Submitted:
29 April 2023
Posted:
30 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction: Oxygen, ROS and Oxidative Stress
2. FCM in Oxidative Stress Research
2.1. Specific Features and Limitations of Functional FCM
2.1.1. Multiparametric Data Acquisition
2.1.2. Multivariate Data Analysis
2.1.3. Fast Analysis of Large Number of Live Cells
2.1.4. Real-Time Flow Cytometry
2.1.5. Individual Cell Sorting
2.1.6. Limitations of Functional FCM
3. General Strategies in Flow Cytometric Analysis of Oxygen and Oxidative Stress
- (a)
- Performing cell-based studies in hypoxic conditions:
- (b) Monitoring intracellular Oxygen in hypoxic conditions:
- (c) Direct detection of ROS, the initiators of the oxidative stress process:
- (d) Detection of more stable oxidized end products:
- (e) Assessment of antioxidant defences, mostly GSH and SH-containing proteins:
3.1. Monitoring intracellular Oxygen in hypoxic conditions
3.2. Direct Detection of ROS using fluorogenic substrates
3.2.1. 1O2 Probes
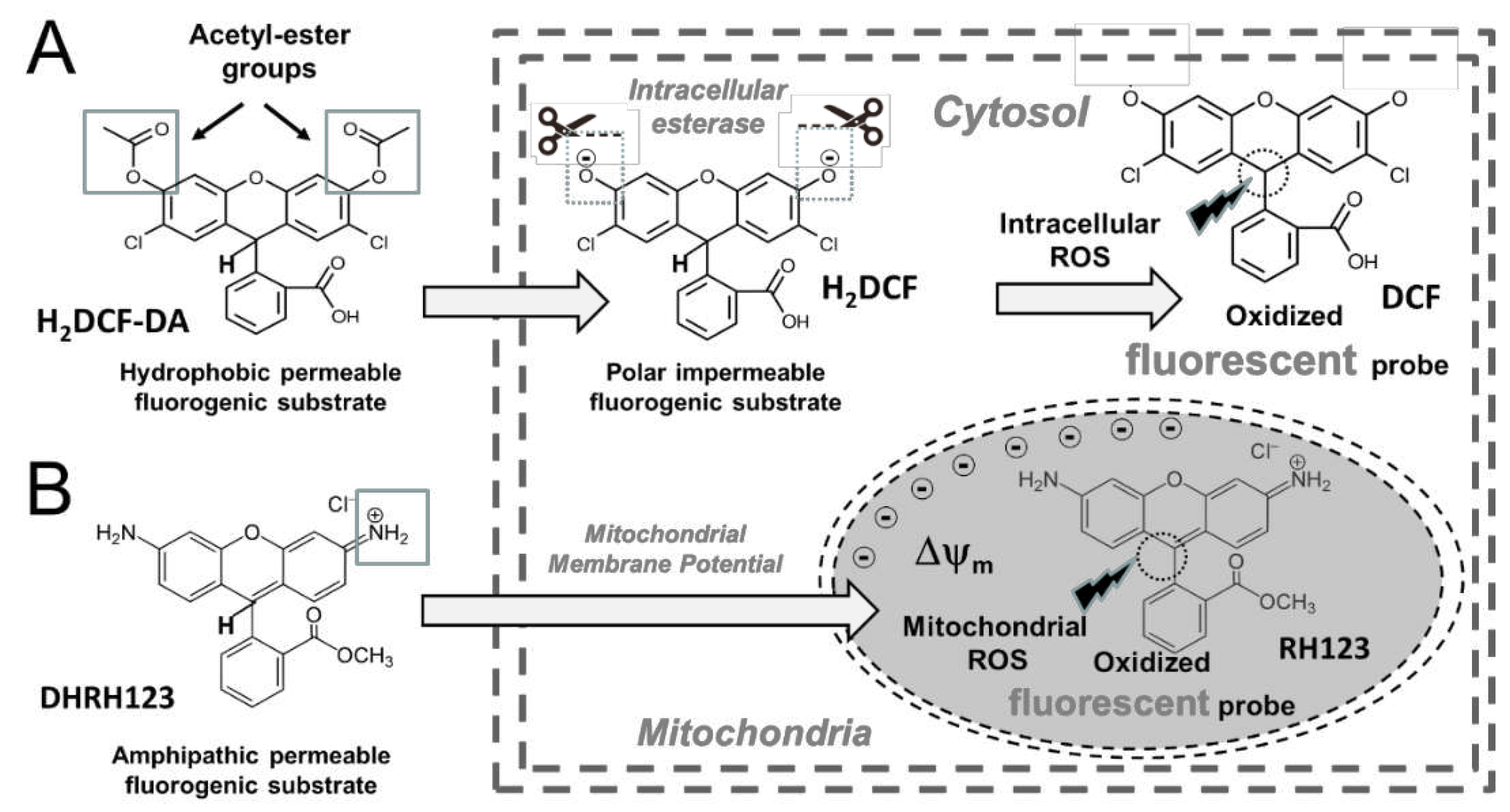
3.2.2. 2’,7’-Dichlorodihydrofluorescein diacetate (H2DCF-DA) and related probes
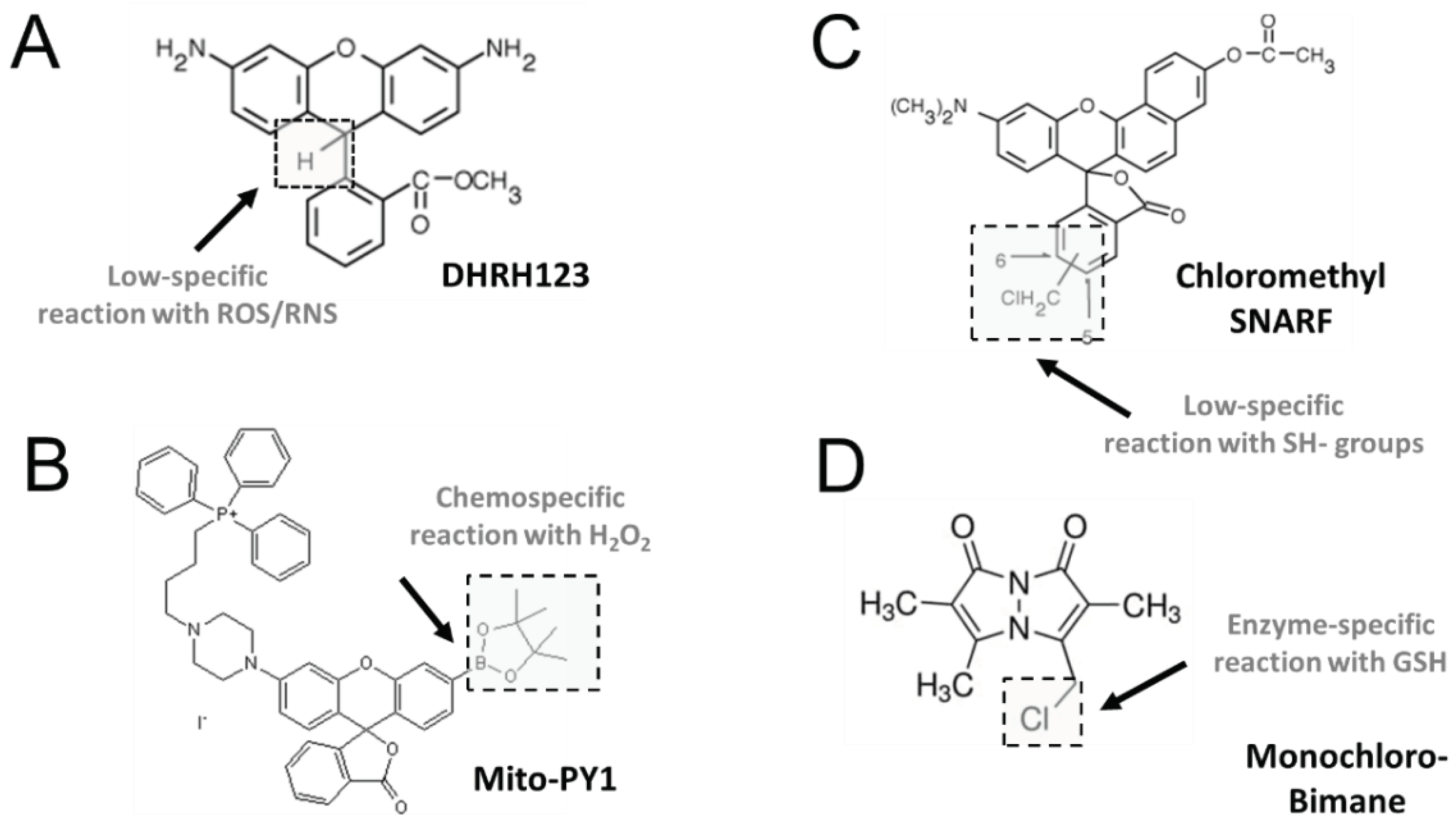
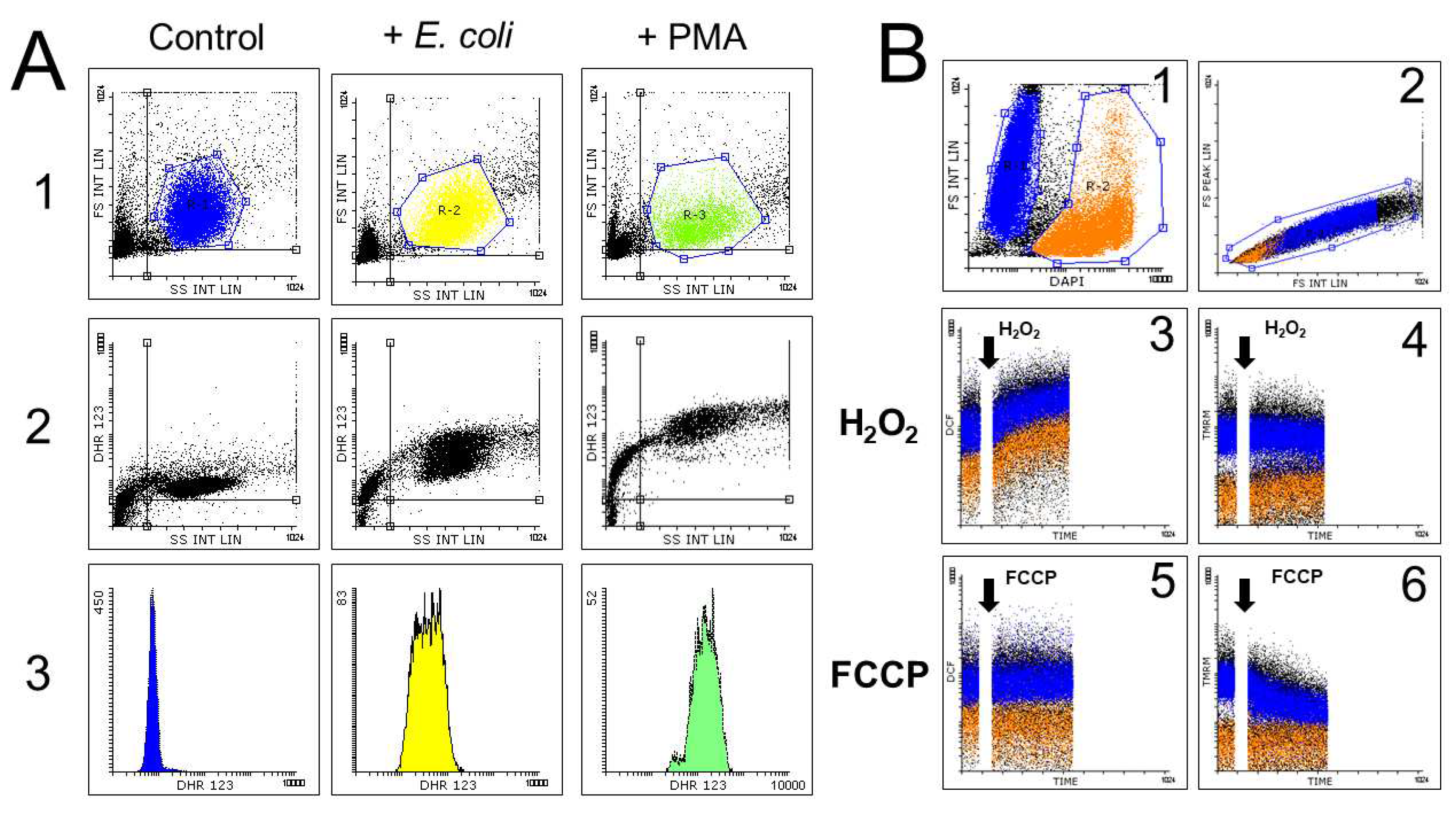
3.2.3. Dihydrorhodamine 123 (DHR123)
3.2.4. Mitochondria peroxy yellow 1 (MitoPY1) and related arylboronate fluorescent probes
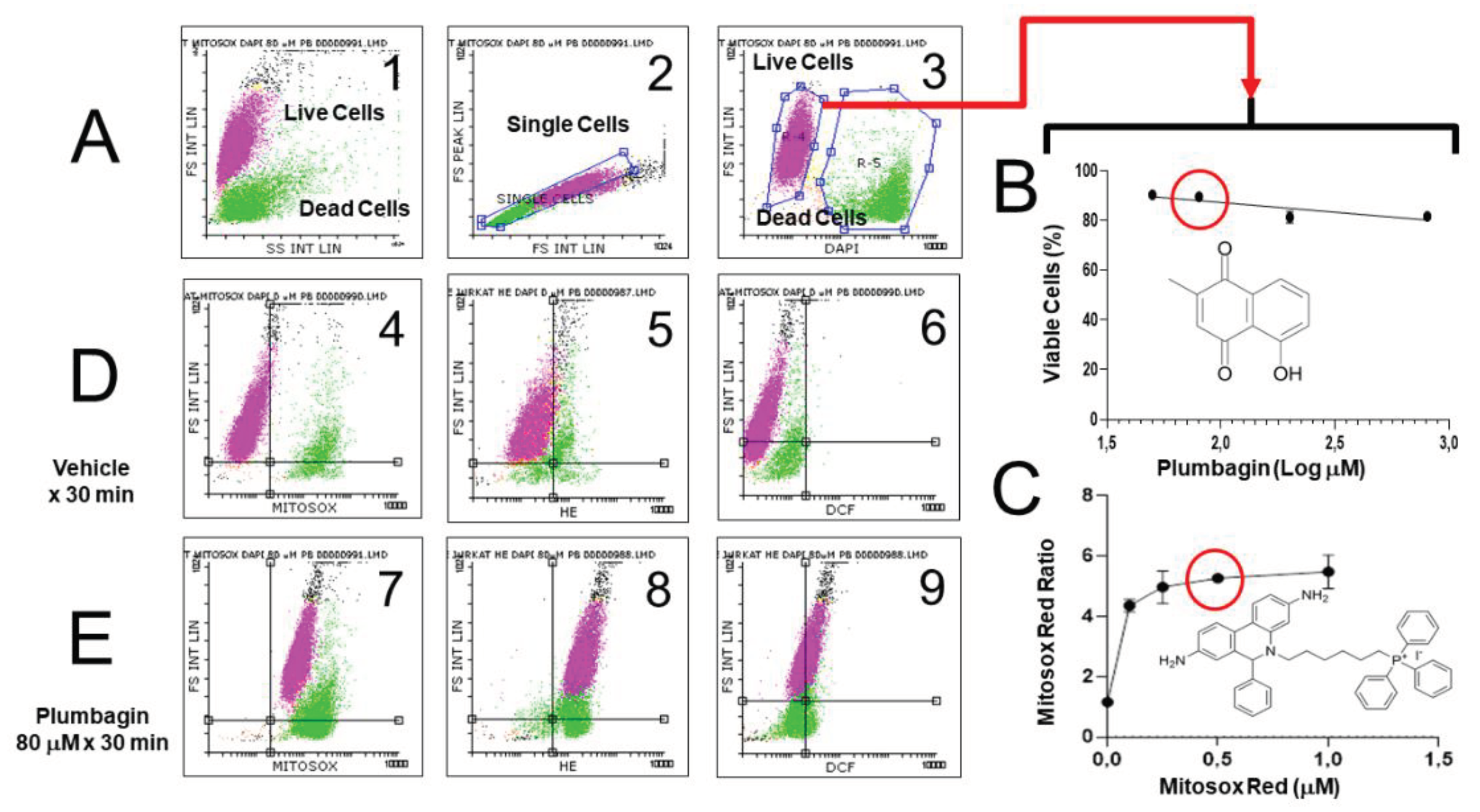
3.2.5. Hydroethidine and MitoSOX mitochondrial O2.- indicators
3.2.6. CellROX® reagents
3.2.7. ROS-ID® reagents
3.3. Detection of more stable products of ROS reaction
3.3.1. Detection of Lipid Peroxidation
cis-Parinaric Acid
Lipophilic Fluorescein derivatives
3.3.2. Detection of Metabolic Derivatives of Peroxidized Lipids
Immunofluorescent detection of 4-hydroxy-2-nonenal (4-HNE)
Immunofluorescent detection of oxidized bases in DNA
3.4. Assessment of Antioxidant Defenses: Glutathione (GSH) and Thiols (SH)
4. FCM in Oxidative Stress Research
4.1. Variability in half-life and intracellular sources of ROS
4.2. Interactions among and between ROS and RNS
4.3. Influence of the probes on the experimental system
4.4. Cell integrity and intracellular retention of probes
4.5. Experimental artifacts
4.6. Intrinsic limitations of fluorogenic substrates and probes
4.6.1. Probes used for detection of H2O2 and organic peroxides
4.6.2. Probes used for detection of O2.-
4.6.3. Probes used for detection of lipid peroxides
4.6.4. Probes used for the determination of GSH
5. Recommendations for performing FCM analysis of ROS, RNS and Oxidative Stress
5.1. Inclusion of Experimental Controls
5.1.1. Positive Controls
5.1.2. Negative Controls
5.1.3. Genetically-Modified organisms as controls
5.2. Choice of Fluorescent Probes
5.3. Fluorescent Probe titration
5.4. Range-finding experiments and Exclusion of dead/injured cells
5.6. Data generation, presentation, data analysis and publication
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Taverne, Y.J.; Merkus, D.; Bogers, A.J.; Halliwell, B.; Duncker, D.J.; Lyons, T.W. Reactive Oxygen Species: Radical Factors in the Evolution of Animal Life. BioEssays. 2018, 40, 1700158. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Fasciolo, G.; Venditti, P. The Ambiguos Aspects of Oxygen. Oxygen. 2022, 2, 382–409. [Google Scholar] [CrossRef]
- Forman, H.J.; Augusto, O.; Brigelius-Flohe, R.; Dennery, P.A.; Kalyanaraman, B.; Ischiropoulos, H.; Mann, G.E.; Radi, R.; Roberts, L.J., 2nd; Vina, J.; et al. Even free radicals should follow some rules: A guide to free radical research terminology and methodology. Free Radic Biol Med. 2015, 78, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Sedlářová, M.; Pospíšil, P. Singlet oxygen imaging using fluorescent probe Singlet Oxygen Sensor Green in photosynthetic organisms. Sci Rep. 2018, 8, 13685. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid Med Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Speckmann, B.; Steinbrenner, H.; Grune, T.; Klotz, L.O. Peroxynitrite: From interception to signaling. Arch Biochem Biophys. 2016, 595, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen. 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Clancy, D.; Birdsall, J. Flies, worms and the Free Radical Theory of ageing. Ageing Res Rev. 2013, 12, 404–412. [Google Scholar] [CrossRef]
