1. Introduction
In the last decade, blood flow-restricted training (BFR) has increased its popularity not only among gym centers, but also in the medical field.[
1] It is based on the combination of impaired blood flow and various exercises and sports such as walking, jogging, cycling, resistance exercises, or even yoga practice.[
2,
3,
4,
5,
6] Originating from the 7,8 Kaatsu technique, it is constantly modified using multiple tools to induce blood flow restriction, such as elastic bands, pressure cuffs, tourniquets, and dedicated instruments such as Kaatsu Master or Vasper devices.[
7,
8] During BFR, muscle mass and strength increase faster than in regular exercises.[
9] It is also often more suitable for people unable to perform full-intensity sports activities due to movement limitations, as BFR seems more efficient and effective.[
10]
Physical activity is one of the most crucial factors influencing the human vascular system. It leads to lower resting heart rate and blood pressure, as well as an increase in blood oxygenation, improved endothelial functions, and stimulation of the production of proangiogenic factors, improving peripheral vascular flow. [
11,
12] Those phenomena are widely used in the rehabilitation of patients after myocardial infarction or ischemic stroke.[
13] Furthermore, walking training is one of the elements of non-invasive treatment in patients with intermittent claudication, accompanied by lifestyle changes and pharmacotherapy[
14].
Despite growing evidence of the significant influence of BFR training on different body functions, its impact on the vascular system, especially the arteries, is a matter of controversy. [
15]
Therefore, the aim of our study was to analyze to date published study results and answer scientific questions. Does and how BFR exercise, compared to other types of exercise without blood flow restriction or other active intervention, influence the vascular endothelium in adults? How does BFR exercise influence angiogenesis in adults compared to different types of exercise without blood flow restriction or other active intervention?
2. Materials and Methods
2.1. Search strategy
The protocol for this study was registered in PROSPERO (CRD42020222257). It has been carried out and reported according to the PRISMA Statement.[
16] We have searched for three main online databases: Cochrane Database of Systematic Reviews, PubMed, and Embase. Experimental studies published from January 2000 up to November 2022 that met inclusion criteria were searched, identified, and included in our analysis. The Cochrane database was searched for similar systematic reviews and for reviews to check their reference lists. We use MeSH terms and Emtree terms related to restricted blood flow training in PubMed and Embase, respectively. The detailed search strategy is presented in Appendix 1. In case MeSH Terms or Emtree were not available, the "All fields search" option was used.
2.2. Inclusion criteria
We included studies that were original articles published in peer review journals in full text, b) randomized or non-randomized controlled trials or cross-over designed studies with a study group (at least one) performing BFR activity including Kaatsu training compared with other active intervention/treatment (other form of BFR as a comparator was also accepted), c) conducted in adult humans, d) presented results related to arterial functions (at least one parameter): Endothelial functions (Flow-mediated dilatation FMD, intima-media thickness IMT, reactive hyperemia index RHI, vascular stiffness AI / SI / RI, NO), angiogenesis (VEGF, CD31 / PECAM-1, CD106 / VCAM-1, Von Willebrand Factor), Other Vascular Functions (Tcpo2, ABI, TBI, CAVI).
2.3. Screening
All matching references were imported into the bibliographic software – Mendeley.[
17] The duplicates were removed. At the screening, two reviewers independently searched through the titles and abstracts, choosing the eligible records. The third reviewer resolved the disagreements. Analysis based on full text was performed in all articles meeting inclusion criteria or of uncertain significance. Two reviewers performed the full text analysis and an independent reviewer made final decisions in case of disagreement.
2.4. Data extraction
The data extraction form with categories of information was used to collect data. It included an assessment of the quality and completeness of the data of the included studies (Supplementary Table 1). It was performed by two independent reviewers, while the third reviewer solved any differences between the extracted data. At this point, review articles on the examined topic were also searched for relevant references that could have been omitted during screening.
2.5. Bias risk assessment
The quality of the included studies was assessed based on the Joanna Briggs Institute critical appraisal tools for randomized and non-randomized prospective studies. [
18] The risk of bias was assessed by independent reviewers. In the event of disagreements, there was a discussion and consensus with additional reviewers.
2.6. Statistical analysis
Analyses were performed in R 4.2.2 (R Core Team, Statistical Foundation, Vienna, Austria) using the Metafor and ESC packages. Studies were included if sufficient data were available to calculate the standardized mean change with raw score standardization (SMCR). [
19,
20] Separate meta-analyses were conducted for each pre-defined outcome measure reflecting the acute hemodynamic response. The SMCR was chosen as an effect size measure (Yi) due to a pretest and posttest control group design that is utilized across studies. Yi is calculated as the difference between the SMCR of treatment and control samples, while the sampling variance is added due to group independence.[
21] A conservative estimate for the correlation between measurements was set at 0.7. We used a random-effects modeling approach with a restricted maximum likelihood (REML) estimator.[
22] A comparison was illustrated with a forest plotted based on the standardized mean change with a 95% confidence interval for each outcome. Heterogeneity across studies was evaluated using the I-squared value and Q test. The P-value was considered statistically significant at <0.05.
3. Results
3.1. Study selection
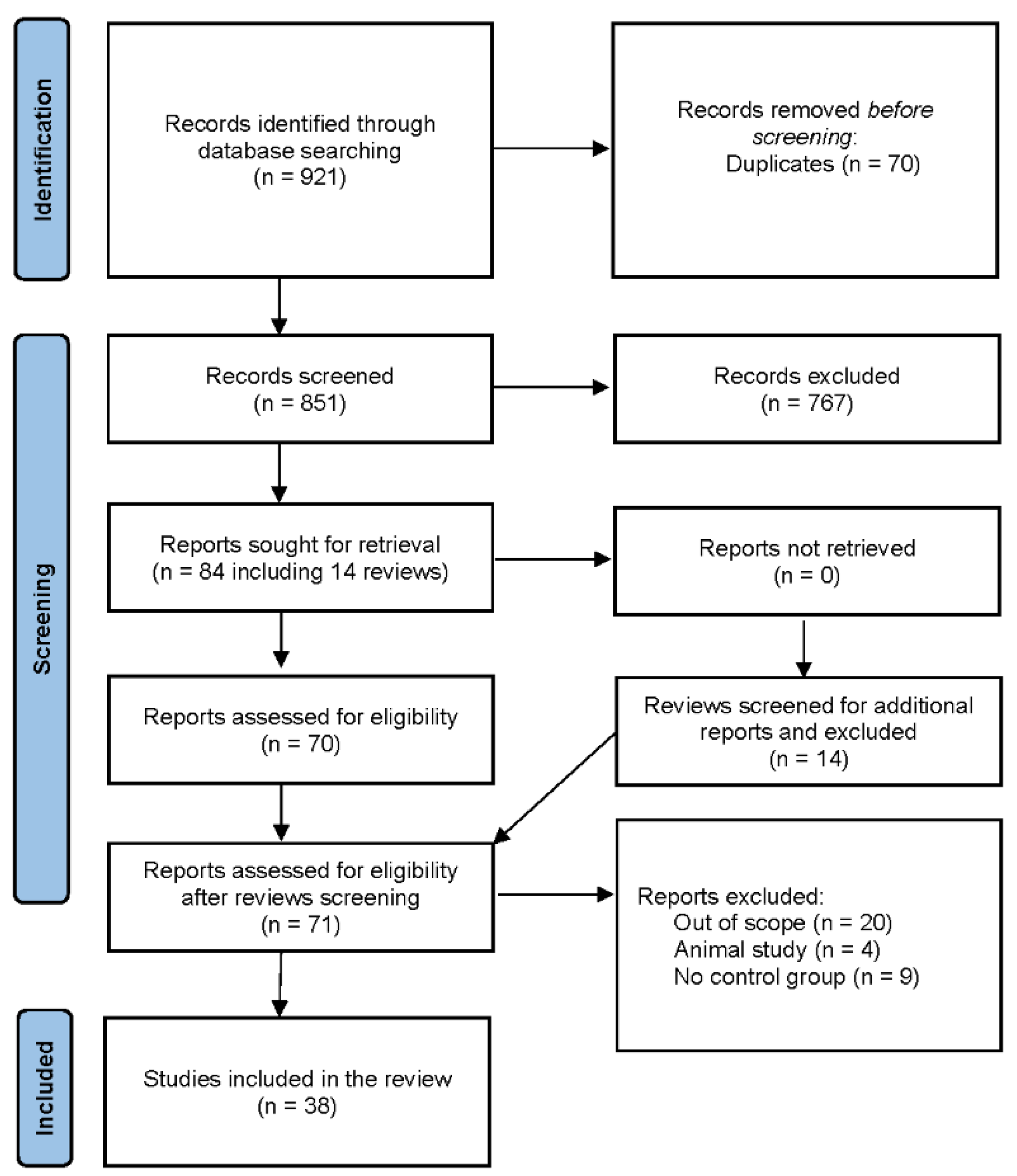
The database search resulted in 921 records found, including 70 duplicates. 851 articles were selected and 84 of them (including 14 review articles) were sought for retrieval. After searching through the reference list of review articles, 1 missing original article was found and 71 articles were evaluated for eligibility. 38 of them were included in the final analysis. (
Figure 1)
3.2. Included studies’ characteristics
The studies were carried out in 12 countries, mainly in the USA (n=13) and Brazil (n = 8) from 2005[
23] until 2022[
24]. Most studies were in a cross-over design (n=21) and RCT (n=13). One prospective non-RCT study and 3 cross-over-like studies that were performed on different extremities but within the same participants were included.
There were significant differences in the type of activities: resistance exercise (n=21), treadmill (n=2), walking (n=2), cycling on an ergometer (n=4), cross-trainer interval exercise (n=1), handgrip (n=5), squats and push-ups (n=1), yoga (n=1). Additionally, the pressure used for blood flow restriction differed between included studies, ranging from 45 mmHg to 220 mmHg, and most cases were equal or above the participant's systolic blood pressure, causing temporary ischemia (n=24). The number of sessions differed, but in 21 studies, the results were obtained based on only one session.
The total number of subjects in all studies included in this systematic review was 658 participants (72% male) mean age of 39.03 (±3.53) years. In most of the studies, the participants did not present any comorbidities, except for 2 studies conducted in female participants with hypertension[
25,
26] and 1 study in patients with coronary arterial disease[
27] (Supplementary Table 1)
3.3. Endothelial functions
3.3.1. Flow-mediated dilatation
14 studies examining FMD changes were identified, but 6 of them were not eligible for statistical analysis due to insufficient data.[
5,
24,
28,
29,
30,
31] In studies excluded from meta-analysis, Maga M. et al.[
24] found FMD increased after BFR, while 2 studies showed a decrease[
28,
30], and the rest did not detect any significant changes.
