1. Introduction
Nowadays toxic metal radionuclide accumulation particularly uranium is rapidly accelerated due to the enormous technological development that continuously strives to satisfy military needs and increasing demands for energy [1-2]. Uranium exhibits a very complex behaviour in water comprising a wide spectrum of transitions between reduced or oxidized species, hydrolysed forms and complexes. Furthermore, precipitate formation, colloid aggregation and sorption generate a multitude of chemical states that cause different side effects in the hydrosphere [1-3].
Adsorption proved the most effective technique for addressing the radioactivity of industrial wastewater and the decontamination of uranium-polluted aquatic systems among the other researched treatment methods (precipitation, solvent extraction, ion exchange). It is mainly based on the complexation of the predominant form of uranium in water i.e. the hexavalent uranyl cation (UO
22+) with the external groups of the adsorbing material (e.g., -NH
2, -COOH, -OH) producing inner-sphere complexes and the electrostatic interactions between the charged substrate surface and the opposite ionic species affording outer sphere analogues
[4-8]. Intensive investigations performed recently led to the discovery of exceptional adsorbing substrates distinguished for both their specificity and effectiveness. The field extends from purely inorganic compounds such as carbon allotropes [
9] metal oxides and minerals [
10] to organic-inorganic hybrids
[9, 11, 12], composites
[13-14], metal-organic frameworks (MOFs)
[15-17] and aerogels
[18-20]. Organic counterparts include conventional polymers
[21-22], biopolymers
[23-24] and dendritic polymers
[25-26].
Hyperbranched polymers represent one of the three major categories of dendritic polymers
[27-33]. Their polydispersity, inherited from their asymmetry is the major difference from the symmetrical dendrimers
[34-38]. Dendrons
[39-40] on the other hand, are fragments of dendrimers or hyperbranched polymers that emanate from a central focal point. Together with the more exotic variants of dendrigrafts [
41] and dendronized polymers
[42-45], they form the family of radially polymerized dendritic polymers
[46-48]: The fourth class of macromolecular architecture next to the linear, the branched and the cross-linked [
49].
Due to their particular irregular architecture, hyperbranched polymers resemble more closely the branched structures commonly encountered in nature. Some examples are the river deltas the roots of the trees the arteries of the blood circulatory system the veins of the leaves, the fractals, and the dendritic cells [
50]. The properties of hyperbranched polymers depend on their three main structural parts. The core or central focal point usually is formed by a similar type of monomers or by a functional moiety designed to introduce a specific functionality. The dense network of branches that generate internal cavities capable of hosting a wide variety of guest molecules or aggregates and the external-terminal groups that are responsible for the organization solubility, and reactivity of the macromolecules that frequently leads to their functionalization. The hyperbranched polymers share the same range of applications with their other dendritic polymers for instance in liquid crystals
[51-52] drug delivery
[53-56], antimicrobial protection
[57-63], diagnostics
[64-69], gene transfection
[70-72], and biosensors
[73-74]. They have a substantial contribution to the therapy of diseases such as cancer
[75-76], conditions of the central nervous system
[77-78] and rheumatoid arthritis
[79-80]. In some of the most important implementations, the hyperbranched polymers are combined with inorganic substrates [
81]. Examples of this category include (photo)catalysts
[82-84], implants [
85] and most notably water purification
[86-88]. In this context, our group has developed biomimetically silica-hyperbranched poly(ethylene imine) (PEI) nanospheres that proved capable of adsorbing a variety of pollutants [
89]. By fine-tuning the inorganic/organic content ratio this hybrid material was further evolved to gels that could be dried to xerogels. Gel formation optimally takes place in the pores of porous in particular ceramic substrates Apart from dispersions, they are thus also capable to formulate coatings and in consequence composite filters for continuous filtration. The scope of this work is to compare the two variants i.e. nanospheres with higher content in hyperbranched nanosponge against the more flexible and applicable xerogels under the demanding conditions required for the adsorption of U(IV) cation.
2. Materials and Methods
Hyperbranched poly(ethylene imines) PEIs (Mn = 2000, 5000, 25000 and 750000) were purchased from BASF, Ludwigshafen, Germany. Tetraethoxysilane, UO2(NO3) 2‧6H2O and P2O5 were obtained from Sigma-Aldrich, Steinheim, Germany, K2HPO4 from Carlo Erba, KH2PO4 from Merck and CoSO4 · 7H2O from Alpha Aesar. All compounds were used as received.
The existence of solubilized adsorbents was determined by UV–vis spectroscopy, employing a Cary 100 spectrophotometer. The samples before and after U(VI) adsorption were characterized by FTIR (KBr) spectroscopy (using an FTIR spectrometer 8900, Shimadzu) after drying the samples overnight in a vacuum oven at 70oC. The morphology of the nanospheres and the xerogels’ was observed by scanning electron microscopy (SEM) using an FEI Quanta Inspect instrument, also capable of Energy Dispersive X-Ray Spectroscopy (SEM-EDS) analysis. A thermal analysis study was conducted on a Setaram SETSYS Evolution 16/18, TGA/DSC analyzer. The samples were heated up to 700oC at a rate of 10oC/min and remained for 3 hours at the terminal temperature. The size of the aggregates at low pH before and after U(IV) sorption was determined in typical DLS experiments with the aid of an AXIOS-150/EX (Triton Hellas) with a He–Ne laser emitting at 658 nm and an Avalance detector at 90◦. Electrostatic interactions were monitored by ζ-potential measurements on a ZetaPlus (Brookhaven Instruments Corporation); the results represent the mean values of 10 measurements collected for each dispersion. N2-sorption experiments were performed on an Autosorb-1 gas analyzer with Krypton upgrade (Quantachrome Corp.) Before the isotherm measurements, the samples were outgassed overnight at 120 °C. The surface areas were calculated according to the Brunauer–Emmett–Teller (BET) equation
2.1. Preparation of Hybrid Silica-PEI Nanoparticles
The PEI core–silica shell composites were synthesized according to the standard method disclosed by Knecht et al. [90-91] for poly(amidoamine) PAMAM and poly(propylene imine) PPI dendrimers adapted for the non-symmetric PEI counterpart. Briefly, 10 ml of 20 mM (concerning the total of primary and secondary amine groups) hyperbranched PEIs solutions (for all 4 different Mws) were buffered at pH 7.5 by phosphates. Silicic acid (1 M) was prepared from the hydrolysis of tetramethyl orthosilicate in 5 mM HCl under intense stirring for 15 min. Silica precipitation was instantaneous after adding 1 ml to the dendritic polymer solution. The product was isolated by centrifugation (10 min 12,000 × g) and washed twice with water (yield 77%).
2.2. Preparation of Silica-PEI Xerogels
Acid hydrolysis of 5 mL tetraethoxysilane solution 1 M with 25µL HCl 1 M under stirring for 15 min afforded 1 M orthosilicic acid as above. Afterwards, 5 mL of PEI solutions (40 mM concerning the total of primary and secondary amine groups) were added, and the pH was adjusted to 7.5 with KHPO4. Following 2 h a hydrogel was formed and submitted to drying overnight under vacuum and over phosphorus pentoxide (P2O5) (Sigma-Aldrich, Steinheim, Germany) to form a silica/PEI xerogel.
2.3. Determination of Sorption Kinetics
Adsorption experiments of UO22+ on the composite nanoparticles and xerogel samples were conducted as described elsewhere [20, 92]. Briefly, aqueous solutions (25 mL) containing 0.01 g (0.4 g/L) of each adsorbate and varying concentrations of U(VI) at pH 3.0 or 4.0 were prepared and the cation adsorption was investigated under ambient conditions. The initial U(VI) concentration was varied between 1×10–6 mol/L and 0.1 mol/L, the contact time was set at 24 h and the determination of UO22+ in the solutions was carried out by UV-Vis spectrophotometry directly at higher concentrations and using Arsenazo-III at lower U(VI) concentrations [20, 92]. The effect of temperature was investigated at 298 K, 308 K and 318 K at a U(VI) concentration of 5×10–5 mol/L, and the kinetic experiments were carried out under similar conditions at 0.01 mol/L. The spectrophotometric method was calibrated before and after each experiment using reference solutions, which were prepared by dissolution of analytical grade UO2(NO3) 2‧6H2O in de-ionized water under similar conditions.
The isothermal data were fitted with the
Langmuir (Equation 1) and
Freundlich (Equation 2) isotherm models.
where q
e is the U(VI) uptake (in mol/kg) at equilibrium, C
e is the U(VI) concentration in solution at equilibrium (in mol/L), q
max is the adsorption capacity (in mol/kg) and K
L is the Langmuir equilibrium constant (in L/mol), K
F is the Freundlich constant, and n is the empirical adsorption intensity of the Freundlich model.
The amount of U(VI) adsorbed at time t, q
t (mol kg
–1), was calculated using Equation (3), where C
i (mol/L) is the initial U(VI) concentration in the solution, C
t (mol/L) is the final U(VI) concentration in the solution at time t, V (L) is the solution volume and m (g) is the weight of xerogel and nanoparticle samples.
The experiments were performed in duplicate and the mean values of the results have been used for data evaluation. The relative uncertainty of the values was below 10%.
3. Results and Discussion
3.1. Xerogel and Nanoparticle Characterization
3.1.1. Thermogravimetry
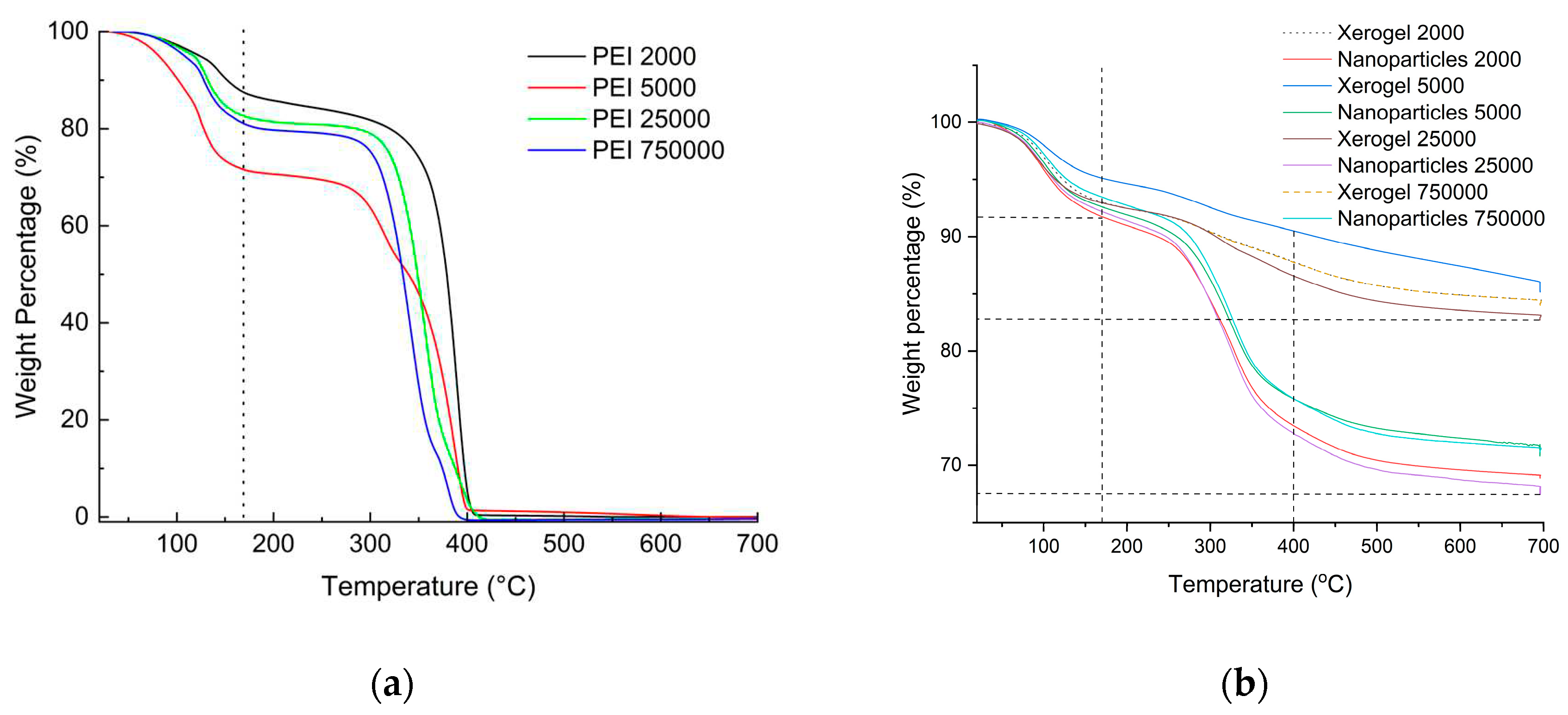
From the experimental section, it can be easily deduced that in the case of nanoparticles fivefold quantity of hyperbranched poly(ethylene imine) is used concerning the xerogels. A first assessment of the final composition of the adsorbing materials is obtained with thermogravimetry and by comparison of the thermal decomposition profiles of the dendritic matrices. As can be seen in
Figure 1a PEIs thermal decomposition profile contain roughly two major steps. The first up to 170
oC corresponds to the loss of humidity and potentially hydrogen-bonded ethanol deriving from the hydrolysis of tetraethoxysilane. The second depicts their total decomposition at 400
oC. The latter is completed with a considerable thermal hysteresis when the dendritic polymers are coupled with silica (
Figure 1b). From the percentages of organic content that derive from the deduction of the moisture-alcoholic fraction from the total weight loss percentages (
Table 1), it can be concluded that the organic content of the hybrid nanoparticles is more than double.
