Submitted:
29 April 2023
Posted:
30 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
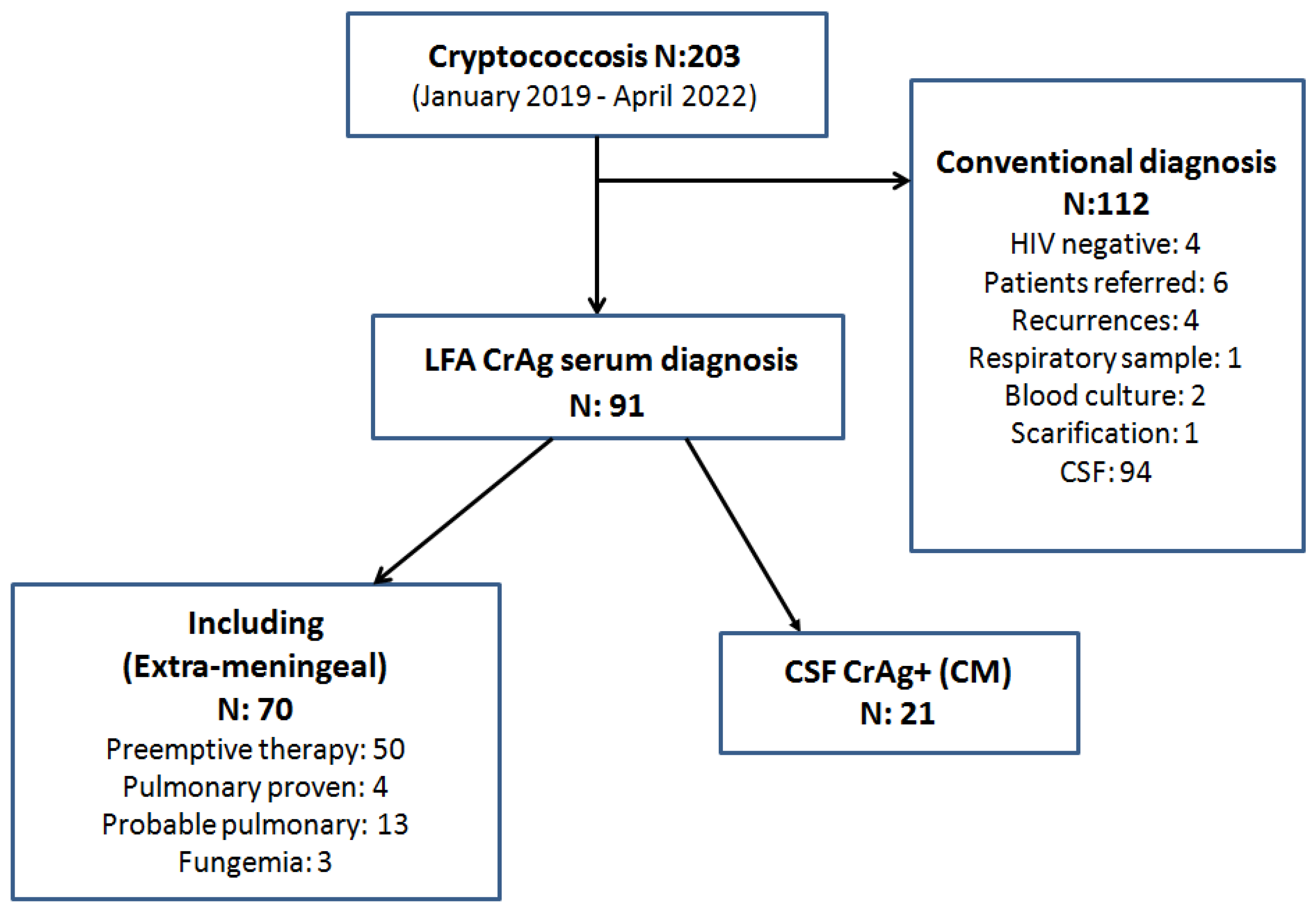
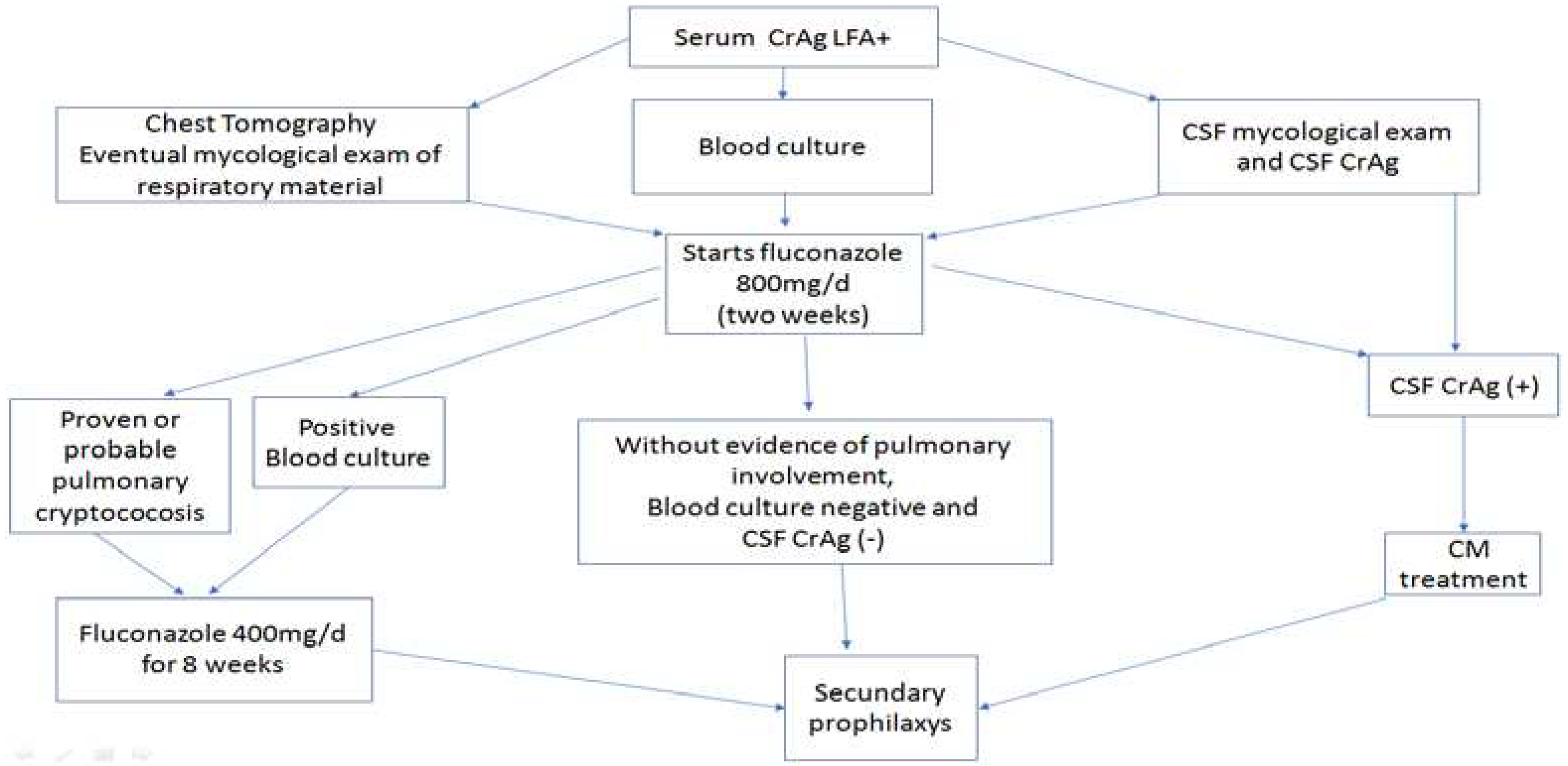
2. Materials and Methods
Inclusion Criteria
- ✓
- All PLHIV hospitalized with initial diagnosis of cryptococcosis by serum CrAg LFA without meningeal involvement.