- Hayashi, G.; Cortopassi, G. Oxidative stress in inherited mitochondrial diseases. Free Radic Biol Med. 2015, 88, 10–17. [Google Scholar] [CrossRef]
- Moulin, M.; Ferreiro, A. Muscle redox disturbances and oxidative stress as pathomechanisms and therapeutic targets in early-onset myopathies. Semin Cell Dev Biol. 2017, 64, 213–223. [Google Scholar] [CrossRef]
- Reula, A.; Pellicer, D.; Castillo, S.; Magallón, M.; Armengot, M.; Herrera, G.; O’Connor, J.E.; Bañuls, L.; Navarro-García, M.M.; Escribano, A.; et al. New Laboratory Protocol to Determine the Oxidative Stress Profile of Human Nasal Epithelial Cells Using Flow Cytometry. J Clin Med. 2021, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, B.; Nos, P.; Dasí, F.; Iborra, M.; Bastida, G.; Martínez, M.; O’Connor, J.E.; Sáez, G.; Moret, I.; Ponce, J. Mitochondrial dysfunction, persistent oxidative damage, and catalase inhibition in immune cells of naïve and treated Crohn’s disease. Inflamm Bowel Dis. 2010, 16, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Battacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int J Mol Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Santilli, F.; D’Ardes, D.; Davì, G. Oxidative stress in chronic vascular disease: From prediction to prevention. Vascul Pharmacol. 2015, 74, 23–37. [Google Scholar] [CrossRef]
- Li, H.; Horke, S.; Förstermann, U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014, 237, 208–219. [Google Scholar] [CrossRef]
- Montezano, A.C.; Dulak-Lis, M.; Tsiropoulou, S.; Harvey, A.; Briones, A.M.; Touyz, R.M. Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol. 2015, 31, 631–641. [Google Scholar] [CrossRef]
- Fuentes, E.; Palomo, I. Role of oxidative stress on platelet hyperreactivity during aging. Life Sci. 2016, 148, 17–23. [Google Scholar] [CrossRef]
- Collado, R.; Ivars, D.; Oliver, I.; Tormos, C.; Egea, M.; Miguel, A.; Sáez, G.T.; Carbonell, F. Increased oxidative damage associated with unfavorable cytogenetic subgroups in chronic lymphocytic leukemia. Biomed Res Int. 2014, 2014, 686392. [Google Scholar] [CrossRef]
- Oh, B.; Figtree, G.; Costa, D.; Eade, T.; Hruby, G.; Lim, S.; Elfiky, A.; Martine, N.; Rosenthal, D.; Clarke, S.; et al. Oxidative stress in prostate cancer patients: A systematic review of case control studies. Prostate Int. 2016, 4, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wen, J.; Huang, Z.; Nice, E.C.; Huang, C.; Zhang, H.; Li, Q. Redox proteomics screening cellular factors associated with oxidative stress in hepatocarcinogenesis. Proteomics Clin Appl. 2017, 11, 1600089. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; Ciucis, C.D.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy. Oxid Med Cell Longev. 2016, 2016, 6235641. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Zhelev, Z.; Aoki, I.; Bakalova, R.; Higashi, T. Overproduction of reactive oxygen species - obligatory or not for induction of apoptosis by anticancer drugs. Chin J Cancer Res. 2016, 28, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid Med Cell Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef] [PubMed]
- Elbim, C.; Pillet, S.; Prevost, M.H.; Preira, A.; Girard, P.M.; Rogine, N.; Hakim, J.; Israel, N.; Gougerot-Pocidalo, M.A. The role of phagocytes in HIV-related oxidative stress. J Clin Virol. 2001, 20, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Henchcliffe, C.; Beal, M. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008, 4, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.; Masters, C.; Busch, A.J. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Forman, H.J. Redox signaling: An evolution from free radicals to aging. Free Radic Biol Med. 2016, 97, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Dugas, B.; Debré, P.; Moncada, S. Nitric oxide, a vital poison inside the immune and inflammatory network. Res Immunol. 