Submitted:
29 April 2023
Posted:
30 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
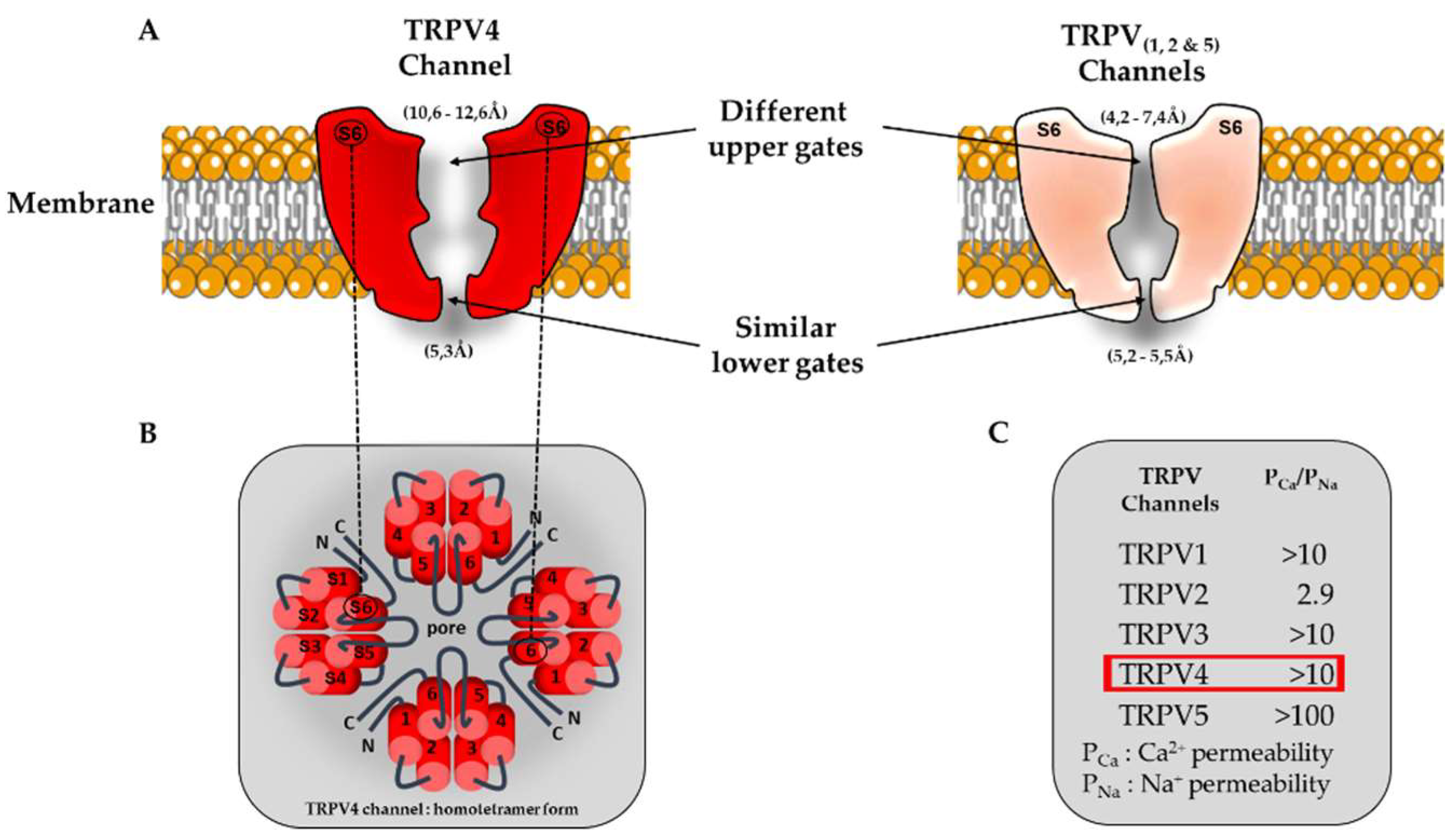
2. Gene, structure, function and electrical properties
3. Available tools to investigate TRPV4 roles
3.1. Pharmacological modulators
3.1.1. TRPV4 agonists
3.1.2. TRPV4 antagonists
3.2. TRPV4 knockout mice
4. Physiological roles in cardiovascular system
4.1. TRPV4 expression profile under physiological condition
4.2. Modulation of ventricular electrical activity
4.3. Modulation of cardiac contractility
4.4. Modulation of vascular tone
5. Pathological implications of TRPV4 channels
5.1. Expression remodelling under pathological condition
5.2. Arrhythmias
5.3. Cardiac Remodelling and fibrosis
6. Conclusions and perspectives
References
- Moran, M.M., H. Xu, and D.E. Clapham, TRP ion channels in the nervous system. Curr Opin Neurobiol, 2004. 14(3): p. 362-9. [CrossRef]
- Moran, M.M., TRP Channels as Potential Drug Targets. Annu Rev Pharmacol Toxicol, 2018. 58: p. 309-330. [CrossRef]
- Hof, T., et al., Transient receptor potential channels in cardiac health and disease. Nat Rev Cardiol, 2019. 16(6): p. 344-360. [CrossRef]
- White, J.P., et al., TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol Rev, 2016. 96(3): p. 911-73. [CrossRef]
- Miller, M., et al., Role of Known Transient Receptor Potential Vanilloid Channels in Modulating Cardiac Mechanobiology. Front Physiol, 2021. 12: p. 734113. [CrossRef]
- Randhawa, P.K. and A.S. Jaggi, TRPV4 channels: physiological and pathological role in cardiovascular system. Basic Res Cardiol, 2015. 110(6): p. 54. [CrossRef]
- Inoue, R., L.H. Kurahara, and K. Hiraishi, TRP channels in cardiac and intestinal fibrosis. Semin Cell Dev Biol, 2019. 94: p. 40-49. [CrossRef]
- Chaigne, S., et al., Transient receptor potential vanilloid 4 channel participates in mouse ventricular electrical activity. Am J Physiol Heart Circ Physiol, 2021. 320(3): p. H1156-H1169. [CrossRef]
- Gorbunov, A.S., et al., Physiological and Pathological Role of TRPV1, TRPV2 and TRPV4 Channels in Heart. Curr Cardiol Rev, 2019. 15(4): p. 244-251. [CrossRef]
- Zhao, Y., et al., Unusual localization and translocation of TRPV4 protein in cultured ventricular myocytes of the neonatal rat. Eur J Histochem, 2012. 56(3): p. e32. [CrossRef]
- Jones, J.L., et al., TRPV4 increases cardiomyocyte calcium cycling and contractility yet contributes to damage in the aged heart following hypoosmotic stress. Cardiovasc Res, 2019. 115(1): p. 46-56. [CrossRef]
- Arniges, M., et al., Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J Biol Chem, 2006. 281(3): p. 1580-6. [CrossRef]
- Shigematsu, H., et al., A 3.5-nm structure of rat TRPV4 cation channel revealed by Zernike phase-contrast cryoelectron microscopy. J Biol Chem, 2010. 285(15): p. 11210-8. [CrossRef]
- Inada, H., et al., Structural and biochemical consequences of disease-causing mutations in the ankyrin repeat domain of the human TRPV4 channel. Biochemistry, 2012. 51(31): p. 6195-206. [CrossRef]
- Takahashi, N., et al., TRPV4 channel activity is modulated by direct interaction of the ankyrin domain to PI(4,5)P(2). Nat Commun, 2014. 5: p. 4994. [CrossRef]
- Deng, Z., et al., Cryo-EM and X-ray structures of TRPV4 reveal insight into ion permeation and gating mechanisms. Nat Struct Mol Biol, 2018. 25(3): p. 252-260. [CrossRef]
- Nilius, B. and T. Voets, The puzzle of TRPV4 channelopathies. EMBO Rep, 2013. 14(2): p. 152-63. [CrossRef]
- Hellwig, N., et al., Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci, 2005. 118(Pt 5): p. 917-28. [CrossRef]
- Jin, X., J. Touhey, and R. Gaudet, Structure of the N-terminal ankyrin repeat domain of the TRPV2 ion channel. J Biol Chem, 2006. 281(35): p. 25006-10. [CrossRef]
- Liao, M., et al., Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature, 2013. 504(7478): p. 107-12. [CrossRef]
- Lishko, P.V., et al., The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron, 2007. 54(6): p. 905-18. [CrossRef]
