Introduction
Polyamines (PAs) including putrescine (PUT), spermidine (SPD) and spermine (SPM) are small, versatile molecules with two or more amino groups and fulfil widespread biological functions. Most living organisms require PAs for survival, but their precise biological functions are only partially understood. The mystery of PA function in molecular and cellular biology [
1] remains unresolved so far.
Many important biological functions of PAs are based on their molecular structure, consisting of two to four potentially charged amino groups with interspaced hydrophobic hydrocarbon chains. This composition offers, among others, two very likely functions: (i) as potential buffer systems and (ii) as interactants with poly-negatively charged macromolecules, like (but not only) DNA and RNA.
Apparently, the presence of simple molecules with multiple positively charged groups is required for important biological functions. The question arises, whether such compounds need to be PAs. Other simple molecules like small peptides with closely spaced positively charged side chains might be suitable as well. The same problem also holds for the interaction with nucleic acids. Are the detailed structures of SPD or SPM really essential for a functional interaction with DNA or RNA molecules?
The present report uses straightforward experiments to provide additional data for answering those questions. Consequently, the behavior of oligolysines (tri-Lys, tetra-Lys, and penta-Lys) was compared to that of PAs (PUT, SPD, and SPM) with respect to buffering capacity and specific interactions with DNA.
Materials and Methods
Polyamine Titrations
Polyamines (PAs) were obtained from Sigma-Aldrich Germany. Putrescine dihydrochloride (PUT), spermidine trihydrochloride (SPD) and spermine tetrahydrochloride (SPM) were each dissolved in 2 mM HCl, yielding a 10 mM solution of the respective PA at a pH-value below 3.0. 500 µl of each PA-solution was then titrated with 2.0 µl of a 500 mM NaOH solution until the pH exceeded a value of 10.0.
Oligolysine Titrations
Oligolysines (LysX) were obtained from Sigma Aldrich Germany. Tri-lysine (Lys3), tetra-lysine (Lys4) and penta-lysine (Lys5) were each dissolved in 5 mM HCl, resulting in a 10 mM solution at a pH-value close to 5.0. 500 µl of each LysX-solution was titrated with 2.0 µl of 500 mM NaOH until the pH-value exceeded 11.0.
Spectroscopic analysis of Polyamine DNA interaction
500 ng/µl herring sperm DNA (Sigma-Aldrich) in a physiological “intracellular” buffer (150 mM KCl, 10 mM NaCl, 10 mM HEPES, 2 mM MgCl2, pH 7.3) was mixed with various concentrations of spermine, spermidine, putrescine and tri-Lys (0 – 45 mM) in a total volume of 20 µl. Samples were incubated at 37°C for 15 min. Finally, absorption spectra between 220 nm and 320 nm were recorded on a TECAN Spark instrument (TECAN group, Männedorf, Switzerland) using the quartz optic NanoQuant plate. Spectra were analysed using the Nano Quant App. Changes in the extinction at 260 nm (E260) were used to detect binding of PAs to DNA.
Results
In general, the intracellular pH value is kept within narrow boundaries in eukaryotic cells [
2,
3]. PAs might be an essential component of this process. The question arises, however, whether other simple molecules like small peptides with closely spaced positively charged side chains might be suitable as well.