3.1.2. IR Spectroscopy
IR of Nanoparticles and Xerogels
Infrared spectra reveal more information about composite nanoparticles and xerogels.
Table 2 summarizes the assignment of the observed absorption bands along with PEI 25000 for comparison purposes. The typical amorphous silica vibrations are present [
93] whereas the majority of the PEI bands are weak and difficult to detect. Some of them appear as shoulders for instance the antisymmetric C-N stretching band at 1105 cm
-1 that is next to the broad Si–O–Si stretching at 1042 cm
−1 for the nanoparticles and 1074 cm
-1 for the xerogels while some others such as the respective symmetric C-N stretching vibration at 1045 cm
-1 and the CH
2 rocking vibrations at 760 cm
-1 are completely overlapped. The latter is under the Si–O–Si bending band at 784 cm
−1. All these observations are consistent with the typical spectra of silicas deriving from both biomimetic
[90, 94-95] and biogenic bio silicification
[96-98]. The reason for the lower absorption intensities of the organic matrices resides in their encapsulation into a ceramic silica entourage. From the few discerned, the N–H asymmetric stretching vibration appears at 3400-3370 cm
−1 and is partially overlapped by the broad absorption of the hydrogen-bonded Si-OH groups that are not transformed into siloxanes (3250 cm
−1). Additionally, both symmetric and asymmetric stretching of CH
2, are present at 2981 and 2820 for nanoparticles, and 2962 and 2853 cm
−1 for the xerogels [
99]. They exhibit a shift to higher wavelengths in comparison to the respective PEI 25000 bands (2935 and 2810 cm
−1 respectively) due to the protonation of the PEI primary and secondary amine groups that balance the negative change of the Si–O− groups (stretching band at 964 cm
−1) of the developing silica gels and the forming nanoparticles. An analogous shift is encountered in the FTIR spectra of PEI–H
2SO
4 complexes [
100]. This formation of ammonium groups additionally affects the N-H stretching, mainly the asymmetric [
101]. The presence of the ammonium groups is further established by two new absorption bands at 1524 and 1473 cm
−1 for the nanoparticles and 1530 cm
-1 for the xerogels that correspond to the NH
x+ symmetric and asymmetric bending of the Si–O
-···NH
x+ associations
[102-103]. The characteristic Si-O-Si rocking (540 cm
-1) band is also present [
104] while a small sharp band observed at 3750 cm
-1 in the case of xerogels is attributed to the SiO-H stretching of the non-hydrogen bonded silanol groups.
3.1.3. BET
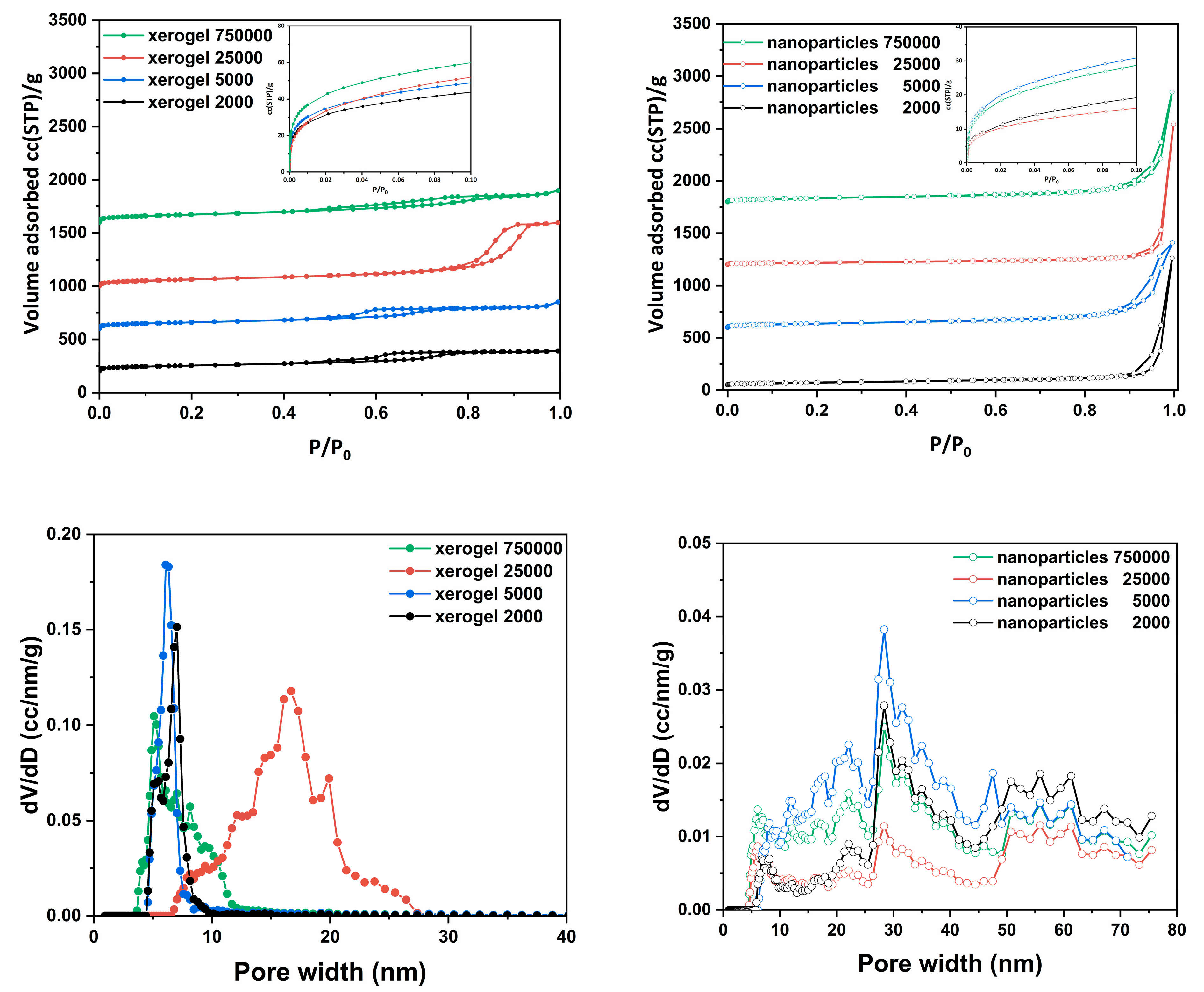
N
2 adsorption isotherms were performed to determine the structural characteristics of the samples, such as pore size distribution, total pore volume and specific surface area. Through the nitrogen adsorption isotherms at 77 K were obtained the amounts of adsorption (
Figure 2a–d), with the BET surface areas [
105] (Brunauer–Emmett–Teller) between ~100 and 270 m
2/g and the measured total adsorbed amount which was fluctuated between 309 and 1900 cc/g (STP) and 1250 and 2850 cc/g (STP) for xerogel and nanospheres samples respectively (
Table 3).
For the pore size distribution, the BJH (Barret, Joyner, and Halenda) model [
106] was applied to the desorption branch of the isotherm. The pore sizes were determined to be between 2 and 100nm for all studied samples. As we can see (
Figure 2) each material’s group presents a different type of N
2 adsorption isotherm. The xerogel samples present similar (
Figure 2a), of type IV
(a) with H4 hysteresis loop, isotherms [
107]. The main difference among the four isotherms is in the different total absorbed amounts and in the case of “xerogel 25000”, this with the highest pore sizes and the largest pore volume, a slightly wider hysteresis loop is also recorded at higher relative pressures. The existence of these, reproducible, permanent hysteresis loops, is generally associated with capillary condensation and in this case, can be attributed to network effects [
107]. The type of the presented hysteresis loop, although seems to be equal to H4, practically is H1 but in an outstretched orientation because of the shape of the adsorption isotherm. This type of loop is found in materials which exhibit a narrow range of uniform mesopores, for instance in templated silicas, some controlled pore glasses and ordered mesoporous carbons. Usually, network effects are minimal and the steep, narrow loop is a clear sign of delayed condensation on the adsorption branch. However, Type H1 hysteresis has also been found in networks of ink-bottle pores where the width of the neck size distribution is similar to the width of the pore/cavity size distribution [
107].
With the exemption of the xerogel 25000, which presents the highest pore size and the largest pore volume for the other xerogel samples the molecular weight seems to be a crucial parameter which determines the total pore volume and the surface area but not the formatted pore size which remains almost the same. The formatted hysteresis loops are classified close to the H3 type and indicate the existence of a pore network consisting of macropores which are not filled with pore condensate [
107]. On the other hand, the nanoparticles samples present lower surface areas but higher total pore volume and pore sizes (
Table 3). The N
2 adsorption isotherms (
Figure 1b) are similar to type I
(b) with a sharp uptake at the higher relative pressures, after P/P
0 > 0.9, which formats a hysteresis loop during the desorption branch. The pore sizes of the nanoparticles samples are significantly larger than those of xerogel samples but overall their specific surface area is considerably smaller.