- McCleverty, C.J., et al., Crystal structure of the human TRPV2 channel ankyrin repeat domain. Protein Sci, 2006. 15(9): p. 2201-6. [CrossRef]
- Michalick, L. and W.M. Kuebler, TRPV4-A Missing Link Between Mechanosensation and Immunity. Front Immunol, 2020. 11: p. 413. [CrossRef]
- D'Hoedt, D., et al., Stimulus-specific modulation of the cation channel TRPV4 by PACSIN 3. J Biol Chem, 2008. 283(10): p. 6272-80. [CrossRef]
- Wang, Y., et al., OS-9 regulates the transit and polyubiquitination of TRPV4 in the endoplasmic reticulum. J Biol Chem, 2007. 282(50): p. 36561-70. [CrossRef]
- Toft-Bertelsen, T.L., B.R. Larsen, and N. MacAulay, Sensing and regulation of cell volume - we know so much and yet understand so little: TRPV4 as a sensor of volume changes but possibly without a volume-regulatory role? Channels (Austin), 2018. 12(1): p. 100-108. [CrossRef]
- Strotmann, R., G. Schultz, and T.D. Plant, Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem, 2003. 278(29): p. 26541-9. [CrossRef]
- Loukin, S.H., J. Teng, and C. Kung, A channelopathy mechanism revealed by direct calmodulin activation of TrpV4. Proc Natl Acad Sci U S A, 2015. 112(30): p. 9400-5. [CrossRef]
- Suzuki, M., A. Hirao, and A. Mizuno, Microtubule-associated [corrected] protein 7 increases the membrane expression of transient receptor potential vanilloid 4 (TRPV4). J Biol Chem, 2003. 278(51): p. 51448-53. [CrossRef]
- Stewart, A.P., et al., Atomic force microscopy reveals the alternating subunit arrangement of the TRPP2-TRPV4 heterotetramer. Biophys J, 2010. 99(3): p. 790-7. [CrossRef]
- Ma, X., et al., Heteromeric TRPV4-C1 channels contribute to store-operated Ca(2+) entry in vascular endothelial cells. Cell Calcium, 2011. 50(6): p. 502-9. [CrossRef]
- Du, J., et al., TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. FASEB J, 2014. 28(11): p. 4677-85. [CrossRef]
- Benfenati, V., et al., An aquaporin-4/transient receptor potential vanilloid 4 (AQP4/TRPV4) complex is essential for cell-volume control in astrocytes. Proc Natl Acad Sci U S A, 2011. 108(6): p. 2563-8. [CrossRef]
- Liu, X., et al., A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J Biol Chem, 2006. 281(22): p. 15485-95. [CrossRef]
- Galizia, L., et al., Functional interaction between AQP2 and TRPV4 in renal cells. J Cell Biochem, 2012. 113(2): p. 580-9. [CrossRef]
- Verkerk, A.O., E.M. Lodder, and R. Wilders, Aquaporin Channels in the Heart-Physiology and Pathophysiology. Int J Mol Sci, 2019. 20(8). [CrossRef]
- Shin, S.H., et al., Phosphorylation on TRPV4 Serine 824 Regulates Interaction with STIM1. Open Biochem J, 2015. 9: p. 24-33. [CrossRef]
- Shukla, A.K., et al., Arresting a transient receptor potential (TRP) channel: beta-arrestin 1 mediates ubiquitination and functional down-regulation of TRPV4. J Biol Chem, 2010. 285(39): p. 30115-25. [CrossRef]
- Ma, X., et al., Electrophysiological properties of heteromeric TRPV4-C1 channels. Biochim Biophys Acta, 2011. 1808(12): p. 2789-97. [CrossRef]
- Kottgen, M., et al., TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol, 2008. 182(3): p. 437-47. [CrossRef]
- Brauchi, S. and P. Orio, Voltage sensing in thermo-TRP channels. Adv Exp Med Biol, 2011. 704: p. 517-30. [CrossRef]
- Long, S.B., E.B. Campbell, and R. Mackinnon, Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science, 2005. 309(5736): p. 903-8. [CrossRef]
- Long, S.B., E.B. Campbell, and R. Mackinnon, Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science, 2005. 309(5736): p. 897-903. [CrossRef]
- Whicher, J.R. and R. MacKinnon, Structure of the voltage-gated K(+) channel Eag1 reveals an alternative voltage sensing mechanism. Science, 2016. 353(6300): p. 664-9. [CrossRef]
- Lee, C.H. and R. MacKinnon, Structures of the Human HCN1 Hyperpolarization-Activated Channel. Cell, 2017. 168(1-2): p. 111-120 e11. [CrossRef]
- Watanabe, H., et al., Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem, 2002. 277(49): p. 47044-51. [CrossRef]
