1. Introduction
Escherichia coli (E. coli) is one of the most important gram-negative bacteria of the
Enterobacteriaceae family.
E. coli is one of the major pathogens for humans and animals with a contamination dose of less than 100 colonies [
1]. The most common way for it to be transmitted to humans and animals is through contaminated water and food such as undercooked meat and contaminated vegetables [
2]. Although the vast majority of
E. Coli serotypes are harmless and exist in the gastrointestinal tract normally, some of them such as
E. coli O157:H7, E. coli O167,… cause infection and are pathogenic to humans and animals [
2,
3]. In recent decades,
E.coli has received a lot of attention as one of the main pathogens causing severe illnesses, such as gastrointestinal diseases, bloody diarrhea, Kidney failure, stillbirth, premature birth, and infection during pregnancy[
4,
5,
6,
7,
8].According to the report of Centers for Disease Control (CDC)
E. coli infection causes approximately 125,000 hospitalizations and 3000 deaths in the USA each year [
2]. Therefore, early detection and prevention of the spread of this pathogen in all countries, including developed countries, is critical.
Given the importance of understanding the mechanism
of E. coli pathogenicity many studies have concentrated on investigating interactions between animal tissues and the bacterium. Thus, Sharon et al. reported that many strains of
E. coli and other bacteria from
Enterobacteriaceae family attach to the host cell by FimH, a two-domain protein at the tip of type I fimbriae (i.e. pili) on the bacterial surface. FimH contains a mannoside-binding lectin domain having a high affinity for D-mannose (MAN). MAN is a six-carbon sugar from the aldohexoses group that is abundant in the human body, especially in epithelial cells [
9,
10,
11].
Traditional methods based on media culture and colony counting are the most common methods of identifying
E. coli. One of the most important limitations of these methods is that they are time-consuming (2 to 5 days). New methods for rapid detection of
E. coli bacteria, such as polymerase chain reaction (PCR), enzyme-linked immunosorbent assay (ELISA), and real-time PCR, have been developed in recent decades [
12]. Although these methods have high accuracy and speed, the high cost and need for specialized knowledge and complex equipment are the disadvantages of these methods [
13]. Several types of biosensors such as aptasensor [
14], immunosensor [
15], carbohydrate biosensor [
16], and enzymatic biosensor [
17] have been developed to identify pathogens including
E. coli bacteria using different techniques such as electrochemical [
18], colorimetric [
19], optical [
20], and quartz crystal microbalance (QCM) methods [
21].
Among various detection methods, electrochemical detection stands out due to its simplicity, cost-effectiveness, and short response time. The electrochemical impedance spectroscopy (EIS) technique, in particular, is a highly sensitive method for accessing changes in electron transfer resistance at the electrode surface [
22]. For example, Wang et al. developed an aptamer-based electrochemical biosensor for the detection of
E. coli in licorice extract with the limit of detection of 80 CFU.mL
−1 [
23]. Zhang et al. fabricated an electrochemical biosensor for
E. coli detection using 16S rDNA as a target biomarker and oligonucleotide probes for
E. coli detection [
24]. Yang et al. reported an impedimetric biosensor based on lectin functionalized mixed self-assembled monolayer for
E. coli detection with the limit of detection of 75 CFU.mL
−1 [
25].
This study is the first carbohydrate-based electrochemical sensor. D-MAN was immobilized on the GCE surface in a simple way, in which immobilized D-MAN on the surface of gold nanoparticles (AuNPs)-modified GCE was used as a receptor for detection of E. coli. Previous works reported using carbohydrate-based sensors were based on QCM, SPR, and optical techniques for E. coli detection; on the other hand, D-MAN is available and cost-effective material and it has advantages comparing to the biological elements such as aptamers or antibodies. The mechanism of bacterial detection in the proposed sensor is based on the interaction between MAN and FimH at the tip of the pili located on the outer membrane of E. coli. The results of this interaction were evaluated using EIS, differential pulse voltammetry (DPV), and cyclic voltammetry (CV). The developed sensor is simple, sensitive, can detect E. coli within 60 min and can discriminate between E. coli and other bacteria.
2. Experimental
2.1. Materials
HAuCl4, pure ethanol (100%), K3Fe(CN)6, K4Fe(CN)6, Dimethyl aminopropyl-3-ethyl carbodiimide (EDC), and N-hydroxysuccinimide (NHS) were purchased from Sigma-Aldrich. Cysteamine (Cyst),4-aminobenzoic acid (PABA), acetic acid, MAN, KH2PO4, K2HPO4, NaOH and alumina powder were purchased from Merck. Deionized water (DI water) was obtained from Bandar Imam Petrochemical company in Iran. The microorganisms E. coli (PTCC 1399), Staphylococcus epidermidis (PTCC 1856), and Citrobacter freundii (PTCC 1600) were collected from Persian Type Culture Collection. The phosphate buffered saline (PBS) solution (pH 7.4) was used to prepare different bacterial concentrations by the serial dilution method for all of the strains. R2A Agar (Scharlau, Microbiology 01-540-500) was used for culture and count and Nutrient Broth (Scharlau, Microbiology 02-1440-500) is used for the regeneration of all types of bacteria.
2.2. Apparatus
Electrochemical measurements were done using the Metrohm Autolab potentiostat (model PGSTATAT302N, Netherlands). Modified GCE, Ag/AgCl electrode, and platinum wire were used as working, reference and counter electrodes, respectively. An ultrasonic bath (Eurosonic 4D) was used to ensure complete cleaning of the GCE surface. Also, colony counter (WTW, BZG 30), Vortex (IKA, WORTEX GENIUS 3), and pH meter (Metrohm, Swiss made) were used. The morphological structure of the modified electrode surface was examined using a Field emission scanning electron microscope (FE-SEM) and EDS spectroscopy (Energy-dispersive X-ray) taken on a Vega-Tescan electron microscope.
2.3. Synthesis of biorecognition element
MAN was modified according to the previously reported method [
26]. MAN was dissolved in water at 100mM and 4-aminobenzoic acid is solved in acetic acid at 100mM.). The mannose with p-amino benzoic acid solutions was mixed and then incubated in seal tubes for 1h at 20 °C for the amination reaction step. Then the reduction step was carried out overnight in fume hood in unsealed tube. As acetic-acid evaporates, p-carboxyphenylamino mannose (PCAM) single crystals were formed and used as a bioreceptor in this work. The resulting bioreceptors were stored at -20 °C under light and moisture blocking conditions. The name and identity of the PCAM were predicted using Chem Draw and confirmed with FTIR spectra.
