Submitted:
29 April 2023
Posted:
29 April 2023
You are already at the latest version
Abstract
Keywords:
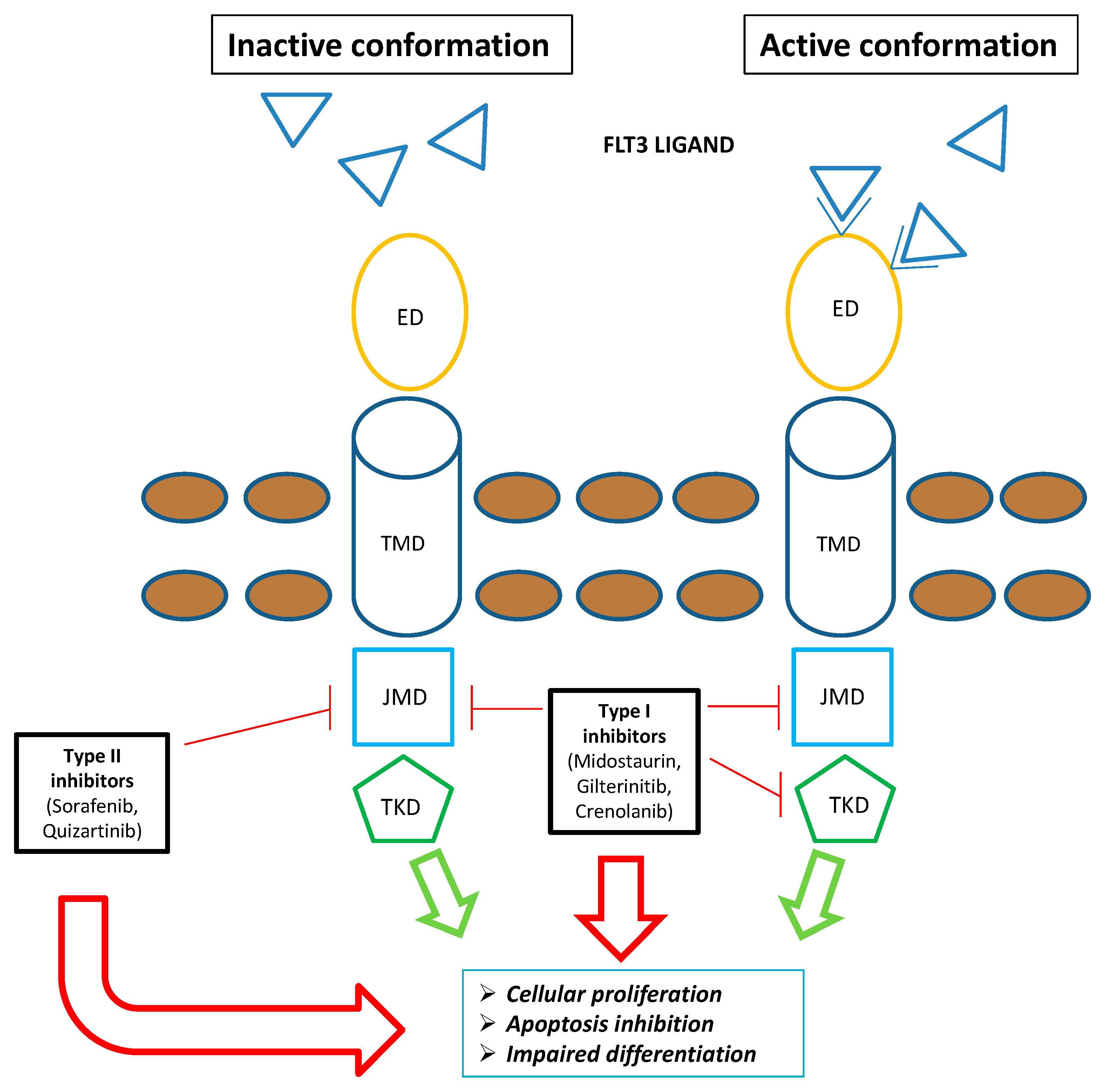
1. Introduction
2. Pharmacodynamics and pharmacokinetics of gilteritinib
3. Clinical trials including gilteritinib as monotherapy
3.1. Chrysalis trial
3.2. ADMIRAL trial
4. Real-life experiences with gilteritinib in R/R AML
5. Safety Profile of gilterinib
6. Combination regimens including gilteritinib in R/R and de novo AML
6.1. Gilteritinib plus azacitidine in FLT3-mutated AML
6.2. Gilteritinib plus venetoclax in R/R AML
6.3. Gilteritinib plus chemotherapy in patients with newly diagnosed AML
7. Maintenance therapy with gilteritinib after allogenic transplant
8. Gilteritinib for extramedullary AML relapse
9. Antifungal prophylaxis in patients treated with gilteritinib
10. Development of resistances to gilteritinib
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gale, R.E.; Green, C.; Allen, C.; Mead, A.J.; Burnett, A.K.; Hills, R.K.; Linch, D.C. ; Medical Research Council Adult Leukaemia Working Party The Impact of FLT3 Internal Tandem Duplication Mutant Level, Number, Size, and Interaction with NPM1 Mutations in a Large Cohort of Young Adult Patients with Acute Myeloid Leukemia. Blood 2008, 111, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Yanada, M.; Matsuo, K.; Suzuki, T.; Kiyoi, H.; Naoe, T. Prognostic Significance of FLT3 Internal Tandem Duplication and Tyrosine Kinase Domain Mutations for Acute Myeloid Leukemia: A Meta-Analysis. Leukemia 2005, 19, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Tiesmeier, J.; Müller-Tidow, C.; Westermann, A.; Czwalinna, A.; Hoffmann, M.; Krauter, J.; Heil, G.; Ganser, A.; Serve, H.; Verbeek, W. Evolution of FLT3-ITD and D835 Activating Point Mutations in Relapsing Acute Myeloid Leukemia and Response to Salvage Therapy. Leuk Res 2004, 28, 1069–1074. [Google Scholar] [CrossRef]
