Submitted:
29 April 2023
Posted:
30 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Lectin isolation
2.2. Electrochemical evaluations of cMoL immobilized on MOF/Platinum electrode surface using MOF, Metal-Organic Framework of [Cu3(BTC)2.(H2O)3]n
2.3. Preparation of the reference electrode
2.4. Washing and preparation of the working electrode
2.5. Immobilization and Potentiometry
- 1)
- Platinum electrode with MOF (Pt/MOF);
- 2)
- Platinum electrode with Pt/MOF/cMoL with 0.15 M NaCl in the electrolytic medium;
- 3)
- Pt/MOF/cMoL with 0.15 M NaCl and different concentrations of galactose: 10, 15 and 20 mM.
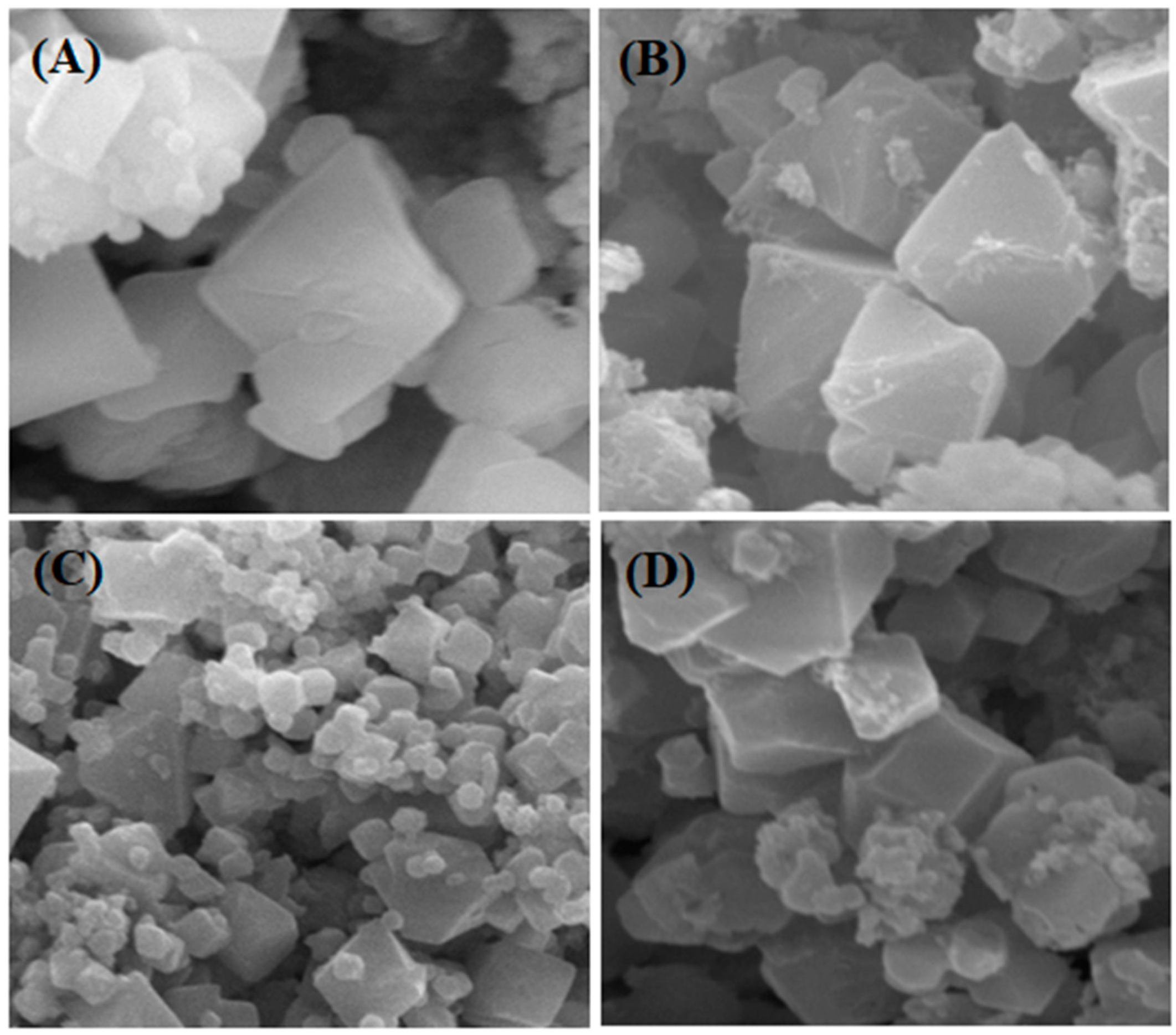
2.6. Scanning electron microscopy
2.7. Electrocoagulation

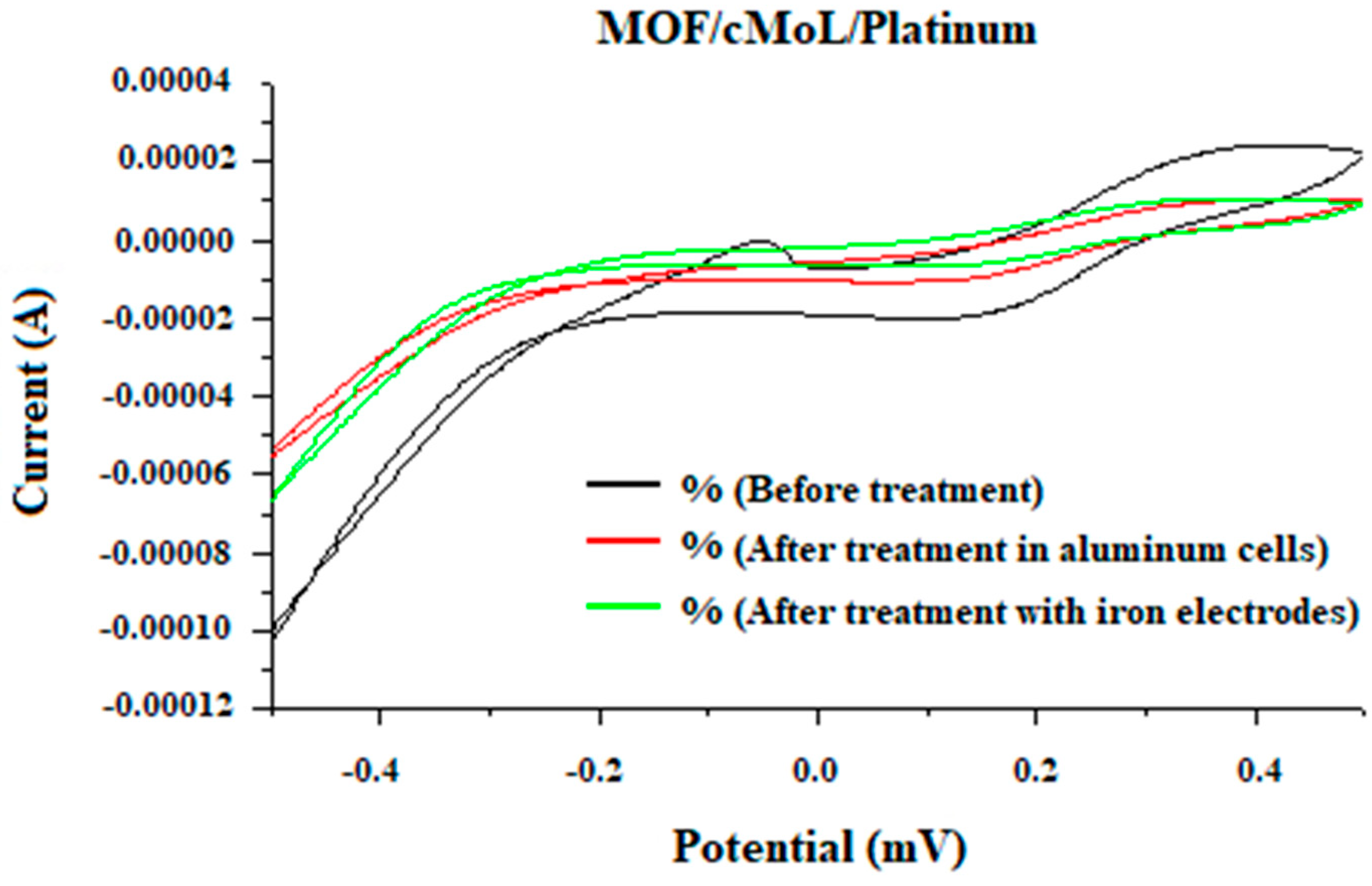
2.8. Cyclic Voltammetry
3. Results and discussion

| Samples with dye | pH |
|---|---|
| Sample without treatment | 5.97 |
| Sample after aluminum electrode treatment | 6.24 |
| Sample after iron electrode treatment | 7.01 |
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Chettri, D.; Boro, M.; Sarkar, L.; Verma, A.K. Lectins: Biological significance to biotechnological application. Carbohydrate Research, 2021, 506, 1–7. [Google Scholar] [CrossRef]
- Coelho, L.C.B.B.; Silva, P.M.; Lima, V.L.; Pontual, E.V.; Paiva, P.M.G.; Napoleão, T.H.; Correia, M.T.S. Lectins, Interconnecting Proteins with Biotechnological/Pharmacological and Therapeutic Applications. Evid. Based. Complement. Alternat. Med. 2017, 2017, 1–23. [Google Scholar] [CrossRef]
- Saleem, M.; Bachmann, R.T. A Contemporary Review on Plant-Based Coagulants for Applications in Water Treatment. J. Ind. Eng. Chem. 2019, 72, 281–297. [Google Scholar] [CrossRef]
- Naithani, S.; Komath, S.S.; Nonomura, A.; Govindjee, G. Plant Lectins and Their Many Roles: Carbohydrate-Binding and Beyond. J. Plant. Physiol. 2021, 266, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Konozy, E.H.E.; Osman, M.E.M. Plant Lectin: A Promising Future Anti-Tumor Drug. Biochimie. 2022, 202, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, L.; Zhao, M.; Yang, X. The Hypoglycemic and Hypolipemic Potentials of Moringa oleifera leaf Polysaccharide and Polysaccharide-flavonoid Complex. Int. J. Biol. Macromol. 2022, 210, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kumar, M.; Waghmare, R.; Suhag, R.; Gupta, O.P.; Lorenzo, J.M.; Prakash, S.; Radha.; Rais, N.; Sampathrajan, V.; Thappa, C.; Anitha, T.; Sayed, A.A.S.; Abdel-Wahab, B.A.; Senapathy, M.; Pandiselvam, R.; Dey, A.; Dhumal, S.; Amarowicz, R.; Kennedy, J.F. Moringa (Moringa oleifera Lam.) Polysaccharides: Extraction, Characterization, Bioactivities, and Industrial Application. Int. J. Biol. Macromol. 2022, 209, 763–778. [CrossRef]