- Voets, T., et al., Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem, 2002. 277(37): p. 33704-10. [CrossRef]
- Nilius, B., et al., TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol, 2004. 286(2): p. C195-205. [CrossRef]
- Lawhorn, B.G., E.J. Brnardic, and D.J. Behm, TRPV4 antagonists: a patent review (2015-2020). Expert Opin Ther Pat, 2021: p. 1-12. [CrossRef]
- Coetzee, W.A., Channel-mediated calcium current in the heart. Cardiovasc Drugs Ther, 1988. 1(5): p. 447-59. [CrossRef]
- Bers, D.M., Cardiac excitation-contraction coupling. Nature, 2002. 415(6868): p. 198-205. [CrossRef]
- Liao, J., et al., TRPV4 blockade suppresses atrial fibrillation in sterile pericarditis rats. JCI Insight, 2020. 5(23). [CrossRef]
- Goyal, N., et al., Clinical Pharmacokinetics, Safety, and Tolerability of a Novel, First-in-Class TRPV4 Ion Channel Inhibitor, GSK2798745, in Healthy and Heart Failure Subjects. Am J Cardiovasc Drugs, 2019. 19(3): p. 335-342. [CrossRef]
- Lawhorn, B.G., E.J. Brnardic, and D.J. Behm, Recent advances in TRPV4 agonists and antagonists. Bioorg Med Chem Lett, 2020. 30(8): p. 127022. [CrossRef]
- Thorneloe, K.S., et al., PROPERTIES OF THE TRPV4 AGONIST GSK1016790A AND the TRPV4 ANTAGONIST GSK2193874. Physiol Rev, 2017. 97(4): p. 1231-1232. [CrossRef]
- Wu, Q.F., et al., Activation of transient receptor potential vanilloid 4 involves in hypoxia/reoxygenation injury in cardiomyocytes. Cell Death Dis, 2017. 8(5): p. e2828. [CrossRef]
- Baratchi, S., et al., The TRPV4 Agonist GSK1016790A Regulates the Membrane Expression of TRPV4 Channels. Front Pharmacol, 2019. 10: p. 6. [CrossRef]
- Atobe, M., et al., Discovery of Novel Transient Receptor Potential Vanilloid 4 (TRPV4) Agonists as Regulators of Chondrogenic Differentiation: Identification of Quinazolin-4(3 H)-ones and in Vivo Studies on a Surgically Induced Rat Model of Osteoarthritis. J Med Chem, 2019. 62(3): p. 1468-1483. [CrossRef]
- Grace, M.S., et al., Modulation of the TRPV4 ion channel as a therapeutic target for disease. Pharmacol Ther, 2017. 177: p. 9-22. [CrossRef]
- Vincent, F., et al., Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun, 2009. 389(3): p. 490-4. [CrossRef]
- Dong, Q., et al., Blockage of transient receptor potential vanilloid 4 alleviates myocardial ischemia/reperfusion injury in mice. Sci Rep, 2017. 7: p. 42678. [CrossRef]
- Hilfiker, M.A., et al., Optimization of a Novel Series of TRPV4 Antagonists with In Vivo Activity in a Model of Pulmonary Edema. ACS Med Chem Lett, 2013. 4(2): p. 293-6. [CrossRef]
- Wei, Z.L., et al., Identification of orally-bioavailable antagonists of the TRPV4 ion-channel. Bioorg Med Chem Lett, 2015. 25(18): p. 4011-5. [CrossRef]
- Brooks, C.A., et al., Discovery of GSK3527497: A Candidate for the Inhibition of Transient Receptor Potential Vanilloid-4 (TRPV4). J Med Chem, 2019. 62(20): p. 9270-9280. [CrossRef]
- Tsuno, N., et al., Discovery of novel 2',4'-dimethyl-[4,5'-bithiazol]-2-yl amino derivatives as orally bioavailable TRPV4 antagonists for the treatment of pain: Part 1. Bioorg Med Chem Lett, 2016. 26(20): p. 4930-4935. [CrossRef]
- Tsuno, N., et al., Discovery of novel 2',4'-dimethyl-[4,5'-bithiazol]-2-yl amino derivatives as orally bioavailable TRPV4 antagonists for the treatment of pain: Part 2. Bioorg Med Chem Lett, 2016. 26(20): p. 4936-4941. [CrossRef]
- Pero, J.E., et al., Design and Optimization of Sulfone Pyrrolidine Sulfonamide Antagonists of Transient Receptor Potential Vanilloid-4 with in Vivo Activity in a Pulmonary Edema Model. J Med Chem, 2018. 61(24): p. 11209-11220. [CrossRef]
- Xu, F., E. Satoh, and T. Iijima, Protein kinase C-mediated Ca2+ entry in HEK 293 cells transiently expressing human TRPV4. Br J Pharmacol, 2003. 140(2): p. 413-21. [CrossRef]
- A.J.Duncton, M., Chapter 12 - Small Molecule Agonists and Antagonists of TRPV4. TRP Channels as Therapeutic Targets From Basic Science to Clinical Use 2015: p. 205-219.