3.1.4. Size (DLS) and Charge (ζ-potential)
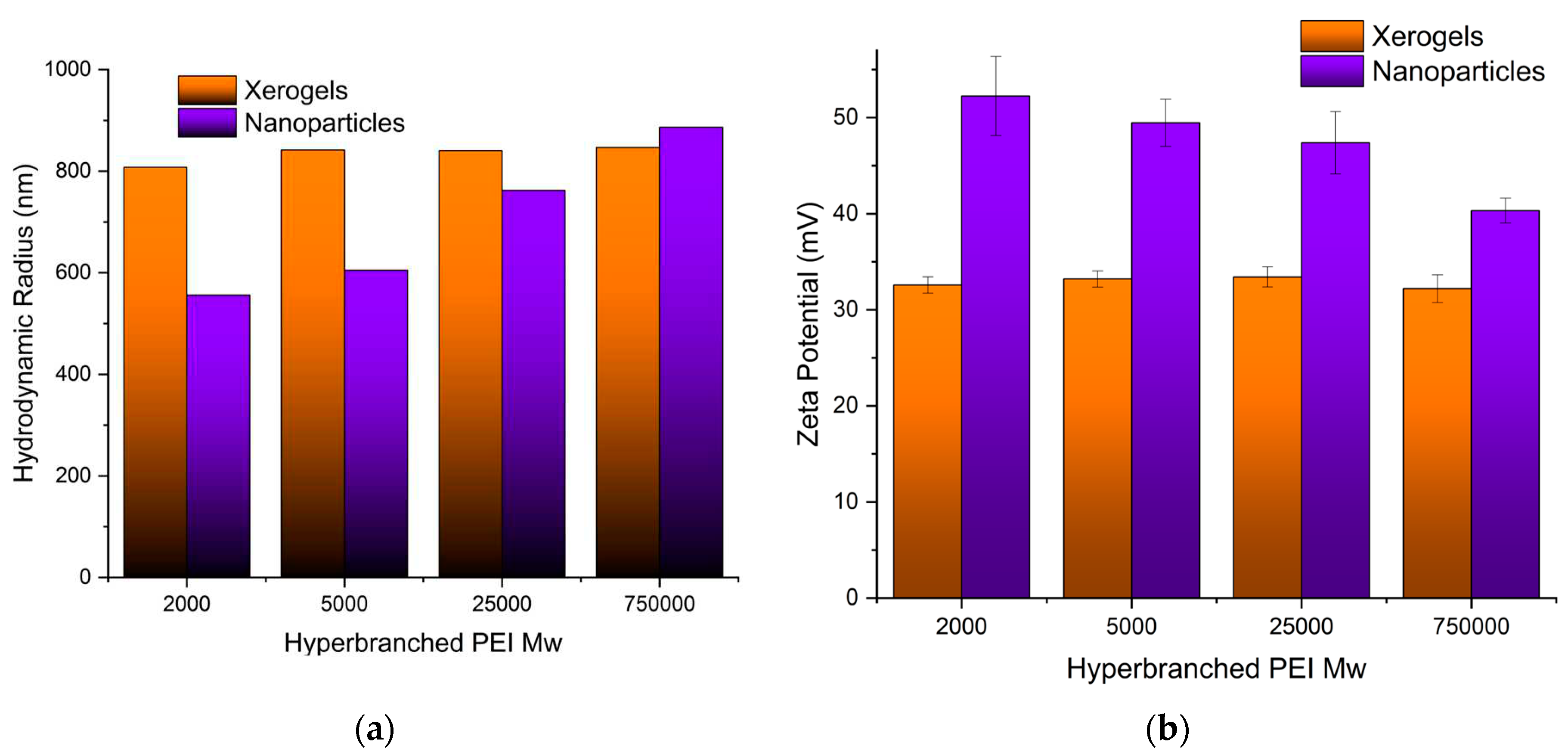
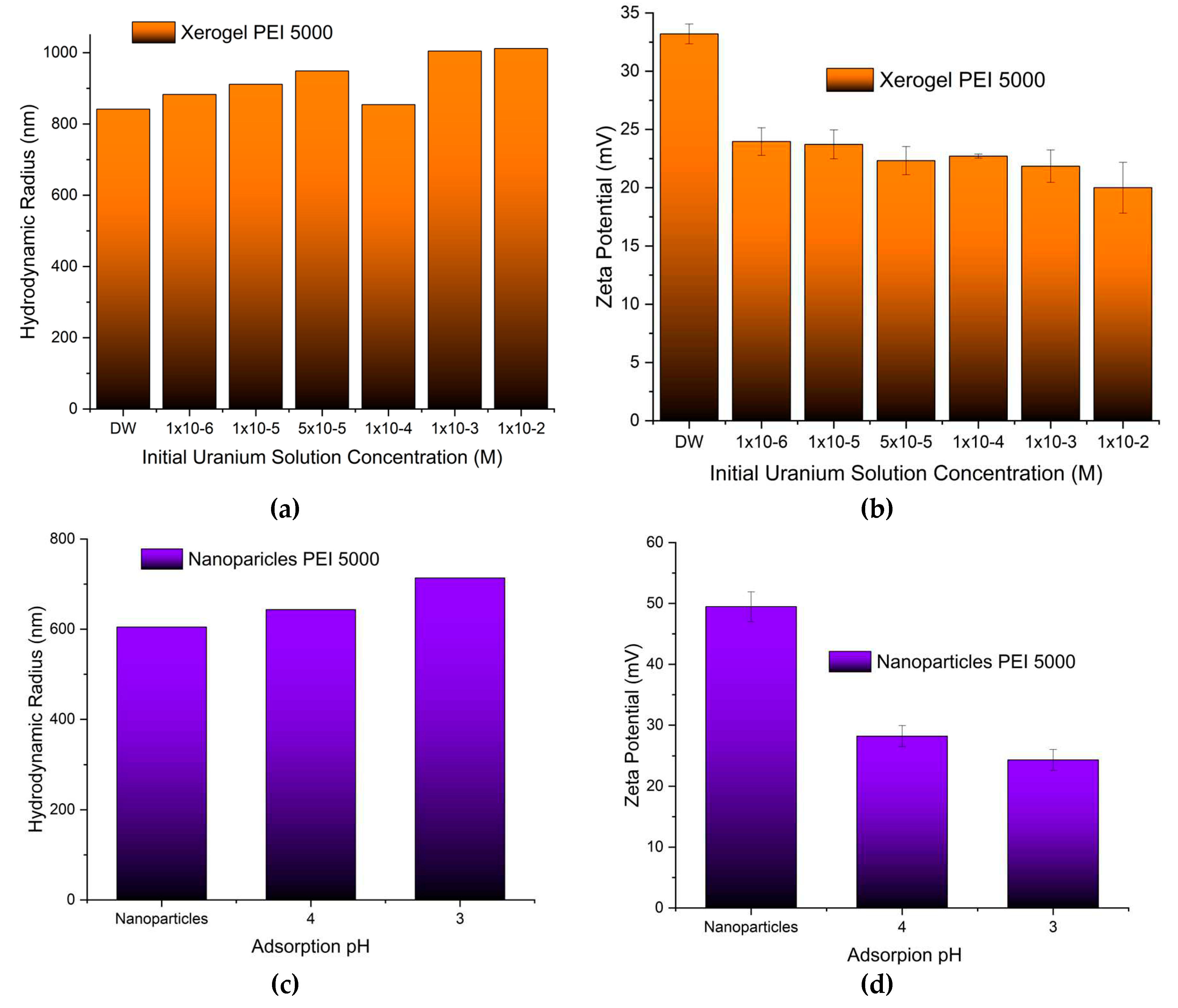
Both the size of the pure adsorbents and the charge that develops at the interface between the surface of their solvation sphere and the liquid were measured at pH 3 obtained by nitric acid to simulate the conditions of the uranyl cation adsorption. Dynamic light scattering measurements of the PEI 5000 nanoparticles (
Figure 3a) revealed aggregates with much bigger hydrodynamic radii (605 nm) than those measured in phosphate buffer pH 7 (384 nm) [
89] indicating the development of bulkier solvation spheres around them most probably due to the larger concentration of the nitrate anions. The nanoparticle size increases by increasing Mw of hyperbranched PEI. On the other hand, the particle’s charge decreases at the bulkier solvation spheres (
Figure 3b) reflecting simply the fact that the same concentration of ammonium cations is distributed in a larger volume. In contrast to the hybrid silica nanoparticles, the size and charge of the xerogel aggregates remain independent of PEI Mw. In this case, there are no separate silica shell polymer core entities but dendritic “islands” dispersed in a continuous ”sea” of silica.
3.2. Adsorption Kinetics
Silica is a well-known heavy metal adsorbent from water and this property has been correlated with the electrostatic attractions between the positively charged metal ions and the negatively charged external silanol groups [108-109]. In this case, though at pH 3, all adsorbing composites are positively charged and electrostatic interactions are unfavourable. Uranium removal is attributed mainly to the presence of the dendritic polymer and the formation of inner-sphere complexes. This behaviour is analogous to that of the negatively charged dichromates and simple and hybrid silica nanoparticles [
89]. Generally, the nanoparticles adsorb faster and about twice as much uranium as xerogels while equilibrium for the former is established in about 2 hours (
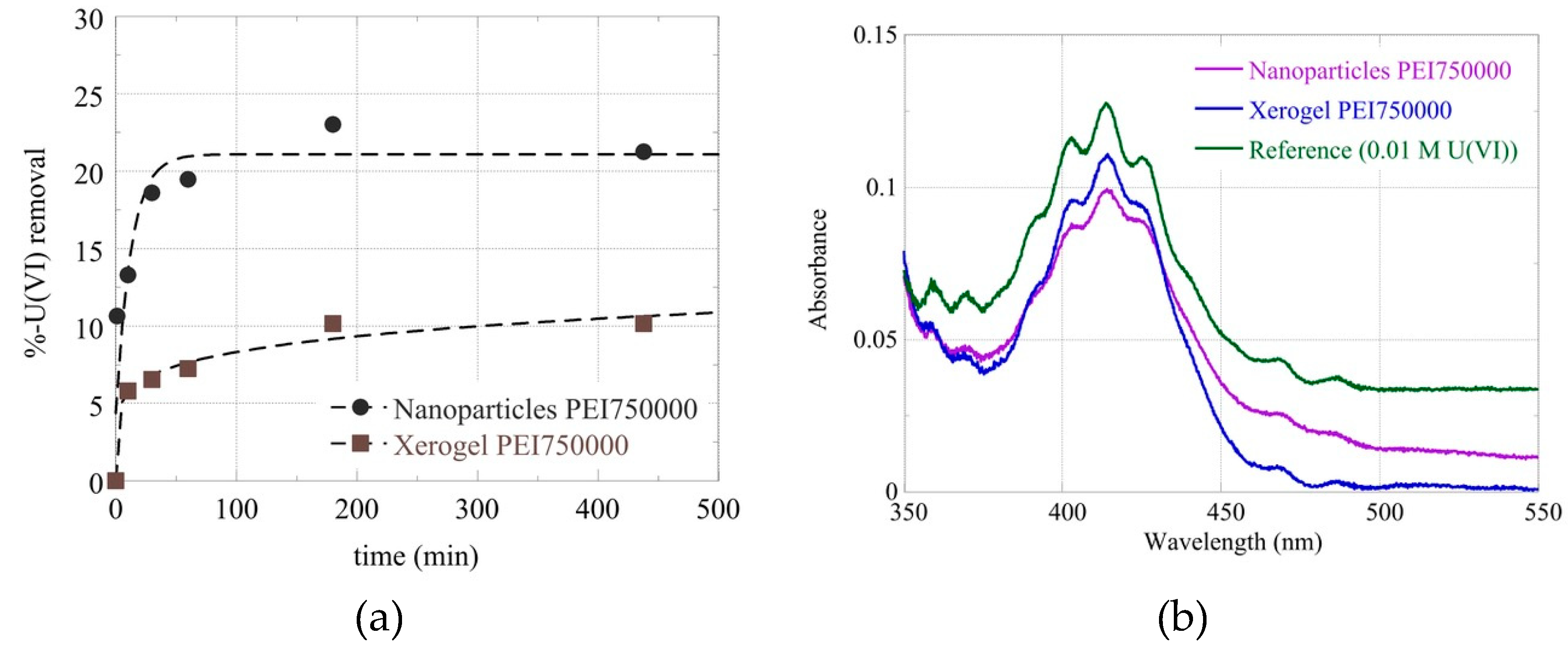
Figure 4) a bit later than what was observed with lead and dichromate ions and at about the same time as mercury and cadmium cations.
3.3. Adsorption Isotherms
To evaluate the adsorption capacity of the dendrimers for the hexavalent uranium cations (UO
22+), batch-type adsorption experiments have been performed at pH 4.0 and pH 3.0 under ambient conditions. The experiments were carried out in the acidic pH range (pH 3.0 and pH 4.0) to avoid U(VI) solid phase precipitation due to relatively high U(VI) concentrations used in the experiments (5×10
–6 mol/L < [U(VI)] < 0.1 mol/L)
[4-6, 110]. The adsorption isotherms obtained at pH 4 are shown in
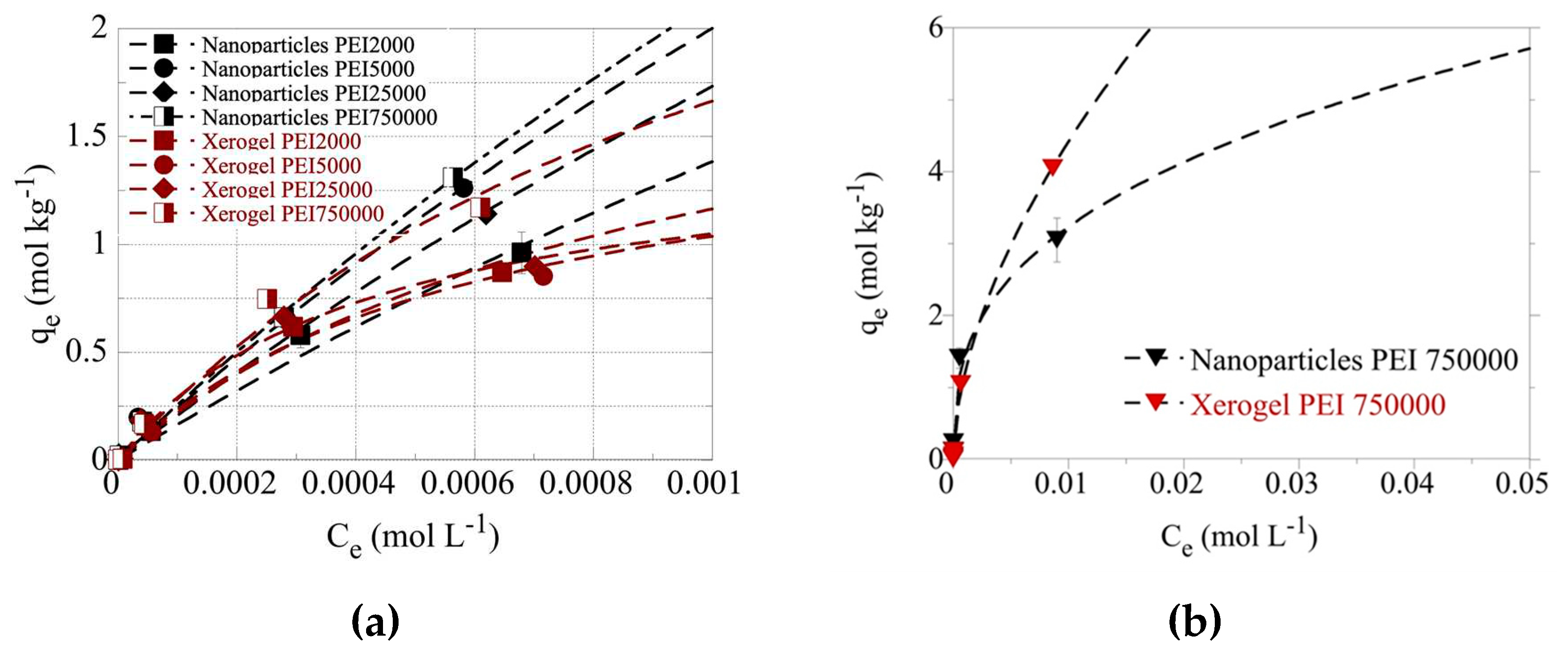
Figure 5a and indicate that for the same size PEIs the nanoparticles present higher adsorption capacities than xerogels as expected due to their higher dendritic polymer content. Additionally, the molecular weight affects the adsorption efficiency. Except for PEI 5000 nanoparticles, which show the second-best adsorption efficiency, matrices of larger polymers result in better performance. This exception may be correlated with PEI 5000 abnormally high BET surface area The highest adsorption efficiency is observed for PEI 750000 nanoparticles and the lowest for PEI 2000 counterparts in conformity with the general tendency of materials with larger BET surface areas to exhibit higher pollutant retention potential.
