1. Introduction
Epithelial cells form a continuous selective barrier that separates the external and internal environments. Tight junctions (TJs) have an important role in preserving and regulating this barrier [
1,
2]. TJs form a wall between the apical and basolateral domains of polarized cells and promote bi-directional signal transduction between the intracellular and extracellular environments [
1,
2]. TJs contain both transmembrane and cytoplasmic structural elements. They are also associated with numerous signal transduction pathways that regulate gene expression, epithelial cell proliferation, differentiation, and morphogenesis [
3]. TJs include bicellular TJs and tricellular TJs (tTJs) [
4,
5]. tTJs are located at the convergence of bicellular TJs, where three epithelial cells encounter polarized epithelia [
6]. Tricellulin is a molecular component of tTJs [
6], and angulin-1/lipolysis-stimulated lipoprotein receptor (LSR) is an integral membrane protein localized at tTJs [
7]. tTJs seal the intercellular space at the meeting point of three epithelial cells. Although tricellulin and angulin family membrane proteins have been identified as constituents of tTJs, the molecular mechanism of tTJ formation remains unknown [
8].
Short- and long-term exposure to air pollution induces and exacerbates respiratory diseases [
9], asthma [
10], and chronic obstructive pulmonary disorder (COPD) [
11]. More than 99% of the world’s population is exposed to unhealthy levels of air pollutants, reinforcing air pollution as a major public health priority worldwide [
12]. Air pollution adversely affects various bodily systems, suggesting there may be common pathways through which short- and long-term exposure to air pollution impact health [13-15]. Exposure to particulate matter (PM) and nitrogen dioxide can lead to systemic oxidative stress [
16], inflammation [
17], and immune activation [
18], contributing to pathophysiology across various organs. Exposure to air pollution has been connected with increased asthma-related mortality risk [
19]. High concentrations of outdoor air pollution have direct inflammatory and irritant effects on the airway epithelium, and at lower concentrations lead to airway hyperresponsiveness and inflammation, both observed in asthma [
20]. The pathological mechanisms of air pollutants include airway remodeling, oxidative damage, immune response induction, and sensitization to aeroallergens [
20].
There are few data [
21] on the impacts of air pollutants on asthma. To clarify the mechanisms by which air pollution impacts asthma, we focused on TJ proteins. Specifically, the aim of this study was to measure LSR expression levels in a mouse model of asthma with and without exposure to air pollution, and to assess the relationship between LSR levels and clinical variables in asthma patients.
Amieva, M. R., Vogelmann, R., Covacci, A., Tompkins, L. S., Nelson, W. J., Falkow, S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300: 1430-1434, 2003.
2. Methods
2.1. Patients
All subjects had a clinical diagnosis of asthma according to Global Initiative for Asthma (GINA) guidelines [
22] that was supported by one or more of the following criteria. This study was approved by the institutional review board of Soonchunhyang University Hospital (approval No. SCHBC 2017-12-013-003). The biospecimens and clinical data were provided by the biobank of Soonchunhyang University Bucheon Hospital, a member of the Korea Biobank Network. The clinical characteristics of patients and healthy individuals are presented in
Table 1.
2.2. Animals
The ethical approval for this study was approved by the Institutional Animal Care and Use Committee in Soonchunhyang University Bucheon Hospital (approval No. SCHBC-Animal-2020-06). Six-week-old female BALB/c mice were sensitized and challenged with ovalbumin (OVA) or TiO
2 as previously described [
23]. Mice in the TiO
2 nanoparticles groups were administered 200 µg/m
3 nanoparticles by inhalation at 1 hour before OVA challenge daily for 3 days. On day 24, Mice were anesthetized with 2.5 mg/kg tiletamine and xylazine (Zoletil and lumpum; Bayer Korea Co., Seoul, Korea) and AHR were assessed following challenges with 0, 6.25, 12.5, or 50 mg/mL methacholine (Sigma-Aldrich). Bronchoalveolar lavage fluid (BALF) was collected, and lung tissue was harvested for protein extraction and Diff-Quick staining, hematoxylin and eosin (H&E) and immunohistochemical (IHC) stain and confocal imaging.
2.3. Western blot
Protein extracts of mouse lung tissue were collected as previously described [
23]. Protein was separated by SDS-PAGE and transferred to p
olyvinylidene fluoride (PVDF) membranes. The membranes were blocked for 5% BSA in 0.1% Tween 20 in TBS for 2h at room temperature. And the membranes incubated with anti-LSR (1:1000, Cell Signaling, Massachusetts, USA), anti-TGFβ (1:1000, Santa Cruz, Dallas, USA) and anti-RAGE (1:1000, Santa Cruz, Dallas, USA) in overnight, 4℃. The next day, the membranes were incubated with horseradish peroxidase (HRP-)-conjugated secondary antibodies. Detection was performed using EzWestLumi plus western blot detection reagent (ATTO Corporation, Tokyo, Japan). The relative protein abundance was determined quantitatively by densitometric analysis after normalization to β-actin (Sigma-Aldrich).
2.4. Immunohistochemistry
Mouse lung sections were deparaffinized and rehydrated in an ethanol series. The IHC staining of LSR (1:400, Cell Signaling, Massachusetts, USA) was carried out as described previously [
23]. The images were captured light microscopy (Olympus DP Controller 70).
2.5. Immunofluorescence
Mouse lung sections was incubated with LSR followed by Donkey polyclonal anti-Rabbit IgG H&L (Alexa Fluor 488) (ab150073, Abcam, Cambridge, MA). Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) (Ab104139, Abcam, Cambridge, MA). The images were generated using the Leica image browser (Leica Microsystems, Milton Keynes, UK). Images were analyzed with the Image J program (National Institutes of Health, Bethesda, MD, USA), and stain density was quantified with an average of LSR arbitrary density numbers from 6–8 fields.
2.6. Enzyme-linked immunosorbent assays
LSR, High mobility group box protein 1 (HMGB1) and receptor for advanced glycation end products (RAGE) levels in plasma samples from patients with asthma were determined by enzyme-linked immunosorbent assay (ELISA; BT LAB, Mybiosource and Invitrogen). IL-1β, IL-4 and TNF-α levels in mouse lung protein samples were determined by ELISA (R&D systems, Inc). The minimum detection limit was set to 1.54 pg/ml, 19.5 pg/ml, 3 pg/ml, 2.31 pg/ml, 2.0 pg/ml or 1.88 pg/ml for LSR, HMGB1, RAGE, IL-1β, IL-4 or TNF-α according to the manufacturer’s recommendations.
