1. Introduction
Lithium-ion batteries (LIBs) are widely used in different kinds of technology as an energy storage source, such as handheld devices (e.g., smartphones, tablets) or electric vehicles (EVs) [
1]. Their wide applicability is due to their superior electrical performance, such as high energy density, long life cycle, and no memory effect and their lighter weight when compared to other types of batteries technology (e.g., lead-acid, nickel-metal hydride, and nickel-cadmium) [
2]. The LIBs concept was proposed by different researchers in the 1970s [
3]. Many innovations had been attributed to Yoshino for the development of rechargeable lithium-ion batteries, who registered the first patent [
4].
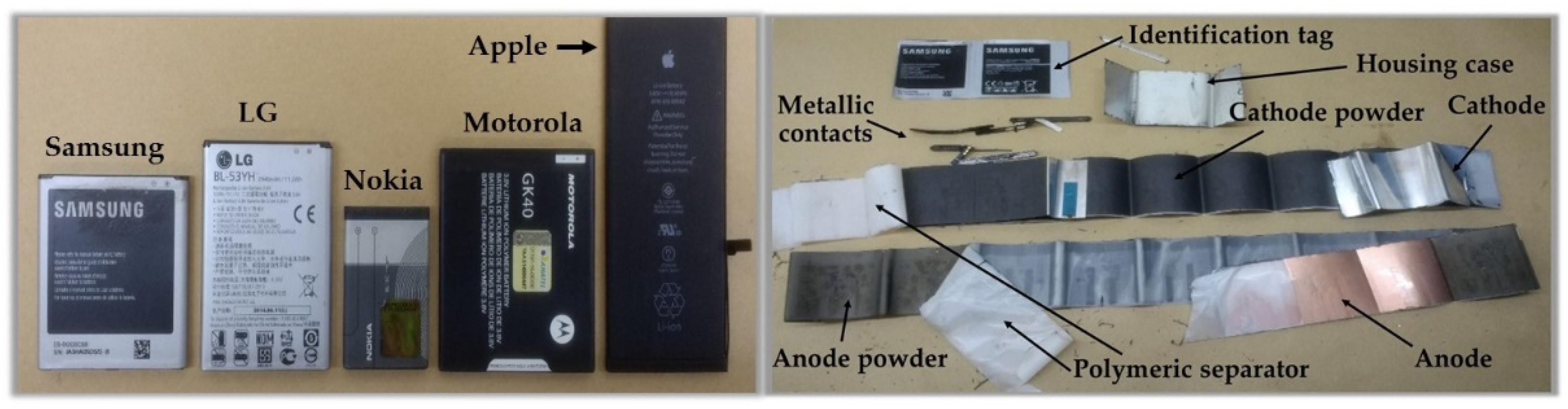
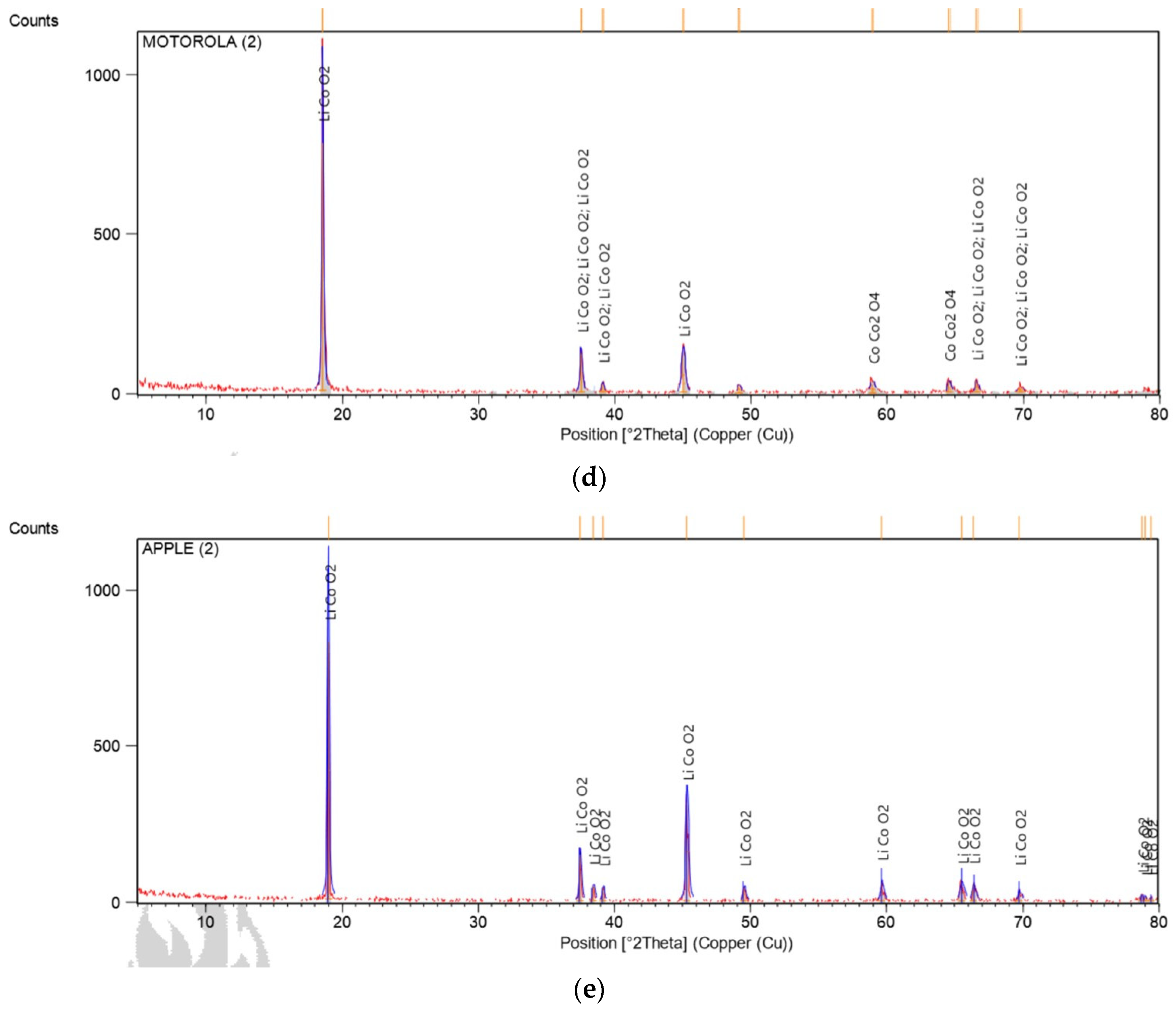
