Submitted:
30 April 2023
Posted:
01 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
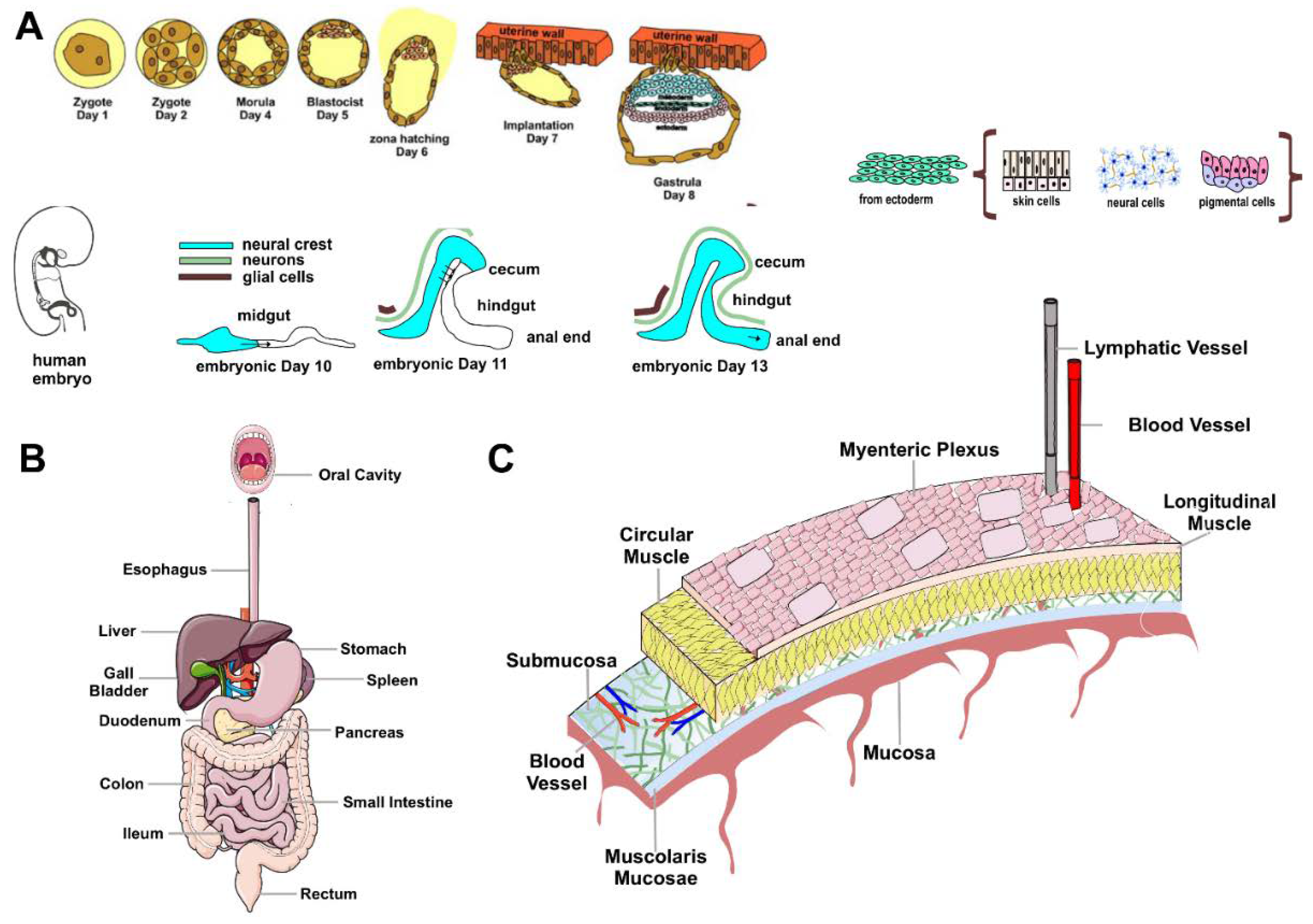
2. Overview of the Enteric Nervous System (ENS): anatomy and function.
3. Evidence of the role of the ENS in animal models of Parkinson's disease
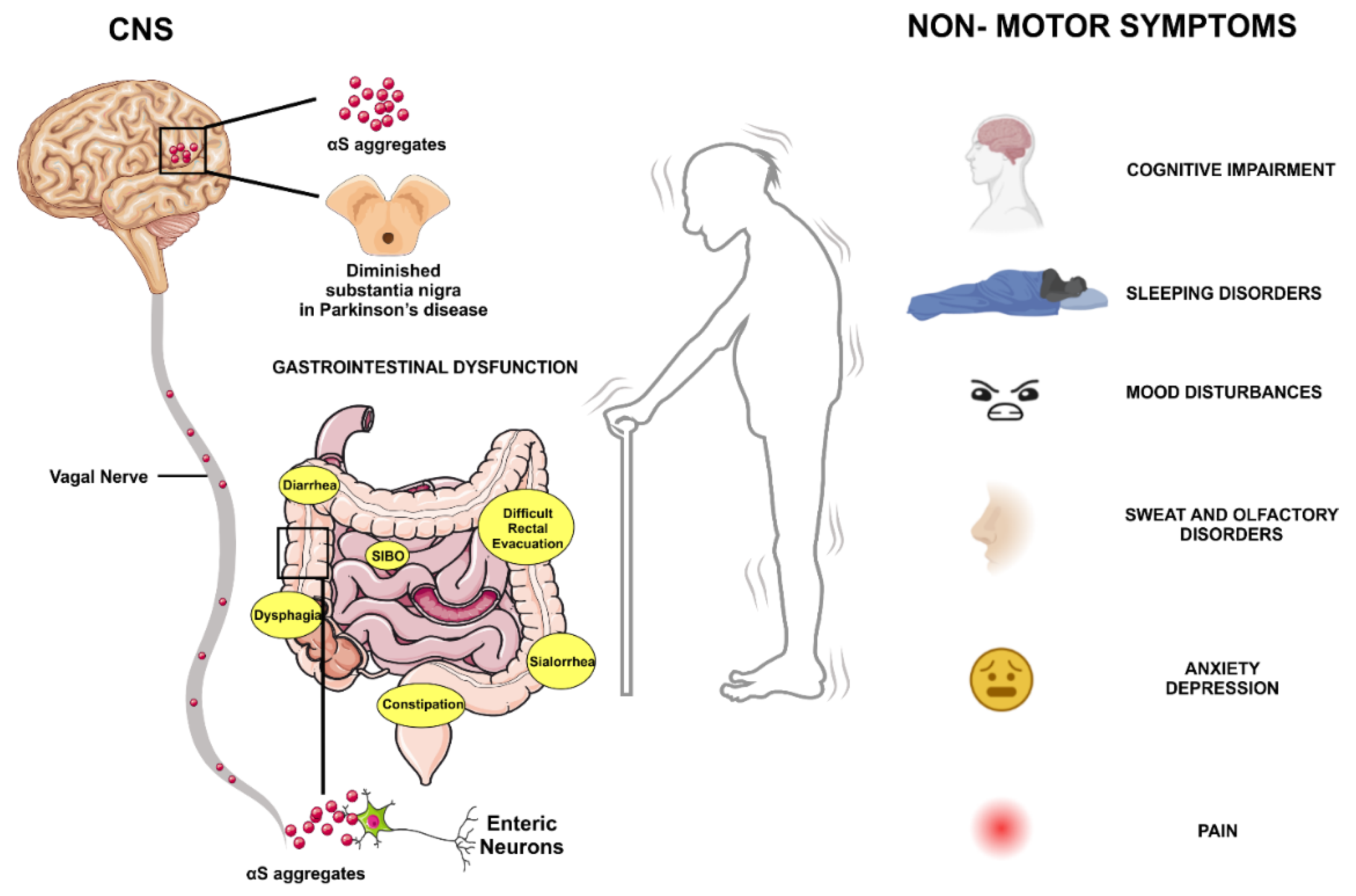
4. The possible role of the ENS in Parkinson's Disease: clinical evidence
| PD symptomps | Affected Neuron types | GI Symptoms | Alteration Biomarker | References |
|---|---|---|---|---|
|
Nd |
Nd |
Gastric emptying Difficult rectal evacuation Slow transit constipation |
Nd |
[73,74,75] |
| Hypo/anosmia Sleep disturbances Rigidity, bradykinesia, tremor, postural instability Cognitive and behavioral disturbances |
Neurodegenerative process starts at the level of the DMV with a pattern of periphery-center (bottom-top) |
Nd |
Increased inclusions of αS and phosphorylated αS |
[80] [78] [80] |
|
Nd |
Nd |
Prolonged intestinal transit, constipation | Minority of cases with Lewy pathology do not have pathological inclusions in the DMV. Limbic-predominant distribution of αS inclusions with less pathology in the brainstem |
[86,87] [89] |
| Motor and cognitive symptoms |
Nd |
Nd |
Decrease in the short-chain fatty acids, including Fusicatenibacter and Faecalibacterium Increase in pro-inflammatory bacteria |
[97] [98] |
|
Nd |
Nd |
Nd |
Systemic and fecal inflammatory markers IFN-γ, TNF-α, and neutrophil gelatinase-associated lipocalin, associated with an elevated expression of Bacteroides and Bifidobacterium. | [99] |
|
Nd |
Nd |
Alteration of intestinal epithelial barrier | Accumulation of enteric αS Activation of immune/inflammatory signaling, including canonical caspase-1- dependent inflammasome pathways Massive release of IL-1β |
[103] [102] [101] |
5. New therapeutic approach targeting the ENS
6. Future perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation LIST
- Acetylcholine (ach)
- α-synuclein (αS)
- Central nervous system (CNS)
- Dopamine (DA)
- Dopamine transporter (DAT)
- Dorsal motor nucleus of the vagus nerve (DMV)
- Enteric nervous system (ENS)
- Enteric neurons (NEs)
- Gastrointestinal tract (GI)
- Gut-brain axis (GBA)
- G-aminobutyric acid (GAB)
- Interferon-gamma (IFN-γ)
- Interleukin -1β (IL-1β)
- Human αs under the thy-1 promoter (Thy1-αS)
- 6-hydroxydopamine (6-OHDA)
- Lewy bodies (LB)
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)
- Nitric oxide (NO)
- Nitric oxide synthase (NOS)
