Submitted:
29 April 2023
Posted:
29 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Persister cells
2.1. Overview
2.2. Bacterial persisters
2.3. Mycobacterium tuberculosis persisters
3. Differentially detectable (DD) Mtb cells
4. In Vitro Killing of AR+NR Mtb Cells by Drug Combinations
5. Drug Combinations Eradicating Mtb from BALB/c and C3HeB/FeJ Mice
5.1. Eradication of Mtb from Balb/c mice
5.2. Eradication of Mtb from C3HeB/FeJ mice
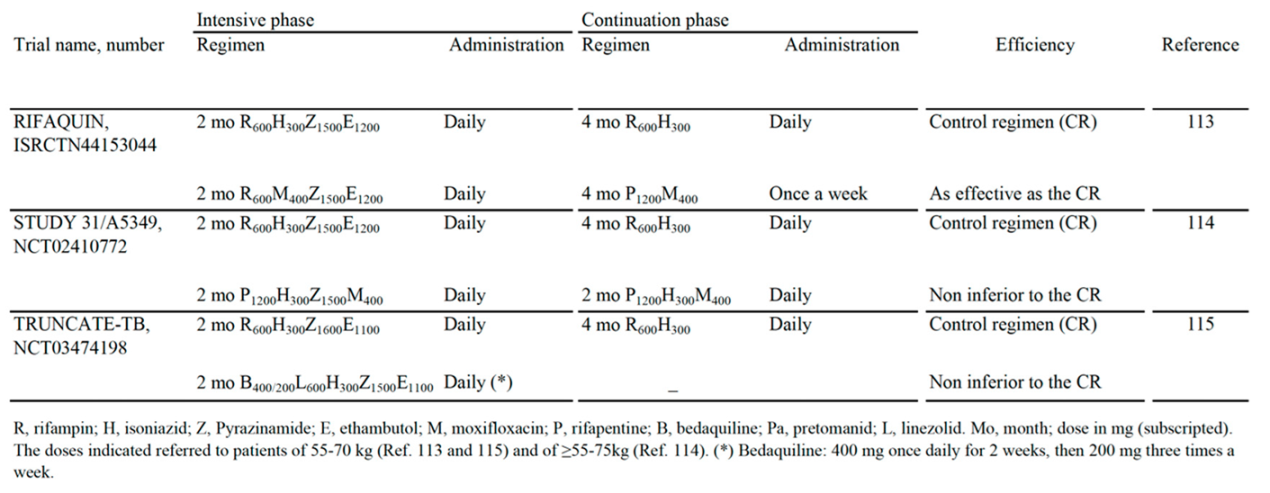
6. New drug combinations for treatment of human TB
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Global tuberculosis report; ; WHO: Geneva, Switzerland, 2020; ISBN 978-92-4-001313-1. [Google Scholar]
- WHO. WHO Global tuberculosis report; ; WHO: Geneva, Switzerland, 2019; ISBN 978-92-4-156571-4. [Google Scholar]
- Barry, C.E. 3rd; Boshoff, H.I.; Dartois, V.; Dick, T.; Ehrt, S.; Flynn, J.; Schnappinger, D.; Wilkinson, RJ.; Young, D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 2009, 7, 845–55. [Google Scholar] [CrossRef] [PubMed]
- Dartois, V. The path of anti-tuberculosis drugs: from blood to lesions to mycobacterial cells. Nat. Rev. Microbiol. 2014, 12, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, A.; Barry, C.E. 3rd.; Dartois, V. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol. Rev. [CrossRef]
- Gengenbacher, M.; Kaufmann, S.H. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol. Rev. 2012, 36, 514–32. [Google Scholar] [CrossRef]
- Daniel, J.; Maamar, H.; Deb, C.; Sirakova, T.D.; Kolattukudy, PE. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. 2011, 7, e1002093. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Wainwright, H.C.; Locketz, M.; Bekker, L.G.; Walther, G.B.; Dittrich, C.; Visser, A.; Wang, W.; Hsu, F.F.; Wiehart, U.; et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol. Med. 2010, 2, 258–74. [Google Scholar] [CrossRef] [PubMed]
- Sarathy, J.; Dartois, V.; Dick, T.; Gengenbacher, M. Reduced drug uptake in phenotypically resistant nutrient-starved nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2013, 57, 1648–53. [Google Scholar] [CrossRef] [PubMed]
- Garton, N.J.; Waddell, S.J.; Sherratt, A.L.; Lee, S.M.; Smith, R.J.; Senner, C.; Hinds, J.; Rajakumar, K.; Adegbola, R.A.; Besra, G.S.; et al. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 2008, 5, e75. [Google Scholar] [CrossRef]
- Iacobino, A.; Piccaro, G.; Giannoni, F.; Mustazzolu, A.; Fattorini, L. Fighting tuberculosis by drugs targeting nonreplicating Mycobacterium tuberculosis bacilli. Int. J. Mycobacteriol. 2017, 6, 213–221. [Google Scholar] [CrossRef]
- Mukamolova, G.V.; Turapov, O.; Malkin, J.; Woltmann, G.; Barer, M.R. Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am. J. Respir. Crit. Care Med. 2010, 181, 174–80. [Google Scholar] [CrossRef]
- Chengalroyen, M.D.; Beukes, G.M.; Gordhan, B.G.; Streicher, E.M.; Churchyard, G.; Hafner, R.; Warren, R.; Otwombe, K.; Martinson, N.; Kana, B.D. Detection and quantification of differentially culturable tubercle bacteria in sputum from patients with tuberculosis. Am. J. Respir. Crit. Care Med. 2016, 194, 1532–1540. [Google Scholar] [CrossRef]
- Saito, K.; Warrier, T.; Somersan-Karakaya, S.; Kaminski, L.; Mi, J.; Jiang, X.; Park, S.; Shigyo, K.; Gold, B.; Roberts, J.; et al. Rifamycin action on RNA polymerase in antibiotic-tolerant Mycobacterium tuberculosis results in differentially detectable populations. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, E4832–E4840. [Google Scholar] [CrossRef]
- McAulay, K.; Saito, K.; Warrier, T.; Walsh, K.F.; Mathurin, L.D.; Royal-Mardi, G.; Lee, M.H.; Ocheretina, O.; Pape, J.W.; Fitzgerald, D.W.; et al. Differentially detectable Mycobacterium tuberculosis cells in sputum from treatment-naive subjects in Haiti and their proportionate increase after initiation of treatment. mBio. 2018, 9, e02192–e18. [Google Scholar] [CrossRef]
- Zainabadi, K.; Walsh, K.F.; Vilbrun, S.C.; Mathurin, L.D.; Lee, M.H.; Saito, K.; Mishra, S.; Ocheretina, O.; Pape, JW.; Nathan, C.; et al. Characterization of differentially detectable Mycobacterium tuberculosis in the sputum of subjects with drug-sensitive or drug-resistant tuberculosis before and after two months of therapy. Antimicrob. Agents Chemother. 2021, 65, e0060821. [Google Scholar] [CrossRef]
- Gordhan, B.G.; Peters, J.S.; McIvor, A.; Machowski, E.E.; Ealand, C.; Waja, Z.; Martinson, N.; Kana, B.D. Detection of differentially culturable tubercle bacteria in sputum using mycobacterial culture filtrates. Sci. Rep. 2021, 11, 6493. [Google Scholar] [CrossRef] [PubMed]
- Zainabadi, K.; Saito, K.; Mishra, S.; Walsh, K.F.; Mathurin, L.D.; Vilbrun, S.C.; Ocheretina, O.; Pape, J.W.; Fitzgerald, D.W.; Nathan, C.F.; et al. Transcriptional biomarkers of differentially detectable Mycobacterium tuberculosis in patient sputum. mBio. 2022, 13, e0270122. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.; Fourie, P.B.; Mitchison, D.A. Persister populations of Mycobacterium tuberculosis in sputum that grow in liquid but not on solid culture media. J. Antimicrob. Chemother. 2014, 69, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, B.; Fauvart, M.; Michiels, J. Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol. Rev. 2017, 41, 219–251. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, J.; Van Dijck, P.; Holtappels, M. Fungal persister cells: The basis for recalcitrant infections? PLoS Pathog. 2018, 14, e1007301. [Google Scholar] [CrossRef]