- ✓
- Age: greater than or equal to 18 years old.
- ✓
- Patients with recurrent cryptococcosis due to treatment or prophylaxis interruption.
- ✓
- Patients whose diagnosis of cryptococcosis was initially made by blood culture, sputum, bronchoalveolar lavage, scarification of skin or mucosal lesions, or deep biopsies.
- ✓
- Meningeal cryptococcosis (MC)
- ✓
- Patients referred from other hospitals or health centers.
- ✓
- HIV-negative patients.
Samples
- Isolation of fungal DNA (extraction by physical heat/cold treatment and subsequent treatment with proteinase K and CTAB (hexa-decyl trimethylammonium bromide) for preparation of the DNA library, which was kept at -20 ºC.
- Amplification of Ura 5 gene by end-point PCR.
- Detection of the amplicon obtained by electrophoretic in 1% agarose gel.
- RFLP of Ura 5 with Sau96I and HhaI restriction enzymes.
- Detection the products with restriction enzymes on 3% agarose gel.
- The RFLP patterns were assigned by comparison with the patterns obtained from reference strains (C. neoformans var. grubii: CBS 10085 VNI and CBS 10084 VNII; from C. neoformans hybrid AD: CBS 10080 VNIII; from C. neoformans var. neoformans: CBS 10079 VNIV; and from C. gattii: CBS 10078 VGI; CBS 10082 VGII; CBS 10081 VGIII and CBS 10101 VGIV) [16].
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seagle EE, Williams SL, Chiller TM. Recent Trends in the epidemiology of fungal infections. Infect Dis Clin North Am. 2021;35:237- 60. [CrossRef]
- Firacative C, Meyer W, Castañeda E. Cryptococcus neoformans and Cryptococcus gattii species complexes in Latin America: A map of molecular types, genotypic diversity, and antifungal susceptibility as reported by the Latin American Cryptococcal Study Group. J Fungi (Basel). 2021 Apr 9;7(4):282. https://doi.org/10.3390/jof7040282. PMID: 33918572; PMCID: PMC8069395. [CrossRef]
- World Health Organization. Rapid advice: Diagnosis, prevention, and management of cryptococcal disease in HIV-infected adults, adolescents and children; World Health Organization: Geneva, Switzerland, 2011.
- Jarvis JN, Percival A, Bauman S, Pelfrey J, Meintjes G, Williams GN et al. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin Infect Dis. 2011; 53:1019–23. [CrossRef]
- Binnicker MJ, Jespersen DJ, Bestrom JE, Rollins LO. Comparison of four assays for the detection of cryptococcal antigen. Clin Vaccine Immunol. 2012;19:1988-90. https://doi.org/10.1128/CVI.00446-12. Epub 2012 Oct 17. PMID: 23081814; PMCID: PMC3535868. [CrossRef]
- Rick F, Niyibizi AA, Shroufi A, Onami K, Steele SJ, Kuleile M, Muleya I, Chiller T, Walker T, Van Cutsem G. Cryptococcal antigen screening by lay cadres using a rapid test at the point of care: A feasibility study in rural Lesotho. PLoS One. 2017 Sep 6;12(9): e0183656. https://doi.org/10.1371/journal.pone.0183656. PMID: 28877182; PMCID: PMC5587318. [CrossRef]
- Li Y, Huang X, Chen H, Qin Y, Hou J, Li A, Wu H, Yan X, Chen Y. The prevalence of cryptococcal antigen (CrAg) and benefits of pre-emptive antifungal treatment among HIV-infected persons with CD4+ T-cell counts < 200 cells/μL: evidence based on a meta-analysis. BMC Infect Dis. 2020 Jun 12;20(1):410. https://doi.org/10.1186/s12879-020-05126-z. Erratum in: BMC Infect Dis. 2021 Jun 24;21(1):604. PMID: 32532212; PMCID: PMC7291520. [CrossRef]
- Greene G, Lawrence DS, Jordan A, Chiller T, Jarvis JN. Cryptococcal meningitis: a review of cryptococcal antigen screening programs in Africa. Expert Rev Anti Infect Ther. 2021;19:233-244. https://doi.org/10.1080/14787210.2020.1785871. Epub 2020 Jul 14. PMID: 32567406. [CrossRef]
- WHO - World Health Organization. Guidelines for diagnosing, preventing and managing cryptococcal disease among adults, adolescents and children living with HIV June 2022. Available in: https://www.who.int/publications/i/item/9789240052178.
