Submitted:
30 April 2023
Posted:
01 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Pathways of oxidative stress
2.1. Production of oxygen radicals
2.2. Antioxidative defense
2.3. Sources of oxidative stress in COPD
3. Oxidative stress – a link between COPD and cardiovascular comorbidities
3.1. COPD and vascular aging, hypertension
3.1.1. Oxidative stress in COPD and vascular aging
3.1.2. The consequences and aggravators of oxidative stress
3.2. COPD and pulmonary arterial hypertension (PAH)
3.3. COPD and accelerated atherosclerosis
3.4. COPD and cardiac diseases
4. Biomarkers of oxidative stress in COPD and cardiovascular diseases
4.1. Biological biomarkers
4.2. Heart rate variability – a potential non-conventional biomarker of oxidative stress in COPD and CVD
| Sample | Biomarker | Finding | Reference |
|---|---|---|---|
| Blood (systemic oxidative stress) | |||
| erythrocytes | reduced GSH | ↓ in COPD patients (n=236) vs. controls (n=150) and correlates with disease severity – all patients are smokers or ex-smokers | [201] |
| ↓ in stable COPD patients (n=41) vs. controls (n=30); and further decreased in exacerbated COPD (n=21) – varying smoking status | [203] | ||
| SOD activity | ↓ in COPD patients (n=140) vs. healthy controls (n=75) – varying smoking status | [75] | |
| ↓ in COPD patients (n=234) vs. healthy controls (n=182) – varying smoking status | [218] | ||
| ↓ in COPD patients (n=82) vs. non-smoking healthy controls (n=22) | [80] | ||
| ↓ in stable COPD patients (n=21) vs. non-smoking healthy controls (n=24) | [82] | ||
| CAT activity | ↓ in COPD patients (n=236) vs. controls (n=150) and correlates with disease severity – all patients are smokers or ex-smokers | [201] | |
| ↓ in COPD patients (n=140) vs. healthy controls (n=75) – varying smoking status | [75] | ||
| → comparable in COPD patients (n=82) and non-smoking healthy controls (n=22) | [80] | ||
| GPx activity | ↓ in COPD patients (n=236) vs. controls (n=150) – all patients are smokers or ex-smokers | [201] | |
| ↓ in COPD patients (n=140) vs. healthy controls (n=75) – varying smoking status | [75] | ||
| ↓ in COPD patients (n=82) vs. non-smoking healthy controls (n=22) | [80] | ||
| ↓ in COPD patients (n=20) vs. healthy controls (n=50) – varying smoking status | [217] | ||
| plasma | MDA | ↑ in COPD patients (n=236) vs. controls (n=150) – and correlates with disease severity all patients are smokers or ex-smokers | [201] |
| ↑ in stable COPD patients (n=41) vs. controls (n=30); and further decreased in exacerbated COPD (n=21) – varying smoking status | [203] | ||
| ↑ in COPD patients (n=140) vs. healthy controls (n=75) – varying smoking status | [75] | ||
| ↑ in COPD patients (n=82) vs. non-smoking healthy controls (n=22) | [80] | ||
| ↑ in COPD patients (n=20) vs. healthy controls (n=50) – varying smoking status | [217] | ||
| ↑ in COPD patients (n=100) vs. controls (n=100) – varying smoking status | [206] | ||
| ↑ in COPD patients (n=100) vs. controls (n=100) – varying smoking status | [207] | ||
| ↑ in healthy smokers (n=30) and in patients with stable (n=7) and exacerbated COPD (n=31) than in healthy non-smokers (n=30) | [208] | ||
| ↑ in COPD patients (n=106) vs. controls (n=45) – varying smoking status | [210] | ||
| ↑ in COPD patients exposed to wood smoke (n = 30) and tobacco smoking (n = 30) vs. healthy controls (n=30) | [211] | ||
| ↑ in COPD patients (n=815) vs. controls (n=530) – varying smoking status - METANALYIS | [212] | ||
| ↑ in severe COPD patients (n=74) vs. controls (n=41) – varying smoking status | [213] | ||
| ↑ in COPD patients (n=26) vs. controls (n=28) –smoking status n.a. | [214] | ||
| ↑ in smoker COPD patients (n=202) vs. smoker controls without COPD (n=136) | [83,215] | ||
| ↑ in patients with exacerbated (n=43) and stable (n=35), and in healthy smokers (n=14) vs. healthy non-smokers (n=14) | [84] | ||
| → comparable in ex-smoker COPD patients (n=11) and non-smoking healthy controls (n=12), exercise induces increase only in COPD | [239] | ||
| AOPP | ↑ in severe COPD patients (n=74) vs. controls (n=41) – varying smoking status | [213] | |
| reduced GSH | ↓ in COPD patients (n=20) vs. healthy controls (n=50) – varying smoking status | [217] | |
| ↓ in chronic smokers with stable COPD (n = 20) and without COPD (n = 20) vs. healthy non-smokers (n = 20) | [240] | ||
| ↓ in smoker COPD patients (n=202) vs. smoker controls without COPD (n=136) | [83,215] | ||
| ↓ in patients with exacerbated (n=43) and stable (n=35), and in healthy smokers (n=14) vs. healthy non-smokers (n=14) | [84] | ||
| SOD activity | ↓ in severe COPD patients (n=74) vs. controls (n=41) – varying smoking status | [213] | |
| ↓ in patients with exacerbated (n=43) and stable (n=35), and in healthy smokers (n=14) vs. healthy non-smokers (n=14) | [84] | ||
| ↓ in patients with stable COPD (n=96) vs. controls without COPD (n=96) – varying smoking status | [216] | ||
| CAT activity | ↓ in smoker COPD patients (n=202) vs. smoker controls without COPD (n=136) | [83,215] | |
| → comparable in patients with stable COPD (n=96) and without COPD (n=96) – varying smoking status | [216] | ||
| GPx activity | ↓ in smoker COPD patients (n=202) vs. smoker controls without COPD (n=136) | [83,215] | |
| ↓ in patients with exacerbated (n=43) and stable (n=35), and in healthy smokers (n=14) vs. healthy non-smokers (n=14) | [84] | ||
| ↓ in COPD patients (n=82) vs. non-smoking healthy controls (n=22) | [80] | ||
| whole blood | total glutathione | ↑ in COPD patients (n=140) vs. healthy controls (n=75) – varying smoking status | [75,80] |
| ↑ in COPD patients (n=82) vs. non-smoking healthy controls (n=22) | [80] | ||
| GPx activity | ↓ in stable COPD patients (n=21) vs. non-smoking healthy controls (n=24) | [82] | |
| Exhaled air (systemic/local oxidative stress) | |||
| CO | ↑ in ex-smokers with COPD (n=15) and in smokers with COPD (n=15) vs. non-smoking healthy controls (n=10) | [241] | |
| ethane | ↑ COPD (n=12) vs. healthy (n=14) (all ex-smokers) | [242] | |
| Exhaled breath condensate (systemic/local oxidative stress) | |||
| hexanal, heptanal, nonanal | ↑ in patients with stable COPD (n=20) vs. non-smoking healthy subjects (n=20), but not vs. smoking controls (n=12) | [205] | |
| ↑ in patients with COPD (n=11; smokers and ex-smokers) vs. non-smoking controls (n=9) | [204] | ||
| MDA | ↑ in patients with stable COPD (n=20) vs. non-smoking healthy subjects (n=20), and also vs. smoking controls (n=12) | [205] | |
| ↑ in patients with COPD (n=11; smokers and ex-smokers) vs. non-smoking controls (n=9) | [204] | ||
| ↑ in patients with COPD (n=73) vs. healthy non-smokers (n=14); an inverse correlation between MDA concentrations and FEV1(%) was found | [202] | ||
| → comparable values in patients with exacerbated COPD (n=34), stable COPD (n=21) and healthy controls (n=20) – all ex-smokers | [63] | ||
| ↑ in patients with COPD (n=53) vs. healthy (n=10); MDA correlates with disease severity - all patients were retired coal miners with varying smoking status | [209] | ||
| H2O2 | ↑ in patients with COPD (n=30) vs. healthy (n=10) and increases with disease severity - all smokers | [243] | |
| ↑ in patients with stable COPD (n=12) and with exacerbated COPD (n=19) (smokers and ex-smokers) vs. healthy never-smokers (n=10) | [244] | ||
| pH | ↓ in COPD exacerbation vs. recovery (n=29) – current and ex-smokers | [245] | |
| condensate pH remained unchanged during COPD exacerbation, both in smokers (n=21) and ex-smokers (n=17 | [246] | ||
| nitrotyrosine | ↑ in patients with COPD (n=53) vs. healthy (n=10) - patients were retired coalminers with varying smoking status | [209] | |
| 8-isoprotane | ↑ in exacerbating COPD patients (n=21) and fell after treatment with antibiotics | [247] | |
| ↑ in patients with COPD (n=30) vs. healthy (n=10) - all smokers | [243] | ||
| LTB4 | ↑ in exacerbating COPD patients (n=21) and fell after treatment with antibiotics | [247] | |
| ↑ in steroid naïve (n=20) and steroid treated patients with COPD (n=25) compared to control subjects (n=15) – all ex-smokers | [248] | ||
| Sputum (local oxidative stress) | |||
| hexanal, heptanal, nonanal | ↑ in patients with COPD (n=11; smokers and ex-smokers) vs. non-smoking controls (n=9) | [204] | |
| MDA | ↑ in patients with stable COPD (n=21) vs. healthy controls (n=20); increased further iv exacerbated COPD patients and decreased during recovery (n=34), – all ex-smokers | [63] | |
| ↑ in patients with COPD (n=11; smokers and ex-smokers) vs. non-smoking controls (n=9) | [204] | ||
| SOD | SOD activity was comparable between stable COPD patients and (n=24) and healthy controls (n=23); but it increased in COPD exacerbation (n=36) – all patients were ex-smokers | [34] | |
| CAT | CAT activity was comparable between stable COPD patients and (n=24) and healthy controls (n=23); but it increased in COPD exacerbation (n=36) – all patients were ex-smokers | [34] | |
| Biomarker | CV disease | Finding | Reference |
|---|---|---|---|
| Reduced GSH | Atherosclerosis, arterial aging | lower GSH is a predictor of intima/media thickness | [249,250] |