1995, 146, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Erusalimsky, J.D.; Moncada, S. Nitric oxide and mitochondrial signaling: from physiology to pathophysiology. Arterioscler Thromb Vasc Biol. 2007, 27, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nature reviews. Molecular cell biology 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.C.; Gougerot-Pocidalo, M.A.; Dang, P.M. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Burhans, W.C.; Heintz, N.H. The cell cycle is a redox cycle: linking phase-specific targets to cell fate. Free Radic Biol Med. 2009, 47, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Lionaki, E.; Markaki, M.; Tavernarakis, N. Autophagy and ageing: insights from invertebrate model organisms. Ageing Res Rev. 2013, 12, 413–428. [Google Scholar] [CrossRef]
- Gibellini, L.; De Biasi, S.; Pinti, M.; Nasi, M.; Riccio, M.; Carnevale, G.; Cavallini, G.M.; Sala de Oyanguren, F.J.; O’Connor, J.E.; Mussini, C.; et al. The protease inhibitor atazanavir triggers autophagy and mitophagy in human preadipocytes. AIDS. 2012, 26, 2017–2026. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed Res Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Measuring reactive species and oxidative damage in vivo and in cell cutures: how should you do it and what do the results mean? Br J Pharmacol. 2004, 142, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Use of spectroscopic probes for detection of reactive oxygen species. Clin Chim Acta. 2006, 368, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Sung, G.; Lin, J.M. Reactive oxygen species and their chemiluminescence-detection methods. Trends Anal Chem. 2006, 25, 985–995. [Google Scholar] [CrossRef]
- Maity, A. Photophysical Detection of Singlet Oxygen. In Reactive Oxygen Species; Ahmad, R., Ed.; Intechopen Limited: London, United Kingdom, 2022. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Singh, S.P.; Häder, D.-P.; Sinha, R.P. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem Biophys Res Commun. 2010, 397, 603–607. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.E.; Callaghan, R.C.; Escudero, M.; Herrera, G.; Martínez, A.; Monteiro, M.C.; Montolíu, H. The relevance of flow cytometry for biochemical analysis. IUBMB Life 2001, 51, 231–239. [Google Scholar] [CrossRef]
- Herrera, G.; Diaz, L.; Martinez, A.; Gomes, A.; Villamón, E.; Callaghan, R.C.; O’Connor, J.E. Cytomics: A multiparametric, dynamic approach to cell research. Toxicol In Vitro. 2007, 21, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Barrientos, A.; O’Connor, J.E.; Nieto-Castillo, R.; Moreno-Moreno, A.B.; Prieto, P. Use of flow cytometry and confocal microscopy techniques to investigate early CdCl2-induced nephrotoxicity in vitro. Toxicol In Vitro. 2001, 15, 407–412. [Google Scholar] [CrossRef]
- Ploppa, A.; George, T.C.; Unertl, K.E.; Nohe, B.; Durieux, M.E. ImageStream cytometry extends the analysis of phagocytosis and oxidative burst. Scand J Clin Lab Invest. 2011, 71, 362–369. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J.; 2nd, Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic Biol Med. 2012, 52, 1–6. [Google Scholar] [CrossRef]
- Debowska, K.; Debski, D.; Hardy, M.; Jakubowska, M.; Kalyanaraman, B.; Marcinek, A.; Michalski, R.; Michalowski, B.; Ouari, O.; Sikora, A.; et al. Toward selective detection of reactive oxygen and nitrogen species with the use of fluorogenic probes--Limitations, progress, and perspectives. Pharmacol Rep. 2015, 67, 756–764. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.E.; Herrera, G.; Sala-de-Oyanguren, F.; Jávega, B.; Martínez-Romero, A. Cytomics of oxidative stress: Probes and problems. In Single Cell Analysis: Contemporary Research and Clinical Applications, Robinson, J.; Cossarizza, A., Ed.; Springer: Singapore, 2017; pp. 83–118. [Google Scholar]
- Shapiro, H. Practical Flow Cytometry, 4th ed, John Wiley and Sons: Hoboken, NJ, USA, 2003.