- Raman, J.K.; Alves, C.M.; Gnansounou, E. A Review on Moringa Tree and Vetiver Grass - Potential Biorefinery Feedstocks. Bioresour. Technol. 2018, 249, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Aldhahrani, A.; Alkhedaide, A.; Nassan, M.A.; Althobaiti, F.; Mohamed, W.A. The Ameliorative Impacts of Moringa oleifera Leaf Extract Against Oxidative Stress and Methotrexate-Induced Hepato-Renal Dysfunction. Biomed. Pharmacother. 2020, 128, 1–10. [Google Scholar] [CrossRef]
- Shan, T.C.; Matar, M.A.; Makky, E.A.; Ali, E.N. The Use of Moringa oleifera Seed as a Natural Coagulant for Wastewater Treatment and Heavy Metals Removal. Appl. Water Sci. 2017, 7, 1369–1376. [Google Scholar] [CrossRef]
- Varsani, V.; Vyas, S.J.; Dudhagara, D.R. Development of Bio-Based Material from the Moringa oleifera and Its Bio-Coagulation Kinetic Modeling-A Sustainable Approach to Treat the Wastewater. Heliyon. 2022, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Boulaadjoul, S.; Zemmouri, H.; Bendjama, Z.; Drouiche, N. A Novel use of Moringa oleifera Seed Powder in Enhancing the Primary Treatment of Paper Mill Effluent. Chemosphere. 2018, 206, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Luz, L.A.; Argolo, A.C.; Teixeira, J.A.; Paiva, P.M.G.; Coelho, L.C.B.B. . Isolation of a Seed Coagulant Moringa oleifera Lectin. Process Biochem. 2009, 44, 504–508. [Google Scholar] [CrossRef]
- Luz, L.A.; Silva, M.C.; Ferreira, R.S.; Santana, L.A.; Silva-Lucca, R.A.; Mentele, R.; Oliva, M.L.; Paiva, P.M.G.; Coelho, L.C.B.B. Structural Characterization of Coagulant Moringa oleifera Lectin and Its Effect on Hemostatic Parameters. Int. J. Biol. Macromol. 2013, 58, 31–36. [Google Scholar] [CrossRef]
- Oliveira, C.F.R.; Luz, L.A.; Paiva, P.M.G.; Coelho, L.C.B.B.; Marangoni, S.; Macedo, M.L.R. Evaluation of Seed Coagulant Moringa oleifera Lectin (cMoL) as a Bioinsecticidal Tool with Potential for the Control of Insects. Process Biochem. 2011, 46, 498–504. [Google Scholar] [CrossRef]
- Medeiros, M.L.S.; Alves, R.R.V.; Oliveira, B.F.; Napoleão, T.H.; Paiva, P.M.G.; Coelho, L.C.B.B.; Bezerra, A.C.D.S.; Silva, M.D.C. In vitro effects of Moringa oleifera Seed Lectins on Haemonchus contortus in Larval and Adult Stages. Exp. Parasitol. 2020, 218, 1–8. [Google Scholar] [CrossRef]