- Brandwein, J.M.; Saini, L.; Geddes, M.N.; Yusuf, D.; Liu, F.; Schwann, K.; Billawala, A.; Westcott, C.; Kurniawan, J.A.; Cheung, W.Y. Outcomes of Patients with Relapsed or Refractory Acute Myeloid Leukemia: A Population-Based Real-World Study. Am J Blood Res 2020, 10, 124–133. [Google Scholar] [PubMed]
- Stahl, M.; DeVeaux, M.; Montesinos, P.; Itzykson, R.; Ritchie, E.K.; Sekeres, M.A.; Barnard, J.D.; Podoltsev, N.A.; Brunner, A.M.; Komrokji, R.S.; et al. Hypomethylating Agents in Relapsed and Refractory AML: Outcomes and Their Predictors in a Large International Patient Cohort. Blood Advances 2018, 2, 923–932. [Google Scholar] [CrossRef]
- Roboz, G.J.; Rosenblat, T.; Arellano, M.; Gobbi, M.; Altman, J.K.; Montesinos, P.; O’Connell, C.; Solomon, S.R.; Pigneux, A.; Vey, N.; et al. International Randomized Phase III Study of Elacytarabine versus Investigator Choice in Patients with Relapsed/Refractory Acute Myeloid Leukemia. J Clin Oncol 2014, 32, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Molica, M.; Mazzone, C.; Niscola, P.; Carmosino, I.; Di Veroli, A.; De Gregoris, C.; Bonanni, F.; Perrone, S.; Cenfra, N.; Fianchi, L.; et al. Identification of Predictive Factors for Overall Survival and Response during Hypomethylating Treatment in Very Elderly (≥75 Years) Acute Myeloid Leukemia Patients: A Multicenter Real-Life Experience. Cancers 2022, 14, 4897. [Google Scholar] [CrossRef]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3 -Mutated AML. N Engl J Med 2019, 381, 1728–1740. [Google Scholar] [CrossRef]
- EMA Astellas Pharma. Xospata (Gilteritinib): EU Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xospata (accessed on 8 April 2023).