Polyamines or oligolysines as potential intracellular buffer systems
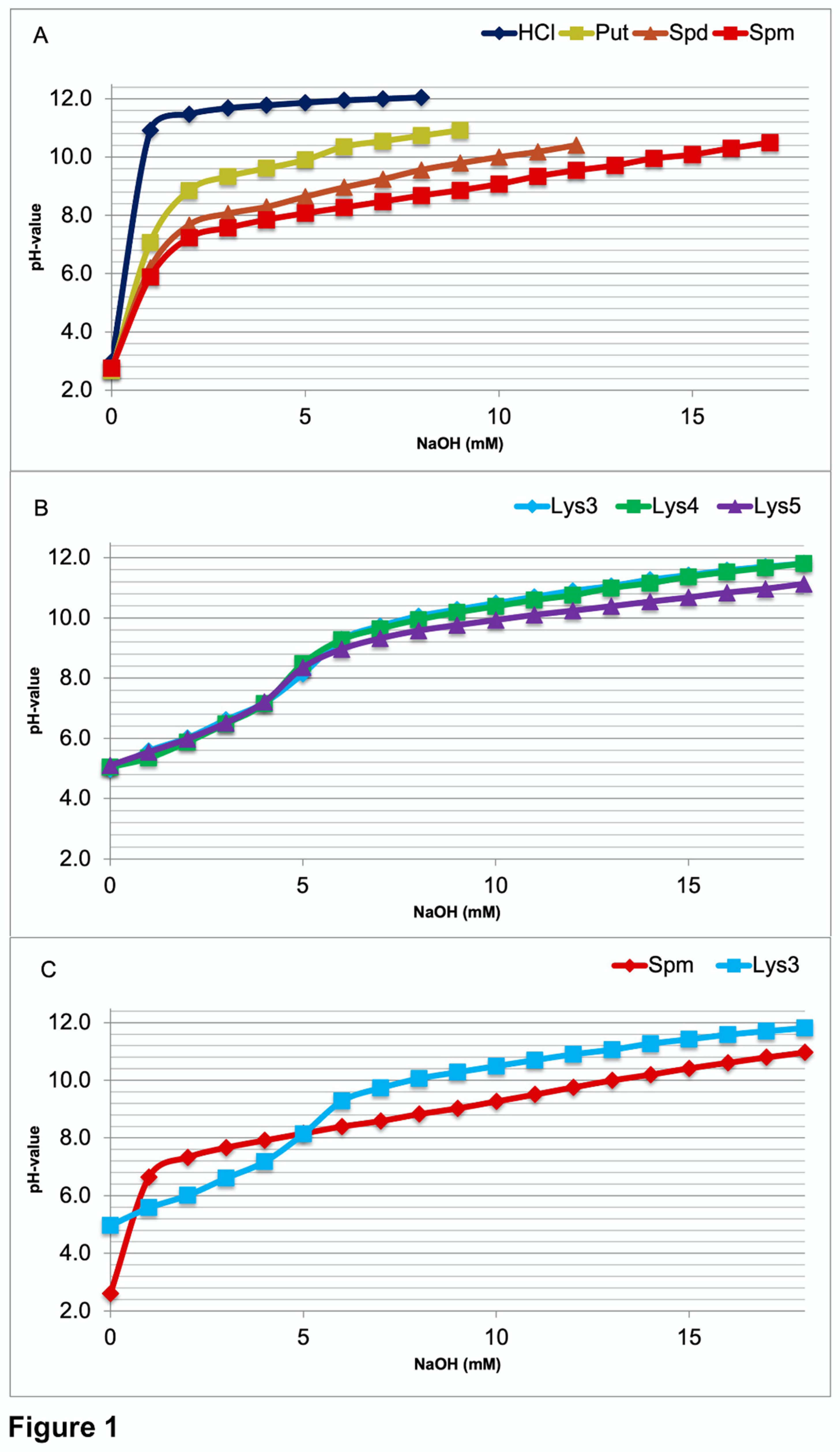
To obtain direct information on the pH range, where intracellular buffering of polyamines or oligolysines might occur, all the compounds were titrated to a pH value above ten. Titration curves of PAs (
Figure 1A) display that buffering of PUT starts at about pH 8.4. Buffering by SPD already begins at about pH 7.6 and by SPM at about pH 7.2, close to their lowest pKa values (Table) as expected. Titration curves of tri-lysine (Lys3), tetra-lysine (Lys4), and penta-lysine (Lys5) all show a first buffering range around pH 6.0 (
Figure 1B). Graphs are closer to each other, those of Lys3 and Lys4 rather identical. In the alkaline pH range buffering starts about 8.8 with Lys5 and about 9.2 with Lys3 and Lys4. The direct comparison between Lys3 and SPM titrations (
Figure 1C) discloses that SPM is a much better buffer at close to neutral pH values.
