Submitted:
28 April 2023
Posted:
01 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
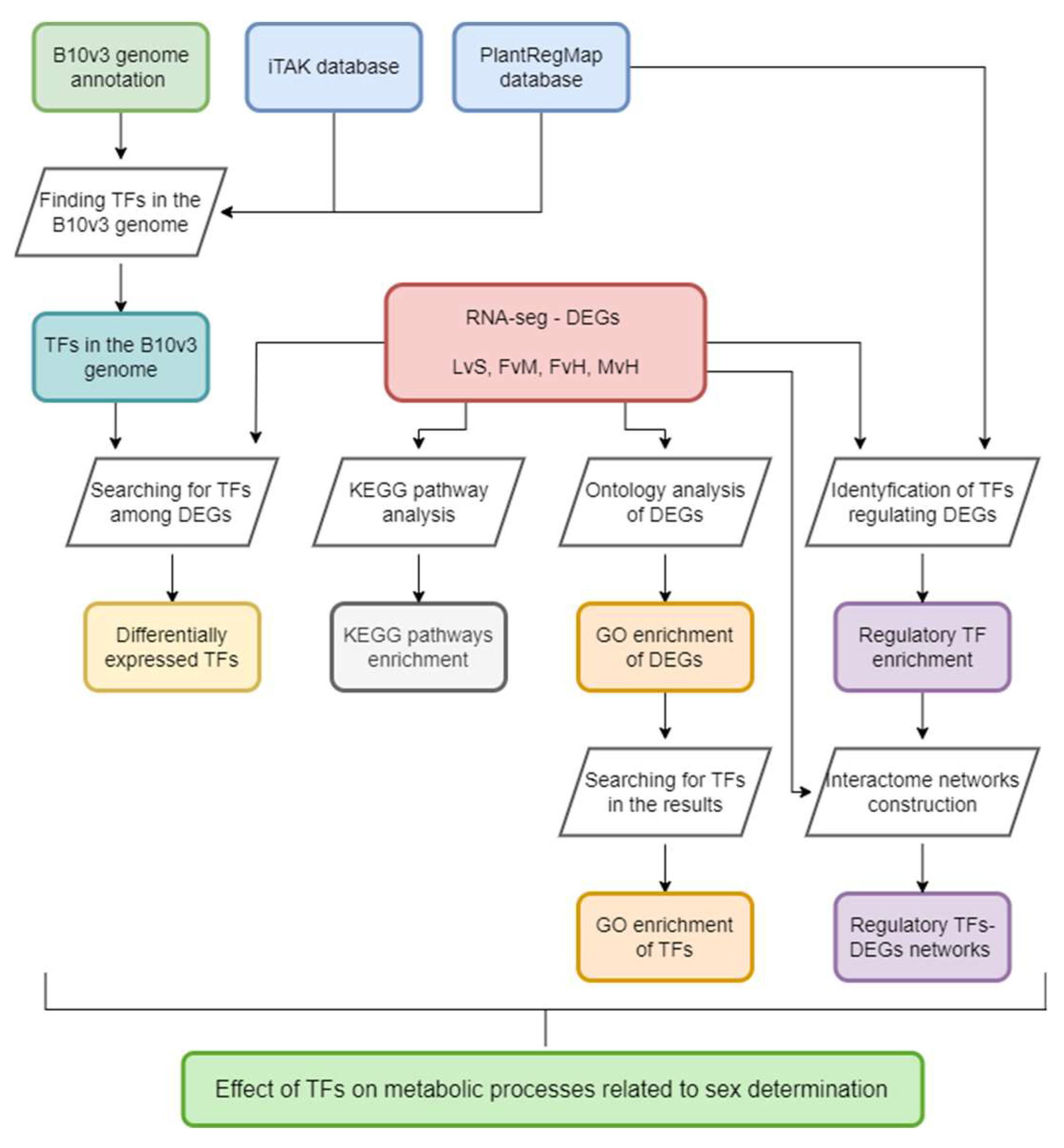
2.1. Finding the transcription factors in the B10v3 genome.
2.2. Tanscription factors among the results of RNA-seq experiments
2.3. Ontology analysis among differentially expressed genes
2.4. KEGG pathways enrichment analysis in DEGs
2.5. Regulatory Transcription Factors enrichment in PlantRegMap for DEGs
3. Results and discussions
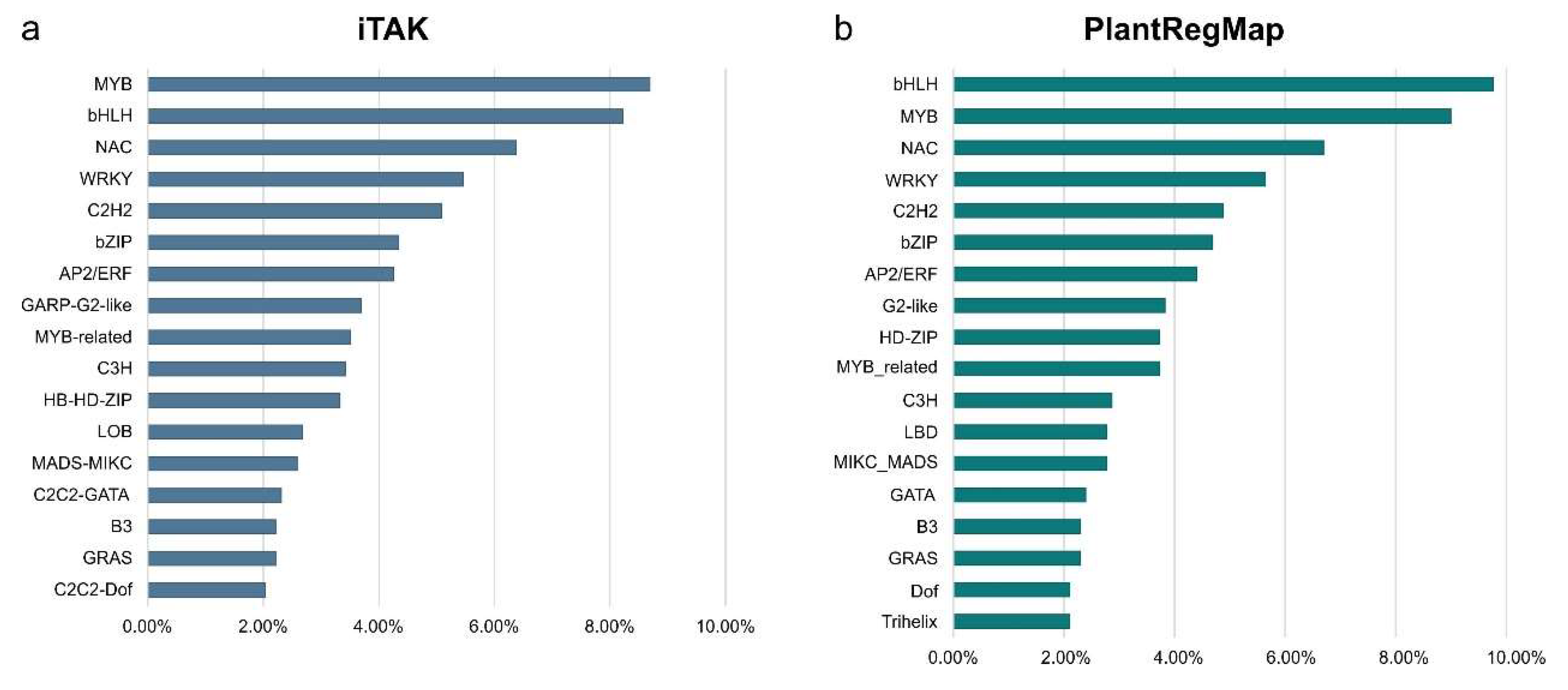
3.1. Transcription factor search results in the B10v3 genome.
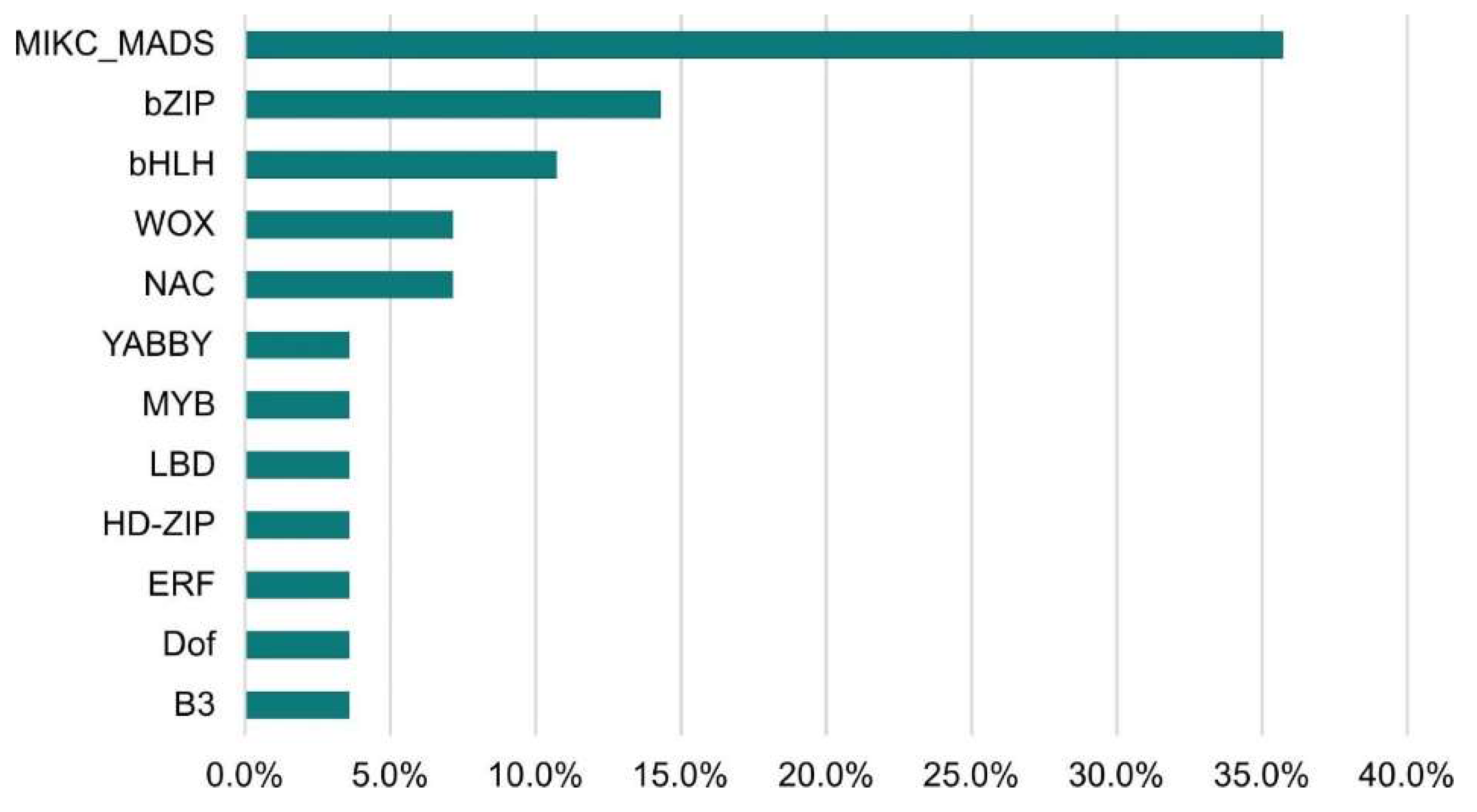
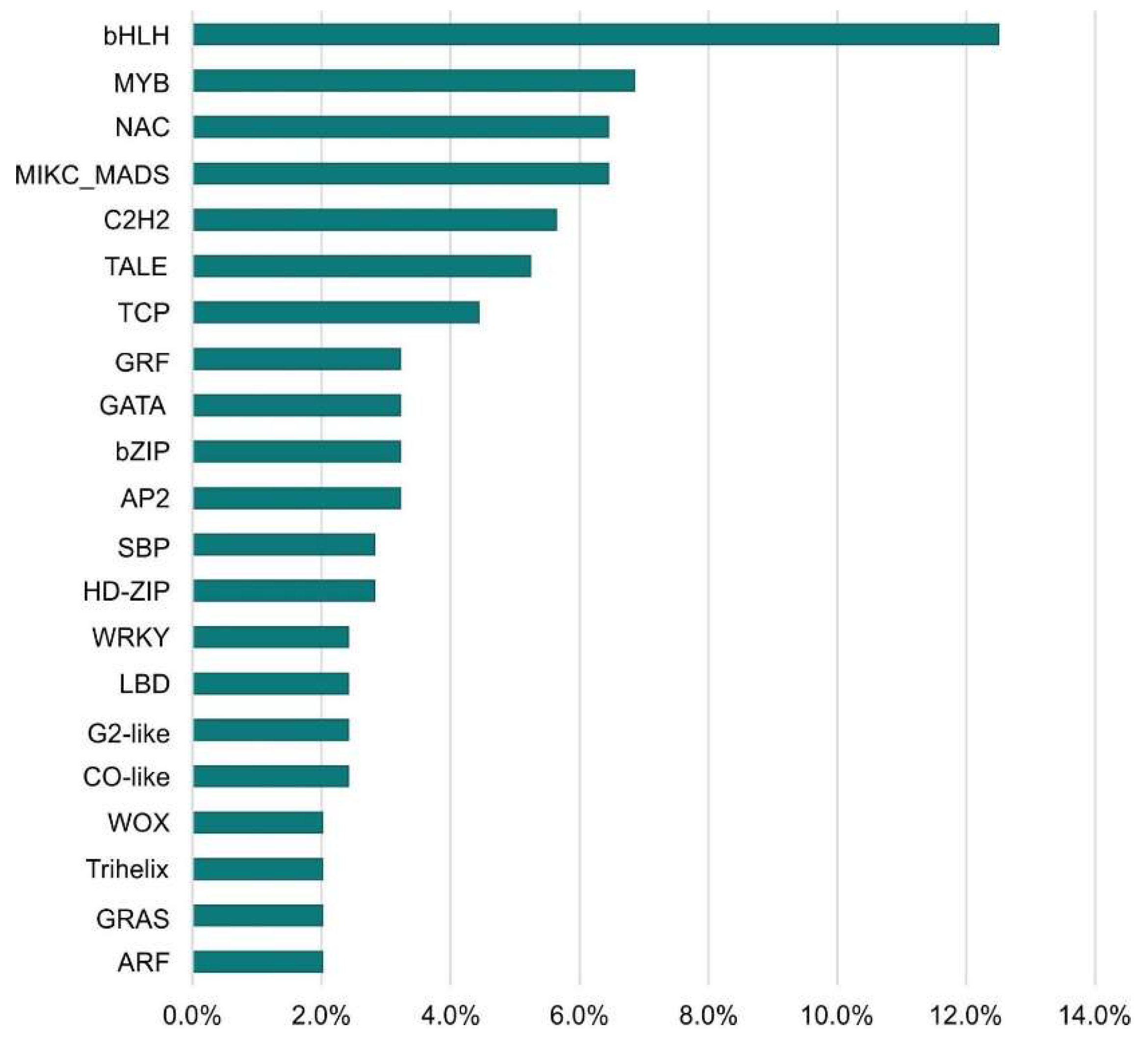
3.2. Transcription factor among DEGs
3.3. Ontology analysis among differentially expressed genes
3.4. KEGG enrichment results in DEGs
3.6. Regulatory Transcription Factors influencing DEGs.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, S.; Li, R.; Zhang, Z.; Li, L.; Gu, X.; Fan, W.; Lucas, W.J.; Wang, X.; Xie, B.; Ni, P.; et al. The Genome of the Cucumber, Cucumis Sativus L. Nature Genetics 2009 41:12 2009, 41, 1275–1281. [Google Scholar] [CrossRef]
- Yang, L.; Koo, D.H.; Li, Y.; Zhang, X.; Luan, F.; Havey, M.J.; Jiang, J.; Weng, Y. Chromosome Rearrangements during Domestication of Cucumber as Revealed by High-Density Genetic Mapping and Draft Genome Assembly. The Plant Journal 2012, 71, 895–906. [Google Scholar] [CrossRef]
- Osipowski, P.; Pawełkowicz, M.; Wojcieszek, M.; Skarzyńska, A.; Przybecki, Z.; Pląder, W. A High-Quality Cucumber Genome Assembly Enhances Computational Comparative Genomics. Molecular Genetics and Genomics 2020, 295, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Turek, S.; Pląder, W.; Hoshi, Y.; Skarzyńska, A.; Pawełkowicz, M. Insight into the Organization of the B10v3 Cucumber Genome by Integration of Biological and Bioinformatic Data. Int J Mol Sci 2023, 24, 4011. [Google Scholar] [CrossRef] [PubMed]
- Biłas, R.; Szafran, K.; Hnatuszko-Konka, K.; Kononowicz, A.K. Cis-Regulatory Elements Used to Control Gene Expression in Plants. Plant Cell, Tissue and Organ Culture (PCTOC) 2016 127:2 2016, 127, 269–287. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, C.; Sun, H.; Rosli, H.G.; Pombo, M.A.; Zhang, P.; Banf, M.; Dai, X.; Martin, G.B.; Giovannoni, J.J.; et al. ITAK: A Program for Genome-Wide Prediction and Classification of Plant Transcription Factors, Transcriptional Regulators, and Protein Kinases. Mol Plant 2016, 9, 1667–1670. [Google Scholar] [CrossRef]
- Fletcher, M. Genome-Scale Characterization of Transcription Factors. Nature Genetics 2023 55:3 2023, 55, 357–357. [Google Scholar] [CrossRef]
- Dai, X.; Sinharoy, S.; Udvardi, M.; Zhao, P.X. PlantTFcat: An Online Plant Transcription Factor and Transcriptional Regulator Categorization and Analysis Tool. BMC Bioinformatics 2013, 14, 1–6. [Google Scholar] [CrossRef]
- Franco-Zorrilla, J.M.; López-Vidriero, I.; Carrasco, J.L.; Godoy, M.; Vera, P.; Solano, R. DNA-Binding Specificities of Plant Transcription Factors and Their Potential to Define Target Genes. Proc Natl Acad Sci U S A 2014, 111, 2367–2372. [Google Scholar] [CrossRef]