2.4. Sensor fabrication
The GCEs were polished with alumina powder (0.3, 0.1, and 0.05 μm) and then carefully washed with DI water. To ensure a well-cleaned surface, the GCEs were sonicated in an ultrasonic bath containing DI water and ethanol in1: 1 ratio for 3 min and then was rinsed again with DI water and dried at room temperature. AuNPs were deposited on the surface of the GCEs by electrochemical deposition. AuNPs on the surface of a GCE increase the effective electrode surface and the sensitivity of electrochemical analyses and provide a unique platform for increased loading of receptors.
A GCE was immersed in a solution containing 5 mmol.L
-1 HAuCl
4 and 0.5 mol.L
-1 H
2SO
4,and the potential of -0.2 V was applied for 200 s [
27]. The AuNPs/GCE electrode was immersed in the 0.001 M solution of Cyst for 17 h. Cyst was used to be as a linker because it could stabilize activated PCAM (bioreceptor) on the surface of AuNPs/GCE. Then, the electrode was rinsed with DI water to remove unbounded Cyst at the electrode surface. PCAM dissolved in 1 mL acetic acid and DI water (1: 1) for 20 min. Then, the electrode was activated using 0.0035 mg.mL
-1 of EDC and 0.0028 mg.mL
-1NHS for 1 h. In the next step, the activated Cyst/AuNPs/GCE electrode was immersed in the PCAM solution for 3 h, and the electrode surface was then thoroughly washed with DI water and PBS (pH 7.4) to remove the unbound bioreceptor and avoid false-positive responses. Finally, the electrode was stabilized in PBS (pH 7.4) for 24 h. All preparation stages of the proposed biosensor were monitored by EIS, DPV, and cyclic voltammetry (CV) methods. Resistance changes (R
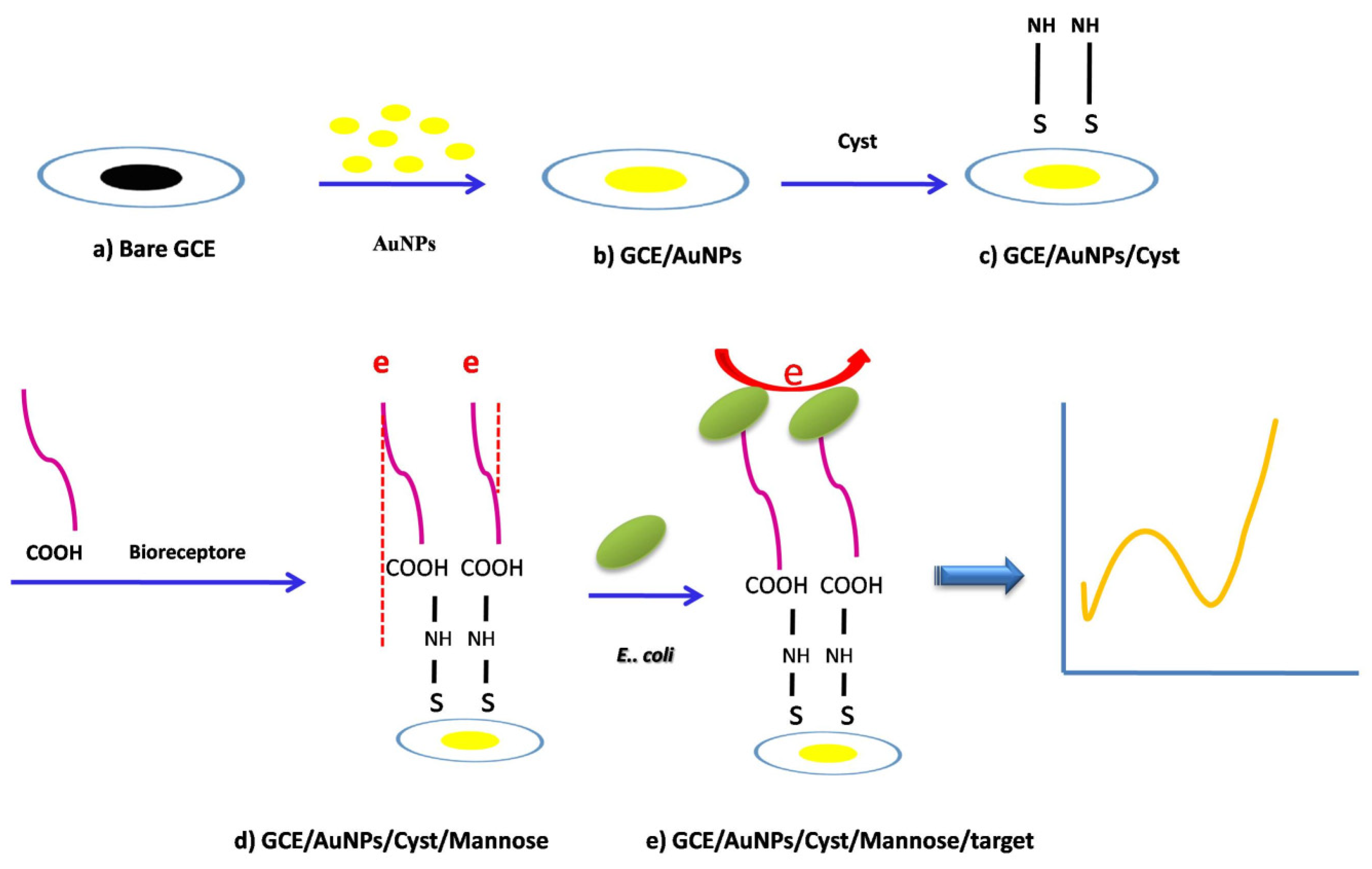
et) were measured at all fabrication steps of the biosensor to evaluate the performance of the proposed biosensor. All measurements were repeated at least three times. The overall process is shown in
Scheme 1.
2.5. Electroanalytical measurements
All electrochemical measurements were done in a standard three-electrode system in a glass cell containing 18 mL of 0.1 mol.L-1 KCl and 5.0 mmol.L-1 K3[Fe(CN)6]/K4[Fe(CN)6] (1:1) as a redox probe. For bacteria detection the modified electrodes were incubated at concentrations of 1.3 × 10 to 1.3 × 106 CFU.mL−1 E. coli for 1 h. After incubation, the electrode was thoroughly rinsed with DI water to wash bacteria that were not attached to the electrode surface or had poor binding to prevent a false positive response.