- Short, N.J.; Kantarjian, H.; Ravandi, F.; Daver, N. Emerging Treatment Paradigms with FLT3 Inhibitors in Acute Myeloid Leukemia. Ther Adv Hematol 2019, 10, 2040620719827310. [Google Scholar] [CrossRef]
- Mori, M.; Kaneko, N.; Ueno, Y.; Yamada, M.; Tanaka, R.; Saito, R.; Shimada, I.; Mori, K.; Kuromitsu, S. Gilteritinib, a FLT3/AXL Inhibitor, Shows Antileukemic Activity in Mouse Models of FLT3 Mutated Acute Myeloid Leukemia. Invest New Drugs 2017, 35, 556–565. [Google Scholar] [CrossRef]
- Levis, M.; Perl, A.E. Gilteritinib: Potent Targeting of FLT3 Mutations in AML. Blood Adv 2020, 4, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T.; Nakazawa, T.; Eguchi, T.; Tsuzuki, H.; Ueno, Y.; Amano, Y.; Suzuki, T.; Mori, M.; Yoshida, T. Effect of Fms-like Tyrosine Kinase 3 (FLT3) Ligand (FL) on Antitumor Activity of Gilteritinib, a FLT3 Inhibitor, in Mice Xenografted with FL-Overexpressing Cells. Oncotarget 2019, 10, 6111–6123. [Google Scholar] [CrossRef] [PubMed]
- Usuki, K.; Sakura, T.; Kobayashi, Y.; Miyamoto, T.; Iida, H.; Morita, S.; Bahceci, E.; Kaneko, M.; Kusano, M.; Yamada, S.; et al. Clinical Profile of Gilteritinib in Japanese Patients with Relapsed/Refractory Acute Myeloid Leukemia: An Open-Label Phase 1 Study. Cancer Sci 2018, 109, 3235–3244. [Google Scholar] [CrossRef] [PubMed]
- James, A.J.; Smith, C.C.; Litzow, M.; Perl, A.E.; Altman, J.K.; Shepard, D.; Kadokura, T.; Souda, K.; Patton, M.; Lu, Z.; et al. Pharmacokinetic Profile of Gilteritinib: A Novel FLT-3 Tyrosine Kinase Inhibitor. Clin Pharmacokinet 2020, 59, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Juvale, I.I.; Abdul Hamid, A.A.; Abd Halim, K.B.; Che Has, A.T. P-Glycoprotein: New Insights into Structure, Physiological Function, Regulation and Alterations in Disease. Heliyon 2022, 8, e09777. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Altman, J.K.; Cortes, J.; Smith, C.; Litzow, M.; Baer, M.R.; Claxton, D.; Erba, H.P.; Gill, S.; Goldberg, S.; et al. Selective Inhibition of FLT3 by Gilteritinib in Relapsed or Refractory Acute Myeloid Leukaemia: A Multicentre, First-in-Human, Open-Label, Phase 1-2 Study. Lancet Oncol 2017, 18, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Larson, R.A.; Podoltsev, N.A.; Strickland, S.; Wang, E.S.; Atallah, E.; Schiller, G.J.; Martinelli, G.; Neubauer, A.; Sierra, J.; et al. Follow-up of Patients with R/R FLT3-Mutation-Positive AML Treated with Gilteritinib in the Phase 3 ADMIRAL Trial. Blood 2022, 139, 3366–3375. [Google Scholar] [CrossRef]
- Smith, C.C.; Levis, M.J.; Perl, A.E.; Hill, J.E.; Rosales, M.; Bahceci, E. Molecular Profile of FLT3-Mutated Relapsed/Refractory Patients with AML in the Phase 3 ADMIRAL Study of Gilteritinib. Blood Adv 2022, 6, 2144–2155. [Google Scholar] [CrossRef]
- Perl, A.E.; Hosono, N.; Montesinos, P.; Podoltsev, N.; Martinelli, G.; Panoskaltsis, N.; Recher, C.; Smith, C.C.; Levis, M.J.; Strickland, S.; et al. Clinical Outcomes in Patients with Relapsed/Refractory FLT3-Mutated Acute Myeloid Leukemia Treated with Gilteritinib Who Received Prior Midostaurin or Sorafenib. Blood Cancer J. 2022, 12, 84. [Google Scholar] [CrossRef]
- Dumas, P.-Y.; Raffoux, E.; Bérard, E.; Bertoli, S.; Hospital, M.-A.; Heiblig, M.; Desbrosses, Y.; Bonmati, C.; Pautas, C.; Lambert, J.; et al. Gilteritinib Activity in Refractory or Relapsed FLT3-Mutated Acute Myeloid Leukemia Patients Previously Treated by Intensive Chemotherapy and Midostaurin: A Study from the French AML Intergroup ALFA/FILO. Leukemia 2023, 37, 91–101. [Google Scholar] [CrossRef]