In most cells the intracellular pH is maintained in the range from 7.0 to 7.4 [
2]. A very similar value (7.03 to 7.46) is also found in hippocampal neurons [
3]. As seen from above (
Figure 1), SPM is the only compound, which may directly contribute to stabilizing the intracellular pH in the required range. Surprisingly, the oligolysines are not suitable for this purpose.
Figure 1.
Comparative titration curves of HCl (control), polyamines, and oligolysins. Before titration all solutions were acidified to a value beneath the lowest pKa of the reactants. When comparing (1A) the polyamines putrescine (Put), spermidine (Spd), and spermine (Spm), the only compound that started buffering below a value of 7.4 was Spm. So, this is the only polyamine (PA), which to some degree may serve to stabilize the intracellular pH value within the physiological range. All the oligolysines (tri-lys, Lys3; tetra-lys, Lys4; penta-lys, Lys5) buffer the first time around pH 6.0 (alpha amino group) and secondly again above 8.0 far outside the physiological range (1B). In addition, the two molecules with four positive charges (Spm and Lys3) are compared directly (1C).
Figure 1.
Comparative titration curves of HCl (control), polyamines, and oligolysins. Before titration all solutions were acidified to a value beneath the lowest pKa of the reactants. When comparing (1A) the polyamines putrescine (Put), spermidine (Spd), and spermine (Spm), the only compound that started buffering below a value of 7.4 was Spm. So, this is the only polyamine (PA), which to some degree may serve to stabilize the intracellular pH value within the physiological range. All the oligolysines (tri-lys, Lys3; tetra-lys, Lys4; penta-lys, Lys5) buffer the first time around pH 6.0 (alpha amino group) and secondly again above 8.0 far outside the physiological range (1B). In addition, the two molecules with four positive charges (Spm and Lys3) are compared directly (1C).
Before titration all solutions were acidified to a value beneath the lowest pKa of the reactants. When comparing (1A) the polyamines putrescine (Put), spermidine (Spd), and spermine (Spm), the only compound that started buffering below a value of 7.4 was Spm. So, this is the only polyamine (PA), which to some degree may serve to stabilize the intracellular pH value within the physiological range. All the oligolysins (tri-lys, Lys3; tetra-lys, Lys4; penta-lys, Lys5) buffer the first time around pH 6.0 (alpha amino group) and secondly again above 8.0 far outside the physiological range (1B). In addition, the two molecules with four positive charges (Spm and Lys3) are compared directly (1C).
Their carboxyl groups buffer around 3.0 (Table), their alpha amino groups around 6.0, and their epsilon amino groups above 8.5, all outside the physiological boundaries.
Table 1.
pKs values of spermidine, spermine [4], di-lysine, and tri-lysine [5].
Table 1.
pKs values of spermidine, spermine [4], di-lysine, and tri-lysine [5].
| Putrescine [4] |
| pK1 |
pK2 |
pK3 |
pK4 |
pK5 |
| Ø |
Ø |
Ø |
9,35 |
10,80 |
| Spermidine [4] |
| pK1 |
pK2 |
pK3 |
pK4 |
pK5 |
| Ø |
Ø |
8,4 |
9,94 |
10,81 |
| Spermine [4] |
| pK1 |
pK2 |
pK3 |
pK4 |
pK5 |
| Ø |
8,1 |
8,9 |
10,1 |
10,9 |
| Lys-Lys [5] |
| pK1 |
pK2 |
pK3 |
pK4 |
pK5 |
| 3,01 |
7,53 |
10,05 |
11,01 |
Ø |
| Lys-Lys-Lys [5] |
| pK1 |
pK2 |
pK3 |
pK4 |
pK5 |
| 3,08 |
7,34 |
9,80 |
10,54 |
11,32 |
Polyamines or oligolysines as potential interactants with nucleic acids
PAs are well known to interact with DNA and RNA [
6,
7,
8] among other polyanionic molecules. The interaction with nucleic acids is of utmost biological importance. It influences the conformation of DNA molecules, determines the density of packed DNA or regulates the translation of RNA molecules (see Derst et al, 2022; this special issue). Again the question arises, whether the detailed structures of SPD or SPM are vital for this functional interaction, or whether oligolysines could serve as potential alternatives.