EIS measurement, which is an accurate and sensitive method to evaluate electron transfer changes at the electrode surface, was performed to evaluate R
et changes at the electrode surface for all steps of fabrication and detection of
E. coli bacteria and obtaining the calibration curve. CV were performed to further evaluate the electrochemical performance of the proposed sensor and also to further confirm the results obtained from EIS.EIS measurements were performed in the frequency range of 0.1Hz to 100 kHz, the potential of 0.24 V, and amplitude of 0.01 V. CV measurements were done within the potential range of -0.4 to 0.8 V at a scan rate of 10 mV.s
-1. All preparation stages of the proposed biosensor were monitored by EIS, DPV, and cyclic voltammetry (CV) methods. R
et was measured at all fabrication steps of the biosensor to evaluate the performance of the proposed biosensor. All measurements were repeated at least three times. The overall process is shown in
Scheme 1.
2.6. Real sample preparation
In order to evaluate the performance of the proposed biosensor in real samples. Tap water and low-fat milk was prepared as real samples. Samples were spiked with E. coli to obtain the final concentrations of 10,104, 105, and 106 CFU.mL−1. EIS was performed for the samples, and according to the calibration plot, the recovery percentage, and RSD% were determined. It is also important to mention that we used the sample of low-fat milk purchased from the supermarket without special preparation or screening
2.7. Bacterial culture and counting methods
E. coli (PTCC 1399) as target bacteria and Staphylococcus epidermidis (PTCC 1856) and Citrobacter freundi (PTCC 1600) as the non-target species were cultivated in nutrient broth (NB) at 37 °C for 20 h. Bacteria cells were harvested by centrifugation for 5 min at 3500 rpm. The cells were then re-suspended in PBS (0.1 mol.L−1, pH 7.4) and washed by centrifugation (3 times). The resulting solution in PBS (0.1 mol.L−1, pH 7.4) was used as a stock solution for E. coli and other bacterial species detection. The serial dilution method in PBS (0.1 mol.L−1, pH 7.4) was used to prepare different concentrations of bacteria from the stock solution. Bacterial populations were counted on the R2A agar plate before use by the conventional colony counting method.
2.8. Sensor concept and design of modified mannose
The concept of the sensor is based on the interaction between type I fimbriae in the outer membrane of
E. coli bacteria and mannose ligands (PCAM) fixed on the surface, which has a strong binding and high selectivity to this bacterium compared to other bacteria. The steps of the synthesis of PCAM using Chem Draw software were shown in
Scheme 2. This method to modify surface is very economic and can be applicable to other receptors such as aptamers, and antibodies.
2.9. Material selection
In this study, AuNPs were used as material to improve the surface of GCE and MAN as capture prob. AuNPs are widely used in many fields for their unique optical and physical properties. AuNPs with unique properties, such as a high specific surface area can provide a wide surface for coating carbohydrate ligands or other ligands. Also, these nanoparticles are highly conductive which can use to improve the transfer of electron current on the surface of the electrode. Au-NPs could be conjugated with many functionalizing agents, such as polymers, ligands, dendrimers, drugs, DNA, RNA, and proteins. Another feature of these nanoparticles is the ability to form strong covalent bonds with thiol groups that provided the possible formation of SAM on the surface whit other materials.
MAN is a carbohydrate that can be chemically modified to contain functional groups such as carboxyl or thiol and create self-assembled monolayers (SAMs) on the electrode surface through reaction with other groups. This carbohydrate has an affinity attached to the adhesion FimH on the tip of type I fimbriae in the outer membrane of Escherichia coli bacteria that was used as a receptor in this work.
3. Results and discussion
3.1. Surface characterization
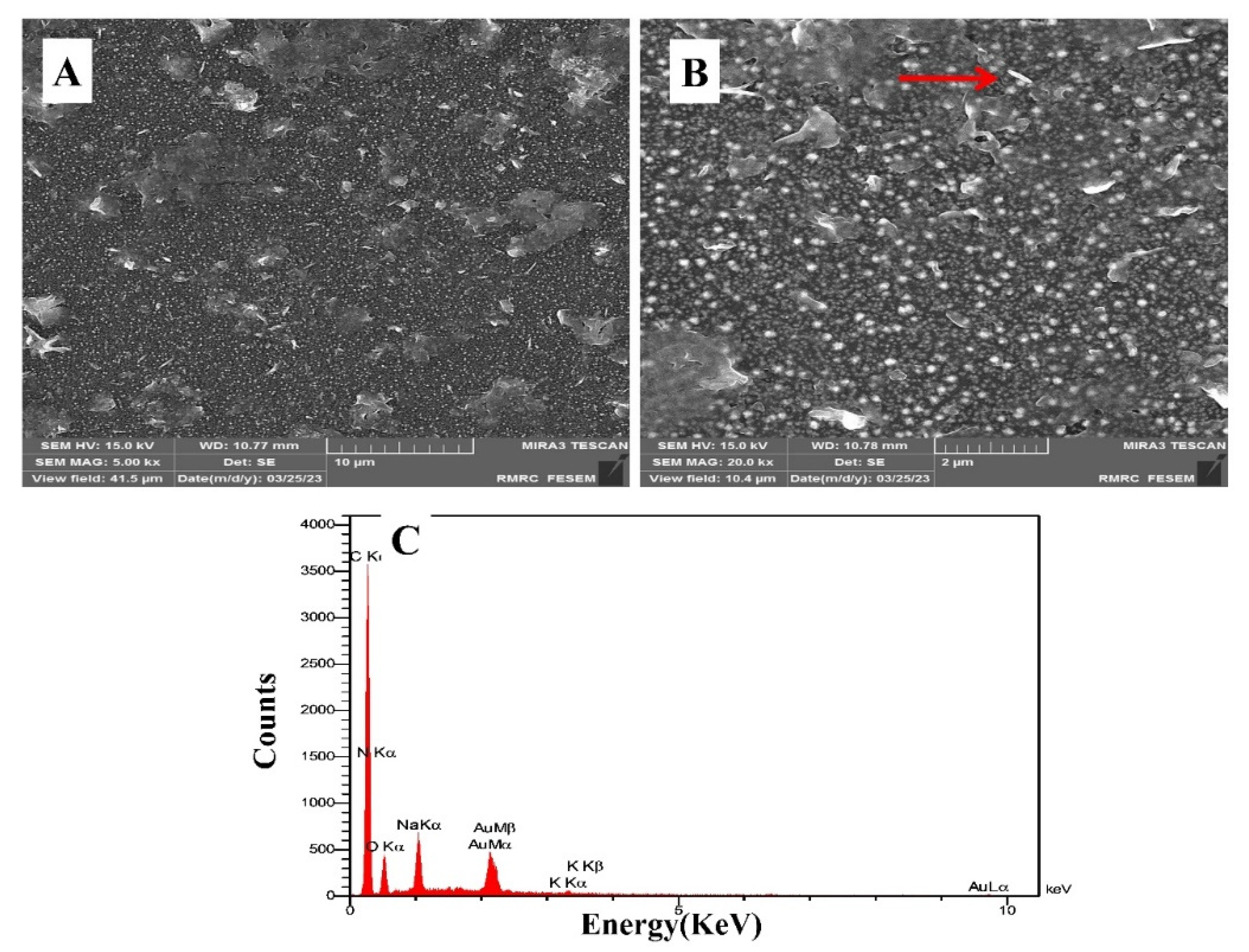
The morphologies of the AuNPs electrodeposited on the surface of GCE, under the deposition conditions mentioned in
Section 2.4, were characterized with FESEM images. The FESEM micrograph in
Figure 1A indicates that an appropriate layer of AuNPs with a nanoparticle size were formed on the surface of GCE; hence, as seen, the FESEM images indicated a satisfactory uniform coverage of the AuNPs on the surface of GCE. Also, to visualize the capture of bacteria by the PCAM-coated electrode, we used FESEM to confirm
E. coli on the sensor surface. The FESEM micrograph in
Figure 1B indicates the captured
E. coli on the electrode surface It is shown by the red arrow in the figure. Furthermore, it is established that Energy-dispersive X-ray spectroscopy (EDS) penetrates much more deeply and hence gives the bulk materials composition. As shown in
Figure 1C, the EDS spectrum confirms the presence of Au on the GCE surface and the peaks of C, N, and O in the EDS spectrum prove the stabilization of mannose on the surface of the electrode.