| Hypertension | ↑ GSH, increased glutathione-related antioxidant defense in treated hypertensives | [251] | |
| CAD | ↓ in angiographically proven CAD | [219] | |
| SOD activity | Arterial aging | negatively correlated with systolic and diastolic blood pressure, low serum SOD activity is an independent predictor carotid intima/media thickening | [252] |
| Hypertension | ↓ in hypertensive patients regardless of BMI | [253] | |
| IHD, CAD | ↑ in angiographically proven CAD and IHD | [219,220,221] | |
| CAT activity | Hypertension | ↓ in hypertensive patients regardless of BMI | [253] |
| IHD | ↑ in men with IHD | [221] | |
| GPx activity | Atherosclerosis | ↓ in prevalent atherosclerosis and lower values are associated with an increased risk of future cardiovascular events | [254] |
| Hypertension | lower levels associated with high blood pressure in black women | [255] | |
| IHD | ↓ in men with IHD | [221] | |
| any cardiovascular events | lower GPx is associated with higher risk of CV events | [256] | |
| MDA | Atherosclerosis, arterial aging | ↑ with carotid intima/media thickening | [250] |
| Hypertension | ↑ in untreated hypertension | [257,258] | |
| CAD | ↑ in angiographically proven CAD | [219] | |
| ox-LDL | Atherosclerosis, arterial aging | ↑ associated with carotid intima/media thickening, and higher arterial stiffness | [250,259] |
| Hypertension | ↑ in hypertensive men, and in prehypertensive subjects of both genders | [260,261] | |
| CAD | ↑ ox-LDL associated with CAD, with severity of CAD and was found to be prognostic for CAD events | [262,263,264,265] | |
| Stroke | higher values are associated with cerebrovascular events and increased risk of recurrent stroke in TIA patients | [266,267,268] |
5. Conclusion and future perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Antus, B.; Kardos, Z. Oxidative stress in COPD: molecular background and clinical monitoring. Curr Med Chem 2015, 22, 627–650. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and Risk of Mortality in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.D.; Zakeri, R.; Quint, J.K. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Therapeutic Advances in Respiratory Disease 2018, 12, 175346581775052. [Google Scholar] [CrossRef]
- Axson, E.L.; Ragutheeswaran, K.; Sundaram, V.; Bloom, C.I.; Bottle, A.; Cowie, M.R.; Quint, J.K. Hospitalisation and mortality in patients with comorbid COPD and heart failure: a systematic review and meta-analysis. Respiratory Research 2020, 21. [Google Scholar] [CrossRef]
- Cavailles, A.; Brinchault-Rabin, G.; Dixmier, A.; Goupil, F.; Gut-Gobert, C.; Marchand-Adam, S.; Meurice, J.C.; Morel, H.; Person-Tacnet, C.; Leroyer, C.; et al. Comorbidities of COPD. European Respiratory Review 2013, 22, 454–475. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cellular & Molecular Immunology 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Azadmanesh, J.; Borgstahl, G.E.O. A Review of the Catalytic Mechanism of Human Manganese Superoxide Dismutase. Antioxidants 2018, 7, 25. [Google Scholar] [CrossRef]
- Arnhold, J. The Dual Role of Myeloperoxidase in Immune Response. International Journal of Molecular Sciences 2020, 21, 8057. [Google Scholar] [CrossRef]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.S.; Mano, J.i. Lipid Peroxide-Derived Reactive Carbonyl Species as Mediators of Oxidative Stress and Signaling. Frontiers in Plant Science 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Medicinal Research Reviews 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Hypochlorite-induced oxidation of proteins in plasma: formation of chloramines and nitrogen-centred radicals and their role in protein fragmentation. Biochem J 1999, 340 ( Pt 2) Pt 2, 539–548. [Google Scholar] [CrossRef]
- Prütz, W.A. Hypochlorous Acid Interactions with Thiols, Nucleotides, DNA, and Other Biological Substrates. Archives of Biochemistry and Biophysics 1996, 332, 110–120. [Google Scholar] [CrossRef]
- van der Vliet, A.; Eiserich, J.P.; O'Neill, C.A.; Halliwell, B.; Cross, C.E. Tyrosine modification by reactive nitrogen species: a closer look. Arch Biochem Biophys 1995, 319, 341–349. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, A.J.A.; Sapey, E. Oxidative Stress in COPD: Sources, Markers, and Potential Mechanisms. Journal of Clinical Medicine 2017, 6, 21. [Google Scholar] [CrossRef]
- Abdala-Valencia, H.; Berdnikovs, S.; Cook-Mills, J.M. Vitamin E Isoforms as Modulators of Lung Inflammation. Nutrients 2013, 5, 4347–4363. [Google Scholar] [CrossRef]
- Iwao, Y.; Ishima, Y.; Yamada, J.; Noguchi, T.; Kragh-Hansen, U.; Mera, K.; Honda, D.; Suenaga, A.; Maruyama, T.; Otagiri, M. Quantitative evaluation of the role of cysteine and methionine residues in the antioxidant activity of human serum albumin using recombinant mutants. IUBMB Life 2012, 64, 450–454. [Google Scholar] [CrossRef]
- Kim, H.J.; Ha, S.; Lee, H.Y.; Lee, K.J. ROSics: chemistry and proteomics of cysteine modifications in redox biology. Mass Spectrom Rev 2015, 34, 184–208. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Mosoni, L.; Berlett, B.S.; Stadtman, E.R. Methionine residues as endogenous antioxidants in proteins. Proceedings of the National Academy of Sciences 1996, 93, 15036–15040. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim Biophys Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid Med Cell Longev 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Church, D.F.; Pryor, W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect 1985, 64, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, S.A.; Braber, S.; Henricks, P.A.J.; Kleinjan, M.; Kamp, V.M.; Georgiou, N.A.; Garssen, J.; Kraneveld, A.D.; Folkerts, G. Cigarette smoke induces β2-integrin-dependent neutrophil migration across human endothelium. Respiratory research 2011, 12, 75–75. [Google Scholar] [CrossRef]
- Schiffers, C.; Reynaert, N.L.; Wouters, E.F.M.; van der Vliet, A. Redox Dysregulation in Aging and COPD: Role of NOX Enzymes and Implications for Antioxidant Strategies. Antioxidants 2021, 10, 1799. [Google Scholar] [CrossRef]
- Schiffers, C.; Reynaert, N.L.; Wouters, E.F.M.; van der Vliet, A. Redox Dysregulation in Aging and COPD: Role of NOX Enzymes and Implications for Antioxidant Strategies. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Hollins, F.; Sutcliffe, A.; Gomez, E.; Berair, R.; Russell, R.; Szyndralewiez, C.; Saunders, R.; Brightling, C. Airway smooth muscle NOX4 is upregulated and modulates ROS generation in COPD. Respiratory Research 2016, 17, 84. [Google Scholar] [CrossRef]
- Liu, X.; Hao, B.; Ma, A.; He, J.; Liu, X.; Chen, J. The Expression of NOX4 in Smooth Muscles of Small Airway Correlates with the Disease Severity of COPD. Biomed Res Int 2016, 2016, 2891810. [Google Scholar] [CrossRef]
- Guo, X.; Fan, Y.; Cui, J.; Hao, B.; Zhu, L.; Sun, X.; He, J.; Yang, J.; Dong, J.; Wang, Y.; et al. NOX4 expression and distal arteriolar remodeling correlate with pulmonary hypertension in COPD. BMC Pulmonary Medicine 2018, 18, 111. [Google Scholar] [CrossRef]
- Gould, N.S.; Min, E.; Gauthier, S.; Martin, R.J.; Day, B.J. Lung glutathione adaptive responses to cigarette smoke exposure. Respiratory Research 2011, 12, 133. [Google Scholar] [CrossRef]
- Cantin, A.M.; North, S.L.; Hubbard, R.C.; Crystal, R.G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987, 63, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Antus, B.; Paska, C.; Simon, B.; Barta, I. Monitoring Antioxidant Enzyme Activity during Exacerbations of Chronic Obstructive Pulmonary Disease. Copd 2018, 15, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Betsuyaku, T.; Fuke, S.; Inomata, T.; Kaga, K.; Morikawa, T.; Odajima, N.; Adair-Kirk, T.; Nishimura, M. Bronchiolar epithelial catalase is diminished in smokers with mild COPD. European Respiratory Journal 2013, 42, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, P.; Raghu, P.S.; Reddy, V.D.; Bulle, S.; Marthadu, S.B.; Maturu, P.; Varadacharyulu, N.C. Chronic cigarette smoking-induced oxidative/nitrosative stress in human erythrocytes and platelets. Molecular & Cellular Toxicology 2018, 14, 27–34. [Google Scholar] [CrossRef]
- Barnes, P.J.; Burney, P.G.J.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F.M. Chronic obstructive pulmonary disease. Nature Reviews Disease Primers 2015, 1, 15076. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C.; Timens, W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 2009, 4, 435–459. [Google Scholar] [CrossRef]
- Harvey, A.; Montezano, A.C.; Touyz, R.M. Vascular biology of ageing-Implications in hypertension. J Mol Cell Cardiol 2015, 83, 112–121. [Google Scholar] [CrossRef]
- Ungvari, Z.; Kaley, G.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci 2010, 65, 1028–1041. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circulation Research 2018, 123, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Sorond, F.; Merkely, B.; Csiszar, A. Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J Am Coll Cardiol 2020, 75, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Vivodtzev, I.; Tamisier, R.; Baguet, J.P.; Borel, J.C.; Levy, P.; Pepin, J.L. Arterial stiffness in COPD. Chest 2014, 145, 861–875. [Google Scholar] [CrossRef]