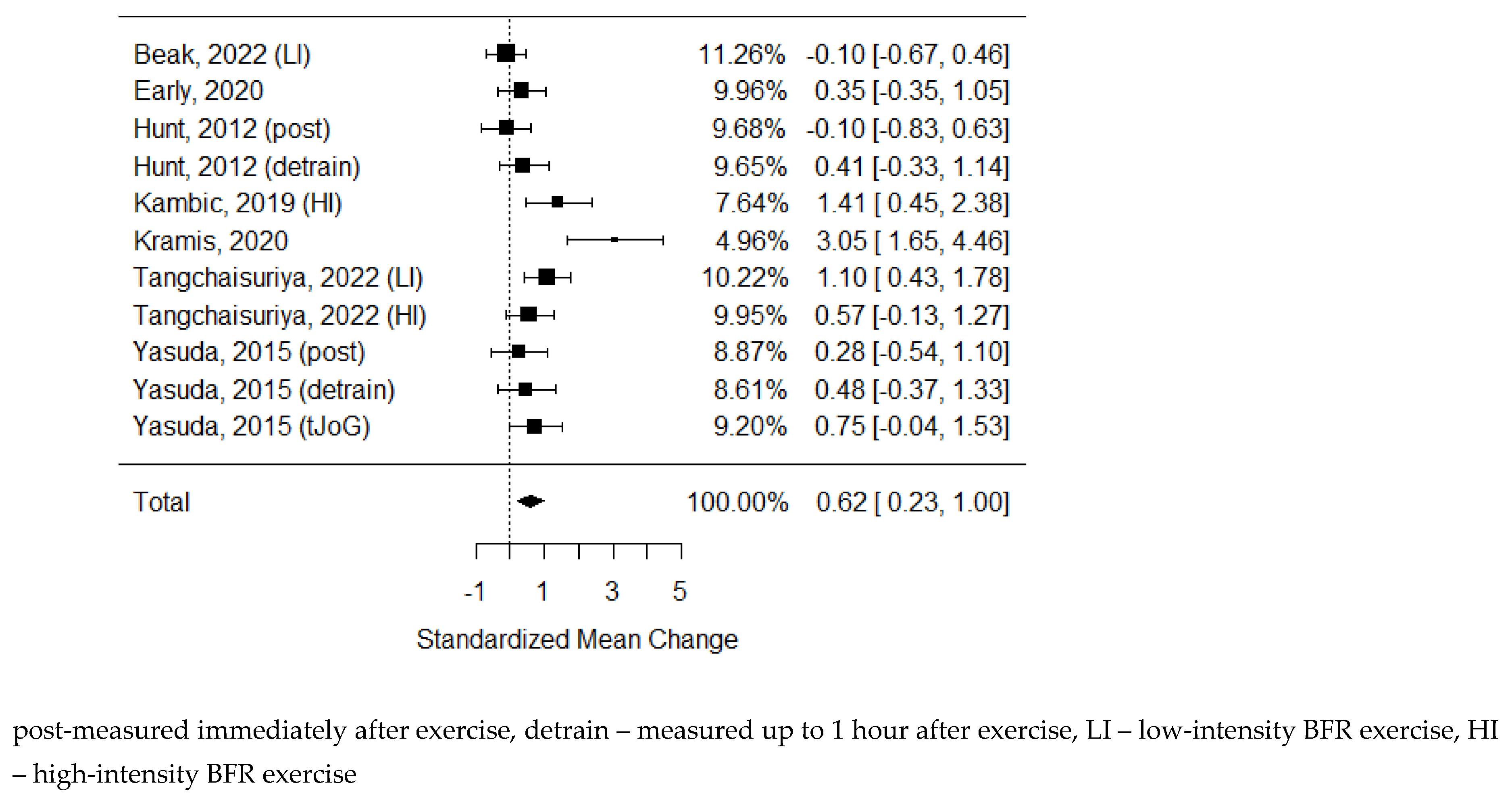
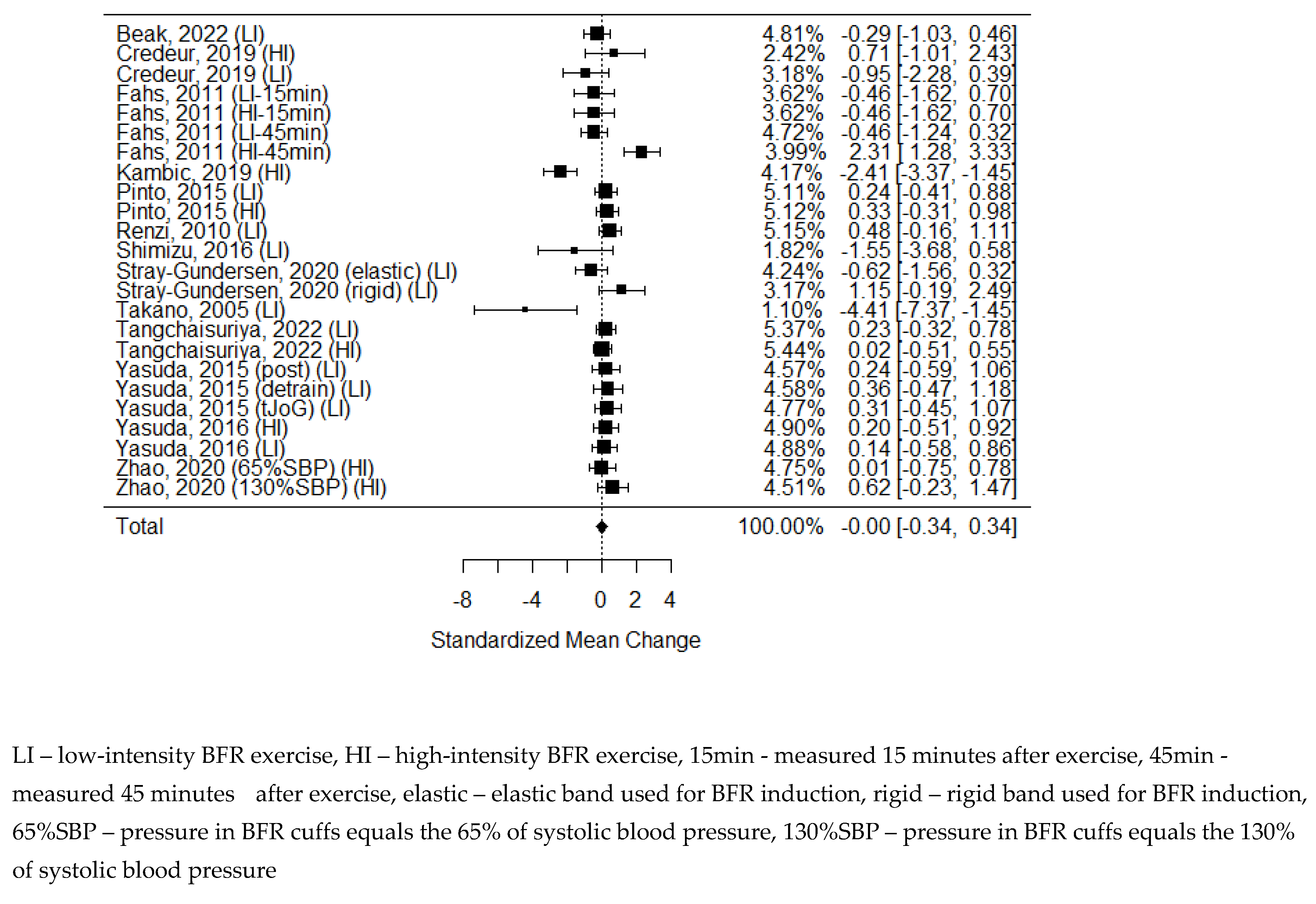
As some of the studies consisted of different subgroups performing different types of exercise or/and were assessed at multiple time points, all variants were pulled, resulting in 11 trials to examine the differences between the effects of BFR and non-BFR on flow-mediated dilation. The model estimate was 0.617 (CI 0.235, 1.000, P-value = 0.002). Heterogeneity was high (I-squared: 62.72%, P-value = 0.003).
Figure 2.
Forest plot on the effects of BFR on FMD values.
Figure 2.
Forest plot on the effects of BFR on FMD values.
3.3.2. Reactive hyperemia index
Only three studies analyzed the impact of BFR on the RHI [
24,
32,
33], but the data provided were insufficient for calculations. None of the studies reported any significant changes in RHI due to BFR exercise.