- Olfactory bulb (OB)
- Parkinson's disease (PD)
- Substantia nigra (SN)
- Substantia nigra pars compacta (SNpc)
- 5-hydroxytryptamine or serotonine (5-HT)
- Tumor necrosis factor-alpha (TNF-α)
- Tyrosine hydroxylase (TH)
- Tyrosine Hydroxylase-Immunoreactive (TH-IR)
- Vasoactive intestinal peptide (VIP)
- Wild-type (WT)
References
- Tan, A.H.; Lim, S.Y.; Lang, A.E. The microbiome-gut-brain axis in Parkinson disease - from basic research to the clinic. Nat. Rev. Neurol. 2022, 18, 476–495. [CrossRef]
- Schirinzi, T.; Martella, G.; Pisani, A. Double hit mouse model of Parkinson’s disease. Oncotarget 2016, 7, 80109–80110. [CrossRef]
- Kline, E.M.; Houser, M.C.; Herrick, M.K.; Seibler, P.; Klein, C.; West, A.; Tansey, M.G. Genetic and environmental factors in parkinson’s disease converge on immune function and inflammation. Mov. Disord. 2021, 36, 25–36. [CrossRef]
- Martella, G.; Madeo, G.; Maltese, M.; Vanni, V.; Puglisi, F.; Ferraro, E.; Schirinzi, T.; Valente, E.M.; Bonanni, L.; Shen, J.; Mandolesi, G.; Mercuri, N.B.; Bonsi, P.; Pisani, A. Exposure to low-dose rotenone precipitates synaptic plasticity alterations in PINK1 heterozygous knockout mice. Neurobiol. Dis. 2016, 91, 21–36. [CrossRef]
- Poewe, W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15 Suppl 1, 14–20. [CrossRef]
- Noyce, A.J.; Bestwick, J.P.; Silveira-Moriyama, L.; Hawkes, C.H.; Giovannoni, G.; Lees, A.J.; Schrag, A. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann. Neurol. 2012, 72, 893–901. [CrossRef]
- Cersosimo, M.G.; Benarroch, E.E. Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol. Dis. 2012, 46, 559–564. [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [CrossRef]
- Lomax, A.E.; Fernández, E.; Sharkey, K.A. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol. Motil. 2005, 17, 4–15. [CrossRef]
- Giaroni, C.; De Ponti, F.; Cosentino, M.; Lecchini, S.; Frigo, G. Plasticity in the enteric nervous system. Gastroenterology 1999, 117, 1438–1458. [CrossRef]
- Leclair-Visonneau, L.; Neunlist, M.; Derkinderen, P.; Lebouvier, T. The gut in Parkinson’s disease: Bottom-up, top-down, or neither? Neurogastroenterol. Motil. 2020, 32, e13777. [CrossRef]
- Horsager, J.; Andersen, K.B.; Knudsen, K.; Skjærbæk, C.; Fedorova, T.D.; Okkels, N.; Schaeffer, E.; Bonkat, S.K.; Geday, J.; Otto, M.; Sommerauer, M.; Danielsen, E.H.; Bech, E.; Kraft, J.; Munk, O.L.; Hansen, S.D.; Pavese, N.; Göder, R.; Brooks, D.J.; Berg, D.; Borghammer, P. Brain-first versus body-first Parkinson’s disease: A multimodal imaging case-control study. Brain 2020. [CrossRef]