- Andrade, L.L.; Rossato, F.A.; Costa, R.A.P.E. , Napoleão, T.H.; Paiva, P.M.G.; Coelho, L.C.B.B. Cytotoxicity of the Coagulant Moringa oleifera Lectin (cMoL) to B16-F10 Melanoma Cells. Toxicol. In Vitro. 2017, 44, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chao, L.; Pang, J.; Li, Z.; Wan, Y.; Jiang, X.; Mao, Z.; Liu, W.; Chen, X.; Zhang, X. A Novel Way for High Value-Added Application of Lignosulfonate: Producing Lignosulfonate Nanosheets/Graphene Ultrathin Film Electrodes for Electrochemical Capacitors. Int. J. Biol. Macromol. 2020, 161, 666–673. [Google Scholar] [CrossRef]
- Andrade, C.A.; Oliveira, M.D.; Melo, C.P.; Coelho, L.C.B.B.; Correia, M.T.S.; Nogueira, M.L.; Singh, P.R.; Zeng, X. Diagnosis of Dengue Infection Using a Modified Gold Electrode with Hybrid Organic-Inorganic Nanocomposite and Bauhinia monandra Lectin. J. Colloid. Interface. Sci. 2011, 362, 517–523. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Villamón, E.; Luna, I.; Ramos, D.; Doménech-Casasús, C.; Cebrián-Torrejón, G. Transmembrane Electrochemistry of Erythrocytes: Direct Electrochemical Test for Detecting Hemolysis in Whole Blood. Sens. Actuators B Chem. 2016, 226, 419–428. [Google Scholar] [CrossRef]
- Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.A.; Soozanipour, A.; Low, Z.X.; Asadnia, M.; Taheri-Kafrani, A.; Razmjou, A. Biosensors for Wastewater Monitoring: A Review. Biosens. Bioelectron. 2018, 118, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, G.; Kim, K. Determination of Equilibrium State and Sn Redox Ratio in Aluminoborosilicate Glass Melts by Potentiometry and Voltammetry. Electrochem. Commun. 2019, 109, 1–5. [Google Scholar] [CrossRef]
- Theyagarajan, K.; Kim, Y.J. Recent Developments in the Design and Fabrication of Electrochemical Biosensors Using Functional Materials and Molecules. Biosensors. 2023, 13, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent Developments in Biosensors for Healthcare and Biomedical Applications: A Review. Measurement. 2021, 167, 1–128. [Google Scholar] [CrossRef]
- Li, M.; Zhan, G.; Boakye, A. Chai, H.; Qu, L.; Zhang, X. Recent Advances in Metal-Organic Framework-Based Electrochemical Biosensing Applications. Front. Bioeng. Biotechnol. 2021, 9, 1–8. [CrossRef]
- Boumya, W.; Taoufik, N.; Achak, M.; Bessbousse, H.; Elhalil, A.; Barka, N. Electrochemical Sensors and Biosensors for the Determination of Diclofenac in Pharmaceutical, Biological and Water Samples. Talanta Open. 2021, 3, 1–13. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamsshidi-Zanjani, A. A review on Industrial Wastewater Treatment via Electrocoagulation Processes. Curr. Opin Electrochem. 2020, 22, 159–169. [Google Scholar] [CrossRef]