- Qvist, L.; Nilsson, U.; Johansson, V.; Larsson, K.; Rönmark, E.; Langrish, J.; Blomberg, A.; Lindberg, A. Central arterial stiffness is increased among subjects with severe and very severe COPD: report from a population-based cohort study. European Clinical Respiratory Journal 2015, 2, 27023. [Google Scholar] [CrossRef]
- McAllister, D.A.; Maclay, J.D.; Mills, N.L.; Mair, G.; Miller, J.; Anderson, D.; Newby, D.E.; Murchison, J.T.; Macnee, W. Arterial Stiffness Is Independently Associated with Emphysema Severity in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2007, 176, 1208–1214. [Google Scholar] [CrossRef]
- Albu, A.; Fodor, D.; Bondor, C.; Suciu, O. Carotid arterial stiffness in patients with chronic obstructive pulmonary disease. Acta Physiol Hung 2011, 98, 117–127. [Google Scholar] [CrossRef]
- Castagna, O.; Boussuges, A.; Vallier, J.M.; Prefaut, C.; Brisswalter, J. Is impairment similar between arm and leg cranking exercise in COPD patients? Respir Med 2007, 101, 547–553. [Google Scholar] [CrossRef]
- Luehrs, R.E.; Newell, J.D., Jr.; Comellas, A.P.; Hoffman, E.A.; Warner, K.; Croghan, A.; DuBose, L.E.; Nopoulos, P.; Magnotta, V.; Arndt, S.; et al. CT-Measured Lung Air-Trapping is Associated with Higher Carotid Artery Stiffness in Individuals with Chronic Obstructive Pulmonary Disease. J Appl Physiol (1985) 2018, 125, 1760–1766. [Google Scholar] [CrossRef]
- Cinarka, H.; Kayhan, S.; Gumus, A.; Durakoglugil, M.E.; Erdogan, T.; Ezberci, I.; Yavuz, A.; Ozkaya, S.; Sahin, U. Arterial Stiffness Measured Via Carotid Femoral Pulse Wave Velocity Is Associated With Disease Severity in COPD. Respiratory Care 2014, 59, 274–280. [Google Scholar] [CrossRef]
- Tarnoki, D.L.; Tarnoki, A.D.; Lazar, Z.; Medda, E.; Littvay, L.; Cotichini, R.; Fagnani, C.; Stazi, M.A.; Nistico, L.; Lucatelli, P.; et al. Genetic and environmental factors on the relation of lung function and arterial stiffness. Respir Med 2013, 107, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Kowlessar, B.S.; Donaldson, G.C.; Mackay, A.J.; Singh, R.; George, S.N.; Garcha, D.S.; Wedzicha, J.A.; Hurst, J.R. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013, 188, 1091–1099. [Google Scholar] [CrossRef]
- Aldabayan, Y.S.; Ridsdale, H.A.; Alrajeh, A.M.; Aldhahir, A.M.; Lemson, A.; Alqahtani, J.S.; Brown, J.S.; Hurst, J.R. Pulmonary rehabilitation, physical activity and aortic stiffness in COPD. Respiratory Research 2019, 20. [Google Scholar] [CrossRef]
- Pako, J.; Barta, I.; Balogh, Z.; Kerti, M.; Drozdovszky, O.; Bikov, A.; Antus, B.; Horvath, I.; Varga, J. Assessment of the Anti-Aging Klotho Protein in Patients with COPD Undergoing Pulmonary Rehabilitation. COPD 2017, 14, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Flenley, D.C. Sleep in chronic obstructive lung disease. Clin Chest Med 1985, 6, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Kapa, S.; Kuniyoshi, F.H.S.; Somers, V.K. Sleep Apnea and Hypertension: Interactions and Implications for Management. Hypertension 2008, 51, 605–608. [Google Scholar] [CrossRef]
- Lavie, L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: implications to the heart and brain. Sleep Med Rev 2015, 20, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, S.; Mao, Y. The mechanisms of vascular aging. AGING MEDICINE 2021, 4, 153–158. [Google Scholar] [CrossRef]
- Krause, K.H. Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp Gerontol 2007, 42, 256–262. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef]
- Puca, A.A.; Carrizzo, A.; Villa, F.; Ferrario, A.; Casaburo, M.; Maciag, A.; Vecchione, C. Vascular ageing: the role of oxidative stress. Int J Biochem Cell Biol 2013, 45, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Bailey-Downs, L.; Gautam, T.; Sosnowska, D.; Wang, M.; Monticone, R.E.; Telljohann, R.; Pinto, J.T.; de Cabo, R.; Sonntag, W.E.; et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-kappaB activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci 2011, 66, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Antus, B.; Harnasi, G.; Drozdovszky, O.; Barta, I. Monitoring oxidative stress during chronic obstructive pulmonary disease exacerbations using malondialdehyde. Respirology 2014, 19, 74–79. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Rodriguez-Manas, L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 2013, 65, 380–401. [Google Scholar] [CrossRef]
- Rybka, J.; Kupczyk, D.; Kedziora-Kornatowska, K.; Pawluk, H.; Czuczejko, J.; Szewczyk-Golec, K.; Kozakiewicz, M.; Antonioli, M.; Carvalho, L.A.; Kedziora, J. Age-related changes in an antioxidant defense system in elderly patients with essential hypertension compared with healthy controls. Redox Rep 2011, 16, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Siedlinski, M.; van Diemen, C.C.; Postma, D.S.; Vonk, J.M.; Boezen, H.M. Superoxide dismutases, lung function and bronchial responsiveness in a general population. Eur Respir J 2009, 33, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.; Bowler, R.P.; Juul, K.; Crapo, J.D.; Levy, S.; Nordestgaard, B.G. Superoxide dismutase 3 polymorphism associated with reduced lung function in two large populations. Am J Respir Crit Care Med 2008, 178, 906–912. [Google Scholar] [CrossRef]
- Sotgia, S.; Paliogiannis, P.; Sotgiu, E.; Mellino, S.; Zinellu, E.; Fois, A.G.; Pirina, P.; Carru, C.; Mangoni, A.A.; Zinellu, A. Systematic Review and Meta-Analysis of the Blood Glutathione Redox State in Chronic Obstructive Pulmonary Disease. Antioxidants 2020, 9, 1146. [Google Scholar] [CrossRef]
- Beeh, K.M.; Beier, J.; Koppenhoefer, N.; Buhl, R. Increased glutathione disulfide and nitrosothiols in sputum supernatant of patients with stable COPD. Chest 2004, 126, 1116–1122. [Google Scholar] [CrossRef]
- Lopes, R.A.; Neves, K.B.; Tostes, R.C.; Montezano, A.C.; Touyz, R.M. Downregulation of Nuclear Factor Erythroid 2-Related Factor and Associated Antioxidant Genes Contributes to Redox-Sensitive Vascular Dysfunction in Hypertension. Hypertension 2015, 66, 1240–1250. [Google Scholar] [CrossRef]
- Alves-Lopes, R.; Neves, K.B.; Montezano, A.C.; Harvey, A.; Carneiro, F.S.; Touyz, R.M.; Tostes, R.C. Internal Pudental Artery Dysfunction in Diabetes Mellitus Is Mediated by NOX1-Derived ROS-, Nrf2-, and Rho Kinase-Dependent Mechanisms. Hypertension 2016, 68, 1056–1064. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and Its Activators in Respiratory Diseases. Oxid Med Cell Longev 2019, 2019, 7090534. [Google Scholar] [CrossRef] [PubMed]
- Mercado, N.; Thimmulappa, R.; Thomas, C.M.; Fenwick, P.S.; Chana, K.K.; Donnelly, L.E.; Biswal, S.; Ito, K.; Barnes, P.J. Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress. Biochem Biophys Res Commun 2011, 406, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Bailey-Downs, L.; Gautam, T.; Jimenez, R.; Losonczy, G.; Zhang, C.; Ballabh, P.; Recchia, F.A.; Wilkerson, D.C.; Sonntag, W.E.; et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol 2011, 300, H1133–H1140. [Google Scholar] [CrossRef]
- Ahmad, A.; Shameem, M.; Husain, Q. Altered oxidant-antioxidant levels in the disease prognosis of chronic obstructive pulmonary disease. Int J Tuberc Lung Dis 2013, 17, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Tavilani, H.; Nadi, E.; Karimi, J.; Goodarzi, M.T. Oxidative stress in COPD patients, smokers, and non-smokers. Respir Care 2012, 57, 2090–2094. [Google Scholar] [CrossRef] [PubMed]
- Betsuyaku, T.; Fuke, S.; Inomata, T.; Kaga, K.; Morikawa, T.; Odajima, N.; Adair-Kirk, T.; Nishimura, M. Bronchiolar epithelial catalase is diminished in smokers with mild COPD. Eur Respir J 2013, 42, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Medicine and Cellular Longevity 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Joppa, P.; Petrasova, D.; Stancak, B.; Dorkova, Z.; Tkacova, R. Oxidative stress in patients with COPD and pulmonary hypertension. Wien Klin Wochenschr 2007, 119, 428–434. [Google Scholar] [CrossRef]
- Nadeem, A.; Raj, H.G.; Chhabra, S.K. Increased oxidative stress and altered levels of antioxidants in chronic obstructive pulmonary disease. Inflammation 2005, 29, 23–32. [Google Scholar] [CrossRef]
- Wozniak, A.; Gorecki, D.; Szpinda, M.; Mila-Kierzenkowska, C.; Wozniak, B. Oxidant-antioxidant balance in the blood of patients with chronic obstructive pulmonary disease after smoking cessation. Oxid Med Cell Longev 2013, 2013, 897075. [Google Scholar] [CrossRef]
- Santos, M.C.; Oliveira, A.L.; Viegas-Crespo, A.M.; Vicente, L.; Barreiros, A.; Monteiro, P.; Pinheiro, T.; Bugalho De Almeida, A. Systemic markers of the redox balance in chronic obstructive pulmonary disease. Biomarkers 2004, 9, 461–469. [Google Scholar] [CrossRef]