- Arotcarena, M.-L.; Dovero, S.; Prigent, A.; Bourdenx, M.; Camus, S.; Porras, G.; Thiolat, M.-L.; Tasselli, M.; Aubert, P.; Kruse, N.; Mollenhauer, B.; Trigo Damas, I.; Estrada, C.; Garcia-Carrillo, N.; Vaikath, N.N.; El-Agnaf, O.M.A.; Herrero, M.T.; Vila, M.; Obeso, J.A.; Derkinderen, P.; Bezard, E. Bidirectional gut-to-brain and brain-to-gut propagation of synucleinopathy in non-human primates. Brain 2020, 143, 1462–1475. [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [CrossRef]
- Chalazonitis, A.; Rao, M. Enteric nervous system manifestations of neurodegenerative disease. Brain Res. 2018, 1693, 207–213. [CrossRef]
- O’Donovan, S.M.; Crowley, E.K.; Brown, J.R.-M.; O’Sullivan, O.; O’Leary, O.F.; Timmons, S.; Nolan, Y.M.; Clarke, D.J.; Hyland, N.P.; Joyce, S.A.; Sullivan, A.M.; O’Neill, C. Nigral overexpression of α-synuclein in a rat Parkinson’s disease model indicates alterations in the enteric nervous system and the gut microbiome. Neurogastroenterol. Motil. 2020, 32, e13726. [CrossRef]
- Dickson, D.W. Parkinson’s disease and parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2012, 2. [CrossRef]
- Ma, C.; Zhang, W.; Cao, M. Role of the peripheral nervous system in PD pathology, diagnosis, and treatment. Front. Neurosci. 2021, 15, 598457. [CrossRef]
- Chalazonitis, A.; Rao, M.; Sulzer, D. Similarities and differences between nigral and enteric dopaminergic neurons unravel distinctive involvement in Parkinson’s disease. npj Parkinsons Disease 2022, 8, 50. [CrossRef]
- Gershon, M.D. The enteric nervous system: A second brain. Hosp Pract (Minneap) 1999, 34, 31–2, 35. [CrossRef]
- The Central Nervous System: Structure and Function - Per Brodal - Google Libri; Oxford University Press, U., 2004, Ed.; ISBN 9780195165609.
- The Enteric Nervous System - John Barton Furness - Google Libri; John Wiley & Sons, 2008, Ed.; ISBN 9781405173445.
- Cussotto, S.; Strain, C.R.; Fouhy, F.; Strain, R.G.; Peterson, V.L.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology (Berl) 2019, 236, 1671–1685. [CrossRef]
- Sasselli, V.; Pachnis, V.; Burns, A.J. The enteric nervous system. Dev. Biol. 2012, 366, 64–73. [CrossRef]
- The enteric nervous system and regulation of intestinal motility - ProQuest. Available online: https://www.proquest.com/docview/222539969?pq-origsite=gscholar&fromopenview=true (accessed on 10 August 2022).