- Tian, F.; Yang, D.C.; Meng, Y.Q.; Jin, J.; Gao, G. PlantRegMap: Charting Functional Regulatory Maps in Plants. Nucleic Acids Res 2020, 48, D1104–D1113. [Google Scholar] [CrossRef]
- Pawełkowicz, M.; Pryszcz, L.; Skarzyńska, A.; Wóycicki, R.K.; Posyniak, K.; Rymuszka, J.; Przybecki, Z.; Pląder, W. Comparative Transcriptome Analysis Reveals New Molecular Pathways for Cucumber Genes Related to Sex Determination. Plant Reproduction 2019 32:2 2019, 32, 193–216. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Jung, D.; Jung, D.; Yao, R. ShinyGO: A Graphical Gene-Set Enrichment Tool for Animals and Plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- GitHub - Christophergandrud/NetworkD3: D3 JavaScript Network Graphs from R Available online:. Available online: https://github.com/christophergandrud/networkD3 (accessed on 25 April 2023).
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.Z.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R.; et al. Arabidopsis Transcription Factors: Genome-Wide Comparative Analysis among Eukaryotes. Science (1979) 2000, 290, 2105–2110. [Google Scholar] [CrossRef]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional Factors Regulate Plant Stress Responses Through Mediating Secondary Metabolism. Genes 2020, Vol. 11, Page 346 2020, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Daher, H.; Galien, A.; Hugouvieux, V.; Zubieta, C. Structural Basis for Plant MADS Transcription Factor Oligomerization. Comput Struct Biotechnol J 2019, 17, 946–953. [Google Scholar] [CrossRef]
- Theißen, G.; Gramzow, L. Structure and Evolution of Plant MADS Domain Transcription Factors. Plant Transcription Factors: Evolutionary, Structural and Functional Aspects 2016, 127–138. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, L.; Song, J.; Jiang, L.; Liu, S. Isolation and Characterization of a MADS-Box Gene in Cucumber (Cucumis Sativus L.) That Affects Flowering Time and Leaf Morphology in Transgenic Arabidopsis. http://mc.manuscriptcentral.com/tbeq 2019, 33, 54–63. [Google Scholar] [CrossRef]
- Theißen, G.; Melzer, R.; Ruümpler, F. MADS-Domain Transcription Factors and the Floral Quartet Model of Flower Development: Linking Plant Development and Evolution. Development 2016, 143, 3259–3271. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Chen, P.; Wu, C.; Zhang, H.; Yuan, J.; Zhou, J.; Li, X. Genome-Wide Identification of APETALA2/ETHYLENE RESPONSIVE FACTOR Transcription Factors in Cucurbita Moschata and Their Involvement in Ethylene Response. Front Plant Sci 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Pawełkowicz, M.E.; Skarzyńska, A.; Pląder, W.; Przybecki, Z. Genetic and Molecular Bases of Cucumber (Cucumis Sativus L.) Sex Determination. Molecular Breeding 2019, 39, 1–27. [Google Scholar] [CrossRef]
