreased cell proliferation. Moreover , Britt et al. 2016 examined the effects of vitamin D on inflammation-induced contractility and remodeling of asthmatic human airway smooth muscle. They found that vitamin D reduced the expression of genes involved in inflammation, such as IL-6 and IL-8, and inhibited the contraction of airway smooth muscle cells in response to inflammatory stimuli.
Overall, these studies suggest that vitamin D may have a protective effect against airway smooth muscle cell contraction and remodeling in asthma. Finally , Kim, Sung-Ho et al.(2017) reported that active vitamin D3 (1,25(OH)2D3) inhibited VEGF-induced ADAM33 expression and proliferation in human airway smooth muscle cells. The researchers suggested that this finding has implications for asthma treatment, as ADAM33 is involved in airway remodeling, a key feature of asthma. They proposed that vitamin D3 supplementation may have potential as an adjunct therapy for asthma by reducing airway remodeling.
3.4. Vitamin D can reduce inflammation and regulate collagen synthesis in the airways.
Two studies suggested that vitamin D can reduce inflammation and regulate collagen synthesis in the airways, as seen in the studies by Song et al. 2013 and Jin et al. 2021. Inflammation is a key feature of asthma and is characterized by the infiltration of immune cells and the production of inflammatory mediators such as cytokines. Song et al. 2013 investigated the effect of vitamin D on nuclear factor kappa B (NF-κB), a transcription factor that plays a crucial role in the regulation of inflammatory responses. The authors found that vitamin D reduced the activation of NF-κB in passively sensitized human airway smooth muscle cells, thereby suppressing the expression of pro-inflammatory cytokines such as IL-6 and IL-8. They suggested that this effect may be mediated by the stabilization of inhibitor IκBα, a protein that prevents NF-κB from entering the nucleus and activating gene expression.
Collagen is a major component of the extracellular matrix in the airways and is responsible for maintaining the structural integrity of the airway wall. However, in asthma, collagen synthesis can be dysregulated, leading to excessive collagen deposition and airway remodeling. Jin et al. 2021 investigated the effect of vitamin D on collagen synthesis in human lung fibroblasts, which are cells that are responsible for producing collagen in the airways. The authors found that vitamin D reduced the expression of collagen type I and the activity of enzymes involved in collagen synthesis, such as protein arginine methyltransferase 1 (PRMT1). They suggested that this effect may be mediated by the inhibition of signal transducer and activator of transcription 3 (STAT3), a transcription factor that regulates collagen synthesis. Taken together, these studies suggest that vitamin D may have anti-inflammatory and anti-fibrotic effects in the airways, which may help to reduce the severity of asthma and prevent airway remodeling.
3.5. Vitamin D can modulate the action of bronchial fibroblasts.
The study by Plesa et al. 2020 investigated the effect of vitamin D on human asthmatic bronchial fibroblasts, which are cells that are involved in airway remodeling and contribute to the development of asthma.
The authors found that treatment with vitamin D (specifically 1,25(OH)2D3) reduced the proliferation and migration of bronchial fibroblasts in a dose-dependent manner, suggesting that vitamin D can inhibit the growth of these cells. They also found that vitamin D reduced the expression of genes involved in extracellular matrix remodeling, such as collagen type I and matrix metalloproteinase 2 (MMP2), indicating that vitamin D can modulate the action of bronchial fibroblasts. The authors further investigated the mechanisms by which vitamin D exerts its effects on bronchial fibroblasts and found that it inhibits the activity of several signaling pathways that are involved in cell proliferation and migration, such as ERK1/2 and Akt. They also found that vitamin D upregulates the expression of genes involved in cell cycle arrest, such as p21 and p27, suggesting that vitamin D can induce cell cycle arrest in bronchial fibroblasts.
Overall, this study suggests that vitamin D can modulate the action of bronchial fibroblasts by inhibiting their proliferation and migration, and by reducing the expression of genes involved in extracellular matrix remodeling. These findings may have important implications for the prevention and treatment of asthma, as airway remodeling is a key feature of the disease.
3.6. TGF-β1 can impair vitamin D-induced and constitutive airway epithelial host defense mechanisms.
The study by Schrumpf et al. 2020 investigated the relationship between two important molecules in the airways: vitamin D and TGF-β1. Vitamin D is known to play a role in regulating immune responses in the airways and promoting host defense against respiratory pathogens, while TGF-β1 is a cytokine that is involved in the development of asthma and other respiratory diseases. The authors found that treatment with TGF-β1 impaired the ability of airway epithelial cells to induce the expression of genes involved in host defense when stimulated with vitamin D. This suggests that TGF-β1 can inhibit the action of vitamin D in promoting host defense mechanisms in the airways. The authors further investigated the mechanisms underlying this effect and found that TGF-β1 interferes with the signaling pathway activated by vitamin D, specifically by reducing the expression of the vitamin D receptor (VDR) and suppressing the activity of the transcription factor NF-κB. These effects lead to a reduced expression of genes involved in host defense and an impaired ability of the airway epithelial cells to fight off respiratory pathogens.