- Messina F, Maiolo E, Negroni R, Arechavala A, Santiso G, Bianchi M. Alternativas terapéuticas de la criptococosis meníngea. Actualizaciones en sida e infectología. 2015; 23: 88:25-32.
- Arechavala A, Negroni R, Messina F, Romero M, Marín E, Depardo R, Walker L, Santiso G. Cryptococcosis in an Infectious Diseases Hospital of Buenos Aires, Argentina. Revision of 2041 cases: Diagnosis, clinical features, and therapeutics. Rev Iberoam Micol. 2018;35(1):1-10. https://doi.org/10.1016/j.riam.2017.04.003. Epub 2017 Nov 10. PMID: 29129578. [CrossRef]
- Arechavala AI, Robles AM, Negroni R, Bianchi MH, Taborda A. Valor de los métodos directos e indirectos de diagnóstico en las micosis sistémicas asociadas al sida. RevInstMedtrop S Paulo, 1993; 35: 163-9.
- Guelfand L, Cataldi S, Arechavala A, Perrone M. Manual práctico de Micología Médica. Acta Bioquim Clín Latinoam, 2015; Supl. 1.
- Bianchi M, Robles AM, Vitale R, Helou S, Arechavala A, Negroni R. The usefulness of blood culture in diagnosing HIV-related systemic mycoses: evaluation of a manual lysis centrifugation method. MedMycol. 2000; 38: 77-80. [CrossRef]
- Negroni R, Arechavala A. Métodos de laboratorio de diagnóstico micológico e interpretación de los resultados. En: Lecciones de clinica micológica. 2da ed. Editorial Ascune 2019; 150-174. [e-book disponible www.editorialascune.com/ebookdetalle/4-lecciones-de-clinica-micologica].
- Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E; IberoAmerican Cryptococcal Study Group. Molecular typing of Ibero American Cryptococcus neoformans isolates. Emerg Infect Dis. 2003; 9:189-195. https://doi.org/10.3201/eid0902.020246. PMID: 12603989; PMCID: PMC2901947. [CrossRef]
- CLSI. Reference method for broth dilution antifungal susceptibility testing of yeast, approved standard, CLSI M27-A 4th Edition. 2017.Clinical and Laboratory Standards Institute, Wayne, PA.
- CLSI M57S, 4th ed. Principles and procedures for the development of epidemiological cutoff values for antifungal susceptibility testing. 2022. Clinical and Laboratory Standards Institute, Wayne, PA.
- CLSI M59S, 4th ed. Epidemiological cutoff values for antifungal susceptibility testing, 2022. Clinical and Laboratory Standards Institute, Wayne, PA.
- CLSI. Performance standards for antifungal susceptibility testing of yeasts, 2nd ed. CLSI supplement M60. 2020. Clinical and Laboratory Standards Institute, Wayne, PA.
- Immy CrAg lateral flow assay REF CR2003 – www.immy.com.