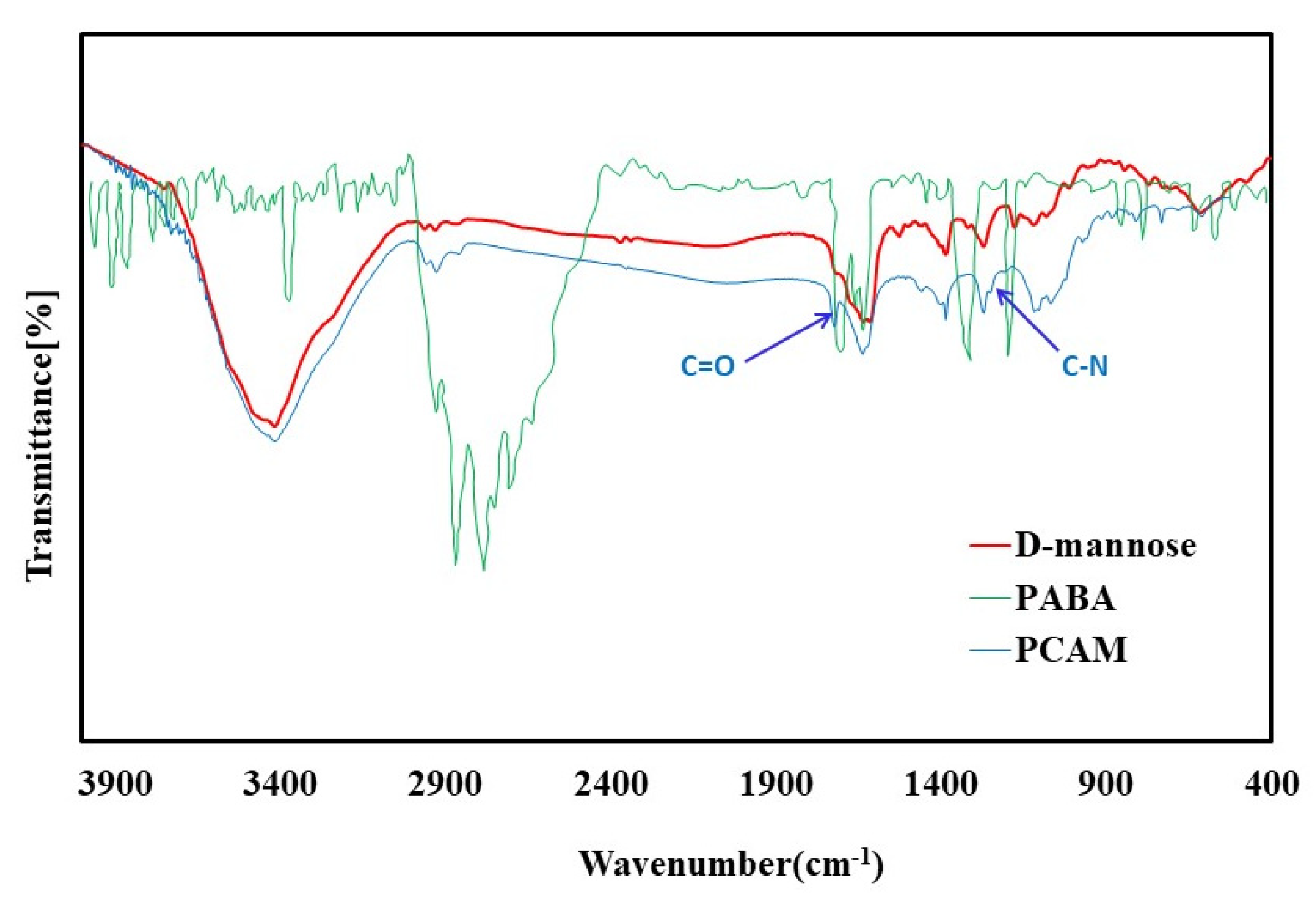
3.2. FTIR Characterizations
FTIR analysis was performed to evaluate the synthesis of the PCAM receptor. PCAM receptor was obtained from the reaction between two substances, para-aminobenzoic acid, and MAN. according to the FTIR spectrum of para-aminobenzoic acid, it is expected that seen in the FTIR spectrum of the PCAM receptor, compared to pure MAN, there is a peak corresponding to C=O at the wavenumber of 1710 cm
-1 Figure 2. in the FTIR of the PCAM receptor, this peak is seen in 1710 cm
-1 and this indicates the PCAM was successfully synthesized.
Also, the peak observed in the wavenumber range of 1342-1266 cm-1 is related to C-N, which is shown the presence of aromatic amine of synthesized PCAM; although, it is not seen in pure MAN and indicates the reaction between para-aminobenzoic acid and MAN.