2.7. Statistical analysis
Data with a normal distribution presented as means ± standard error of the mean (SEM) or median (range) using SPSS version 22 (SPSS, Chicago, IL) [
23]. Group differences were compared by two-sample t-tests, Mann-Whitney tests, or Pearson’s χ2 tests for normally distributed, distorted, and categorical data, respectively. Correlations between outcome measures were evaluated by calculating Pearson or Spearman correlation coefficients analysis. Values of
p<0.05 were deemed to indicate statistical significance.
3. Results
3.1. Patients with asthma characteristics
Forty-two patients with asthma (mean age, 72 years) and 9 healthy individuals (mean age, 50 years) were enlisted, the clinical feature of whom are shown in
Table 1. The initial FEV
1 and FVC, as well as the FEV
1/FVC ratio, were lower in patients with asthma compared with healthy individuals. Total IgE and eosinophil blood count were also higher in patients with asthma. Percent-predicted FEV
1, percent-predicted FVC, and FEV
1/FVC were lower in patients with asthma compared with patients with control subjects, while neutrophil blood count was higher.
3.2. Tricellular TJ protein LSR levels related with the clinicopathological characteristics of patients with asthma
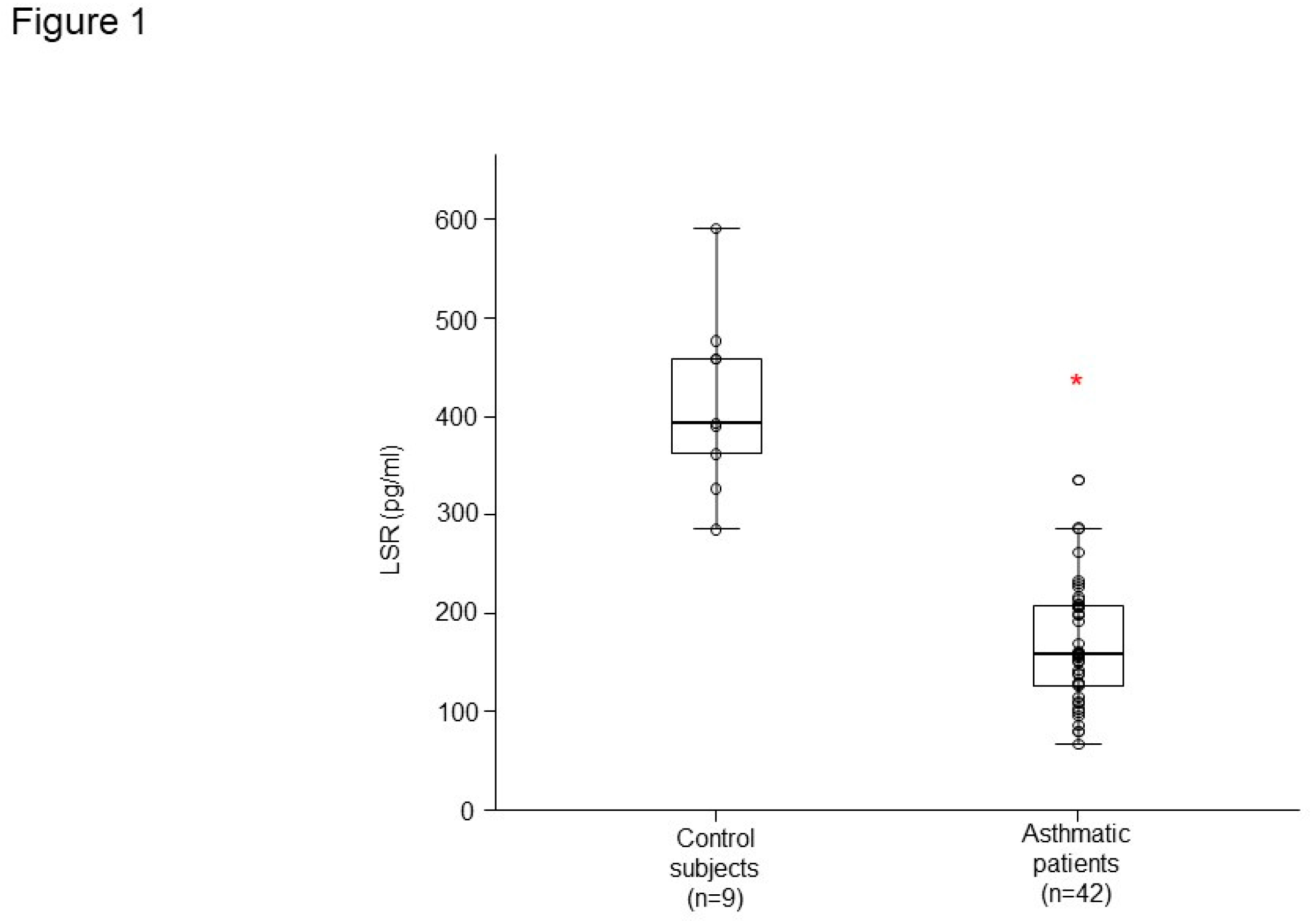
LSR level had lower concentrations in plasma from patients with asthma (n = 42) than that of healthy controls (n = 9) (
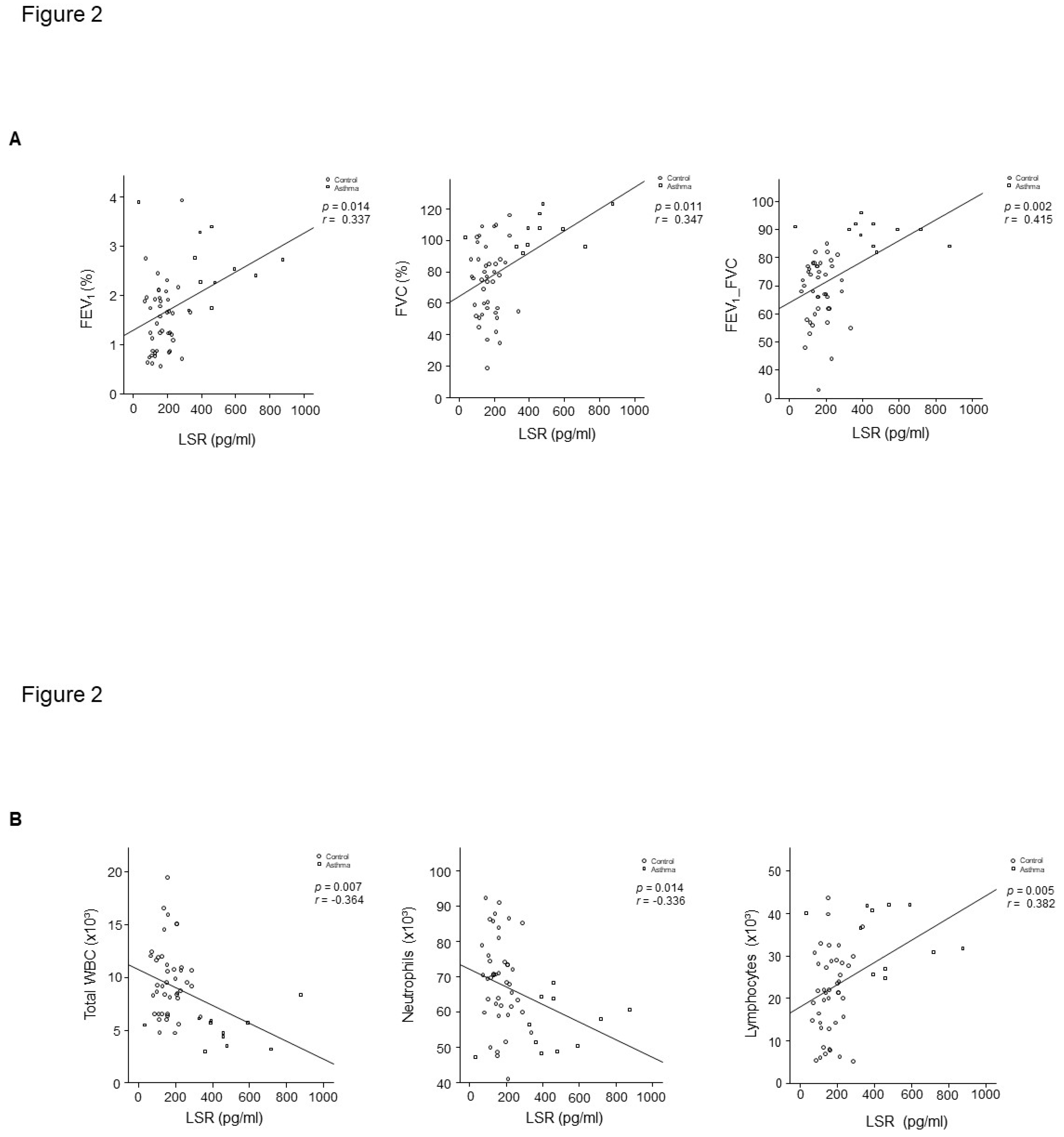
Figure 1). LSR level was correlated with percent-predicted FVC (r =0.347,
p = 0.011), FEV
1/FVC (r =0.415,
p = 0.002), WBC (r =- 0.364,
p = 0.007), and blood lymphocyte percent (r =0.382,
p = 0.005) in controls and patients with asthma (
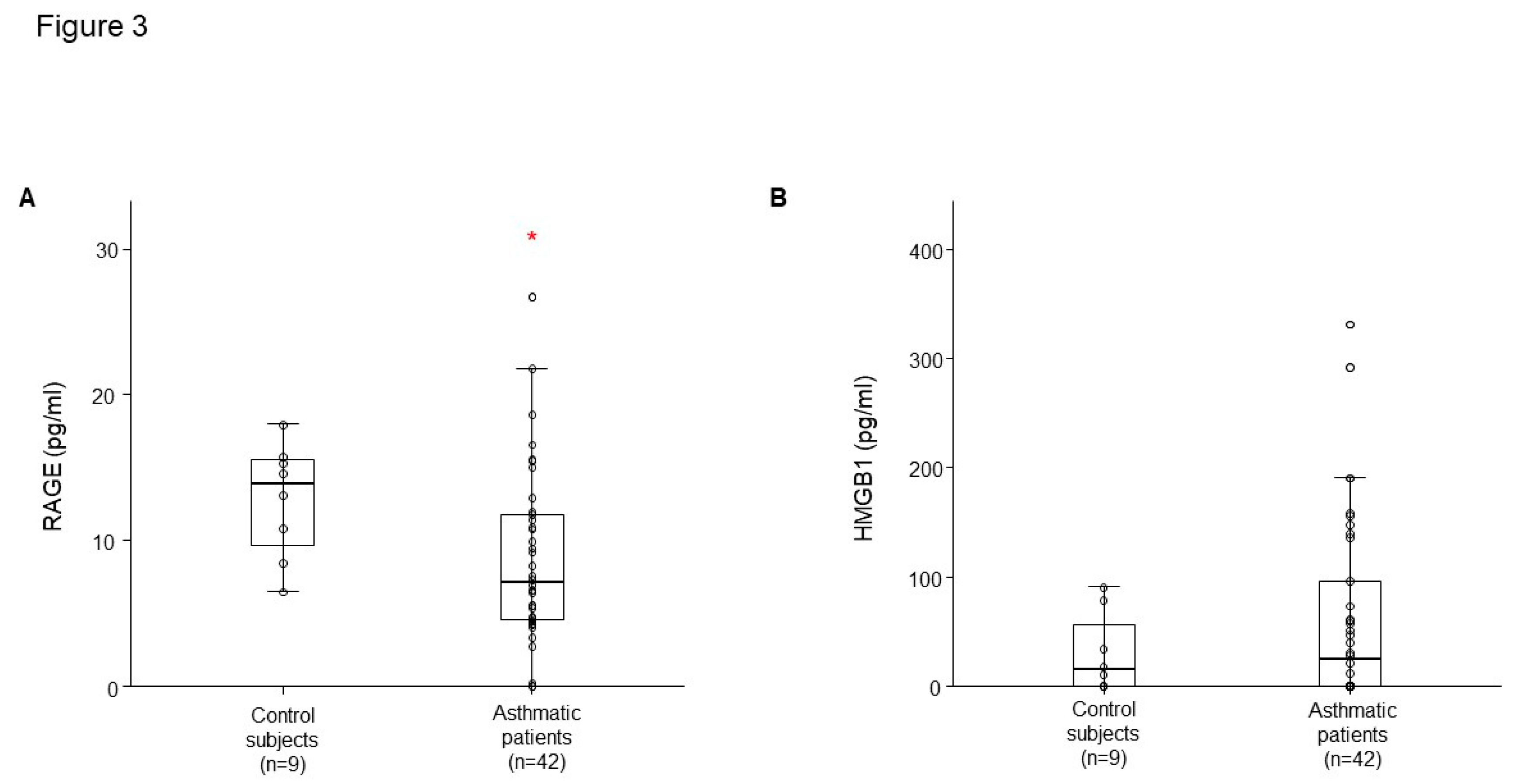
Figure 2A,B). RAGE level had lower concentrations in plasma from patients with asthma than that of healthy controls (
Figure 3A), HMGB1 level was not different (
Figure 3B).