- Alexander, R., et al., 4alpha-phorbol 12,13-didecanoate activates cultured mouse dorsal root ganglia neurons independently of TRPV4. Br J Pharmacol, 2013. 168(3): p. 761-72. [CrossRef]
- Dahan, D., et al., Implication of the ryanodine receptor in TRPV4-induced calcium response in pulmonary arterial smooth muscle cells from normoxic and chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol, 2012. 303(9): p. L824-33. [CrossRef]
- Watanabe, H., et al., Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature, 2003. 424(6947): p. 434-8. [CrossRef]
- Katragadda, D., et al., Epoxyeicosatrienoic acids limit damage to mitochondrial function following stress in cardiac cells. J Mol Cell Cardiol, 2009. 46(6): p. 867-75. [CrossRef]
- Lai, J. and C. Chen, The Role of Epoxyeicosatrienoic Acids in Cardiac Remodeling. Front Physiol, 2021. 12: p. 642470. [CrossRef]
- Yang, L., et al., The role of epoxyeicosatrienoic acids in the cardiovascular system. Br J Clin Pharmacol, 2015. 80(1): p. 28-44. [CrossRef]
- Thorneloe, K.S., et al., An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med, 2012. 4(159): p. 159ra148. [CrossRef]
- Xu, S., et al., A novel TRPV4-specific agonist inhibits monocyte adhesion and atherosclerosis. Oncotarget, 2016. 7(25): p. 37622-37635. [CrossRef]
- Pankey, E.A., et al., Analysis of responses to the TRPV4 agonist GSK1016790A in the pulmonary vascular bed of the intact-chest rat. Am J Physiol Heart Circ Physiol, 2014. 306(1): p. H33-40. [CrossRef]
- Zhang, S., et al., Activation of transient receptor potential vanilloid 4 exacerbates myocardial ischemia-reperfusion injury via JNK-CaMKII phosphorylation pathway in isolated mice hearts. Cell Calcium, 2021. 100: p. 102483. [CrossRef]
- Willette, R.N., et al., Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther, 2008. 326(2): p. 443-52. [CrossRef]
- Adapala, R.K., et al., TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol, 2013. 54: p. 45-52. [CrossRef]
- Ahn, M.S., et al., Transient receptor potential channel TRPV4 mediates TGF-beta1-induced differentiation of human ventricular fibroblasts. Cardiol J, 2020. [CrossRef]
- Donate-Macian, P., et al., Structural determinants of TRPV4 inhibition and identification of new antagonists with antiviral activity. Br J Pharmacol, 2022. 179(14): p. 3576-3591. [CrossRef]
- Filosa, J.A., X. Yao, and G. Rath, TRPV4 and the regulation of vascular tone. J Cardiovasc Pharmacol, 2013. 61(2): p. 113-9. [CrossRef]
- Greenberg, H.Z.E., et al., Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced nitric oxide production and vasorelaxation in rabbit mesenteric arteries. Vascul Pharmacol, 2017. 96-98: p. 53-62. [CrossRef]
- Lu, J., et al., An abnormal TRPV4-related cytosolic Ca(2+) rise in response to uniaxial stretch in induced pluripotent stem cells-derived cardiomyocytes from dilated cardiomyopathy patients. Biochim Biophys Acta Mol Basis Dis, 2017. 1863(11): p. 2964-2972. [CrossRef]
- Xia, Y., et al., TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am J Physiol Cell Physiol, 2013. 305(7): p. C704-15. [CrossRef]
- Gevaert, T., et al., Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest, 2007. 117(11): p. 3453-62. [CrossRef]
- Vizin, R.C., et al., TRPV4 activates autonomic and behavioural warmth-defence responses in Wistar rats. Acta Physiol (Oxf), 2015. 214(2): p. 275-89. [CrossRef]
- Jia, X., et al., TRPV4 Mediates Cardiac Fibrosis via the TGF-beta1/Smad3 Signaling Pathway in Diabetic Rats. Cardiovasc Toxicol, 2020. [CrossRef]
- Veteto, A.B., et al., TRPV4 Contributes to Stretch-Induced Hypercontractility and Time-Dependent Dysfunction in the Aged Heart. Cardiovasc Res, 2019. [CrossRef]
- Cheung, M., et al., Discovery of GSK2193874: An Orally Active, Potent, and Selective Blocker of Transient Receptor Potential Vanilloid 4. ACS Med Chem Lett, 2017. 8(5): p. 549-554. [CrossRef]
- O'Brien, F., C.A. Staunton, and R. Barrett-Jolley, Systemic application of the transient receptor potential vanilloid-type 4 antagonist GSK2193874 induces tail vasodilation in a mouse model of thermoregulation. Biol Lett, 2022. 18(6): p. 20220129. [CrossRef]
- Pero, J.E., et al., Identification, Synthesis, and Characterization of a Major Circulating Human Metabolite of TRPV4 Antagonist GSK2798745. ACS Med Chem Lett, 2021. 12(9): p. 1498-1502. [CrossRef]