- Ortolani, C. Flow Cytometry Today: Everything You Need to Know about Flow Cytometry; Springer Nature Switzerland: Cham, Switzerland, 2022. [Google Scholar]
- Robinson, J.P. Spectral flow cytometry—Quo vadimus? Cytometry 2019, 95, 823–824. [Google Scholar] [CrossRef] [PubMed]
- Schmid, I. (Ed.) Flow Cytometry: Recent Perspectives; Intechopen Limited: London, United Kingdom, 2012. [Google Scholar] [CrossRef]
- Cossarizza, A.; Chang, H.D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur J Immunol. 2019, 49, 1457–1973. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, C. Flow Cytometry of Hematological Malignancies, 2nd ed.; Wiley Blackwell: Hoboken, NJ, USA, 2021. [Google Scholar]
- Robinson, J.P. Flow cytometry: past and future. Biotechniques. 2022, 72, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Spidlen, J.; Moore, W.; Parks, D.; Goldberg, M.; Bray, C.; Bierre, P.; Gorombey, P.; Hyun, B.; Hubbard, M.; Lange, S.; et al. Data File Standard for Flow Cytometry, version FCS 3.1. Cytometry A. 2010, 77, 97–100. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.E.; Herrera, G.; Corrochano, V. Flow versus Flux: Functional Assays by Flow Cytometry. In Fluorescence and Fluorescent Probes II, Slavík, J.; ed.; Plenum Press: New York, USA, 1998; pp. 47–54. [Google Scholar]
- Sun, G.; Qu, L.; Azi, F.; Liu, Y.; Li, J.; Lv, X.; Du, G.; Chen, J.; Chen, C.H.; Liu, L. Recent progress in high-throughput droplet screening and sorting for bioanalysis. Biosens Bioelectron. 2023, 225, 115107. [Google Scholar] [CrossRef] [PubMed]
- Elbim, C.; Lizard, G. Flow cytometric investigation of neutrophil oxidative burst and apoptosis in physiological and pathological situations. Cytometry A. 2009, 75, 475–481. [Google Scholar] [CrossRef]
- De Gaetano, A.; Gibellini, L.; Zanini, G.; Nasi, M.; Cossarizza, A.; Pinti, M. Mitophagy and Oxidative Stress: The Role of Aging. Antioxidants 2021, 10, 794. [Google Scholar] [CrossRef]
- Gibellini, L.; Pinti, M.; Nasi, M.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cossarizza, A. Interfering with ROS Metabolism in Cancer Cells: The Potential Role of Quercetin. Cancers (Basel). 2010, 2, 1288–1311. [Google Scholar] [CrossRef]
- Petit, P.X.; Ardilla-Osorio, H.; Penalvia, L.; Rainey, N.E. Tafazzin Mutation Affecting Cardiolipin Leads to Increased Mitochondrial Superoxide Anions and Mitophagy Inhibition in Barth Syndrome. Cells. 2020, 9, 2333. [Google Scholar] [CrossRef]
- Ivars, D.; Orero, M.T.; Javier, K.; Díaz-Vico, L.; García-Giménez, J.L.; Mena, S.; Tormos, C.; Egea, M.; Pérez, P.L.; Arrizabalaga, B.; et al. Oxidative imbalance in low/intermediate-1-risk myelodysplastic syndrome patients: The influence of iron overload. Clin Biochem. 2017, 50, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, V.; Jávega, B.; Gasperini, S.; O’Connor, J.E.; Lenzi, M.; Hrelia, P. 6-(Methylsulfonyl) Hexyl Isothiocyanate: A Chemopreventive Agent Inducing Autophagy in Leukemia Cell Lines. Biomolecules. 2022, 12, 1485. [Google Scholar] [CrossRef] [PubMed]
- Urios, A.; López-Gresa, M.P.; González, M.C.; Primo, J.; Martínez, A.; Herrera, G.; Escudero, J.C.; O’Connor, J.E.; Blanco, M. Nitric oxide promotes strong cytotoxicity of phenolic compounds against Escherichia coli: the influence of antioxidant defenses. Free Radic Biol Med. 2003, 35, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K. Basic Biology of Hypoxic Responses Mediated by the Transcription Factor HIFs and its Implication for Medicine. Biomedicines 2020, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Godet, I.; Doctorman, S.; Wu, F.; Gilkes, D.M. Detection of Hypoxia in Cancer Models: Significance, Challenges, and Advances. Cells 2022, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Shrivastava, V.; Joshi, D.; Singal, C.M.S.; Tyagi, S.; Bhat, M.A.; Jaiswal, P.; Sharma, J.B.; Palanichamy, J.K.; Sinha, S.; et al. Hypoxia Induces Early Neurogenesis in Human Fetal Neural Stem Cells by Activating the WNT Pathway. Mol Neurobiol. 2023, 60, 2910–2921. [Google Scholar] [CrossRef] [PubMed]
- Pachón-Peña, G.; Serena, C.; Ejarque, M.; Petriz, J.; Duran, X.; Oliva-Olivera, W.; Simó, R.; Tinahones, F.J.; Fernández-Veledo, S.; Vendrell, J. Obesity Determines the Immunophenotypic Profile and Functional Characteristics of Human Mesenchymal Stem Cells From Adipose Tissue. Stem Cells Transl Med. 2016, 5, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Espinosa, C.; Ferreira, I.; Ríos-Kristjánsson, J.G.; Rizo-Roca, D.; García Godoy, M.D.; Rico, L.G.; Rubi-Sans, G.; Torrella, J.R.; Pagès, T.; Petriz, J.; et al. Effects of intermittent hypoxia and light aerobic exercise on circulating stem cells and side population, after strenuous eccentric exercise in trained rats. Curr Stem Cell Res Ther. 2015, 10, 132–139. [Google Scholar] [CrossRef]
- Synowiec, A.; Brodaczewska, K.; Wcisło, G.; Majewska, A.; Borkowska, A.; Filipiak-Duliban, A.; Gawrylak, A.; Wilkus, K.; Piwocka, K.; Kominek, A.; et al. Hypoxia, but Not Normoxia, Reduces Effects of Resveratrol on Cisplatin Treatment in A2780 Ovarian Cancer Cells: A Challenge for Resveratrol Use in Anticancer Adjuvant Cisplatin Therapy. Int J Mol Sci. 2023, 24, 5715. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Jin, Y.; Lin, J.; Gong, L.; Xu, Y. Hypoxia upregulates the expression of lncRNA H19 in non-small cell lung cancer cells and induces drug resistance. Transl Cancer Res. 2022, 11, 2876–2886. [Google Scholar] [CrossRef]
- Such, L.; O’Connor, J.E.; Sáez, G.T.; Gil, F.; Beltrán, J.F.; Moya, A.; Alberola, A. Flow cytometric analysis of peroxidative activity in granulocytes from coronary and peripheral blood in acute myocardial ischemia and reperfusion in dogs: protective effect of methionine. Cytometry. 1999, 37, 140–146. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Y.; Xu, C.; Gao, S. A Flow Cytometry-based Assay for Measuring Mitochondrial Membrane Potential in Cardiac Myocytes After Hypoxia/Reoxygenation. J Vis Exp. 2018, 137, 57725. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Hao, Y.; Hou, D.; Yang, R. Lycium barbarum polysaccharide protects cardiomyocytes from hypoxia/reoxygenation injury via activation of SIRT3/CypD signaling. Ann Transl Med. 2023, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; He, W.; Xia, J.; Huang, Q.; Yang, J.; Gu, W.P.; Zhang, N.; Liu, Y.H. Human umbilical cord mesenchymal stem cells-derived exosomal circDLGAP4 promotes angiogenesis after cerebral ischemia-reperfusion injury by regulating miR-320/KLF5 axis. FASEB J. 2023, 37, e22733. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Wang, M.; Cui, M.; Fang, Y.; Li, H.; Zheng, C.; Li, Z.; Xu, Y.; Hua, H.; Li, D. Small-molecule probes for fluorescent detection of cellular hypoxia-related nitroreductase. J Pharm Biomed Anal. 2021, 203, 114199. [Google Scholar] [CrossRef] [PubMed]
- Wallabregue, A.L.D.; Bolland, H.; Faulkner, S.; Hammond, E.M.; Conway, S.J. Two Color Imaging of Different Hypoxia Levels in Cancer Cells. J Am Chem Soc. 2023, 145, 2572–2583. [Google Scholar] [CrossRef] [PubMed]
- Nordsmark, M.; Loncaster, J.; Aquino-Parsons, C.; Chou, S.C.; Ladekarl, M.; Havsteen, H.; Lindegaard, J.C.; Davidson, S.E.; Varia, M.; West, C.; et al. Measurements of hypoxia using pimonidazole and polarographic oxygen-sensitive electrodes in human cervix carcinomas. Radiother Oncol. 2003, 67, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.J. Measurement of absolute oxygen levels in cells and tissues using oxygen sensors and 2-nitroimidazole EF5. Methods Enzymol. 2002, 352, 3–31. [Google Scholar] [CrossRef]
- Nakajima, K.; Homma, M.; Suzuki, M.; Yokouchi, Y.; Matsuda, T.; Takakura, H.; Hirata, K.; Kuge, Y.; Ogawa, M. Reduction of tumor hypoxia by anti-PD-1 therapy assessed using pimonidazole and [18F]FMISO. Nucl Med Biol. 2022, 108-109, 85–92. [Google Scholar] [CrossRef]
- Gravelle, P.; Jean, C.; Familiades, J.; Decaup, E.; Blanc, A.; Bezombes-Cagnac, C.; Laurent, C.; Savina, A.; Fournié, J.J.; Laurent, G. Cell growth in aggregates determines gene expression, proliferation, survival, chemoresistance, and sensitivity to immune effectors in follicular lymphoma. Am J Pathol. 2014, 184, 282–295. [Google Scholar] [CrossRef]
- Chang, Q.; Ornatsky, O.I.; Koch, C.J.; Chaudary, N.; Marie-Egyptienne, D.T.; Hill, R.P.; Tanner, S.D.; Hedley, D.W. Single-cell measurement of the uptake, intratumoral distribution and cell cycle effects of cisplatin using mass cytometry. Int J Cancer. 2015, 136, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Document Connect (thermofisher.com). Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2FMAN0017632_Hypoxia_Green_for_Flow_Cytometry_UG.pdf (accessed on 27 April 2023).