3.3. Electrochemical characterization
The results of electrochemical measurements of EIS at each stage of the sensor fabrication in the K
3[Fe(CN)
6]/K
4[Fe(CN)
6] solution as a redox probe are shown in
Figure 2A. The R
et of bare GCE was obtained to be 250 Ω. After electrochemical deposition of AuNPs, R
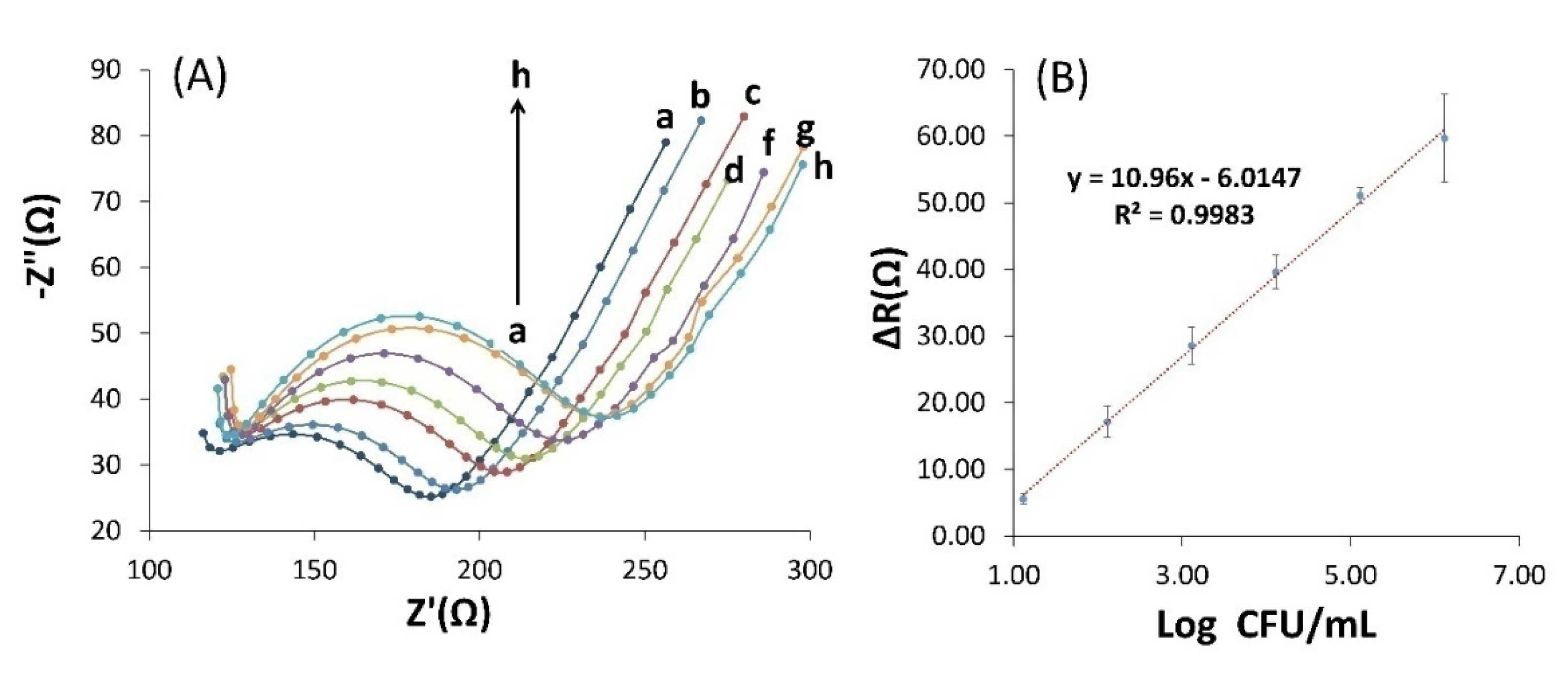
et was greatly reduced, and the resulting spectrum became almost linear, indicating that the charge transfer resistance was significantly reduced due to electrode coverage with high-conductive AuNPs. When the AuNPs/GCE electrode was immersed in the Cyst solution for stabilizing of PCAM (bioreceptor) on the surface of AuNPs/GCE, the diameter of the semicircle in the Nyquist diagram was increased that indicates an enhanced surface resistance to 60 Ω and successful stabilization of PCAM on the electrode surface according to Figure3A.
According to Figure3A, EIS measurements with increasing concentration of
E. coli showed an increase in R
et up to 120 Ω, which confirmed the detection of bacteria by the proposed biosensor. To further ensure the performance of the designed biosensor, DPV and CV measurements were performed simultaneously for different bacterial concentrations.CV was performed for further characterization of electrode surface modifications. The cyclic voltammograms were recorded within the potential range of −0.4 to 0.8 V. At recorded cyclic voltammograms for the different steps of the modified electrode showed the conductivity of bare GCE (a) that the conductivity of surface electrode extremely increased after electrodeposition of AuNPs on GCE surface (b); but after immobilizing of receptor (MAN) (c) and also, exposing the sensor to
E. coli bacteria (d) conductivity of sensor was decreased. These measurements are in good agreement with their results obtained from EIS, both sets confirm the successful fabrication and operation of biosensor (
Figure 3B). DPV results showed a current response decreased by increasing the concentration of