Even at 0.001 M U(VI) concentration in solution, the adsorption data did not reach a plateau indicating extremely high adsorption capacities of the dendrimers for U(VI). To avoid U(VI) surface precipitation, which could interfere with the U(VI) sorption by the dendrimers, the adsorption experiments have been performed also at pH 3 using the PEI 750000 nanoparticle and xerogel samples. The corresponding data, which are presented in
Figure 5b, show even at 0.01 M U(VI) concentration in solution the adsorption data do not reach a plateau assuming extraordinary adsorption capacities (q
max > 6 mol/kg), which are supreme sorption capacity values and comparable to those reported for aerogel materials [
110] and dendritic fibrous nano-silica bearing hyperbranched poly(amidoamine) [
111].
3.3.1. Effect of Temperature and Calcination
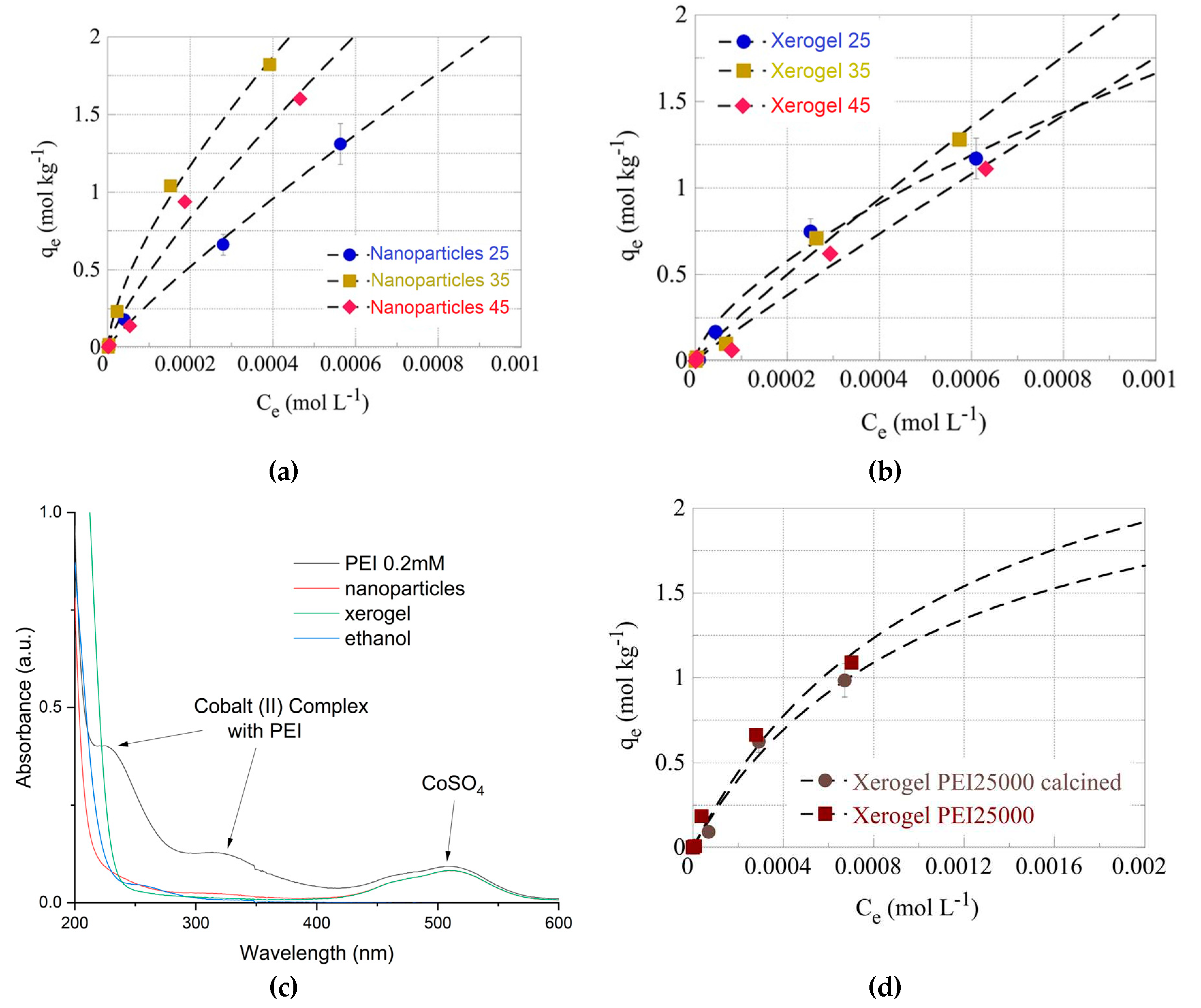
The effect of temperature has been studied at three different temperatures (e.g. 25, 35 and 45
oC) for the U(VI) adsorption by the PEI 750000 of nanoparticles (
Figure 6a) and xerogels (
Figure 6b). From the data summarized therein it is obvious that for both material types, the adsorption efficiency increases significantly when the temperature increases from 25
oC to 35
oC assuming that the adsorption is an endothermic, entropy-driven process. At 45
oC the adsorption efficiency decreases and this is in contradiction with the previous statement. However, this could be attributed to an observed partial dissolution of the solid phase at 45
oC, which could explain the decline in the adsorption efficiency. The overall behaviour of the U(VI) sorption by the hybrid silica dendritic polymer composites differs from what is generally observed for adsorption of U(VI) by aerogel materials [
110] dendritic fibrous nano-silica bearing hyperbranched poly(amidoamine) [
111] and oxidized biochar fibres [4-7], which was found to be a purely endothermic, entropy-driven process.
In other to further investigate this discrepancy and inquire about the macroscopic material loss; 100 mg of the two samples (xerogel-nanoparticles) were left under constant stirring at 45
oC for a week. Then following centrifugation, we measured the supernatant’s UV-Vis absorbance. A broad peak at 230-200 nm (
Figure 6c) could be attributed to PEI but is not characteristic. To verify PEI elusion into aqueous solutions at 45
oC we attempted complexation with Co(II). The formation of a yellow complex permitted easy detection of PEI up to 0.2 mM (
Figure 6c) (in primary and secondary amines) 200 times less than the initial concentration in the xerogels. Nevertheless, no such absorbance was observed in either supernatant. A possible explanation for the above-mentioned weight loss could be the release of the ethoxy silane hydrolysis byproduct: ethanol which has a similar absorbance spectrum (
Figure 6c). Probably some ethanol molecules were firmly incorporated in the amine-silanol hydrogen bond network and did not evaporate during drying. Thus U(VI) adsorption to silica xerogels and nanoparticles is still endothermic and the decrease in capacity at 45
oC is due to material dissolution that disrupts the pore structure of the adsorbent. Finally, elimination of the organic matrix by 3-hour protracted heating at 700
oC reduces pollutant retention but this decrease is not dramatic as proven for the PEI 25000 nanoparticles (
Figure 6d)
3.4. Adsorbent Characterization Posterior to the Uranyl Adsorption
3.4.1. Scanning Electron Microscopy SEM
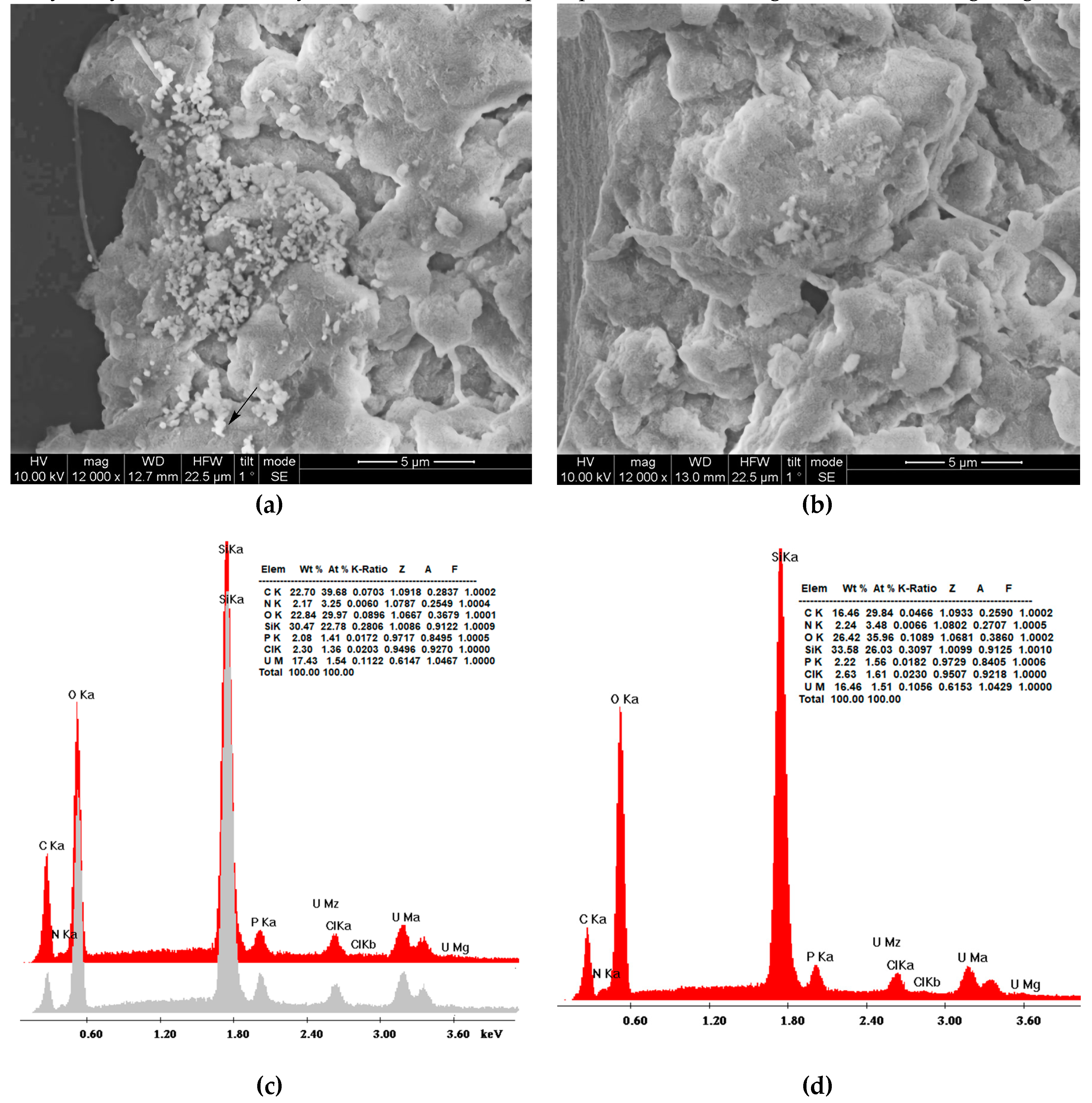
Figure 7a and
Figure 7b depict SEM micrographs of the hybrid nanoparticles and xerogel adsorbents respectively and provide a first perception of their morphology. The only difference between silica samples obtained through precipitation and gel-drying is the quasi-spherical structures that are present in
Figure 7a although to a lesser extent than previously recorded [112-113]. Uranium cubic crystals or other accumulation forms were not detected in contrast to the previous observations on uranyl adsorption on polyamide microplastics [
114]. The formation of SiO
2 was established by energy-dispersive X-ray spectroscopy (
Figure 7c for the nanoparticles,
Figure 7d for the xerogels). The oxygen to silicon atomic ratio is about the same for the two samples (1,32 nanoparticles 1,36 xerogels) however the weight percentage of carbon is much larger in the nanoparticles (22,70 against 16.46) indicating the larger content of the dendritic matrix as registered at the thermogravimetry profiles. A slight increase in the uranium weight percentage in the nanoparticles corroborates the isotherm adsorption data on their better adsorption capacity. The concentration of the electron beam on the bright spots did not cause an increase in the uranium percentage (
Figure 7c grey profile) verifying they originate from silica. The small percentages of chlorine are due to the HCl used for the hydrolysis of tetraethoxy silane whereas the phosphorus has its origin in the buffering reagents.
3.4.2. Size (DLS) and Charge (ζ-potential)
To get a first impression of the effects of uranyl cation adsorption two additional experiments were designed. The size and the charge of the xerogel aggregates adsorbing in a gradually more concentrated uranyl cation solution have been measured at pH 3. As is naturally expected xerogel aggregates became bigger when exposed to gradually higher uranium concentrations (
Figure 8a). Furthermore, in conformity, to the behaviour of the polyamide microplastics adsorbing europium and uranium [
114] a decrease of the charge that develops at the interface between the liquid and the solvation sphere surface by increasing uranium concentration has been observed (
Figure 8b). This decrease though is less intense and the hybrid adsorbents maintain a positive charge throughout the entire concentration range. This phenomenally unexpected tendency has been attributed to the development of an anion solvation sphere with hydroxyl and nitrate anions that counterbalance the positive charge of the adsorbed uranyl cations. The observed considerable increase in hydrodynamic radii is thus not attributed solely to the accumulation of uranyl cations but also to the formation of larger solvation spheres. Analogous behaviour has been encountered during the investigation of the size and the charge of the silica nanoparticles adsorbing at different pH (
Figure 8c). The increase in the hydrodynamic radius indicates a higher adsorption capacity-accumulation of uranyl cations at pH 3 as was verified for nanoparticles 75000 (compare
Figure 5a with
Figure 5b) and in parallel a larger solvation sphere as discussed above. The “unanticipated” lower charge of the nanoparticles at pH 3 (
Figure 8d) corroborates the theory of the larger concentration of counter anions to their solvation sphere.