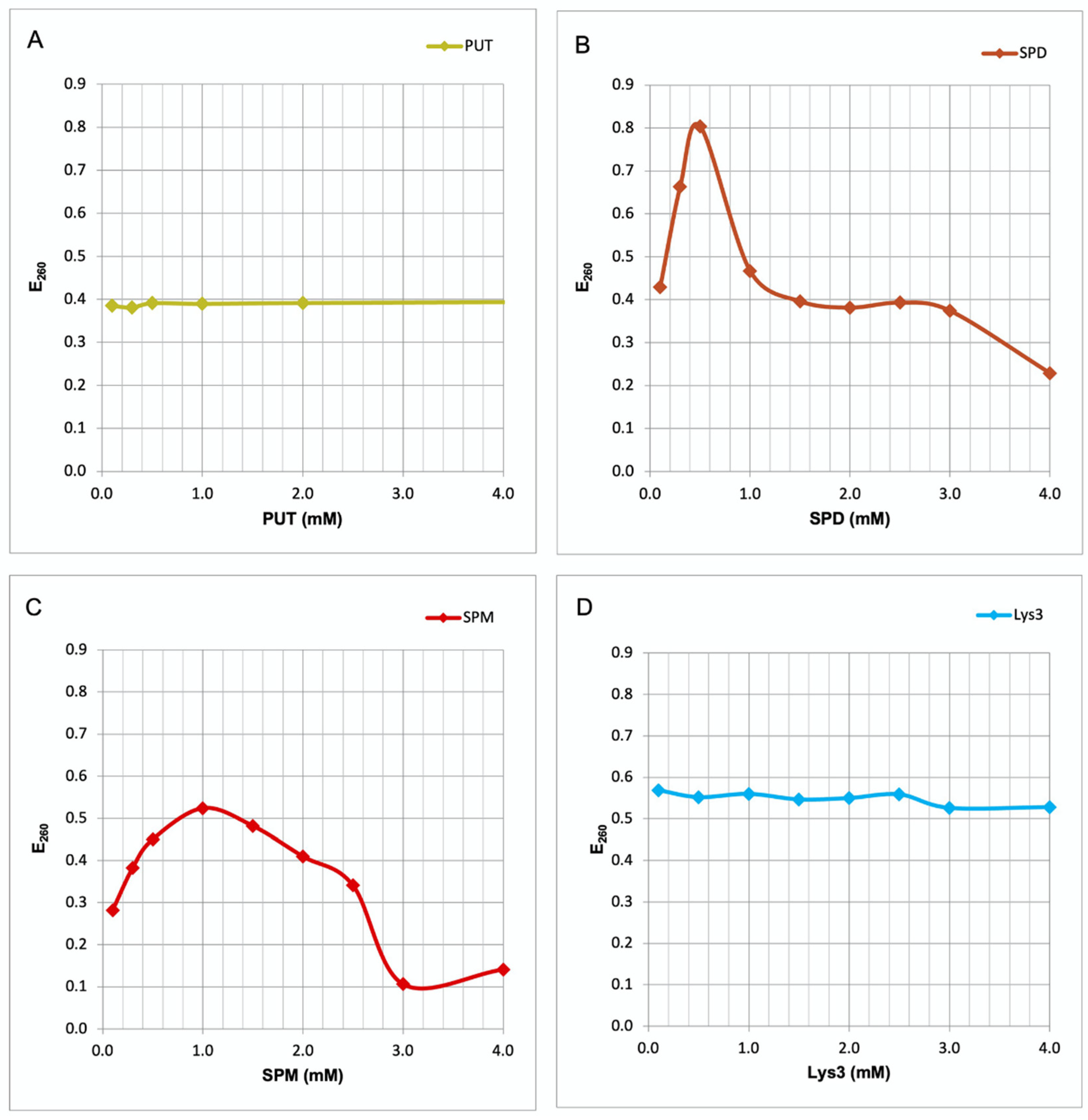
In the present study we measured changes in the UV absorption at 260 nm (E
260) of herring sperm DNA. Addition of PUT did not produce any alteration in the E
260 of the DNA (
Figure 2A). In contrast, SPD and SPM appear to interact with the DNA, as both PAs strongly heighten E
260 values. With SPD the increase is highest at 0.5 mM, followed by a steep decrease in extinction (
Figure 2B). This decrease simply is the result of DNA precipitation at higher SPD concentrations. With SPM the maximum increase occurs at 1 mM (
Figure 2C), followed by precipitation. Again, the elevated E
260 values above 1 mM SPM may be due to turbidity produced by the precipitate. Addition of Lys3, which like SPD bears three positively charged groups, results in no increase at E
260 (
Figure 2D), even at concentrations up to 45 mM. Thus, only the longer PAs like SPD and SPM, and neither PUT nor Lys3 are able to interact and produce conformational changes in double stranded DNA molecules.