- BioTracker 520 Green Hypoxia Dye Live Cell Imaging Millipore (sigmaaldrich.com). Available online: https://www.sigmaaldrich.com/ES/es/product/mm/sct033 (accessed on 27 April 2023).
- Document Connect (thermofisher.com). Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2FMAN0013497_Image_iT_Hypoxia_Reagents_UG.pdf (accessed on 27 April 2023).
- Zhang, S.; Hosaka, M.; Yoshihara, T.; Negishi, K.; Iida, Y.; Tobita, S.; Takeuchi, T. Phosphorescent light–emitting iridium complexes serve as a hypoxia-sensing probe for tumor imaging in living animals. Cancer Res. 2010, 70, 4490–4498. [Google Scholar] [CrossRef] [PubMed]
- Vordermark, D.; Shibata, T.; Brown, J.M. Green fluorescent protein is a suitable reporter of tumor hypoxia despite an oxygen requirement for chromophore formation. Neoplasia. 2001, 3, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Gately, D.P.; Hom, D.; Mishima, M.; Los, G.; Howell, S.B. Quantification of tumor cell injury in vitro and in vivo using expression of green fluorescent protein under the control of the GADD153 promoter. Int J Cancer. 2001, 91, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Erapaneedi, R.; Belousov, V.V.; Schäfers, M.; Kiefer, F. A novel family of fluorescent hypoxia sensors reveal strong heterogeneity in tumor hypoxia at the cellular level. EMBO J. 2016, 35, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, A.; Huang, L.; Zou, Y.; Gu, Y.; Chen, X.; Zhao, Y.; Yang, Y. Monitoring cellular redox state under hypoxia using a fluorescent sensor based on eel fluorescent protein. Free Radic Biol Med. 2018, 120, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Miura, T.; Umezawa, N.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Nagano, T.; Rational design of fluorescein-based fluorescence probes. Mechanism-based design of a maximum fluorescence probe for singlet oxygen. J Am Chem Soc. 2001, 123, 2530–2536. [Google Scholar] [CrossRef] [PubMed]
- Ragas, X.; Jimenez-Banzo, A.; Sanchez-Garcia, D.; Batllori, X.; Nonell, S. Singlet oxygen photosensitisation by the fluorescent probe Singlet Oxygen Sensor Green. Chem Commun (Camb). 2009, 20, 2920–2922. [Google Scholar] [CrossRef]
- Song, D.; Cho, S.; Han, Y.; You, Y.; Nam, W. Ratiometric Fluorescent Probes for Detection of Intracellular Singlet Oxygen. Org Lett. 2013, 15, 3582–3585. [Google Scholar] [CrossRef]
- Umezawa, N.; Tanaka, K.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Nagano, T. Novel Fluorescent Probes for Singlet Oxygen. Angew Chem Int Ed Engl 1999, 38, 2899–2901. [Google Scholar] [CrossRef]
- Hideg, E.; Kalai, T.; Kos, P.B.; Asada, K.; Hideg, K. Singlet oxygen in plants- Its significance and possible detection with double (fluorescent and spin) indicator reagents. Photochem Photobiol. 2006, 82, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Gollmer, A.; Arnbjerg, J.; Blaikie, F.H.; Pedersen, B.W.; Breitenbach, T.; Daasbjerg, K.; Glasius, M.; Ogilby, P.R. Singlet Oxygen Sensor Green®: photochemical behavior in solution and in a mammalian cell. Photochem Photobiol. 2011, 87, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Fujitsuka, M.; Majima, T. Photochemistry of Singlet Oxygen Sensor Green. J Phys Chem B. 2013, 117, 13985–13992. [Google Scholar] [CrossRef] [PubMed]
- Flors, C.; Fryer, M.J.; Waring, J.; Reeder, B.; Bechtold, U.; Mullineaux, P.M.; Nonell, S.; Wilson, M.T.; Baker, N.R. Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green®. J Exp Bot. 2006, 57, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Carter, P.J.H.; Laan, A.C.; Eelkema, R.; Denkova, A.G. Singlet Oxygen Sensor Green is not a Suitable Probe for 1O2 in the Presence of Ionizing Radiation. Sci Rep. 2019, 9, 8393. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.K.; Holmehave, J.; Blaikie, F.H.; Gollmer, A.; Breitenbach, T.; Jensen, H.H.; Ogilby, P.R. Aarhus Sensor Green: A Fluorescent Probe for Singlet Oxygen. J Org Chem. 2014, 79, 3079–3087. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods. 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef]
- Keston, A.S.; Brandt, R. The fluorometric analysis of ultramicro quantities of hydrogen peroxide. Anal Biochem. 1965, 11, 1–5. [Google Scholar] [CrossRef]
- van Eeden, S.F.; Klut, M.E.; Walker, B.A.; Hogg, J.C. The use of flow cytometry to measure neutrophil function. J Immunol Methods. 1999, 232, 23–43. [Google Scholar] [CrossRef]
- Caldefie-Chézet, F.; Walrand, S.; Moinard, C.; Tridon, A.; Chassagne, J.; Vasson, M.P. Is the neutrophil reactive oxygen species production measured by luminol and lucigenin chemiluminescence intra or extracellular? Comparison with DCFH-DA flow cytometry and cytochrome c reduction. Clin Chim Acta 2002, 319, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bourré, L.; Thibaut, S.; Briffaud, A.; Rousset, N.; Eléouet, S.; Lajat, Y.; Patrice, T. Indirect detection of photosensitizer ex vivo. J Photochem Photobiol, B Biol 2002, 67, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.R.; Pereira-Da-Silva, L.; Juel, C.; Hellsten, Y. Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radic Biol Med. 2003, 35, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Tampo, Y.; Kotamraju, S.; Chitambar, C.R.; Kalivendi, S.V.; Keszler, A.; Joseph, J.; Kalyanaraman, B. Oxidative stress-induced iron signaling is responsible for peroxide-dependent oxidation of dichlorodihydrofluorescein in endothelial cells: role of transferrin receptor-dependent iron uptake in apoptosis. Circ Res. 2003, 92, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Kotamraju, S.; Tampo, Y.; Keszler, A.; Chitambar, C.R.; Joseph, J.; Haas, A.L.; Kalyanaraman, B. Nitric oxide inhibits H2O2-induced transferrin receptor-dependent apoptosis in endothelial cells: role of ubiquitin–proteasome pathway. Proc Natl Acad Sci U S A. 2003, 100, 10653–10658. [Google Scholar] [CrossRef] [PubMed]
- Kotamraju, S.; Kalivendi, S.V.; Konorev, E.; Chitambar, C.R.; Joseph, J.; Kalyanaraman, B. Oxidant induced iron signaling in doxorubicin-mediated apoptosis. Methods Enzymol. 2004, 378, 362–382. [Google Scholar] [CrossRef] [PubMed]
- Probes for Reactive Oxygen Species, Including Nitric Oxide—Chapter 18 | Thermo Fisher Scientific – ES. Available online: https://www.thermofisher.com/es/es/home/references/molecular-probes-the-handbook/probes-for-reactive-oxygen-species-including-nitric-oxide.html (accessed on 27 April 2023).