3.3. OVA and TiO2-activated inflammation and AHR in mice
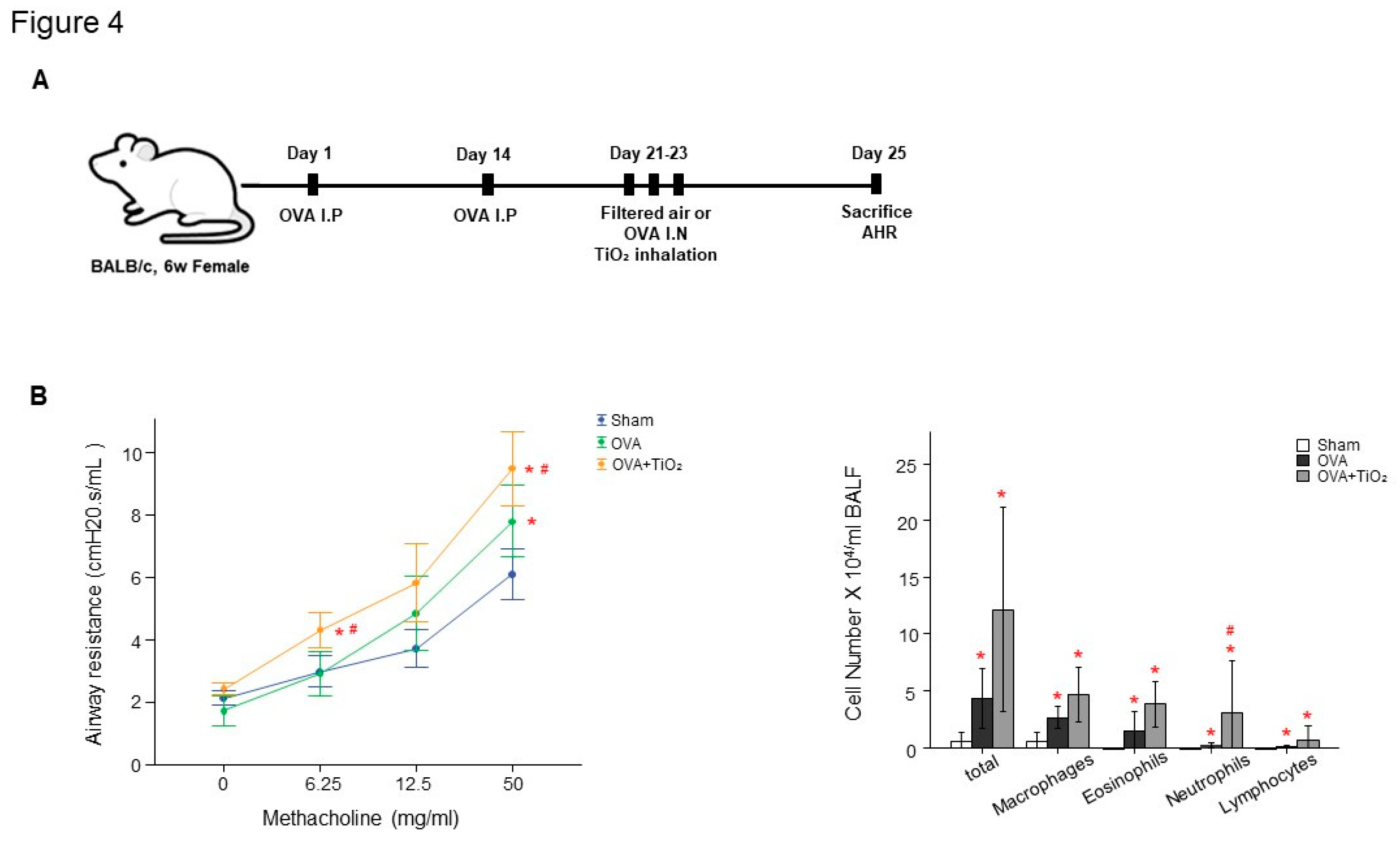
There was increased AHR in the OVA-sensitized/challenged and OVA/TiO
2 group compared to control mice (
Figure 4B). The OVA-sensitized/challenged mice and OVA/TiO
2 mice had increase in inflammatory cells in BALF compared to control mice (
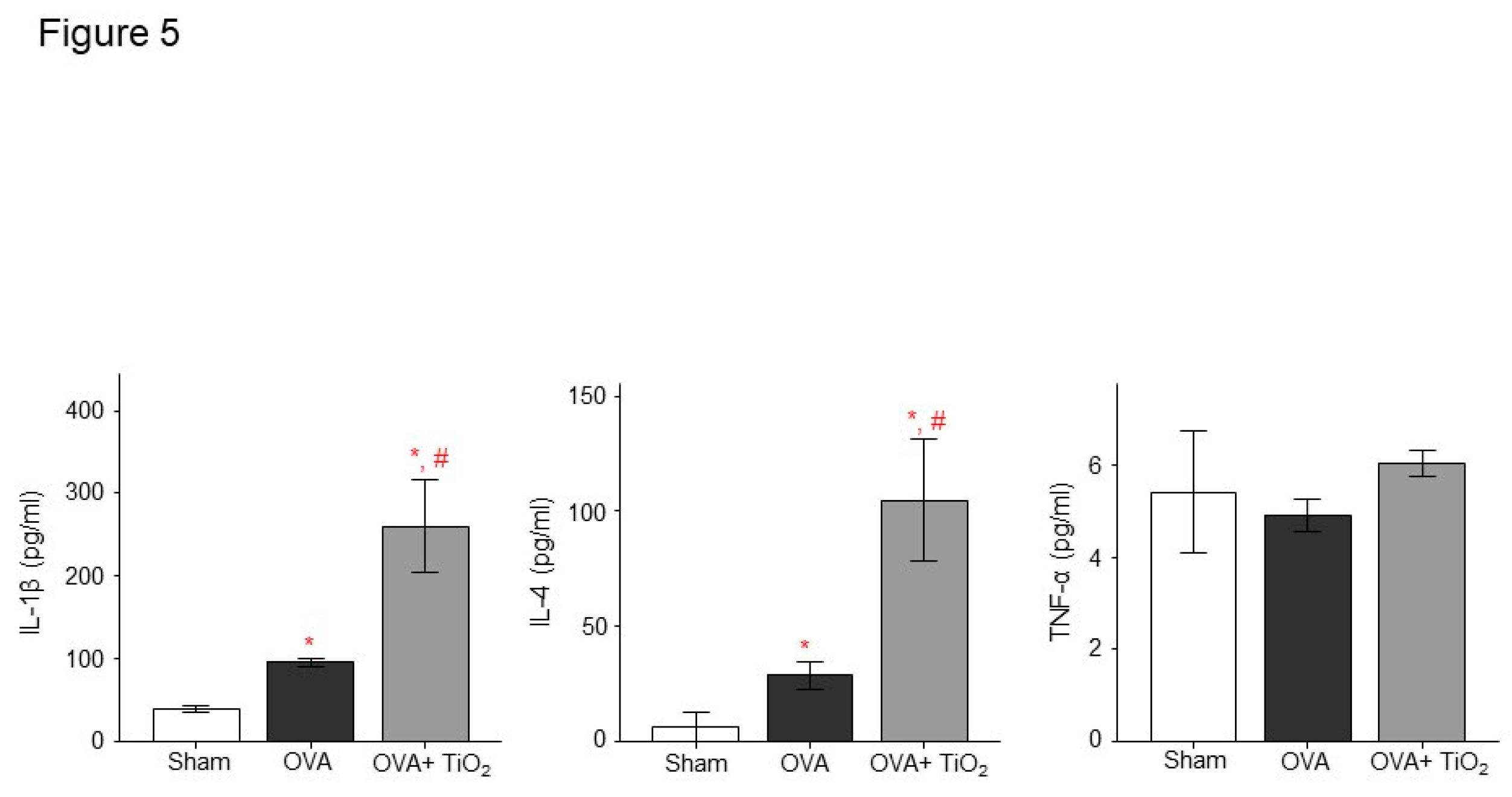
Figure 4C). OVA-sensitized/challenged mice group had resulted in increase of IL-1β and IL-4, and more increase in OVA/TiO
2 mice (
Figure 5) by ELISA using protein lysates from the lungs. Upon histologic examination, the OVA-sensitized/challenged mice and OVA/TiO
2 mice exhibited increase in inflammatory cell infiltration and exudative changes in the peribronchial layers and intraluminal areas of the bronchi (
Figure 6C).