- Vibhuti, A.; Arif, E.; Mishra, A.; Deepak, D.; Singh, B.; Rahman, I.; Mohammad, G.; Pasha, M.A. CYP1A1, CYP1A2 and CYBA gene polymorphisms associated with oxidative stress in COPD. Clin Chim Acta 2010, 411, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Li, Y.; Jiang, Y.; Lu, G.; Huang, X.; Guan, K. Local and systemic oxidative stress status in chronic obstructive pulmonary disease patients. Can Respir J 2013, 20, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Oelze, M.; Kröller-Schön, S.; Steven, S.; Lubos, E.; Doppler, C.; Hausding, M.; Tobias, S.; Brochhausen, C.; Li, H.; Torzewski, M.; et al. Glutathione peroxidase-1 deficiency potentiates dysregulatory modifications of endothelial nitric oxide synthase and vascular dysfunction in aging. Hypertension 2014, 63, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.E.; Guo, H.; Fedarko, N.; DeZern, A.; Fried, L.P.; Xue, Q.L.; Leng, S.; Beamer, B.; Walston, J.D. Glutathione peroxidase enzyme activity in aging. J Gerontol A Biol Sci Med Sci 2008, 63, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I. , Donnelly, L. E., Kiss, A., Paredi, P., Kharitonov, S. A., & Barnes, P. J. Raised levels of exhaled carbon monoxide are associated with an increased expression of heme oxygenase-1 in airway macrophages in asthma: a new marker of oxidative stress. Thorax 1998, 53, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Polverino, F.; Celli, B.R.; Owen, C.A. COPD as an endothelial disorder: endothelial injury linking lesions in the lungs and other organs? (2017 Grover Conference Series). Pulmonary Circulation 2018, 8, 1–18. [Google Scholar] [CrossRef]
- Mei, D.; Tan, W.S.D.; Liao, W.; Heng, C.K.M.; Wong, W.S.F. Activation of angiotensin II type-2 receptor protects against cigarette smoke-induced COPD. Pharmacol Res 2020, 161, 105223. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nature Reviews Immunology 2007, 7, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ Res 2018, 123, 825–848. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, T.M.; Ismail, R.S.; Mansoor, F.A.; Zweier, J.R.; Lowe, F.; Zweier, J.L. Cigarette smoke constituents cause endothelial nitric oxide synthase dysfunction and uncoupling due to depletion of tetrahydrobiopterin with degradation of GTP cyclohydrolase. Nitric Oxide 2018, 76, 113–121. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, Y.; Hou, C.; He, W.; Chen, P. Cigarette Smoke Extract Changes Expression of Endothelial Nitric Oxide Synthase (eNOS) and p16(INK4a) and is Related to Endothelial Progenitor Cell Dysfunction. Med Sci Monit 2017, 23, 3224–3231. [Google Scholar] [CrossRef]
- Stanisavljevic, N.; Stojanovich, L.; Marisavljevic, D.; Djokovic, A.; Dopsaj, V.; Kotur-Stevuljevic, J.; Martinovic, J.; Memon, L.; Radovanovic, S.; Todic, B.; et al. Lipid peroxidation as risk factor for endothelial dysfunction in antiphospholipid syndrome patients. Clin Rheumatol 2016, 35, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- Tejovathi, B.; Suchitra, M.M.; Suresh, V.; Reddy, V.S.; Sachan, A.; Srinivas Rao, P.V.; Bitla, A.R. Association of lipid peroxidation with endothelial dysfunction in patients with overt hypothyroidism. Exp Clin Endocrinol Diabetes 2013, 121, 306–309. [Google Scholar] [CrossRef]
- Watson, A.M.D.; Soro-Paavonen, A.; Jandeleit-Dahm, K.A. AGE-RAGE signalling in endothelial dysfunction and atherosclerosis in diabetes. In Endothelial Dysfunction and Inflammation, Dauphinee, S., Karsan, A., Eds.; Springer Basel: Basel, 2010; pp. 161–174. [Google Scholar]
- Goven, D.; Boutten, A.; Lecon-Malas, V.; Marchal-Somme, J.; Amara, N.; Crestani, B.; Fournier, M.; Leseche, G.; Soler, P.; Boczkowski, J.; et al. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax 2008, 63, 916–924. [Google Scholar] [CrossRef]
- Yamada, K.; Asai, K.; Nagayasu, F.; Sato, K.; Ijiri, N.; Yoshii, N.; Imahashi, Y.; Watanabe, T.; Tochino, Y.; Kanazawa, H.; et al. Impaired nuclear factor erythroid 2-related factor 2 expression increases apoptosis of airway epithelial cells in patients with chronic obstructive pulmonary disease due to cigarette smoking. BMC Pulm Med 2016, 16, 27. [Google Scholar] [CrossRef]
- Nana-Sinkam, S.P.; Lee, J.D.; Sotto-Santiago, S.; Stearman, R.S.; Keith, R.L.; Choudhury, Q.; Cool, C.; Parr, J.; Moore, M.D.; Bull, T.M.; et al. Prostacyclin prevents pulmonary endothelial cell apoptosis induced by cigarette smoke. Am J Respir Crit Care Med 2007, 175, 676–685. [Google Scholar] [CrossRef]
- Voelkel, N.F. Cigarette Smoke Is an Endothelial Cell Toxin. Am J Respir Crit Care Med 2018, 197, 274. [Google Scholar] [CrossRef]
- Green, C.E.; Turner, A.M. The role of the endothelium in asthma and chronic obstructive pulmonary disease (COPD). Respiratory Research 2017, 18, 20. [Google Scholar] [CrossRef]
- Kasahara, Y.; Tuder, R.M.; Taraseviciene-Stewart, L.; Le Cras, T.D.; Abman, S.; Hirth, P.K.; Waltenberger, J.; Voelkel, N.F. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000, 106, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Weissmann, N.; Lobo, B.; Pichl, A.; Parajuli, N.; Seimetz, M.; Puig-Pey, R.; Ferrer, E.; Peinado, V.I.; Dominguez-Fandos, D.; Fysikopoulos, A.; et al. Stimulation of soluble guanylate cyclase prevents cigarette smoke-induced pulmonary hypertension and emphysema. Am J Respir Crit Care Med 2014, 189, 1359–1373. [Google Scholar] [CrossRef]
- Chun, P. Role of sirtuins in chronic obstructive pulmonary disease. Archives of Pharmacal Research 2015, 38, 1–10. [Google Scholar] [CrossRef]
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The sirtuin family in health and disease. Signal Transduction and Targeted Therapy 2022, 7, 402. [Google Scholar] [CrossRef]
- Rajendrasozhan, S.; Yang, S.R.; Kinnula, V.L.; Rahman, I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008, 177, 861–870. [Google Scholar] [CrossRef]
- Takasaka, N.; Araya, J.; Hara, H.; Ito, S.; Kobayashi, K.; Kurita, Y.; Wakui, H.; Yoshii, Y.; Yumino, Y.; Fujii, S. Autophagy induction by SIRT6 through attenuation of insulin-like growth factor signaling is involved in the regulation of human bronchial epithelial cell senescence. The Journal of Immunology 2014, 192, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-R.; Wright, J.; Bauter, M.; Seweryniak, K.; Kode, A.; Rahman, I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-κB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. American Journal of Physiology-Lung Cellular and Molecular Physiology 2007, 292, L567–L576. [Google Scholar] [CrossRef] [PubMed]
- Rajendrasozhan, S.; Yang, S.-R.; Kinnula, V.L.; Rahman, I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 177, 861–870. [Google Scholar] [CrossRef]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 2013, 25, 1939–1948. [Google Scholar] [CrossRef]
- Yao, H.; Chung, S.; Hwang, J.W.; Rajendrasozhan, S.; Sundar, I.K.; Dean, D.A.; McBurney, M.W.; Guarente, L.; Gu, W.; Rönty, M.; et al. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest 2012, 122, 2032–2045. [Google Scholar] [CrossRef]
- Gu, C.; Li, Y.; Liu, J.; Ying, X.; Liu, Y.; Yan, J.; Chen, C.; Zhou, H.; Cao, L.; Ma, Y. LncRNA-mediated SIRT1/FoxO3a and SIRT1/p53 signaling pathways regulate type II alveolar epithelial cell senescence in patients with chronic obstructive pulmonary disease. Mol Med Rep 2017, 15, 3129–3134. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, S.; Araya, J.; Numata, T.; Nojiri, S.; Hara, H.; Yumino, Y.; Kawaishi, M.; Odaka, M.; Morikawa, T.; Nishimura, S.L.; et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2011, 300, L391–L401. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.; Goligorsky, M.S. Sirtuins, Cell Senescence, and Vascular Aging. Can J Cardiol 2016, 32, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Ghaeni, L.; Baldessari, D.; Mostoslavsky, R.; Rossig, L.; Dequiedt, F.; Haendeler, J.; Mione, M.; Dejana, E.; Alt, F.W.; et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 2007, 21, 2644–2658. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Gao, P.; Zhou, S.; Zhang, Z.Q.; Hao, D.L.; Lian, L.S.; Li, Y.J.; Chen, H.Z.; Liu, D.P. SIRT1-mediated epigenetic downregulation of plasminogen activator inhibitor-1 prevents vascular endothelial replicative senescence. Aging Cell 2014, 13, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.N.; Chen, H.Z.; Gao, P.; Zhu, L.H.; Li, H.L.; Lv, X.; Zhang, Q.J.; Zhang, R.; Wang, Z.; et al. SIRT1 acts as a modulator of neointima formation following vascular injury in mice. Circ Res 2011, 108, 1180–1189. [Google Scholar] [CrossRef]
- Gorenne, I.; Kumar, S.; Gray, K.; Figg, N.; Yu, H.; Mercer, J.; Bennett, M. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation 2013, 127, 386–396. [Google Scholar] [CrossRef]
- Gao, P.; Xu, T.T.; Lu, J.; Li, L.; Xu, J.; Hao, D.L.; Chen, H.Z.; Liu, D.P. Overexpression of SIRT1 in vascular smooth muscle cells attenuates angiotensin II-induced vascular remodeling and hypertension in mice. J Mol Med (Berl) 2014, 92, 347–357. [Google Scholar] [CrossRef]
- Kuro, O.M. The FGF23 and Klotho system beyond mineral metabolism. Clin Exp Nephrol 2017, 21, 64–69. [Google Scholar] [CrossRef]
- Chen, C.D.; Podvin, S.; Gillespie, E.; Leeman, S.E.; Abraham, C.R. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 2007, 104, 19796–19801. [Google Scholar] [CrossRef] [PubMed]