- Brnardic, E.J., et al., Discovery of Pyrrolidine Sulfonamides as Selective and Orally Bioavailable Antagonists of Transient Receptor Potential Vanilloid-4 (TRPV4). J Med Chem, 2018. 61(21): p. 9738-9755. [CrossRef]
- Suzuki, M., et al., Impaired pressure sensation in mice lacking TRPV4. J Biol Chem, 2003. 278(25): p. 22664-8. [CrossRef]
- Liedtke, W. and J.M. Friedman, Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci U S A, 2003. 100(23): p. 13698-703. [CrossRef]
- Liu, L., et al., Role of Transient Receptor Potential Vanilloid 4 in Vascular Function. Front Mol Biosci, 2021. 8: p. 677661. [CrossRef]
- Zhang, D.X., et al., Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension, 2009. 53(3): p. 532-8. [CrossRef]
- Vriens, J., et al., Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res, 2005. 97(9): p. 908-15. [CrossRef]
- Mendoza, S.A., et al., TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol, 2010. 298(2): p. H466-76. [CrossRef]
- Sonkusare, S.K., et al., Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science, 2012. 336(6081): p. 597-601. [CrossRef]
- Earley, S., et al., TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol, 2009. 297(3): p. H1096-102. [CrossRef]
- Loot, A.E., et al., Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res, 2008. 80(3): p. 445-52. [CrossRef]
- Hartmannsgruber, V., et al., Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One, 2007. 2(9): p. e827. [CrossRef]
- Mizuno, A., et al., Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol, 2003. 285(1): p. C96-101. [CrossRef]
- Tabuchi, K., et al., Hearing impairment in TRPV4 knockout mice. Neurosci Lett, 2005. 382(3): p. 304-8. [CrossRef]
- Alvarez, D.F., et al., Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res, 2006. 99(9): p. 988-95. [CrossRef]
- Taniguchi, J., et al., TRPV4 as a flow sensor in flow-dependent K+ secretion from the cortical collecting duct. Am J Physiol Renal Physiol, 2007. 292(2): p. F667-73. [CrossRef]
- Gualdani, R., et al., Mechanical activation of TRPV4 channels controls albumin reabsorption by proximal tubule cells. Sci Signal, 2020. 13(653). [CrossRef]
- Masuyama, R., et al., TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab, 2008. 8(3): p. 257-65. [CrossRef]
- Seghers, F., et al., TRPV4 participates in pressure-induced inhibition of renin secretion by juxtaglomerular cells. J Physiol, 2016. 594(24): p. 7327-7340. [CrossRef]
- Kunert-Keil, C., et al., Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics, 2006. 7: p. 159. [CrossRef]
- Veteto, A.B., et al., Transient receptor potential vanilloid-4 contributes to stretch-induced hypercontractility and time-dependent dysfunction in the aged heart. Cardiovasc Res, 2020. 116(11): p. 1887-1896. [CrossRef]
- Qi, Y., et al., Uniaxial cyclic stretch stimulates TRPV4 to induce realignment of human embryonic stem cell-derived cardiomyocytes. J Mol Cell Cardiol, 2015. 87: p. 65-73. [CrossRef]
- Thodeti, C.K., S. Paruchuri, and J.G. Meszaros, A TRP to cardiac fibroblast differentiation. Channels (Austin), 2013. 7(3): p. 211-4. [CrossRef]
- Ahn, M.S., et al., Transient receptor potential channel TRPV4 mediates TGF-beta1-induced differentiation of human ventricular fibroblasts. Cardiol J, 2020. 27(2): p. 162-170. [CrossRef]
- Hatano, N., Y. Itoh, and K. Muraki, Cardiac fibroblasts have functional TRPV4 activated by 4alpha-phorbol 12,13-didecanoate. Life Sci, 2009. 85(23-26): p. 808-14. [CrossRef]
- Du, J., et al., TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res, 2010. 106(5): p. 992-1003. [CrossRef]
- Kohler, R., et al., Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol, 2006. 26(7): p. 1495-502. [CrossRef]
- Sullivan, M.N., et al., Optical recording reveals novel properties of GSK1016790A-induced vanilloid transient receptor potential channel TRPV4 activity in primary human endothelial cells. Mol Pharmacol, 2012. 82(3): p. 464-72. [CrossRef]
- Sukumaran, S.V., et al., TRPV4 channel activation leads to endothelium-dependent relaxation mediated by nitric oxide and endothelium-derived hyperpolarizing factor in rat pulmonary artery. Pharmacol Res, 2013. 78: p. 18-27. [CrossRef]
- Bubolz, A.H., et al., Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. Am J Physiol Heart Circ Physiol, 2012. 302(3): p. H634-42. [CrossRef]
- Barbeau, S., et al., Mechanosensitivity in Pulmonary Circulation: Pathophysiological Relevance of Stretch-Activated Channels in Pulmonary Hypertension. Biomolecules, 2021. 11(9). [CrossRef]