- Othman, J.; Afzal, U.; Amofa, R.; Austin, M.J.; Bashford, A.; Belsham, E.; Byrne, J.; Coats, T.; Dang, R.; Dennis, M.; et al. Gilteritinib for Relapsed Acute Myeloid Leukaemia with FLT3 Mutation during the COVID-19 Pandemic: Real World Experience from the UK National Health Service. Blood 2021, 138, 1254. [Google Scholar] [CrossRef]
- Numan, Y.; Abdel Rahman, Z.; Grenet, J.; Boisclair, S.; Bewersdorf, J.P.; Collins, C.; Barth, D.; Fraga, M.; Bixby, D.L.; Zeidan, A.M.; et al. Gilteritinib Clinical Activity in Relapsed/Refractory FLT3 Mutated acute myeloid leukemia Previously Treated with FLT3 Inhibitors. American J Hematol 2022, 97, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Shimony, S.; Canaani, J.; Kugler, E.; Nachmias, B.; Ram, R.; Henig, I.; Frisch, A.; Ganzel, C.; Vainstein, V.; Moshe, Y.; et al. Gilteritinib Monotherapy for Relapsed/Refractory FLT3 Mutated Acute Myeloid Leukemia: A Real-World, Multi-Center, Matched Analysis. Ann Hematol 2022, 101, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Montesinos, P.; Minden, M.D.; Lee, J.-H.; Heuser, M.; Naoe, T.; Chou, W.-C.; Laribi, K.; Esteve, J.; Altman, J.K.; et al. Phase 3 Trial of Gilteritinib plus Azacitidine vs Azacitidine for Newly Diagnosed FLT3 Mut+ AML Ineligible for Intensive Chemotherapy. Blood 2022, 140, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Montesinos, P.; Ivanov, V.; DiNardo, C.D.; Novak, J.; Laribi, K.; Kim, I.; Stevens, D.A.; Fiedler, W.; Pagoni, M.; et al. Venetoclax plus LDAC for Newly Diagnosed AML Ineligible for Intensive Chemotherapy: A Phase 3 Randomized Placebo-Controlled Trial. Blood 2020, 135, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.; Hogdal, L.; Busman, T.; McKeegan, E.; Salem, A.H.; Zhu, M.; Ricker, J.L.; et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov 2016, 6, 1106–1117. [Google Scholar] [CrossRef]
- Singh Mali, R.; Zhang, Q.; DeFilippis, R.A.; Cavazos, A.; Kuruvilla, V.M.; Raman, J.; Mody, V.; Choo, E.F.; Dail, M.; Shah, N.P.; et al. Venetoclax Combines Synergistically with FLT3 Inhibition to Effectively Target Leukemic Cells in FLT3-ITD+ Acute Myeloid Leukemia Models. Haematologica 2021, 106, 1034–1046. [Google Scholar] [CrossRef]
- Brinton, L.T.; Zhang, P.; Williams, K.; Canfield, D.; Orwick, S.; Sher, S.; Wasmuth, R.; Beaver, L.; Cempre, C.; Skinner, J.; et al. Synergistic Effect of BCL2 and FLT3 Co-Inhibition in Acute Myeloid Leukemia. J Hematol Oncol 2020, 13, 139. [Google Scholar] [CrossRef]
- Daver, N.; Perl, A.E.; Maly, J.; Levis, M.; Ritchie, E.; Litzow, M.; McCloskey, J.; Smith, C.C.; Schiller, G.; Bradley, T.; et al. Venetoclax Plus Gilteritinib for FLT3 -Mutated Relapsed/Refractory Acute Myeloid Leukemia. JCO 2022, 40, 4048–4059. [Google Scholar] [CrossRef]
- Short, N.; DiNardo, C.D.; Daver, N.; Macaron, W.; Yilmaz, M.; Borthakur, G.; Montalban-Bravo, G.; Garcia-Manero, G.; Issa, G.C.; Sasaki, K.; et al. Updated Results from a Phase I/II Study of the Triplet Combination of Azacitidine, Venetoclax and Gilteritinib for Patients with FLT3 -Mutated Acute Myeloid Leukemia. Blood 2022, 140, 2007–2009. [Google Scholar] [CrossRef]
- Pratz, K.W.; Cherry, M.; Podoltsev, N.A.; Altman, J.K.; Perl, A.E.; Cooper, B.W.; Jurcic, J.G.; Lin, T.L.; Schiller, G.J.; Wu, R.; et al. AML-256 A Phase 1 Study of Gilteritinib in Combination With Induction and Consolidation Chemotherapy in Patients With Newly Diagnosed Acute Myeloid Leukemia: Final Study Results. Clinical Lymphoma Myeloma and Leukemia 2022, 22, S230. [Google Scholar] [CrossRef]
- Levis, M.J.; Hamadani, M.; Logan, B.; Rosales, M.; Perl, A.E.; Devine, S.M.; Bahceci, E.; Chen, Y.-B.A. A Phase 3, Trial of Gilteritinib, as Maintenance Therapy after Allogeneic Hematopoietic Stem Cell Transplantation in Patients with FLT3- ITD + AML. JCO 2018, 36, TPS7075. [Google Scholar] [CrossRef]