E. coli (
Figure 3C). In contrast to CV measurements, increasing the concentration of
E. coli decreased the redox current at the surface. The results of all three measurement methods confirmed each other.
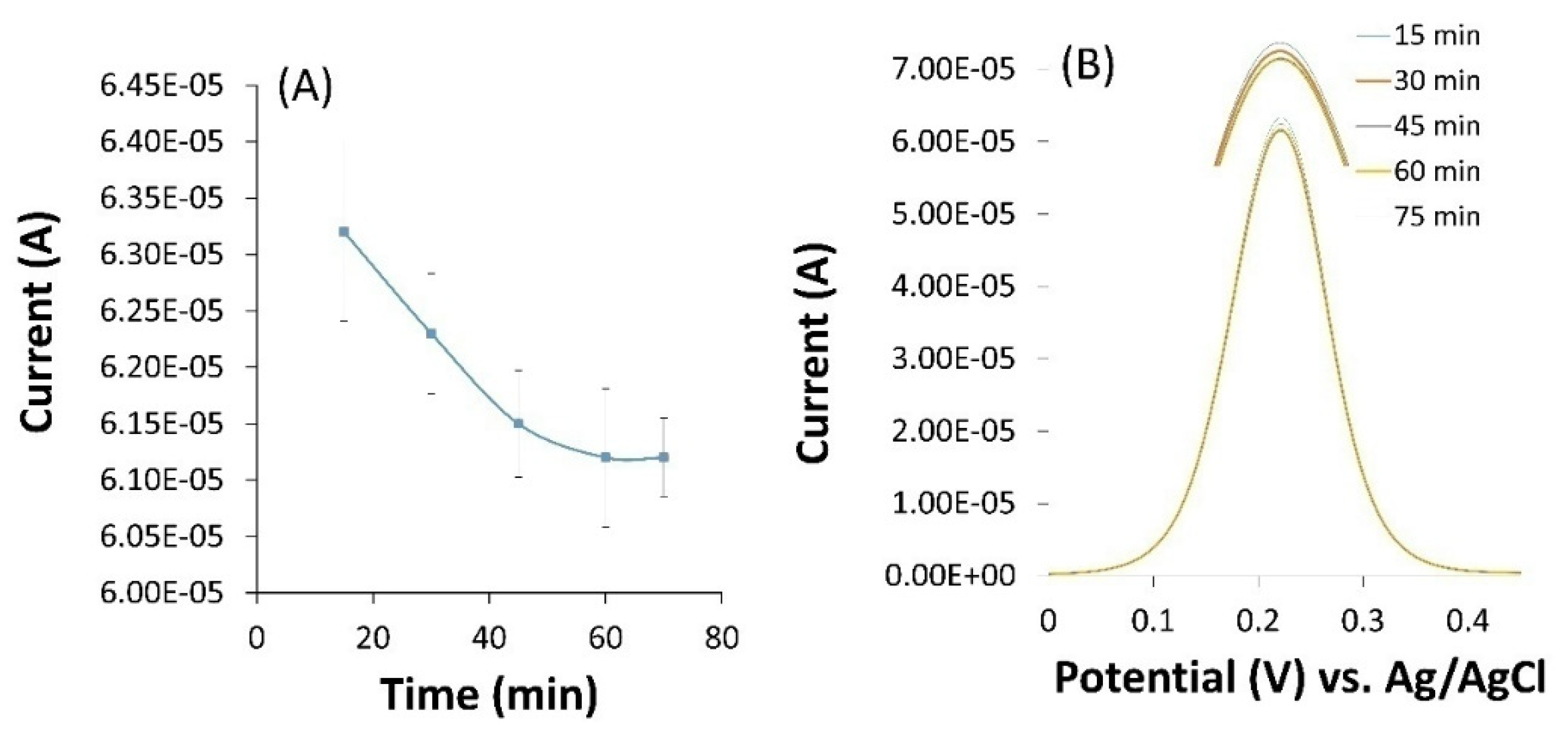
3.4. Optimization of incubation time
One of the advantages of biosensors over conventional methods of detecting bacteria such as culture medium is their high speed in detecting bacteria. In order to prove this and also to evaluate the proposed biosensor, the optimal incubation time to attach bacteria to the electrode surface was calculated. The modified electrode was incubated in
E. coli solution with 10
5 CFU.mL
−1 concentration. According to
Figure 4, the electrochemical response of the biosensor was increased until the electrochemical response of the biosensor was stable after 1 h incubation. Therefore, 1 h was chosen as the optimal incubation time for
E. coli in all experimental steps. This step of the experiment was repeated at least three times. The shorter incubation time means that the identification of bacteria by the biosensor will be done in a shorter period of time, which is one of the most important advantages of biosensors compared to conventional methods such as culture medium.
3.5. Biosensor calibration curve and limit of detection
The calibration curve was obtained using EIS at different concentrations of
E. coli bacteria. Changes in the EIS and DPV responses of the fabricated biosensor were evaluated at 1.3 × 10
-1 - 10 × 10
-6 CFU.mL
−1 concentrations of
E. coli. According to the results of EIS measurement, R
et values were obtained for each concentration, and ΔR
et was calculated from the obtained values. Then, ΔR
et versus logarithmic concentration of
E. coli was plotted in the range of 1.3 × 10
-1 - 10 × 10
-6 CFU.mL
−1. According to
Figure 5B, the calibration curve shows a linear range with the correlation equation y = 10.96x - 6.014 (R
2 = 0.998). The limit of detection (LOD) for biosensor was determined to be 2 CFU.mL
−1 (3 times the standard detection of the blank experiment: incubation in the absence of
E. coli) based on EIS measurement before and after the addition of
E. coli bacteria.
Compared to the previous works done with carbohydrate receptors to identify pathogenic
E. coli bacteria, the results of this study show a high linear range at 1.3 × 10
-1 - 10 × 10
-6 CFU.mL
−1. Further, the previous works were done with more expensive and complicated methods such as QCM or SPR. Although they obtained good accuracy and sensitivity, these methods were expensive and complicated. In addition, the use of intermediary materials caused more complexity and higher cost. In this research, a simple electrochemical method and an inexpensive electrode GCE as a working electrode and support for MAN were used for the first time.
E. coli bacteria were accurately and sensitively identified with 2 CFU.mL
−1 with a great linear range which shows the excellent performance of the proposed biosensor compared to previous works (
Table 1). Also, the proposed biosensor was evaluated after 23 days of storage in phosphate buffer, and no decrease in the efficiency of bacteria identification was observed by the proposed biosensor, which indicates the excellent stability of the biosensor.
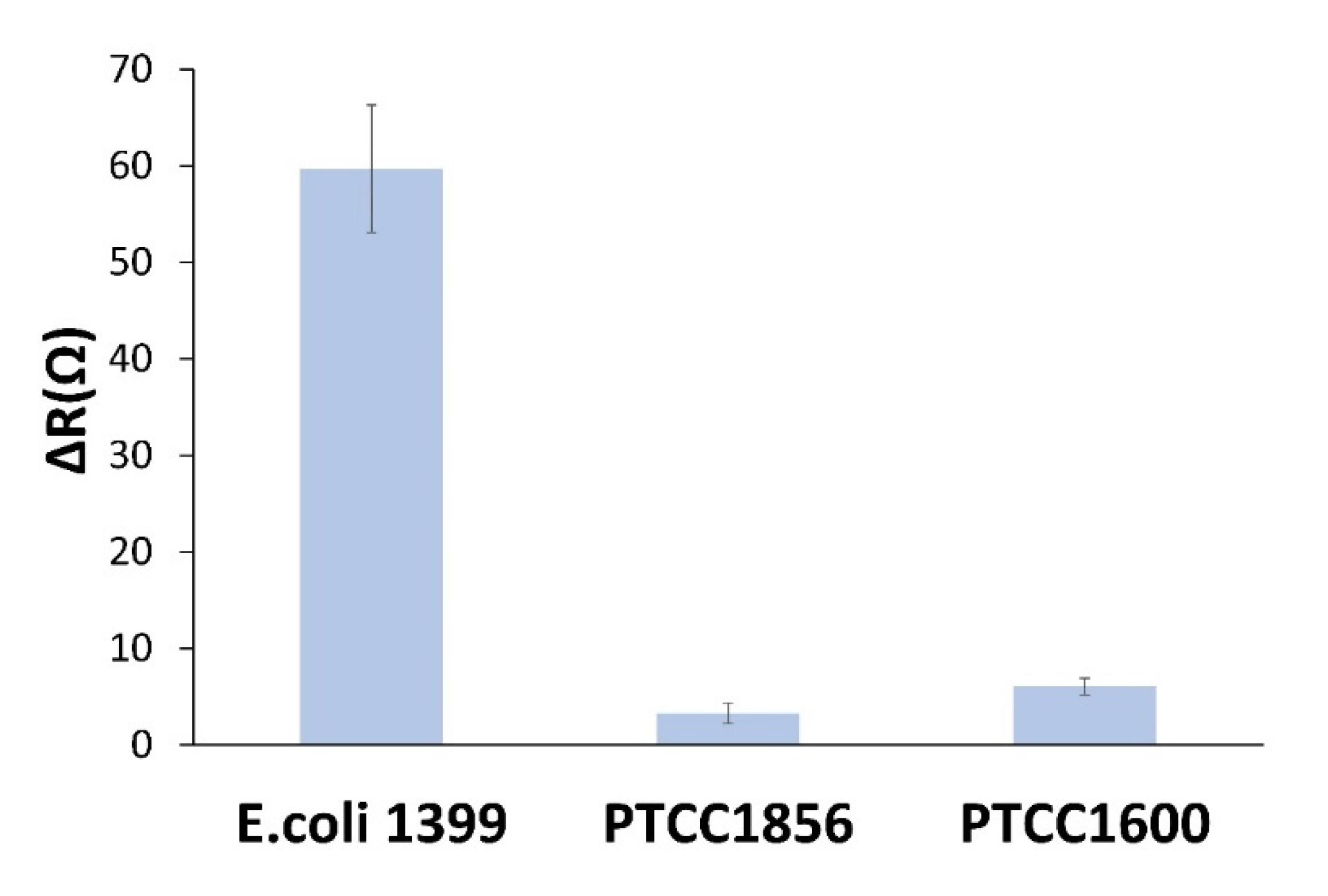
3.6. Selectivity of the biosensor
One of the most important parameters for evaluating the performance of a biosensor is the ability of the sensor to distinguish between target and non-target samples. The sensor specificity was evaluated in
E. coli samples of target and two non-target strains, including
Staphylococcus epidermidis (PTCC 1856) and
Citrobacter freundii (PTCC 1600), which tend to bind to MAN. The biosensor was incubated in the 10
6 CFU.mL
−1solution of each bacterium for 1 h, and EIS measurements were performed for all bacterial strains. As shown in
Figure 6, the impedimetric biosensor did not show a significant response to the two non-target bacterial species.
3.7. Real sample measurement
To evaluate the performance of the proposed biosensor in real samples, owing to the importance of water and food safety, the biosensor was tested in tap water sample and low-fat milk. Samples were spiked at target concentrations of 10,10
4, 10
5, and 10
6 CFU.mL
-1. The biosensors were then incubated in spiked samples. The results of biosensor recovery for water samples are reported in
Table 2. As can be seen from
Table 2, the obtained recoveries for lower concentrations of bacteria are more acceptable.