Overall, this study suggests that TGF-β1 can impair the action of vitamin D in promoting host defense mechanisms in the airways, by interfering with the signaling pathway activated by vitamin D and reducing the expression of key genes involved in host defense. These findings may have important implications for the development of new therapies for respiratory diseases, as targeting the interaction between vitamin D and TGF-β1 may be a promising approach for improving airway host defense.
4. Discussion
In our study, the outcomes of the included studies on the identification effect of vitamin D on asthma airway remodeling suggested that vitamin D has a potential role in regulating several key processes involved in respiratory diseases, such as airway smooth muscle cell contraction and remodeling, inflammation, collagen synthesis, and the action of bronchial fibroblasts. Specifically, the studies included in this review provide evidence that vitamin D can inhibit airway smooth muscle cell contraction and remodeling, which are key features of asthma and other respiratory diseases. The studies by Bossé et al. (2007), Song et al. (2007), Damera et al. (2009), Britt et al. (2016) and Kim, Sung-Ho et al.(2017) all demonstrate the ability of vitamin D to modulate these processes, suggesting that it may be a promising therapeutic target for respiratory diseases. These findings are in line with other studies have demonstrated that lower levels of serum vitamin D are associated with increased airway smooth muscle (ASM) mass, airway hyperresponsiveness (AHR), poorer asthma control, and increased exacerbations in children, adolescents, and adults [
23,
24,
25]. These studies suggest that vitamin D can be a potential therapeutic agent for asthma and other airway diseases. By inhibiting airway smooth muscle cell contraction and remodeling, vitamin D may reduce airway hyperresponsiveness and improve airway function. However, further studies are necessary to investigate the underlying mechanisms by which vitamin D exerts its effects on airway smooth muscle cells. Additionally, clinical trials are needed to determine the efficacy of vitamin D supplementation in treating airway diseases.
In addition, other studies such as Song et al. (2013) and Jin et al. (2021) support the notion that vitamin D can reduce inflammation and regulate collagen synthesis in the airways, which are critical processes in airway remodeling. Previously, it was believed that airway remodeling was a consequence of chronic inflammation over a long period of time. However, recent evidence indicates that the process of remodeling begins in early childhood, even before the age of three years [
26]. Observational studies suggest that vitamin D may have a protective role in severe asthma. The immune-modulating potential of vitamin D has been implicated in asthma, and there is increasing evidence to support the role of the vitamin D pathway in regulating immune function. The vitamin D receptor (VDR) has been found on almost all immune cells, including macrophages, dendritic cells, T-cells, and B-cells [
6,
27,
28,
29,
30]. In mouse models, studies have shown that VDR knockout mice do not develop experimental asthma, suggesting that vitamin D is required for the generation of T-helper (Th)2-driven inflammation in the airways [
31].
Furthermore, Plesa et al. (2020) found that vitamin D can modulate the action of bronchial fibroblasts, which play a crucial role in the development of airway remodeling. They found that treatment with 1,25(OH)2D3 led to a reduction in the proliferation of asthmatic bronchial fibroblasts, as well as a decrease in the production of extracellular matrix proteins and pro-inflammatory cytokines. Several studies have reported on the antifibrotic effects of vitamin D in various disease models. In VDR knockout mice, an increase in inflammatory cell infiltration, upregulation of metalloproteinases, and phosphoacetylation of NF-κB were observed in the lung, which was associated with emphysema and a decline in lung function along with formation of lymphoid aggregates [
32]. This highlights the importance of vitamin D in regulating these processes. Moreover, in tuberculosis, 1,25(OH)2D3 was shown to suppress production of MMPs while enhancing TIMP-1 levels [
33]. In a human squamous carcinoma cell line, vitamin D3 was found to significantly reduce the production of MMP-9 and MMP-13 mRNA and proteins in a dose-dependent manner [
34]. Similarly, in a Crohn’s disease model, a vitamin D analog attenuated the profibrotic response of colonic myofibroblasts to high matrix stiffness [
35]. These findings suggest that vitamin D may have a potential therapeutic effect in preventing fibrosis in various disease conditions.
However, it’s important to note that the review also highlights the potential for TGF-β1 to impair vitamin D-induced and constitutive airway epithelial host defense mechanisms, as shown by Schrumpf et al. (2020). The deposition of collagen is excessively increased when fibroblasts are activated by tumor growth factor-beta-1 (TGF-β1), which is a profibrotic cytokine. This activation plays a crucial role in asthma, as TGF-β is synthesized by various cells such as macrophages, lymphocytes, eosinophils, fibroblasts, and airway epithelial cells [
36]. Additionally, Th2-derived cytokines, including IL-4, are essential in airway remodeling. TGF-β also induces the expression of TIMP-1, and this process is dependent on Th2 [
37,
38].This suggests that the relationship between vitamin D and airway remodeling is complex and further research is needed to fully understand the mechanisms involved.