- Boualem, A.; Troadec, C.; Camps, C.; Lemhemdi, A.; Morin, H.; Sari, M.A.; Fraenkel-Zagouri, R.; Kovalski, I.; Dogimont, C.; Perl-Treves, R.; et al. A Cucurbit Androecy Gene Reveals How Unisexual Flowers Develop and Dioecy Emerges. Science 2015, 350, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Boualem, A.; Troadec, C.; Kovalski, I.; Sari, M.A.; Perl-Treves, R.; Bendahmane, A. A Conserved Ethylene Biosynthesis Enzyme Leads to Andromonoecy in Two Cucumis Species. PLoS One 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Trebitsh, T.; Staub, J.E.; O’Neill, S.D. Identification of a 1-Aminocyclopropane-1-Carboxylic Acid Synthase Gene Linked to the Female (F) Locus That Enhances Female Sex Expression in Cucumber. Plant Physiol 1997, 113, 987. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Fujii, N.; Takahashi, H. Characterization of Ethylene Effects on Sex Determination in Cucumber Plants. Sex Plant Reprod 2003, 16, 103–111. [Google Scholar] [CrossRef]
- BHOWMICK, B.K.; JHA, S.U.M.I.T.A. Dynamics of Sex Expression and Chromosome Diversity in Cucurbitaceae: A Story in the Making. J Genet 2015, 94, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, X.; Liu, S.; Lin, W.; Li, Y.; Zhang, X. Genome-Wide Analysis of AP2/ERF Transcription Factors in Pineapple Reveals Functional Divergence during Flowering Induction Mediated by Ethylene and Floral Organ Development. Genomics 2021, 113, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Chen, S.; Jiang, J.; Chen, F.; Chen, Y.; Gu, C.; Li, P.; Song, A.; Zhu, X.; Gao, H.; et al. Heterologous Expression of the Chrysanthemum R2R3-MYB Transcription Factor CmMYB2 Enhances Drought and Salinity Tolerance, Increases Hypersensitivity to ABA and Delays Flowering in Arabidopsis Thaliana. Mol Biotechnol 2012, 51, 160–173. [Google Scholar] [CrossRef]
- Pei, H.; Ma, N.; Tian, J.; Luo, J.; Chen, J.; Li, J.; Zheng, Y.; Chen, X.; Fei, Z.; Gao, J. An NAC Transcription Factor Controls Ethylene-Regulated Cell Expansion in Flower Petals. Plant Physiol 2013, 163, 775. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Yu, Y.; Quan, R.; Zhang, Z.; Zhang, H.; Huang, R. The Ethylene Response Factor AtERF11 That Is Transcriptionally Modulated by the BZIP Transcription Factor HY5 Is a Crucial Repressor for Ethylene Biosynthesis in Arabidopsis. Plant J 2011, 68, 88–99. [Google Scholar] [CrossRef]
- Alessio, V.M.; Cavaçana, N.; Dantas, L.L.D.B.; Lee, N.; Hotta, C.T.; Imaizumi, T.; Menossi, M. The FBH Family of BHLH Transcription Factors Controls ACC Synthase Expression in Sugarcane. J Exp Bot 2018, 69, 2511–2525. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, X.; Wang, Y.; Sun, M.; Zhao, P.; Wang, Q.; Yang, B.; Li, J.; Jiang, Y.Q. WRKY29 Transcription Factor Regulates Ethylene Biosynthesis and Response in Arabidopsis. Plant Physiol Biochem 2023, 194, 134–145. [Google Scholar] [CrossRef] [PubMed]
- van Es, S.W.; Silveira, S.R.; Rocha, D.I.; Bimbo, A.; Martinelli, A.P.; Dornelas, M.C.; Angenent, G.C.; Immink, R.G.H. Novel Functions of the Arabidopsis Transcription Factor TCP5 in Petal Development and Ethylene Biosynthesis. Plant J 2018, 94, 867–879. [Google Scholar] [CrossRef]