- Barrett, M.P.; Kyle, D.E.; Sibley, L.D.; Radke, J.B.; Tarleton, R.L. Protozoan persister-like cells and drug treatment failure. Nat. Rev. Microbiol. 2019, 17, 607–620. [Google Scholar] [CrossRef]
- Dhanyamraju, P.K.; Schell, T.D.; Amin, S.; Robertson, G.P. Drug-tolerant persister cells in cancer therapy resistance. Cancer Res. 2022, 82, 2503–2514. [Google Scholar] [CrossRef]
- Oren, Y.; Tsabar, M.; Cuoco, M.S.; Amir-Zilberstein, L.; Cabanos, H.F.; Hutter, J.C.; Hu, B.; Thakore, P.I.; Tabaka, M.; Fulco, C.P.; et al. Cycling cancer persister cells arise from lineages with distinct programs. Nature. 2021, 596, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Bigger, J.W. Treatment of staphylococcal infections with penicillin by intermittent sterilization. The Lancet. 1944, 244, 497–500. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Moyed, H.S.; Bertrand, K.P. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J. Bacteriol. 1983, 155, 768–75. [Google Scholar] [CrossRef] [PubMed]
- Black, D.S.; Irwin, B.; Moyed, H.S. Autoregulation of hip, an operon that affects lethality due to inhibition of peptidoglycan or DNA synthesis. J. Bacteriol. 1994, 176, 4081–91. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, JJ.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Orman, M.A.; Brynildsen, M.P. Dormancy is not necessary or sufficient for bacterial persistence. Antimicrob. Agents Chemother. 2013, 57, 3230–9. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Merrin, J.; Chait, R.; Kowalik, L.; Leibler, S. Bacterial persistence as a phenotypic switch. Science. 2004, 305, 1622–5. [Google Scholar] [CrossRef]
- Levin-Reisman, I.; Balaban, N.Q. Quantitative measurement of Type I and Type II persisters using ScanLag. In: Bacterial Persistence, Methods and Protocols. Methods in Molecular Biology. Volume 1333. Edited by Michiels J and Fauvart M. Humana Press. 2016, Pages 75-81. [CrossRef]
- Carvalho, G.; Guilhen, C.; Balestrino, D.; Forestier, C.; Mathias, J.D. Relating switching rates between normal and persister cells to substrate and antibiotic concentrations: a mathematical modelling approach supported by experiments. Microb. Biotechnol. 2017, 10, 1616–1627. [Google Scholar] [CrossRef]
- Ju, Y.; Long, H.; Zhao, P.; Xu, P.; Sun, L.; Bao, Y.; Yu, P.; Zhang, Y. The top 100 cited studies on bacterial persisters: A bibliometric analysis. Front. Pharmacol. 2022, 13, 1001861. [Google Scholar] [CrossRef]
- Mandal, S.; Njikan, S.; Kumar, A.; Early, J.V.; Parish, T. The relevance of persisters in tuberculosis drug discovery. Microbiology (Reading). 2019, 165, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.; Fourie, P.B.; Mitchison, D.A. Persister populations of Mycobacterium tuberculosis in sputum that grow in liquid but not on solid culture media. J. Antimicrob. Chemother. 2014, 69, 437–40. [Google Scholar] [CrossRef] [PubMed]
- Ayyappan, J.P.; Vinnard, C.; Subbian, S.; Nagajyothi, J.F. Effect of Mycobacterium tuberculosis infection on adipocyte physiology. Microbes Infect. 2018, 20, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Iacobino, A.; Fattorini, L.; Giannoni, F. Drug-resistant tuberculosis 2020. Where we stand. Appl. Sci. 2020, 10, 2153. [Google Scholar] [CrossRef]
- Wayne, L.G.; Hayes, L.G. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 1996, 64, 2062–9. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.C.; Lukey, P.T.; Robb, L.C.; McAdam, R.A.; Duncan, K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002, 43, 717–31. [Google Scholar] [CrossRef]
- Piccaro, G.; Poce, G.; Biava, M.; Giannoni, F.; Fattorini, L. Activity of lipophilic and hydrophilic drugs against dormant and replicating Mycobacterium tuberculosis. J Antibiot (Tokyo). 2015, 68, 711–4. [Google Scholar] [CrossRef]
- Iona, E.; Pardini, M.; Mustazzolu, A.; Piccaro, G.; Nisini, R.; Fattorini, L.; Giannoni, F. Mycobacterium tuberculosis gene expression at different stages of hypoxia-induced dormancy and upon resuscitation. J Microbiol. 2016, 54, 565–72. [Google Scholar] [CrossRef]
- Iacobino, A.; Piccaro, G.; Giannoni, F.; Mustazzolu, A.; Fattorini, L. Mycobacterium tuberculosis is selectively killed by rifampin and rifapentine in hypoxia at neutral pH. Antimicrob. Agents Chemother. [CrossRef]
- Gold, B.; Nathan, C. Targeting Phenotypically Tolerant Mycobacterium tuberculosis. Microbiol. Spectr. 2017. [Google Scholar] [CrossRef]
- Chung, E.S.; Johnson, W.C.; Aldridge, B.B. Types and functions of heterogeneity in mycobacteria. Nat. Rev. Microbiol. 2022, 20, 529–541. [Google Scholar] [CrossRef]
- Vaubourgeix, J.; Lin, G, Dhar, N. ; Chenouard, N.; Jiang, X.; Botella, H.; Lupoli, T.; Mariani, O.; Yang, G.; Ouerfelli, O.; et al. Stressed mycobacteria use the chaperone ClpB to sequester irreversibly oxidized proteins asymmetrically within and between cells. Cell Host Microbe. 2015, 17, 178–90. [Google Scholar] [CrossRef] [PubMed]
- Quigley, J.; Lewis, K. Noise in a metabolic pathway leads to persister formation in Mycobacterium tuberculosis. Microbiol. Spectr. 2022, 10, e0294822. [Google Scholar] [CrossRef]
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016; 18, 16051. [Google Scholar] [CrossRef]
- Manuse, S.; Shan, Y.; Canas-Duarte, S.J.; Bakshi, S.; Sun, W.S.; Mori, H.; Paulsson, J.; Lewis, K. Bacterial persisters are a stochastically formed subpopulation of low-energy cells. PLoS Biol. 2021, 19, e3001194. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, W.; Rudel, T.; Heesemann, J.; Goebel, W. Link between antibiotic persistence and antibiotic resistance in bacterial pathogens. Front. Cell. Infect. Microbiol. 2022; 12, 900848. [Google Scholar] [CrossRef]