- Structure of Enteric Neurons - Axel Brehmer - Google Libri; Springer Science & Business Media, 2006, Ed.; ISBN 9783540328742.
- Costa, M.; Furness, J.B.; Gibbins, I.L. Chapter 15 Chemical coding of enteric neurons. In; Progress in brain research; Elsevier, 1986; Vol. 68, pp. 217–239 ISBN 9780444807625.
- Furness, J.B.; Costa, M. Types of nerves in the enteric nervous system. In Commentaries in the neurosciences; Elsevier, 1980; pp. 235–252 ISBN 9780080255019.
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.-J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [CrossRef]
- Shirazi-Beechey, S.P.; Moran, A.W.; Batchelor, D.J.; Daly, K.; Al-Rammahi, M. Glucose sensing and signalling; regulation of intestinal glucose transport. Proc. Nutr. Soc. 2011, 70, 185–193. [CrossRef]
- Saffrey, M.J. Cellular changes in the enteric nervous system during ageing. Dev. Biol. 2013, 382, 344–355. [CrossRef]
- McQuade, R.M.; Singleton, L.M.; Wu, H.; Lee, S.; Constable, R.; Di Natale, M.; Ringuet, M.T.; Berger, J.P.; Kauhausen, J.; Parish, C.L.; Finkelstein, D.I.; Furness, J.B.; Diwakarla, S. The association of enteric neuropathy with gut phenotypes in acute and progressive models of Parkinson’s disease. Sci. Rep. 2021, 11, 7934. [CrossRef]
- Lama, J.; Buhidma, Y.; Fletcher, E.J.R.; Duty, S. Animal models of Parkinson’s disease: A guide to selecting the optimal model for your research. Neuronal Signal. 2021, 5, NS20210026. [CrossRef]
- Tieu, K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2011, 1, a009316. [CrossRef]
- Zhang, X.; Li, Y.; Liu, C.; Fan, R.; Wang, P.; Zheng, L.; Hong, F.; Feng, X.; Zhang, Y.; Li, L.; Zhu, J. Alteration of enteric monoamines with monoamine receptors and colonic dysmotility in 6-hydroxydopamine-induced Parkinson’s disease rats. Transl. Res. 2015, 166, 152–162. [CrossRef]
- Anderson, G.; Noorian, A.R.; Taylor, G.; Anitha, M.; Bernhard, D.; Srinivasan, S.; Greene, J.G. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Exp. Neurol. 2007, 207, 4–12. [CrossRef]
- Chaumette, T.; Lebouvier, T.; Aubert, P.; Lardeux, B.; Qin, C.; Li, Q.; Accary, D.; Bézard, E.; Bruley des Varannes, S.; Derkinderen, P.; Neunlist, M. Neurochemical plasticity in the enteric nervous system of a primate animal model of experimental Parkinsonism. Neurogastroenterol. Motil. 2009, 21, 215–222. [CrossRef]
- Zhu, H.C.; Zhao, J.; Luo, C.Y.; Li, Q.Q. Gastrointestinal dysfunction in a Parkinson’s disease rat model and the changes of dopaminergic, nitric oxidergic, and cholinergic neurotransmitters in myenteric plexus. J. Mol. Neurosci. 2012, 47, 15–25. [CrossRef]
- Tian, Y.M.; Chen, X.; Luo, D.Z.; Zhang, X.H.; Xue, H.; Zheng, L.F.; Yang, N.; Wang, X.M.; Zhu, J.X. Alteration of dopaminergic markers in gastrointestinal tract of different rodent models of Parkinson’s disease. Neuroscience 2008, 153, 634–644. [CrossRef]
- Singaram, C.; Ashraf, W.; Gaumnitz, E.A.; Torbey, C.; Sengupta, A.; Pfeiffer, R.; Quigley, E.M. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet 1995, 346, 861–864. [CrossRef]
- Li, Z.S.; Schmauss, C.; Cuenca, A.; Ratcliffe, E.; Gershon, M.D. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: Analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J. Neurosci. 2006, 26, 2798–2807. [CrossRef]
- Walker, J.K.; Gainetdinov, R.R.; Mangel, A.W.; Caron, M.G.; Shetzline, M.A. Mice lacking the dopamine transporter display altered regulation of distal colonic motility. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G311-8. [CrossRef]
- Bové, J.; Prou, D.; Perier, C.; Przedborski, S. Toxin-induced models of Parkinson’s disease. NeuroRx 2005, 2, 484–494. [CrossRef]
- Jackson-Lewis, V.; Jakowec, M.; Burke, R.E.; Przedborski, S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration 1995, 4, 257–269. [CrossRef]