3.3.3. Vascular stiffness parameters
Studies analyzing six parameters of vascular stiffness were identified: augmentation index (AI), corrected AI (AI75), Systemic Vascular Resistance (SVR), Pulse Wave Analysis (PWV), Large Artery Elasticity Index (LAEI), Small Artery Elasticity Index (SAEI). In the case of AI, AI75, SVR, LAEI, and SAEI data were not sufficient to perform calculations:
AI: 4 studies were identified[
34,
35,
36,
37], but only Amorim S. et al. presented a more decisive influence of BFR over nonBFR.[
34]
AI75: None of the identified three studies reported any significance in the change of AI75 between BFR and nonBFR [
24,
34,
36]
SVR: According to Karabulut et al., the BFR exercise is more effective in decreasing SVR than low-intensity nonBFR exercise but less effective compared to high-intensity nonBFR training.[
38] The rest of the studies presented no significant differences regardless of exercise type. [
26,
39]
LAEI: None of the studies analyzing changes in large artery stiffness presented any significant changes [
38,
39]
SAEI: Small artery elasticity improved significantly more after BFR compared only to high-intensity nonBFR, but not low-intensity nonBFR exercise[
39]. Also, in case of push-up and squat exercises, the BFR training was more effective in increasing SAEI[
38]
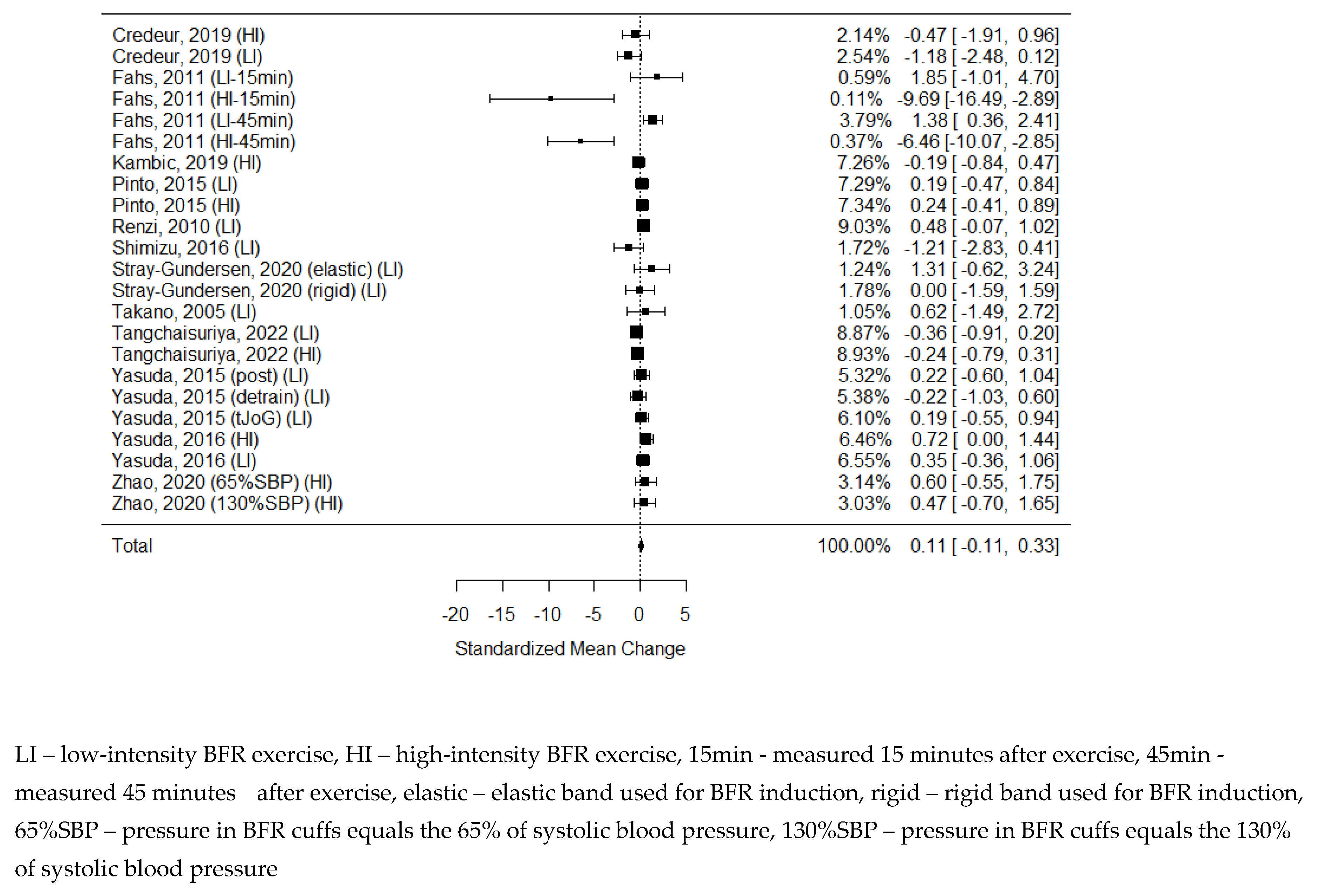
6 studies examining the changes in PWV were identified, but 1 of them was not eligible for statistical analysis due to insufficient data. [
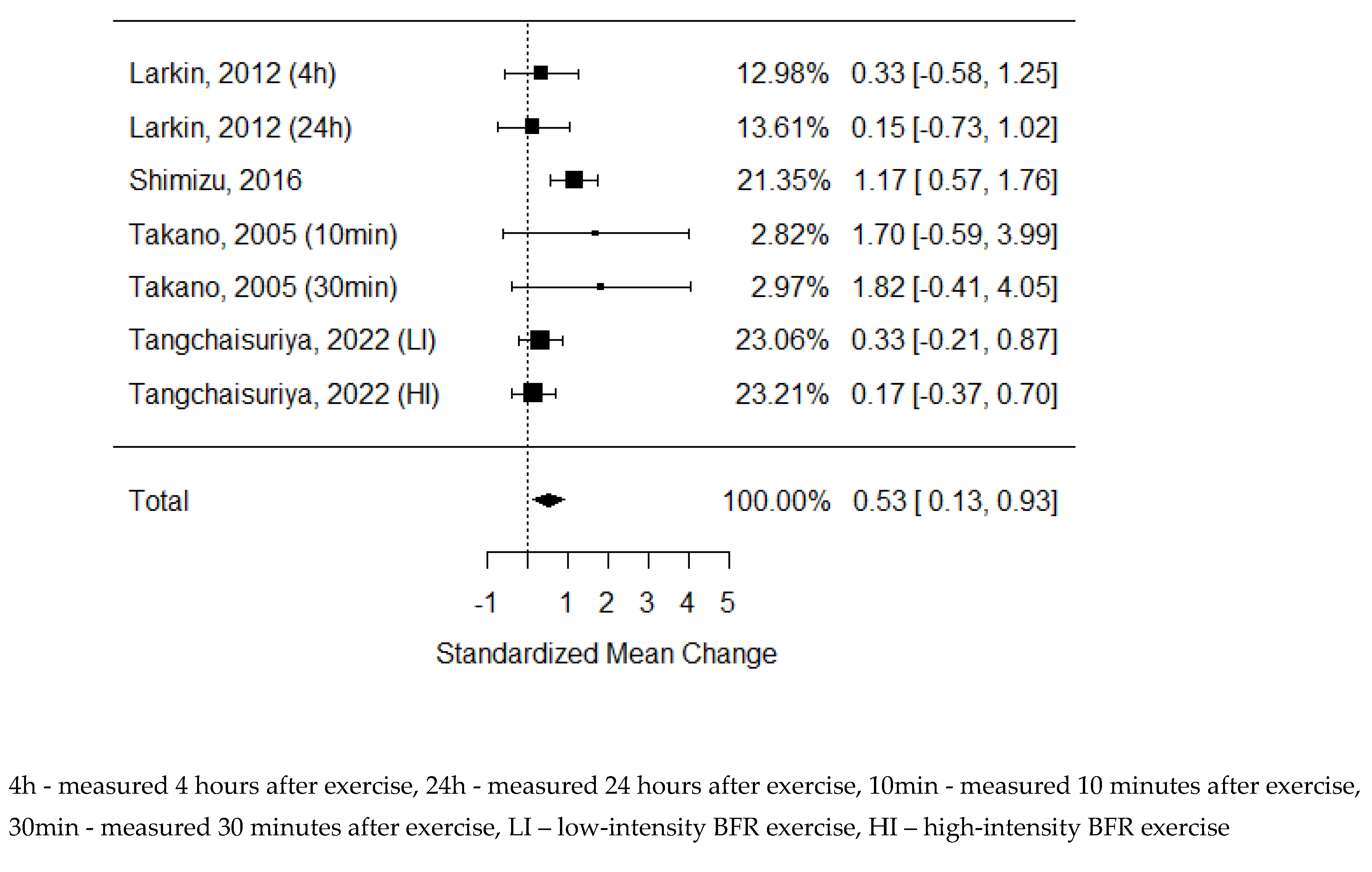
34] As some of the studies consisted of different subgroups performing different types of exercise, all variants were pooled, resulting in 7 trials pooled to examine the effects of exercise on pulse-wave velocity. The model estimate was 0.230 (CI -0.093, 0.554, P value = 0.163). Heterogeneity was moderate (I-squared: 41.87%, P value = 0.083).
3.3.4. Intima-media thickness
Only the study of Tangchaisuriya P. et al.[
33] examined the IMT values. Still, it did not present any significant changes after BFR exercise, also without a difference to nonBFR high-intensity and low-intensity training.
3.3.5. Nitric oxide
Boneo et al.[
40] reported that an increase of NOx is more significant after BFR exercise than high-intensity nonBFR but not different from low-intensity nonBFR. The other 3 studies showed no differences in NO concentrations between BFR and nonBFR trainings.[
25,
41,
42], but Remis et al. reported significant elevation of NO after both forms of training.[
42] Obtained data were insufficient for calculations.
3.4. Angiogenesis
3.4.1. Vascular endothelial growth factor and its variations
15 trials were analyzing vascular endothelial growth factor in multiple forms and its receptors: serum VEGF (n=9), VEGF mRNA (n=5), and VEGF-R (n=6).
Figure 3.
Forest plot on the effects of BFR exercise on PWV values.
Figure 3.
Forest plot on the effects of BFR exercise on PWV values.
Serum VEGF: Only four studies examining serum VEGF were eligible for statistical analysis[
23,
32,
33,
41]. The rest did not present sufficient data for calculations, but they showed the trend of higher VEGF concentrations after BFR compared to nonBFR.[
2,
43,
44,
45] Only in the study of Christiansen D. et. al [
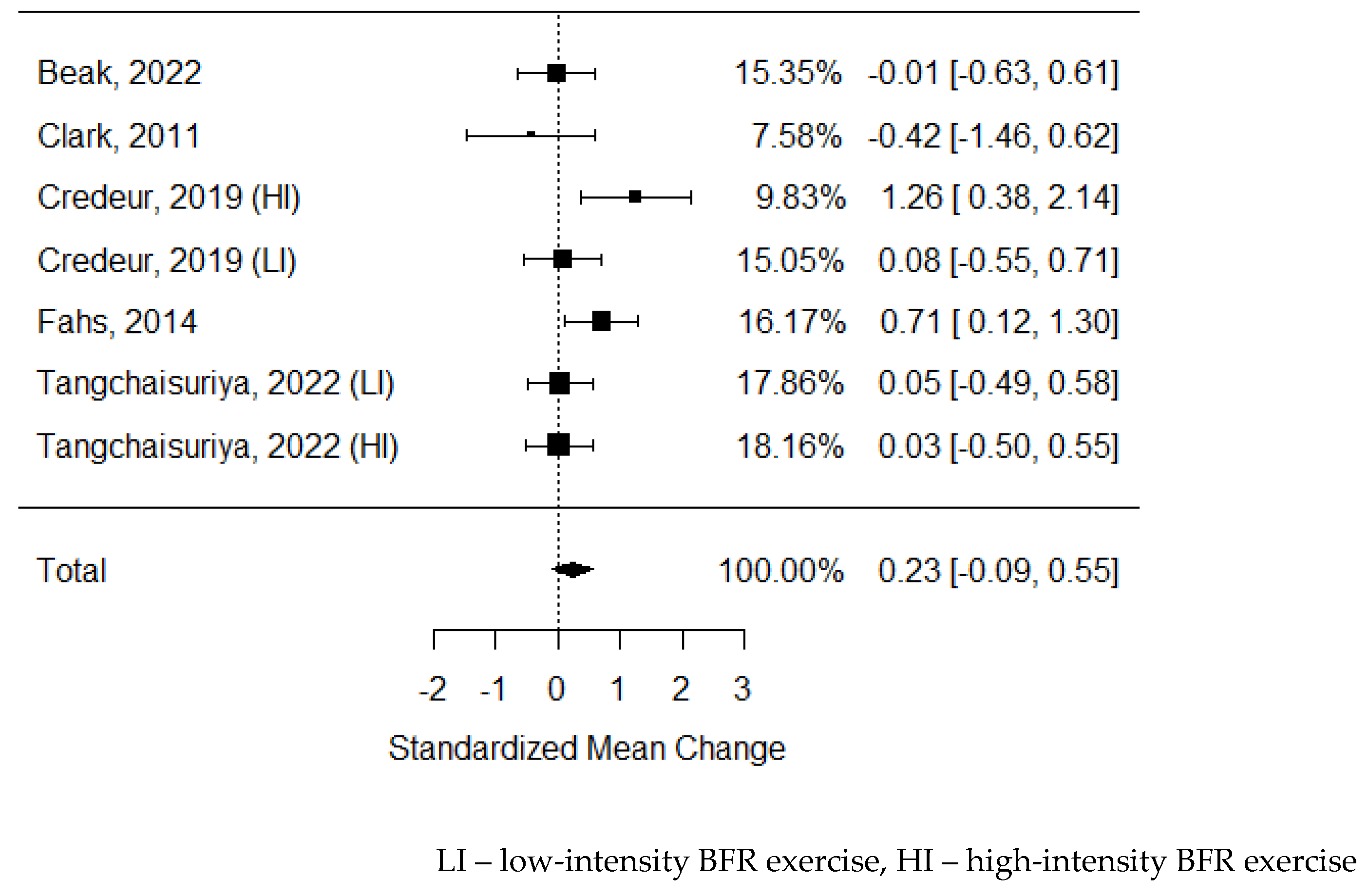
46] no differences were observed. As some of the studies consisted of different subgroups performing different types of exercise or/and were assessed at multiple time points, all of the variants were pooled, resulting in 7 trials to be examined the effects of exercise on circulating VEGF concentrations. The model estimate was 0.529 (CI 0.130, 0.928, P value = 0.009). Heterogeneity was low (I-squared: 39.41%, P value = 0.130).