For the milk sample, in this study, we used the low-fat milk sample purchased from the supermarket without pre-preparation or screening. The graph obtained from the impedance measurement based on the logarithm of the bacterial concentration against the resistance changes for the final concentrations of 101 and 106 is drawn. The slope of the resulting line shows the change in resistance caused by the change in the number of bacteria, from the comparison of the slope of the resulting line of 11.593 with the slope of the curve calibration graph line of 10.96, the amount of deviation from the standard state of the biosensor was calculated as 5.46%. On the other hand, we know that the width from the origin of the graph depends on the properties of the fluid and as it is clear from the graph, the width from the origin in the graph of the valve is 584.58 Ω which is larger than the width from the origin in the graph of the sensor calibration curve is 6.014 Ω which can be due to the properties of proteins and impurities in milk.
4. Conclusion
A label-free carbohydrate-based biosensor was proposed for the sensitive and accurate identification of E. coli bacteria using MAN and AuNPs. FE-SEM, FTIR, EDS, EIS, and cyclic voltammetry were performed to characterize and confirm the electrode surface fabrication. The fabricated biosensor with advantages such as excellent low LOD 2 CFU.mL−1 was able to identify this pathogenic bacterium in a very short time of 1 hour and did not show a significant response to the non-target bacterial species, indicating the high selectivity of the proposed biosensor. The manufactured biosensor was used in a tap water sample and low-fat milk successfully with acceptable recoveries. which shows the prospect for fast and accurate on-site assay of environmental and food products. Another advantage of this biosensor is the use of a very inexpensive and available carbohydrate bioreceptor compared to other biosensors such as antibodies and aptamers. Also, the proposed method in this work can be used for other bacteria by changing the bioreceptor.
Acknowledgments
We: the authors, would like to thank the Petrochemical research center and development in Bandar Imam Iran (BIPC) for its financial and spiritual support.
Author Contributions
Sakineh Hargolzadeh: Conceptualization, Investigation, Writing – original draft. Soheila Kashanian: Supervision. Maryam Nazari: Data curation, Visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Petrochemical research center and development in Bandar Imam Iran (BIPC).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Doyle, M.P. Food Safety: Bacterial Contamination. In Encyclopedia of Human Nutrition (Third Edition); Caballero, B., Ed.; Academic Press: Waltham, 2013; pp. 322–330. [Google Scholar]
- Mead, P.S.; Slutsker, L.; Dietz, V.; McCaig, L.F.; Bresee, J.S.; Shapiro, C.; Griffin, P.M.; Tauxe, R.V. Food-related illness and death in the United States. Emerging infectious diseases 1999, 5, 607. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.V.; Rowe, B.; McConnell, M.M. In strains of Escherichia coli O167 a single plasmid encodes for the coli surface antigens CS5 and CS6 of putative colonization factor PCF8775, heat-stable enterotoxin, and colicin Ia. Infection and immunity 1987, 55, 1929–1931. [Google Scholar] [CrossRef] [PubMed]
- Krohn, M.A.; Thwin, S.S.; Rabe, L.K.; Brown, Z.; Hillier, S.L. Vaginal colonization by Escherichia coli as a risk factor for very low birth weight delivery and other perinatal complications. Journal of Infectious Diseases 1997, 175, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Banatvala, N.; Griffin, P.M.; Greene, K.D.; Barrett, T.J.; Bibb, W.F.; Green, J.H.; Wells, J.G. The United States National Prospective Hemolytic Uremic Syndrome Study: Microbiologic, Serologic, Clinical, and Epidemiologic Findings. The Journal of Infectious Diseases 2001, 183, 1063–1070. [Google Scholar] [CrossRef]
- Li, S.; Konoval, H.M.; Marecek, S.; Lathrop, A.A.; Feng, S.; Pokharel, S. Control of Escherichia coli O157: H7 using lytic bacteriophage and lactic acid on marinated and tenderized raw pork loins. Meat Science 2023, 196, 109030. [Google Scholar] [CrossRef]
- Dester, E.; Kao, K.; Alocilja, E.C. Detection of unamplified E. coli O157 DNA extracted from large food samples using a gold nanoparticle colorimetric biosensor. Biosensors 2022, 12, 274. [Google Scholar] [CrossRef]
- Chan, M.Y.; Smith, M.A. Infections in pregnancy. Comprehensive Toxicology 2018, 232. [Google Scholar]
- Mayer, K.; Eris, D.; Schwardt, O.; Sager, C.P.; Rabbani, S.; Kleeb, S.; Ernst, B. Urinary tract infection: Which conformation of the bacterial lectin FimH is therapeutically relevant? Journal of Medicinal Chemistry 2017, 60, 5646–5662. [Google Scholar] [CrossRef]
- Firon, N.; Ofek, I.; Sharon, N. Carbohydrate-binding sites of the mannose-specific fimbrial lectins of enterobacteria. Infection and immunity 1984, 43, 1088–1090. [Google Scholar] [CrossRef]
- McClure, E.M.; Goldenberg, R.L. Infection and stillbirth; Elsevier: 2009; pp. 182–189.
