1. Introduction
The red palm weevil (RPW), Rhynchophorus ferrugineus Olivier, 1790 (Coleoptera Curculionidae), is one of the most harmful palm pests and its damages to date palms was recorded since the mid-1980s in Saudi Arabia and then in Egypt in the 1990s (Cox 1993). The RPW then spread since 2000 in the Mediterranean basin (EPPO Reporting Service 1996, 1999a, b, 2004, 2006a, b, c, 2007a, b, c, d, e, 2008a, b, 2009, 2010a, b; EPPO/OEPP 2013), infesting palms essentially belonging to the genus Phoenix, used here for ornamental purposes.
The fight against the red palm weevil is unfortunately still an open question; early detection of the RPW is challenging because larvae (the most damaging stage) are endophytic (Soroker et al. 2017). Several control methods have been used and tested so far, including biological control by means of entomopathogenic nematodes (e.g., Steinernematidae and Heterorhabditidae), fungi (e.g., Beauveria bassiana, Metarhizium anisopliae), virus (Baculovirus oryctes, Cytoplasmic Polyhedrosis Virus) (Wraight et al., 2001), bacteria (Bacillus sp., Pseudomonas aeruginosa) and mass trapping (Rochat et al. 2006; Faleiro et al. 2012; Masilamany et al. 2012; Mazza et al. 2014; Giblin-Davis et al. 2013; Lin et al. 2017). Nevertheless, insecticide applications are, at present, the most effective method for protecting palms from attack by palm weevils (Milosavljević et al. 2019). Insecticides can be applied outside the apical part of the plant (cover high pressure sprays or low-pressure solutions) (Rochat et al. 2006; Masilamany & Tang 2013; Al-Shawaf et al. 2013; Peri et al. 2013; Al-Dosary et al. 2016; Llopis et al. 2018; DOA 2016) or inside it (trunk injection method) (Kielbaso et al. 1979; Dembilio et al. 2014). In the first case, treatment should be repeated periodically, depending on the persistence of the sprayed insecticide (Ferry and Gomez 2012) and local climate; in the second one, treatment may be more scattered over time (Chihaoui-Meridja et al. 2020). For these reasons, endotherapy is regarded as a safer method rather than spray diffusion, with low adverse effects on non-targeted species, humans and environment (Chihaoui-Meridja et al. 2020).
In the Marche Region, chemical control of Rhynchophorus ferrugineus started in 2008 and it was carried out using 15–20 litres per palm of an insecticide spray solution, containing as main chemical active substances chlorpyrifos (CPF) or acetamiprid (ACE)/imidacloprid (IMI). At least one treatment application per year was conducted during the last years (Nardi et al. 2011). Thus, considering the widespread use of these synthetic pesticides, concerns arise regarding the environmental fate and potential toxic effects of these compounds within our study area. ACE and IMI are two neonicotinoid insecticides that act as selective agonists of type-2 nicotinic acetylcholine receptors in insects (Gervais et al. 2010). However, distribution of these compounds into the plant's pollen and nectar (Stoner and Eitzer 2012) contributes to expose other non-target invertebrates such as pollinating honeybees (Apis mellifera) (Johnson and Pettis 2014; Chagnon et al. 2015). In addition, both soil drench and seed treatment may contribute to spreading the contamination, also exposing vertebrate species including humans (Rogers et al. 2019). CPF is one of the most widely used chlorinated organophosphate (OP) pesticides and it is well known as a highly persistent chemical, especially in aquatic ecosystems (EPA 2000; Watts 2012). CPF toxicity has been shown to vary, ranging from neurological dysfunctions to endocrine disruption, in both human and animal studies (Rahman et al. 2021).