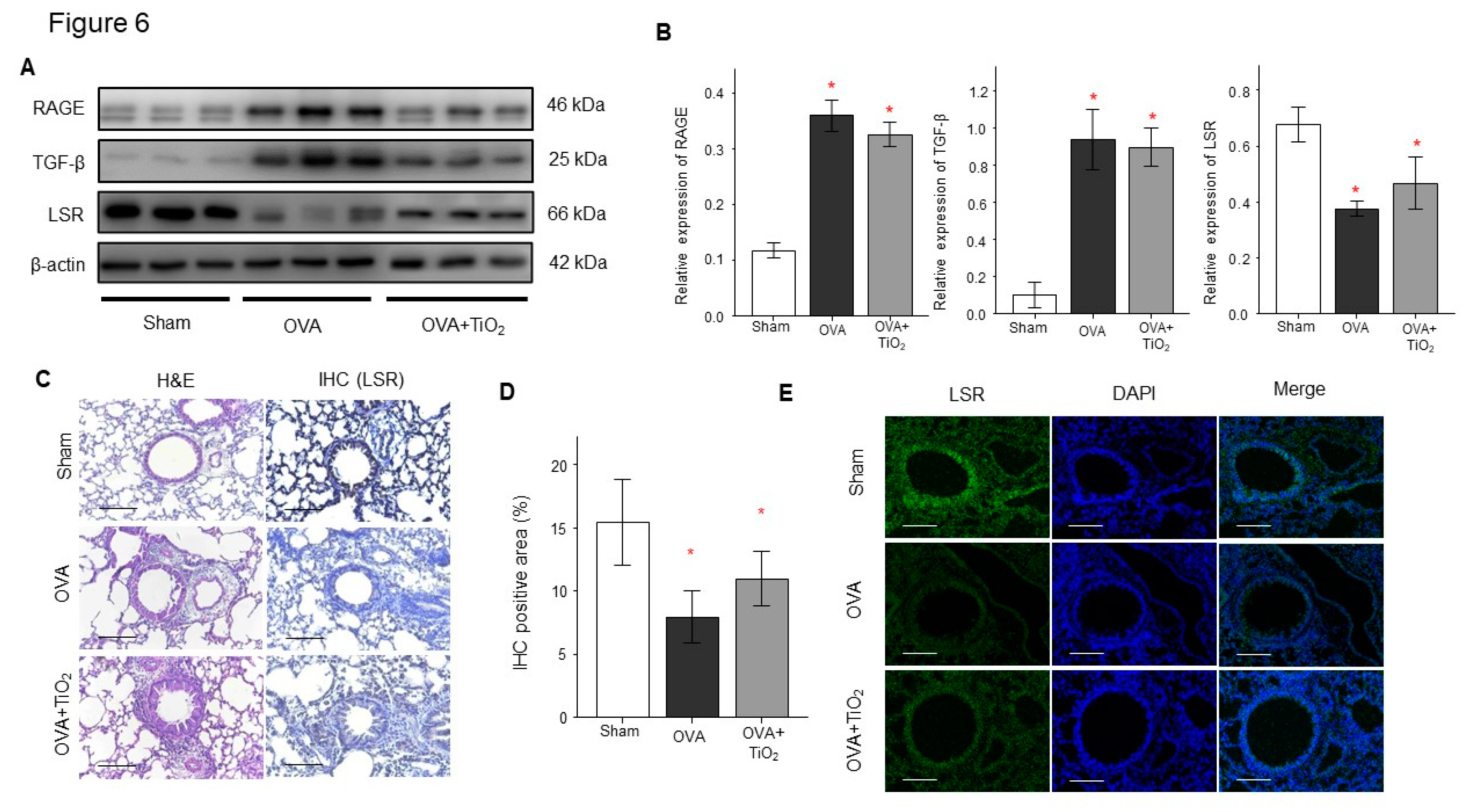
3.4. Change in RAGE, LSR and TGFβ in the lungs of OVA-sensitized/challenged mice and OVA/TiO2 mice
OVA-sensitized/challenged mice group had resulted in decrease of LSR and in increase of RAGE and TGFβ (
Figure 6 A,B) by Western blotting using protein lysates from the lungs. OVA/TiO
2 mice group resulted in change in RAGE, LSR and TGFβ by Western blotting using protein lysates from the lungs of mice (
Figure 6A,B). Additionally, IHC staining and immunofluorescence revealed that LSR expression were decreased in the lungs of OVA-sensitized/challenged mice compared with control mice, and changed in OVA/TiO
2 mice (
Figure 6C,D,E).
4. Discussion
Epithelial cells function as barriers, protecting the internal body from the external environment, and also forming diverse fluid compartments within various organs. TJs limit solute leakage via the paracellular pathway, contributing to the epithelial barrier function [24-26]. To maintain the full function of epithelial cells and paracellular barriers, there must be no extracellular space at tricellular contacts (TCs), i.e., the meeting point of three cells [27-32].
tTJs are supported by tricellulin and angulin family proteins [
33]. Tricellulin is a four-transmembrane protein, anatomically similar to occludin [
34]. Angulins are type I transmembrane proteins with a single immunoglobulin-like domain [33, 35]. Tricellulin and angulins are located alongside the central sealing elements of tTJs [33, 34]. Because angulins work on tricellulin to TCs [
33,
35], the angulin–tricellulin axis has a key role in tTJ formation [
36]. Despite an accumulation of research on the physiological roles of tTJ-related proteins, the molecular mechanism of tTJ formation remains to be fully defined. LSR is associated with the plasma membrane seal at TCs, separate from tricellulin and claudins [
37].