- Shiraki-Iida, T.; Aizawa, H.; Matsumura, Y.; Sekine, S.; Iida, A.; Anazawa, H.; Nagai, R.; Kuro-o, M.; Nabeshima, Y. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett 1998, 424, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Yuan, C.; Zhang, J.; Li, L.; Yu, L.; Wiegman, Coen H. ; Barnes, Peter J.; Adcock, Ian M.; Huang, M.; Yao, X. Klotho expression is reduced in COPD airway epithelial cells: effects on inflammation and oxidant injury. Clinical Science 2015, 129, 1011–1023. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho as a regulator of oxidative stress and senescence. Biol Chem 2008, 389, 233–241. [Google Scholar] [CrossRef]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 2005, 280, 38029–38034. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Patel, M.S.; Lewis, A.; Natanek, S.A.; Martolini, D.; Bruijnzeel, P.; Anikin, V.; McGonigle, N.; Jordan, S.; Hopkinson, N.S.; Man, W.D.-C.; et al. Klotho expression is reduced in COPD. European Respiratory Journal 2013, 42, P3708. [Google Scholar]
- Pako, J.; Kunos, L.; Meszaros, M.; Tarnoki, D.L.; Tarnoki, A.D.; Horvath, I.; Bikov, A. Decreased Levels of Anti-Aging Klotho in Obstructive Sleep Apnea. Rejuvenation Res 2020, 23, 256–261. [Google Scholar] [CrossRef]
- Pákó, J.; Barta, I.; Kerti, M.; Balogh, Z.; Drozdovszky, O.; Antus, B.; Varga, J.; Horvath, I. Assessment of plasma klotho concentration in stable COPD. European Respiratory Journal 2016, 48, PA1001. [Google Scholar] [CrossRef]
- Lim, K.; Halim, A.; Lu, T.-S.; Ashworth, A.; Chong, I. Klotho: A Major Shareholder in Vascular Aging Enterprises. International Journal of Molecular Sciences 2019, 20, 4637. [Google Scholar] [CrossRef]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Crasto, C.; Guralnik, J.M.; Ferrucci, L. Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc 2011, 59, 1596–1601. [Google Scholar] [CrossRef]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Crasto, C.; Guralnik, J.M.; Ferrucci, L. Plasma klotho and mortality risk in older community-dwelling adults. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences 2011, 66, 794–800. [Google Scholar] [CrossRef]
- Arking, D.E.; Atzmon, G.; Arking, A.; Barzilai, N.; Dietz, H.C. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res 2005, 96, 412–418. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Quiñones, H.; Griffith, C.; Kuro-o, M.; Moe, O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. Journal of the American Society of Nephrology 2011, 22, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Kawarazaki, W.; Mizuno, R.; Nishimoto, M.; Ayuzawa, N.; Hirohama, D.; Ueda, K.; Kawakami-Mori, F.; Oba, S.; Marumo, T.; Fujita, T. Salt causes aging-associated hypertension via vascular Wnt5a under Klotho deficiency. Journal of Clinical Investigation 2020. [Google Scholar] [CrossRef] [PubMed]
- Lanzani, C.; Citterio, L.; Vezzoli, G. Klotho: a link between cardiovascular and non-cardiovascular mortality. Clin Kidney J 2020, 13, 926–932. [Google Scholar] [CrossRef]
- Horvath, I.; Canotilho, M.; Chlumsky, J.; Chorostowska-Wynimko, J.; Corda, L.; Derom, E.; Ficker, J.H.; Kneussl, M.; Miravitlles, M.; Sucena, M.; et al. Diagnosis and management of alpha(1)-antitrypsin deficiency in Europe: an expert survey. ERJ Open Res 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.M.; Marciniak, S.J.; Teckman, J.; Ferrarotti, I.; Brantly, M.L.; Lomas, D.A.; Stoller, J.K.; McElvaney, N.G. α1-Antitrypsin deficiency. Nature Reviews Disease Primers 2016, 2, 16051. [Google Scholar] [CrossRef]
- Strnad, P.; McElvaney, N.G.; Lomas, D.A. Alpha1-Antitrypsin Deficiency. New England Journal of Medicine 2020, 382, 1443–1455. [Google Scholar] [CrossRef]
- Winther, S.V.; Ahmed, D.; Al-Shuweli, S.; Landt, E.M.; Nordestgaard, B.G.; Seersholm, N.; Dahl, M. Severe α1-antitrypsin deficiency associated with lower blood pressure and reduced risk of ischemic heart disease: a cohort study of 91,540 individuals and a meta-analysis. Respiratory Research 2022, 23, 55. [Google Scholar] [CrossRef]
- Pini, L.; Peroni, M.; Zanotti, C.; Pini, A.; Bossoni, E.; Giordani, J.; Bargagli, E.; Perger, E.; Ferrarotti, I.; Vizzardi, E.; et al. Investigating the Link between Alpha-1 Antitrypsin Deficiency and Abdominal Aortic Aneurysms. Ann Vasc Surg 2021, 77, 195–201. [Google Scholar] [CrossRef]
- Dako, F.; Zhao, H.; Mulvenna, A.; Gupta, S.; Simpson, S.; Kueppers, F. Relationship between alpha-1-antitrypsin deficiency and ascending aortic distention. European Respiratory Journal 2019, 54, PA5391. [Google Scholar] [CrossRef]
- Schievink, W.I.; Björnsson, J.; Parisi, J.E.; Prakash, U.B. Arterial fibromuscular dysplasia associated with severe alpha 1-antitrypsin deficiency. Mayo Clin Proc 1994, 69, 1040–1043. [Google Scholar] [CrossRef]
- Bofinger, A.; Hawley, C.; Fisher, P.; Daunt, N.; Stowasser, M.; Gordon, R. Alpha-1-antitrypsin phenotypes in patients with renal arterial fibromuscular dysplasia. J Hum Hypertens 2000, 14, 91–94. [Google Scholar] [CrossRef]
- Dasí, F.; Amor, M.; Sanz, F.; Codoñer-Franch, P.; Navarro-García, M.M.; Escribano, A. Oxidative stress in serum of patients with alpha-1 antitrypsin deficiency. European Respiratory Journal 2013, 42, 1488. [Google Scholar]
- Escribano, A.; Amor, M.; Pastor, S.; Castillo, S.; Sanz, F.; Codoñer-Franch, P.; Dasí, F. Decreased glutathione and low catalase activity contribute to oxidative stress in children with α-1 antitrypsin deficiency. Thorax 2015, 70, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Marcus, N.Y.; Blomenkamp, K.; Ahmad, M.; Teckman, J.H. Oxidative stress contributes to liver damage in a murine model of alpha-1-antitrypsin deficiency. Experimental Biology and Medicine 2012, 237, 1163–1172. [Google Scholar] [CrossRef]
- Rubio, M.L.; Martin-Mosquero, M.C.; Ortega, M.; Peces-Barba, G.; González-Mangado, N. Oral N-acetylcysteine attenuates elastase-induced pulmonary emphysema in rats. Chest 2004, 125, 1500–1506. [Google Scholar] [CrossRef]
- Hilde, J.M.; Skjorten, I.; Hansteen, V.; Melsom, M.N.; Hisdal, J.; Humerfelt, S.; Steine, K. Haemodynamic responses to exercise in patients with COPD. Eur Respir J 2013, 41, 1031–1041. [Google Scholar] [CrossRef]
- Ferrer, E.; Peinado, V.I.; Castaneda, J.; Prieto-Lloret, J.; Olea, E.; Gonzalez-Martin, M.C.; Vega-Agapito, M.V.; Diez, M.; Dominguez-Fandos, D.; Obeso, A.; et al. Effects of cigarette smoke and hypoxia on pulmonary circulation in the guinea pig. Eur Respir J 2011, 38, 617–627. [Google Scholar] [CrossRef]
- Peinado, V.I.; Barbera, J.A.; Abate, P.; Ramirez, J.; Roca, J.; Santos, S.; Rodriguez-Roisin, R. Inflammatory reaction in pulmonary muscular arteries of patients with mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999, 159, 1605–1611. [Google Scholar] [CrossRef]
- Seimetz, M.; Parajuli, N.; Pichl, A.; Veit, F.; Kwapiszewska, G.; Weisel, F.C.; Milger, K.; Egemnazarov, B.; Turowska, A.; Fuchs, B.; et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell 2011, 147, 293–305. [Google Scholar] [CrossRef]
- Gao, Y.; Du, X.; Qin, W.; Li, K. Assessment of the right ventricular function in patients with chronic obstructive pulmonary disease using MRI. Acta Radiol 2011, 52, 711–715. [Google Scholar] [CrossRef]
- Vonk-Noordegraaf, A.; Marcus, J.T.; Holverda, S.; Roseboom, B.; Postmus, P.E. Early changes of cardiac structure and function in COPD patients with mild hypoxemia. Chest 2005, 127, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Piccari, L.; Barbera, J.A. Pulmonary vasculature in COPD: The silent component. Respirology 2016, 21, 984–994. [Google Scholar] [CrossRef]
- Weir-Mccall, J.R.; Struthers, A.D.; Lipworth, B.J.; Houston, J.G. The role of pulmonary arterial stiffness in COPD. Respiratory Medicine 2015, 109, 1381–1390. [Google Scholar] [CrossRef]
- Barbera, J.A. Mechanisms of development of chronic obstructive pulmonary disease-associated pulmonary hypertension. Pulm Circ 2013, 3, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Peinado, V.I.; Ramirez, J.; Melgosa, T.; Roca, J.; Rodriguez-Roisin, R.; Barbera, J.A. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J 2002, 19, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Scott, D.E.; Shandas, R.; Stenmark, K.R.; Tan, W. High pulsatility flow induces adhesion molecule and cytokine mRNA expression in distal pulmonary artery endothelial cells. Ann Biomed Eng 2009, 37, 1082–1092. [Google Scholar] [CrossRef]
- Scott, D.; Tan, Y.; Shandas, R.; Stenmark, K.R.; Tan, W. High pulsatility flow stimulates smooth muscle cell hypertrophy and contractile protein expression. Am J Physiol Lung Cell Mol Physiol 2013, 304, L70–L81. [Google Scholar] [CrossRef]
- Pak, O.; Aldashev, A.; Welsh, D.; Peacock, A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J 2007, 30, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Zepeda, A.B.; Pessoa, A., Jr.; Castillo, R.L.; Figueroa, C.A.; Pulgar, V.M.; Farías, J.G. Cellular and molecular mechanisms in the hypoxic tissue: role of HIF-1 and ROS. Cell Biochem Funct 2013, 31, 451–459. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, F. Role of the HIF-1 signaling pathway in chronic obstructive pulmonary disease. Exp Ther Med 2018, 16, 4553–4561. [Google Scholar] [CrossRef] [PubMed]
- Rong, B.; Liu, Y.; Li, M.; Fu, T.; Gao, W.; Liu, H. Correlation of serum levels of HIF-1α and IL-19 with the disease progression of COPD: a retrospective study. Int J Chron Obstruct Pulmon Dis 2018, 13, 3791–3803. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Leopold, J.A.; Hemnes, A.R. Metabolic syndrome, neurohumoral modulation, and pulmonary arterial hypertension. British Journal of Pharmacology 2020, 177, 1457–1471. [Google Scholar] [CrossRef] [PubMed]