VEGF mRNA: 5 studies analyzed levels of VEGF mRNA, and 4 showed a significant increase after BFR and more effective compared to nonBFR exercise regardless of training types.[
41,
47,
48,
49] Only Conceicao M. et al., in their 2016 study, did not observe significant changes in VEGF mRNA concentrations.[
50] Unfortunately, the provided data were insufficient for calculations.
VEGF-R: Serum VEGF-R concentrations were measured only by two studies, but they both confirmed its significant elevation after BFR, which was greater compared to nonBFR.[
24,
45] VEGF-R mRNA was assessed in 3 studies[
41,
48,
49], and all of them confirmed its peak due to BFR exercise, but only in 2 of them, it was significantly different compared to nonBFR.
Figure 4.
Forest plot on the effects of BFR exercise on serum VEGF values.
Figure 4.
Forest plot on the effects of BFR exercise on serum VEGF values.
3.4.2. CD31 (PECAM-1) and CD34
Only Maga M. et al.[
24] analyzed the concentration of CD31 and CD34. They both showed significant elevation after BFR, which was higher compared to nonBFR. Montgomery R. et al. [
51] examined CD34+CD45dim cells that did not significantly change their count after BFR. They also assessed CD34+VEGFR2+ and CD34+CD45dimVEGFR2+; both counts changed after BFR but were considerably lower than nonBFR.
3.4.1. CD106 / VCAM-1
We did not identify any study analyzing concentrations of VCAM-1 that could be included in this systematic review.
3.4.1. Von Willebrand factor
According to Shimizu R. [
32] serum concentration of vWF decreased significantly after BFR, but there was no difference compared to nonBFR exercise.
3.5. Other vascular functions
3.5.1. Ankle-brachial index and toe-brachial index
Six studies examining the changes in ABI were identified.[
3,
30,
37,
52,
53,
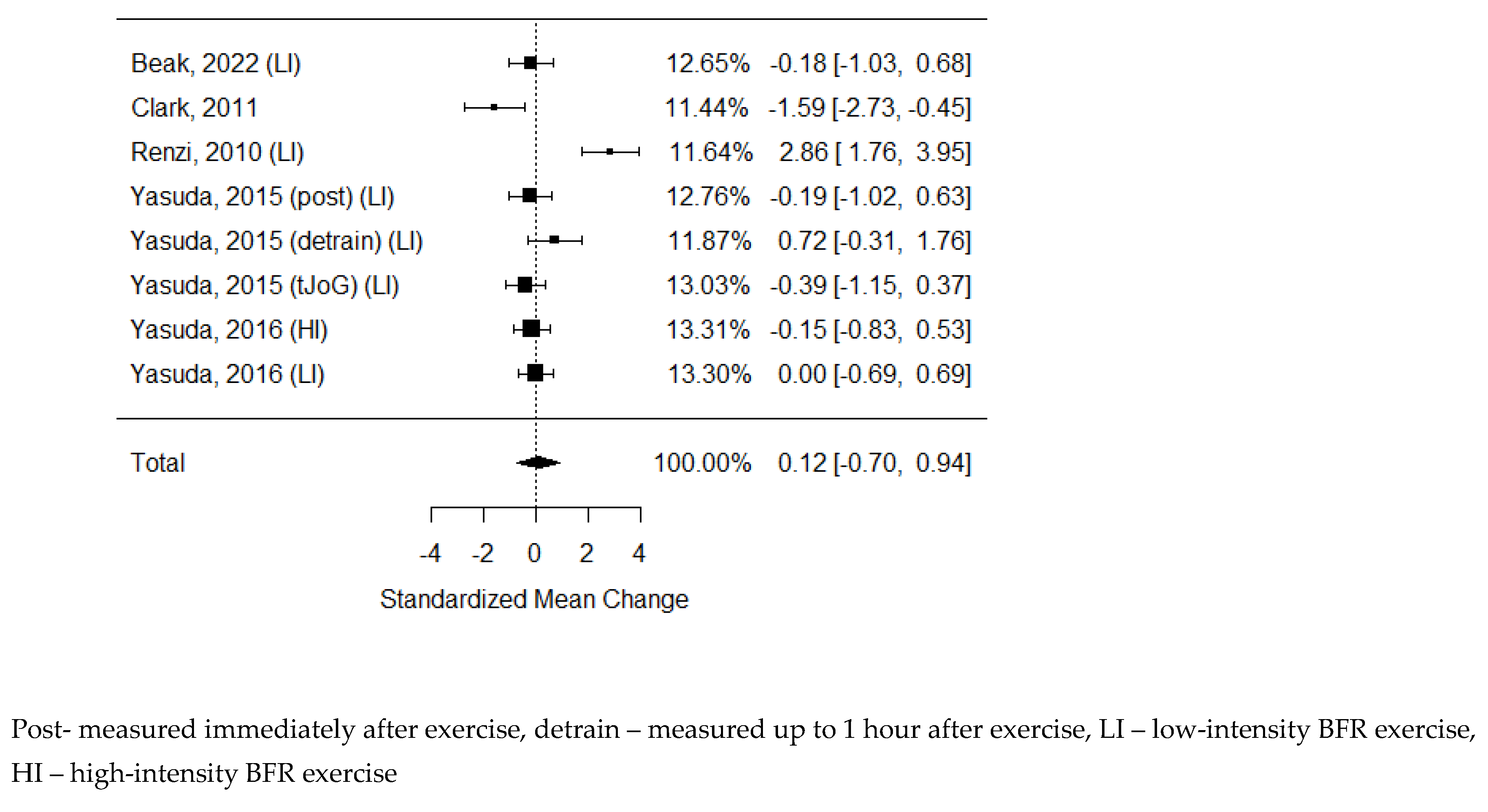
54] As some of the studies consisted of different subgroups performing different types of exercise, all variants were pooled, resulting in 8 trials pooled to examine the effects of exercise on the ankle-brachial index. None of the publications presented any significant changes after the BFR exercise. The model estimate was 0.119 (CI -0.703, 0.941, P value = 0.776). Heterogeneity was high (I-squared: 86.55%, P value <0.001).
None of the studies enrolled in this systematic review assessed Toe-Brachial Index.
Figure 5.
Forest plot on the effects of BFR exercise on ABI values.
Figure 5.
Forest plot on the effects of BFR exercise on ABI values.
3.5.2. Cardio-ankle vascular index
Only six studies analyzed the impact of BFR on the CAVI[
5,
31,
37,
53,
54], but the data provided were insufficient for calculations. Only 1 of them presented a significant reduction of CAVI after BFR exercise [
31]. Still, none of the studies showed significant differences between BFR and nonBFR exercise regarding CAVI reduction, regardless of activity type and intensity.
3.5.3. TcPO2
Only Shimizu R. et al.[
32] analyzed the impact of BFR on TcPO2. The results suggest the increase of oxygen pressure after BFR exercise, but it was not different from nonBFR activity.
3.5.4. Systolic blood pressure
Among the studies included in the systematic review, 20 examined changes in systolic blood pressure after exercise with BFR and nonBFR exercise[
3,
5,
23,
25,
26,
27,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
45,
53,
54,
55], from which 14 were eligible for calculations. As some of the studies consisted of different subgroups performing different types of exercise or/and were assessed at multiple time points, all variants were pooled, resulting in 24 trials examined on the effects of exercise on SBP. The model estimate is -0.002 (CI -0.343, 0.339; P value = 0.990). Heterogeneity was present in all studies (I-squared: 72.08%, P value <0.001). In 8 studies, SBP increased significantly after BFR exercise[
5,
23,
25,
31,
32,
35,
36,
56], while in 2 the value decreased significantly[
27,
55]. Only in 4 studies was the elevation of SBP more significant after BFR than nonBFR.[
23,
26,
31,
32]
Figure 6.
Forest plot on the effects of BFR exercise on systolic blood pressure values.
Figure 6.
Forest plot on the effects of BFR exercise on systolic blood pressure values.
3.5.5. Heart rate
Eighteen studies examining heart rate changes after BFR and nonBFR exercise were included in this systematic review[
5,
23,
25,
26,
27,
30,
31,
32,
33,
35,
36,
37,
38,
39,
45,
53,
54,
55] . 13 of them were eligible for calculations. As some of the studies consisted of different subgroups performing different types of exercise or/and were assessed in multiple time points, all variants were pooled, resulting in 23 trials examined on the effects of exercise on HR. The model estimate was 0.113 (CI -0.109, 0.335, P value = 0.319). Heterogeneity was moderate (I-squared: 24.34%, P value <0.001).
In 9 studies, significant elevation of HR after BFR exercise was observed[
5,
23,
25,
31,
32,
35,
36,
38,
39], while in only a single study, the value decreased significantly[
45]. In 5 studies elevation of HR was more significant after BFR compared to nonBFR.[
23,
25,
26,
31,
32] According only to Fahs C. et al. [
39], the elevation of HR was less intense after BFR than after nonBFR but only compared to high-intensity training.
Figure 7.
Forest plot on the effects of BFR exercise on heart rate values.
Figure 7.
Forest plot on the effects of BFR exercise on heart rate values.
4. Discussion
4.1. Impact of blood flow restriction on exercise performance
Improvements in exercise techniques remain one of the greatest challenges of physiotherapy and sports medicine. With increasing expectations for better performance, there is a growing expectation that training methods are more efficient. Modern training should provide the same or even better results in shorter exercise time and lower physical effort. BFR training was meant to be one of the answers to this urgent matter.[
57,
58] There is an extensive number of publications demonstrating an increase in muscle hypertrophy and strength through resistance exercise combined with BFR. Numerous systematic reviews and meta-analyses have illustrated the effectiveness of this combination in enhancing skeletal muscle strength, including dynamic isotonic, isometric, and isokinetic strength, as well as the rate of force development strength capacity.[
59,
60] It is also well established that muscle hypertrophy and strength adaptations achieved by resistance exercise with BFR are generally more significant than resistance exercise with low load alone. It is comparable with the increases in strength observed in high-load resistance exercise.[
61] Improvements are observed in a relatively short period, even 2-10 days after training, [
62,
63], which can also be the result of the better frequency of exercise, which may be unavailable in high-load training, where recovery time is prolonged. In the case of regular low-load resistance training, the time needed to achieve such results is much longer, from 3 up to even 8 weeks. [
37,
64,
65]
On the contrary, the BFR method is still a matter of controversy. [
66,
67] The variety of BFR devices leads to confusion about the restriction of blood flow with total arterial occlusion. Not all types of training, especially high-load resistance exercise, should be performed in complete ischemia. In addition, too much compression of the veins can cause increased venous pressure. It can damage vein valves and ultimately result in chronic venous insufficiency.[
68] Furthermore, the elevation of a mean systolic arterial blood pressure during and after BFR sessions (
Figure 6) raises concerns, as it is a decisive risk factor for potential cardiovascular adverse events.