- Adou, K.E.; Kouakou, A.R.; Ehouman, A.D.; Tyagi, R.D.; Drogui, P.; Adouby, K. Coupling Anaerobic Digestion Process and Electrocoagulation Using Iron and Aluminium Electrodes for Slaughterhouse Wastewater Treatment. Sci. Afr. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Idusuyi, N.; Ajide, O. O.; Abu, R.; Okewole, O. A.; Ibiyemi, O.O. Low Cost Electrocoagulation Process for Treatment of Contaminated Water Using Aluminium Electrodes from Recycled Cans. Mater. Today: Proc. 2022, 56, 1712–1716. [Google Scholar] [CrossRef]
- Silva, J. R.; Carvalho, F.; Vicente, C.; Santos, A. D.; Quinta-Ferreira, R.M.; Castro, L.M. . Electrocoagulation Treatment of Cork Boiling Wastewater. J. Environ. Chem. Eng. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Ebba, M.; Asaithambi, P.; Alemayehu, E. Development of Electrocoagulation Process for Wastewater Treatment: Optimization by Response Surface Methodology. Heliyon. 2022, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kothai, A.; Sathishkumar, C.; Muthupriya, R.; Dharchana, R. Experimental Investigation of Textile Dyeing Wastewater Treatment Using Aluminium in Electro Coagulation Process And Fenton’s Reagent in Advanced Oxidation Process. Mater. Today: Proc. 2021, 45, 1411–1416. [Google Scholar] [CrossRef]
- Song, D.; Kadier, A.; Peralta-Hernández, J.M.; Xie, H.; Hao, B.; Ma, P.C. Separation of oil-water emulsions by a novel packed bed electrocoagulation (EC) process using anode from recycled aluminum beverage cans. J. Clean. Prod. 2022, 379, 1–13. [Google Scholar] [CrossRef]
- Sohrabi, H.; Maleki, F.; Khaaki, P.; Kadhom, M.; Kudaibergenov, N.; Khataee, A. Electrochemical-Based Sensing Platforms for Detection of Glucose and H2O2 by Porous Metal-Organic Frameworks: A Review of Status and Prospects. Biosensors. 2023, 13, 1–20. [Google Scholar] [CrossRef]
- Ahmad, N.; Younus, H.A.; Chughtai, A.H.; Verpoort, F. Metal-Organic Molecular Cages: Applications of Biochemical Implications. Chem. Soc. Rev. 2015, 44, 9–25. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors. 2022, 13, 1–32. [Google Scholar] [CrossRef]
- Green, A.A.; Hughes, W.L. Protein Fractionation on the Basis of Solubility in Aqueous Solutions of Salts and Organic Solvents. Meth. Enzymol. 1955, 1, 67–90. [Google Scholar] [CrossRef]