3.4.3. IR Spectroscopy
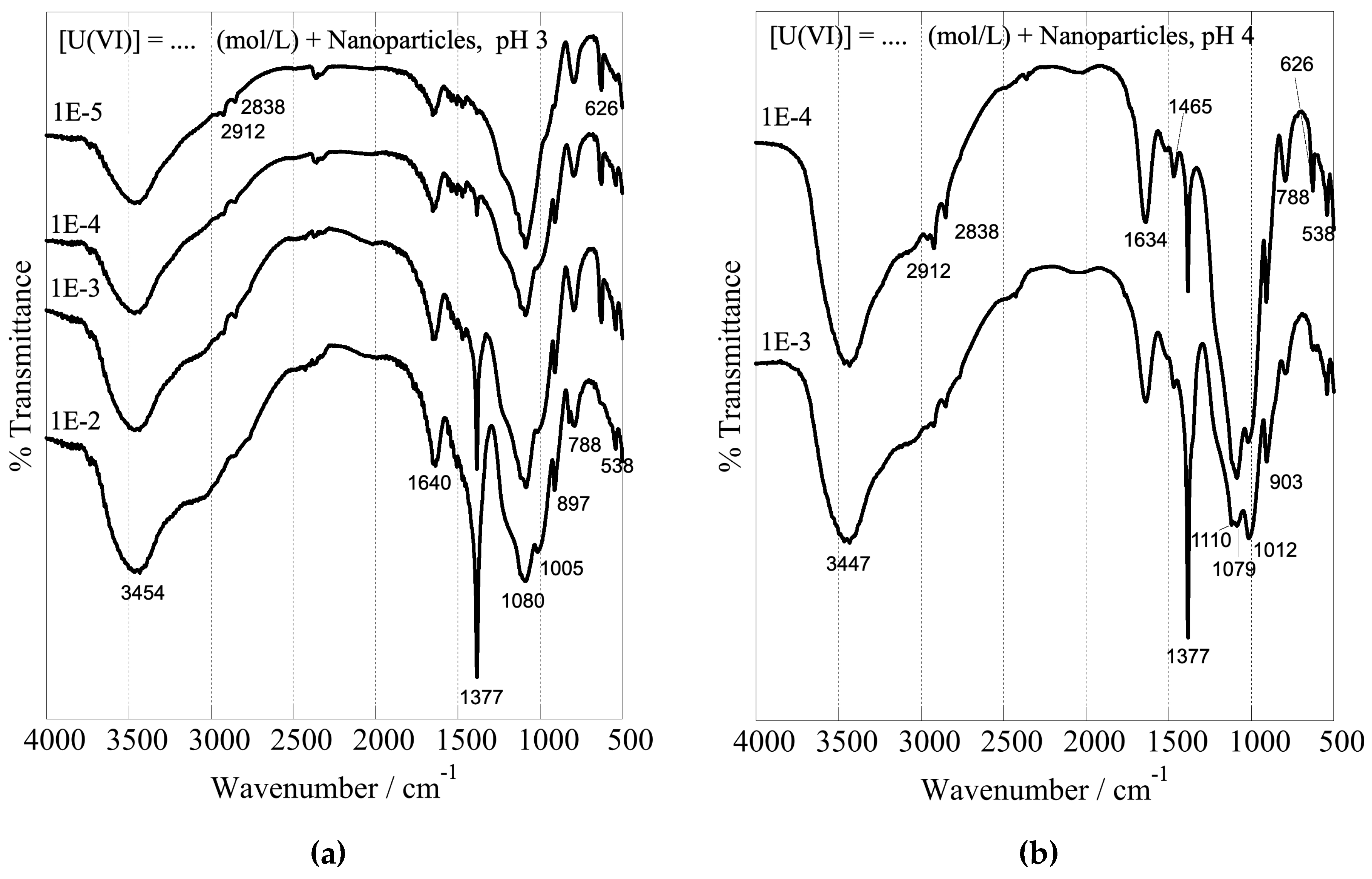
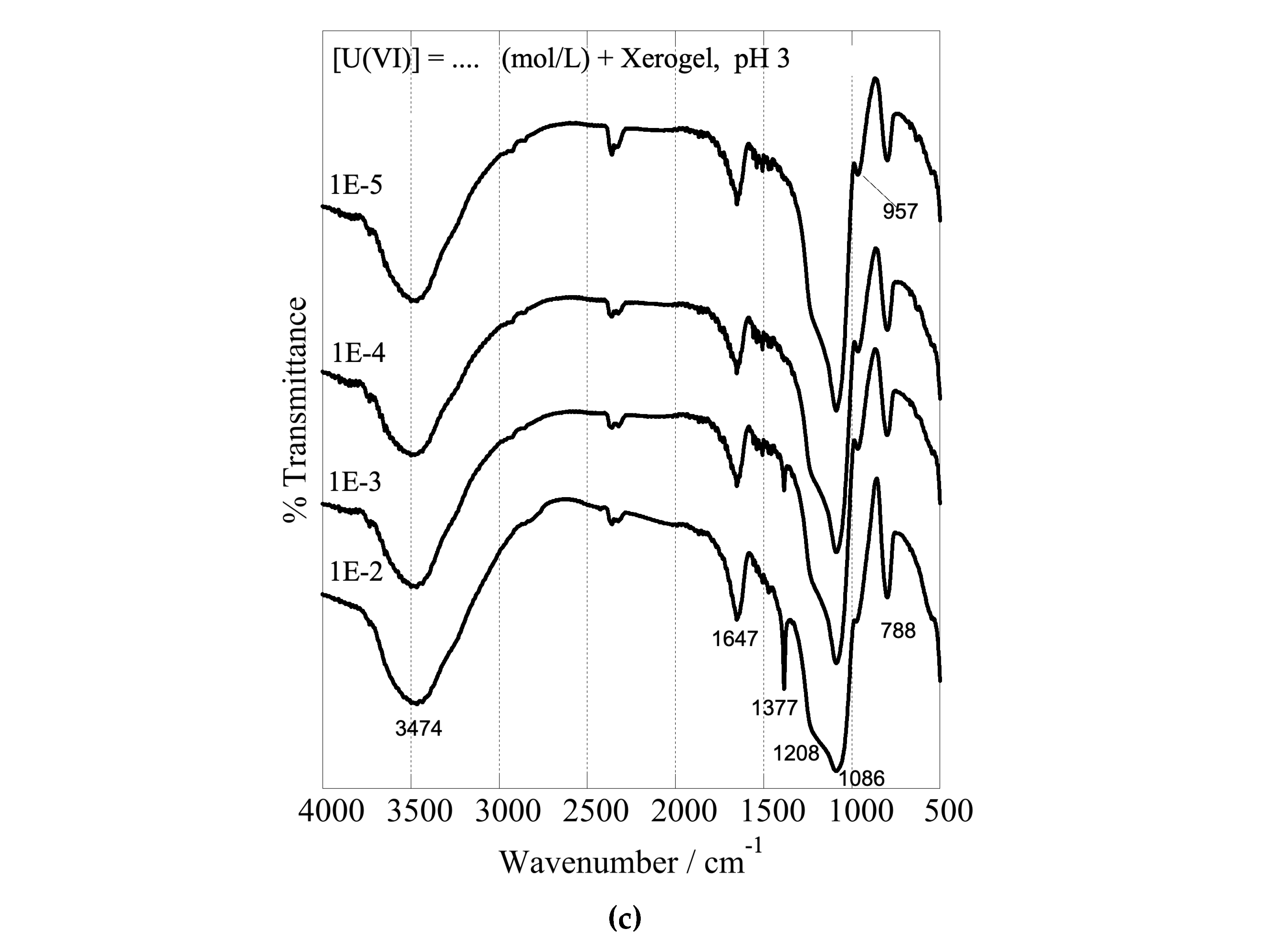
The formation of inner-sphere complexes between uranium and the composites with the hyperbranched poly(ethylene imine) matrix is indicated by the associated FTIR spectra, which have been obtained after U(VI) adsorption at different initial concentrations and are shown in
Figure 9. The spectra show significant changes in the structure and intensity ratio of the absorption bands related to the surface-active groups of the dendritic polymers (peaks in the range of 1700 and 1000 cm
–1) after interaction with U(VI) [
89]. These changes are related to the formation of inner-sphere complexes between U(VI) and the surface-active groups affecting the geometry and bonding strength of the interacting groups. There is also a significant shift of the silanol stretching bands from 960 to 900 cm
-1. Moreover, the peak which appears at 1010 cm
–1 and 970 cm
–1 in the FTIR spectra of the nanoparticles and xerogels, respectively, is attributed to the asymmetric band (ν3) of the [O=U=O]
2+ moiety [
115] and its intensity increases with the amount of U(VI) adsorbed, indicating the progressing and supreme U(VI) adsorption by the dendrimers. Moreover, the sharp peak at 1380 cm
-1 is ascribed to nitrate anions (NO
3-) [
116], which act as counter ions for the U(VI) cationic surface complexes. It is very interesting to note in all cases the profound escalation in the intensity of this NO
3- band by increasing uranium concentration. This is another piece of evidence that supports the accumulation of nitrates in the solvation sphere and explains the previously discussed ζ-potential decrease. It further indicates that the anions are adsorbed into the composite surface and remain in the dried samples.