- Ohta, M.; Matsui, K.; Hiratsu, K.; Shinshi, H.; Ohme-Takagi, M. Repression Domains of Class II ERF Transcriptional Repressors Share an Essential Motif for Active Repression. Plant Cell 2001, 13, 1959. [Google Scholar] [CrossRef]
- Hamant, O.; Nogué, F.; Belles-Boix, E.; Jublot, D.; Grandjean, O.; Traas, J.; Pautot, V. The KNAT2 Homeodomain Protein Interacts with Ethylene and Cytokinin Signaling. Plant Physiol 2002, 130, 657. [Google Scholar] [CrossRef]
- Brian, L.; Warren, B.; McAtee, P.; Rodrigues, J.; Nieuwenhuizen, N.; Pasha, A.; David, K.M.; Richardson, A.; Provart, N.J.; Allan, A.C.; et al. A Gene Expression Atlas for Kiwifruit (Actinidia Chinensis) and Network Analysis of Transcription Factors. BMC Plant Biol 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Trebitsh, T.; Rudich, J.; Riov, J. Auxin, Biosynthesis of Ethylene and Sex Expression in Cucumber (Cucumis Sativus). Plant Growth Regul 1987, 5, 105–113. [Google Scholar] [CrossRef]
- Shannon, S.; De La Guardia, M.D. Sex Expression and the Production of Ethylene Induced by Auxin in the Cucumber (Cucumis Sativum L.). Natur 1969, 223, 186. [Google Scholar] [CrossRef]
- He, X.J.; Mu, R.L.; Cao, W.H.; Zhang, Z.G.; Zhang, J.S.; Chen, S.Y. AtNAC2, a Transcription Factor Downstream of Ethylene and Auxin Signaling Pathways, Is Involved in Salt Stress Response and Lateral Root Development. Plant J 2005, 44, 903–916. [Google Scholar] [CrossRef]
- Reyes-Olalde, J.I.; Zúñiga-Mayo, V.M.; Serwatowska, J.; Chavez Montes, R.A.; Lozano-Sotomayor, P.; Herrera-Ubaldo, H.; Gonzalez-Aguilera, K.L.; Ballester, P.; Ripoll, J.J.; Ezquer, I.; et al. The BHLH Transcription Factor SPATULA Enables Cytokinin Signaling, and Both Activate Auxin Biosynthesis and Transport Genes at the Medial Domain of the Gynoecium. PLoS Genet 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Wu, M.F.; Winter, C.M.; Wagner, D. LEAFY and Polar Auxin Transport Coordinately Regulate Arabidopsis Flower Development. Plants 2014, 3, 251. [Google Scholar] [CrossRef] [PubMed]
- Shanks, C.M.; Hecker, A.; Cheng, C.; Brand, L.; Collani, S.; Schmid, M.; Schaller, G.E.; Wanke, D.; Harter, K.; Kieber, J.J. Role of BASIC PENTACYSTEINE Transcription Factors in a Subset of Cytokinin Signaling Responses. Plant J 2018, 95, 458–473. [Google Scholar] [CrossRef]
- Theune, M.L.; Bloss, U.; Brand, L.H.; Ladwig, F.; Wanke, D. Phylogenetic Analyses and GAGA-Motif Binding Studies of BBR/BPC Proteins Lend to Clues in GAGA-MOTIF Recognition and a Regulatory Role in Brassinosteroid Signaling. Front Plant Sci 2019, 10, 466. [Google Scholar] [CrossRef]