5. Conclusions
Based on the studies mentioned, it can be concluded that Vitamin D has various beneficial effects on the airways. More than half of the studies reviewd here suggested that Vitamin D inhibits airway smooth muscle cell contraction and remodeling. Other studies indicated that Vitamin D can reduce inflammation and regulate collagen synthesis in the airways. Some of the studies showed that Vitamin D can modulate the action of bronchial fibroblasts. However, one of the studies suggested that TGF-β1 can impair Vitamin D-induced and constitutive airway epithelial host defense mechanisms. Overall, Vitamin D seems to influence much of the pathways that appear to be within the core af asthma progression. This positive effect on airway remodeling and inflammation has a promising potential in the management of asthma and preventaion of asthma progression.
Author Contributions
Conceptualization, L.S.,W.M. and B.M.; methodology, L.S, K.M.,M.L. and R.H; software,R.H.; Studies review, L.S. and B.M.; data curation,L.S, K.M., L.M. and S.S.; writing—original draft preparation, L.S, K.M., M.L.and S.S.; writing—review and editing, L.S., R.H. and W.M.; visualization, W.M. and R.H. ; supervision, B.M.; funding acquisition, L.S.,B.M. and R.H. All authors have contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
L.S.: B.M. and R.H. are funded by the University of Sharjah (grant code: 22010902103).
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma (Primer). Nature Reviews: Disease Primers. 2015;1(1). [CrossRef]
- Network GA. The Global Asthma Report 2018 Auckland NZ. 2018.
- Ullmann N, Mirra V, Di Marco A, Pavone M, Porcaro F, Negro V, et al. Asthma: differential diagnosis and comorbidities. Frontiers in pediatrics. 2018;6:276. [CrossRef]
- Hall SC, Agrawal DK. Vitamin D and Bronchial Asthma: An Overview of Data From the Past 5 Years. Clin Ther. 2017;39(5):917-29. [CrossRef]
- Kerley CP, Elnazir B, Faul J, Cormican L. Vitamin D as an adjunctive therapy in asthma. Part 1: A review of potential mechanisms. Pulmonary pharmacology & therapeutics. 2015;32:60-74. [CrossRef]
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocrine reviews. 2005;26(5):662-87. [CrossRef]
- Bozzetto S, Carraro S, Giordano G, Boner A, Baraldi E. Asthma, allergy and respiratory infections: the vitamin D hypothesis. Allergy. 2012;67(1):10-7. [CrossRef]
- Yawn J, Lawrence LA, Carroll WW, Mulligan JK. Vitamin D for the treatment of respiratory diseases: is it the end or just the beginning? The Journal of steroid biochemistry and molecular biology. 2015;148:326-37.
- Gupta A, Bush A, Hawrylowicz C, Saglani S. Vitamin D and asthma in children. Paediatric respiratory reviews. 2012;13(4):236-43. [CrossRef]
- Thacher TD, Clarke BL, editors. Vitamin D insufficiency. Mayo Clinic Proceedings; 2011: Elsevier.
- Barragan M, Good M, Kolls JK. Regulation of dendritic cell function by vitamin D. Nutrients. 2015;7(9):8127-51. [CrossRef]
- Hall SC, Fischer KD, Agrawal DK. The impact of vitamin D on asthmatic human airway smooth muscle. Expert Rev Respir Med. 2016;10(2):127-35. [CrossRef]
- Pain M, Bermudez O, Lacoste P, Royer P-J, Botturi K, Tissot A, et al. Tissue remodelling in chronic bronchial diseases: from the epithelial to mesenchymal phenotype. European Respiratory Review. 2014;23(131):118-30. [CrossRef]
- Jin A, Tang X, Zhai W, Li Y, Sun Q, Liu L, et al. TSLP-induced collagen type-I synthesis through STAT3 and PRMT1 is sensitive to calcitriol in human lung fibroblasts. Biochim Biophys Acta Mol Cell Res. 2021;1868(10):119083. [CrossRef]
- Song Y, Hong J, Liu D, Lin Q, Lai G. 1,25-dihydroxyvitamin D3 inhibits nuclear factor kappa B activation by stabilizing inhibitor IκBα via mRNA stability and reduced phosphorylation in passively sensitized human airway smooth muscle cells. Scand J Immunol. 2013;77(2):109-16. [CrossRef]
- Song Y, Qi H, Wu C. Effect of 1,25-(OH)2D3 (a vitamin D analogue) on passively sensitized human airway smooth muscle cells. Respirology. 2007;12(4):486-94. [CrossRef]