- Sebastian, J.; Swaminath, S.; Nair, R.R.; Jakkala, K.; Pradhan, A.; Ajitkumar, P. De novo emergence of genetically resistant mutants of Mycobacterium tuberculosis from the persistence phase cells formed against antituberculosis drugs in vitro. Antimicrob. Agents Chemother. 2017, 61, e01343–16. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Nair, R.R.; Jakkala, K.; Pradhan, A.; Ajitkumar, P. Elevated levels of three reactive oxygen species and Fe(II) in the antibiotic-surviving population of mycobacteria facilitate de novo emergence of genetic resisters to antibiotics. Antimicrob. Agents Chemother. 2022, 66, e0228521. [Google Scholar] [CrossRef]
- Piccaro, G.; Pietraforte, D.; Giannoni, F.; Mustazzolu, A.; Fattorini, L. Rifampin induces hydroxyl radical formation in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 7527–33. [Google Scholar] [CrossRef]
- Iacobino, A.; Piccaro, G.; Pardini, M.; Fattorini, L.; Giannoni, F. Moxifloxacin activates the SOS response in Mycobacterium tuberculosis in a dose- and time-dependent manner. Microorganisms. 2021, 9, 255. [Google Scholar] [CrossRef]
- Shee, S.; Singh, S.; Tripathi, A.; Thakur, C.; Kumar, T.A.; Das, M.; Yadav, V.; Kohli, S.; Rajmani, R.S.; Chandra, N.; et al. Moxifloxacin-mediated killing of Mycobacterium tuberculosis involves respiratory downshift, reductive stress, and accumulation of reactive oxygen species. Antimicrob. Agents Chemother. 2022, 66, e0059222. [Google Scholar] [CrossRef]
- Singh, A.; Zhao, X.; Drlica, K. Fluoroquinolone heteroresistance, antimicrobial tolerance, and lethality enhancement. Front. Cell. Infect. Microbiol. 2022, 12, 938032. [Google Scholar] [CrossRef]
- Ehrt, S.; Schnappinger, D.; Rhee, K.Y. Metabolic principles of persistence and pathogenicity in Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2018, 16, 496–507. [Google Scholar] [CrossRef]
- Keren, I.; Minami, S.; Rubin, E.; Lewis, K. Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. mBio. 2011, 2, e00100–11. [Google Scholar] [CrossRef]
- Torrey, H.L.; Keren, I.; Via, L.E.; Lee, J.S.; Lewis, K. High persister mutants in Mycobacterium tuberculosis. PLoS One. 2016, 11, e0155127. [Google Scholar] [CrossRef]
- Hu, Y.; Pertinez, H.; Ortega-Muro, F.; Alameda-Martin, L.; Liu, Y.; Schipani, A.; Davies, G.; Coates, A. Investigation of elimination rate, persistent subpopulation removal, and relapse rates of Mycobacterium tuberculosis by using combinations of first-line drugs in a modified Cornell mouse model. Antimicrob. Agents Chemother. 2016, 60, 4778–85. [Google Scholar] [CrossRef]
- Salina, E.G.; Makarov, V. Mycobacterium tuberculosis dormancy: how to fight a hidden danger. Microorganisms. 2022, 10, 2334. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R.; Venugopal, U.; Chandra, G.; Bharti, S.; Maurya, R.K.; Krishnan, M.Y. Effect of various drugs on differentially detectable persisters of Mycobacterium tuberculosis generated by long-term lipid diet. Tuberculosis (Edinb). 2019, 115, 89–95. [Google Scholar] [CrossRef]
- Mesman, A.W.; Baek, S.H.; Huang, C.C.; Kim, Y.M.; Cho, S.N.; Ioerger, T.R.; Barreda, N.N.; Calderon, R.; Sassetti, C.M.; Murray, M.B. Characterization of drug-resistant lipid-dependent differentially detectable Mycobacterium tuberculosis. J. Clin. Med. 2021, 10, 3249. [Google Scholar] [CrossRef] [PubMed]
- Gordhan, B.G.; Sewcharran, A.; Letsoalo, M.; Chinappa, T.; Yende-Zuma, N.; Padayatchi, N.; Naidoo, K.; Kana, B.D. Detection of differentially culturable tubercle bacteria in sputum from drug-resistant tuberculosis patients. Antimicrob. Agents Chemother. 2022, 12, 949370. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, D.V.; Salina, E.G.; Fursov, M.V.; Skvortsov, T.A.; Azhikina, T.L.; Kaprelyants, A.S. Dormant non-culturable Mycobacterium tuberculosis retains stable low-abundant mRNA. BMC Genomics. 2015, 16, 954. [Google Scholar] [CrossRef]
- Turapov, O.; O’Connor, B.D.; Sarybaeva, A.A.; Williams, C.; Patel, H.; Kadyrov, A.S.; Sarybaev, A.S.; Woltmann, G.; Barer, M.R.; Mukamolova, G.V. Phenotypically adapted Mycobacterium tuberculosis populations from sputum are tolerant to first-line drugs. Antimicrob Agents Chemother. 2016, 60, 2476–83. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Mishra, S.; Warrier, T.; Cicchetti, N.; Mi, J.; Weber, E.; Jiang, X.; Roberts, J.; Gouzy, A.; Kaplan, E.; et al. Oxidative damage and delayed replication allow viable Mycobacterium tuberculosis to go undetected. Sci Transl Med. 2021, 13, eabg2612. [Google Scholar] [CrossRef]
- Hong, Y.; Zeng, J.; Wang, X.; Drlica, K.; Zhao, X. Post-stress bacterial cell death mediated by reactive oxygen species. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 10064–10071. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.Y.; Kim, P.; Zhang, L.; Kang, S.; Keller, T.H.; Jiricek, J.; Barry, C. E 3rd. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science. 2008, 322, 1392–5. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, J.; Sun, M.; Zhang, X.; Cook, G.M.; Zhang, T. Nitric oxide-dependent electron transport chain inhibition by the cytochrome bc1 inhibitor and pretomanid combination kills Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2021, 65, e0095621. [Google Scholar] [CrossRef] [PubMed]