- Crow, J.P. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide. 1997, 1, 145–157. [Google Scholar] [CrossRef]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med. 1994, 16, 149–156. [Google Scholar] [CrossRef]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H. Oxidation of 2,7-dichlorofluorescin by peroxynitrite. Free Radic Res. 1997, 27, 245–254. [Google Scholar] [CrossRef]
- Miller, E.W.; Albers, A.E.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J Am Chem Soc. 2005, 127, 16652–16659. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Huynh, C.; Chang, C.J. A palette of fluorescent probes with varying emission colors for imaging hydrogen peroxide signaling in living cells. J Am Chem Soc. 2010, 132, 5906–5915. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Aleyasin, H.; Dickinson, B.C.; Haskew-Layton, R.E.; Ratan, R.R. Recent advances in hydrogen peroxide imaging for biological applications. Cell Biosci. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Aleyasin, H.; Howard, S.S.; Dickinson, B.C.; Lin, V.S.; Haskew-Layton, R.E.; Xu, C.; Chen, Y.; Ratan, R.R. Two-photon fluorescence imaging of intracellular hydrogen peroxide with chemoselective fluorescent probes. J Biomed Opt. 2013, 18, 106002. [Google Scholar] [CrossRef] [PubMed]
- Albers, A.E.; Okreglak, V.S.; Chang, C.J. A FRET-based approach to ratiometric fluorescence detection of hydrogen peroxide. J Am Chem Soc. 2006, 128, 9640–9641. [Google Scholar] [CrossRef] [PubMed]
- Jávega, B.; Herrera, G.; O’Connor, J.E. Flow Cytometric Analysis of Oxidative Stress in Escherichia coli B Strains Deficient in Genes of the Antioxidant Defence. Int J Mol Sci. 2022, 10, 6537. [Google Scholar] [CrossRef] [PubMed]
- Benov, L.; Sztejnberg, L.; Fridovich, I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radic Biol Med 1998, 25, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Rothe, G.; Valet, G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2,7-dichlorofluorescin. J Leukoc Biol. 47:440-448.
- Walrand, S.; Valeix, S.; Rodriguez, C.; Ligot, P.; Chassagne, J.; Vasson, M.P. Flow cytometry study of polymorphonuclear neutrophil oxidative burst: a comparison of three fluorescent probes. Clin Chim Acta. 2003, 331, 103–110. [Google Scholar] [CrossRef]
- Carter, W.O.; Narayanan, P.K.; Robinson, J.P. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J Leukoc Biol. 1994, 55, 253–258. [Google Scholar] [CrossRef]
- Barbacanne, M.A.; Souchard, J.P.; Darblade, B.; Iliou, J.P.; Nepveu, F.; Pipy, B.; Bayard, F.; Arnal, J.F. Detection of superoxide anion released extracellularly by endothelial cells using cytochrome c reduction, ESR, fluorescence and lucigenin-enhanced chemiluminescence techniques. Free Radic Biol Med. 2000, 29, 388–396. [Google Scholar] [CrossRef]
- Münzel, T.; Afanas’ev, I.B.; Kleschyov, A.L.; Harrison, D.G. Detection of superoxide in vascular tissue. Arterioscler Thromb Vasc Biol. 2002, 22, 1761–1768. [Google Scholar] [CrossRef]
- Tarpey, M.M.; Wink, D.A.; Grisham, M.B. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004, 286, R431–R444. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.L.; Miller, M.A.; Shapiro, I.M.; Shenker, B.J. Mercuric chloride induces apoptosis in human T lymphocytes: evidence of mitochondrial dysfunction. Toxicol Appl Pharmacol. 1998, 153, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Le, S.B.; Hailer, M.K.; Buhrow, S.; Wang, Q.; Flatten, K.; Pediaditakis, P.; Bible, K.C.; Lewis, L.D.; Sausville, E.A.; Pang, Y.P.; et al. Inhibition of mitochondrial respiration as a source of adaphostin-induced reactive oxygen species and cytotoxicity. J Biol Chem. 2007, 282, 8860–8872. [Google Scholar] [CrossRef] [PubMed]
- De Biasi, S.; Gibellini, L.; Bianchini, E.; Nasi, M.; Pinti, M.; Salvioli, S.; Cossarizza, A. Quantification of mitochondrial reactive oxygen species in living cells by using multi-laser polychromatic flow cytometry. Cytometry A. 2016, 89, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.M.; Janes, M.S.; Beckman, J.S. The selective detection of mitochondrial superoxide by live cell imaging. Nat Protoc. 2008, 3, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Kalyanaraman, B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med. 2010, 48, 983–1001. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, G.; Yang, T.; Gan, J.; Xu, L.; Yang, H. A flow-cytometry-based protocol for detection of mitochondrial ROS production under hypoxia. STAR Protoc. 2021, 2, 100466. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Fairfull-Smith, K.E.; Morrow, B.J.; Lussini, V.; Kim, B.; Bondar, M.V.; Bottle, S.E.; Belfield, K.D. Two-photon fluorescence microscopy imaging of cellular oxidative stress using profluorescent nitroxides. J Am Chem Soc. 2012, 134, 4721–4730. [Google Scholar] [CrossRef]
- Davison, C.A.; Durbin, S.M.; Thau, M.R.; Zellmer, V.R.; Chapman, S.E.; Diener, J.; Wathen, C.; Leevy, W.M.; Schafer, Z.T. Antioxidant Enzymes Mediate Survival of Breast Cancer Cells Deprived of Extracellular Matrix. Cancer Res. 2013, 73, 3704–3715. [Google Scholar] [CrossRef]
- DeLoughery, Z.; Luczak, M.W.; Zhitkovich, A. Monitoring Cr intermediates and reactive oxygen species with fluorescent probes during chromate reduction. Chem Res Toxicol. 2014, 27, 843–851. [Google Scholar] [CrossRef]
- Plaza Davila, M.; Martin Muñoz, P.; Tapia, J.A.; Ortega Ferrusola, C.; Balao da Silva, C.C.; Peña, F.J. Inhibition of Mitochondrial Complex I Leads to Decreased Motility and Membrane Integrity Related to Increased Hydrogen Peroxide and Reduced ATP Production, while the Inhibition of Glycolysis Has Less Impact on Sperm Motility. PLoS One 2015, 10, e0138777. [Google Scholar] [CrossRef] [PubMed]
- mp10422.pdf (fishersci.com). Available online: https://assets.fishersci.com/TFS-Assets/LSG/manuals/mp10422.pdf?_ga=2.231040351.1726096505.1681672212-1827320652.1681672212 (accessed on 27 April 2023).
- ROS-ID® Total ROS/Superoxide detection kit - ENZ-51010 - Enzo Life Sciences. Available online: https://www.enzolifesciences.com/ENZ-51010/ros-id-total-ros-superoxide-detection-kit/ (accessed on 27 April 2023).
- Egawa, N.; Tanaka, T.; Matsufuji, S.; Yamada, K.; Ito, K.; Kitagawa, H.; Okuyama, K.; Kitajima, Y.; Noshiro, H. Antitumor effects of low-dose tipifarnib on the mTOR signaling pathway and reactive oxygen species production in HIF-1α-expressing gastric cancer cells. FEBS Open Bio. 2021, 11, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Sun, C.; Wang, Y.; Mehta, J.L. Hemodynamic Shear Stress via ROS Modulates PCSK9 Expression in Human Vascular Endothelial and Smooth Muscle Cells and Along the Mouse Aorta. Antioxid Redox Signal. 2015, 22, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, F.A.; van den Berg, J.J.; Schalkwijk, C.; Roelofsen, B.; Op den Kamp, J.A. Parinaric acid as a sensitive fluorescent probe for the determination of lipid peroxidation. Biochim Biophys Acta. 1987, 921, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Hedley, D.; Chow, S. Flow cytometric measurement of lipid peroxidation in vital cells using parinaric acid. Cytometry. 1992, 13, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Drummen, G.P.; Makkinje, M.; Verkleij, A.J.; Op den Kamp, J.A.; Post, J.A. Attenuation of lipid peroxidation by antioxidants in rat-1 fibroblasts: comparison of the lipid peroxidation reporter molecules cis-parinaric acid and C11-BODIPY(581/591) in a biological setting. Biochim Biophys Acta. 2004, 1636, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Shimakawa, S.; Itoh, N.; Niki, E. Action of DCFH and BODIPY as a probe for radical oxidation in hydrophilic and lipophilic domain. Free Radic Res. 2003, 37, 861–872. [Google Scholar] [CrossRef]
- Brouwers, J.F.; Gadella, B. M In situ detection and localization of lipid peroxidation in individual bovine sperm cells. Free Radic Biol Med. 2003, 35, 1382–1391. [Google Scholar] [CrossRef]
- Cheloni, G.; Slaveykova, V.I. Optimization of the C11-BODIPY(581/591) dye for the determination of lipid oxidation in Chlamydomonas reinhardtii by flow cytometry. Cytometry A. 2013, 83, 952–961. [Google Scholar] [CrossRef]
- Peluso, I.; Adorno, G.; Raguzzini, A.; Urban, L.; Ghiselli, A.; Serafini, M. A new flow cytometry method to measure oxidative status: the Peroxidation of Leukocytes Index Ratio (PLIR). J Immunol Methods. 2013, 390, 113–120. [Google Scholar] [CrossRef]
- Donato, M.T.; Martínez-Romero, A.; Jiménez, N.; Negro, A.; Herrera, G.; Castell, J.V.; O’Connor, J.E.; Gómez-Lechón, M.J. Cytometric analysis for drug-induced steatosis in HepG2 cells. Chem Biol Interact. 2009, 181, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Makrigiorgos, G.M.; Kassis, A.I.; Mahmood, A.; Bump, E.A.; Savvides, P. Novel fluorescein-based flow–cytometric method for detection of lipid peroxidation. Free Radic Biol Med. 1997, 22, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Maulik, G.; Kassis, A.I.; Savvides, P.; Makrigiorgos, G.M. Fluoresceinated phosphoethanolamine for flow–cytometric measurement of lipid peroxidation. Free Radic Biol Med. 1998, 26, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Blair, I.A. Characterization of 4-oxo-2-nonenal as a novel product of lipid peroxidation. Chem Res Toxicol. 2000, 13, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the role of 4-hydroxynonenal in health and disease. Biochim Biophys Acta. 2015, 1852, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S.; Miyake, N.; Hiai, H.; Hagiwara, M.; Kawakishi, S.; Osawa, T.; Uchida, K. The monoclonal antibody specific for the 4-hydroxy-2-nonenal histidine adduct. FEBS Lett. 1995, 359, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Martin Muñoz, P.; Ortega Ferrusola, C.; Vizuete, G.; Plaza Dávila, M.; Rodriguez Martinez, H.; Peña, F.J. Depletion of Intracellular Thiols and Increased Production of 4-Hydroxynonenal that Occur During Cryopreservation of Stallion Spermatozoa Lead to Caspase Activation, Loss of Motility, and Cell Death. Biol Reprod. 2015, 93, 143. [Google Scholar] [CrossRef]
- Fortini, P.; Pascucci, B.; Parlanti, E.; D’Errico, M.; Simonelli, V.; Dogliotti, E. 8-Oxoguanine DNA damage: at the crossroad of alternative repair pathways. Mutat Res. 2003, 531, 127–139. [Google Scholar] [CrossRef]
- Barregard, L.; Møller, P.; Henriksen, T.; Mistry, V.; Koppen, G.; Rossner, P. ; Jr, Sram, R. J.; Weimann, A.; Poulsen, H.E.; Nataf, R.; et al. Human and methodological sources of variability in the measurement of urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine. Antioxid Redox Signal. 2013, 18, 2377–2391. [Google Scholar] [CrossRef]
- Oxy dna assay kit | Sigma-Aldrich (sigmaaldrich.com). Available online: https://www.sigmaaldrich.com/ES/en/search/oxy-dna-assay-kit?focus=products&page=1&perpage=30&sort=relevance&term=oxy%20dna%20assay%20kit&type=product (accessed on 27 April 2023).