4.2. Other BFR vascular-related studies
At first, similar reviews as ours were looked for, and three publications were identified, but none covered the whole of our search area or were not up to date.[
69,
70,
71] Pereira-Neto et al. excluded the number of articles that met the inclusion criteria set by them.[
69] What is more, the analysis methodology was questionable as the studies were grouped by the type of vascular function assessed, even though multiple outcomes were measured in different ways yet pulled into the same analysis. In our opinion, it could distorted the readers, leading to too far-reaching conclusions and assumptions. Li S et al. focused on angiogenesis-related factors but limited only to skeletal muscles while circulating pro- and anti-angiogenesis factors were not analyzed.[
70] On the other hand, analysis conducted by Liu et al. includes a broad spectrum of vascular outcomes but only after resistance BFR exercise.[
71] This means numerous other types of exercises combined with BFR were excluded, and their impact on vascular functions remains unanalyzed.
4.3. Vascular parameters
Although physical training is widely recognized to have a positive impact on endothelial functions, specific effects on endothelial vasodilation abilities are still uncertain, particularly regarding increasing FMD and reducing vascular stiffness, depending on the intensity and type of training, and the optimal balance between these factors and endothelial responses.[
72] The complex assessment of vascular properties should include endothelial functions and angiogenesis. The combination of these two groups of parameters characterizes the performance of the main vessels and the whole process of creating new vasculature and expanding the arterial network. Additionally, clinical hemodynamic parameters, such as systolic/diastolic blood pressure or heart rate, do not directly reflect either of these categories, but give critical clinical data on the condition of the whole cardiovascular system. Last but not least, there are the microcirculation parameters, that is, TcPO2, that also need to be mentioned in the analysis of vascular parameters.
4.3.1. Endothelium
The parameter of endothelium most commonly used in clinical practice is IMT. It describes the thickness of the intima-media layer of the carotid arteries. Its enlargement used to be interpreted as a subclinical phase of atherosclerosis, but has recently been recognized as an increased cardiovascular risk factor.[
73,
74] Only one single study of this systematic review analyzed this parameter. As in the young and healthy population, which in most cases were the subjects of the studies, the IMT is mainly within the normal range, there is no field for improvement.
From a physiological point of view, the most popular endothelial function assessment is FMD. This index is based on the difference between the diameter of the artery before, during and after the release of brachial artery occlusionn[
75]. Increased FMD represents an improvement in the vasodilatory functions of the arterial wall, leading to better pressure control and greater oxygenation of tissue and organ oxygenation.[
76] This phenomenon has also been confirmed in other forms of training.[
77] Our results show that BFR is the type of exercise that also stimulates changes in vasodilatation, improving blood flow within the main arteries. Furthermore, BFR improves FMD more significantly compared to regular activity (
Figure 2). However due to large heterogeneity index we have to treat this result with caution.
The reactive hyperemia index is also used to assess endothelial vasodilatation but within the microvascular system. [
78] It is strictly related to atherosclerosis-based cardiovascular disease. [
79] Its noninvasive character and high sensitivity make this method quite popular in the sports and rehabilitation field. According to Higashi et al., even daily aerobics significantly improves vasodilatation within micro vessels. [
80] Surprisingly, we found only a few studies that examined the impact of exercise in the BFR on this parameter, which did not produce any conclusive results. [
24,
32,
33] This could again be due to the characteristics of the population, mostly young and healthy with generally proper baseline RHI values.
Vascular stiffness is a physical phenomenon and can be defined by multiple parameters. Its reduction is beneficial for the reduction of blood pressure and reduces the risk of atherosclerosis.[
81] In this systematic review, we focused on the most commonly used parameters, but only the Pulse Wave Velocity data were homogeneous enough and presented a sufficient quality to be included in the meta-analysis. [
3,
33,
35,
52,
82] Unfortunately, this parameter did not yield any conclusive results. There were also no differences between BFR and non-BFR activities in the Augmentation Index or its corrected heart rate version (AI75), except in the study by Amorim et al. [
34] However, Karabulut et al. confirmed that SVR was reduced more efficiently after BFR, it was highly dependent on the intensity of the exercise performed. [
38]
Nitric oxide is the primary biochemical biomarker of endothelial vasodilatation. It is responsible for vasodilatation regulation and is crucial in arteries' adaptation to stress or sports activities. [
83] Physical activities are generally accountable for their elevation not only in acute reaction but also in form of long-term outcome of regular exercise. [
84] This is in opposition to the results of our analysis, as only in 1 study elevation of NO was observed.[
42] We hypothesize that this could be due to the analyzed studies' questionable methodology, as the blood samples were taken immediately after exercise. What is more two studies were based only on single sessions.[
25,
41] Additionally, in Barilli et al. study, the participants were suffering from hypertension, so we assume that production of endothelial NO in this population was already impaired by comorbidities. [
25]
4.3.2. Angiogenesis
Physical activity is one of the crucial factors for stimulation of angiogenesis, even after short-term exercise, but performed frequently.85 During physical exercise, especially in the anaerobic phase, the production of proangiogenic factors such as VEGF, soluble endoglin, hypoxia-inducible factor 1 (HIF-1), or peroxisome proliferator-activated receptor gamma coactivator (PGC-1α) is stimulated. 86,87 This phenomenon is widely used in the rehabilitation of patients with intermittent claudication in the course of peripheral arterial disease.88,89 As a result, pain reduction and extension of the claudication distance are observed, combined with the progression of wound healing in patients with chronic limb-threatening ischemia. 90
The matter of BFR training's influence on angiogenesis has been poorly examined. So far, only one meta-analysis has been performed, analysing only the muscle concentrations of angiogenic factors. 70 Few of the included articles were not eligible for our study as they did not meet the methodology inclusion criteria, ie, blood flow restriction applied after but not during training 91, blood flow restriction induced by gravity, but not any restriction tool. 92
Our results are most of all significant for evaluating changes in VEGF concentration. Most of the studies included in this analysis confirmed that BFR induces VEGF production. The meta-analysis showed that exercise with restriction of blood flow is more effective in stimulating angiogenesis than regular activity (
Figure 4), but the confidence interval is not far from “0” value and after data sensitivity subgroups analysis the result could be different. Also, increased expression of VEGF mRNA was observed after training in BFR, and in all cases, the elevation was more significant after training in BFR compared to regular training.
43,49–51 We found only three studies that examined different angiogenic factors than VEGF. Shimizu et al. showed a decrease in the von Willebrand Factor, but comparable to nonBFR exercise. PECAM-1 and CD-34 concentrations increased significantly more than those of exercise without BFR
26, but cells CD34 + CD45dim, CD34 + VEGFR2 +, and CD34 + CD45dim VEGFR2 + did not show superiority in count after BFR vs. non-BFR
53.
4.3.3 Other vascular functions
The ankle-brachial index is the most basic and yet most commonly used lower extremity ischemia diagnosis method. 16 Stenosis of peripheral artery occlusion leads to a reduction in peripheral arterial pressure, which causes ischemia of muscles and skin. This index remains one of the most important scores for assessing therapy outcomes.93 In this analysis, we did not present a significant improvement in ABI after BFR training or its superiority over non-BFR exercise. It should be emphasised that in most studies, participants were relatively young and healthy, and the baseline ABI results were already within the normal range. Studies in patients with impaired peripheral circulation are needed to assess the real impact of BFR on changes in ABI. The same lack of improvement was observed in the CAVI score, and we hypothesise that the reason was the same background. TcPO2 was examined only in one study with a small sample size. Its result suggests that it is stimulated similarly regardless of exercise type, but improved compared to baseline level. It is inconclusive, and further studies are needed to examine this phenomenon.
Systolic blood pressure and heart rate are variables that describe the dynamics of arterial blood flow. During physical activity, the heart rate increases to supply the muscles and brain with additional oxygen-rich blood. After the activity, HR decreases, and people regularly perform sport are lowered at rest. 94 Frequently performed physical activity also reduces blood pressure. 95 The European Society of Cardiology recommends it as one of the elements of hypertension treatment and rehabilitation after myocardial infarction. 96 The results of this meta-analysis did not confirm those tendencies. It could be due to the short time between the completion of the training and the measurement, which occurred in most of the included studies. Although there was a trend of elevated heart rate after exercise, it was not statistically significant.
4.4. Study limitations
There are a limited number of randomized clinical trials that study the impact of BFR training on vascular functions. Most studies are underpowered, which can influence the results obtained. To overcome this issue, we decided to treat the comparison of different groups as separate studies. Such a solution also has a drawback – the same population is included multiple times and may bias the population characteristics. Another issue is the lack of standardization in activity protocols: the length of exercise in studies included in this analysis varies from single sessions to five sessions per week for 8 weeks.[
45] There are multiple studies that indicate that acute improvements in endothelial functions after exercise are temporary and long-term training is needed to obtain a permanent effect.[
97,
98] Another thing is the difference in the type of exercise, as there are studies indicating that the type of exercise is a critical factor for changes in endothelial functions. [
99] It also influences the activation of angiogenesis.[
100] Also, the difference in the degree of blood flow restriction is not without significance and could bias the analysis. All these differences make these studies hardly comparable, and the results of this meta-analysis must be taken with great caution. Last but not least, bias analysis revealed that almost none of the studies was blinded, neither for participants nor researchers. [Supplementary
Figure 2] This is a common problem in experimental studies involving physical therapy, as it is difficult or even impossible to blind the form of intervention when it is an activity. Even if patients are not told which branch is included, they can easily estimate the type of activity they perform. [
101] Most of the studies analyzed did not involve long-term observation after finishing the training program. [Supplementary
Figure 2] This limits our conclusions on the durability of the effect of BFR on the measured vascular parameters. It should also be mentioned that the three studies included in the analysis did not have a typical control group – the comparison was made between different extremities but within the same patients. [
28,
82,
102] This is a significant methodological issue, as the endothelium is currently considered a single endocrine organ. [
103] This implies that even local stimulation impacts the whole endothelium in a similar way. Despite this, we decided to include those studies due to the already low number of other randomized clinical trials and typical cross-over studies.
5. Conclusions
The results obtained present trends, suggesting the significant impact of BFR training on endothelial functions and angiogenesis. Mainly arterial vasodilatation functions and VEGF-based angiogenesis seems to be influenced more by BFR than regular activity without restriction of blood flow. There is still a lack of multicenter randomized clinical trials that include a large number of participants. More studies, addressed not only to young, healthy subjects, are needed to confirm the advantage of BFR over non-BFR activity in the aspect of arterial function and angiogenesis stimulation.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Graphs of risk of bias in accordance to Joanna Briggs Institute guidelines.; Table S1: Data extraction table.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, M.M. and A.Ś.; methodology, M.M, A.W-M., K.B. and A.Ś..; software, M.M.; validation, A.Ś.; formal analysis, K.B.; investigation, M.M., A.W-M., A.W., P.K., J.K., N.S.; resources, N.S.; data curation, M.M., A.W-M.; writing—original draft preparation, M.M., A.W-M., K.B., A.W., P.K., J.K.,; writing—review and editing, M.M., A.Ś.; visualization, M.M., A.Ś.; supervision, A.Ś.; project administration, M.M. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Table A1.