- Romero M, Messina F, Depardo R, Marín E, Arechavala A, Lista N, Rodríguez A, Santiso G. Problemas clínicos en Micología Médica: problema número 55 [Clinical problems in Medical Mycology: problema number 55]. Rev Iberoam Micol. 2021; 38:23-26. Spanish. https://doi.org/10.1016/j.riam.2020.10.004. Epub 2021 Jan 21. PMID: 33485778. [CrossRef]
- Fishman JA. Infection in organ transplantation. Am J Transplant 2017; 17: 856-879. [CrossRef]
- Temfack E, Rim JJB, Spijker R, Loyse A, Chiller T, Pappas PG, Perfect J, Sorell TE, Harrison TS, Cohen JF, Lortholary O. Cryptococcal antigen in serum and cerebrospinal fluid for detecting cryptococcal meningitis in adults living with HIV: systematic review and meta-analysis of diagnostic test accuracy studies. Clin Infect Dis, 2021: 2: 1268-1278. https://doi.org/10.1093/cid/ciaa1243. [CrossRef]
- Vijayan T, Chiller T, Klausner JD. Sensitivity and specificity of a new cryptococcal antigen lateral flow assay in serum and cerebrospinal fluid. MLO Med Lab Obs. 2013 Mar;45(3):16, 18, 20. PMID: 23822028; PMCID: PMC4119400.
- Skipper C, Abassi M, Boulware DR. Diagnosis, and management of central nervous system cryptococcal infections in HIV-infected adults. J.Fungi 2019;5, 65;. https://doi.org/10.3390/jof5030065. [CrossRef]
- Meya DB, Kiragga AN, Nalintya E, Morawski BM, Rajasingham R, Park BJ, Mubiru A, Kaplan JE, Manabe YC, Boulware DR. Reflexive laboratory-bases cryptococcal antigen screening and preemptive fluconazole therapy for cryptococcal antigenemia in HIV-infected individuals with CD4<100 cell/µL: a stepped-wedge, cluster-randomized trial. J Acquir Immune Defic Syndr. 2019; 80: 182-189.
- Montoya MC, Magwene PM, Perfect JR. Associations between Cryptococcus genotypes, phenotypes, and clinical parameters of human disease: A review. J. Fungi 2021, 7, 260. https://doi.org/10.3390/jof7040260. [CrossRef]
- Firacative C, Lizarazo J, Illnait-Zaragozi MT, Castañeda E, Latin American Study Group. The status of cryptococcosis in Latin America. Mem Inst Oswaldo Cruz, 2018; 113 (7): e170554. [CrossRef]
- Kaplan, J.E.; Vallabhaneni, S.; Smith, R.M.; Chideya-Chihota, S.; Chehab, J.; Park, B. Cryptococcal antigen screening and early antifungal treatment to prevent cryptococcal meningitis: A review of the literature. J Acquir. Immune Defic. Syndr. 2015, 68, S331–S339. [CrossRef]
- Meya DB, Kiragga AN, Nalintya E, Morawski BM, Rajasingham R, Park BJ, Mubiru A, Kaplan JE, Manabe YC, Boulware DR. Reflexive Laboratory-based cryptococcal antigen screening and preemptive fluconazole therapy for cryptococcal antigenemia in HIV-infected individuals with CD4 <100 cells/µL: A stepped-wedge, cluster-randomized trial. J Acquir Immune DeficSyndr. 2019 Feb 1;80(2):182-189. https://doi.org/10.1097/QAI.0000000000001894. PMID: 30399034; PMCID: PMC6339522. [CrossRef]
- Wake RM, Govender NP, Omar T, Nel C, Mazanderani AH, Karat AS, Ismail NA, Tiemessen CT, Jarvis JN, Harrison TS. Cryptococcal-related mortality despite fluconazole preemptive treatment in a cryptococcal antigen screen-and-treat program. Clin Infect Dis. 2020;70(8):1683-1690. https://doi.org/10.1093/cid/ciz485. Erratum in: Clin Infect Dis. 2020;70(11):2459. PMID: 31179488; PMCID: PMC7346756. [CrossRef]
- Longley, N.; Jarvis, J. N.; Meintjes, G.; et al. Cryptococcal Antigen Screening in Patients Initiating ART in South Africa: A Prospective Cohort Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am., 2016, 62 (5), 581–587. https://doi.org/10.1093/cid/civ936. [CrossRef]
- Messina F, Romero M, Marin E, Benchetrit A, Arechavala A, Depardo R, Negroni R, Santiso G. Característicasclínicas, métodosdiagnósticos y evolución de la criptococosis extrameníngea en personas viviendo con VIH. Actualizaciones en sida e infectología. 2022; 30: 109:1-47.