- Yin, T.; Quinn, J.A. Tests of a Mechanistic Model of One Hormone Regulating Both Sexes in Cucumis Sativus (Cucurbitaceae). Am J Bot 1995, 82, 1537–1546. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, G.; Li, Y.; Mo, N.; Zhang, J.; Liang, Y. Transcriptomic Analysis Implies That GA Regulates Sex Expression via Ethylene-Dependent and Ethylene-Independent Pathways in Cucumber (Cucumis Sativus L.). Front Plant Sci 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Winter, C.M.; Wu, M.F.; Kanno, Y.; Yamaguchi, A.; Seo, M.; Wagner, D. Gibberellin Acts Positively Then Negatively to Control Onset of Flower Formation in Arabidopsis. Science 2014, 344, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ma, Y.; Li, J. The Rice YABBY4 Gene Regulates Plant Growth and Development through Modulating the Gibberellin Pathway. J Exp Bot 2016, 67, 5545–5556. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Zhao, Y.; Ma, Q.; Hu, Y.; Hedden, P.; Zhang, Q.; Zhou, D.X. The Rice YABBY1 Gene Is Involved in the Feedback Regulation of Gibberellin Metabolism. Plant Physiol 2007, 144, 121. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Liu, B.; Wang, W.; Liu, X.; Chen, C.; Liu, X.; Yang, S.; Ren, H. A GAMYB Homologue CsGAMYB1 Regulates Sex Expression of Cucumber via an Ethylene-Independent Pathway. J Exp Bot 2014, 65, 3201. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Xing, L.; Zhang, C.; Chen, H.; Li, Y.; Zhang, D.; Ma, J.; Zhao, C.; Han, M.; Ren, X.; et al. MdKNOX15, a Class I Knotted-like Transcription Factor of Apple, Controls Flowering and Plant Height by Regulating GA Levels through Promoting the MdGA2ox7 Transcription. Environ Exp Bot 2021, 185. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, X.; Liu, S.; Lin, W.; Li, Y.; Zhang, X. Genome-Wide Analysis of AP2/ERF Transcription Factors in Pineapple Reveals Functional Divergence during Flowering Induction Mediated by Ethylene and Floral Organ Development. Genomics 2021, 113, 474–489. [Google Scholar] [CrossRef]
- Lin, R.C.; Park, H.J.; Wang, H.Y. Role of Arabidopsis RAP2.4 in Regulating Light- and Ethylene-Mediated Developmental Processes and Drought Stress Tolerance. Mol Plant 2008, 1, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhao, L.; Chong, K.; Wang, T. Overexpression of OsERF1, a Novel Rice ERF Gene, up-Regulates Ethylene-Responsive Genes Expression besides Affects Growth and Development in Arabidopsis. J Plant Physiol 2008, 165, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Niu, H.; Wang, Z.; Zhang, W.; Wang, H.; Wang, S.; Zhang, X.; Li, Z. Ethylene Responsive Factor ERF110 Mediates Ethylene-Regulated Transcription of a Sex Determination-Related Orthologous Gene in Two Cucumis Species. J Exp Bot 2018, 69, 2953–2965. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Luo, Y.; Zhang, Z.; Xu, M.; Wang, W.; Zhao, Y.; Zhang, L.; Fan, Y.; Wang, L. ZmSOC1, an MADS-Box Transcription Factor from Zea Mays, Promotes Flowering in Arabidopsis. Int J Mol Sci 2014, 15, 19987. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhuo, S.; Liu, X.; Che, G.; Wang, Z.; Gu, R.; Shen, J.; Song, W.; Zhou, Z.; Han, D.; et al. The MADS-Box Gene CsSHP Participates in Fruit Maturation and Floral Organ Development in Cucumber. Front Plant Sci 2020, 10, 1781. [Google Scholar] [CrossRef]
- Sharma, N.; Xin, R.; Kim, D.H.; Sung, S.; Lange, T.; Huq, E. NO FLOWERING IN SHORT DAY (NFL) Is a BHLH Transcription Factor That Promotes Flowering Specifically under Short-Day Conditions in Arabidopsis. Development (Cambridge) 2016, 143, 682–690. [Google Scholar] [CrossRef]