The detailed search strategy.
Table A1.
The detailed search strategy.
| PUBMED: |
| (("blood flow restricted training") OR ("blood flow restriction training") OR ("blood flow restricted exercise") OR ("blood flow restriction exercise") OR ("blood flow restriction") OR (BFR) OR (“BFR-RT”) OR ("ischemic training") OR ("ischemic exercise") OR (kaatsu) OR (katsu) OR ("kaatsu exercise") OR ("kaatsu training") OR ("vascular occlusion exercise") OR ("vascular occlusion training")) AND (("vascular function") OR ("endothelium"[MeSH Terms]) OR ("endothelium, vascular"[MeSH Terms]) OR (vasodilatation[MeSH Terms]) OR ("flow mediated dilatation") OR ("flow-mediated dilatation") OR (FMD) OR ("reactive hyperemia index") OR (RHI) OR („intima-media thickness”) OR (IMT) OR („transcutaneous oximetry”) OR (tcpo2) OR („arterial stiffness”) OR ("ankle brachial index"[MeSH Terms]) OR ("toe brachial index") OR ("toe- brachial index") OR (TBI) OR ("cardio ankle vascular index"[MeSH Terms]) OR ("vascular endothelial growth factor a"[MeSH Terms]) OR ("vascular endothelial growth factor b"[MeSH Terms]) OR (vascular stiffness[MeSH Terms]) OR ("vascular remodeling"[MeSH Terms]) OR (Angiogenesis effect[MeSH Terms]) OR (angiogenesis factor[MeSH Terms]) OR ("angiogenesis inducing agents"[MeSH Terms]) OR (angiogenesis) OR (cd31 antigen[MeSH Terms]) OR (vcam-1) OR (CD106) OR (CD-106) OR (von willebrand factor[MeSH Terms]) OR "nitric oxide"[MeSH Terms])) |
| EMBASE: |
| ('blood flow restriction training'/de OR 'blood flow restriction exercise'/de OR 'blood flow restriction'/de OR 'ischemic training' OR 'ischemic exercise' OR 'kaatsu' OR 'katsu' OR 'kaatsu training' OR 'kaatsu exercise' OR 'blood vessel occlusion'/de OR 'vascular occlusion exercise' OR 'vascular occlusion training') AND ('vascular function'/de OR 'endothelium'/de OR 'vascular endothelium'/de OR 'vasodilatation'/de OR 'flow mediated dilatation'/de OR 'flow-mediated dilation test'/de OR 'flow mediated vasodilation'/de OR 'reactive hyperemia index'/de OR 'arterial wall thickness'/de OR 'intima-media thickness' OR 'transcutaneous oximetry'/de OR tcpo2 OR 'ankle brachial index'/de OR 'toe brachial index'/de OR 'cardio-ankle vascular index'/de OR 'vasculotropin'/de OR 'vascular endothelial growth factor' OR 'arterial stiffness'/de OR 'vascular remodeling'/de OR 'angiogenesis'/de OR 'angiogenic factor'/de OR 'angiogenesis modulator'/de OR 'cd31 antibody'/de OR 'platelet endothelial cell adhesion molecule 1'/de OR 'vascular cell adhesion molecule 1'/de OR 'von willebrand factor'/de OR 'nitric oxide'/de) AND [english]/lim AND 'human'/de |
References
- Cognetti, D.J.; Sheean, A.J.; Owens, J.G. Blood Flow Restriction Therapy and Its Use for Rehabilitation and Return to Sport: Physiology, Application, and Guidelines for Implementation. Arthrosc. Sport. Med. Rehabil. 2022, 4. [Google Scholar] [CrossRef] [PubMed]
- Barjaste, A.; Mirzaei, B.; Rahmani-nia, F.; Haghniyaz, R.; Brocherie, F. Concomitant Aerobic- and Hypertrophy-Related Skeletal Muscle Cell Signaling Following Blood Flow-Restricted Walking. Sci. Sports 2020. [Google Scholar] [CrossRef]
- Beak, H.J.; Park, W.; Yang, J.H.; Kim, J. Effect of Low-Intensity Aerobic Training Combined with Blood Flow Restriction on Body Composition, Physical Fitness, and Vascular Responses in Recreational Runners. Healthcare 2022, 10, 1789. [Google Scholar] [CrossRef] [PubMed]
- Kilgas, M.A.; Yoon, T.; McDaniel, J.; Phillips, K.C.; Elmer, S.J. Physiological Responses to Acute Cycling With Blood Flow Restriction. Front. Physiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Wooten, S. V; Stray-Gundersen, S.; Tanaka, H. Hemodynamic and Pressor Responses to Combination of Yoga and Blood Flow Restriction. Int. J. Sports Med. 2020, 41, 759–765. [Google Scholar] [CrossRef]
- Wortman, R.J.; Brown, S.M.; Savage-Elliott, I.; Finley, Z.J.; Mulcahey, M.K. Blood Flow Restriction Training for Athletes: A Systematic Review. Am. J. Sports Med. 2021, 49. [Google Scholar] [CrossRef]
- Gladden, J.; Wernecke, C.; Rector, S.; Tecson, K.; McCullough, P. Pilot Safety Study: The Use of VasperTM, a Novel Blood Flow Restriction Exercise in Healthy Adults. J. Exerc. Physiol. Online 2016, 19, 99–106. [Google Scholar]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G.; et al. Blood Flow Restriction Exercise Position Stand: Considerations of Methodology, Application, and Safety. Front. Physiol. 2019, 10. [Google Scholar]
- Ferguson, R.A.; Mitchell, E.A.; Taylor, C.W.; Bishop, D.J.; Christiansen, D. Blood-Flow-Restricted Exercise: Strategies for Enhancing Muscle Adaptation and Performance in the Endurance-Trained Athlete. Exp. Physiol. 2021, 106. [Google Scholar] [CrossRef]
- Patterson, S.D.; Hughes, L.; Head, P.; Warmington, S.; Brandner, C. Blood Flow Restriction Training: A Novel Approach to Augment Clinical Rehabilitation: How to Do It. Br. J. Sports Med. 2017, 51. [Google Scholar] [CrossRef]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Januszek, R.; Mika, P.; Nowobilski, R.; Maga, P.; Niżankowski, R. The Improvement of Walking Abilities and Endothelial Function after the Supervised Training Treadmill Program (STTP) in Patients with Peripheral Artery Disease (PAD) Is Not Related to Prostacyclin and Thromboxane Release. Int. J. Cardiol. 2016, 222, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by Representatives of 10 Societies and by Invited Experts) Developed with the Special Contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016, 252. [Google Scholar] [CrossRef]
- Frank, U.; Nikol, S.; Belch, J.; Boc, V.; Brodmann, M.; Carpentier, P.H.; Chraim, A.; Canning, C.; Dimakakos, E.; Gottsäter, A.; et al. ESVM Guideline on Peripheral Arterial Disease. Vasa 2019, 48, 1–79. [Google Scholar] [CrossRef] [PubMed]
- Stanford, D.M.; Mouser, J.G.; Chatlaong, M.A.; Jessee, M.B. A Narrative Review of the Effects of Blood Flow Restriction on Vascular Structure and Function. Physiol. Int. 2022, 109, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. ; PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269, W64. [Google Scholar] [CrossRef] [PubMed]
- Lo Russo, G.; Spolveri, F.; Ciancio, F.; Mori, A. Mendeley: An Easy Way to Manage, Share, and Synchronize Papers and Citations. Plast. Reconstr. Surg. 2013, 131. [Google Scholar] [CrossRef] [PubMed]
- Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, M.P.-F. Systematic Reviews of Etiology and Risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris E, M.Z., Ed.; The Joanna Briggs Institute, 2017.