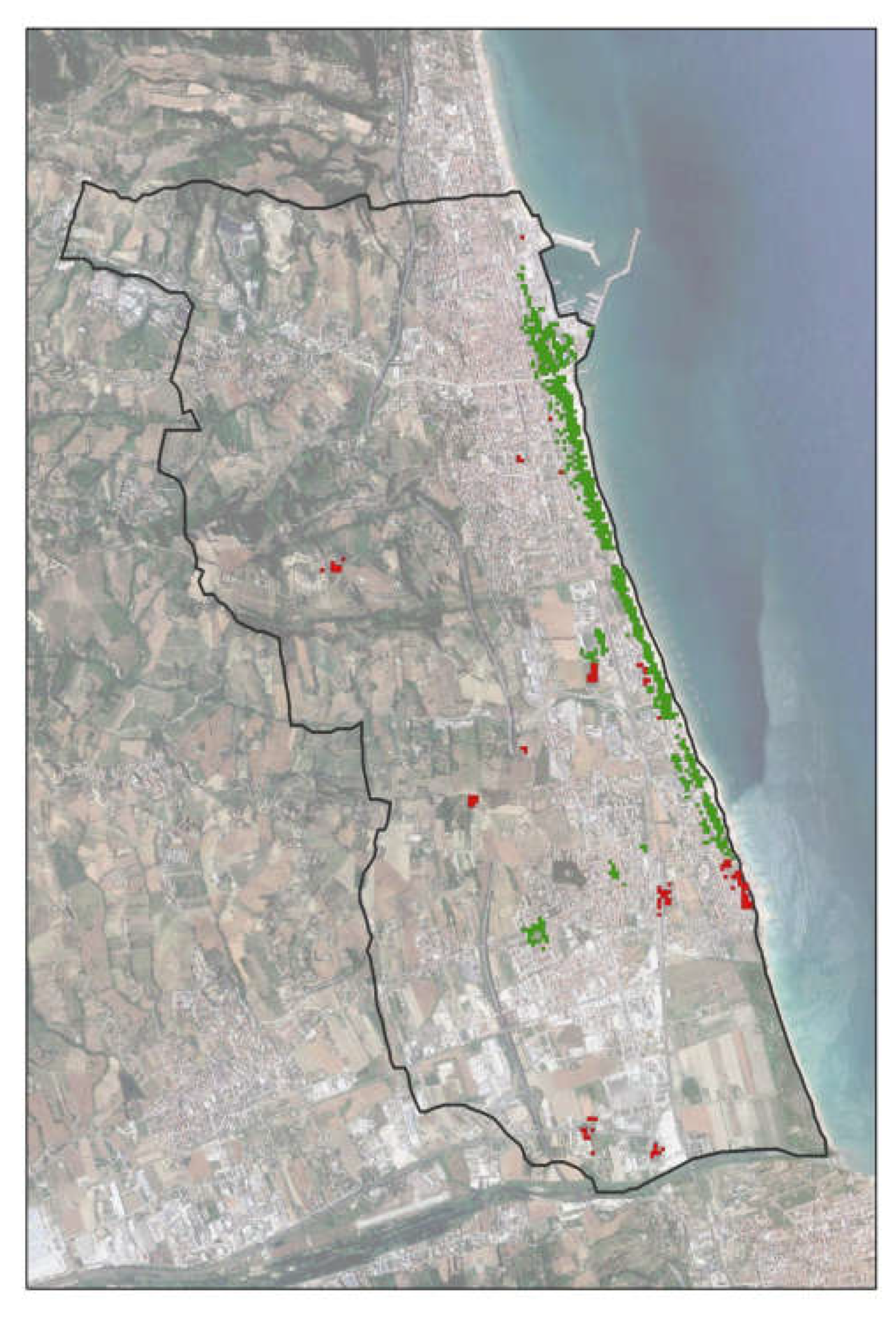
Urban areas look like specific challenges for the management of pest infestations such as RPW, as infested palms are placed often on private land which may complicate control programs (Milosavljević et al. 2019) and because pesticide use is highly regulated to preserve public health. In this regard, a sequential photo-surveillance approach may be very useful to monitoring the evolution of pest because allows the quantification of mortality rates and spread patterns (Cohen et al. 2012; Hogan et al. 2017); usually photos refer to satellite (Cinnirella et al. 2020) or drones (Milosavljević et al. 2019). Many of these territories link their image precisely to the presence of palms and therefore have to face this great problem. For instance, the municipal area of San Benedetto del Tronto is known as the Palm Tree Coast; hence the importance of preserving its palm tree heritage at all costs for tourism purposes.
In this paper, we present the evolution of the RPW spread in the San Benedetto del Tronto Municipality (at 2020) with respect to the previous status (2007 - 2012) (Cinnirella et al. 2020) using available remote sensing images, field surveys and GIS. Moreover, we predict the potential adverse health effects that may result from exposure to chemicals used for RPW control by carrying out a systems toxicology method. The specific objectives of this work were: a) to evaluate the effectiveness of the chemical treatments carried out by the municipality of San Benedetto del Tronto; b) to analyse the new RPW spatial distribution in this urban area; c) to predict signalling pathways targeted by insecticides that are potentially associated to adverse consequences; d) to suggest specific management urban measures. As far as we know, this was the first study that considers the multiple aspects of the fight against the RPW in an urban area.