- Yang, X.R., et al., Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol, 2012. 302(6): p. L555-68. [CrossRef]
- Parpaite, T., et al., Effect of hypoxia on TRPV1 and TRPV4 channels in rat pulmonary arterial smooth muscle cells. Pflugers Arch, 2016. 468(1): p. 111-130. [CrossRef]
- Baylie, R.L. and J.E. Brayden, TRPV channels and vascular function. Acta Physiol (Oxf), 2011. 203(1): p. 99-116. [CrossRef]
- Ducret, T., et al., Serotonin-induced activation of TRPV4-like current in rat intrapulmonary arterial smooth muscle cells. Cell Calcium, 2008. 43(4): p. 315-23. [CrossRef]
- Song, S., et al., Flow shear stress enhances intracellular Ca2+ signaling in pulmonary artery smooth muscle cells from patients with pulmonary arterial hypertension. Am J Physiol Cell Physiol, 2014. 307(4): p. C373-83. [CrossRef]
- Ottolini, M., et al., Mechanisms underlying selective coupling of endothelial Ca(2+) signals with eNOS vs. IK/SK channels in systemic and pulmonary arteries. J Physiol, 2020. 598(17): p. 3577-3596. [CrossRef]
- Doleschal, B., et al., TRPC3 contributes to regulation of cardiac contractility and arrhythmogenesis by dynamic interaction with NCX1. Cardiovasc Res, 2015. 106(1): p. 163-73. [CrossRef]
- Qi, Z., et al., TRPC3 regulates the automaticity of embryonic stem cell-derived cardiomyocytes. Int J Cardiol, 2016. 203: p. 169-81. [CrossRef]
- Guinamard, R., et al., TRPM4 in cardiac electrical activity. Cardiovasc Res, 2015. 108(1): p. 21-30. [CrossRef]
- Hof, T., et al., TRPM4 non-selective cation channels influence action potentials in rabbit Purkinje fibres. J Physiol, 2016. 594(2): p. 295-306. [CrossRef]
- Hof, T., et al., Implication of the TRPM4 nonselective cation channel in mammalian sinus rhythm. Heart Rhythm, 2013. 10(11): p. 1683-9. [CrossRef]
- Sah, R., et al., Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc Natl Acad Sci U S A, 2013. 110(32): p. E3037-46. [CrossRef]
- Saito, Y., et al., TRPM4 Mutation in Patients With Ventricular Noncompaction and Cardiac Conduction Disease. Circ Genom Precis Med, 2018. 11(5): p. e002103. [CrossRef]
- Bianchi, B., et al., Four TRPM4 Cation Channel Mutations Found in Cardiac Conduction Diseases Lead to Altered Protein Stability. Front Physiol, 2018. 9: p. 177. [CrossRef]
- Liu, H., et al., Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet, 2010. 3(4): p. 374-85. [CrossRef]
- Stallmeyer, B., et al., Mutational spectrum in the Ca(2+)--activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum Mutat, 2012. 33(1): p. 109-17. [CrossRef]
- Palladino, A., et al., The Role of TRPM4 Gene Mutations in Causing Familial Progressive Cardiac Conduction Disease: A Further Contribution. Genes (Basel), 2022. 13(2). [CrossRef]
- Demion, M., et al., Trpm4 gene invalidation leads to cardiac hypertrophy and electrophysiological alterations. PLoS One, 2014. 9(12): p. e115256. [CrossRef]
- Jin, M., et al., Determinants of TRPV4 activity following selective activation by small molecule agonist GSK1016790A. PLoS One, 2011. 6(2): p. e16713. [CrossRef]
- Eisner, D.A., et al., Calcium and Excitation-Contraction Coupling in the Heart. Circ Res, 2017. 121(2): p. 181-195. [CrossRef]
- Abi-Samra, F. and D. Gutterman, Cardiac contractility modulation: a novel approach for the treatment of heart failure. Heart Fail Rev, 2016. 21(6): p. 645-660. [CrossRef]
- Ezeani, M., TRP Channels Mediated Pathological Ca2+-Handling and Spontaneous Ectopy. Front Cardiovasc Med. 2019; 6: 83. , 2020. [CrossRef]
- Freichel, M., et al., TRP Channels in the Heart, in Neurobiology of TRP Channels, T.L.R. Emir, Editor. 2017: Boca Raton (FL). p. 149-185.
- Li, J., et al., Role of transient receptor potential vanilloid 4 in the effect of osmotic pressure on myocardial contractility in rat. Sheng Li Xue Bao, 2008. 60(2): p. 181-8.
- Wu, Q., et al., Blockade of Transient Receptor Potential Vanilloid 4 Enhances Antioxidation after Myocardial Ischemia/Reperfusion. Oxid Med Cell Longev, 2019. 2019: p. 7283683. [CrossRef]
- Gregory H Turner1, W.B., Beat M Jucker2, John J Lepore2, Robert N Willette2, and Kevin S Thorneloe2, Preservation of Cardiac Function and Attenuation of Remodelling in Transient Receptor Potential Vanilloid 4 Knockout Mice Following Myocardial Infarction. Clinical & Experimental Cardiology, 2015. 6(366).