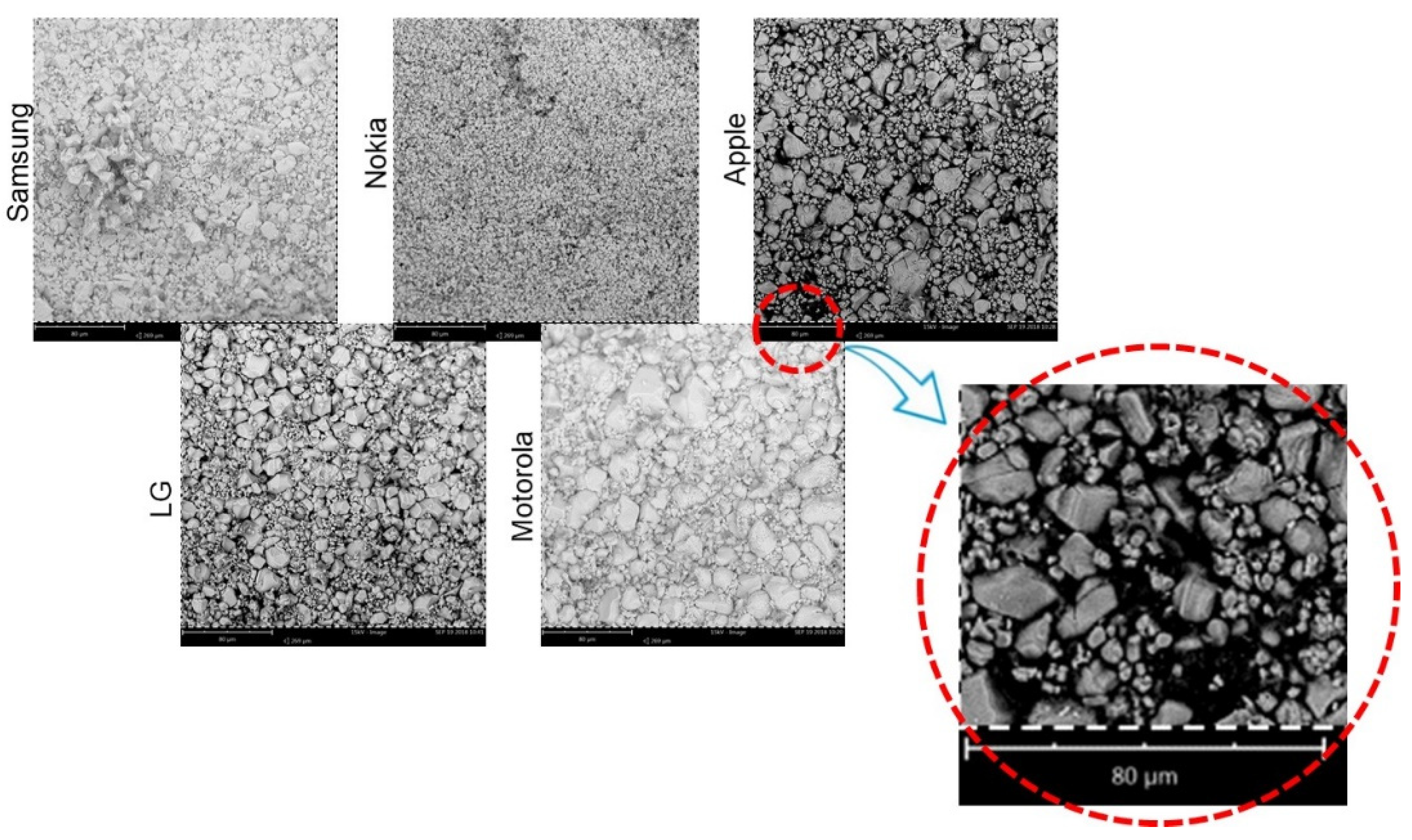
The lithium-ion batteries are composed of cathode – transition metal powders such as cobalt, manganese, and nickel are usually used –, graphite anode, a porous polymeric membrane, which can allow the electrons to flow during charge and discharge processes, and an electrolyte – means by which the flow of electrons occurs. This electron flow is generated by the movement of lithium ions between the cathodic and anodic powders. The cathode and anode collectors are composed of aluminum and copper, respectively. Polyvinylidene fluoride (PVDF) is a polymer used as a binder to adhere cathode (lithium-cobalt oxide) and anode (graphite) powders into the support sheets [
5,
6], which can be a major obstacle to the efficiency improvement of hydrometallurgical routes aiming to recover lithium and cobalt for recycling.
The largest lithium producers in 2020 were Australia and Chile, responsible for 48.1%and 26.0% of the total lithium production, respectively. Among the various industrial applications, 74% of lithium is destined for the manufacture of batteries and 14% for the ceramic and glass industry [
7]. Regarding cobalt, the majority of the world production in 2020 was attributed to the Democratic Republic of Congo with 68.9%, while Russia and Australia were responsible for 6.3% and 4.0%, respectively [
7]. Around 46% of the cobalt produced in 2018 was used in the manufacture of batteries, and according to a report by the German Mineral Resources Agency (DERA)[
8], this demand is due to the intense EV development.