Conformational changes of nucleic acids may be recognized by UV spectroscopy [
9]. Consequently, in the present study we measured changes in the UV absorption at 260 nm (E
260) of herring sperm DNA in the presence of PAs or tri-lysine (Lys3). Addition of PUT, the smallest PA with only two charged groups, did not produce any alteration in the E
260 of the DNA (
Figure 2A). In contrast, SPD and SPM appear to interact with the DNA, as both PAs strongly heighten E
260 values. With SPD the increase is highest at 0.5 mM, followed by a steep decrease in extinction. This decrease, however, must not be interpreted as conformational change. It simply is the result of DNA precipitation at higher SPD concentrations (
Figure 2B), which also is abserved visually. With SPM the maximum increase occurs at 1 mM (
Figure 2C), again followed by precipitation. The elevated E
260 values at SPM concentrations above 1 mM may be due to turbidity produced by the precipitate. Interestingly, addition of Lys3, which like SPD also bears three positively charged groups, results in no increase at E
260 (
Figure 2D), even at concentrations up to 45 mM. This fact indicates that Lys3 is not able to interact with the DNA molecule. Taking together, only the longer PAs like SPD and SPM, and neither PUT nor Lys3 are able to interact and produce conformational changes in double stranded DNA molecules.
Discussion
Biologial functions of PAs are largely based on their molecular structure with two to four charged amino groups and interspaced hydrophobic hydrocarbon chains. These structures suggest are two likely biological functions: (i) as potential buffer systems and (ii) as interactants with poly-negatively charged nucleic acids, proteins, or lipid complexes.
PAs do contribute to intracellular pH homeostasis
PAs often are considered as potential buffer systems to directly stabilize the intracellular pH value [
15,
16]. This, however, appears rather unlikely from our data (