- Heikkila, R.E.; Hess, A.; Duvoisin, R.C. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science 1984, 224, 1451–1453. [CrossRef]
- Li, Z.S.; Pham, T.D.; Tamir, H.; Chen, J.J.; Gershon, M.D. Enteric dopaminergic neurons: Definition, developmental lineage, and effects of extrinsic denervation. J. Neurosci. 2004, 24, 1330–1339. [CrossRef]
- Wakabayashi, K.; Takahashi, H.; Ohama, E.; Ikuta, F. Parkinson’s disease: An immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990, 79, 581–583. [CrossRef]
- Colucci, M.; Cervio, M.; Faniglione, M.; De Angelis, S.; Pajoro, M.; Levandis, G.; Tassorelli, C.; Blandini, F.; Feletti, F.; De Giorgio, R.; Dellabianca, A.; Tonini, S.; Tonini, M. Intestinal dysmotility and enteric neurochemical changes in a Parkinson’s disease rat model. Auton. Neurosci. 2012, 169, 77–86. [CrossRef]
- Zheng, L.F.; Song, J.; Fan, R.F.; Chen, C.L.; Ren, Q.Z.; Zhang, X.L.; Feng, X.Y.; Zhang, Y.; Li, L.S.; Zhu, J.X. The role of the vagal pathway and gastric dopamine in the gastroparesis of rats after a 6-hydroxydopamine microinjection in the substantia nigra. Acta Physiol (Oxf) 2014, 211, 434–446. [CrossRef]
- Rota, L.; Pellegrini, C.; Benvenuti, L.; Antonioli, L.; Fornai, M.; Blandizzi, C.; Cattaneo, A.; Colla, E. Constipation, deficit in colon contractions and alpha-synuclein inclusions within the colon precede motor abnormalities and neurodegeneration in the central nervous system in a mouse model of alpha-synucleinopathy. Transl. Neurodegener. 2019, 8, 5. [CrossRef]
- Qualman, S.J.; Haupt, H.M.; Yang, P.; Hamilton, S.R. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Gastroenterology 1984, 87, 848–856. [CrossRef]
- Braak, H.; de Vos, R.A.I.; Bohl, J.; Del Tredici, K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett. 2006, 396, 67–72. [CrossRef]
- Kuo, Y.-M.; Li, Z.; Jiao, Y.; Gaborit, N.; Pani, A.K.; Orrison, B.M.; Bruneau, B.G.; Giasson, B.I.; Smeyne, R.J.; Gershon, M.D.; Nussbaum, R.L. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum. Mol. Genet. 2010, 19, 1633–1650. [CrossRef]
- Gispert, S.; Del Turco, D.; Garrett, L.; Chen, A.; Bernard, D.J.; Hamm-Clement, J.; Korf, H.-W.; Deller, T.; Braak, H.; Auburger, G.; Nussbaum, R.L. Transgenic mice expressing mutant A53T human alpha-synuclein show neuronal dysfunction in the absence of aggregate formation. Mol. Cell. Neurosci. 2003, 24, 419–429. [CrossRef]
- Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Parkinsonism Relat. Disord. 2011, 17, 10–15. [CrossRef]
- Noorian, A.R.; Rha, J.; Annerino, D.M.; Bernhard, D.; Taylor, G.M.; Greene, J.G. Alpha-synuclein transgenic mice display age-related slowing of gastrointestinal motility associated with transgene expression in the vagal system. Neurobiol. Dis. 2012, 48, 9–19. [CrossRef]
- Vance, J.M.; Ali, S.; Bradley, W.G.; Singer, C.; Di Monte, D.A. Gene-environment interactions in Parkinson’s disease and other forms of parkinsonism. Neurotoxicology 2010, 31, 598–602. [CrossRef]
- Wang, L.; Fleming, S.M.; Chesselet, M.-F.; Taché, Y. Abnormal colonic motility in mice overexpressing human wild-type alpha-synuclein. Neuroreport 2008, 19, 873–876. [CrossRef]
- Lam, H.A.; Wu, N.; Cely, I.; Kelly, R.L.; Hean, S.; Richter, F.; Magen, I.; Cepeda, C.; Ackerson, L.C.; Walwyn, W.; Masliah, E.; Chesselet, M.-F.; Levine, M.S.; Maidment, N.T. Elevated tonic extracellular dopamine concentration and altered dopamine modulation of synaptic activity precede dopamine loss in the striatum of mice overexpressing human α-synuclein. J. Neurosci. Res. 2011, 89, 1091–1102. [CrossRef]
- Chesselet, M.-F.; Richter, F. Modelling of Parkinson’s disease in mice. Lancet Neurol. 2011, 10, 1108–1118. [CrossRef]