- Appukuttan, P.S.; Surolia, A.; Bachawat, B.K. Isolation of Two Galactose-Binding Proteins from Ricinus Communis by Affinity Chromatography. Indian J. Biochem. Biophys. 1977, 14, 382–384. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Paiva, P.M.G.; Coelho, L.C.B.B. Purification and Partial Characterization of Two Lectin Isoforms from Cratylia mollis Mart. (camaratu bean). Appl. Biochem. Biotechnol. 1992, 36, 113–118. [Google Scholar] [CrossRef]
- Silva, G.G.; Machado, F.L.A.; Alves Junior, S.; Padrón-Hernández, E. Metal-Organic Framework: Structure and Magnetic Properties of [Cu3(BTC)2 (L)x·(CuO)y]n (L=H2O, DMF). J. Solid State Chem. 2017, 253, 1–5. [Google Scholar] [CrossRef]
- Abreu, D.S.; Sousa, T.P.; Castro, C.B.; Sousa, M.N.; Silva, T.T.; Almeida-Neto, F.W.Q.; Queiros, M.V.A.; Rodrigues, B.S.F.; Oliveira, M.C.F.; Paulo, T.F.; Cavada, B.S.; Nascimento, K.S.; Temperini, M.L.A.; Diógenes, I.C.N. SAM of Gliotoxin on Gold: A Natural Product Platform for Sugar Recognition Based on the Immobilization of Canavalia brasiliensis Lectin (ConBr). Electrochim. Acta. 2017, 241, 116–123. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme Immobilized Nanomaterials as Electrochemical Biosensors for Detection of Biomolecules. Enzyme Microb. Technol. 2022, 156, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.R.; Correia, M.T.S.; Pessoa, M.M.A.; Kennedy, J.F.; Lima-Filho, J.L.; Coelho, L.C.B.B. A Novel Model to Characterize the Electric Double Layer of Lectins from Cratylia mollis (Camaratu bean) and Canavalia ensiformis Adsorbed on Metallic Surface. Carbohydr. Polym. 2001, 46, 191–193. [Google Scholar] [CrossRef]
- Carvalho, M.H.; Araújo, H.D.A.; Silva, R.P.; Correia, M.T.; Freitas, K.C.S.; Souza, S.R.; Coelho, L.C.B.B. Biosensor Characterization from Cratylia mollis Seed Lectin (Cramoll)-MOF and Specific Carbohydrate Interactions in an Electrochemical Model. Chem. Biodivers. 2022, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Selzer, S.M.; Vico, R.V.; Ferreyra, N.F. Immobilization of Concanavalin A on Iron Oxide Magnetic Nanoparticles. Effect of Bovine Serum Albumin in the Recognition Interactions of the Lectin. Surf. Interfaces. 2022, 30, 1–11. [Google Scholar] [CrossRef]
- Abrantes-Coutinho, V.E.; Santos, A.O.; Moura, R.B.; Pereira-Junior, F.N.; Mascaro, L.H.; Morais, S.; Oliveira, T.M.B.F. Systematic Review on Lectin-Based Electrochemical Biosensors for Clinically Relevant Carbohydrates and Glycoconjugates. Colloids Surf. B. 2021, 208, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yuan, R.; Chai, Y.; Zhong, H.; Wang, Y. . Study of the Biosensor Based on Platinum Nanoparticles Supported on Carbon Nanotubes and Sugar–Lectin Biospecific Interactions for the Determination of Glucose. Electrochim. Acta. 2011, 56, 4203–4208. [Google Scholar] [CrossRef]