- Ye, Y.; Xin, H.; Gu, X.; Ma, J.; Li, L. Genome-Wide Identification and Functional Analysis of the Basic Helix-Loop-Helix (Bhlh) Transcription Family Reveals Candidate Ptfbh Genes Involved in the Flowering Process of Populus Trichocarpa. Forests 2021, 12, 1439. [Google Scholar] [CrossRef]
- Li, X.; Tian, X.; He, M.; Liu, X.; Li, Z.; Tang, J.; Mei, E.; Xu, M.; Liu, Y.; Wang, Z.; et al. BZIP71 Delays Flowering by Suppressing Ehd1 Expression in Rice. J Integr Plant Biol 2022, 64, 1352–1363. [Google Scholar] [CrossRef]
- Pei, H.; Ma, N.; Tian, J.; Luo, J.; Chen, J.; Li, J.; Zheng, Y.; Chen, X.; Fei, Z.; Gao, J. An NAC Transcription Factor Controls Ethylene-Regulated Cell Expansion in Flower Petals. Plant Physiol 2013, 163, 775. [Google Scholar] [CrossRef]
- Hendelman, A.; Stav, R.; Zemach, H.; Arazi, T. The Tomato NAC Transcription Factor SlNAM2 Is Involved in Flower-Boundary Morphogenesis. J Exp Bot 2013, 64, 5497–5507. [Google Scholar] [CrossRef]
- Guo, S.; Dai, S.; Singh, P.K.; Wang, H.; Wang, Y.; Tan, J.L.H.; Wee, W.; Ito, T. A Membrane-Bound NAC-like Transcription Factor OsNTL5 Represses the Flowering in Oryza Sativa. Front Plant Sci 2018, 9, 555. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, G.; Jia, J.; Zhao, G.; Xia, C.; Zhang, L.; Li, F.; Zhang, Q.; Dong, C.; Gao, S.; et al. The Wheat MYB-Related Transcription Factor TaMYB72 Promotes Flowering in Rice. J Integr Plant Biol 2016, 58, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kobayashi, Y.; Yamamoto, S.; Daimon, Y.; Yamaguchi, A.; Ikeda, Y.; Ichinoki, H.; Notaguchi, M.; Goto, K.; Araki, T. FD, a BZIP Protein Mediating Signals from the Floral Pathway Integrator FT at the Shoot Apex. Science 2005, 309, 1052–1056. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Z.; Wang, L.; Kim, S.G.; Seo, P.J.; Qiao, M.; Wang, N.; Li, S.; Cao, X.; Park, C.M.; et al. WRKY71 Accelerates Flowering via the Direct Activation of FLOWERING LOCUS T and LEAFY in Arabidopsis Thaliana. Plant J 2016, 85, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Li, N.; Su, D.; Chen, J.; Li, Z. Overexpression of a Basic Helix-Loop-Helix Transcription Factor Gene, SlbHLH22, Promotes Early Flowering and Accelerates Fruit Ripening in Tomato (Solanum Lycopersicum L.). Planta 2019, 250, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Verhoeff, N.I.; Chen, Z.; Chen, S.; Wang, M.; Zhu, Z.; Ouwerkerk, P.B.F. Functions of OsDof25 in Regulation of OsC4PPDK. Plant Mol Biol 2015, 89, 229. [Google Scholar] [CrossRef] [PubMed]
- Noguero, M.; Atif, R.M.; Ochatt, S.; Thompson, R.D. The Role of the DNA-Binding One Zinc Finger (DOF) Transcription Factor Family in Plants. Plant Sci 2013, 209, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Lorrai, R.; Gandolfi, F.; Boccaccini, A.; Ruta, V.; Possenti, M.; Tramontano, A.; Costantino, P.; Lepore, R.; Vittorioso, P. Genome-Wide RNA-Seq Analysis Indicates That the DAG1 Transcription Factor Promotes Hypocotyl Elongation Acting on ABA, Ethylene and Auxin Signaling. Sci Rep 2018, 8. [Google Scholar] [CrossRef]