- Schaffernicht, G.; Shang, Q.; Stievenard, A.; Bötzel, K.; Dening, Y.; Kempe, R.; Toussaint, M.; Gündel, D.; Kranz, M.; Reichmann, H.; Vanbesien-Mailliot, C.; Brust, P.; Dieterich, M.; Funk, R.H.W.; Ravens, U.; Pan-Montojo, F. Pathophysiological Changes in the Enteric Nervous System of Rotenone-Exposed Mice as Early Radiological Markers for Parkinson’s Disease. Front. Neurol. 2021, 12, 642604. [CrossRef]
- Pan-Montojo, F.; Schwarz, M.; Winkler, C.; Arnhold, M.; O’Sullivan, G.A.; Pal, A.; Said, J.; Marsico, G.; Verbavatz, J.-M.; Rodrigo-Angulo, M.; Gille, G.; Funk, R.H.W.; Reichmann, H. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci. Rep. 2012, 2, 898. [CrossRef]
- Klingelhoefer, L.; Reichmann, H. Pathogenesis of Parkinson disease--the gut-brain axis and environmental factors. Nat. Rev. Neurol. 2015, 11, 625–636. [CrossRef]
- Pan-Montojo, F.J.; Funk, R.H.W. Oral administration of rotenone using a gavage and image analysis of alpha-synuclein inclusions in the enteric nervous system. J. Vis. Exp. 2010. [CrossRef]
- Arnhold, M.; Dening, Y.; Chopin, M.; Arévalo, E.; Schwarz, M.; Reichmann, H.; Gille, G.; Funk, R.H.W.; Pan-Montojo, F. Changes in the sympathetic innervation of the gut in rotenone treated mice as possible early biomarker for Parkinson’s disease. Clin. Auton. Res. 2016, 26, 211–222. [CrossRef]
- Sharrad, D.F.; Chen, B.N.; Gai, W.P.; Vaikath, N.; El-Agnaf, O.M.; Brookes, S.J.H. Rotenone and elevated extracellular potassium concentration induce cell-specific fibrillation of α-synuclein in axons of cholinergic enteric neurons in the guinea-pig ileum. Neurogastroenterol. Motil. 2017, 29. [CrossRef]
- Paillusson, S.; Tasselli, M.; Lebouvier, T.; Mahé, M.M.; Chevalier, J.; Biraud, M.; Cario-Toumaniantz, C.; Neunlist, M.; Derkinderen, P. α-Synuclein expression is induced by depolarization and cyclic AMP in enteric neurons. J. Neurochem. 2010, 115, 694–706. [CrossRef]
- Camilleri, M.; Cowen, T.; Koch, T.R. Enteric neurodegeneration in ageing. Neurogastroenterol. Motil. 2008, 20, 185–196. [CrossRef]
- Phillips, R.J.; Powley, T.L. Innervation of the gastrointestinal tract: Patterns of aging. Auton. Neurosci. 2007, 136, 1–19. [CrossRef]
- Phillips, R.J.; Walter, G.C.; Ringer, B.E.; Higgs, K.M.; Powley, T.L. Alpha-synuclein immunopositive aggregates in the myenteric plexus of the aging Fischer 344 rat. Exp. Neurol. 2009, 220, 109–119. [CrossRef]
- Braak, H.; Ghebremedhin, E.; Rüb, U.; Bratzke, H.; Del Tredici, K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134. [CrossRef]
- Wolters, E.C.; Braak, H. Parkinson’s disease: Premotor clinico-pathological correlations. J. Neural Transm. Suppl. 2006, 309–319. [CrossRef]
- Krogh, K.; Christensen, P. Neurogenic colorectal and pelvic floor dysfunction. Best Pract. Res. Clin. Gastroenterol. 2009, 23, 531–543. [CrossRef]
- Marrinan, S.; Emmanuel, A.V.; Burn, D.J. Delayed gastric emptying in Parkinson’s disease. Mov. Disord. 2014, 29, 23–32. [CrossRef]
- Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003, 2, 107–116. [CrossRef]
- Lebouvier, T.; Neunlist, M.; Bruley des Varannes, S.; Coron, E.; Drouard, A.; N’Guyen, J.-M.; Chaumette, T.; Tasselli, M.; Paillusson, S.; Flamand, M.; Galmiche, J.-P.; Damier, P.; Derkinderen, P. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS ONE 2010, 5, e12728. [CrossRef]
- Taguchi, T.; Ikuno, M.; Yamakado, H.; Takahashi, R. Animal model for prodromal parkinson’s disease. Int. J. Mol. Sci. 2020, 21. [CrossRef]
- Liepelt-Scarfone, I.; Ophey, A.; Kalbe, E. Cognition in prodromal Parkinson’s disease. Prog. Brain Res. 2022, 269, 93–111. [CrossRef]
- Solla, P.; Wang, Q.; Frau, C.; Floris, V.; Loy, F.; Sechi, L.A.; Masala, C. Olfactory impairment is the main predictor of higher scores at REM sleep behavior disorder (RBD) screening questionnaire in parkinson’s disease patients. Brain Sci. 2023, 13, 599. [CrossRef]
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018, 10. [CrossRef]