4. Conclusions
Dendritic PEI proved an ideal matrix for biomimetic silicification. Furthermore, besides the beneficial fusion of an organic and an inorganic adsorbent, an adequate alternative to the tedious processes required for the chemical attachment of a dendritic polymer to silica particles was provided. A simple variation of the orthosilicic acid/ hyperbranched poly(ethylene imine) ratio yields two materials with different properties and capabilities: silica hydrogels-xerogels and precipitated silica nanoparticles. Both are potent adsorbing materials and can be used in the form of dispersed powders. Gelation though in contrast to precipitation may take place into porous substrates This implementation of xerogels as coatings offers many more possibilities such as enhancement of the pollutant removal potential of an already porous adsorbent and immobilization to an appropriate scaffold for easy removal and reuse. On the other hand, precipitated nanoparticles contain double quantities of PEI. This larger organic content enhances their capacity. The performance of these hybrid materials equals and even surpasses their conventionally synthesized counterparts. They are far cheaper and environmentally friendly since their production does not require high temperatures or toxic organic solvents. Their most promising property though is the ability of PEI to combine silicification with biomimetic mineralization [
117]. Transformation of noble metal ions to nanoparticles allows synchronous adsorption and (photo)catalytic decomposition of many toxic pollutants [
118] Both composites may benefit from this possibility and investigation is currently underway with promising results
Author Contributions
Conceptualization, I.P., M.A., D.G. and K.T.; methodology, E.F. and S.P.; validation, K.G.; formal analysis, G.T.; investigation, A.G, E.G. and G.T.; resources, M.V.; data curation, E.F.; writing—original draft preparation, M.A. and I.P.; writing—review and editing, M.A., I.P., D.G.; visualization, D.G. and E.G; supervision, M.A., I.P. and K.T.; project administration, M.V.; funding acquisition, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was co-financed by Greece/Greek General Secretariat for Research and Technology and European Union under the frame of EPAnEK 2014-2020 Operational Programme Competitiveness, Entrepreneurship Innovation, project “MEDNANOLEAT” grant number Τ6ΥΒΠ-00081.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Markich, S.J. Uranium Speciation and Bioavailability in Aquatic Systems: An Overview. Sci. World J. 2002, 2, 707–729. [Google Scholar] [CrossRef] [PubMed]
- Mühr-Ebert, E.L.; Wagner, F.; Walther, C. Speciation of Uranium: Compilation of a Thermodynamic Database and Its Experimental Evaluation Using Different Analytical Techniques. Appl. Geochem. 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Fanghänel, T.; Neck, V. Aquatic Chemistry and Solubility Phenomena of Actinide Oxides/Hydroxides. Pure Appl. Chem. 2002, 74, 1895–1907. [Google Scholar] [CrossRef]
- Philippou, K.; Savva, I.; Pashalidis, I. Uranium(VI) Binding by Pine Needles Prior and after Chemical Modification. J. Radioanal. Nucl. Chem. 2018, 318, 2205–2211. [Google Scholar] [CrossRef]
- Liatsou, I.; Michail, G.; Demetriou, M.; Pashalidis, I. Uranium Binding by Biochar Fibres Derived from Luffa Cylindrica after Controlled Surface Oxidation. J. Radioanal. Nucl. Chem. 2017, 311, 871–875. [Google Scholar] [CrossRef]
- Hadjittofi, L.; Pashalidis, I. Uranium Sorption from Aqueous Solutions by Activated Biochar Fibres Investigated by FTIR Spectroscopy and Batch Experiments. J. Radioanal. Nucl. Chem. 2015, 304, 897–904. [Google Scholar] [CrossRef]
- Stasi, C.; Georgiou, E.; Ioannidis, I.; Pashalidis, I. Uranium Removal from Laboratory and Environmental Waters by Oxidised Biochar Prepared from Palm Tree Fibres. J. Radioanal. Nucl. Chem. 2022, 331, 375–381. [Google Scholar] [CrossRef]
- Bhalara, P.D.; Punetha, D.; Balasubramanian, K. A Review of Potential Remediation Techniques for Uranium(VI) Ion Retrieval from Contaminated Aqueous Environment. J. Environ. Chem. Eng. 2014, 2, 1621–1634. [Google Scholar] [CrossRef]
- Guo, H.; Mei, P.; Xiao, J.; Huang, X.; Ishag, A.; Sun, Y. Carbon Materials for Extraction of Uranium from Seawater. Chemosphere 2021, 278, 130411. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Anastopoulos, I.; Barczak, M.; Antoniou, Ε.; Terpiłowski, K.; Mohammadi, E.; Shams, M.; Coy, E.; Bakandritsos, A.; Katsoyiannis, I.A.; et al. Enhanced Uranium Removal from Acidic Wastewater by Phosphonate-Functionalized Ordered Mesoporous Silica: Surface Chemistry Matters the Most. J. Hazard. Mater. 2021, 413, 125279. [Google Scholar] [CrossRef]
- Guo, D.; Song, X.; Zhang, L.; Chen, W.; Chu, D.; Tan, L. Recovery of Uranium (VI) from Aqueous Solutions by the Polyethyleneimine-Functionalized Reduced Graphene Oxide/Molybdenum Disulfide Composition Aerogels. J. Taiwan Inst. Chem. Eng. 2020, 106, 198–205. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Z.; Zheng, L.; Zhou, L.; Chai, Z.; Wang, X.; Shi, W. Interaction Mechanism of Uranium(VI) with Three-Dimensional Graphene Oxide-Chitosan Composite: Insights from Batch Experiments, IR, XPS, and EXAFS Spectroscopy. Chem. Eng. J. 2017, 328, 1066–1074. [Google Scholar] [CrossRef]
- Ioannou, K.; Hadjiyiannis, P.; Liatsou, I.; Pashalidis, I. U(VI) Adsorption by Biochar Fiber–MnO2 Composites. J. Radioanal. Nucl. Chem. 2019, 320, 425–432. [Google Scholar] [CrossRef]
- Philippou, K.; Christou, C.N.; Socoliuc, V.; Vekas, L.; Tanasă, E.; Miclau, M.; Pashalidis, I.; Krasia-Christoforou, T. Superparamagnetic Polyvinylpyrrolidone/Chitosan/Fe3O4 Electrospun Nanofibers as Effective U(VI) Adsorbents. J. Appl. Polym. Sci. 2021, 138, 50212. [Google Scholar] [CrossRef]
- Panagiotou, N.; Liatsou, I.; Pournara, A.; Angeli, G.K.; Giappa, R.M.; Tylianakis, E.; Manos, M.J.; Froudakis, G.E.; Trikalitis, P.N.; Pashalidis, I.; et al. Water-Stable 2-D Zr MOFs with Exceptional UO22+ Sorption Capability. J. Mater. Chem. A 2020, 8, 1849–1857. [Google Scholar] [CrossRef]
- Liu, H.; Fu, T.; Mao, Y. Metal–Organic Framework-Based Materials for Adsorption and Detection of Uranium(VI) from Aqueous Solution. ACS Omega 2022, 7, 14430–14456. [Google Scholar] [CrossRef]
- Koppula, S.; Manabolu Surya, S.; Katari, N.K.; Dhami, P.S.; Sivasankaran Nair, R.K. Mesoporous MOF Composite for Efficient Removal of Uranium, Methyl Orange, Methylene Blue, and Congo Red Dyes from Aqueous Solutions. Appl. Organomet. Chem. 2022, 36, e6554. [Google Scholar] [CrossRef]
- Li, N.; Yang, L.; Wang, D.; Tang, C.; Deng, W.; Wang, Z. High-Capacity Amidoxime-Functionalized β-Cyclodextrin/Graphene Aerogel for Selective Uranium Capture. Environ. Sci. Technol. 2021, 55, 9181–9188. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Chen, F.; Wang, H.; Wang, Q.; Liang, K. Efficiency and Mechanism of Adsorption of Low-Concentration Uranium from Water by a New Chitosan/Aluminum Sludge Composite Aerogel. Dalton Trans. 2020, 49, 3209–3221. [Google Scholar] [CrossRef]
- Georgiou, E.; Raptopoulos, G.; Papastergiou, M.; Paraskevopoulou, P.; Pashalidis, I. Extremely Efficient Uranium Removal from Aqueous Environments with Polyurea-Cross-Linked Alginate Aerogel Beads. ACS Appl. Polym. Mater. 2022, 4, 920–928. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Milojković, J.V.; Tsigkou, K.; Zafiri, C.; Lopičić, Z.R.; Kornaros, M.; Pashalidis, I. A Nappies Management By-Product for the Treatment of Uranium-Contaminated Waters. J. Hazard. Mater. 2021, 404, 124147. [Google Scholar] [CrossRef] [PubMed]
- Liatsou, I.; Savva, I.; Vasile, E.; Vekas, L.; Marinica, O.; Mpekris, F.; Pashalidis, I.; Krasia-Christoforou, T. Magnetoresponsive Polymer Networks as Adsorbents for the Removal of U(VI) Ions from Aqueous Media. Eur. Polym. J. 2017, 97, 138–146. [Google Scholar]
- Yin, J.; Yang, S.; He, W.; Zhao, T.; Li, C.; Hua, D. Biogene-Derived Aerogels for Simultaneously Selective Adsorption of Uranium(VI) and Strontium(II) by Co-Imprinting Method. Sep. Purif. Technol. 2021, 271, 118849. [Google Scholar] [CrossRef]
- Yu, J. Wang, J.; Jiang, Y. Removal of Uranium from Aqueous Solution by Alginate Beads. Nucl. Eng. Technol. 2017, 49, 534–540. [Google Scholar] [CrossRef]
- Ilaiyaraja, P.; Deb, A.S.; Ponraju, D.; Ali, S.M.; Venkatraman, B. Surface engineering of PAMAM-SDB chelating resin with diglycolamic acid (DGA) functional group for efficient sorption of U (VI) and Th (IV) from aqueous medium. Journal of hazardous materials 2017, 328, 1–11. [Google Scholar] [CrossRef]
- Douloudi, M.; Nikoli, E.; Katsika, T.; Arkas, M. Dendritic polymers for water resources remediation. In Novel Materials for Environmental Remediation Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 435–490. [Google Scholar]
- Jikei, M.; Kakimoto, M.A. Hyperbranched polymers: a promising new class of materials. Progress in Polymer Science 2001, 26, 1233–1285. [Google Scholar] [CrossRef]
- Kim, Y.H. Hyperbranched polymers 10 years after. Journal of Polymer Science Part A: Polymer Chemistry 1998, 36, 1685–1698. [Google Scholar] [CrossRef]
- Malmström, E.; Hult, A. Hyperbranched polymers. Journal of Macromolecular Science, Part C: Polymer Reviews 1997, 37, 555–579. [Google Scholar] [CrossRef]
- Sunder, A.; Heinemann, J.; Frey, H. Controlling the growth of polymer trees: concepts and perspectives for hyperbranched polymers. Chemistry–A European Journal 2000, 6, 2499–2506. [Google Scholar] [CrossRef]
- Voit, B.I. Hyperbranched polymers: a chance and a challenge. Comptes Rendus Chimie 2003, 6, 821–832. [Google Scholar] [CrossRef]
- Yates, C.R.; Hayes, W. Synthesis and applications of hyperbranched polymers. European Polymer Journal 2004, 40, 1257–1281. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: advances from synthesis to applications. Chemical Society Reviews 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Ardoin, N.; Astruc, D. Molecular trees: from syntheses towards applications. Bulletin de la Société chimique de France 1995, 9, 875–909. [Google Scholar]
- Bosman, D.A.; Janssen, H.M.; Meijer, E.W. About dendrimers: structure, physical properties, applications. Chemical reviews 1999, 99, 1665–1688. [Google Scholar] [CrossRef] [PubMed]
- Dvornic, P.R.; Tomalia, D.A. November. Starburst® dendrimers: a conceptual approach to nanoscopic chemistry and architecture. In Macromolecular Symposia; Hüthig & Wepf Verlag: Basel, 1994. [Google Scholar] [CrossRef]
- Tully, D.C.; Fréchet, J.M. Dendrimers at surfaces and interfaces: chemistry and applications. Chemical Communications 2001 (14), pp.1229-1239. [CrossRef]
- Zeng, F.; Zimmerman, S.C. Dendrimers in supramolecular chemistry: from molecular recognition to self-assembly. Chemical reviews 1997, 97, 1681–1712. [Google Scholar] [CrossRef] [PubMed]
- Grayson, S.M.; Frechet, J.M. Convergent dendrons and dendrimers: from synthesis to applications. Chemical Reviews 2001, 101, 3819–3868. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.M.; Wilson, C.J.; Wilson, D.A.; Peterca, M.; Imam, M.R.; Percec, V. Dendron-mediated self-assembly, disassembly, and self-organization of complex systems. Chemical Reviews 2009, 109, 6275–6540. [Google Scholar] [CrossRef] [PubMed]
- Teertstra, S.J.; Gauthier, M. Dendrigraft polymers: macromolecular engineering on a mesoscopic scale. Progress in polymer science 2004, 29, 277–327. [Google Scholar] [CrossRef]
- Afang, Z. Synthesis, characterization and applications of dendronized polymers. Prog. Chem. 2005, 17, 157–171. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, X. Tailoring dendronized polymers. Chemical Communications 2010, 46, 5049–5060. [Google Scholar] [CrossRef]
- Frauenrath, H. , 2005. Dendronized polymers—building a new bridge from molecules to nanoscopic objects. Progress in polymer science, 30(3-4), pp.325-384. [CrossRef]
- Schlüter, A.D.; Rabe, J.P. Dendronized polymers: synthesis, characterization, assembly at interfaces, and manipulation. Angewandte Chemie International Edition 2000, 39, 864–883. [Google Scholar] [CrossRef]
- Buhleier, E.; Wehner, W.; Vogtle, F. “Cascade”- and “nonskid-chain-like” syntheses of molecular cavity topologies. Synthesis 1978, 1978, 155–158. [Google Scholar] [CrossRef]