- Levis, M.J.; Perl, A.E.; Altman, J.K.; Gocke, C.D.; Bahceci, E.; Hill, J.; Liu, C.; Xie, Z.; Carson, A.R.; McClain, V.; et al. A Next-Generation Sequencing–Based Assay for Minimal Residual Disease Assessment in AML Patients with FLT3-ITD Mutations. Blood Advances 2018, 2, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Burchert, A.; Bug, G.; Fritz, L.V.; Finke, J.; Stelljes, M.; Röllig, C.; Wollmer, E.; Wäsch, R.; Bornhäuser, M.; Berg, T.; et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3 –Internal Tandem Duplication Mutation (SORMAIN). JCO 2020, 38, 2993–3002. [Google Scholar] [CrossRef]
- Maziarz, R.T.; Levis, M.; Patnaik, M.M.; Scott, B.L.; Mohan, S.R.; Deol, A.; Rowley, S.D.; Kim, D.D.H.; Hernandez, D.; Rajkhowa, T.; et al. Midostaurin after Allogeneic Stem Cell Transplant in Patients with FLT3-Internal Tandem Duplication-Positive Acute Myeloid Leukemia. Bone Marrow Transplant 2021, 56, 1180–1189. [Google Scholar] [CrossRef]
- Perl, A.E.; Larson, R.A.; Podoltsev, N.A.; Strickland, S.; Wang, E.S.; Atallah, E.; Schiller, G.J.; Martinelli, G.; Neubauer, A.; Sierra, J.; et al. Outcomes in Patients with FLT3-Mutated Relapsed/ Refractory Acute Myelogenous Leukemia Who Underwent Transplantation in the Phase 3 ADMIRAL Trial of Gilteritinib versus Salvage Chemotherapy. Transplant Cell Ther 2023, 29, 265.e1–265.e10. [Google Scholar] [CrossRef]
- Yeh, J.; Saliba, R.M.; Wang, C.; Fang, Z.; Figgins, B.; Ahmed, S.; Yilmaz, M.; Daver, N.; Mehta, R.S.; Alatrash, G.; et al. Efficacy and Safety of Gilteritinib Vs. Sorafenib As Post-Transplant Maintenance in Patients with FLT3-ITD Acute Myeloid Leukemia. Blood 2022, 140, 7686–7688. [Google Scholar] [CrossRef]
- Terao, T.; Matsuoka, K.; Ueda, H.; Matsumura, A.; Matsubara, C.; Kondo, K.; Kondo, T.; Fujiwara, H.; Asada, N.; Ennishi, D.; et al. Gilteritinib Maintenance Therapy Post-Allogenic Stem-Cell Transplantation Improves the Prognosis of Patients with FLT3-Mutated AML. Blood 2022, 140, 3290–3291. [Google Scholar] [CrossRef]
- Fukuda, S.; Onishi, C.; M., L. Trafficking of Acute Leukemia Cells – Chemokine Receptor Pathways That Modulate Leukemia Cell Dissemination. In Acute Leukemia - The Scientist’s Perspective and Challenge; Antica, M., Ed.; InTech, 2011 ISBN 978-953-307-553-2.
- Mohammadiasl, J.; Khosravi, A.; Shahjahani, M.; Azizidoost, S.; Saki, N. Molecular and Cellular Aspects of Extramedullary Manifestations of Acute Myeloid Leukemia. J Cancer Metastasis Treat 2015, 0, 0. [Google Scholar] [CrossRef]
- Perrone, S.; Ortu La Barbera, E.; Viola, F.; Cipollone, E.; Scerpa, M.C.; Siniscalchi, R.; Ottone, T.; Voso, M.T.; Cimino, G. A Relapsing Meningeal Acute Myeloid Leukaemia FLT3-ITD+ Responding to Gilteritinib. Chemotherapy 2021, 66, 134–138. [Google Scholar] [CrossRef]
- Vignal, N.; Kelly, L.; Lengline, E.; Cabannes-Hamy, A.; Siavellis, J.; Ghez, D.; Sauvageon, H.; Braun, T.; Jacqz-Aigrain, E.; Kohn, M.; et al. Favorable Pharmacokinetics and Pharmacodynamics Properties of Gilteritinib in Cerebrospinal Fluid: A Potential Effective Treatment in Relapsing Meningeal Acute Myeloid Leukaemia FLT3-ITD Patients. haematol 2023. [Google Scholar] [CrossRef]