- Braak, H.; Del Tredici, K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J Parkinsons Dis 2017, 7, S71–S85. [CrossRef]
- Yilmaz, R.; Hopfner, F.; van Eimeren, T.; Berg, D. Biomarkers of Parkinson’s disease: 20 years later. J. Neural Transm. 2019, 126, 803–813. [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [CrossRef]
- Liu, B.; Fang, F.; Pedersen, N.L.; Tillander, A.; Ludvigsson, J.F.; Ekbom, A.; Svenningsson, P.; Chen, H.; Wirdefeldt, K. Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology 2017, 88, 1996–2002. [CrossRef]
- Kelly, M.J.; Breathnach, C.; Tracey, K.J.; Donnelly, S.C. Manipulation of the inflammatory reflex as a therapeutic strategy. Cell Rep. Med. 2022, 3, 100696. [CrossRef]
- Parkkinen, L.; Pirttilä, T.; Alafuzoff, I. Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol. 2008, 115, 399–407. [CrossRef]
- Frigerio, R.; Fujishiro, H.; Ahn, T.-B.; Josephs, K.A.; Maraganore, D.M.; DelleDonne, A.; Parisi, J.E.; Klos, K.J.; Boeve, B.F.; Dickson, D.W.; Ahlskog, J.E. Incidental Lewy body disease: Do some cases represent a preclinical stage of dementia with Lewy bodies? Neurobiol. Aging 2011, 32, 857–863. [CrossRef]
- Koga, S.; Sekiya, H.; Kondru, N.; Ross, O.A.; Dickson, D.W. Neuropathology and molecular diagnosis of Synucleinopathies. Mol. Neurodegener. 2021, 16, 83. [CrossRef]
- Macefield, V.G.; Henderson, L.A. Identification of the human sympathetic connectome involved in blood pressure regulation. Neuroimage 2019, 202, 116119. [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [CrossRef]
- Dash, S.; Syed, Y.A.; Khan, M.R. Understanding the role of the gut microbiome in brain development and its association with neurodevelopmental psychiatric disorders. Front. Cell Dev. Biol. 2022, 10, 880544. [CrossRef]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20. [CrossRef]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [CrossRef]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell 2019, 6, 454–481. [CrossRef]
- Kim, S.; Kwon, S.-H.; Kam, T.-I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; Shen, C.; Lee, H.; Kulkarni, S.; Pasricha, P.J.; Lee, G.; Pomper, M.G.; Dawson, V.L.; Dawson, T.M.; Ko, H.S. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627-641.e7. [CrossRef]
- Wallen, Z.D.; Demirkan, A.; Twa, G.; Cohen, G.; Dean, M.N.; Standaert, D.G.; Sampson, T.R.; Payami, H. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat. Commun. 2022, 13, 6958. [CrossRef]
- Zhu, M.; Liu, X.; Ye, Y.; Yan, X.; Cheng, Y.; Zhao, L.; Chen, F.; Ling, Z. Gut microbiota: A novel therapeutic target for parkinson’s disease. Front. Immunol. 2022, 13, 937555. [CrossRef]
- Chen, S.-J.; Lin, C.-H. Gut microenvironmental changes as a potential trigger in Parkinson’s disease through the gut-brain axis. J. Biomed. Sci. 2022, 29, 54. [CrossRef]
- Zeng, J.; Wang, X.; Pan, F.; Mao, Z. The relationship between Parkinson’s disease and gastrointestinal diseases. Front. Aging Neurosci. 2022, 14, 955919. [CrossRef]
- Misera, A.; Łoniewski, I.; Palma, J.; Kulaszyńska, M.; Czarnecka, W.; Kaczmarczyk, M.; Liśkiewicz, P.; Samochowiec, J.; Skonieczna-Żydecka, K. Clinical significance of microbiota changes under the influence of psychotropic drugs. An updated narrative review. Front. Microbiol. 2023, 14, 1125022. [CrossRef]
- Horsager, J.; Knudsen, K.; Sommerauer, M. Clinical and imaging evidence of brain-first and body-first Parkinson’s disease. Neurobiol. Dis. 2022, 164, 105626. [CrossRef]
- Molla, M.D.; Akalu, Y.; Geto, Z.; Dagnew, B.; Ayelign, B.; Shibabaw, T. Role of Caspase-1 in the Pathogenesis of Inflammatory-Associated Chronic Noncommunicable Diseases. J. Inflamm. Res. 2020, 13, 749–764. [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [CrossRef]