- de Gennes, P.G.; Hervet, H. Statistics of «starburst» polymers. Journal de Physique Lettres 1983, 44, 351–360. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Fréchet, J.M. Discovery of dendrimers and dendritic polymers: A brief historical perspective. Journal of Polymer Science Part A: Polymer Chemistry 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Vögtle, F.; Gestermann, S.; Hesse, R.; Schwierz, H.; Windisch, B. Functional dendrimers. Progress in Polymer Science 2000, 25, 987–1041. [Google Scholar] [CrossRef]
- Caminade, A.M.; Turrin, C.O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. (Eds.) Dendrimers: towards catalytic, material, and biomedical uses; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Marcos, M.; Martín-Rapún, R.; Omenat, A.; Serrano, J.L. Highly congested liquid crystal structures: dendrimers, dendrons, dendronized and hyperbranched polymers. Chemical Society Reviews 2007, 36, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Tsiourvas, D.; Arkas, M. Columnar and smectic self-assembly deriving from non-ionic amphiphilic hyperbranched polyethylene imine polymers and induced by hydrogen bonding and segregation into polar and non-polar parts. Polymer 2013, 54, 1114–1122. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Hosseini, S.H.; Alizadeh, M.; Bennett, C. , 2014. Magnetic pH-responsive nanocarrier with long spacer length and high colloidal stability for controlled delivery of doxorubicin. Colloids and Surfaces B: Biointerfaces, 116, pp. [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.T.; Nguyen, T.N.Q.; Cao, V.D.; Hoang, D.T.; Ngo, V.C.; Hoang Thi, T.T. Recent progress and advances of multi-stimuli-responsive dendrimers in drug delivery for cancer treatment. Pharmaceutics 2019, 11, 591. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, Z.; Tziveleka, L.A. Drug delivery using multifunctional dendrimers and hyperbranched polymers. Expert opinion on drug delivery 2010, 7, 1387–1398. [Google Scholar] [CrossRef]
- Calabretta, M.K.; Kumar, A.; McDermott, A.M.; Cai, C. Antibacterial activities of poly (amidoamine) dendrimers terminated with amino and poly (ethylene glycol) groups. Biomacromolecules 2007, 8, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Stach, M.; Siriwardena, T.N.; Köhler, T.; Van Delden, C.; Darbre, T.; Reymond, J.L. Combining Topology and Sequence Design for the Discovery of Potent Antimicrobial Peptide Dendrimers against Multidrug-Resistant Pseudomonas aeruginosa. Angewandte Chemie 2014, 126, 13041–13045. [Google Scholar] [CrossRef]
- Pérez-Anes, A.; Spataro, G.; Coppel, Y.; Moog, C.; Blanzat, M.; Turrin, C.O.; Caminade, A.M.; Rico-Lattes, I.; Majoral, J.P. Phosphonate terminated PPH dendrimers: influence of pendant alkyl chains on the in vitro anti-HIV-1 properties. Organic & biomolecular chemistry 2009, 7, 3491–3498. [Google Scholar] [CrossRef]
- Wang, S.K.; Liang, P.H.; Astronomo, R.D.; Hsu, T.L.; Hsieh, S.L.; Burton, D.R.; Wong, C.H. Targeting the carbo-hydrates on HIV-1: Interaction of oligomannose dendrons with human monoclonal antibody 2G12 and DC-SIGN. Proceedings of the National Academy of Sciences 2008, 105, 3690–3695. [Google Scholar] [CrossRef] [PubMed]
- Meyers, S.R.; Juhn, F.S.; Griset, A.P.; Luman, N.R.; Grinstaff, M.W. Anionic amphiphilic dendrimers as antibacterial agents. Journal of the American Chemical Society 2008, 130, 14444–14445. [Google Scholar] [CrossRef]
- Chen, C.Z.; Beck-Tan, N.C.; Dhurjati, P.; van Dyk, T.K.; LaRossa, R.A.; Cooper, S.L. Quaternary ammonium functionalized poly (propylene imine) dendrimers as effective antimicrobials: Structure-activity studies. Biomacromolecules 2000, 1, 473–480. [Google Scholar] [CrossRef]
- Arkas, M.; Kythreoti, G.; Favvas, E.P.; Giannakopoulos, K.; Mouti, N.; Arvanitopoulou, M.; Athanasiou, A.; Douloudi, M.; Nikoli, E.; Vardavoulias, M.; Dimitriou, M. Hydrophilic Antimicrobial Coatings for Medical Leathers from Silica-Dendritic Polymer-Silver Nanoparticle Composite Xerogels. Textiles 2022, 2, 464–485. [Google Scholar] [CrossRef]
- Shi, X.; Wang, S.H.; Van Antwerp, M.E.; Chen, X.; Baker, J.R., Jr. Targeting and detecting cancer cells using spontaneously formed multifunctional dendrimer-stabilized gold nanoparticles. Analyst 2009, 134, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, J.; Zhu, H.; Wu, H.; Zhang, H.; Gu, Z.; Luo, K. , 2021. Recent advances in development of dendritic polymer-based nanomedicines for cancer diagnosis. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 13(2), p.e1670. [CrossRef]
- Wiener, E.; Brechbiel, M.W.; Brothers, H.; Magin, R.L.; Gansow, O.A.; Tomalia, D.A.; Lauterbur, P.C. Dendrimer-based metal chelates: a new class of magnetic resonance imaging contrast agents Magn. Reson. Med. 1994, 31, 1–8. [Google Scholar] [CrossRef]
- Nwe, K.; Bryant Jr, L.H.; Brechbiel, M.W. Poly (amidoamine) dendrimer based MRI contrast agents exhibiting enhanced relaxivities derived via metal preligation techniques. Bioconjugate Chemistry 2010, 21, 1014–1017. [Google Scholar] [CrossRef]
- Ma, Y.; Mou, Q.; Wang, D.; Zhu, X.; Yan, D. , 2016. Dendritic polymers for theranostics. Theranostics, 6(7), p.930. [CrossRef]
- Korake, S.; Shaikh, A.; Salve, R.; Gajbhiye, K.R.; Gajbhiye, V.; Pawar, A. , 2021. Biodegradable dendritic Boltorn™ nano-constructs: A promising avenue for cancer theranostics. International Journal of Pharmaceutics, 594, p.120177. [CrossRef]
- Haensler, J.; Szoka, F.C., Jr. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjugate Chemistry 1993, 4, 372–379. [Google Scholar] [CrossRef]
- Kabanov, V.A.; Sergeyev, V.G.; Pyshkina, O.A.; Zinchenko, A.A.; Zezin, A.B.; Joosten, J.G.H.; Brackman, J.; Yoshikawa, K. , 2000. Interpolyelectrolyte complexes formed by DNA and astramol poly (propylene imine) dendrimers.; Macromolecules, 33(26), pp.9587-9593. [CrossRef]
- Patil, M.L.; Zhang, M.; Taratula, O.; Garbuzenko, O.B.; He, H.; Minko, T. Internally cationic polyamidoamine PAMAM-OH dendrimers for siRNA delivery: effect of the degree of quaternization and cancer targeting. Biomacromolecules 2009, 10, 258–266. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. , 2022. Development and practical application of glucose biosensor based on dendritic gold nanostructures modified by conducting polymers. Biosensors, 12(8), p.641. [CrossRef]
- Jimenez, A.; Armada, M.P.G.; Losada, J.; Villena, C.; Alonso, B.; Casado, C.M. , 2014. Amperometric biosensors for NADH based on hyperbranched dendritic ferrocene polymers and Pt nanoparticles. Sensors and Actuators B: Chemical, 190, pp.111-119. [CrossRef]
- Shukla, R.; Singh, A.; Pardhi, V.; Sunil Dubey, P.K. Dendrimer (polyamidoamine, polypropylene imine, poly-L-lysine, carbosilane dendrimers, triazine dendrimers) as promising tool for anticancer therapeutics. In Polymeric Nanoparticles as a Promising Tool for Anti-Cancer Therapeutics; Wolff, A.G., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 233–255. ISBN 9780128169636. [Google Scholar]
- Wang, B.Y.; Liao, M.L.; Hong, G.C.; Chang, W.W.; Chu, C.C. , 2017. Near-infrared-triggered photodynamic therapy toward breast cancer cells using dendrimer-functionalized upconversion nanoparticles. Nanomaterials, 7(9), p.269. [CrossRef]
- Dhanikula, R.S.; Argaw, A.; Bouchard, J.F.; Hildgen, P. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: enhanced efficacy and intratumoral transport capability. Molecular pharmaceutics 2008, 5, 105–116. [Google Scholar] [CrossRef]
- Klajnert, B.; Cangiotti, M.; Calici, S.; Majoral, J.P.; Caminade, A.M.; Cladera, J.; Bryszewska, M.; Ottaviani, M.F. EPR study of the interactions between dendrimers and peptides involved in Alzheimer's and prion diseases. Macromolecular bioscience 2007, 7, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Fruchon, S.; Poupot, M.; Martinet, L.; Turrin, C.O.; Majoral, J.P.; Fournié; JJ; Caminade, A. M.; Poupot, R. Anti-inflammatory and immunosuppressive activation of human monocytes by a bioactive dendrimer. Journal of leukocyte biology 2009, 85, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, D.; Sistla, R.; Ahmad, F.J.; Khar, R.K.; Diwan, P.V. Folate-coupled poly (ethyleneglycol) conjugates of anionic poly (amidoamine) dendrimer for inflammatory tissue-specific drug delivery. Journal of Biomedical Materials Research Part A 2007, 82, 92–103. [Google Scholar] [CrossRef]
- Douloudi, M.; Nikoli, E.; Katsika, T.; Vardavoulias, M.; Arkas, M. Dendritic polymers as promising additives for the manufacturing of hybrid organoceramic nanocomposites with ameliorated properties suitable for an extensive diversity of applications. Nanomaterials 2020, 11, 19. [Google Scholar] [CrossRef]
- Kitsou, I.; Panagopoulos, P.; Maggos, T.; Arkas, M.; Tsetsekou, A. Development of SiO2@TiO2 core-shell nanospheres for catalytic applications. Appl. Surf. Sci. 2018, 441, 223–231. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Papavasiliou, A.; Deze, E.G.; Papageorgiou, S.K.; Katsaros, F.K.; Romanos, G.E.; Poulakis, E.; Philippopoulos, C.J.; Xin, Q.; Cool, P. A green route to copper loaded silica nanoparticles using hyperbranched poly(Ethylene imine) as a biomimetic template: Application in heterogeneous catalysis. Catalysts 2017, 7, 390. [Google Scholar] [CrossRef]
- Kitsou, I.; Arkas, M.; Tsetsekou, A. Synthesis and characterization of ceria-coated silica nanospheres: Their application in heterogeneous catalysis of organic pollutants. SN Appl. Sci. 2019, 1, 1–12. [Google Scholar] [CrossRef]
- Jensen, L.K.; Jensen, H.E.; Blirup-Plum, S.A.; Bue, M.; Hanberg, P.; Kvich, L.; Aalbæk, B.; López, Y.; Soto, S.M.; Douloudi, M.; Papageorgiou, M. Coating of bone implants with silica, hyperbranched polyethyleneimine, and gentamicin prevents development of osteomyelitis in a porcine model. Materialia 2022, 24, 101473. [Google Scholar] [CrossRef]
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Xie, G. PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 2011, 281, 278–284. [Google Scholar] [CrossRef]
- Tsetsekou, A.; Arkas, M.; Kritikaki, A.; Simonetis, S.; Tsiourvas, D. Optimization of hybrid hyperbranched polymer/ceramic filters for the efficient absorption of polyaromatic hydrocarbons from water. Journal of Membrane Science 2008, 311, 128–135. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Organosilicon dendritic networks in porous ceramics for water purification. Chemistry of materials 2005, 17, 3439–3444. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D. Organic/inorganic hybrid nanospheres based on hyperbranched poly (ethylene imine) encapsulated into silica for the sorption of toxic metal ions and polycyclic aromatic hydrocarbons from water. Journal of hazardous materials 2009, 170, 35–42. [Google Scholar] [CrossRef]
- Knecht, M.R.; Wright, D.W. Amine-terminated dendrimers as biomimetic templates for silica nanosphere formation. Langmuir 2004, 20, 4728–4732. [Google Scholar] [CrossRef]
- Knecht, M.R.; Sewell, S.L.; Wright, D.W. Size control of dendrimer-templated silica. Langmuir 2005, 21, 2058–2061. [Google Scholar] [CrossRef]
- Dimitrios, A. Giannakoudakis, Ioannis Anastopoulos, Mariusz Barczak, Εvita Antoniou, Konrad Terpiłowski, Elmira Mohammadi, Mahmoud Shams, Emerson Coy, Aristides Bakandritsos, Ioannis A. Katsoyiannis, Juan Carlos Colmenares, Ioannis Pashalidis. Enhanced uranium removal from acidic wastewater by phosphonate-functionalized ordered mesoporous silica: Surface chemistry matters the most. Journal of Hazardous Materials 2021, 413, 125279. [Google Scholar] [CrossRef]
- Yoshino, H.; Kamiya, K.; Nasu, H. IR study on the structural evolution. of sol–gel derived SiO2 gels in the early stage of conversion to glasses. J. Non-Cryst. Solids 1990, 126, 68–78. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Meyer, R.L.; Poulsen, C.H.; Kragh, K.M.; Besenbacher, F.; Laursen, B.S. Biomimetic Silica Encapsulation of Enzymes for Replacement of Biocides in Antifouling Coatings. Green Chem. 2010. [Google Scholar] [CrossRef]
- Knecht, M.R.; Wright, D.W. Functional Analysis of the Biomimetic Silica Precipitating Activity of the R5 Peptide from Cylindrotheca Fusiformis. Chem. Commun. 2003, 3038–3039. [Google Scholar] [CrossRef] [PubMed]
- Kroger, N.; Deutzmann, R.; Sumper, M. Polycationic Peptides from Diatom Biosilica That Direct Silica Nanosphere Formation. Science 1999, 286, 1129–1132. [Google Scholar] [CrossRef]