- Becker, B.J. Synthesizing Standardized Mean-change Measures. Br. J. Math. Stat. Psychol. 1988, 41. [Google Scholar] [CrossRef]
- Morris, S.B. Estimating Effect Sizes from Pretest-Posttest-Control Group Designs. Organ. Res. Methods 2008, 11. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor. J. Stat. Softw. 2010, 36. [Google Scholar] [CrossRef]
- Viechtbauer, W. Bias and Efficiency of Meta-Analytic Variance Estimators in the Random-Effects Model. J. Educ. Behav. Stat. 2005, 30. [Google Scholar] [CrossRef]
- Takano, H.; Morita, T.; Iida, H.; Asada, K.; Kato, M.; Uno, K.; Hirose, K.; Matsumoto, A.; Takenaka, K.; Hirata, Y.; et al. Hemodynamic and Hormonal Responses to a Short-Term Low-Intensity Resistance Exercise with the Reduction of Muscle Blood Flow. Eur. J. Appl. Physiol. 2005, 95, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Maga, M.; Schönborn, M.; Wachsmann-Maga, A.; Śliwka, A.; Krężel, J.; Włodarczyk, A.; Olszewska, M.; Nowobilski, R. Stimulation of the Vascular Endothelium and Angiogenesis by Blood-Flow-Restricted Exercise. Int. J. Environ. Res. Public Health 2022, 19, 15859. [Google Scholar] [CrossRef] [PubMed]
- Barili, A.; Corralo, V. da S.; Cardoso, A.M.; Mânica, A.; Bonadiman, B. da S.R.; Bagatini, M.D.; Da Silva-Grigoletto, M.E.; de Oliveira, G.G.; De Sá, C.A. Acute Responses of Hemodynamic and Oxidative Stress Parameters to Aerobic Exercise with Blood Flow Restriction in Hypertensive Elderly Women. Mol. Biol. Rep. 2018, 45. [Google Scholar] [CrossRef]
- Pinto, R.R.; Polito, M.D. Haemodynamic Responses during Resistance Exercise with Blood Flow Restriction in Hypertensive Subjects. Clin. Physiol. Funct. Imaging 2016, 36. [Google Scholar] [CrossRef] [PubMed]
- Kambič, T.; Novaković, M.; Tomažin, K.; Strojnik, V.; Jug, B. Blood Flow Restriction Resistance Exercise Improves Muscle Strength and Hemodynamics, but Not Vascular Function in Coronary Artery Disease Patients: A Pilot Randomized Controlled Trial. Front. Physiol. 2019, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Credeur, D.P.; Hollis, B.C.; Welsch, M.A. Effects of Handgrip Training with Venous Restriction on Brachial Artery Vasodilation. Med. Sci. Sports Exerc. 2010, 42, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Paiva, F.M.; Vianna, L.C.; Fernandes, I.A.; Nóbrega, A.C.; Lima, R.M. Effects of Disturbed Blood Flow during Exercise on Endothelial Function: A Time Course Analysis. Brazilian J. Med. Biol. Res. = Rev. Bras. Pesqui. medicas e Biol. 2016, 49, e5100. [Google Scholar] [CrossRef]
- Renzi, C.P.; Tanaka, H.; Sugawara, J. Effects of Leg Blood Flow Restriction during Walking on Cardiovascular Function. Med. Sci. Sports Exerc. 2010, 42, 726–732. [Google Scholar] [CrossRef]
- Stray-Gundersen, S.; Wooten, S.; Tanaka, H. Walking With Leg Blood Flow Restriction: Wide-Rigid Cuffs vs. Narrow-Elastic Bands. Front. Physiol. 2020, 11, 568. [Google Scholar] [CrossRef]
- Shimizu, R.; Hotta, K.; Yamamoto, S.; Matsumoto, T.; Kamiya, K.; Kato, M.; Hamazaki, N.; Kamekawa, D.; Akiyama, A.; Kamada, Y.; et al. Low-Intensity Resistance Training with Blood Flow Restriction Improves Vascular Endothelial Function and Peripheral Blood Circulation in Healthy Elderly People. Eur. J. Appl. Physiol. 2016, 116, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Tangchaisuriya, P.; Chuensiri, N.; Tanaka, H.; Suksom, D. Physiological Adaptations to High-Intensity Interval Training Combined with Blood Flow Restriction in Masters Road Cyclists. Med. Sci. Sports Exerc. 2022, 54. [Google Scholar] [CrossRef] [PubMed]
- Amorim, S.; Gaspar, A.P.; Degens, H.; Cendoroglo, M.S.; de Mello Franco, F.G.; Ritti-Dias, R.M.; Cucato, G.G.; Rolnick, N.; de Matos, L.D.N.J. The Effect of a Single Bout of Resistance Exercise with Blood Flow Restriction on Arterial Stiffness in Older People with Slow Gait Speed: A Pilot Randomized Study. J. Cardiovasc. Dev. Dis. 2022, 9. [Google Scholar] [CrossRef]
- Credeur, D.P.; Jones, R.; Stanford, D.; Stoner, L.; McCoy, S.; Jessee, M. Central Cardiovascular Hemodynamic Response to Unilateral Handgrip Exercise with Blood Flow Restriction. Eur. J. Appl. Physiol. 2019, 119, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.L.; Marshall, E.M.; Parks, J.C.; Kingsley, J.D. Hemodynamic Response and Pulse Wave Analysis after Upper- and Lower-Body Resistance Exercise with and without Blood Flow Restriction. Eur. J. Sport Sci. 2022, 22. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Fukumura, K.; Tomaru, T.; Nakajima, T. Thigh Muscle Size and Vascular Function after Blood Flow-Restricted Elastic Band Training in Older Women. Oncotarget 2016, 7, 33595–33607. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, U.; Karabulut, M.; James, E.G. Small Arteries Stay Stiff for a Longer Period Following Vibration Exercises in Combination with Blood Flow Restriction. Clin. Physiol. Funct. Imaging 2018, 38. [Google Scholar] [CrossRef]
- Fahs, C.A.; Rossow, L.M.; Seo, D.-I.; Loenneke, J.P.; Sherk, V.D.; Kim, E.; Bemben, D.A.; Bemben, M.G. Effect of Different Types of Resistance Exercise on Arterial Compliance and Calf Blood Flow. Eur. J. Appl. Physiol. 2011, 111, 2969–2975. [Google Scholar] [CrossRef]
- Boeno, F.P.; Ramis, T.R.; Farinha, J.B.; de Lemos, L.S.; Medeiros, N. da S.; Ribeiro, J.L. Acute Effects of Strength Exercise with Blood Flow Restriction on Vascular Function of Young Healthy Males. J. Vasc. Bras. 2018, 17. [Google Scholar] [CrossRef]
- Larkin, K.A.; Macneil, R.G.; Dirain, M.; Sandesara, B.; Manini, T.M.; Buford, T.W. Blood Flow Restriction Enhances Post-Resistance Exercise Angiogenic Gene Expression. Med. Sci. Sports Exerc. 2012, 44, 2077–2083. [Google Scholar] [CrossRef]
- Ramis, T.R.; Muller, C.H. de L.; Boeno, F.P.; Teixeira, B.C.; Rech, A.; Pompermayer, M.G.; Medeiros, N. da S.; Oliveira, Á.R. de; Pinto, R.S.; Ribeiro, J.L. Effects of Traditional and Vascular Restricted Strength Training Program With Equalized Volume on Isometric and Dynamic Strength, Muscle Thickness, Electromyographic Activity, and Endothelial Function Adaptations in Young Adults. J. Strength Cond. Res. 2020, 34, 689–698. [Google Scholar] [CrossRef]
- Patterson, S.D.; Leggate, M.; Nimmo, M.A.; Ferguson, R.A. Circulating Hormone and Cytokine Response to Low-Load Resistance Training with Blood Flow Restriction in Older Men. Eur. J. Appl. Physiol. 2013, 113, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Shill, D.D.; Polley, K.R.; Willingham, T.B.; Call, J.A.; Murrow, J.R.; McCully, K.K.; Jenkins, N.T. Experimental Intermittent Ischemia Augments Exercise-Induced Inflammatory Cytokine Production. J. Appl. Physiol. 2017, 123, 434–441, http://resolver.ebscohost.com/openurl?sid=EMBASE&issn=15221601&id=doi:10.1152%2Fjapplphysiol.01006.2016&atitle=Experimental+intermittent+ischemia+augments+exerciseinduced+inflammatory+cytokine+production&stitle=J.+Appl.+Physiol.&title=Journal+of+applied+physiology+%28Bethesda%2C+Md.+%3A+1985%29&volume=123&issue=2&spage=434&epage=441&aulast=Shill&aufirst=Daniel+D.&auinit=D.D.&aufull=Shill+D.D.&coden=&isbn=&pages=434-441&date=2017&auinit1=D&auinitm=D.. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lin, A.; Jiao, L. Eight Weeks of Resistance Training with Blood Flow Restriction Improve Cardiac Function and Vascular Endothelial Function in Healthy Young Asian Males. Int. Health 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, D.; Eibye, K.H.; Hostrup, M.; Bangsbo, J. Blood Flow-Restricted Training Enhances Thigh Glucose Uptake during Exercise and Muscle Antioxidant Function in Humans. Metabolism 2019, 98, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Conceição, M.S.; Junior, E.M.M.; Telles, G.D.; Libardi, C.A.; Castro, A.; Andrade, A.L.L.; Brum, P.C.; Urias, Ú.; Kurauti, M.A.; Júnior, J.M.C.; et al. Augmented Anabolic Responses after 8-Wk Cycling with Blood Flow Restriction. Med. Sci. Sports Exerc. 2019, 51, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.A.; Hunt, J.E.A.; Lewis, M.P.; Martin, N.R.W.; Player, D.J.; Stangier, C.; Taylor, C.W.; Turner, M.C. The Acute Angiogenic Signalling Response to Low-Load Resistance Exercise with Blood Flow Restriction. Eur. J. Sport Sci. 2018, 18, 397–406. [Google Scholar] [CrossRef]
- Gustafsson, T.; Ameln, H.; Fischer, H.; Sundberg, C.J.; Timmons, J.A.; Jansson, E. VEGF-A Splice Variants and Related Receptor Expression in Human Skeletal Muscle Following Submaximal Exercise. J. Appl. Physiol. 2005, 98, 2137–2146. [Google Scholar] [CrossRef]
- CONCEIÇÃO, M.S.; CHACON-MIKAHIL, M.P.T.; TELLES, G.D.; LIBARDI, C.A.; JÚNIOR, E.M.M.; VECHIN, F.C.; DE ANDRADE, A.L.L.; GÁSPARI, A.F.; BRUM, P.C.; CAVAGLIERI, C.R.; et al. Attenuated PGC-1α Isoforms Following Endurance Exercise with Blood Flow Restriction. Med. Sci. Sport. Exerc. 2016, 48, 1699–1707. [Google Scholar] [CrossRef]
- Montgomery, R.; Paterson, A.; Williamson, C.; Florida-James, G.; Ross, M.D. Blood Flow Restriction Exercise Attenuates the Exercise-Induced Endothelial Progenitor Cell Response in Healthy, Young Men. Front. Physiol. 2019, 10, 447. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M.; Hoffman, R.L.; Williams, P.S.; Guiler, M.K.; Knutson, M.J.; McGLynn, M.L.; Kushnick, M.R. Relative Safety of 4 Weeks of Blood Flow-Restricted Resistance Exercise in Young, Healthy Adults. Scand. J. Med. Sci. Sports 2011, 21, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Fukumura, K.; Iida, H.; Nakajima, T. Effects of Detraining after Blood Flow-Restricted Low-Load Elastic Band Training on Muscle Size and Arterial Stiffness in Older Women. Springerplus 2015, 4, 348. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Fukumura, K.; Uchida, Y.; Koshi, H.; Iida, H.; Masamune, K.; Yamasoba, T.; Sato, Y.; Nakajima, T. Effects of Low-Load, Elastic Band Resistance Training Combined With Blood Flow Restriction on Muscle Size and Arterial Stiffness in Older Adults. J. Gerontol. A. Biol. Sci. Med. Sci. 2015, 70, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Early, K.S.; Rockhill, M.; Bryan, A.; Tyo, B.; Buuck, D.; McGinty, J. EFFECT OF BLOOD FLOW RESTRICTION TRAINING ON MUSCULAR PERFORMANCE, PAIN AND VASCULAR FUNCTION. Int. J. Sports Phys. Ther. 2020, 15, 892–900. [Google Scholar] [CrossRef] [PubMed]
- S, A.; PGaspar, A.; Degens, H.; DNJ de Matos, L. The Effects of Blood Flow Restriction Exercise on Vascular Function in the Elderly: A Systematic Review. Integr. Clin. Med. 2019, 3. [Google Scholar] [CrossRef]
- Sato, Y. The History and Future of KAATSU Training. Int. J. KAATSU Train. Res. 2005, 1, 1–5. [Google Scholar] [CrossRef]