There have been no reports on the role of LSR in asthma. Therefore, the present study examined the roles of LSR in asthma using a mouse model and blood from asthma patients. LSR levels were decreased in the plasma of asthma patients, and were associated with clinical variables such as lung function, white blood cell count, and blood lymphocyte proportion. Similarly, LSR expression levels were decreased in asthmatic mice. These findings indicate that LSR has a pivotal role in the pathogenesis of asthma, and suggest that LSR may be a marker for asthma and allergic inflammation.
High mobility group box protein 1 (HMGB1), receptor for advanced glycation end-products (RAGE), and toll-like receptor 4 (TLR4) are key pathways for the formation of cigarette smoke-induced COPD inflammation. HMGB1 is a typical damage-associated molecular pattern protein, which mainly exerts its activity by binding to RAGE and TLR4, and is implicated in airway inflammation [38, 39]. RAGE, as a member of the immunoglobulin superfamily, is highly expressed in the lung (mostly in type I alveolar epithelial cells) but presents low expression in other organs and cells; it plays an important role in lung maturation and function [
40]. Under certain pathological conditions, RAGE is upregulated in response to the accumulation of ligands [
41]. In the present study, we found that plasma RAGE levels were decreased (similar to LSR levels) in asthma patients compared to healthy controls; But RAGE expression levels were increased in asthmatic mice. There was discrepancy of RAGE expression between blood level in asthmatics and lung tissue in mice, suggesting that may be different by target tissue and secretary form in the blood. HMGB1 levels did not differ between the two groups. These findings indicate that LSR and RAGE together are involved in airway inflammation in asthma. HMGB1 levels did not differ between the two groups. These findings indicate that LSR and RAGE together are involved in airway inflammation in asthma.
Air pollution represents a considerable risk factor for human health, and may cause many lung and respiratory diseases. Air pollutants can be categorized as either major (emitted directly into the atmosphere) or subsidiary (formed within the atmosphere) air pollutants [
42]. Asthma is a multiplex disease caused and exacerbated by increased exposure to various chemical, physical, and biological agents from diverse indoor and outdoor sources [
43,
44]. For instance, asthma symptoms such as coughing, wheezing, and shortness of breath have been implicated with short-term exposure to ambient PM
2.5 and PM
10 in prospective cohorts, particularly in children with atopy [
45]. In addition, exposure to traffic-related air pollution was associated with both persistent and new-onset asthma in adults in a cohort study [
46]. Moreover, living less than 200 m from a main roadway was correlated with greater odds of developing new asthma in middle-aged people who had had no asthma by age 45 [
46]. In the same study, asthmatic participants at 45 years had an increased risk of persistent asthma up to 53 years if they lived less than 200 m from a main roadway compared with asthmatic participants living farther than 200 m from a main roadway [
46]. Several studies have also found that short-term exposure to PM
2.5 produced by combustion can cause asthma attacks and exacerbate COPD [47-51]. In our study OVA-sensitized/challenged mice group had resulted in increase of IL-1β and IL-4 not TNF-α in mice lungs, and more increase in mice following TiO2 exposure as one of air pollutants.
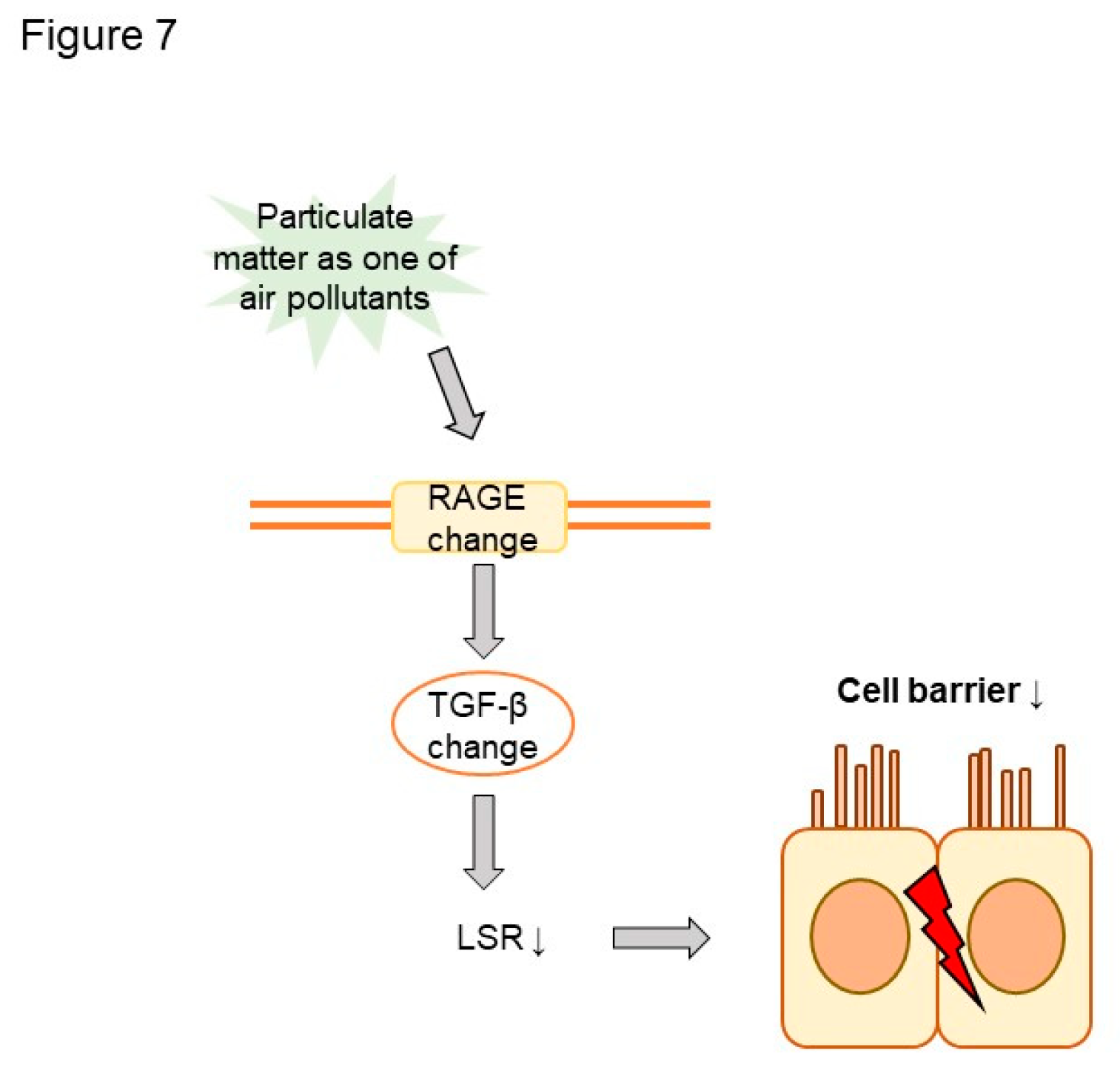
In this study, we examined the impacts of air pollution on LSR in asthma (
Figure 7). Plasma LSR levels were decreased in asthma patients. Moreover, LSR protein levels were decreased, and transforming growth factor beta (TGFβ) increased, in asthmatic mouse lung. Both LSR and TGFβ levels in asthmatic mice were altered by exposure to the air pollutant titanium dioxide, suggesting that air pollutant exposure can affect asthma phenotypes. Further studies are required to elucidate the precise role of LSR in air pollutant-induced airway inflammation. This study had limitations. First, it did not assess cell barrier function, such as transepithelial electrical resistance. Second, it relied on data with a small number of control subjects. Regardless, the present findings highlight the involvement of LSR in the pathogenesis of asthma and asthma phenotypes under air pollutant exposure.