- Kroger, N.; Deutzmann, R.; Bergsdorf, C.; Sumper, M. Species-Specific Polyamines from Diatoms Control Silica Morphology. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 14133–14138. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Lorenz, S.; Brunner, E.; Sumper, M. Self-Assembly of Highly Phosphorylated Silaffins and Their Function in Biosilica Morphogenesis. Science 2002. [Google Scholar] [CrossRef] [PubMed]
- Dillon, R.E.A.; Shriver, D.F. Ion transport and vibrational spectra of branched polymer and dendrimer electrolytes, Chem. Mater. 2001, 13, 1369–1373. [Google Scholar]
- Hu, H.; Ortíz-Aguilar, B.E.; Hechavarría, L.E. Effect of pH value of poly(ethylenimine)–H2SO4 electrolyte on electrochromic response of polyaniline thin films. Opt. Mater. 2007, 29, 579–584. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules, 3rd ed.; Chapman and Hall: London, UK, 1975; pp. 16–17. [Google Scholar]
- Kłonkowski, A.M.; Grobelna, B.; Widernik, T.; Jankowska-Frydel, A.; Mozgawa, W. Coordination state of copper(II) complexes anchored and grafted onto the surface of organically modified silicates. Langmuir 1999, 15, 5814–5819. [Google Scholar] [CrossRef]
- Shimizu, I.; Okabayashi, H.; Taga, K.; Nishio, E.; O’Connor, C.J. Diffuse reflectance infrared Fourier transform spectral study of the thermal and adsorbed-water effects of a 3-aminopropyltriethoxysilane layer modified onto the surface of silica gel. Vib. Spectrosc. 1997, 14, 113–123. [Google Scholar] [CrossRef]
- Yoshino, H.; Kamiya, K.; Nasu, H. IR Study on the Structural Evolution of Sol-Gel Derived SiO2 Gels in the Early Stage of Conversion to Glasses. J. Non. Cryst. Solids 1990. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Amer. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Lantenois, S.; Prélot, B.; Douillard, J.-M.; Szczodrowski, K.; Charbonnel, M.-C. Flow microcalorimetry: experimental development and application to adsorption of heavy metal cations on silica. Appl. Surf. Sci. 2007, 253, 5807–5813. [Google Scholar] [CrossRef]
- da Silva, L.C.C.; Santos, L.B.O.D.; Abate, G.; Cosentino, I.C.; Fantini, M.C.A.; Masini, J.C.; Matos, J.R. Adsorption of Pb2+, Cu2+ and Cd2+ in FDU-1 silica and FDU-1 silica modified with humic acid. Micropor. Mesopor. Mater. 2008, 110, 250–259. [Google Scholar] [CrossRef]
- Georgiou, E.; Raptopoulos, G.; Anastopoulos, I.; Giannakoudakis, D.A.; Arkas, M.; Paraskevopoulou, P.; Pashalidis, I. Uranium Removal from Aqueous Solutions by Aerogel-Based Adsorbents—A Critical Review. Nanomaterials 2023, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liao, Y.; Xia, L. Poly (amidoamine) dendrimer decorated dendritic fibrous nano-silica for efficient removal of uranium (VI). Journal of Solid State Chemistry 2021, 303, 122511. [Google Scholar] [CrossRef]
- Arkas, M.; Douloudi, M.; Nikoli, E.; Karountzou, G.; Kitsou, I.; Kavetsou, E.; Korres, D.; Vouyiouka, S.; Tsetsekou, A.; Giannakopoulos, K.; Papageorgiou, M. Investigation of two bioinspired reaction mechanisms for the optimization of nano catalysts generated from hyperbranched polymer matrices. Reactive and Functional Polymers 2022, 174, 105238. [Google Scholar] [CrossRef]
- Arkas, M.; Douloudi, M.; Nikoli, E.; Karountzou, G.; Kitsou, I.; Kavetsou, E.; Korres, D.; Vouyiouka, S.; Tsetsekou, A.; Giannakopoulos, K.; Papageorgiou, M. Additional data on the investigation of the reaction mechanisms for the production of silica hyperbranched polyethylene imine silver nanoparticle composites. Data in Brief 2022, 43, 108374. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Giannakopoulos, K.; Arkas, M.; Dosche, C.; Pashalidis, I. A comprehensive investigation on the sorption of U (VI) and Eu (III) by polyamide microplastics: Surface-assisted microparticle formation. Journal of Molecular Liquids 2022, 368, 120757. [Google Scholar] [CrossRef]
- Gary, S. Groenewold, Anita K. Gianotto, Michael E. McIlwain, Michael J. Van Stipdonk, Michael Kullman, David T. Moore, Nick Polfer, Jos Oomens, Ivan Infante, Lucas Visscher, Bertrand Siboulet, Wibe A. de Jong. Infrared Spectroscopy of Discrete Uranyl Anion Complexes. J. Phys. Chem. A 2008, 112, 3–508. [Google Scholar]
- Gan, F.; Wu, K.; Ma, F.; Du, C. In Situ Determination of Nitrate in Water Using Fourier Transform Mid-Infrared Attenuated Total Reflectance Spectroscopy Coupled with Deconvolution Algorithm. Molecules 2020, 25, 5838. [Google Scholar] [CrossRef] [PubMed]
- Arkas, M.; Kithreoti, G.; Boukos, N.; Kitsou, I.; Petrakli, F.; Panagiotaki, K. Two completely different biomimetic reactions mediated by the same matrix producing inorganic/organic/inorganic hybrid nanoparticles. Nano-Structures & Nano-Objects 2018, 14, 138–148. [Google Scholar]
- Arkas, M.; Anastopoulos, I.; Giannakoudakis, D.A.; Pashalidis, I.; Katsika, T.; Nikoli, E.; Panagiotopoulos, R.; Fotopoulou, A.; Vardavoulias, M.; Douloudi, M. Catalytic Neutralization of Water Pollutants Mediated by Dendritic Polymers. Nanomaterials 2022, 12, 445. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Thermogravimetry profiles for the dendritic matrices. Adapted by permission from [
112] (
a); and the hybrid adsorbents (
b).
Figure 1.
Thermogravimetry profiles for the dendritic matrices. Adapted by permission from [
112] (
a); and the hybrid adsorbents (
b).
Figure 2.
N2 adsorption (77 K) of the prepared: (a) xerogels and (b) nanoparticles; in insets, N2 adsorption (77 K) of all samples in the low-pressure region is depicted. Pore size distributions determined by the NLDFT method of (c) xerogels and (d) nanospheres.
Figure 2.
N2 adsorption (77 K) of the prepared: (a) xerogels and (b) nanoparticles; in insets, N2 adsorption (77 K) of all samples in the low-pressure region is depicted. Pore size distributions determined by the NLDFT method of (c) xerogels and (d) nanospheres.
Figure 3.
(a) Hydrodynamic Radii and (b) Surface Charges of all adsorbing materials as measured by Dynamic Light Scattering and ζ-Potential respectively.
Figure 3.
(a) Hydrodynamic Radii and (b) Surface Charges of all adsorbing materials as measured by Dynamic Light Scattering and ζ-Potential respectively.
Figure 4.
(a) Removal percentage of Uranyl cations from Nanoparticles and Xerogel Mw 750000 as a function of time and respective absorbance curves after 2 hours (b).
Figure 4.
(a) Removal percentage of Uranyl cations from Nanoparticles and Xerogel Mw 750000 as a function of time and respective absorbance curves after 2 hours (b).
Figure 5.
(a) Isotherms of U(VI) adsorbed by xerogel and nanoparticle adsorbents at 283 K and varying initial U(VI) levels in solution (1×10–5 – 0.1 mol/L). The experiments were performed at pH 4 (left) (a) and pH 3 (b), at an adsorbent dosage of 0.4 g/L, under ambient conditions (298 K), 24 h contact time, and an agitation rate of 125 rpm.
Figure 5.
(a) Isotherms of U(VI) adsorbed by xerogel and nanoparticle adsorbents at 283 K and varying initial U(VI) levels in solution (1×10–5 – 0.1 mol/L). The experiments were performed at pH 4 (left) (a) and pH 3 (b), at an adsorbent dosage of 0.4 g/L, under ambient conditions (298 K), 24 h contact time, and an agitation rate of 125 rpm.
Figure 6.
Isotherms of U(VI) adsorbed by PEI 750000 nanoparticles (a) and xerogel (b) adsorbents at various temperatures (298 K, 308 K and 318 K). (c) UV-Visible Spectra of CoSO4 solution, respective complex with PEI, supernatants of 100 mg xerogel and nanoparticles after 1 week at 45oC and ethanol. (d) Isotherms of U(VI) adsorbed by calcinated and non-calcinated PEI 25000 nanoparticles.
Figure 6.
Isotherms of U(VI) adsorbed by PEI 750000 nanoparticles (a) and xerogel (b) adsorbents at various temperatures (298 K, 308 K and 318 K). (c) UV-Visible Spectra of CoSO4 solution, respective complex with PEI, supernatants of 100 mg xerogel and nanoparticles after 1 week at 45oC and ethanol. (d) Isotherms of U(VI) adsorbed by calcinated and non-calcinated PEI 25000 nanoparticles.
Figure 7.
SEM micrographs of composite PEI–silica nanoparticles (a) and silica xerogels (b) and respective Energy Dispersive Spectroscopy (EDS) diagrams (c) and (d) after uranyl cation adsorption. The grey diagram indicates the EDS spectrum in the bright spot indicated by the arrow.
Figure 7.
SEM micrographs of composite PEI–silica nanoparticles (a) and silica xerogels (b) and respective Energy Dispersive Spectroscopy (EDS) diagrams (c) and (d) after uranyl cation adsorption. The grey diagram indicates the EDS spectrum in the bright spot indicated by the arrow.
Figure 8.
Hydrodynamic radii (a) and surface charge (b) of xerogels adsorbing uranyl cations as a function of the initial solution concentration. Hydrodynamic radii (c) and surface charge (d) of nanoparticles adsorbing uranyl cations as a function of the solution pH.
Figure 8.
Hydrodynamic radii (a) and surface charge (b) of xerogels adsorbing uranyl cations as a function of the initial solution concentration. Hydrodynamic radii (c) and surface charge (d) of nanoparticles adsorbing uranyl cations as a function of the solution pH.
Figure 9.
FTIR spectra of nanoparticles at pH 3 (a) and pH 4 (b) and xerogels at pH 3 (c) after U(VI) adsorption at varying initial U(VI) levels in the suspension. The associated experiments were performed in a U(VI) initial concentration range between 1×10–5 and 1×10–2 mol L–1, adsorbent dosage 0.4 g/L, under ambient conditions (298 K), 24 h contact time, and an agitation rate of 125 rpm.
Figure 9.
FTIR spectra of nanoparticles at pH 3 (a) and pH 4 (b) and xerogels at pH 3 (c) after U(VI) adsorption at varying initial U(VI) levels in the suspension. The associated experiments were performed in a U(VI) initial concentration range between 1×10–5 and 1×10–2 mol L–1, adsorbent dosage 0.4 g/L, under ambient conditions (298 K), 24 h contact time, and an agitation rate of 125 rpm.
Table 1.
Weight loss percentages of the hybrid adsorbents.
Table 1.
Weight loss percentages of the hybrid adsorbents.
| Adsorbent |
Weight Loss 170oC
(Water/Ethanol) (%) |
Total Weight Loss
3h 700oC (%) |
Organic Content
(%) |
| Xerogel 2000 |
6.99 |
15.97 |
8.98 |
| Xerogel 5000 |
4.92 |
14.89 |
9.91 |
| Xerogel 25000 |
7.15 |
17.28 |
10.13 |
| Xerogel 750000 |
7.05 |
16.01 |
8.96 |
| Nanoparticles 2000 |
8.28 |
31.08 |
22.80 |
| Nanoparticles 5000 |
7.40 |
28.90 |
21.50 |
| Nanoparticles 25000 |
7.80 |
32.23 |
24.43 |
| Nanoparticles 750000 |
6.53 |
28.84 |
22.31 |
Table 2.
FTIR vibrational band assignments (in cm-1) for hyperbranched poly(ethylene imine), and composite silica xerogels and nanospheres (nanoparticles).
Table 2.
FTIR vibrational band assignments (in cm-1) for hyperbranched poly(ethylene imine), and composite silica xerogels and nanospheres (nanoparticles).
| Band assignment1
|
PEI25000 |
Xerogels |
Nanoparticles |
|
νs SiO-H free |
- |
3750 (vw) |
- |
|
νs SiO-H Hydrogen bonded |
- |
3450 (w/b) |
3450 (w/b) |
|
νas NH (primary, secondary) |
3350 (m) |
3400 (w) |
3370 (sh) |
|
νs NH (primary, secondary) |
3276 (m) |
op |
3200 (w) |
|
νas CH2
|
2935 (m) |
2981 (vw) |
2962 (vw) |
|
νs CH2
|
2810 (s) |
2820 (vw) |
2853 (vw) |
|
δ NH, NH2
|
1585 (m) |
1640 (vw) |
- |
|
δas NH2+, NH3+
|
- |
1530 (vw) |
1524 (vw) |
|
δs NH2+, NH3+
|
- |
- |
1473 (vw) |
|
νas C-N |
1105(m) |
sh |
sh |
|
νs C-N |
1045 (m) |
op |
op |
|
ν Si-O-Si |
- |
1074 (s) |
1042 (s) |
|
ν Si-OH, Si-O-
|
- |
960 (m) |
964 (m) |
|
δ Si-O-Si |
- |
788 (m) |
785 (m) |
|
ρ CH2
|
760 (s) |
op |
op |
| δ Si-OH |
- |
540 (m) |
540 (m) |
Table 3.
Pore characteristics and particle sizes of all samples.
Table 3.
Pore characteristics and particle sizes of all samples.
| Sample |
TPV1
|
SBET
|
dmean2
|
dBJH3
|
dDFT4
|
| (ml/g) |
(m2/g) |
(nm) |
(nm) |
(nm) |
| nanoparticles 750000 |
0.640 |
139.0 |
18.4 |
32.0 |
28.4 |
| nanoparticles 25000 |
0.326 |
77.9 |
16.7 |
53.5 |
55.8 |
| nanoparticles 5000 |
0.880 |
148.3 |
23.7 |
31.0 |
28.4 |
| nanoparticles 2000 |
0.508 |
98.5 |
20.6 |
53.6 |
28.4 |
| xerogel 750000 |
0.461 |
271.4 |
6.8 |
3.8 |
5.1 |
| xerogel 25000 |
0.925 |
242.0 |
15.3 |
14.1 |
16.7 |
| xerogel 5000 |
0.390 |
220.3 |
7.1 |
4.7 |
6.1 |
| xerogel 2000 |
0.297 |
194.3 |
6.1 |
5.4 |
7.0 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).