- Rothe, C.; Sloan, D. J.; Goodson, P.; et al. A Prospective longitudinal study of the clinical outcomes from cryptococcal meningitis following treatment induction with 800 mg oral fluconazole in Blantyre, Malawi. PLOS ONE, 2013, 8 (6), e67311. https://doi.org/10.1371/journal.pone.0067311. [CrossRef]
- Rajasingham R, Boulware DR. Cryptococcal antigen screening and preemptive treatment-how can we improve survival? Clin Infect Dis. 2020;70:1691-1694. https://doi.org/10.1093/cid/ciz488. PMID: 31179484; PMCID: PMC7145997. [CrossRef]
- Ford, N.; Shubber, Z.; Jarvis, J. N.; et al. CD4 cell count threshold for cryptococcal antigen screening of HIV-infected individuals: A systematic review and meta-analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am., 2018; 66 (Suppl 2), S152–9. https://doi.org/10.1093/cid/cix1143. [CrossRef]
- Wake RM, Britz E, Sriruttan C, Rukasha I, Omar T, Spencer DC, Nel JS, Mashamaite S, Adelekan A, Chiller TM, Jarvis JN, Harrison TS, Govender NP. High cryptococcal antigen titers in blood are predictive of subclinical cryptococcal meningitis among human immunodeficiency virus-infected patients. Clin Infect Dis. 2018;66:686-692. https://doi.org/10.1093/cid/cix872. PMID: 29028998; PMCID: PMC6220350. [CrossRef]
- Batzlaff CM, Limper AH. When to consider the possibility of a fungal infection: An overview of clinical diagnosis and laboratory approaches. Clin Chest Med. 2017;38:385-391. https://doi.org/10.1016/j.ccm.2017.04.002. Epub 2017 May 17. PMID: 28797483. [CrossRef]
- Wake RM, Molloy SF, Jarvis JN, Harrison TS, Govender NP. Cryptococcal antigenemia in advanced human immunodeficiency virus disease: Pathophysiology, epidemiology, and clinical implications. Clin Infect Dis. 2023;76:764-770. https://doi.org/10.1093/cid/ciac675. PMID: 35986670; PMCID: PMC9938740. [CrossRef]
- Borges MASB, de Araújo Filho JA, Oliveira BJS, Moreira IS, de Paula VV, de Bastos AL, et al. Prospective cohort of AIDS patients screened for cryptococcal antigenaemia, pre-emptively treated and followed in Brazil. PLoS One. 2019;14(7): e0219928. [CrossRef]
- Kapoor SW, Magambo KA, Kalluvya SE, Fitzgerald DW, Peck RN, Downs JA. Sixmonth outcomes of HIV-infected patients given short-course fluconazole therapy for asymptomatic cryptococcal antigenemia. AIDS 2015; 29:2473–8. [CrossRef]
- Skolnik K, Huston S, Mody CH. Cryptococcal lung infections. Clin Chest Med 2017; 38(3): 451-64.
- Yoon HA, Nakouzi A, Chang CC, et al. Association between plasma antibody responses and risk for Cryptococcus-associated immune reconstitution inflammatory syndrome. J Infect Dis 2019; 219(3): 420-8. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