Figure 1), as the only PA is SPM, which is able to directly exert minor buffering capacity at intracellular pH values.
Indirect contribution of PAs to intracellular pH homeostasis
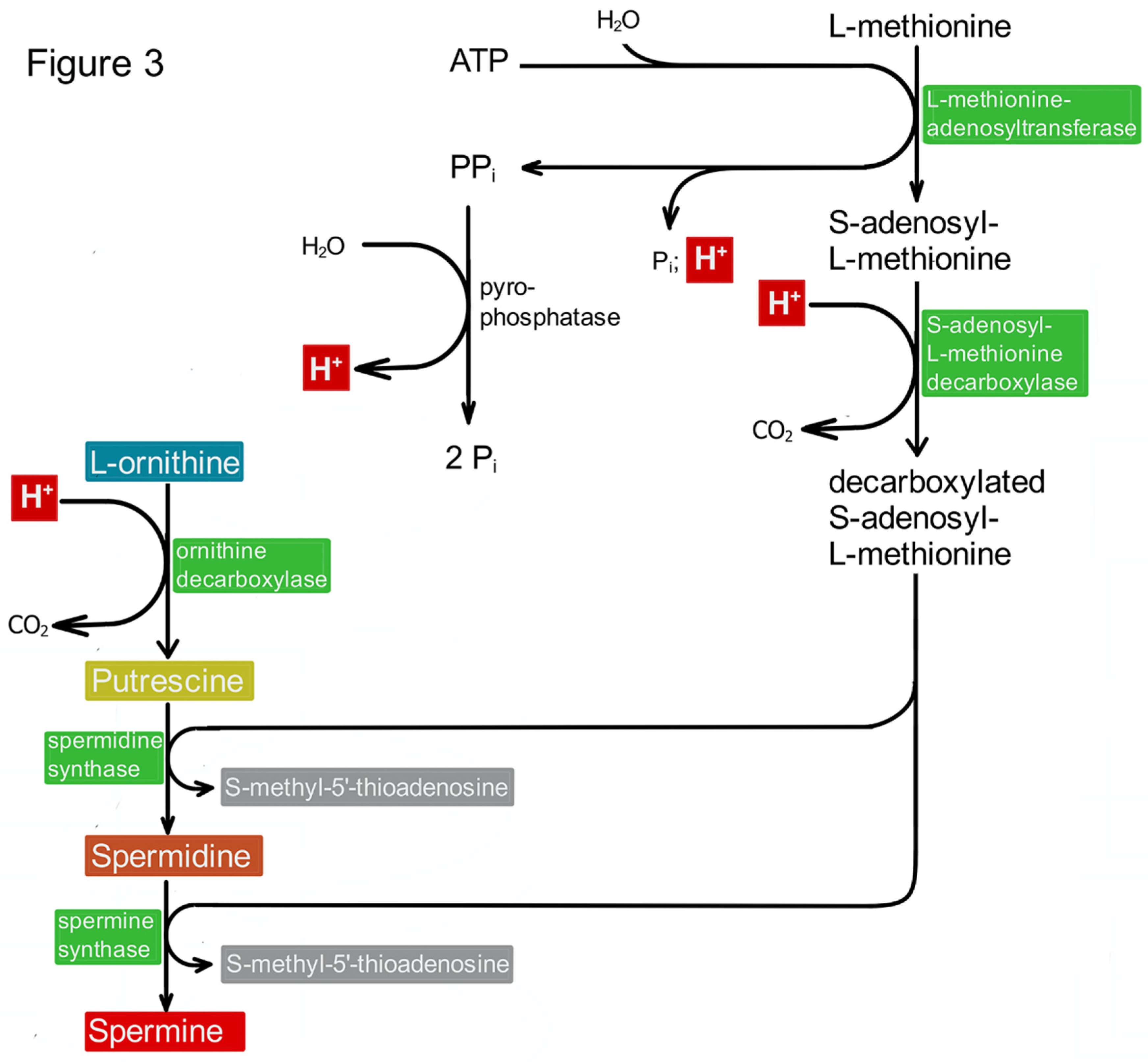
PAs, however, may indirectly contribute to the stabilization of the intracellular pH value. The biosynthesis of PAs includes several steps, which are thought to increase the intracellular pH value. Enzymes like S-adenosylmethionine decarboxylase and ornithine decarboxylase (ODC) consume H
+-ions (see
Figure 3). Consequently, blocking their activity as shown in the case of ODC should decrease the intracellular pH value, and this is actually the case [
15,
16]. Restoring PA synthesis in these cells by the addition of PUT leads to an increase of the pH value again [
16]. This effect may be due to the consumption of two more protons, as the decarboxylation of S-adenosymethionine (SAM) requires one proton, and two molecules of decarboxylated SAM are necessary for the biosynthesis of SPM (
Figure 3). These reflections, however, may be questionable. When the production and the necessary amount of SAM is included in the consideration it turns out that its biosynthesis (methionine plus ATP yields SAM plus three inorganic phosphates) should produce more H
+-ions than had been consumed before.
Proteins also contribute to intracellular pH homeostasis
In addition to small molecular buffers like bicarbonate, phosphate, and PAs, the cell contains a very large amount (20-30%) of proteins [
17]. The corresponding concentrations are estimated to 160 to 310 mg/ml [
18]. Assuming an average molecular weight of 50 kD and a 10% share of side chain carboxylated amino acids (aspartate or glutamate) for cellular proteins [
19], this results in a buffering capacity of about 20 to 30 mM, which is not that much but more that the potential contribution of SPM (intracellular 1 mM[
20]) and may be fundamental.