Author Contributions
Conceptualization, A.S.J.; methodology, D.Y.H.; M.H.A.; investigation, A.S.J.; P.H.L.; J.H.K.; resources, S.P.; A.R.B.; data curation, Y.N.; writing—original draft preparation, A.S.J.; D.Y.H.; writing—review and editing, A.S.J.; P.H.L.; D.Y.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the institutional review board of Soonchunhyang University Hospital (approval No. SCHBC 2017-12-013-003). The care of animals was approved by the Institutional Animal Care and Use Committee in Soonchunhyang University (approval No. 2022-06).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2020R1A2C1006506) and Soonchunhyang University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb Perspect Biol. 2018, 10, a029181. [Google Scholar] [CrossRef]
- Kohno, T.; Konno, T.; Kojima, T. Role of Tricellular Tight Junction Protein Lipolysis-Stimulated Lipoprotein Receptor (LSR) in Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3555. [Google Scholar] [CrossRef] [PubMed]
- Matter, K.; Balda, M.S. Signalling to and from tight junctions. Nat. Rev. Mol. Cell Biol. 2003, 4, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Izumi, Y.; Oda, Y.; Higashi, T.; Iwamoto, N. Molecular organization of tricellular tight junctions. Tissue Barriers 2014, 2, e28960. [Google Scholar] [CrossRef] [PubMed]
- Guillot, C.; Lecuit, T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science 2013, 340, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Kenouchi, J.; Furuse, M.; Furuse, K.; Sasaki, H.; Tsukita, S.; Tsukita, S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005, 171, 939–945. [Google Scholar] [CrossRef]
- Masuda, S.; Oda, Y.; Sasaki, H.; Ikenouchi, J.; Higashi, T.; Akashi, M.; Nishi, E.; Furuse, M. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J Cell Sci. 2011, 124, 548–555. [Google Scholar] [CrossRef]
- Sugawara, T.; Furuse, K.; Otani, T.; Wakayama, T.; Furuse, M. LSR seals tricellular contacts independently of tricellulin and claudins. J Cell Biol. 2021, 220, e202005062. [Google Scholar] [CrossRef]
- Bălă, G-P. ; Râjnoveanu, R-M.; Tudorache, E.; Motiṣan, R.; Oancea, C. Air pollution exposure-the (in)visible risk factor for respiratory diseases. Environ. Sci. Pollu.t Res. Int. 2021, 28, 19615–19628.
- Jacquemin, B.; Siroux, V.; Sanchez, M. ; Carsin, A-E. ; Schikowski, T.; Adam, M, Bellisario, V.; Buschka, A.; Bono, R.; Brunekreef, B.; et al. Ambient air pollution and adult asthma incidence in six European cohorts (ESCAPE). Environ. Health Perspect. 2015, 123, 613–621. [Google Scholar]
- Park, J. ; Kim, H-J. ; Lee, C-H.; Lee, C.H.; Lee, H.W. Impact of long-term exposure to ambient air pollution on the incidence of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Environ. Res. 2021, 194, 110703. [Google Scholar]
- World Health Organisation WHO Global Air Quality Guidelines: Particulate Matter, Ozone, Nitrogen Dioxide, Sulfur Dioxide And Carbon Monoxide. 2021. Available online at: https://apps.who.int/iris/handle/10665/345334 (accessed April 19, 2022).
- Lee, P.H.; Park, S.; Lee, Y.G.; Choi, S.M.; An, M.H.; Jang, A.S. The Impact of Environmental Pollutants on Barrier Dysfunction in Respiratory Disease. Allergy Asthma Immunol. Res. 2021, 13, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, P.H.; Baek, A.R.; Park, J.S.; Lee, J.; Park, S.W.; Kim, D,J. ; Jang, A.S. Association of the Tight Junction Protein Claudin-4 with Lung Function and Exacerbations in Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Lee, P.H.; Choi, S.M.; An, M.H.; Jang, A.S. Effects of Air Pollutants on Airway Diseases. Int. J. Environ. Res. Public Health. 2021. 18(18):9905. [Google Scholar]
- Lodovici, M.; Bigagli, E. Oxidative stress and air pollution exposure. J. Toxicol. 2011, e487074. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, W.; Liu, Q.; Li, Z.; Lei, L. ; Ren, L,; Deng, F. ; Guo, X.; Wu, S. Association between gaseous air pollutants and biomarkers of systemic inflammation: a systematic review and meta-analysis. Environ. Pollut. 2022, 292, 118336. [Google Scholar]
- Glencross, DA. ; Ho, T-R. ; Camiña, N.; Hawrylowicz, CM.; Pfeffer, PE. Air pollution and its effects on the immune system. Free Radical Biol. Med. 2020, 151, 56–68. [Google Scholar]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet, CA, USA, 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, J.; Zhang, H.; Shi, C.; Li, G; Peng, Z. ; Ma, J; Zhang, L. Short-Term Exposure to Ambient Air Pollution and Asthma Mortality. Am. J. Respir. Crit Care Med. 2019, 200, 24–32. [Google Scholar] [CrossRef]
- Amieva, M. R.; Vogelmann, R.; Covacci, A.; Tompkins, L. S.; Nelson, W. J.; Falkow, S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 2003, 300, 1430–1434. [Google Scholar] [CrossRef]
- Kaplan, A.; van Boven, J.; Ryan, D.; Tsiligianni, I.; Bosnic-Anticevich, S. REG Adherence Working Group. GINA 2020: potential impacts, opportunities and challenges for primary care. J. Allergy Clin. Immunol. Pract. 2021, 9, 1516–1519. [Google Scholar] [CrossRef]
- Lee, Y.G.; Lee, S.H.; Hong, J.; Lee, P.H.; Jang, A.S. Titanium dioxide particles modulate epithelial barrier protein, Claudin 7 in asthma. Mol Immunol 2021, 132, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M.; Itoh, M. 2001. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Anderson, J.M. Architecture of tight junctions and principles of molecular composition. Semin. Cell Dev. Biol. 2014, 36, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: from simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Furuse, K.; Otani, T.; Wakayama, T.; Furuse, M. LSR seals tricellular contacts independently of tricellulin and claudins. J Cell Biol. 2021, 220, e202005062. [Google Scholar] [CrossRef] [PubMed]