- Nagy, S.; Kakasi, B.; Bercsényi, M. Flow cytometric detection of oxidative DNA damage in fish spermatozoa exposed to cadmium—short communication. Acta Vet Hung. 2016, 64, 120–124. [Google Scholar] [CrossRef]
- Esperanza, M.; Cid, Á.; Herrero, C.; Rioboo, C. Acute effects of a prooxidant herbicide on the microalga Chlamydomonas reinhardtii: Screening cytotoxicity and genotoxicity endpoints. Aquat Toxicol. 2015, 165, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Cambi, M.; Tamburrino, L.; Marchiani, S.; Olivito, B.; Azzari, C.; Forti, G.; Baldi, E.; Muratori, M. Development of a specific method to evaluate 8-hydroxy, 2-deoxyguanosine in sperm nuclei: relationship with semen quality in a cohort of 94 subjects. Reproduction. 2013, 145, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, C.; Meseguer, M.; García-Herrero, S.; Gil-Salom, M.; O’Connor, J.E.; Garrido, N. Relevance of testicular sperm DNA oxidation for the outcome of ovum donation cycles. Fertil Steril. 2010, 94, 979–988. [Google Scholar] [CrossRef]
- Meseguer, M.; Martínez-Conejero, J.A.; O’Connor, J.E.; Pellicer, A.; Remohí, J.; Garrido, N. The significance of sperm DNA oxidation in embryo development and reproductive outcome in an oocyte donation program: a new model to study a male infertility prognostic factor. Fertil Steril. 2008, 89, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Balao da Silva, C.M.; Ortega-Ferrusola, C.; Morrell, J.M.; Rodriguez Martínez, H.; Peña, F.J. Flow Cytometric Chromosomal Sex Sorting of Stallion Spermatozoa Induces Oxidative Stress on Mitochondria and Genomic DNA. Reprod Domest Anim. 2016, 51, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Durand, R.E.; Olive, P.L. Flow cytometry techniques for studying cellular thiols. Radiat Res 1983, 95, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Treumer, J.; Valet, G. Flow-cytometric determination of glutathione alterations in vital cells by o-phthaldialdehyde (OPT) staining. Exp Cell Res. 1986, 163, 518–524. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.E.; Kimler, B.F.; Morgan, M.C.; Tempas, K.J. A flow cytometric assay for intracellular nonprotein thiols using mercury orange. Cytometry. 1988, 9, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Cell Viability Assays | Thermo Fisher Scientific – ES. Availble online: https://www.thermofisher.com/es/es/home/life-science/cell-analysis/cell-viability-and-regulation/cell-viability. (accessed on 27 April 2023).
- Nair, S.; Singh, S.V.; Krishan, A. Flow cytometric monitoring of glutathione content and anthracycline retention in tumor cells. Cytometry 1991, 12, 336–342. [Google Scholar] [CrossRef]
- Hedley, D.W.; Chow, S. Evaluation of methods for measuring cellular glutathione content using flow cytometry. Cytometry. 1994, 15, 349–358. [Google Scholar] [CrossRef]
- Chow, S.; Hedley, D. Flow cytometric determination of glutathione in clinical samples. Cytometry. 1995, 21, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Skindersoe, M.E.; Kjaerulff, S. Comparison of three thiol probes for determination of apoptosis-related changes in cellular redox status. Cytometry A. 2014, 85, 179–187. [Google Scholar] [CrossRef]
- Keller, A.; Mohamed, A.; Drose, S.; Brandt, U.; Fleming, I.; Brandes, R.P. Analysis of dichlorodihydrofluorescein and dihydrocalcein as probes for the detection of intracellular reactive oxygen species. Free Radic Res. 2004, 38, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Swift, L.M.; Sarvazyan, N. Localization of dichlorofluorescin in cardiac myocytes: implications for assessment of oxidative stress. Am J Physiol Heart Circ Physiol. [CrossRef]
- Balaguer, S.; Diaz, L.; Gomes, A.; Herrera, G.; O’Connor, J.E.; Urios, A.; Felipo, V.; Montoliu, C. Real-time cytometric assay of nitric oxide and superoxide interaction in peripheral blood monocytes: A no-wash, no-lyse kinetic method. Cytometry B Clin Cytom. 2017, 92, 211–217. [Google Scholar] [CrossRef]
- Saengkhae, C.; Loetchutinat, C.; Garnier-Suillerot, A. Kinetic analysis of fluorescein and dihydrofluorescein effluxes in tumour cells expressing the multidrug resistance protein, MRP1. Biochem Pharmacol. 2003, 65, 969–977. [Google Scholar] [CrossRef]
- Grzelak, A.; Rychlik, B.; Bartosz, G. Reactive oxygen species are formed in cell culture media. Acta Biochim Pol. 2000, 47, 47,1197–1198. [Google Scholar] [PubMed]
- Petasne, R.G.; Zika, R.G. Fate of superoxide in coastal sea water. Nature. 1987, 325, 516–518. [Google Scholar] [CrossRef]
- Hopwood, M.J.; Rapp, I.; Schlosser, C.; Achterberg, E.P. Hydrogen peroxide in deep waters from the Mediterranean Sea, South Atlantic and South Pacific Oceans. Sci Rep. 2017, 7, 43436. [Google Scholar] [CrossRef]
- Subramaniam, R.; Fan, X.J.; Scivittaro, V.; Yang, J.; Ha, C.E.; Petersen, C.E.; Surewicz, W.K.; Bhagavan, N.V.; Weiss, M.F.; Monnier, V.M. Cellular oxidant stress and advanced glycation endproducts of albumin: caveats of the dichlorofluorescein assay. Arch Biochem Biophys. 2002, 400, 15–25. [Google Scholar] [CrossRef]
- Chignell, C.F.; Sik, R.H. A photochemical study of cells loaded with 2,7-dichlorofluorescin: implications for the detection of reactive oxygen species generated during UVA irradiation. Free Radic Biol Med. 2003, 34, 1029–1034. [Google Scholar] [CrossRef]
- Sikora, A.; Zielonka, J.; Lopez, M.; Joseph, J.; Kalyanaraman, B. Direct oxidation of boronates by peroxynitrite: mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic Biol Med. 2009, 47, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Kalivendi, S.; Zhang, H.; Joseph, J.; Nithipatikom, K.; Vásquez-Vivar, J.; Kalyanaraman, B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003, 34, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Joseph, J.; Fales, H.M.; Sokoloski, E.A.; Levine, R.L.; Vasquez-Vivar, J.; Kalyanaraman, B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci USA. 2005, 102, 5727–5732. [Google Scholar] [CrossRef] [PubMed]
- Papapostolou, I.; Patsoukis, N.; Georgiou, C.D. The fluorescence detection of superoxide radical using hydroethidine could be complicated by the presence of heme proteins. Anal Biochem. 2004, 332, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, S.; Luthe, A.; Keilhoff, G.; Wolf, G.; Horn, T.F. Oxidative stress in glial cultures: detection by DAF-2 fluorescence used as a tool to measure peroxynitrite rather than nitric oxide. Glia. 2002, 38, 103–114. [Google Scholar] [CrossRef] [PubMed]
- van der Ven, A.J.; Mier, P.; Peters, W.H.; Dolstra, H.; van Erp, P.E.; Koopmans, P.P.; van der Meer, J.W. Monochlorobimane does not selectively label glutathione in peripheral blood mononuclear cells. Anal Biochem. 1994, 217, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Palb, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Woolley, J.F.; Stanicka, J.; Cotter, T.G. Recent advances in reactive oxygen species measurement in biological systems. Trends Biochem Sci. 2013, 38, 556–565. [Google Scholar] [CrossRef]
- Jávega, B. Aplicación de la citometría de flujo al estudio en tiempo real de las interacciones entre especies reactivas de oxígeno y nitrógeno en el estrés oxidativo inducido por xenobióticos. PhD Thesis, Universitat de València, Valencia, Spain.