- Rosenbaum, T., et al., TRPV4: A Physio and Pathophysiologically Significant Ion Channel. Int J Mol Sci, 2020. 21(11). [CrossRef]
- Chen, Y.L. and S.K. Sonkusare, Endothelial TRPV4 channels and vasodilator reactivity. Curr Top Membr, 2020. 85: p. 89-117. [CrossRef]
- Geng, L., et al., Physiological levels of fluid shear stress modulate vascular function through TRPV4 sparklets. Acta Biochim Biophys Sin (Shanghai), 2022. 54(9): p. 1268-1277. [CrossRef]
- Barbeau, S., et al., Cell Confluence Modulates TRPV4 Channel Activity in Response to Hypoxia. Biomolecules, 2022. 12(7). [CrossRef]
- Earley, S., et al., TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res, 2005. 97(12): p. 1270-9. [CrossRef]
- Suresh, K., et al., Reactive oxygen species induced Ca(2+) influx via TRPV4 and microvascular endothelial dysfunction in the SU5416/hypoxia model of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol, 2018. 314(5): p. L893-L907. [CrossRef]
- Morine, K.J., et al., Endoglin selectively modulates transient receptor potential channel expression in left and right heart failure. Cardiovasc Pathol, 2016. 25(6): p. 478-482. [CrossRef]
- Zou, Y., et al., Activation of transient receptor potential vanilloid 4 is involved in pressure overload-induced cardiac hypertrophy. Elife, 2022. 11. [CrossRef]
- Connell, P., T.A. Word, and X.H.T. Wehrens, Targeting pathological leak of ryanodine receptors: preclinical progress and the potential impact on treatments for cardiac arrhythmias and heart failure. Expert Opin Ther Targets, 2020. 24(1): p. 25-36. [CrossRef]
- Peana, D., L. Polo-Parada, and T.L. Domeier, Arrhythmogenesis in the aged heart following ischaemia-reperfusion: role of transient receptor potential vanilloid 4. Cardiovasc Res, 2022. 118(4): p. 1126-1137. [CrossRef]
- van Nieuwenhoven, F.A. and N.A. Turner, The role of cardiac fibroblasts in the transition from inflammation to fibrosis following myocardial infarction. Vascul Pharmacol, 2013. 58(3): p. 182-8. [CrossRef]
- Banerjee, I., et al., Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Ann N Y Acad Sci, 2006. 1080: p. 76-84. [CrossRef]
- Disertori, M., M. Mase, and F. Ravelli, Myocardial fibrosis predicts ventricular tachyarrhythmias. Trends Cardiovasc Med, 2017. 27(5): p. 363-372. [CrossRef]
- Adapala, R.K., et al., TRPV4 Mechanotransduction in Fibrosis. Cells, 2021. 10(11). [CrossRef]
- Biernacka, A. and N.G. Frangogiannis, Aging and Cardiac Fibrosis. Aging Dis, 2011. 2(2): p. 158-173.
- Frangogiannis, N.G., Cardiac fibrosis. Cardiovasc Res, 2021. 117(6): p. 1450-1488.
- Adapala, R.K., et al., TRPV4 deletion protects heart from myocardial infarction-induced adverse remodeling via modulation of cardiac fibroblast differentiation. Basic Res Cardiol, 2020. 115(2): p. 14. [CrossRef]
- Cussac, L.A., et al., TRPV4 channel mediates adventitial fibroblast activation and adventitial remodeling in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol, 2020. 318(1): p. L135-L146. [CrossRef]
- Verheule, S., et al., Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ Res, 2004. 94(11): p. 1458-65. [CrossRef]
- Verheule, S., et al., Fibrillatory conduction in the atrial free walls of goats in persistent and permanent atrial fibrillation. Circ Arrhythm Electrophysiol, 2010. 3(6): p. 590-9. [CrossRef]
- Choi, E.K., et al., Triggered firing and atrial fibrillation in transgenic mice with selective atrial fibrosis induced by overexpression of TGF-beta1. Circ J, 2012. 76(6): p. 1354-62. [CrossRef]
- Verheule, S. and U. Schotten, Electrophysiological Consequences of Cardiac Fibrosis. Cells, 2021. 10(11). [CrossRef]

| Molecules | Year of identification | EC50/IC50 | Other targets | Features | Cardiovascular effects | Clinical trials / uses | References |
|---|---|---|---|---|---|---|---|
| Agonists | |||||||
| 4α-PDD | 2003 | 50 µM | ● Dorsal root ganglia neurons independently of TRPV4 | ● Negative control for phorbol esters (PKC inhibitors) | ● Ca2+ influx in CF & myocytes ● Ca2+ entry in pulmonary artery smooth muscle cells and increased isometric tension in artery rings |
● None | [68], [69], [70] & [71] |
| 5,6-EET | 2003 | 0.13 µM | ● mPTP | ● Metabolite of arachidonic acid by cytochrome P450 | ● Reduction in: vascular tone, inflammatory response, pathological cardiac remodeling (fibrosis, hypertrophy) & apoptosis ● Improvement in cardiomyocytes function ● Cardioprotection ● Promotes angiogenesis |
● None because of poor solubility and short half life | [72], [73], [74] & [75] |
| RN-1747 | 2009 | 5.9 - 7.7 µM | ● TRPM8 antagonist (IC50 = 4 µM) | ● Benzenesulfonamide derivative | ● None reported | ● None | [60] |
| GSK1016790A | 2008 | 1-18 nM | ● Unknown | ● Oral administration ● iv |
● Endothelial failure and circulatory collapse ● Reduction of TNF-α induced monocyte adhesion to human endothelial cells and atherosclerosis ● Cationic non-selective current activation in rat atrial fibroblasts ● Ca2+ influx in CF and differentiation into myofibroblasts and cardiomyocytes ● Worsening of ishemia-reperfusion injuries in isolated mice hearts and in H9c2 cell line and neonatal rat myocytes ● Decrease in systemic arterial pressure, small decrease in pulmonary arterial pressure, and small increase in cardiac output |
● None | [8], [52], [56], [76], [77], [78], [79], [80], [81] & [82] |
| Quinazolin-4(3H) | 2019 | 280 nM | ● Unknown | ● Orally bioactive ? | None reported | ● None in cardiovascular diseases |