- Singh, A.; Poshtiban, S.; Evoy, S. Recent advances in bacteriophage based biosensors for food-borne pathogen detection. Sensors 2013, 13, 1763–1786. [Google Scholar] [CrossRef]
- Spagnolo, S.; De La Franier, B.; Davoudian, K.; Hianik, T.; Thompson, M. Detection of E. coli bacteria in milk by an acoustic wave aptasensor with an anti-fouling coating. Sensors 2022, 22, 1853. [Google Scholar] [CrossRef] [PubMed]
- Sobhan, A.; Jia, F.; Kelso, L.C.; Biswas, S.K.; Muthukumarappan, K.; Cao, C.; Wei, L.; Li, Y. A Novel Activated Biochar-Based Immunosensor for Rapid Detection of E. coli O157: H7. Biosensors 2022, 12, 908. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Rehman, A.; Liu, H.; Zhang, J.; Zhu, S.; Zeng, X. Glycosylation of quinone-fused polythiophene for reagentless and label-free detection of E. coli. Analytical chemistry 2015, 87, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Choudhary, S.; Chaudhari, R.; Jayant, R.D.; Joshi, A. 9 - Enzyme-based biosensors. In Bioelectronics and Medical Devices, Pal, K.; Kraatz, H.-B., Khasnobish, A., Bag, S., Banerjee, I., Kuruganti, U., Eds.; Woodhead Publishing, 2019; pp. 211–240. [Google Scholar]
- Yao, W.; Shi, J.; Ling, J.; Guo, Y.; Ding, C.; Ding, Y. SiC-functionalized fluorescent aptasensor for determination of Proteus mirabilis. Microchimica Acta 2020, 187, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Yang, X.; Wu, J.; Liu, Q.; Li, D.; Huang, S.; Xie, H.; Yu, Z.; Gan, N. Reusable electrochemical biosensing platform based on egg yolk antibody-labeled magnetic covalent organic framework for on-site detection of Escherichia coli in foods. Sensors and Actuators B: Chemical 2022, 369, 132320. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, H.; Deng, S.; Xiao, X.; Xiong, Y.; Peng, J.; Lai, W. An integrated colorimetric and photothermal lateral flow immunoassay based on bimetallic Ag–Au urchin-like hollow structures for the sensitive detection of E. coli O157: H7. Biosensors and Bioelectronics, 2023; 115090. [Google Scholar]
- Gupta, A.; Garg, M.; Singh, S.; Deep, A.; Sharma, A.L. Highly sensitive optical detection of escherichia coli using terbium-based metal–organic framework. ACS applied materials & interfaces 2020, 12, 48198–48205. [Google Scholar]
- Ngo, V.K.T.; Nguyen, D.G.; Nguyen, H.P.U.; Nguyen, T.K.M.; Huynh, T.P.; Lam, Q.V.; Huynh, T.D.; Truong, T.N.L. Quartz crystal microbalance (QCM) as biosensor for the detecting of Escherichia coli O157: H7. Advances in Natural Sciences: Nanoscience and Nanotechnology 2014, 5, 045004. [Google Scholar]
- Kaur, K.; Chelangat, W.; Druzhinin, S.I.; Karuri, N.W.; Müller, M.; Schönherr, H. Quantitative E. coli enzyme detection in reporter hydrogel-coated paper using a smartphone camera. Biosensors 2021, 11, 25. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Bie, S.; Suo, T.; Jia, G.; Liu, B.; Ye, R.; Li, Z. Development of an electrochemical biosensor for rapid and effective detection of pathogenic Escherichia coli in licorice extract. Applied Sciences 2019, 9, 295. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhang, X.; He, F. Rapid detection of Escherichia coli based on 16S rDNA nanogap network electrochemical biosensor. Biosensors and Bioelectronics 2018, 118, 9–15. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, H.; Hao, H.; Gong, Q.; Nie, K. Detection of Escherichia coli with a label-free impedimetric biosensor based on lectin functionalized mixed self-assembled monolayer. Sensors and Actuators B: Chemical 2016, 229, 297–304. [Google Scholar] [CrossRef]
- Yazgan, I.; Noah, N.M.; Toure, O.; Zhang, S.; Sadik, O.A. Biosensor for selective detection of E. coli in spinach using the strong affinity of derivatized mannose with fimbrial lectin. Biosensors and Bioelectronics 2014, 61, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Shahdost-Fard, F. Fabrication of an ultrasensitive ibuprofen nanoaptasensor based on covalent attachment of aptamer to electrochemically deposited gold-nanoparticles on glassy carbon electrode. Talanta 2015, 144, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Kulkarni, A.; Doepke, A.; Halsall, H.B.; Iyer, S.; Heineman, W.R. Carbohydrate-based label-free detection of Escherichia coli ORN 178 using electrochemical impedance spectroscopy. Analytical chemistry 2012, 84, 241–246. [Google Scholar] [CrossRef]
- Shaibani, P.M.; Etayash, H.; Jiang, K.; Sohrabi, A.; Hassanpourfard, M.; Naicker, S.; Sadrzadeh, M.; Thundat, T. Portable nanofiber-light addressable potentiometric sensor for rapid Escherichia coli detection in orange juice. ACS sensors 2018, 3, 815–822. [Google Scholar] [CrossRef]
- Shen, Z.; Huang, M.; Xiao, C.; Zhang, Y.; Zeng, X.; Wang, P.G. Nonlabeled quartz crystal microbalance biosensor for bacterial detection using carbohydrate and lectin recognitions. Analytical chemistry 2007, 79, 2312–2319. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).