- Elisia, I.; Lam, V.; Cho, B.; Hay, M.; Li, M.Y.; Yeung, M.; Bu, L.; Jia, W.; Norton, N.; Lam, S.; et al. The effect of smoking on chronic inflammation, immune function and blood cell composition. Sci Rep 2020, 10, 19480. [Google Scholar] [CrossRef]
- Llinàs, L.; Peinado, V.I.; Ramon Goñi, J.; Rabinovich, R.; Pizarro, S.; Rodriguez-Roisin, R.; Barberà, J.A.; Bastos, R. Similar gene expression profiles in smokers and patients with moderate COPD. Pulm Pharmacol Ther 2011, 24, 32–41. [Google Scholar] [CrossRef]
- Melgosa, M.; Peinado, V.; Santos, S.; Morales, J.; Ramirez, J.; Roca, J.; Rodriguez-Roisin, R.; Barbera, J. Expression of endothelial nitric oxide synthase (eNOS) and endothelin-1 (ET-1) in pulmonary arteries of patients with severe COPD. Eur Respir J 2003, 22, 20s. [Google Scholar]
- Wang, Y.; Li, X.; Niu, W.; Chen, J.; Zhang, B.; Zhang, X.; Wang, Y.; Dang, S.; Li, Z. The alveolar epithelial cells are involved in pulmonary vascular remodeling and constriction of hypoxic pulmonary hypertension. Respiratory Research 2021, 22, 134. [Google Scholar] [CrossRef]
- Gao, W.; Li, L.; Wang, Y.; Zhang, S.; Adcock, I.M.; Barnes, P.J.; Huang, M.; Yao, X. Bronchial epithelial cells: The key effector cells in the pathogenesis of chronic obstructive pulmonary disease? Respirology 2015, 20, 722–729. [Google Scholar] [CrossRef]
- Maclay, J.D.; MacNee, W. Cardiovascular disease in COPD: mechanisms. Chest 2013, 143, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.D.; Zakeri, R.; Quint, J.K. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis 2018, 12, 1753465817750524. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Gupta, A.; Strollo, P.J., Jr.; Fuhrman, C.R.; Leader, J.K.; Bon, J.; Slivka, W.A.; Shoushtari, A.H.; Avolio, J.; Kip, K.E.; et al. Airflow Limitation and Endothelial Dysfunction. Unrelated and Independent Predictors of Atherosclerosis. Am J Respir Crit Care Med 2016, 194, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Topsakal, R.; Kalay, N.; Ozdogru, I.; Cetinkaya, Y.; Oymak, S.; Kaya, M.G.; Dogan, A.; Inanc, M.T.; Ergin, A. Effects of chronic obstructive pulmonary disease on coronary atherosclerosis. Heart Vessels 2009, 24, 164–168. [Google Scholar] [CrossRef]
- Brassington, K.; Selemidis, S.; Bozinovski, S.; Vlahos, R. Chronic obstructive pulmonary disease and atherosclerosis: common mechanisms and novel therapeutics. Clin Sci (Lond) 2022, 136, 405–423. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circulation research 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Arnold, N.; Lechner, K.; Waldeyer, C.; Shapiro, M.D.; Koenig, W. Inflammation and Cardiovascular Disease: The Future. European Cardiology Review 2021;16:e20. [CrossRef]
- Guarner, V.; Rubio-Ruiz, M.E. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip Top Gerontol 2015, 40, 99–106. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Balbirsingh, V.; Mohammed, A.S.; Turner, A.M.; Newnham, M. Cardiovascular disease in chronic obstructive pulmonary disease: a narrative review. Thorax 2022. [Google Scholar] [CrossRef]
- Freixa, X.; Portillo, K.; Paré, C.; Garcia-Aymerich, J.; Gomez, F.P.; Benet, M.; Roca, J.; Farrero, E.; Ferrer, J.; Fernandez-Palomeque, C.; et al. Echocardiographic abnormalities in patients with COPD at their first hospital admission. European Respiratory Journal 2013, 41, 784–791. [Google Scholar] [CrossRef]
- Hilde, J.M.; Skjorten, I.; Grotta, O.J.; Hansteen, V.; Melsom, M.N.; Hisdal, J.; Humerfelt, S.; Steine, K. Right ventricular dysfunction and remodeling in chronic obstructive pulmonary disease without pulmonary hypertension. J Am Coll Cardiol 2013, 62, 1103–1111. [Google Scholar] [CrossRef]
- Rabe, K.F.; Hurst, J.R.; Suissa, S. Cardiovascular disease and COPD: dangerous liaisons? Eur Respir Rev 2018, 27. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, K.M.; Dransfield, M.T.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.A.; Celli, B.R.; Crim, C.; Hartley, B.F.; Martinez, F.J.; Newby, D.E.; et al. Exacerbations of Chronic Obstructive Pulmonary Disease and Cardiac Events. A Post Hoc Cohort Analysis from the SUMMIT Randomized Clinical Trial. Am J Respir Crit Care Med 2018, 198, 51–57. [Google Scholar] [CrossRef]
- Park, J.F.; Liang, J.; Umar, S. Electrical Remodeling in Right Ventricular Failure Due to Pulmonary Hypertension: Unraveling Novel Therapeutic Targets. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Boussuges, A.; Pinet, C.; Molenat, F.; Burnet, H.; Ambrosi, P.; Badier, M.; Sainty, J.M.; Orehek, J. Left atrial and ventricular filling in chronic obstructive pulmonary disease. An echocardiographic and Doppler study. Am J Respir Crit Care Med 2000, 162, 670–675. [Google Scholar] [CrossRef]
- Sievi, N.A.; Clarenbach, C.F.; Camen, G.; Rossi, V.A.; van Gestel, A.J.; Kohler, M. High prevalence of altered cardiac repolarization in patients with COPD. BMC Pulm Med 2014, 14, 55. [Google Scholar] [CrossRef]
- Ivanics, T.; Miklos, Z.; Dezsi, L.; Ikrenyi, K.; Toth, A.; Roemen, T.H.; Van der Vusse, G.J.; Ligeti, L. Concomitant accumulation of intracellular free calcium and arachidonic acid in the ischemic-reperfused rat heart. Mol Cell Biochem 2001, 226, 119–128. [Google Scholar] [CrossRef]
- Miklos, Z.; Ivanics, T.; Roemen, T.H.; van der Vusse, G.J.; Dezsi, L.; Szekeres, M.; Kemecsei, P.; Toth, A.; Kollai, M.; Ligeti, L. Time related changes in calcium handling in the isolated ischemic and reperfused rat heart. Mol Cell Biochem 2003, 250, 115–124. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Shah, A.K.; Adameova, A.; Bartekova, M. Role of Oxidative Stress in Cardiac Dysfunction and Subcellular Defects Due to Ischemia-Reperfusion Injury. Biomedicines 2022, 10, 1473. [Google Scholar] [CrossRef]
- Pelà, G.; Li Calzi, M.; Pinelli, S.; Andreoli, R.; Sverzellati, N.; Bertorelli, G.; Goldoni, M.; Chetta, A. Left ventricular structure and remodeling in patients with COPD. International journal of chronic obstructive pulmonary disease 2016, 11, 1015–1022. [Google Scholar] [CrossRef]
- Chantler, P.D.; Lakatta, E.G. Arterial-ventricular coupling with aging and disease. Frontiers in physiology 2012, 3, 90–90. [Google Scholar] [CrossRef] [PubMed]
- Karch, A.; Vogelmeier, C.; Welte, T.; Bals, R.; Kauczor, H.U.; Biederer, J.; Heinrich, J.; Schulz, H.; Glaser, S.; Holle, R.; et al. The German COPD cohort COSYCONET: Aims, methods and descriptive analysis of the study population at baseline. Respir Med 2016, 114, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Trinkmann, F.; Saur, J.; Borggrefe, M.; Akin, I. Cardiovascular Comorbidities in Chronic Obstructive Pulmonary Disease (COPD)-Current Considerations for Clinical Practice. J Clin Med 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Antus, B. Oxidative Stress Markers in Sputum. Oxid Med Cell Longev 2016, 2016, 2930434. [Google Scholar] [CrossRef]
- Gopcevic, K.R.; Gkaliagkousi, E.; Nemcsik, J.; Acet, O.; Bernal-Lopez, M.R.; Bruno, R.M.; Climie, R.E.; Fountoulakis, N.; Fraenkel, E.; Lazaridis, A.; et al. Pathophysiology of Circulating Biomarkers and Relationship With Vascular Aging: A Review of the Literature From VascAgeNet Group on Circulating Biomarkers, European Cooperation in Science and Technology Action 18216. Front Physiol 2021, 12, 789690. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, E.; Zinellu, A.; Fois, A.G.; Carru, C.; Pirina, P. Circulating biomarkers of oxidative stress in chronic obstructive pulmonary disease: a systematic review. Respir Res 2016, 17, 150. [Google Scholar] [CrossRef]
- Kiss, H.; Orlos, Z.; Gellert, A.; Megyesfalvi, Z.; Mikaczo, A.; Sarkozi, A.; Vasko, A.; Miklos, Z.; Horvath, I. Exhaled Biomarkers for Point-of-Care Diagnosis: Recent Advances and New Challenges in Breathomics. Micromachines (Basel) 2023, 14. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin Chem 2006, 52, 601–623. [Google Scholar] [CrossRef]
- Arja, C.; Surapaneni, K.M.; Raya, P.; Adimoolam, C.; Balisetty, B.; Kanala, K.R. Oxidative stress and antioxidant enzyme activity in South Indian male smokers with chronic obstructive pulmonary disease. Respirology 2013, 18, 1069–1075. [Google Scholar] [CrossRef]
- Bartoli, M.L.; Novelli, F.; Costa, F.; Malagrinò, L.; Melosini, L.; Bacci, E.; Cianchetti, S.; Dente, F.L.; Di Franco, A.; Vagaggini, B.; et al. Malondialdehyde in exhaled breath condensate as a marker of oxidative stress in different pulmonary diseases. Mediators Inflamm 2011, 2011, 891752. [Google Scholar] [CrossRef]
- Calikoglu, M.; Unlu, A.; Tamer, L.; Ercan, B.; Bugdayci, R.; Atik, U. The levels of serum vitamin C, malonyldialdehyde and erythrocyte reduced glutathione in chronic obstructive pulmonary disease and in healthy smokers. Clin Chem Lab Med 2002, 40, 1028–1031. [Google Scholar] [CrossRef]
- Corradi, M.; Pignatti, P.; Manini, P.; Andreoli, R.; Goldoni, M.; Poppa, M.; Moscato, G.; Balbi, B.; Mutti, A. Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. European Respiratory Journal 2004, 24, 1011–1017. [Google Scholar] [CrossRef]