- Rojas-Gracia, P.; Roque, E.; Medina, M.; López-Martín, M.J.; Cañas, L.A.; Beltrán, J.P.; Gómez-Mena, C. The DOF Transcription Factor Sldof10 Regulates Vascular Tissue Formation during Ovary Development in Tomato. Front Plant Sci 2019, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, K.; Park, C.; Hwang, H.J.; Lee, I. SUPPRESSOR OF FRIGIDA4, Encoding a C2H2-Type Zinc Finger Protein, Represses Flowering by Transcriptional Activation of Arabidopsis FLOWERING LOCUS C. Plant Cell 2006, 18, 2985–2998. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; You, C.; Li, C.; Long, T.; Chen, G.; Byrne, M.E.; Zhang, Q. RID1, Encoding a Cys2/His2-Type Zinc Finger Transcription Factor, Acts as a Master Switch from Vegetative to Floral Development in Rice. Proc Natl Acad Sci U S A 2008, 105, 12915–12920. [Google Scholar] [CrossRef] [PubMed]
- Han, Y. chao; Fu, C. chun; Kuang, J. fei; Chen, J. ye; Lu, W. jin Two Banana Fruit Ripening-Related C2H2 Zinc Finger Proteins Are Transcriptional Repressors of Ethylene Biosynthetic Genes. Postharvest Biol Technol 2016, 116, 8–15. [Google Scholar] [CrossRef]
- Huang, R.; Liu, D.; Huang, M.; Ma, J.; Li, Z.; Li, M.; Sui, S. CpWRKY71, a WRKY Transcription Factor Gene of Wintersweet (Chimonanthus Praecox), Promotes Flowering and Leaf Senescence in Arabidopsis. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ning, K.; Che, G.; Yan, S.; Han, L.; Gu, R.; Li, Z.; Weng, Y.; Zhang, X. CsSPL Functions as an Adaptor between HD-ZIP III and CsWUS Transcription Factors Regulating Anther and Ovule Development in Cucumis Sativus (Cucumber). Plant J 2018, 94, 535–547. [Google Scholar] [CrossRef]
- Unte, U.S.; Sorensen, A.M.; Pesaresi, P.; Gandikota, M.; Leister, D.; Saedler, H.; Huijser, P. SPL8, an SBP-Box Gene That Affects Pollen Sac Development in Arabidopsis. Plant Cell 2003, 15, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.K.; Skinner, D.J.; Gallagher, T.L.; Gasser, C.S. Integument Development in Arabidopsis Depends on Interaction of YABBY Protein INNER NO OUTER with Coactivators and Corepressors. Genetics 2017, 207, 1489–1500. [Google Scholar] [CrossRef]
- Jang, S.; Hur, J.; Kim, S.J.; Han, M.J.; Kim, S.R.; An, G. Ectopic Expression of OsYAB1 Causes Extra Stamens and Carpels in Rice. Plant Mol Biol 2004, 56, 133–143. [Google Scholar] [CrossRef]
- Gross, T.; Broholm, S.; Becker, A. CRABS CLAW Acts as a Bifunctional Transcription Factor in Flower Development. Front Plant Sci 2018, 9, 835. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Jeong, C.W.; Nole-Wilson, S.; Krizek, B.A.; Wagner, D. AINTEGUMENTA and AINTEGUMENTA-LIKE6/PLETHORA3 Induce LEAFY Expression in Response to Auxin to Promote the Onset of Flower Formation in Arabidopsis. Plant Physiol 2016, 170, 283–293. [Google Scholar] [CrossRef]
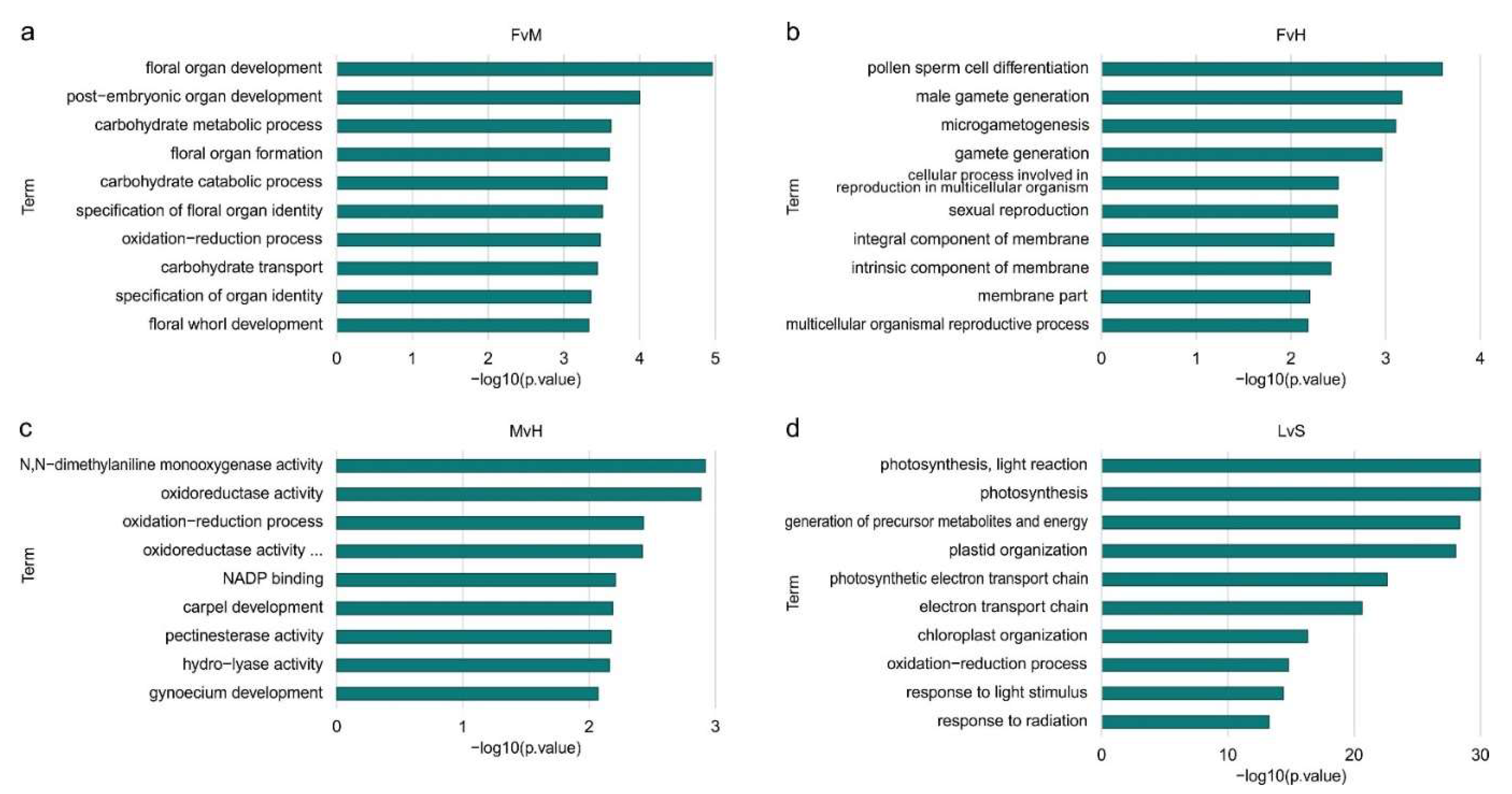
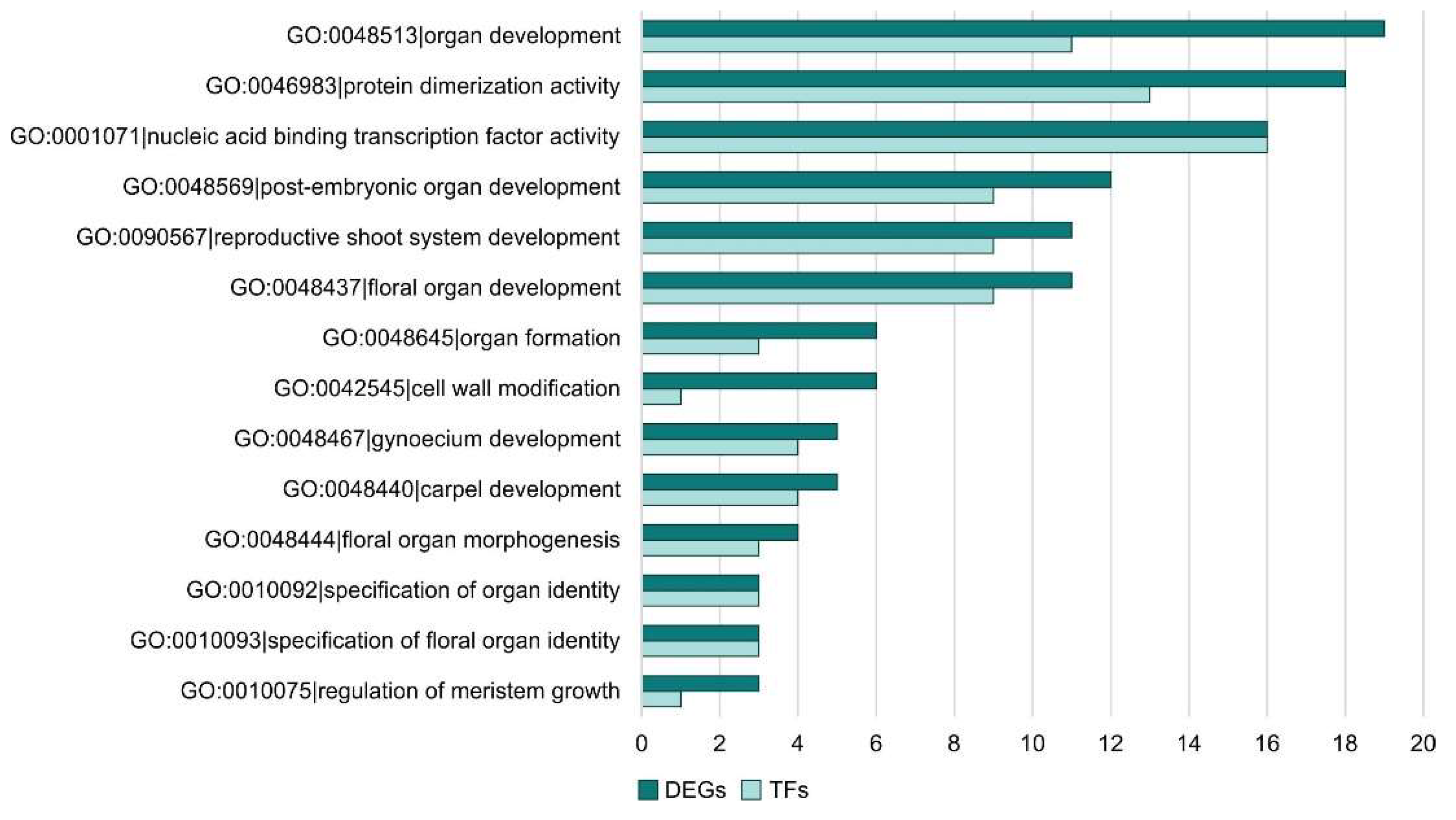
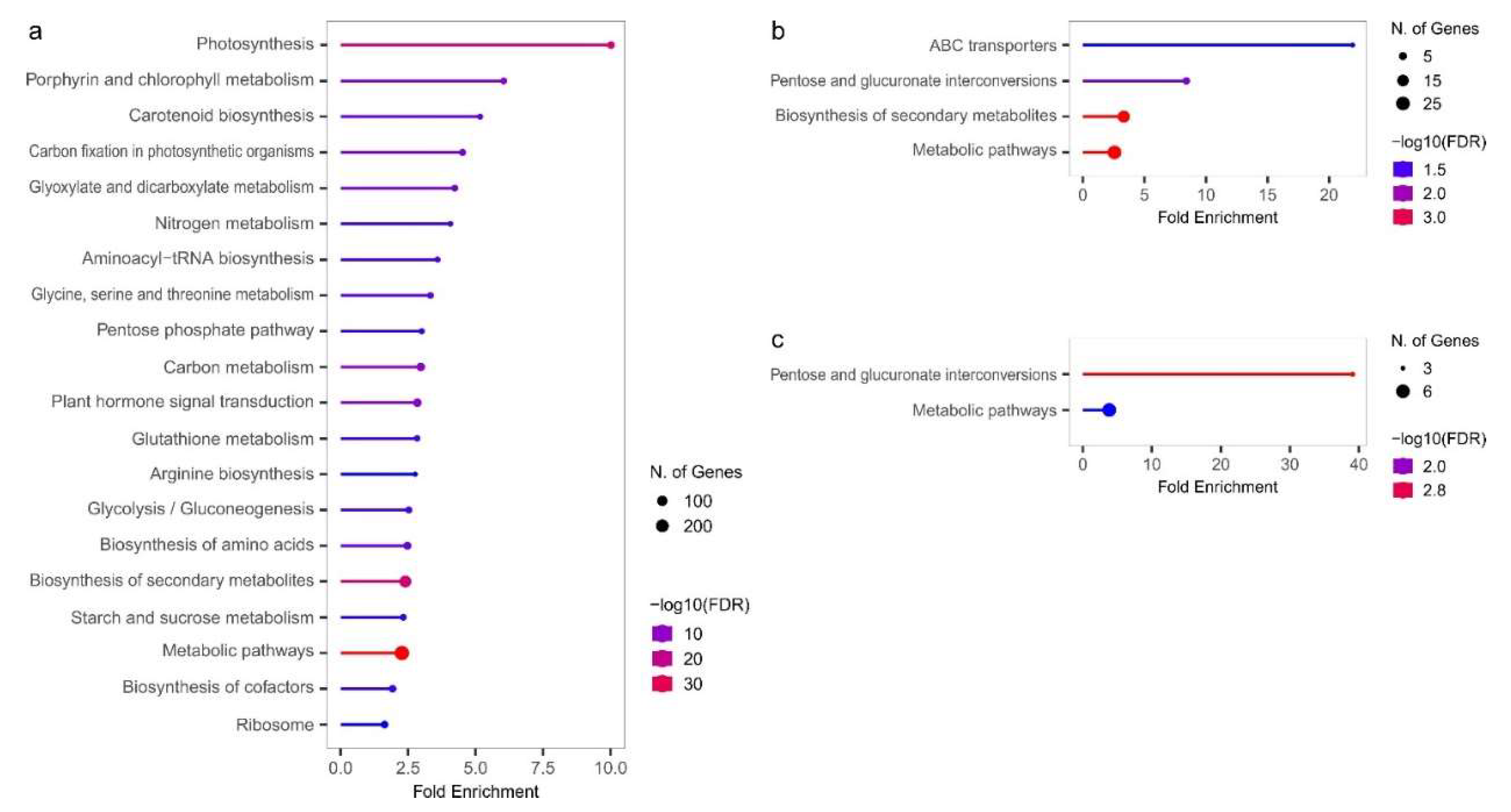