- Diacon, A.H.; Maritz, J.S.; Venter, A.; van Helden, P.D.; Andries, K.; McNeeley, D.F.; Donald, P.R. Time to detection of the growth of Mycobacterium tuberculosis in MGIT 960 for determining the early bactericidal activity of antituberculosis agents. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1561–5. [Google Scholar] [CrossRef]
- Bowness, R.; Boeree, M.J.; Aarnoutse, R.; Dawson, R.; Diacon, A.; Mangu, C.; Heinrich, N.; Ntinginya, N.E.; Kohlenberg, A.; Mtafya, B.; et al. The relationship between Mycobacterium tuberculosis MGIT time to positivity and cfu in sputum samples demonstrates changing bacterial phenotypes potentially reflecting the impact of chemotherapy on critical sub-populations. J Antimicrob Chemother. 2015, 70, 448–55. [Google Scholar] [CrossRef]
- Filippini, P.; Iona, E.; Piccaro, G.; Peyron, P.; Neyrolles, O.; Fattorini, L. Activity of drug combinations against dormant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2010, 54, 2712–5. [Google Scholar] [CrossRef]
- Maitra, A.; Solanki, P.; Sadouki, Z.; McHugh, T.D.; Kloprogge, F. Improving the drug development pipeline for mycobacteria: modelling antibiotic exposure in the hollow fibre infection model. Antibiotics 2021, 10, 1515. [Google Scholar] [CrossRef]
- Gumbo, T.; Srivastava, S.; Deshpande, D.; Pasipanodya, J.G.; Berg, A.; Romero, K.; Hermann, D.; Hanna, D. Hollow-fibre system model of tuberculosis reproducibility and performance specifications for best practice in drug and combination therapy development. J. Antimicrob. Chemother. 2023, dkad029. [Google Scholar] [CrossRef]
- Gumbo, T.; Sherman, CM.; Deshpande, D.; Alffenaar, J.W.; Srivastava, S. Mycobacterium tuberculosis sterilizing activity of faropenem, pyrazinamide and linezolid combination and failure to shorten the therapy duration. Int. J. Infect. Dis. 2021, 104, 680–684. [Google Scholar] [CrossRef]
- Piccaro, G.; Giannoni, F.; Filippini, P.; Mustazzolu, A.; Fattorini, L. Activities of drug combinations against Mycobacterium tuberculosis grown in aerobic and hypoxic acidic conditions. Antimicrob. Agents Chemother. 2013, 57, 1428–33. [Google Scholar] [CrossRef]
- Iacobino, A.; Giannoni, F.; Pardini, M.; Piccaro, G.; Fattorini, L. The combination rifampin-nitazoxanide, but not rifampin-isoniazid-pyrazinamide-ethambutol, kills dormant Mycobacterium tuberculosis in hypoxia at neutral pH. Antimicrob. Agents Chemother. 2019, 63, e00273–19. [Google Scholar] [CrossRef]
- Lanni, A.; Borroni, E.; Iacobino, A.; Russo, C.; Gentile, L.; Fattorini, L.; Giannoni, F. Activity of drug combinations against Mycobacterium abscessus grown in aerobic and hypoxic conditions. Microorganisms. 2022, 10, 1421. [Google Scholar] [CrossRef]
- Olender, D.; Żwawiak, J.; Zaprutko, L. Multidirectional efficacy of biologically active nitro compounds included in medicines. Pharmaceuticals (Basel). 2018, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Lee, H.Y.; Liou, J.P. Nitro-group-containing drugs. J. Med. Chem. 2019, 62, 2851–2893. [Google Scholar] [CrossRef] [PubMed]
- Nuermberger, E.; Rosenthal, I.; Tyagi, S.; Williams, K.N.; Almeida, D.; Peloquin, C.A.; Bishai, W.R.; Grosset, J.H. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2006, 50, 2621–5. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Yan, B.S.; Rojas, M.; Shebzukhov, Y.V.; Zhou, H.; Kobzik, L.; Higgins, D.E.; Daly, M.J.; Bloom, B.R.; Kramnik, I. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005, 434, 767–72. [Google Scholar] [CrossRef] [PubMed]
- Driver, E.R.; Ryan, G.J.; Hoff, D.R.; Irwin, S.M.; Basaraba, R.J.; Kramnik, I.; Lenaerts, A.J. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2012, 56, 3181–95. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, I.M.; Tasneen, R.; Peloquin, C.A.; Zhang, M.; Almeida, D.; Mdluli, K.E.; Karakousis, P.C.; Grosset, J.H.; Nuermberger, E.L. Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob. Agents Chemother. 2012, 56, 4331–40. [Google Scholar] [CrossRef] [PubMed]
- Tasneen, R.; Li, S.Y.; Peloquin, C.A.; Taylor, D.; Williams, K.N.; Andries, K.; Mdluli, K.E.; Nuermberger, EL. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2011, 55, 5485–92. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, I.M.; Williams, K.; Tyagi, S.; Peloquin, C.A.; Vernon, A.A.; Bishai, W.R.; Grosset, J.H.; Nuermberger, E.L. Potent twice-weekly rifapentine-containing regimens in murine tuberculosis. Am. J. Respir. Crit. Care Med. 2006, 174, 94–101. [Google Scholar] [CrossRef]
- Rosenthal, I.M.; Zhang, M.; Williams, K.N.; Peloquin, C.A.; Tyagi, S.; Vernon, A.A.; Bishai, W.R.; Chaisson, R.E.; Grosset, J.H.; Nuermberger, E.L. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 2007, 4, e344. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, I.M.; Zhang, M.; Almeida, D.; Grosset, J.H.; Nuermberger, E.L. Isoniazid or moxifloxacin in rifapentine-based regimens for experimental tuberculosis? Am. J. Respir. Crit. Care Med. 2008, 178, 989–93. [Google Scholar] [CrossRef] [PubMed]
- Nuermberger, E.; Tyagi, S.; Tasneen, R.; Williams, K.N.; Almeida, D.; Rosenthal, I.; Grosset, J.H. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2008, 52, 1522–4. [Google Scholar] [CrossRef] [PubMed]