- Corradi, M.; Rubinstein, I.; Andreoli, R.; Manini, P.; Caglieri, A.; Poli, D.; Alinovi, R.; Mutti, A. Aldehydes in exhaled breath condensate of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003, 167, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, N.; Lamsal, M.; Baral, N.; Shrestha, S.; Dhakal, S.S.; Bhatta, N.; Dubey, R.K. Oxidative stress and nutritional status in chronic obstructive pulmonary disease. J Clin Diagn Res 2015, 9, BC01–BC04. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.F.; Braz, M.G.; de Arruda, N.M.; Caram, L.; Nogueira, D.L.; Tanni, S.E.; de Godoy, I.; Ferrari, R. DNA damage and antioxidant capacity in COPD patients with and without lung cancer. PLoS One 2022, 17, e0275873. [Google Scholar] [CrossRef] [PubMed]
- Hanta, I.; Kocabas, A.; Canacankatan, N.; Kuleci, S.; Seydaoglu, G. Oxidant-antioxidant balance in patients with COPD. Lung 2006, 184, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Shin, J.H.; Hwang, J.H.; Baek, J.E.; Choi, B.S. Malondialdehyde and 3-nitrotyrosine in exhaled breath condensate in retired elderly coal miners with chronic obstructive pulmonary disease. Saf Health Work 2014, 5, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Milevoj Kopcinovic, L.; Domijan, A.M.; Posavac, K.; Cepelak, I.; Zanic Grubisic, T.; Rumora, L. Systemic redox imbalance in stable chronic obstructive pulmonary disease. Biomarkers 2016, 21, 692–698. [Google Scholar] [CrossRef]
- Montano, M.; Cisneros, J.; Ramirez-Venegas, A.; Pedraza-Chaverri, J.; Mercado, D.; Ramos, C.; Sansores, R.H. Malondialdehyde and superoxide dismutase correlate with FEV(1) in patients with COPD associated with wood smoke exposure and tobacco smoking. Inhal Toxicol 2010, 22, 868–874. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Fois, A.G.; Sotgia, S.; Mangoni, A.A.; Zinellu, E.; Pirina, P.; Carru, C.; Zinellu, A. Circulating malondialdehyde concentrations in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. Biomark Med 2018, 12, 771–781. [Google Scholar] [CrossRef]
- Stanojkovic, I.; Kotur-Stevuljevic, J.; Milenkovic, B.; Spasic, S.; Vujic, T.; Stefanovic, A.; Llic, A.; Ivanisevic, J. Pulmonary function, oxidative stress and inflammatory markers in severe COPD exacerbation. Respir Med 2011, 105 Suppl 1, S31–37. [Google Scholar] [CrossRef]
- Sunnetcioglu, A.; Alp, H.H.; Sertogullarindan, B.; Balaharoglu, R.; Gunbatar, H. Evaluation of Oxidative Damage and Antioxidant Mechanisms in COPD, Lung Cancer, and Obstructive Sleep Apnea Syndrome. Respir Care 2016, 61, 205–211. [Google Scholar] [CrossRef]
- Vibhuti, A.; Arif, E.; Deepak, D.; Singh, B.; Qadar Pasha, M.A. Correlation of oxidative status with BMI and lung function in COPD. Clin Biochem 2007, 40, 958–963. [Google Scholar] [CrossRef]
- Ambade, V.N.; Sontakke, A.N.; Barthwal, M.S.; Tyagi, R.; Basannar, D.R. Diagnostic Utility of Biomarkers in COPD. Respir Care 2015, 60, 1729–1742. [Google Scholar] [CrossRef]
- Cristovao, C.; Cristovao, L.; Nogueira, F.; Bicho, M. Evaluation of the oxidant and antioxidant balance in the pathogenesis of chronic obstructive pulmonary disease. Rev Port Pneumol 2013, 19, 70–75. [Google Scholar] [CrossRef]
- Lakhdar, R.; Denden, S.; Mouhamed, M.H.; Chalgoum, A.; Leban, N.; Knani, J.; Lefranc, G.; Miled, A.; Ben Chibani, J.; Khelil, A.H. Correlation of EPHX1, GSTP1, GSTM1, and GSTT1 genetic polymorphisms with antioxidative stress markers in chronic obstructive pulmonary disease. Exp Lung Res 2011, 37, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Bridges, A.B.; Scott, N.A.; Belch, J.J.F.; Pringle, T.H.; Mcneill, G.P. Relationship between the extent of coronary artery disease and indicators of free radical activity. Clinical Cardiology 1992, 15, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-R.; Lu, T.-T.; Chang, H.-T.; Ge, X.; Huang, B.; Li, W.-M. Elevated Levels of Plasma Superoxide Dismutases 1 and 2 in Patients with Coronary Artery Disease. BioMed Research International 2016, 2016, 3708905. [Google Scholar] [CrossRef] [PubMed]
- Shramko, V.S.; Striukova, E.V.; Polonskaya, Y.V.; Stakhneva, E.M.; Volkova, M.V.; Kurguzov, A.V.; Kashtanova, E.V.; Ragino, Y.I. Associations of Antioxidant Enzymes with the Concentration of Fatty Acids in the Blood of Men with Coronary Artery Atherosclerosis. Journal of Personalized Medicine 2021, 11, 1281. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, J.; Nijs, J.; Ickmans, K.; Godderis, L.; Ghosh, M.; Polli, A. The Interplay between Oxidative Stress, Exercise, and Pain in Health and Disease: Potential Role of Autonomic Regulation and Epigenetic Mechanisms. Antioxidants 2020, 9, 1166. [Google Scholar] [CrossRef]
- Lenard, Z.; Studinger, P.; Mersich, B.; Kocsis, L.; Kollai, M. Maturation of Cardiovagal Autonomic Function From Childhood to Young Adult Age. Circulation 2004, 110, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Promsrisuk, T.; Boonla, O.; Kongsui, R.; Sriraksa, N.; Thongrong, S.; Srithawong, A. Oxidative stress associated with impaired autonomic control and severity of lung function in chronic obstructive pulmonary disease patients. J Exerc Rehabil 2023, 19, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.; Argay, K.; Herjavecz, I.; Kollai, M. Relation between bronchial and cardiac vagal tone in healthy humans. Chest 1995, 108, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, J.S.; Aldhahir, A.M.; Alghamdi, S.M.; Al Ghamdi, S.S.; AlDraiwiesh, I.A.; Alsulayyim, A.S.; Alqahtani, A.S.; Alobaidi, N.Y.; Al Saikhan, L.; AlRabeeah, S.M.; et al. A systematic review and meta-analysis of heart rate variability in COPD. Front Cardiovasc Med 2023, 10, 1070327. [Google Scholar] [CrossRef]
- Antonelli Incalzi, R.; Corsonello, A.; Trojano, L.; Pedone, C.; Acanfora, D.; Spada, A.; D'Addio, G.; Maestri, R.; Rengo, F.; Rengo, G. Heart rate variability and drawing impairment in hypoxemic COPD. Brain Cogn 2009, 70, 163–170. [Google Scholar] [CrossRef]
- MacDonald, D.M.; Mkorombindo, T.; Ling, S.X.; Adabag, S.; Casaburi, R.; Connett, J.E.; Helgeson, E.S.; Porszasz, J.; Rossiter, H.B.; Stringer, W.W.; et al. Heart Rate Variability on 10-Second Electrocardiogram and Risk of Acute Exacerbation of COPD: A Secondary Analysis of the BLOCK COPD Trial. Chronic Obstr Pulm Dis 2022, 9, 226–236. [Google Scholar] [CrossRef]
- Coviello, I.; Pinnacchio, G.; Laurito, M.; Stazi, A.; Battipaglia, I.; Barone, L.; Mollo, R.; Russo, G.; Villano, A.; Sestito, A.; et al. Prognostic role of heart rate variability in patients with ST-segment elevation acute myocardial infarction treated by primary angioplasty. Cardiology 2013, 124, 63–70. [Google Scholar] [CrossRef]
- Karp, E.; Shiyovich, A.; Zahger, D.; Gilutz, H.; Grosbard, A.; Katz, A. Ultra-short-term heart rate variability for early risk stratification following acute ST-elevation myocardial infarction. Cardiology 2009, 114, 275–283. [Google Scholar] [CrossRef]
- Kienzle, M.G.; Ferguson, D.W.; Birkett, C.L.; Myers, G.A.; Berg, W.J.; Mariano, D.J. Clinical, hemodynamic and sympathetic neural correlates of heart rate variability in congestive heart failure. Am J Cardiol 1992, 69, 761–767. [Google Scholar] [CrossRef]
- Stein, P.K.; Domitrovich, P.P.; Huikuri, H.V.; Kleiger, R.E.; Cast, I. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. J Cardiovasc Electrophysiol 2005, 16, 13–20. [Google Scholar] [CrossRef]
- Chahine, T.; Baccarelli, A.; Litonjua, A.; Wright, R.O.; Suh, H.; Gold, D.R.; Sparrow, D.; Vokonas, P.; Schwartz, J. Particulate air pollution, oxidative stress genes, and heart rate variability in an elderly cohort. Environ Health Perspect 2007, 115, 1617–1622. [Google Scholar] [CrossRef]
- Chan, C.C.; Lin, L.Y.; Lai, C.H.; Chuang, K.J.; Wu, M.T.; Pan, C.H. Association of Particulate Matter from Cooking Oil Fumes with Heart Rate Variability and Oxidative Stress. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Wang, C.; Lin, J.; Niu, Y.; Wang, W.; Wen, J.; Lv, L.; Liu, C.; Du, X.; Zhang, Q.; Chen, B.; et al. Impact of ozone exposure on heart rate variability and stress hormones: A randomized-crossover study. J Hazard Mater 2022, 421, 126750. [Google Scholar] [CrossRef]
- Antali, F.; Kulin, D.; Lucz, K.I.; Szabo, B.; Szucs, L.; Kulin, S.; Miklos, Z. Multimodal Assessment of the Pulse Rate Variability Analysis Module of a Photoplethysmography-Based Telemedicine System. Sensors (Basel) 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Couillard, A.; Koechlin, C.; Cristol, J.P.; Varray, A.; Prefaut, C. Evidence of local exercise-induced systemic oxidative stress in chronic obstructive pulmonary disease patients. Eur Respir J 2002, 20, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Premanand, R.; Kumar, S.; Mohan, A. Study of thiobarbituric reactive substances and total reduced glutathione as indices of oxidative stress in chronic smokers with and without chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci 2007, 49, 9–12. [Google Scholar] [PubMed]
- Montuschi, P.; Kharitonov, S.A.; Barnes, P.J. Exhaled carbon monoxide and nitric oxide in COPD. Chest 2001, 120, 496–501. [Google Scholar] [CrossRef]