- Pellegrini, C.; D’Antongiovanni, V.; Miraglia, F.; Rota, L.; Benvenuti, L.; Di Salvo, C.; Testa, G.; Capsoni, S.; Carta, G.; Antonioli, L.; Cattaneo, A.; Blandizzi, C.; Colla, E.; Fornai, M. Enteric α-synuclein impairs intestinal epithelial barrier through caspase-1-inflammasome signaling in Parkinson’s disease before brain pathology. npj Parkinsons Disease 2022, 8, 9. [CrossRef]
- Muenter, M.D.; Tyce, G.M. L-dopa therapy of Parkinson’s disease: Plasma L-dopa concentration, therapeutic response, and side effects. Mayo Clin. Proc. 1971, 46, 231–239.
- Poewe, W.; Antonini, A. Novel formulations and modes of delivery of levodopa. Mov. Disord. 2015, 30, 114–120. [CrossRef]
- Amara, A.W.; Memon, A.A. Effects of Exercise on Non-motor Symptoms in Parkinson’s Disease. Clin. Ther. 2018, 40, 8–15. [CrossRef]
- Ouchi, Y.; Kanno, T.; Okada, H.; Yoshikawa, E.; Futatsubashi, M.; Nobezawa, S.; Torizuka, T.; Tanaka, K. Changes in dopamine availability in the nigrostriatal and mesocortical dopaminergic systems by gait in Parkinson’s disease. Brain 2001, 124, 784–792. [CrossRef]
- Bhidayasiri, R.; Phuenpathom, W.; Tan, A.H.; Leta, V.; Phumphid, S.; Chaudhuri, K.R.; Pal, P.K. Management of dysphagia and gastroparesis in Parkinson’s disease in real-world clinical practice - Balancing pharmacological and non-pharmacological approaches. Front. Aging Neurosci. 2022, 14, 979826. [CrossRef]
- Mukherjee, A.; Biswas, A.; Das, S.K. Gut dysfunction in Parkinson’s disease. World J. Gastroenterol. 2016, 22, 5742–5752. [CrossRef]
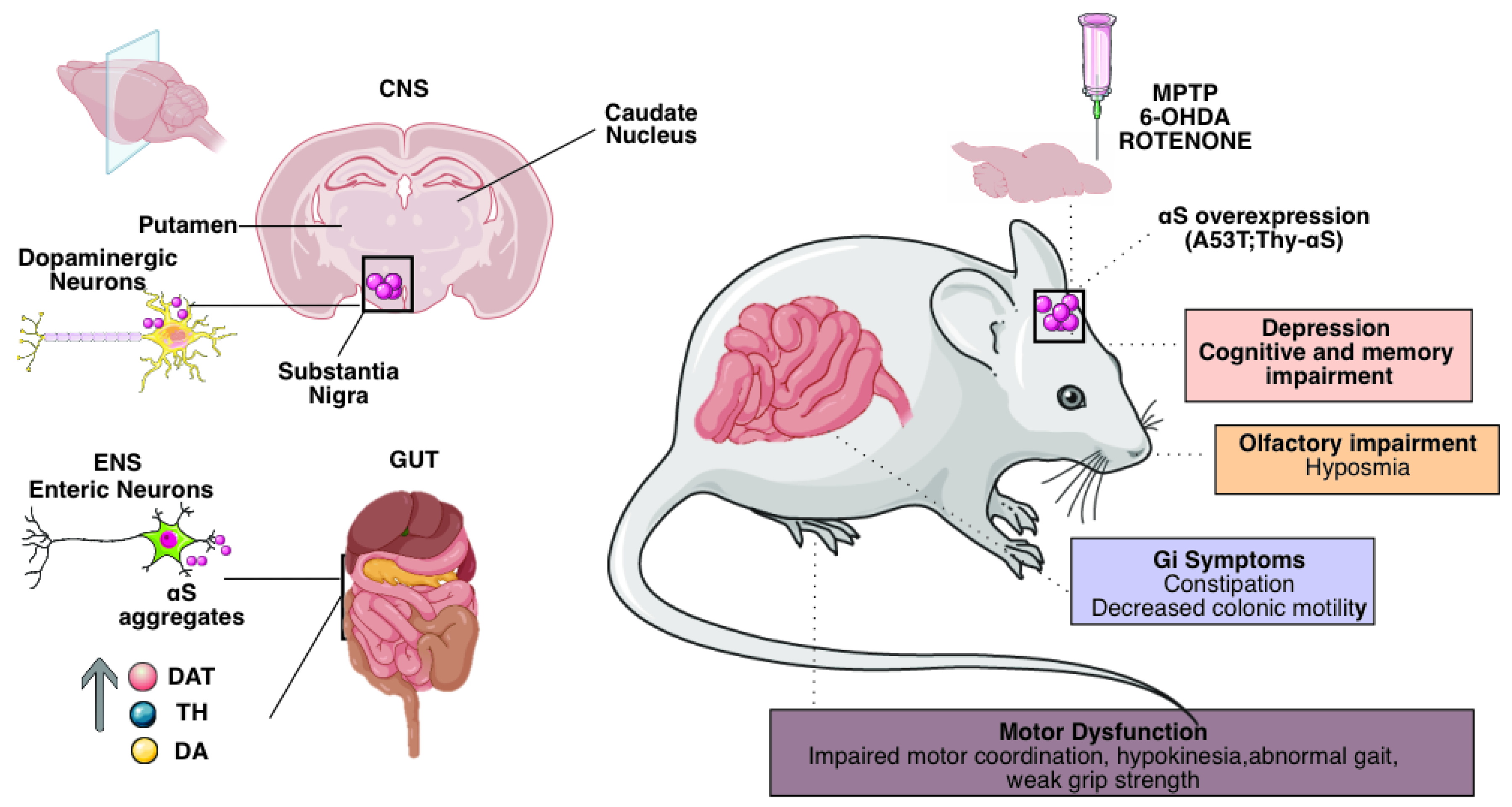
| PD Model | Affected Neuron types | GI Symptoms | Alteration Biomarker | References |
|---|---|---|---|---|
|
MPTP mice |
Loss of dopaminergic neurons in the myenteric plexus | Absence of severe defects in gastrointestinal motility. increased contraction and decreased relaxation of colon muscle in response to electric field stimulation of NEs |
Nd |
[36,37] |
|
MPTP rats (Peripheral administration) |
Unaltered number of dopaminergic neurons in the SNpc Presence of TH-IR neurons in the GI tract |
Nd |
Unaltered expression of dopaminergic markers in the SNpc |
[39] [40] |
|
6-OHDA rats |
Alterations in the monoaminergic and cholinergic system | Delayed gastric emptying and constipation, which could be related to increased gastrointestinal TH and decreased NOS. Increased DA concentration in the colon, which is more likely to cause constipation. Decreased colonic motility. |
Unaltered cholinergic transmitters. Elevated protein levels of TH and DAT both in the epithelium and neurons of the gastrointestinal tract, resulting in increased DA content in the gut and delayed gastric emptying |
[35] [38,39] [47,48,49] |
|
A53T mice (Expressing a mutant form of human αS) |
Disruption of efferent vagal processes that project from the DMV to the gastrointestinal tract | Related slowing of gastrointestinal motility caused by expression of human αS in the DMV | Accumulation of αS aggregates in the ENS before changes in the CNS. | [53] [8,55] [56] |
|
Thy1-αS mice |
Nd |
Striatal dopamine loss only after 14 months: manifesting motor and non-motor deficits, such as olfactory disturbances, as early as 2-3 months of age | Increased transit time and colonic content. Overexpression of αS in the colonic myenteric nervous system. Reduced response to defecation stimuli. |
[57] [58] [59,60] |
|
Rotenone mice model |
Reduced sympathetic noradrenergic and vagal cholinergic gut innervation | Progressive αS deposition both in ENS and CNS neurons affected by PD such as neurons in the myenteric plexus, the DMV, the spinal cord, and the SNS |
Nd |
[61] [64] [65] [66] |
|
Fischer 344 rat |
Neuronal loss and changes in neurochemical phenotype in the ENS | Dystrophic enteric neurons that contain αS aggregates reminiscent of Lewy pathology | Motility disorders | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