- Tasneen, R.; Tyagi, S.; Williams, K.; Grosset, J.; Nuermberger, E. Enhanced bactericidal activity of rifampin and/or pyrazinamide when combined with PA-824 in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2008, 52, 3664–8. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Minkowski, A.; Amoabeng, O.; Peloquin, CA.; Taylor, D.; Andries, K.; Wallis, R.S.; Mdluli, K.E.; Nuermberger, E.L. Sterilizing activities of novel combinations lacking first- and second-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2012, 56, 3114–20. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Tyagi, S.; Minkowski, A.; Peloquin, C.A.; Grosset, J.H.; Nuermberger, E.L. Contribution of moxifloxacin or levofloxacin in second-line regimens with or without continuation of pyrazinamide in murine tuberculosis. Am. J. Respir. Crit. Care Med. 2013, 188, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Tasneen, R.; Williams, K.; Amoabeng, O.; Minkowski, A.; Mdluli, K.E.; Upton, A.M.; Nuermberger, E.L. Contribution of the nitroimidazoles PA-824 and TBA-354 to the activity of novel regimens in murine models of tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 129–35. [Google Scholar] [CrossRef]
- Tasneen, R.; Betoudji, F.; Tyagi, S.; Li, S.Y.; Williams, K.; Converse, P.J.; Dartois, V.; Yang, T.; Mendel, C.M.; Mdluli, K.E.; et al. Contribution of oxazolidinones to the efficacy of novel regimens containing bedaquiline and pretomanid in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 2015, 60, 270–7. [Google Scholar] [CrossRef]
- Lanoix, J.P.; Betoudji, F.; Nuermberger, E. Sterilizing activity of pyrazinamide in combination with first-line drugs in a C3HeB/FeJ mouse model of tuberculosis. Antimicrob. Agents Chemother. 2015, 60, 1091–6. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, A.; Ortega-Muro, F.; Alameda-Martin, L.; Mitchison, D.; Coates, A. High-dose rifampicin kills persisters, shortens treatment duration, and reduces relapse rate in vitro and in vivo. Front. Microbiol. 2015, 6, 641. [Google Scholar] [CrossRef]
- Li, S.Y.; Tasneen, R.; Tyagi, S.; Soni, H.; Converse, P.J.; Mdluli, K.; Nuermberger, E.L. Bactericidal and sterilizing activity of a novel regimen with bedaquiline, pretomanid, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2017, 61, e00913–17. [Google Scholar] [CrossRef] [PubMed]
- Ammerman, N.C.; Swanson, R.V.; Bautista, E.M.; Almeida, D.V.; Saini, V.; Omansen, T.F.; Guo, H.; Chang, Y.S.; Li, S.Y.; Tapley, A.; et al. Impact of clofazimine dosing on treatment shortening of the first-line regimen in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e00636–18. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, S.Y.; Almeida, D.V.; Tasneen, R.; Barnes-Boyle, K.; Converse, P.J.; Upton, A.M.; Mdluli, K.; Fotouhi, N.; Nuermberger, E.L. Contribution of pretomanid to novel regimens containing bedaquiline with either linezolid or moxifloxacin and pyrazinamide in murine models of tuberculosis. Antimicrob. Agents Chemother. 2019, 63, e00021–19. [Google Scholar] [CrossRef]
- Saini, V.; Ammerman, N.C.; Chang, Y.S.; Tasneen, R.; Chaisson, R.E.; Jain, S.; Nuermberger, E.; Grosset, J.H. Treatment-shortening effect of a novel regimen combining clofazimine and high-dose rifapentine in pathologically distinct mouse models of tuberculosis. Antimicrob. Agents Chemother. 2019, 63, e00388–19. [Google Scholar] [CrossRef] [PubMed]
- Tasneen, R.; Garcia, A.; Converse, P.J.; Zimmerman, MD.; Dartois, V.; Kurbatova, E.; Vernon, A.A.; Carr, W.; Stout, J.E.; Dooley, K.E.; et al. Novel regimens of bedaquiline-pyrazinamide combined with moxifloxacin, rifabutin, delamanid and/or OPC-167832 in murine tuberculosis models. Antimicrob. Agents Chemother. 2022, 66, e0239821. [Google Scholar] [CrossRef]
- Hibma, J.E.; Radtke, K.K.; Dorman, S.E.; Jindani, A.; Dooley, K.E.; Weiner, M.; McIlleron, HM.; Savic, RM. Rifapentine population pharmacokinetics and dosing recommendations for latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 2020, 202, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Benator, D.; Bhattacharya, M.; Bozeman, L.; Burman, W.; Catanzaro, A.; Chaisson, R.; Gordin, F.; Horsburgh, C.R.; Horton, J.; et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet. 2002, 360, 528–534. [Google Scholar] [CrossRef]
- 106. Centers for Disease Control and Prevention (CDC); American Thoracic Society. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treatment of latent tuberculosis infection--United States. MMWR Morb. Mortal. Wkly Rep. 2003; 52, 735-9.
- Svensson, E.M.; Murray, S.; Karlsson, M.O.; Dooley, K.E. Rifampicin and rifapentine significantly reduce concentrations of bedaquiline, a new anti-TB drug. J. Antimicrob. Chemother. 2015, 70, 1106–14. [Google Scholar] [CrossRef]
- Gopal, P.; Grüber, G.; Dartois, V, Dick, T. Pharmacological and molecular mechanisms behind the sterilizing activity of pyrazinamide. Trends Pharmacol. Sci. 2019, 40, 930–940. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, W. ; Zhang, W, Mitchison, D. Mechanisms of pyrazinamide action and resistance. Microbiol. Spectr. 2014; 18, 10–128. [Google Scholar] [CrossRef]
- Sarathy, J.P.; Via, L.E.; Weiner, D.; Blanc, L.; Boshoff, H.; Eugenin, E.A.; Barry, C.E. 3rd.; Dartois, V.A. Extreme drug tolerance of Mycobacterium tuberculosis in caseum. Antimicrob Agents Chemother. 2018, 62, e02266–17. [Google Scholar] [CrossRef]
- Iseman, M.D. Tuberculosis therapy: past, present and future. Eur. Respir. J. Suppl. 2002, 36, 87s–94s. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO consolidated guidelines on tuberculosis. Module 4: treatment—drug-susceptible tuberculosis treatment. ISBN 978-92-4-004812-6. WHO: Geneva, Switzerland, 2022.