- Aitken, R.J.; Buckingham, D.; Harkiss, D. Use of a xanthine oxidase free radical generating system to investigate the cytotoxic effects of reactive oxygen species on human spermatozoa. J Reprod Fertil. 1993, 97, 441–450. [Google Scholar] [CrossRef]
- Alexandre, J.; Nicco, C.; Chéreau, C.; Laurent, A.; Weill, B.; Goldwasser, F.; Batteux, F. Improvement of the therapeutic index of anticancer drugs by the superoxide dismutase mimic mangafodipir. J Natl Cancer Inst 2006, 98, 236–244. [Google Scholar] [CrossRef]
- Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.J.; Forbes, A.; Perkins, A.V.; Davey, A.K.; Chess-Williams, R.; Kiefel, M.J.; Arora, D.; et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa. Toxins 2016, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.; Hansen, J.M. Oxidative stress, thiols, and redox profiles. Methods Mol Biol. 2012, 889, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Siwik, D.A.; Tzortzis, J.D.; Pimental, D.R.; Chang, D.L.; Pagano, P.J.; Singh, K.; Sawyer, D.B.; Colucci, W.S. Inhibition of copper-zinc superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro. Circ Res. 1999, 85, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Titov, V.Y.; Osipov, A.N. Nitrite and nitroso compounds can serve as specific catalase inhibitors. Redox Rep. 2017, 22, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Serafim, R.A.; Primi, M.C.; Trossini, G.H.; Ferreira, E.I. Nitric oxide: state of the art in drug design. Curr Med Chem. 2012, 19, 386–405. [Google Scholar] [CrossRef] [PubMed]
- Hrušková, K.; Potůčková, E.; Hergeselová, T.; Liptáková, L.; Hašková, P.; Mingas, P.; Kovaříková, P.; Šimůnek, T.; Vávrová, K. Aroylhydrazone iron chelators: Tuning antioxidant and antiproliferative properties by hydrazide modifications. Eur J Med Chem. 2016, 120, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Herrera, G.; Martinez, A.; Blanco, M.; O’Connor, J.E. Assessment of Escherichia coli B with enhanced permeability to fluorochromes for flow cytometric assays of bacterial cell function. Cytometry. 2002, 49, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Herrera, G.; Martinez, A.; O’Connor, J.E.; Blanco, M. Functional Assays of Oxidative Stress Using Genetically Engineered Escherichia coli Strains. Curr Protoc Cytom. 2003, 24, 11–16. [Google Scholar] [CrossRef]
- Yoon, S.H.; Jeong, H.; Kwon, S.K.; Kim, J.F. Genomics, biological features, and biotechnological applications of Escherichia coli B: “Is B for better?!”. In Systems Biology and Biotechnology of Escherichia coli, Lee, S.Y.; Ed.; Springer, Dordrecht, 2009, pp. 1–17. doi.org/10.1007/978-1-4020-9394-4_1.
- Blanco, M.; Martı́nez, A.; Urios, A.; Herrera, G.; O’Connor, J.E. Detection of oxidative mutagenesis by isoniazid and other hydrazine derivatives in Escherichia coli WP2 tester strain IC203, deficient in OxyR: strong protective effects of rat liver S9. Mutat Res Genet Toxicol Environ Mutagen. 1998, 417, 39–46. [Google Scholar] [CrossRef]
- Blanco, M.; Urios, A.; Martı́nez, A. New Escherichia coli WP2 tester strains highly sensitive to reversion by oxidative mutagens. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1998, 413, 95–101. [Google Scholar] [CrossRef]
- Martı́nez, A.; Urios, A.; Blanco, M. Mutagenicity of 80 chemicals in Escherichia coli tester strains IC203, deficient in OxyR, and its oxyR+ parent WP2 uvrA/pKM101: detection of 31 oxidative mutagens. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 467, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Han, M.J.; Jeong, H.; Lee, C.H.; Xia, X.X.; Lee, D.H.; Shim, J.H.; Lee, S.Y.; Oh, T.K.; Kim, J.F. Comparative multi-omics systems analysis of Escherichia coli strains B and K-12. Genome Biol. 2012, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kim, S.; Mukhopadhyay, P.; Cho, S.; Woo, J.; Storz, G.; Ryu, S.E. Structural basis of redox switch in the OxyR transcription factor. Cell, 2001; 105, 103–113. [Google Scholar] [CrossRef]
- Wardman, P. Methods to measure the reactivity of peroxynitrite-derived oxidants toward reduced fluoresceins and rhodamines. Methods Enzymol. 2008, 441, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Choi, H.J.; Zhu, B.T. Rapid generation of mitochondrial superoxide induces mitochondrion-dependent but caspase-independent cell death in hippocampal neuronal cells that morphologically resembles necroptosis. Toxicol Appl Pharmacol. 2012, 262, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Clothier, R.; Gómez-Lechón, M.J.; Kinsner-Ovaskainen, A.; Kopp-Schneider, A.; O’Connor, J.E.; Prieto, P.; Stanzel, S. Comparative analysis of eight cytotoxicity assays evaluated within the ACuteTox Project. Toxicol In Vitro. 2013, 27, 1347–1356. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Rooprai, H.K.; Lawrence, P.; Keshavarz, S.; Yashod, P.; Gullan, R.W.; Selway, R.P.; Davies, D. DRAQ7 as an Alternative to MTT Assay for Measuring Viability of Glioma Cells Treated With Polyphenols. Anticancer Res. 2020, 40, 5427–5436. [Google Scholar] [CrossRef]
- McBee, M.E.; Chionh, Y.H.; Sharaf, M.L.; Ho, P.; Cai, M.W.L.; Dedon, P.C. Production of Superoxide in Bacteria Is Stress- and Cell State-Dependent: A Gating-Optimized Flow Cytometry Method that Minimizes ROS Measurement Artifacts with Fluorescent Dyes. Front Microbiol. 2017, 8, 459. [Google Scholar] [CrossRef]
- Spidlen, J.; Brinkman, R.R. Use FlowRepository to share your clinical data upon study publication. Cytometry B Clin Cytom. 2018, 94, 196–198. [Google Scholar] [CrossRef]
- Spidlen, J.; Breuer, K.; Brinkman, R. Preparing a Minimum Information about a Flow Cytometry Experiment (MIFlowCyt) compliant manuscript using the International Society for Advancement of Cytometry (ISAC) FCS file repository (FlowRepository. org). Curr Protoc Cytom. 2012; 61, 10–18. [Google Scholar] [CrossRef]
| CRITICAL POINTS AND LIMITATIONS |
|---|
| Identification of blood cells in whole blood samples without lysis of erythrocytes |
| Preparation of single-cell suspensions from adherent cell models |
| Maintenance of viability and functional competence of the cells along sample preparation and experiment performance |
| Identification of the optimal incubation time and concentration for staining |
| Access of fluorogenic substrates to intracellular sites or intracellular processes |
| Retention of fluorogenic substrates and oxidized fluorescent probes |
| Preparation of single-cell suspensions from adherent cell models |
| Lack of absolute specificity of fluorogenic substrates for specific RONS |
| Interference of the probes with ROS biology or ROS-relevant cell functions |
| Interference of the probes with ROS biology or ROS-relevant cell functions |
| Interference of the probes with ROS biology or ROS-relevant cell functions |
| Interference of the probes with ROS biology or ROS-relevant cell functions |
| Selection of the time window for kinetic assays |
| Assay calibration for data expression in biochemical units |
| Adapted from ref. [47] |
| Biological Process of Interest and Experimental In Vitro Setting | ||||||
|---|---|---|---|---|---|---|
| Peroxidative Activity | Redox Cycling | Antioxidant Defense | ||||
| Probe | MitoPY1 | H2DCF-DA | DHRH123 | MitoSOX Red | HE | Monochlorobimane |
| Viability Stain | DAPI or PI |
DAPI or PI |
DAPI or PI |
DAPI | DAPI | PI |
| Positive control | H2O2 | t-BOOH | CHP | Menadione | Plumbagin | N-Acetyl cysteine GSH-ester |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