Proton transport is the most important mechanism for intracellular pH homeostasis
The most important mechanism of intracellular pH homeostasis in neurons [
3] is represented by the metabolic generation of H
+ ions [
21,
22], the effect of the sodium hydrogen antiporter (NHE1 or SLC9A1[
10,
23]), and the action of the bicarbonate-chloride antiporter (AE3 or SLC4A3[
11,
24]). The NHE1 protein possesses a characteristic intracellular domain, which is phosphorylated, when the internal pH-value is decreased below a given set point. Then the transporter activity is increased, and thereby the set point defines the actual internal pH value of the neuron [
25].
In astrocytes intracellular pH is predominantly maintained by the NBCe1 transporter (Natrium Bicarbonate Cotransporter electrogenic 1; SLCA4A), which also influences the extracellular pH in the brain [
12]. PAs are believed to exert direct action on the NHE1 set point thereby regulating the intracellular pH value [
15]. Most likely, therefore, the role of PAs in intracellular pH homeostasis is not due to a direct or indirect buffering mechanism, but on their interaction with the sodium-hydrogen antiporter system.
Conclusion
In this work we investigated two plausible functions of Polyamines (PAs): (i) as potential buffer systems and (ii) as interactants with poly-negatively charged nucleic acids. The report focuses on the question, whether PAs are essential for the assessed functions, or whether other simple molecules like small peptides with closely spaced positively charged side chains might be suitable as well.
In fact, no members of these two groups are optimal for intracellular buffering at physiological pH values. The in vivo contribution of PAs to the stability of the intracellular pH values, therefore, is more likely due to their effect on the set point of the sodium hydrogen antiporter system.
In contrast, SPD and SPM, but not PUT or oligolysines are able to interact with DNA molecules. Obviously, the precise spacing of the positively charged groups is important for biological activity. The present observations contribute to the growing insight into specific roles of PAs in cellular homeostasis and emphasize the importance of their distinct molecular structures.
Author contributions
RWV and CD planned the research and the article. RWV, JR, and CD performed the research, made the figures, wrote the initial draft, and finalized the manuscript. RWV provided resources and acquired funding. All authors reviewed and revised the manuscript.
Funding
This work was supported by the Forschungskommission of the Charité – Universitätsmedizin Berlin.
Data availability statement
Additional data put in the Supplement section and the raw data supporting the conclusions of this review will be made available without undue reservation.
Ethics statement
The animal study was reviewed and approved by the Animal Welfare Committees at the Charité – Universitätsmedizin Berlin, Germany, and at the Universidad Central del Caribe, Puerto Rico, USA.
Acknowledgements
Particular thanks go to Ina Wolter for guaranteeing a perfectly organized laboratory and her excellent technical help.
References
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. Journal of Molecular Biology 2015, 427, 3389–3406. [Google Scholar] [CrossRef] [PubMed]
- Madshus, I.H. Regulation of intracellular pH in eukaryotic cells. Biochemical journal 1988, 250, 1. [Google Scholar] [CrossRef] [PubMed]
- Ruffin, V.; Salameh, A.; Boron, W.; Parker, M. Intracellular pH regulation by acid-base transporters in mammalian neurons. Frontiers in Physiology 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Blagbrough, I.S.; Metwally, A.A.; Geall, A.J. Measurement of Polyamine pKa Values. In Polyamines: Methods and Protocols, Pegg, A.E., Casero, J.R.A., Eds.; Humana Press: Totowa, NJ, 2011; pp. 493–503. [Google Scholar]
- Belitz, H.-D.; Grosch, W. Lehrbuch der Lebensmittelchemie; Springer-Verlag: 2013.