- Jindani, A.; Harrison, T.S.; Nunn, A.J.; Phillips, P.P.; Churchyard, G.J.; Charalambous, S. ; Hatherill, M, Geldenhuys, H.; McIlleron, H.M.; Zvada, S.P.; et al. High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N. Engl. J. Med. 2014; 371, 1599–1608. [Google Scholar] [CrossRef]
- Dorman, S.E.; Nahid, P.; Kurbatova, E.V.; Phillips, P.P.J.; Bryant, K.; Dooley, K.E.; Engle, M.; Goldberg, S.V.; Phan, H.T.T.; Hakim, J.; et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N. Engl. J. Med. 2021, 384, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Paton, N.I.; Cousins, C.; Suresh, C.; Burhan, E.; Chew, K.L.; Dalay, V.B. ; Lu, Q, Kusmiati, T.; Balanag, V.M.; Lee, S.L.; et al. Treatment strategy for rifampin-susceptible tuberculosis. N. Engl. J. Med. 2023; 388, 873–887. [Google Scholar] [CrossRef]
- WHO. Treatment of drug-susceptible tuberculosis: rapid communication (June 2021). ISBN 978-92-4-002867-8. WHO: Geneva, Switzerland, 2021.
- Dartois, V.; Rubin, E.J. Shortening tuberculosis treatment—a strategic retreat. N. Engl. J. Med. 2023, 388, 939–941. [Google Scholar] [CrossRef]
- http://www.drugbank.ca.
- Sarathy, J.P.; Zuccotto, F.; Hsinpin, H.; Sandberg, L.; Via, L.E.; Marriner, G.A.; Masquelin, T.; Wyatt, P.; Ray, P.; Dartois, V. Prediction of drug penetration in tuberculosis lesions. ACS Infect Dis. 2016, 2, 552–63. [Google Scholar] [CrossRef]
- Sarathy, J.P.; Dartois, V. Caseum: a niche for Mycobacterium tuberculosis drug-tolerant persisters. Clin Microbiol Rev. 2020, 33, e00159-19. [Google Scholar] [CrossRef] [PubMed]
- Sarathy, J.P.; Xie, M.; Jones, R.M.; Chang, A.; Osiecki, P.; Weiner, D.; Tsao, W.S.; Dougher, M. ; Blanc, L, Fotouhi, N.; Via, L.E, Barry, C.E. 3rd.; et al. A novel tool to identify bactericidal compounds against vulnerable targets in drug-tolerant M. tuberculosis found in caseum. mBio. 2023; 5, e0059823. [Google Scholar] [CrossRef]
- Kempker, R.R.; Heinrichs, M.T.; Nikolaishvili, K.; Sabulua, I.; Bablishvili, N.; Gogishvili, S.; Avaliani, Z.; Tukvadze, N.; Little, B.; Bernheim, A.; et al. Lung tissue concentrations of pyrazinamide among patients with drug-resistant pulmonary tuberculosis. Antimicrob. Agents Chemother. 2017, 61, e00226–17. [Google Scholar] [CrossRef]
- McLeay, S.C.; Vis, P.; van Heeswijk, R.P.; Green, B. Population pharmacokinetics of bedaquiline (TMC207), a novel antituberculosis drug. Antimicrob. Agents Chemother. 2014, 58, 5315–5324. [Google Scholar] [CrossRef]
- Irwin, S.M.; Prideaux, B.; Lyon, E.R.; Zimmerman, M.D.; Brooks, E.J.; Schrupp, C.A.; Chen, C.; Reichlen, M.J.; Asay, B.C. ; Voskui,l M.I.; et al. Bedaquiline and pyrazinamide treatment responses are affected by pulmonary lesion eterogeneity in Mycobacterium tuberculosis infected C3HeB/FeJ mice. ACS Infect Dis. 2016; 2, 251–267. [Google Scholar] [CrossRef]
- Conradie, F.; Bagdasaryan, T.R.; Borisov, S.; Howell, P.; Mikiashvili, L.; Ngubane, N.; Samoilova, A.; Skornykova, S.; Tudor, E.; Variava, E.; et al. Bedaquiline-pretomanid-linezolid regimens for drug-resistant tuberculosis. N. Engl. J. Med. 2022, 387, 810–823. [Google Scholar] [CrossRef]
- Nyang’wa, B.T.; Berry, C.; Kazounis, E.; Motta, I.; Parpieva, N.; Tigay, Z.; Solodovnikova, V.; Liverko, I.; Moodliar, R.; Dodd, M.; et al. A 24-week, all-oral regimen for rifampin-resistant ruberculosis. N. Engl. J. Med. 2022, 387, 2331–2343. [Google Scholar] [CrossRef]
- Goodall, R.L.; Meredith, S.K.; Nunn, A.J.; Bayissa, A.; Bhatnagar, A.K.; Bronson, G.; Chiang, C.Y.; Conradie, F.; Gurumurthy, M.; Kirenga, B.; et al. Evaluation of two short standardised regimens for the treatment of rifampicin-resistant tuberculosis (STREAM stage 2): an open-label, multicentre, randomised, non-inferiority trial. Lancet. 2022, 400, 1858–1868. [Google Scholar] [CrossRef]
- WHO. WHO consolidated guidelines on tuberculosis. Module 4: treatment—drug-resistant tuberculosis treatment, 2022 update. ISBN 978-92-4-006312-9. WHO: Geneva, Switzerland, 2022.