- Freitas, E.D.S.; Karabulut, M.; Bemben, M.G. The Evolution of Blood Flow Restricted Exercise. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef]
- Lixandrão, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceição, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of Muscle Strength and Mass Adaptations Between High-Load Resistance Training Versus Low-Load Resistance Training Associated with Blood-Flow Restriction: A Systematic Review and Meta-Analysis. Sport. Med. 2018, 48, 361–378. [Google Scholar] [CrossRef]
- Slysz, J.; Stultz, J.; Burr, J.F. The Efficacy of Blood Flow Restricted Exercise: A Systematic Review & Meta-Analysis. J. Sci. Med. Sport 2016, 19, 669–675. [Google Scholar] [CrossRef]
- Damas, F.; Phillips, S.M.; Lixandrão, M.E.; Vechin, F.C.; Libardi, C.A.; Roschel, H.; Tricoli, V.; Ugrinowitsch, C. Early Resistance Training-Induced Increases in Muscle Cross-Sectional Area Are Concomitant with Edema-Induced Muscle Swelling. Eur. J. Appl. Physiol. 2016, 116. [Google Scholar] [CrossRef]
- Nielsen, J.L.; Aagaard, P.; Bech, R.D.; Nygaard, T.; Hvid, L.G.; Wernbom, M.; Suetta, C.; Frandsen, U. Proliferation of Myogenic Stem Cells in Human Skeletal Muscle in Response to Low-Load Resistance Training with Blood Flow Restriction. J. Physiol. 2012, 590. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.J.; Hussain, S.R. A Review on the Mechanisms of Blood-Flow Restriction Resistance Training-Induced Muscle Hypertrophy. Sport. Med. 2015, 45, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Ladlow, P.; Coppack, R.J.; Dharm-Datta, S.; Conway, D.; Sellon, E.; Patterson, S.D.; Bennett, A.N. Low-Load Resistance Training with Blood Flow Restriction Improves Clinical Outcomes in Musculoskeletal Rehabilitation: A Single-Blind Randomized Controlled Trial. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Manimmanakorn, A.; Hamlin, M.J.; Ross, J.J.; Taylor, R.; Manimmanakorn, N. Effects of Low-Load Resistance Training Combined with Blood Flow Restriction or Hypoxia on Muscle Function and Performance in Netball Athletes. J. Sci. Med. Sport 2013, 16. [Google Scholar] [CrossRef] [PubMed]
- Spranger, M.D.; Krishnan, A.C.; Levy, P.D.; O’Leary, D.S.; Smith, S.A. Blood Flow Restriction Training and the Exercise Pressor Reflex: A Call for Concern. Am. J. Physiol. Circ. Physiol. 2015, 309, H1440–H1452. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Wilson, J.M.; Wilson, G.J.; Pujol, T.J.; Bemben, M.G. Potential Safety Issues with Blood Flow Restriction Training. Scand. J. Med. Sci. Sport. 2011, 21. [Google Scholar] [CrossRef]
- Santler, B.; Goerge, T. Chronic Venous Insufficiency – a Review of Pathophysiology, Diagnosis, and Treatment. JDDG - J. Ger. Soc. Dermatology 2017, 15. [Google Scholar] [CrossRef]
- Pereira-Neto, E.A.; Lewthwaite, H.; Boyle, T.; Johnston, K.; Bennett, H.; Williams, M.T. Effects of Exercise Training with Blood Flow Restriction on Vascular Function in Adults: A Systematic Review and Meta-Analysis. PeerJ 2021, 7. [Google Scholar] [CrossRef]
- Li, S.; Li, S.; Wang, L.; Quan, H.; Yu, W.; Li, T.; Li, W. The Effect of Blood Flow Restriction Exercise on Angiogenesis-Related Factors in Skeletal Muscle Among Healthy Adults: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, N.; Pang, F.; Chen, T. Resistance Training with Blood Flow Restriction on Vascular Function: A Meta-Analysis. Int. J. Sports Med. 2021, 42. [Google Scholar] [CrossRef]
- Ceciliato, J.; Costa, E.C.; Azevêdo, L.; Sousa, J.C.; Fecchio, R.Y.; Brito, L.C. Effect of Resistance Training on Arterial Stiffness in Healthy Subjects: A Systematic Review and Meta-Analysis. Curr. Hypertens. Rep. 2020, 22, 51. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Stein, J.H. Carotid Intima-Media Thickness Should Not Be Referred to as Subclinical Atherosclerosis: A Recommended Update to the Editorial Policy at Atherosclerosis. Atherosclerosis 2020, 312, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk. Circulation 2020, 142, 621–642. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Newby, D.E.; Mills, N.L.; Fujisawa, T.; Cruden, N.L.M. Reproducibility of Radial Artery Flow-Mediated Dilatation and Feasibility as a Model of Mechanical Vascular Injury in Man. Atherosclerosis 2015, 241, e49. [Google Scholar] [CrossRef]
- Pinheiro, V. dos S.; da Silva Tavares, A.C.F.; Volino-Souza, M.; de Oliveira, G.V.; Alvares, T.S. Association between Femoral Artery Flow-Mediated Dilation and Muscle Oxygen Saturation Parameters in Healthy, Young Individuals. J. Cardiovasc. Dev. Dis. 2023, 10. [Google Scholar] [CrossRef]
- O’Brien, M.W.; Liu, H.; Shivgulam, M.E.; Langley, J.E.; Bray, N.W.; Kimmerly, D.S. The Impact of Exercise Training Interventions on Flow-Mediated Dilation: An Umbrella Review Protocol. Heal. Popul. J. 2022, 2. [Google Scholar] [CrossRef]
- Rosenberry, R.; Nelson, M.D. Reactive Hyperemia: A Review of Methods, Mechanisms, and Considerations. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2020, 318.
- Motozato, K.; Suematsu, Y.; Norimatsu, K.; Kusumoto, T.; Miura, S. Reactive Hyperemia Index Associated With Atherosclerotic Cardiovascular Disease Under Treatment for Lifestyle Diseases. J. Clin. Med. Res. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Sasaki, S.; Sasaki, N.; Nakagawa, K.; Ueda, T.; Yoshimizu, A.; Kurisu, S.; Matsuura, H.; Kajiyama, G.; Oshima, T. Daily Aerobic Exercise Improves Reactive Hyperemia in Patients with Essential Hypertension. Hypertension 1999, 33. [Google Scholar] [CrossRef]
- Palombo, C.; Kozakova, M. Arterial Stiffness, Atherosclerosis and Cardiovascular Risk: Pathophysiologic Mechanisms and Emerging Clinical Indications. Vascul. Pharmacol. 2016, 77. [Google Scholar] [CrossRef]
- Fahs, C.A.; Rossow, L.M.; Thiebaud, R.S.; Loenneke, J.P.; Kim, D.; Abe, T.; Beck, T.W.; Feeback, D.L.; Bemben, D.A.; Bemben, M.G. Vascular Adaptations to Low-Load Resistance Training with and without Blood Flow Restriction. Eur. J. Appl. Physiol. 2014, 114, 715–724. [Google Scholar] [CrossRef]
- Suvorava, T.; Metry, S.; Pick, S.; Kojda, G. Alterations in Endothelial Nitric Oxide Synthase Activity and Their Relevance to Blood Pressure. Biochem. Pharmacol. 2022, 205. [Google Scholar] [CrossRef] [PubMed]
- Arefirad, T.; Seif, E.; Sepidarkish, M.; Mohammadian Khonsari, N.; Mousavifar, S.A.; Yazdani, S.; Rahimi, F.; Einollahi, F.; Heshmati, J.; Qorbani, M. Effect of Exercise Training on Nitric Oxide and Nitrate/Nitrite (NOx) Production: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13. [Google Scholar] [CrossRef]
- Song, B.X.; Azhar, L.; Koo, G.K.Y.; Marzolini, S.; Gallagher, D.; Swardfager, W.; Chen, C.; Ba, J.; Herrmann, N.; Lanctôt, K. The Effect of Exercise on Blood Concentrations of Angiogenesis Markers in Older Adults: A Systematic Review and Meta-Analysis. BMC Geriatr. 2023, RS Preprint.
- Januszek, R.; Mika, P.; Nowobilski, R.; Nowak, W.; Kusienicka, A.; Klóska, D.; Maga, P.; Niżankowski, R. Soluble Endoglin as a Prognostic Factor of the Claudication Distance Improvement in Patients with Peripheral Artery Disease Undergoing Supervised Treadmill Training Program. J. Am. Soc. Hypertens. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Atakan, M.M.; Kuang, J.; Hu, Y.; Bishop, D.J.; Yan, X. The Molecular Adaptive Responses of Skeletal Muscle to High-Intensity Exercise/Training and Hypoxia. Antioxidants 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S. Changes in Vascular and Inflammatory Biomarkers after Exercise Rehabilitation in Patients with Symptomatic Peripheral Artery Disease. J. Vasc. Surg. 2019, 70, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Noumairi, M.; Bouallala, A.; EL Mir, S.; Allam, A.; EL Oumri, A.A. Rehabilitation of Patients with Peripheral Arterial Disease. Ann. Med. Surg. 2021, 70. [Google Scholar] [CrossRef] [PubMed]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic Strategies for Enhancing Angiogenesis in Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.W.; Ingham, S.A.; Ferguson, R.A. Acute and Chronic Effect of Sprint Interval Training Combined with Postexercise Blood-Flow Restriction in Trained Individuals. Exp. Physiol. 2016, 101, 143–154. [Google Scholar] [CrossRef]
- Preobrazenski, N.; Islam, H.; Drouin, P.J.; Bonafiglia, J.T.; Tschakovsky, M.E.; Gurd, B.J. A Novel Gravity-Induced Blood Flow Restriction Model Augments ACC Phosphorylation and PGC-1α MRNA in Human Skeletal Muscle Following Aerobic Exercise: A Randomized Crossover Study. Appl. Physiol. Nutr. Metab. 2020, 45. [Google Scholar] [CrossRef]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and Unknown. Pathol. Int. 2022, 72. [Google Scholar] [CrossRef]
- Reimers, A.K.; Knapp, G.; Reimers, C.D. Effects of Exercise on the Resting Heart Rate: A Systematic Review and Meta-Analysis of Interventional Studies. J. Clin. Med. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Carpio-Rivera, E.; Moncada-Jiménez, J.; Salazar-Rojas, W.; Solera-Herrera, A. Acute Effects of Exercise on Blood Pressure: A Meta-Analytic Investigation. Arq. Bras. Cardiol. 2016, 106. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef] [PubMed]
- Mika, P.; Konik, A.; Januszek, R.; Petriczek, T.; Mika, A.; Nowobilski, R.; Nizankowski, R.; Szczeklik, A. Comparison of Two Treadmill Training Programs on Walking Ability and Endothelial Function in Intermittent Claudication. Int. J. Cardiol. 2013, 168. [Google Scholar] [CrossRef] [PubMed]
- Pagan, L.U.; Gomes, M.J.; Okoshi, M.P. Endothelial Function and Physical Exercise. Arq. Bras. Cardiol. 2018, 111. [Google Scholar] [CrossRef]
- Boeno, F.P.; Farinha, J.B.; Ramis, T.R.; MacEdo, R.C.O.; Rodrigues-Krause, J.; Queiroz, J.D.N.; Lopez, P.; Pinto, R.S.; Reischak-Oliveira, A. Effects of a Single Session of High-and Moderate-Intensity Resistance Exercise on Endothelial Function of Middle-Aged Sedentary Men. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Abdalla, D.R. Influence of Exercise or Physical Activity in the Angiogenesis Process: Integrative Review. Online J. Cardiol. Res. Reports 2020, 3. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Fuentes, J.; Da Costa, B.R.; Saltaji, H.; Ha, C.; Cummings, G.G. Blinding in Physical Therapy Trials and Its Association with Treatment Effects: A Meta-Epidemiological Study. Am. J. Phys. Med. Rehabil. 2017, 96. [Google Scholar] [CrossRef]
- Hunt, J.E.A.; Walton, L.A.; Ferguson, R.A. Brachial Artery Modifications to Blood Flow-Restricted Handgrip Training and Detraining. J. Appl. Physiol. 2012, 112, 956–961. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).