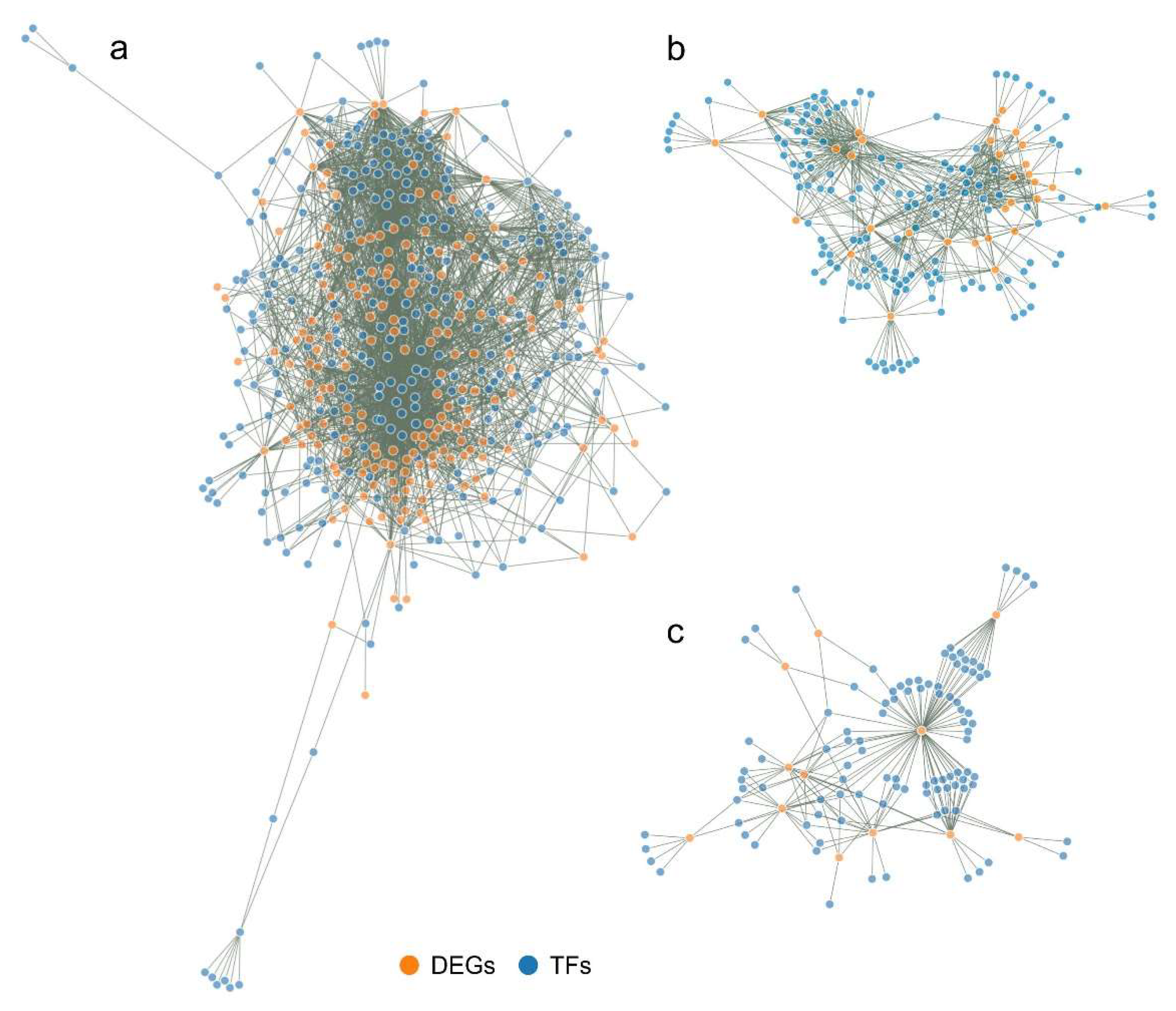
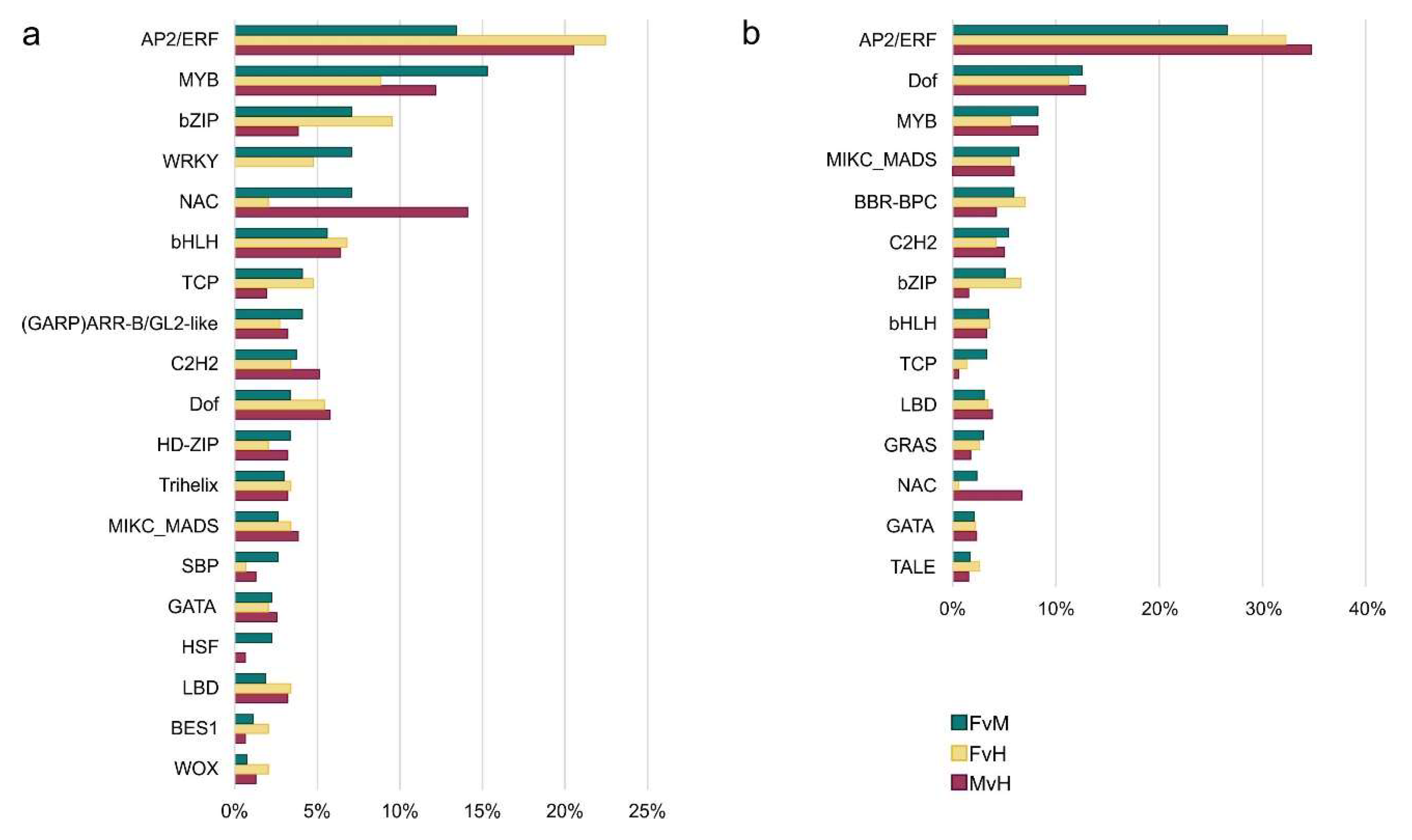
| Comparison | Number of DEGs | Number of TFs found for the comparison: | % of all DEGs |
|---|---|---|---|
| LvS | 2852 | 248 | 8,70% |
| FvM | 260 | 28 | 10,77% |
| FvH | 36 | 2 | 5,88% |
| MvH | 55 | 6 | 10,91% |
| FvM | FvH | MvH | LvS | |
|---|---|---|---|---|
| DEG | 186 | 31 | 35 | 244 |
| TF | 282 | 147 | 156 | 2785 |
| Comparison | Transcription factor | No. of edges |
|---|---|---|
| FvM | Cucsa.362960|MIKC_MADS|MADS box transcription factor | 116 |
| Cucsa.277740|AP2|AP2-like ethylene-responsive transcription factor | 109 | |
| Cucsa.026600|BBR-BPC|GAGA-binding transcriptional activator | 93 | |
| Cucsa.307870|C2H2|Transcription factor IIIA | 82 | |
| Cucsa.102120|Dof|Dof zinc finger protein | 81 | |
| Cucsa.280310|GRAS|DELLA protein GAI | 81 | |
| Cucsa.213830|Dof|Dof zinc finger protein | 76 | |
| Cucsa.159750|BBR-BPC|GAGA-binding transcriptional activator | 69 | |
| Cucsa.341290|Dof|Dof zinc finger protein | 60 | |
| Cucsa.098430|Dof|Dof zinc finger protein | 48 | |
| FvH | Cucsa.362960|MIKC_MADS|MADS box transcription factor | 23 |
| Cucsa.026600|BBR-BPC|GAGA-binding transcriptional activator | 21 | |
| Cucsa.277740|AP2|AP2-like ethylene-responsive transcription factor | 19 | |
| Cucsa.307870|C2H2|Transcription factor IIIA | 17 | |
| Cucsa.159750|BBR-BPC|GAGA-binding transcriptional activator | 14 | |
| Cucsa.280310|GRAS|DELLA protein GAI | 13 | |
| Cucsa.353140|TALE|Homeobox protein knotted-1-like 1 | 13 | |
| Cucsa.102120|Dof|Dof zinc finger protein | 12 | |
| Cucsa.213830|Dof|Dof zinc finger protein | 12 | |
| Cucsa.098430|Dof|Dof zinc finger protein | 9 | |
| MvH | Cucsa.362960|MIKC_MADS|MADS box transcription factor | 21 |
| Cucsa.102120|Dof|Dof zinc finger protein | 17 | |
| Cucsa.277740|AP2|AP2-like ethylene-responsive transcription factor | 16 | |
| Cucsa.213830|Dof|Dof zinc finger protein | 12 | |
| Cucsa.307870|C2H2|Transcription factor IIIA | 12 | |
| Cucsa.341290|Dof|Dof zinc finger protein | 12 | |
| Cucsa.026600|BBR-BPC|GAGA-binding transcriptional activator | 11 | |
| Cucsa.159750|BBR-BPC|GAGA-binding transcriptional activator | 11 | |
| Cucsa.136780|ERF|Dehydration responsive element binding transcription factor | 9 | |
| Cucsa.237150|ERF|Ethylene-responsive transcription factor ERF021 | 9 | |
| LvS | Cucsa.362960|MIKC_MADS|MADS box transcription factor | 1579 |
| Cucsa.277740|AP2|AP2-like ethylene-responsive transcription factor | 1485 | |
| Cucsa.026600|BBR-BPC|GAGA-binding transcriptional activator | 1324 | |
| Cucsa.307870|C2H2|Transcription factor IIIA | 1205 | |
| Cucsa.280310|GRAS|DELLA protein GAI | 1090 | |
| Cucsa.102120|Dof|Dof zinc finger protein | 1076 | |
| Cucsa.213830|Dof|Dof zinc finger protein | 1031 | |
| Cucsa.159750|BBR-BPC|GAGA-binding transcriptional activator | 1000 | |
| Cucsa.353140|TALE|Homeobox protein knotted-1-like 1 | 920 | |
| Cucsa.341290|Dof|Dof zinc finger protein | 823 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