[58] |
| Molecules | Year of identification | EC50/IC50 | Other targets | Features | Cardiovascular effects | Clinical trials / uses | References |
|---|---|---|---|---|---|---|---|
| Antagonists | |||||||
| RN-1734 | 2009 | 2 to 6 µM | ● Poor pharmacokinetics and toxicity | ● Highly selective | ● Prevention of Ca2+ entry mediated vasorelaxation of mesenteric arteries ● Abolition of stretch-activated Ca2+-entry in human induced pluripotent stem cells-derived cardiomyocytes ● Inhibition of the phenylephrine-induced contraction in pulmonary artery smooth muscle cells but nonspecific off-target effects |
● None | [60], [83], [84], [85], [86] & [87] |
| HC-067047 | 2010 | 17 to 133 nM | ● ROS production, depolarization of mitochondrial membrane potential (Δψm) and mPTP opening during H/R | ● iv administration ● Intraperitonealy injected ● Potent ● Selective? |
● Cardioprotection (significantly reduced infarct size, decreased troponin T levels and improved cardiac function in murine model myocardial I/R) [57] ● Anti-apoptotic effects via the activation of RISK pathway ● Reduced TRPV4-related mechanosensitive Ca2+ signaling in DCM-hiPSC-CMs ● Prevent entry of divalent cation in response to myocyte-stretch & hypoosmotic stress-induced cardiomyocyte death and ischemia/reperfusion-induced cardiac damage ● Reduced significantly diabetes-induced cardiac fibrosis ● Inhibition of the PE-induced contraction in pulmonary artery smooth muscle cells |
● None | [11], [56] , [62], [86], [87], [88], [89] & [90] |
| RN-9893 | 2015 | 320 to 660 nM | ● Exhibits >15-fold selectivity for TRPV4 over TRPV1&V3 and TRPM8 | ● Moderate oral bioavailability ● Potent ● Selective |
● Cardioprotection (blocked collagen production following stretch in human valve interstitial cells) ● Reduced cardiac fibrosis |
● None | [82], [63], [89], [88], [89] & [91] |
| GSK2193874 | 2017 | 2 to 50 nM | ● Unknown | ● Orally active ● Potent ● Selective |
● Abolition of pulmonary edema associated with heart failure and enhanced arterial oxygenation ● Increased tail blood flow |
● None | [76], [87], [92] & [93] |
| GSK3527497 | 2019 | 12 nM | ● Unknown | ● Suitable for oral and iv administration ● Reduced bioavailability ● Poor pharmacokinetics and low solubility |
● Unknown | ● None | [88] & [64] |
| GSK2798745 | 2019 | 2 to 16 nM | ● Without any clinically significant safety concerns | ● Highly potent ● Selective ● Orally active |
● Resolve pulmonary edema in heart failure models and attenuate lung damage induced by chemical agents | ● Cardiac heart failure and respiratory diseases ● Diabetic macular edema and cough: https://clinicaltrials.gov/ |
[49], [53], [61], [76], [87] [88], [89] & [94] |
| GSK3395879 | 2018 | 1 nM | ● IC50 > 10µM for TRPA1, TRPV1, TRPM2, TRPM4, TRPM8, TRPC3, TRPC4, TRPC5, TRPC6 | ● Orally bioactive ● Highly potent | ● Abolition of pulmonary edema associated with heart failure | ● None | [11], [91], [67] & [95] |
| Ref. | Ref. | |||
|---|---|---|---|---|
| Outcomes | 129/SvJ trpv4-/- | [96] | C57bl/6J trpv4-/- | [97] |
| Generation method | 129/SvJ strain via a cassette insertion mutagenesis of exon 5 | C57bl/6J strain with a Cre-lox-mediated excision of exon 12 | ||
| Cardiovacular phenotypes | Impaired vasorelaxation, endothelial calcium response, systemic tonicity | [98] [99] [100] [101] | Impaired vasorelaxation | [98] [102] [103] |
| Altered flow-induced vasodilatation | [104] | Loss of shear stress-induced vasodilation | [105] | |
| - | Cardiac electrophysiological changes | [8] | ||
| - | Absence of VGIC remodelling (Na+, Ca2+ and K+ VGIC) in the left ventricle | [8] | ||
| Extracardiac phenotypes | Viable and fertile | Viable and fertile | [97] | |
| Normal appearance, growth, size, and temperature and no obvious behavioral (including drinking) abnormalities | [106] | Tendency to a lower body weight | [8] | |
| Reduced response to harmful stimuli caused by pressure | [96] | Reduction in water intake and serum osmolality changes | [97] | |
| Intact heat detection but abnormal sensory phenotype | [96] | Reduced response to noxious mechanical stimuli and impaired response to mechanical stimulation | [97] | |
| Altered hearing | [107] | Intact thermal sensing | [97] | |
| Inability to thermoregulate | [100] | Loss of the permeability response in lungs, alveolar barrier | [108] | |
| Deficits in renal tubular K+ secretion | [109] | Proximal tubule defect | [110] | |
| Increased bone mass, decreased osteoclast differentiation | [111] | Blood metabolite changes | [112] | |
| - | Increased bladder capacity | [88] |
| TRPV4 | Atrium | Ventricles | Fibroblasts | Endothelial Cells | Smooth Muscle Cells |
|---|---|---|---|---|---|
| • mRNA | - | Mouse [61], neo rat [10], [56], rat [56] | Mouse [119] , rat [118], human [119], [82] | Mouse [101], rat [120], [122], human [121], [123] | Rat [122], [125], [126], [127] |
| • Protein | Rat [52] | Mouse [8], [11], [61], neo rat [56], [10], rat [56] | Rat [81], neo rat [90], human [82], [119] | Mouse [101], [130] , rat [120], [122], human [121], [123] | Rat [122], [71], [125], [127], [128], human [128], [129] |
| • Function | Rat [52] | Mouse [8], [11], [52], [61], [117], neo rat [10], rat [52] | Rat [52], [81], [90], human [82], [119] | Mouse [101], [130], rat [120], [122], human [121], [123] | Mouse [83], rat [122], [71], [124], [125], [128], human [128], [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