- Wade, J.B.; Karnovsky, M.J. The structure of the zonula occludens. A single fibril model based on freeze-fracture. J. Cell Biol. 1974, 60, 168–180. [Google Scholar] [CrossRef]
- Walker, D.C. ; MacKenzie. A.; Hulbert, W.C.; Hogg, J.C. A re-assessment of the tricellular region of epithelial cell tight junctions in trachea of guinea pig. Acta Anat. (Basel). 1985, 122, 35–38. [Google Scholar]
- Staehelin, L.A. Further observations on the fine structure of freeze-cleaved tight junctions. J. Cell Sci. 1973, 13, 763–786. [Google Scholar] [CrossRef]
- Staehelin, L.A.; Mukherjee, T.M.; Williams, A.W. Freeze-etch appearance of the tight junctions in the epithelium of small and large intestine of mice. Protoplasma. 1969, 67, 165–184. [Google Scholar] [CrossRef]
- Ikenouchi, J.; Furuse, M.; Furuse, K.; Sasaki, H.; Tsukita, S.; Tsukita, S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 2005, 171, 939–945. [Google Scholar] [CrossRef]
- Masuda, S.; Oda, Y.; Sasaki, H.; Ikenouchi, J.; Higashi, T.; Akashi, M.; Nishi, E.; and Furuse, M. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J. Cell Sci. 2011, 124, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, J.; Sasaki, H.; Tsukita, S.; Furuse, M.; and Tsukita, S. Loss of occludin affects tricellular localization of tricellulin. Mol. Biol. Cell. 2008, 19, 4687–4693. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Tokuda, S.; Kitajiri, S.; Masuda, S.; Nakamura, H.; Oda, Y.; Furuse, M. Analysis of the ‘angulin’ proteins LSR, ILDR1 and ILDR2—tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J. Cell Sci. 2013, 126, 966–977. [Google Scholar] [CrossRef]
- Furuse, M.; Izumi, Y.; Oda, Y.; Higashi, T.; Iwamoto, N. Molecular organization of tricellular tight junctions. Tissue Barriers. 2014, 2, e28960. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Furuse, K.; Otani, T.; Wakayama, T.; Furuse, M. LSR seals tricellular contacts independently of tricellulin and claudins. J. Cell Biol. 2021, 220, e202005062. [Google Scholar] [CrossRef]
- Chen, L.; Sun, X.; Zhong, X. Role of RAGE and its ligand HMGB1 in the development of COPD. Postgrad. Med. 2022, 134, 763–775. [Google Scholar] [CrossRef]
- Lin, L.; Li, J.; Song, Q.; Cheng, W.; Chen, P. The role of HMGB1/RAGE/TLR4 signaling pathways in cigarette smoke-induced inflammation in chronic obstructive pulmonary disease. Immun. Inflamm. Dis. 2022, 10, e711. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet. 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Yonchuk, J.G.; Silverman, E.; Bowler, R.P.; Agusti, A.; Lomas, D.A.; Miller, B.E.; Tal-Singer, R.; Mayer, R.J. Circulating soluble receptor for advanced glycation end products (sRAGE) as a biomarker of emphysema and the RAGE axis in the lung. Am. J. Respir. Crit. Care Med. 2015, 192, 785–792. [Google Scholar] [CrossRef]
- Bowatte, G.; Lodge, C.J.; Knibbs, L.D.; Erbas, B.; Perret, J.L.; Jalaludin, B.; Morgan, G.G.; Bui, D.S.; Giles, G.G.; Hamiltion, G.S. Traffic related air pollution and development and persistence of asthma and low lung function. Environment International. 2018, 113, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Paterson, C.A.; Sharpe, R.A.; Taylor, T.; Morrissey, K. Indoor PM2.5, VOCs and asthma outcomes: A systematic review in adults and their home environments. Environ. Res. 2021, 202, 111631. [Google Scholar] [CrossRef] [PubMed]
- Misiukiewicz-Stepien, P.; Paplinska-Goryca, M. Biological effect of PM10 on airway epithelium-focus on obstructive lung diseases. Clin. Immunol. 2021, 227, 108754. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, P.M.; Harari, S.; Martinelli, I.; Franchini, M. Effects on health of air pollution: a narrative review. Intern. Emergency Medicine. 2015, 10, 657–662. [Google Scholar] [CrossRef]
- Adar, S.D.; Filigrana, P.A.; Clements, N.; Peel, J.L. Ambient coarse particulate matter and human health: a systematic review and meta-analysis. Current Environmental Health Reports. 2014, 1, 258–274. [Google Scholar] [CrossRef]
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.G.; Han, M. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: The GOLD Science Committee Report 2019. Eur. Respir. Journal. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Tian, Y.; Xiang, X.; Juan, J.; Song, J.; Cao, Y.; Huang, C.; Li, M.; Yonghua, H. Short-term effects of ambient fine particulate matter pollution on hospital visits for chronic obstructive pulmonary disease in Beijing, China. Environmental Health: A Global Access Science Source. 2018, 17, 1–8. [Google Scholar] [CrossRef]
- Nishida, C.; Yatera, K. The Impact of Ambient Environmental and Occupational Pollution on Respiratory Diseases. Int. J. Environ. Res. Public Health. 2022, 19, 2788. [Google Scholar] [CrossRef]
- Lee, Y.G.; Lee, P.H.; Choi, S.M.; An, M.H.; Jang, A.S. Effects of Air Pollutants on Airway Diseases. Int. J. Environ. Res. Public Health. 2021, 18, 9905. [Google Scholar] [CrossRef]
- Lee, P.H.; Park, S.; Lee, Y.G.; Choi, S.M.; An, M.H.; Jang, A.S. The Impact of Environmental Pollutants on Barrier Dysfunction in Respiratory Disease. Allergy Asthma Immunol Res. 2021, 13, 850–862. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).