- Nuermberger, E.L. Preclinical efficacy testing of new drug candidates. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Clary, J.; Hanna, D.; Nuermberger, E.; Lenaerts, A.; Ammerman, N.; Ramey, M.; Hartley, D.; Hermann, D. Model-based meta-analysis of relapsing mouse model studies from the critical path to tuberculosis drug regimens initiative database. Antimicrob. Agents Chemother. 2022, 66, e0179321. [Google Scholar] [CrossRef] [PubMed]
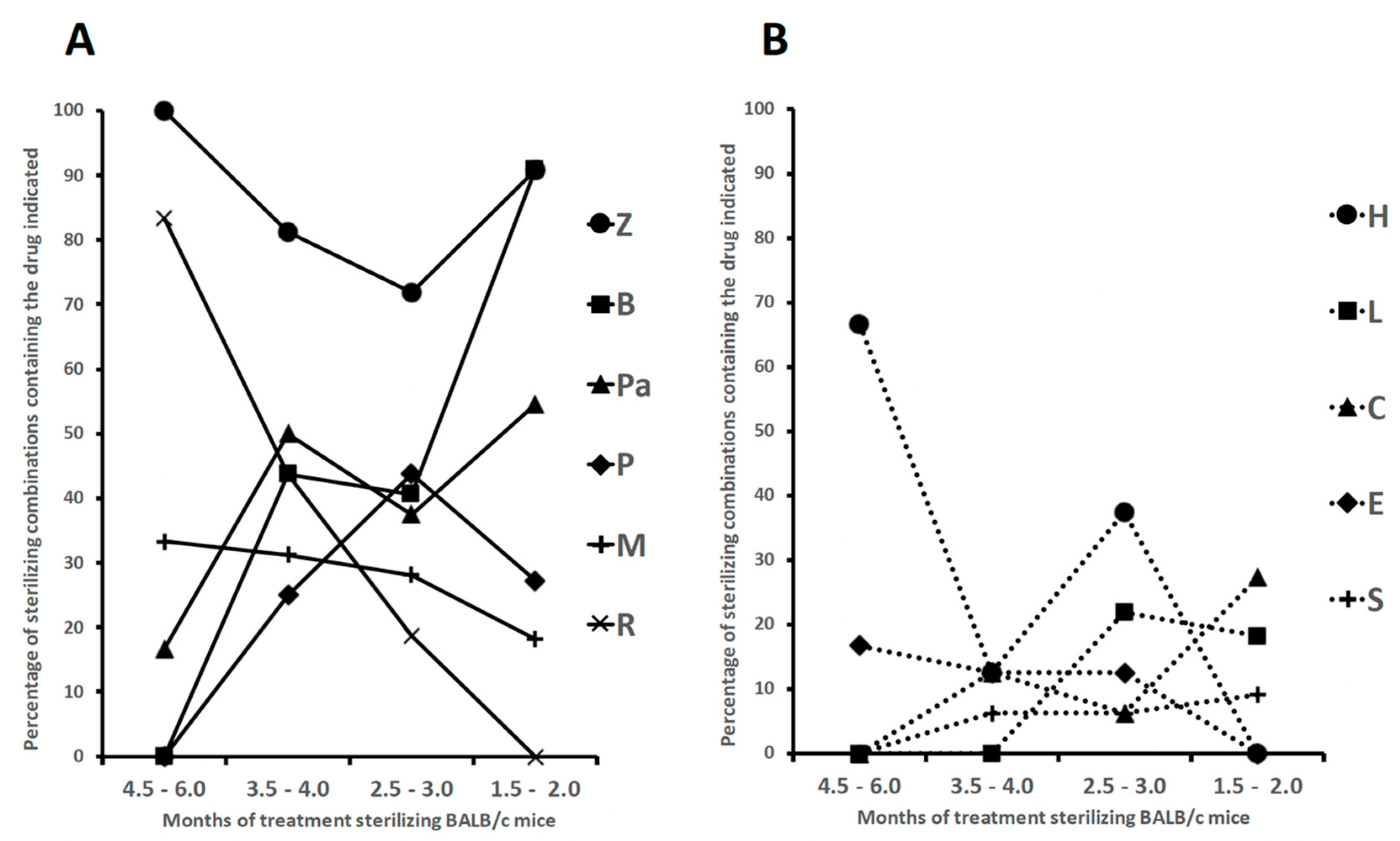
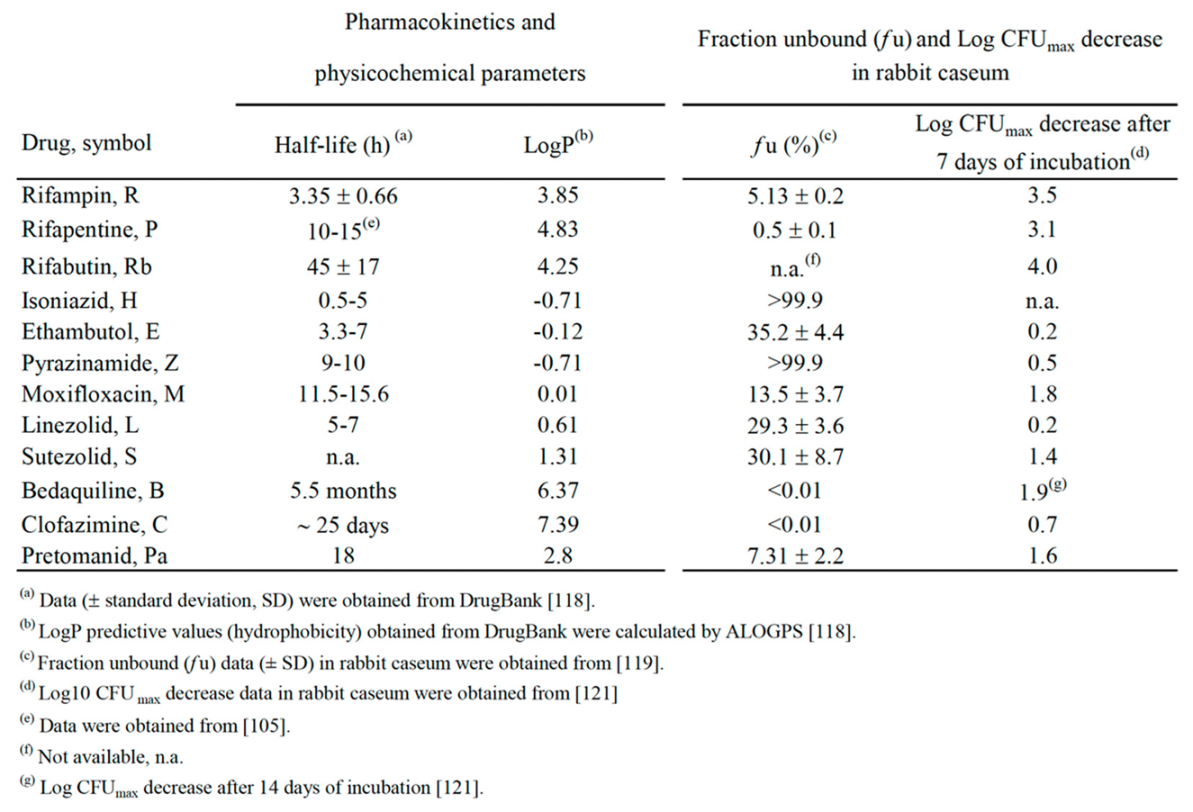
| part A |
 |
| part B |
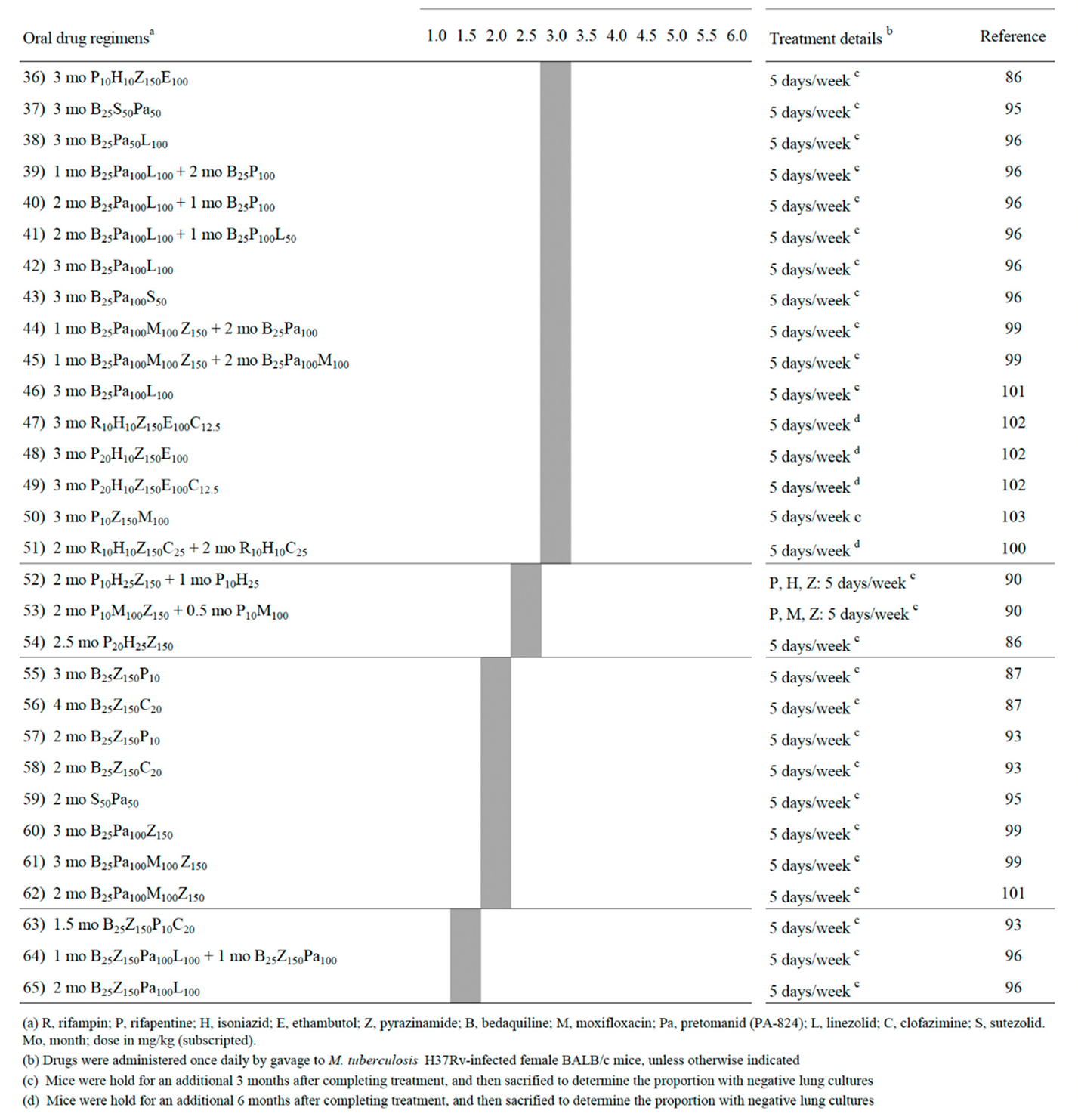 |
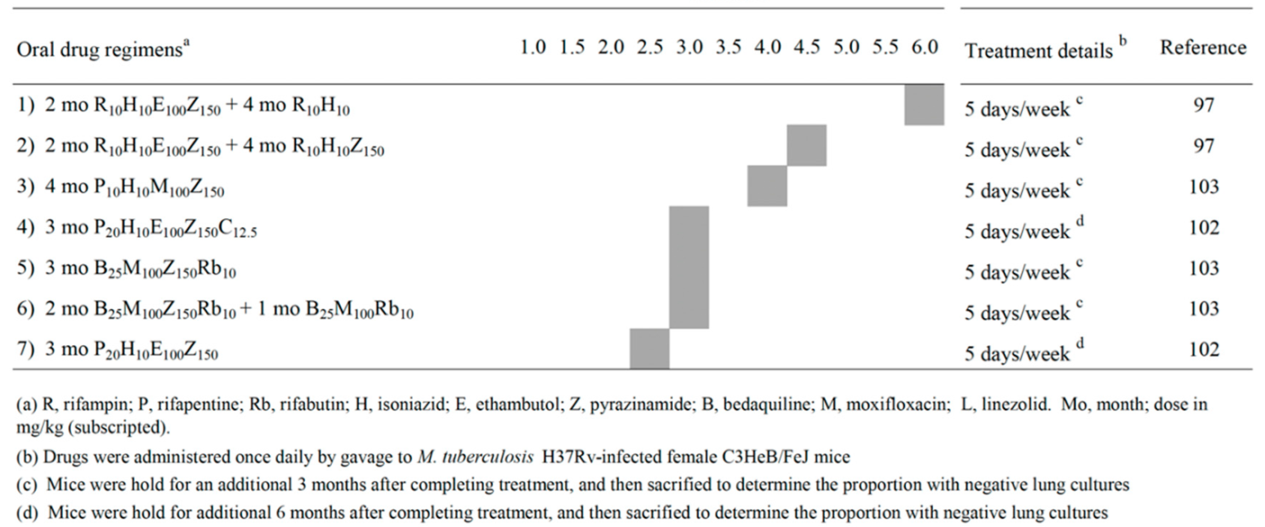 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).