- Igarashi, K.; Sakamoto, I.; Goto, N.; Kashiwagi, K.; Honma, R.; Hirose, S. Interaction between polyamines and nucleic acids or phospholipids. Archives of Biochemistry and Biophysics 1982, 219, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, H.L.; Hall, J. Endogenous polyamine function—the RNA perspective. Nucleic Acids Research 2014, 42, 11275–11290. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.M.; Tolokh, I.S.; Pabit, S.A.; Baker, N.; Onufriev, A.V.; Pollack, L. Spermine Condenses DNA, but Not RNA Duplexes. Biophysical Journal 2017, 112, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Alam, M.K.; Ali, R. Polyamine induced Z-conformation of native calf thymus DNA. FEBS Letters 1995, 368, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Grinstein, S.; Rotin, D.; Mason, M.J. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes 1989, 988, 73–97. [Google Scholar] [CrossRef]
- Reinertsen, K.V.; Tønnessen, T.I.; Jacobsen, J.; Sandvig, K.; Olsnes, S. Role of chloride/bicarbonate antiport in the control of cytosolic pH. Cell-line differences in activity and regulation of antiport. Journal of Biological Chemistry 1988, 263, 11117–11125. [Google Scholar] [CrossRef]
- Gourine, A.V.; Dale, N. Brain H+/CO2 sensing and control by glial cells. Glia 2022, 70, 1520–1535. [Google Scholar] [CrossRef]
- Gourine, A.V.; Kasymov, V.; Marina, N.; Tang, F.; Figueiredo, M.F.; Lane, S.; Teschemacher, A.G.; Spyer, K.M.; Deisseroth, K.; Kasparov, S. Astrocytes Control Breathing Through pH-Dependent Release of ATP. Science 2010, 329, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Theparambil, S.M.; Hosford, P.S.; Ruminot, I.; Kopach, O.; Reynolds, J.R.; Sandoval, P.Y.; Rusakov, D.A.; Barros, L.F.; Gourine, A.V. Astrocytes regulate brain extracellular pH via a neuronal activity-dependent bicarbonate shuttle. Nature Communications 2020, 11, 5073. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R.; Pegg, A.E. Stable intracellular acidification upon polyamine depletion induced by α-difluoromethylornithine or N1,N12-bis(ethyl)spermine in L1210 leukaemia cells. Biochemical Journal 1995, 312, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Miska, J.; Rashidi, A.; Lee-Chang, C.; Gao, P.; Lopez-Rosas, A.; Zhang, P.; Burga, R.; Castro, B.; Xiao, T.; Han, Y.; et al. Polyamines drive myeloid cell survival by buffering intracellular pH to promote immunosuppression in glioblastoma. Science Advances 2021, 7, eabc8929. [Google Scholar] [CrossRef]
- Ellis, R.J. Macromolecular crowding: obvious but underappreciated. Trends in Biochemical Sciences 2001, 26, 597–604. [Google Scholar] [CrossRef]
- Albe, K.R.; Butler, M.H.; Wright, B.E. Cellular concentrations of enzymes and their substrates. Journal of Theoretical Biology 1990, 143, 163–195. [Google Scholar] [CrossRef]
- Kozlowski, L.P. Proteome-pI 2.0: proteome isoelectric point database update. Nucleic Acids Research 2021, 50, D1535–D1540. [Google Scholar] [CrossRef]
- Rieck, J.; Skatchkov, S.N.; Derst, C.; Eaton, M.J.; Veh, R.W. Unique Chemistry, Intake, and Metabolism of Polyamines in the Central Nervous System (CNS) and Its Body. Biomolecules 2022, 12, 501. [Google Scholar] [CrossRef]
- Chesler, M.; Kaila, K. Modulation of pH by neuronal activity. Trends in Neurosciences 1992, 15, 396–402. [Google Scholar] [CrossRef]
- CHESLER, M. Regulation and Modulation of pH in the Brain. Physiological Reviews 2003, 83, 1183–1221. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, Y.; Ilie, A.; Kim, D.; Boucher, A.; Li, B.; Zhang, X.C.; Orlowski, J.; Zhao, Y. Structure and mechanism of the human NHE1-CHP1 complex. Nature Communications 2021, 12, 3474. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, J.; Chen, L.-M. Structure and Function of SLC4 Family HCO3-Transporters. Frontiers in Physiology 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Vallés, P.G.; Bocanegra, V.; Gil Lorenzo, A.; Costantino, V.V. Physiological Functions and Regulation of the Na<sup>+</sup>/H<sup>+</sup> Exchanger [NHE1] in Renal Tubule Epithelial Cells. Kidney and Blood Pressure Research 2015, 40, 452–466. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).