- Zhao, S.; Shi, C.; Hu, H.; Li, Z.; Xiao, G.; Yang, Q.; Sun, P.; Cheng, L.; Niu, W.; Bi, J.; Yue, Z. ISFET and Dex-AgNPs Based Portable Sensor for Reusable and Real-Time Determinations of concanavalin A and Glucose on Smartphone. Biosens. Bioelectron. 2020, 151, 1–9. [Google Scholar] [CrossRef]
- Martínez-Pérez-Cejuela, H.; Calabretta, M.M.; Bocci, V.; D’Elia, M.; Michelini, E. Super-Stable Metal–Organic Framework (MOF)/Luciferase Paper-Sensing Platform for Rapid ATP Detection. Biosensors. 2023, 13, 1–14. [Google Scholar] [CrossRef]
- Cui, H.; Cui, S.; Zhang, S.; Tian, Q.; Liu, Y.; Zhang, P.; Wang, M.; Zhang, J.; Li, X. Cu–MOF/Hemin: A Bionic Enzyme with Excellent Dispersity for the Determination of Hydrogen Peroxide Released from Living Cells. Analyst. 2021, 146, 5951–5961. [Google Scholar] [CrossRef] [PubMed]
- Meneses, J.M.D.; Vasconcelos, R.D.F.; Fernandes, T.D.F.; Araújo, G.T.D. Tratamento do Efluente do Biodiesel Utilizando a Eletrocoagulação/Flotação: Investigação dos Parâmetros Operacionais. Química Nova. 2012, 35, 235–240. [Google Scholar] [CrossRef]
- Criado, S.P.; Gonçalves, M.J.; Tavares, L.B.B.; Bertoli, S.L. Optimization of Electrocoagulation Process for Disperse and Reactive Dyes Using the Response Surface Method with Reuse Application. J. Clean. Prod. 2020, 275, 1–14. [Google Scholar] [CrossRef]
- Kalivel, P.; Singh, R.P.; Kavitha, S.; Padmanabhan, D.; Krishnan, S.K.; Palanichamy, J. Elucidation of Electrocoagulation Mechanism in the Removal of Blue SI Dye from Aqueous Solution Using Al-Al, Cu-Cu Electrodes - A comparative Study. Ecotoxicol. Environ. Saf. 2020, 201, 1–9. [Google Scholar] [CrossRef]
- Hendaoui, K.; Trabelsi-Ayadi, M.; Ayari, F. Optimization and Mechanisms Analysis of Indigo Dye Removal Using Continuous Electrocoagulation. Chin. J. Chem. Eng. 2021, 29, 242–252. [Google Scholar] [CrossRef]
- Özyonar, F.; Gökkuş, Ö.; Sabuni, M. Removal of Disperse and Reactive Dyes from Aqueous Solutions Using Ultrasound-Assisted Electrocoagulation. Chemosphere. 2020, 258, 1–11. [Google Scholar] [CrossRef]
- Munteanu, I. G.; Apetrei, C. Tyrosinase-Based Biosensor-A New Tool for Chlorogenic Acid Detection in Nutraceutical Formulations. Materials 2022, 15, 1–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