- Kumode, T.; Rai, S.; Tanaka, H.; Espinoza, J.L.; Kakutani, H.; Watatani, Y.; Minamoto, S.; Taniguchi, Y.; Nakayama, S.; Morita, Y.; et al. Targeted Therapy for Medullary and Extramedullary Relapse of FLT3-ITD Acute Myeloid Leukemia Following Allogeneic Hematopoietic Stem Cell Transplantation. Leuk Res Rep 2020, 14, 100219. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.S.; Yaghy, A.; Wilde, L.R.; Shields, C.L. An Iridociliochoroidal Myeloid Sarcoma Associated With Relapsed Acute Myeloid Leukemia With FLT3-ITD Mutation, Treated With Gilteritinib, an FLT3 Inhibitor. JAMA Ophthalmol 2020, 138, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Aleissa, M.M.; Alshehri, B.S.; Gonzalez-Bocco, I.H.; McDonnell, A.M.; Leblebjian, H.; Marty, F.M.; Luskin, M.R. Triazole Antifungal Use for Prophylaxis and Treatment of Invasive Fungal Diseases for Patients Receiving Gilteritinib. Leuk Res 2021, 108, 106610. [Google Scholar] [CrossRef]
- Stemler, J.; Cornely, O.A. Antifungal Prophylaxis in Acute Myeloid Leukemia: New Drugs, New Challenges?: Summary of the EHA Guideline on Antifungal Prophylaxis in Adult Patients With Acute Myeloid Leukemia Treated With Novel-Targeted Therapies. Hemasphere 2022, 6, e742. [Google Scholar] [CrossRef]
- Alotaibi, A.S.; Yilmaz, M.; Kanagal-Shamanna, R.; Loghavi, S.; Kadia, T.M.; DiNardo, C.D.; Borthakur, G.; Konopleva, M.; Pierce, S.A.; Wang, S.A.; et al. Patterns of Resistance Differ in Patients with Acute Myeloid Leukemia Treated with Type I versus Type II FLT3 Inhibitors. Blood Cancer Discov 2021, 2, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Peretz, C.A.C.; McGary, L.H.F.; Kumar, T.; Jackson, H.; Jacob, J.; Durruthy-Durruthy, R.; Levis, M.J.; Perl, A.; Huang, B.J.; Smith, C.C. Single-Cell DNA Sequencing Reveals Complex Mechanisms of Resistance to Quizartinib. Blood Adv 2021, 5, 1437–1441. [Google Scholar] [CrossRef]
- Parmar, A.; Marz, S.; Rushton, S.; Holzwarth, C.; Lind, K.; Kayser, S.; Döhner, K.; Peschel, C.; Oostendorp, R.A.J.; Götze, K.S. Stromal Niche Cells Protect Early Leukemic FLT3-ITD+ Progenitor Cells against First-Generation FLT3 Tyrosine Kinase Inhibitors. Cancer Res 2011, 71, 4696–4706. [Google Scholar] [CrossRef]
- Chen, F.; Ishikawa, Y.; Kiyoi, H.; Naoe, T. Mechanism of FLT3 Ligand Dependent Resistance to FLT3 Inhibitors. Blood 2014, 124, 908–908. [Google Scholar] [CrossRef]
- Smith, C.C.; Levis, M.J.; Perl, A.E.; Martinelli, G.; Neubauer, A.; Berman, E.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Chou, W.-C.; et al. Emerging Mutations at Relapse in Patients with FLT3-Mutated Relapsed/Refractory Acute Myeloid Leukemia Who Received Gilteritinib Therapy in the Phase 3 Admiral Trial. Blood 2019, 134, 14–14. [Google Scholar] [CrossRef]
- Levis, M.J.; Perl, A.E.; Altman, J.K.; Cortes, J.E.; Smith, C.C.; Baer, M.R.; Claxton, D.F.; Jurcic, J.G.; Ritchie, E.K.; Strickland, S.A.; et al. Evaluation of the Impact of Minimal Residual Disease, FLT3 Allelic Ratio, and FLT3 Mutation Status on Overall Survival in FLT3 Mutation-Positive Patients with Relapsed/Refractory (R/R) Acute Myeloid Leukemia (AML) in the Chrysalis Phase 1/2 Study. Blood 2017, 130, 2705. [Google Scholar] [CrossRef]
- McMahon, C.M.; Ferng, T.; Canaani, J.; Wang, E.S.; Morrissette, J.J.D.; Eastburn, D.J.; Pellegrino, M.; Durruthy-Durruthy, R.; Watt, C.D.; Asthana, S.; et al. Clonal Selection with RAS Pathway Activation Mediates Secondary Clinical Resistance to Selective FLT3 Inhibition in Acute Myeloid Leukemia. Cancer Discovery 2019, 9, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, T.; Nakatani, T.; Uda, K.; Ogura, H.; Shin, W.; Kurokawa, N.; Saito, K.; Fujikawa, N.; Date, T.; Takasaki, M.; et al. A Novel Irreversible FLT3 Inhibitor, FF-10101, Shows Excellent Efficacy against AML Cells with FLT3 Mutations. Blood 2018, 131, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Ferng, T.T.; Terada, D.; Ando, M.; Tarver, T.C.; Chaudhary, F.; Lin, K.C.; Logan, A.C.; Smith, C.C. The Irreversible FLT3 Inhibitor FF-10101 Is Active Against a Diversity of FLT3 Inhibitor Resistance Mechanisms. Mol Cancer Ther 2022, 21, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Levis, M.J.; Smith, C.C.; Perl, A.E.; Schiller, G.J.; Fathi, A.T.; Roboz, G.J.; Wang, E.S.; Altman, J.K.; Ando, M.; Suzuki, T.; et al. Phase 1 First-in-Human Study of Irreversible FLT3 Inhibitor FF-10101-01 in Relapsed or Refractory Acute Myeloid Leukemia. JCO 2021, 39, 7008–7008. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Wang, Y.; Shen, Y. Sitravatinib as a Potent FLT3 Inhibitor Can Overcome Gilteritinib Resistance in Acute Myeloid Leukemia. Biomark Res 2023, 11, 8. [Google Scholar] [CrossRef]
- McMahon, C.M.; Canaani, J.; Rea, B.; Sargent, R.L.; Qualtieri, J.N.; Watt, C.D.; Morrissette, J.J.D.; Carroll, M.; Perl, A.E. Gilteritinib Induces Differentiation in Relapsed and Refractory FLT3-Mutated Acute Myeloid Leukemia. Blood Advances 2019, 3, 1581–1585. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Shih, L.-Y.; Huang, C.-F.; Wu, J.-H.; Lin, T.-L.; Dunn, P.; Wang, P.-N.; Kuo, M.-C.; Lai, C.-L.; Hsu, H.-C. Internal Tandem Duplication of FLT3 in Relapsed Acute Myeloid Leukemia: A Comparative Analysis of Bone Marrow Samples from 108 Adult Patients at Diagnosis and Relapse. Blood 2002, 100, 2387–2392. [Google Scholar] [CrossRef]
- Schranz, K.; Hubmann, M.; Harin, E.; Vosberg, S.; Herold, T.; Metzeler, K.H.; Rothenberg-Thurley, M.; Janke, H.; Bräundl, K.; Ksienzyk, B.; et al. Clonal Heterogeneity of FLT3 -ITD Detected by High-Throughput Amplicon Sequencing Correlates with Adverse Prognosis in Acute Myeloid Leukemia. Oncotarget 2018, 9, 30128–30145. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Allen, C.; Mirza, A.-S.; Grimshaw, A.A.; Giri, S.; Podoltsev, N.A.; Gowda, L.; Cho, C.; Tallman, M.S.; Zeidan, A.M.; et al. Hypomethylating Agents and FLT3 Inhibitors As Maintenance Treatment for Acute Myeloid Leukemia and Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation-A Systematic Review and Meta-Analysis. Transplant Cell Ther 2021, 27, 997.e1–997.e11. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xuan, L.; Lin, R.; Deng, L.; Fan, Z.; Nie, D.; Li, X.; Liang, X.; Xu, D.; Zhang, Y.; et al. A New Pre-Emptive TKIs Strategy for Preventing Relapse Based on BCR/ABL Monitoring for Ph+ALL Undergoing Allo-HCT: A Prospective Clinical Cohort Study. Leukemia 2021, 35, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Najima, Y. Overcoming Relapse: Prophylactic or Pre-Emptive Use of Azacitidine or FLT3 Inhibitors after Allogeneic Transplantation for AML or MDS. Int J Hematol 2023. [Google Scholar] [CrossRef] [PubMed]
- Heuser, M.; Freeman, S.D.; Ossenkoppele, G.J.; Buccisano, F.; Hourigan, C.S.; Ngai, L.L.; Tettero, J.M.; Bachas, C.; Baer, C.; Béné, M.-C.; et al. 2021 Update on MRD in Acute Myeloid Leukemia: A Consensus Document from the European LeukemiaNet MRD Working Party. Blood 2021, 138, 2753–2767. [Google Scholar] [CrossRef]
- Erba, H.; Montesinos, P.; Vrhovac, R.; Patkowska, E.; Kim, H.-J.; Zak, P.; Wang, P.-N.; Mitov, T.; Hanyok, J.; Liu, L. S100: Quizartinib prolonged survival vs placebo plus intensive induction and consolidation therapy followed by single-agent continuation in patients aged 18-75 years with newly diagnosed FLT3-ITD+ AML. HemaSphere 2022, 6, 1–2. [Google Scholar] [CrossRef]
| Response data | |
|---|---|
Overall Response in FLT3 mutated AML1 (n=247)
|
67.7 34 21.1 26.7 11 |
Rate of response in FLT3 mutated AML previously treated with TKIs7 (n=33)
|
17 8.9 57 47 3.7 12.9 |
Rate of response in FLT3 mutated AML1 by baseline co-mutations (n=239)
|
29 17.2 12.8 20 28.6 27 29.3 30.9 13.3 28.2 |
| Outcomes data | |
Outcomes of FLT3 mutated AML1 (n=247)