When LIBs are discarded, alone or together with some equipment (e.g., smartphone, notebook, tablet, GPS, etc), they are denominated as Waste from Electrical and Electronic Equipment (WEEE). In general, the WEEEs contain about sixty metals, such as copper, cobalt, gold, platinum, lithium, silver, palladium, etc. Developing efficient methodologies for recovering these metals, in addition to avoiding the cost of extracting them from the ore, would a more environmentally favorable bias by reducing the impact of mining and by reducing the pollution due to incorrect destination given to WEEEs [
9,
10]. The useful life of LIBs, used as a power source for smartphones, is about 2 years or 300 to 500 cycles [
6,
7], and represents a significant contribution to the generation of WEEEs [
5]. In case of incorrect disposal, after their useful life cycle, LIBs can cause harmful environmental impact due to the materials they are made of, moreover, an important source of raw material for the recovery of metals with economic added value [
13]. Pre-treatment processes, hydrometallurgy, and pyrometallurgy are the most extensively studied/employed kinds of recycling processes [
14].
There is no single definition of pretreatment for recycling LIBs. Some authors [
15] [
16] [
17] usually divide it into mechanical separation, mechanical-chemical process, thermal treatment, and dissolution process. The pretreatment was divided by Yao
et al. [
18] into unloading, disassembly, and cathodic material separation, while Zhang
et al. [
19] specified such as manual treatment, disassembly and classification, comminution (mechanical treatment), sieving, separation, and mechanical-chemical treatment. They are widely used as a previously step in hydrometallurgy to improve the yelds recovering.
Hydrometallurgical processes consist of dissolving metals in acidic or basic leaching solutions to extract them from the waste. The most consolidated methodologies use strong inorganic acids as leaching agents (e.g., HNO
3, H
2SO
4, and HCl). These processes are not considered environmentally friendly, as they release vapors and gases (NOx, SO
3, and Cl
2) and the solutions can permeate the soil when poorly managed or in cases of accidents, , causing damages to water resources and biodiversity, including human beings [
20]. Alternative proposals to replace inorganic acids for organic acids, which are less harmful to the environment, have been studied. Musariri
et al. used citric acid (C
6H
8O
7) and DL-malic acid (C
4H
6O
5), both 1.5 M, with the addition of H
2O
2 2% v/v as an oxidizing agent, and temperature of 95 °C. Citric acid was the most efficient agent, and dissolved up to 95% of lithium and Cobalt [
21]. With an aqueous mixture of citric and ascorbic acid (C
6H
8O
6), Nayaka
et al.[
22] leached obsolete LIBs. Copper and Lithium were obtained in the form of cobalt oxalate and lithium fluoride by selective precipitation, and the addition of oxalic acid (C
2H
2O
4) and ammonium fluoride, respectively.
The presence of H
2O
2 in the leaching process for some metals results in valence decrease, such as Co
3+, which becomes more soluble Co
2+. Some studies corroborate that the presence of an oxidizing agent improves the performance of metal recovery, for both organic and inorganic acids [
23]. In their work, Sattar
et al. carried out leaching with 3M H
2SO
4 at 90 °C and recovered 92% of Li, 68% of Co, and 34.8% of Mn without adding H
2O
2. After the addition of 4% v/v H
2O
2, the metal leaching efficiency increased by more than 98% [
24].
In pyrometallurgy, the use of furnaces at high temperatures aims to reduce the oxides to a metallic alloy. Gases and slag also result from this process. In recycling LIBs, the great advantage to perform a pyrometallurgical process is that, in addition to being processed in a single batch, it does not require pre-treatment to concentrate the material, as is usually carried out in hydrometallurgy [
19] [
25]. LIBs recycling operations, on an industrial scale, are more common via pyrometallurgy. The main plants are located in North America, Europe, and Asia. Umicore has two pyrometallurgical processing plants in Belgium and China with a capacity of 7000 t/year and 5000 t/year, respectively, while Retriev has a plant using the hydrometallurgy process in the USA/Canada, with a capacity of 4500 t/year. All pyrometallurgical routes adopt high temperatures, each one according to specific process parameters.