- Paredi, P.; Kharitonov, S.A.; Leak, D.; Ward, S.; Cramer, D.; Barnes, P.J. Exhaled ethane, a marker of lipid peroxidation, is elevated in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000, 162, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Kostikas, K.; Papatheodorou, G.; Psathakis, K.; Panagou, P.; Loukides, S. Oxidative stress in expired breath condensate of patients with COPD. Chest 2003, 124, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Dekhuijzen, P.N.; Aben, K.K.; Dekker, I.; Aarts, L.P.; Wielders, P.L.; van Herwaarden, C.L.; Bast, A. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996, 154, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Warwick, G.; Thomas, P.S.; Yates, D.H. Non-invasive biomarkers in exacerbations of obstructive lung disease. Respirology 2013, 18, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Antus, B.; Barta, I.; Kullmann, T.; Lazar, Z.; Valyon, M.; Horvath, I.; Csiszer, E. Assessment of exhaled breath condensate pH in exacerbations of asthma and chronic obstructive pulmonary disease: A longitudinal study. Am J Respir Crit Care Med 2010, 182, 1492–1497. [Google Scholar] [CrossRef]
- Biernacki, W.A.; Kharitonov, S.A.; Barnes, P.J. Increased leukotriene B4 and 8-isoprostane in exhaled breath condensate of patients with exacerbations of COPD. Thorax 2003, 58, 294–298. [Google Scholar] [CrossRef]
- Montuschi, P.; Kharitonov, S.A.; Ciabattoni, G.; Barnes, P.J. Exhaled leukotrienes and prostaglandins in COPD. Thorax 2003, 58, 585–588. [Google Scholar] [CrossRef]
- Ashfaq, S.; Abramson, J.L.; Jones, D.P.; Rhodes, S.D.; Weintraub, W.S.; Hooper, W.C.; Vaccarino, V.; Harrison, D.G.; Quyyumi, A.A. The Relationship Between Plasma Levels of Oxidized and Reduced Thiols and Early Atherosclerosis in Healthy Adults. Journal of the American College of Cardiology 2006, 47, 1005–1011. [Google Scholar] [CrossRef]
- Huang, Y.S.; Wang, L.X.; Sun, L.; Wu, Y.; Lu, J.M.; Zhao, S.C.; Dai, F.M.; Xu, B.S.; Wang, S.R. Elevated peroxidative glutathione redox status in atherosclerotic patients with increased thickness of carotid intima media. Chin Med J (Engl) 2009, 122, 2827–2832. [Google Scholar]
- Rybka, J.; Kupczyk, D.; Kędziora-Kornatowska, K.; Motyl, J.; Czuczejko, J.; Szewczyk-Golec, K.; Kozakiewicz, M.; Pawluk, H.; Carvalho, L.A.; Kędziora, J. Glutathione-Related Antioxidant Defense System in Elderly Patients Treated for Hypertension. Cardiovascular Toxicology 2011, 11, 1–9. [Google Scholar] [CrossRef]
- Isogawa, A.; Yamakado, M.; Yano, M.; Shiba, T. Serum superoxide dismutase activity correlates with the components of metabolic syndrome or carotid artery intima-media thickness. Diabetes Res Clin Pract 2009, 86, 213–218. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Gómez-Hermosillo, L.; Casillas-Moreno, J.; Pacheco-Moisés, F.; Campos-Bayardo, T.I.; Román-Rojas, D.; Miranda-Díaz, A.G. Prevalence of Hypertension and Obesity: Profile of Mitochondrial Function and Markers of Inflammation and Oxidative Stress. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Espinola-Klein, C.; Rupprecht, H.J.; Bickel, C.; Schnabel, R.; Genth-Zotz, S.; Torzewski, M.; Lackner, K.; Munzel, T.; Blankenberg, S. Glutathione peroxidase-1 activity, atherosclerotic burden, and cardiovascular prognosis. Am J Cardiol 2007, 99, 808–812. [Google Scholar] [CrossRef]
- van Zyl, C.; Huisman, H.W.; Mels, C.M.C. Antioxidant enzyme activity is associated with blood pressure and carotid intima media thickness in black men and women: The SABPA study. Atherosclerosis 2016, 248, 91–96. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Torzewski, M.; Hafner, G.; Tiret, L.; Smieja, M.; Cambien, F.; Meyer, J.; Lackner, K.J. Glutathione Peroxidase 1 Activity and Cardiovascular Events in Patients with Coronary Artery Disease. New England Journal of Medicine 2003, 349, 1605–1613. [Google Scholar] [CrossRef]
- Armas-Padilla, M.C.; Armas-Hernández, M.J.; Sosa-Canache, B.; Cammarata, R.; Pacheco, B.; Guerrero, J.; Carvajal, A.R.; Hernández-Hernández, R.; Israili, Z.H.; Valasco, M. Nitric oxide and malondialdehyde in human hypertension. Am J Ther 2007, 14, 172–176. [Google Scholar] [CrossRef]
- Zuin, M.; Capatti, E.; Borghi, C.; Zuliani, G. Serum Malondialdehyde Levels in Hypertensive Patients: A Non-invasive Marker of Oxidative Stress. A Systematic Review and Meta-analysis. High Blood Pressure & Cardiovascular Prevention 2022, 29, 263–273. [Google Scholar] [CrossRef]
- Brinkley, T.E.; Nicklas, B.J.; Kanaya, A.M.; Satterfield, S.; Lakatta, E.G.; Simonsick, E.M.; Sutton-Tyrrell, K.; Kritchevsky, S.B. Plasma oxidized low-density lipoprotein levels and arterial stiffness in older adults: the health, aging, and body composition study. Hypertension (Dallas, Tex. : 1979) 2009, 53, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Skoumas, J.; Economou, M.; Papadimitriou, L.; Stefanadis, C. The association between pre-hypertension status and oxidative stress markers related to atherosclerotic disease: The ATTICA study. Atherosclerosis 2007, 192, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, J.; Wu, R.; Lemne, C.; Thulin, T.; Witztum, J.L.; de Faire, U. Circulating oxidized low-density lipoprotein is increased in hypertension. Clin Sci (Lond) 2003, 105, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, P.; Vanhaecke, J.; Janssens, S.; Van De Werf, F.; Collen, D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation 1998, 98, 1487–1494. [Google Scholar] [CrossRef]
- Ehara, S.; Ueda, M.; Naruko, T.; Haze, K.; Itoh, A.; Otsuka, M.; Komatsu, R.; Matsuo, T.; Itabe, H.; Takano, T.; et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001, 103, 1955–1960. [Google Scholar] [CrossRef]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation 2005, 112, 651–657. [Google Scholar] [CrossRef]
- Holvoet, P.; Mertens, A.; Verhamme, P.; Bogaerts, K.; Beyens, G.; Verhaeghe, R.; Collen, D.; Muls, E.; Werf, F.V.d. Circulating Oxidized LDL Is a Useful Marker for Identifying Patients With Coronary Artery Disease. Arteriosclerosis, Thrombosis, and Vascular Biology 2001, 21, 844–848. [Google Scholar] [CrossRef]
- Yan, Z.; Fu, B.; He, D.; Zhang, Y.; Liu, J.; Zhang, X. The relationship between oxidized low-density lipoprotein and related ratio and acute cerebral infarction. Medicine (Baltimore) 2018, 97, e12642–e12642. [Google Scholar] [CrossRef] [PubMed]
- Uno, M.; Kitazato, K.T.; Nishi, K.; Itabe, H.; Nagahiro, S. Raised plasma oxidised LDL in acute cerebral infarction. Journal of Neurology, Neurosurgery & Psychiatry 2003, 74, 312. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Dai, L.; Zhang, N.; Lin, J.; Chen, G.; Zuo, Y.; Li, H.; Wang, Y.; Meng, X.; Wang, Y. Oxidized low-density lipoprotein (LDL) and LDL cholesterol are associated with outcomes of minor stroke and TIA. Atherosclerosis 2020, 297, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nature Reviews Drug Discovery 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kang, P.M. Oxidative Stress and Antioxidant Treatments in Cardiovascular Diseases. Antioxidants (Basel) 2020, 9. [Google Scholar] [CrossRef]
- Fang, T.; Yang, J.; Liu, L.; Xiao, H.; Wei, X. Nicotinamide mononucleotide ameliorates senescence in alveolar epithelial cells. MedComm (2020) 2021, 2, 279–287. [Google Scholar] [CrossRef]
- Kiss, T.; Balasubramanian, P.; Valcarcel-Ares, M.N.; Tarantini, S.; Yabluchanskiy, A.; Csipo, T.; Lipecz, A.; Reglodi, D.; Zhang, X.A.; Bari, F.; et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: a potential mechanism for the prevention of vascular cognitive impairment. Geroscience 2019, 41, 619–630. [Google Scholar] [CrossRef]
- Tarantini, S.; Valcarcel-Ares, M.N.; Toth, P.; Yabluchanskiy, A.; Tucsek, Z.; Kiss, T.; Hertelendy, P.; Kinter, M.; Ballabh, P.; Süle, Z.; et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biology 2019, 24, 101192. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhu, C.-L.; Li, P.; Li, H.-R.; Liu, Q.; Deng, X.-M.; Wang, J.-F. Nicotinamide Mononucleotide Attenuates LPS-Induced Acute Lung Injury With Anti-Inflammatory, Anti-Oxidative and Anti-Apoptotic Effects. Journal of Surgical Research 2023, 283, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Sun, A.; Ge, J. The effects of nicotinamide adenine dinucleotide in cardiovascular diseases: Molecular mechanisms, roles and therapeutic potential. Genes Dis 2022, 9, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Batlahally, S.; Franklin, A.; Damianos, A.; Huang, J.; Chen, P.; Sharma, M.; Duara, J.; Keerthy, D.; Zambrano, R.; Shehadeh, L.A.; et al. Soluble Klotho, a biomarker and therapeutic strategy to reduce bronchopulmonary dysplasia and pulmonary hypertension in preterm infants. Scientific Reports 2020, 10, 12368. [Google Scholar] [CrossRef]
- Takenaka, T.; Kobori, H.; Miyazaki, T.; Suzuki, H.; Nishiyama, A.; Ishii, N.; Yamashita, M.; Hayashi, M. Klotho protein supplementation reduces blood pressure and renal hypertrophy in db/db mice, a model of type 2 diabetes. Acta Physiol (Oxf) 2019, 225, e13190. [Google Scholar] [CrossRef]
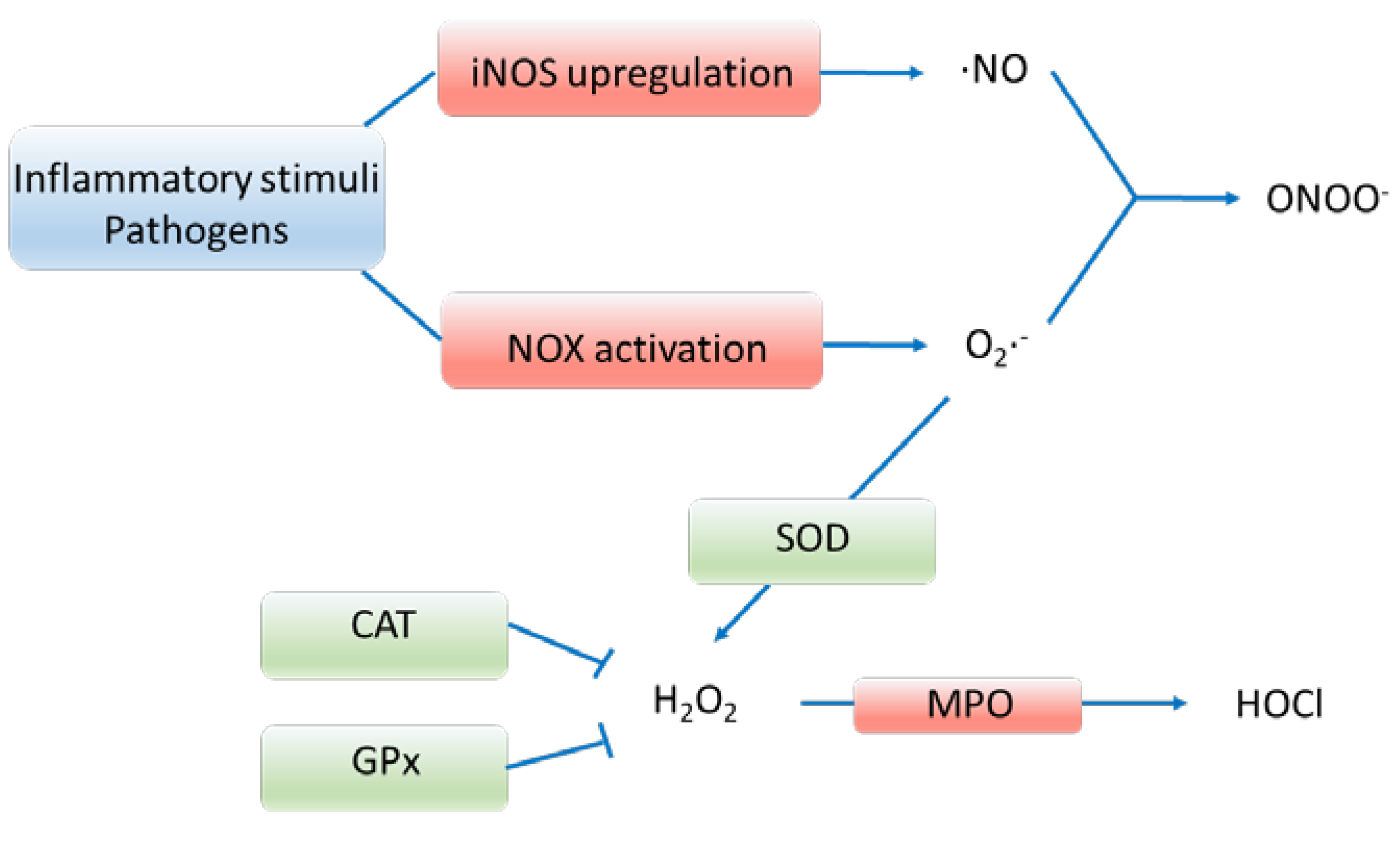
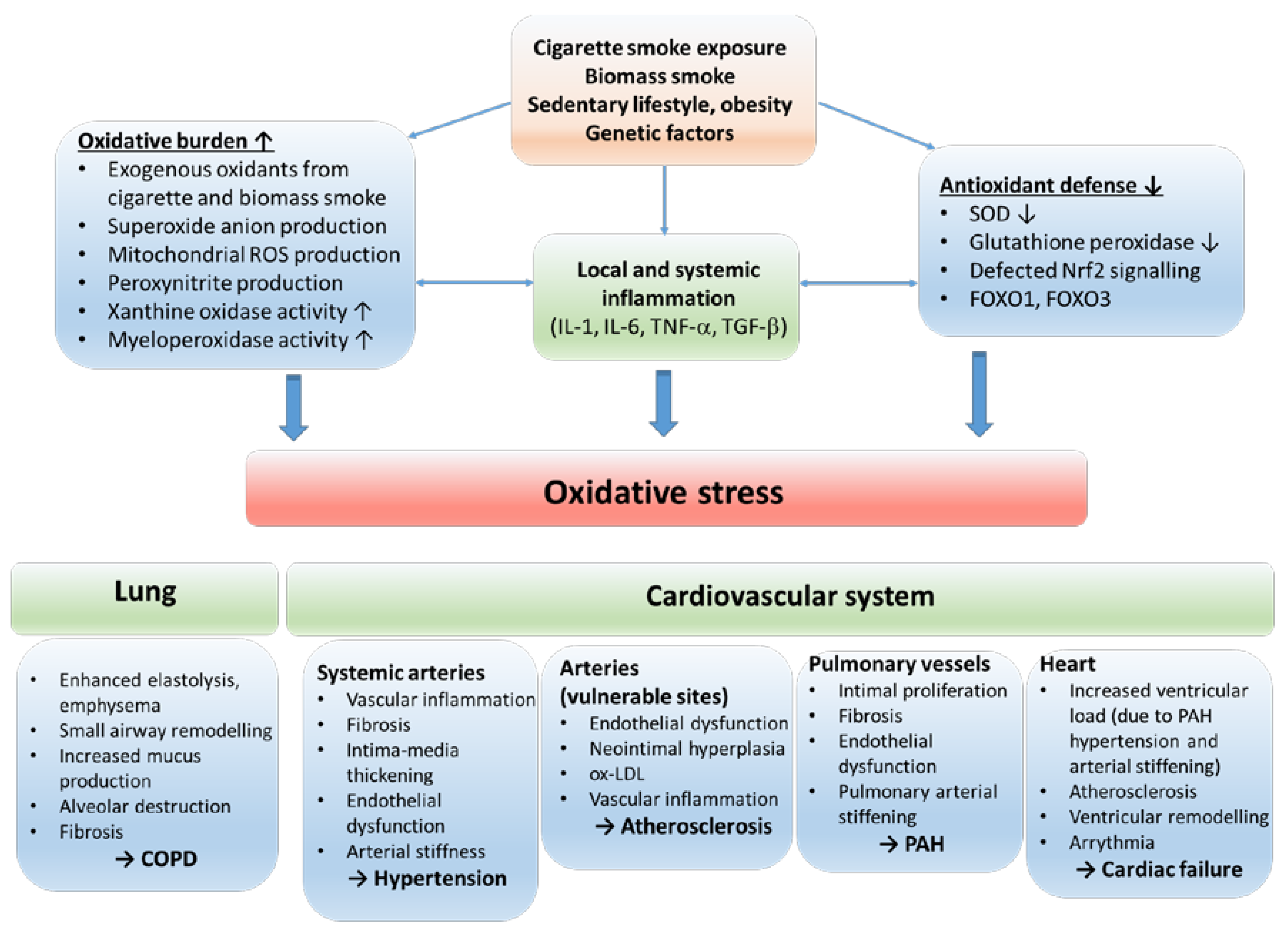
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