|
9.3 36.6 20.6 15.8 52.6 75.7 |
Outcomes of FLT3 mutated AML1 according to previous TKIs7 therapy
|
9.5 8.7 |
Overall survival in FLT3 mutated AML by baseline co-mutations (n=239)
|
11.4 9.6 7.1 4.6 10.6 8.6 11 15.1 8.3 15.4 |
Outcomes of FLT3 mutated AML1 according FLT3-ITD length, multiple FLT3-ITD mutations and FLT3-ITD allelic ratio
|
10.4 8.9 9.3 7.1 10.6 |
| Reference | Number of patients | Composite complete remission | Median overall survival | Comment |
|---|---|---|---|---|
| Dumas et al.21 | 140 (cohort B) 67 previously treated by intensive chemotherapy and midostaurin (cohort C) |
25.4% (cohort B) 27.5% (cohort C) |
6.4 months (cohort B) 7.8 months (cohort C) |
prognostic factors associated with OS identified female gender (HR 1.61), adverse cytogenetic risk (HR 2.52), and allogenic transplant after gilteritinib (HR 0.13) |
| Othman et al.22 | 50 (86% received previous intensive chemotherapy) | 27% | 6.7 months (95%CI 4.5 - not reached) | the rate of composite complete response did not differ in those with previous exposure to FLT3 inhibitors (23% vs 32%, p=0.6) or with past allogeneic transplant (29% vs 27%, p=0.3) |
| Numan et al.23 | 113 (62.8% received gilteritinib as monotherapy, while the remaining patients received gilteritinib in combination with other agents) | 48.7% | 7.4 months for transplant group 7.1 months for none-transplant 7.8 months in patients treated with prior midostaurin 5 months in patients treated with prior sorafenib |
The presence of PTPN11 and NRAS had a significant inferior impact on composite complete remission rate (59% vs. 37.5%) and median overall survival (4.9 months vs. 7.8 months; HR 2.4–95% CI 1. 1–5.4 -p = .0057) |
| Shimony et al.24 | 25 (80% treated with prior intensive chemotherapy and 40% previously treated with tyrosine kinase inhibitor therapy) | 48% | 8 months | Prior tyrosine kinase inhibitor exposure did not negatively impact on overall survival and was associated with superior event-free survival (p = 0.016) |
| Number of the study | Protocol regimen | Eligible patients |
|---|---|---|
| NCT04027309 | gilteritinib versus midostaurin in combination with induction and consolidation therapy followed by one-year maintenance | newly diagnosed acute myeloid leukemia or myelodysplastic syndromes with excess blasts-2 with FLT3 mutations |
| NCT04140487 | azacitidine, venetoclax, and gilteritinib | relapsed/refractory FLT3-mutated acute myeloid leukemia, chronic myelomonocytic leukemia, or high-risk myelodysplastic syndrome/myeloproliferative neoplasm |
| NCT04240002 | gilteritinib combined with chemotherapy | children, adolescents and young adults FLT3-ITD positive relapsed/refractory acute myeloid leukemia |
| NCT05546580 | iadademstat and gilteritinib | relapsed/refractory acute myeloid leukemia with FLT3-ITD mutation |
| NCT05520567 | gilteritinib, venetoclax and azacitidine | newly diagnosed with acute myeloid leukemia with FLT3 mutations |
| NCT05028751 | lanraplenib (lanra) in combination with gilteritinib | FLT3 mutated relapsed/refractory acute myeloid leukemia |
| NCT05010122 | astx727, venetoclax, and gilteritinib | newly Diagnosed, relapsed/refractory FLT3-Mutated acute myeloid leukemia or high-risk myelodysplastic syndrome |
| NCT04293562 | standard chemotherapy versus therapy with cpx-351 and/or gilteritinib | newly diagnosed acute myeloid leukemia with or without FLT3 Mutations |
| NCT05010772 | decitabine alone or in combination with venetoclax, gilteritinib, enasidenib, or ivosidenib as maintenance therapy | acute myeloid leukemia in remission |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