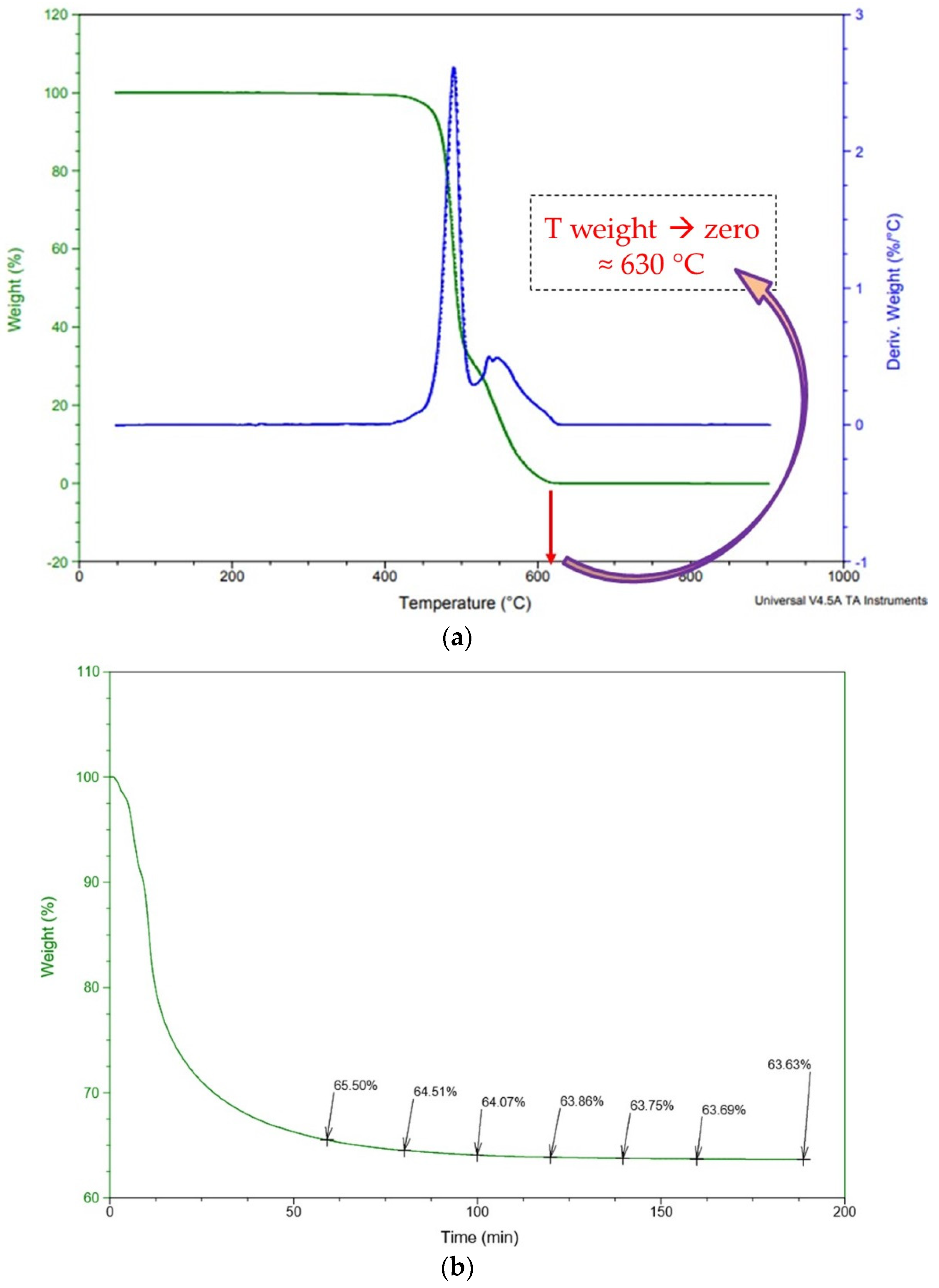
This work aimed to develop a hybrid route, mixing a heat pretreatment step – around 650 °C, not so high as pyrometallurgy because it intended only to decompose the PVDF binder – and a following hydrometallurgical step that should be more environmentally favorable due to the use of DL-malic acid 1.5 M instead of an inorganic acid. The hydrometallurgical process with 2 M sulfuric acid was used as a control. The addition of hydrogen peroxide H
2O
2 10% v/v as an oxidizing agent was also evaluated – an optimized condition according to the work of Dutta
et al. [
26]. This hybrid condition makes this route innovative, as the vast majority of studies focus either on hydrometallurgy or pyrometallurgy exclusively.