- Kim S-H, Pei Q-M, Jiang P, Yang M, Qian X-J, Liu J-B. Effect of active vitamin D3 on VEGF-induced ADAM33 expression and proliferation in human airway smooth muscle cells: implications for asthma treatment. Respiratory Research. 2017;18(1):7. [CrossRef]
- Schrumpf JA, Ninaber DK, van der Does AM, Hiemstra PS. TGF-β1 Impairs Vitamin D-Induced and Constitutive Airway Epithelial Host Defense Mechanisms. J Innate Immun. 2020;12(1):74-89. [CrossRef]
- Britt RD, Jr., Thompson MA, Freeman MR, Stewart AL, Pabelick CM, Prakash YS. Vitamin D Reduces Inflammation-induced Contractility and Remodeling of Asthmatic Human Airway Smooth Muscle. Ann Am Thorac Soc. 2016;13 Suppl 1(Suppl 1):S97-8. [CrossRef]
- Plesa M, Gaudet M, Mogas A, Olivenstein R, Al Heialy S, Hamid Q. Action of 1,25(OH)(2)D(3) on Human Asthmatic Bronchial Fibroblasts: Implications for Airway Remodeling in Asthma. J Asthma Allergy. 2020;13:249-64. [CrossRef]
- Bossé Y, Maghni K, Hudson TJ. 1alpha,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol Genomics. 2007;29(2):161-8. [CrossRef]
- Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, et al. Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol. 2009;158(6):1429-41. [CrossRef]
- Niruban SJ, Alagiakrishnan K, Beach J, Senthilselvan A. Association between vitamin D and respiratory outcomes in Canadian adolescents and adults. J Asthma. 2015;52(7):653-61. [CrossRef]
- Somashekar AR, Prithvi AB, Gowda MN. Vitamin d levels in children with bronchial asthma. J Clin Diagn Res. 2014;8(10):Pc04-7. [CrossRef]
- Gupta A, Sjoukes A, Richards D, Banya W, Hawrylowicz C, Bush A, et al. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med. 2011;184(12):1342-9. [CrossRef]
- Proud D. Role of rhinovirus infections in asthma. Asian Pacific journal of allergy and immunology. 2011;29(3):201.
- Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Archives of biochemistry and biophysics. 2012;523(1):123-33. [CrossRef]
- Hewison M. Vitamin D and the intracrinology of innate immunity. Molecular and cellular endocrinology. 2010;321(2):103-11. [CrossRef]
- Baeke F, Korf H, Overbergh L, van Etten E, Verstuyf A, Gysemans C, et al. Human T lymphocytes are direct targets of 1, 25-dihydroxyvitamin D3 in the immune system. The Journal of steroid biochemistry and molecular biology. 2010;121(1-2):221-7. [CrossRef]
- Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1, 25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181-3. [CrossRef]
- Wittke A, Weaver V, Mahon BD, August A, Cantorna MT. Vitamin D receptor-deficient mice fail to develop experimental allergic asthma. The journal of immunology. 2004;173(5):3432-6. [CrossRef]
- Sundar IK, Hwang J-W, Wu S, Sun J, Rahman I. Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation. Biochemical and biophysical research communications. 2011;406(1):127-33. [CrossRef]
- Anand SP, Selvaraj P. Effect of 1, 25 dihydroxyvitamin D3 on matrix metalloproteinases MMP-7, MMP-9 and the inhibitor TIMP-1 in pulmonary tuberculosis. Clinical immunology. 2009;133(1):126-31. [CrossRef]
- Meephansan J, Komine M, Tsuda H, Ohtsuki M. Suppressive effect of calcipotriol on the induction of matrix metalloproteinase (MMP)-9 and MMP-13 in a human squamous cell carcinoma cell line. Clinical and experimental dermatology. 2012;37(8):889-96. [CrossRef]
- Johnson LA, Sauder KL, Rodansky ES, Simpson RU, Higgins PD. CARD-024, a vitamin D analog, attenuates the pro-fibrotic response to substrate stiffness in colonic myofibroblasts. Experimental and molecular pathology. 2012;93(1):91-8. [CrossRef]
- Cao Y, Zeng D, Song Q, Cao C, Xie M, Liu X, et al. The effects of antisense interleukin-4 gene transferred by recombinant adeno-associated virus vector on the airway remodeling in allergic rats. Journal of Asthma. 2010;47(9):951-8. [CrossRef]
- Poon AH, Mahboub B, Hamid Q. Vitamin D deficiency and severe asthma. Pharmacology & therapeutics. 2013;140(2):148-55. [CrossRef]
- Yamauchi K, Inoue H. Airway Remodeling in Asthma and Irreversible Airflow Limitation—ECM Deposition in Airway and Possible Therapy for Remodeling—. Allergology International. 2007;56(4):321-9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).