2. Materials and Methods
2.1. The study area and collected data
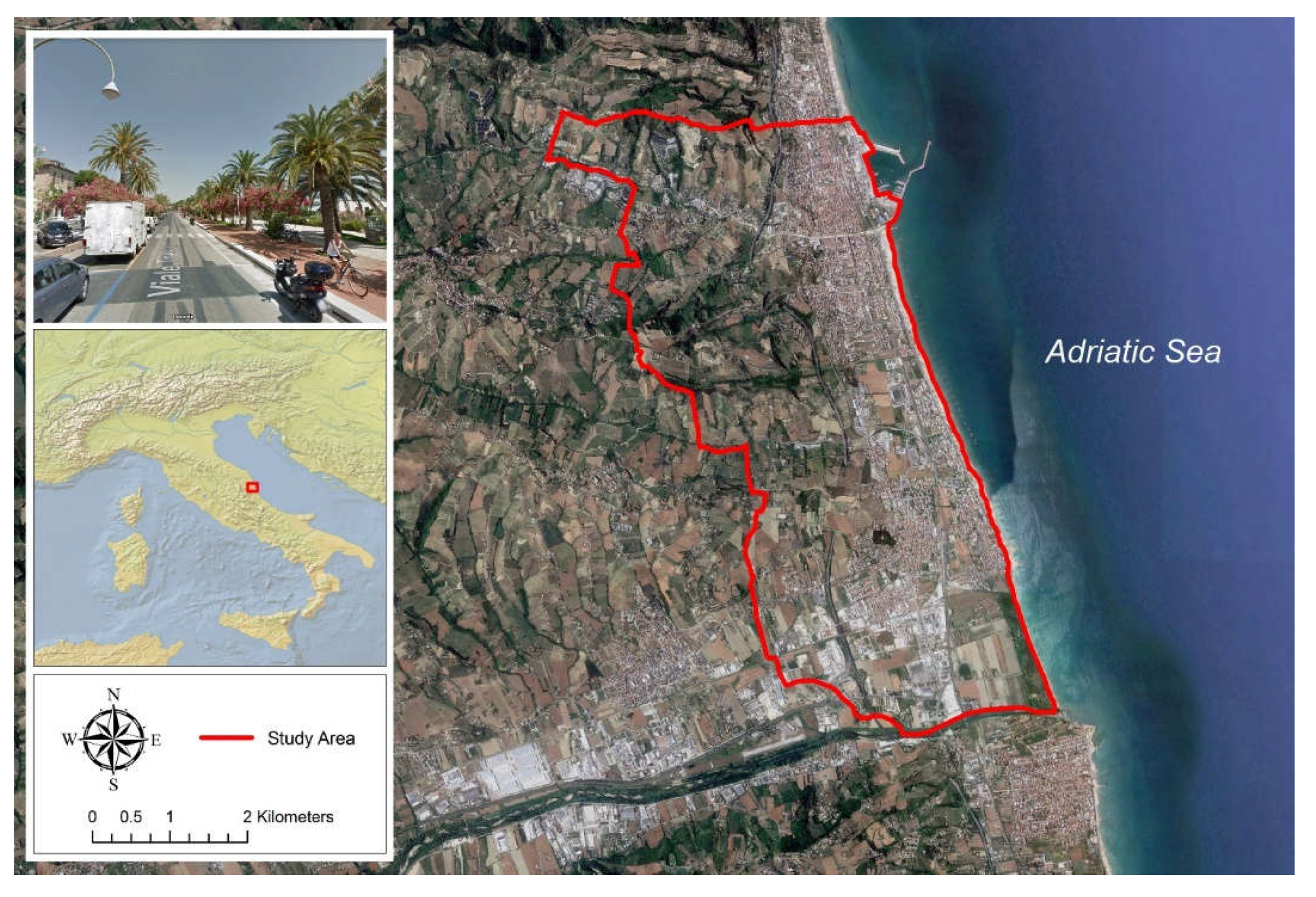
The Municipality of San Benedetto del Tronto is our study area and it’s located along the Adriatic coast of Central Italy (
Figure 1). The bioclimate is Mediterranean (Upper meso-mediterranean thermotype) (Pesaresi et al. 2017) with: i) annual rainfall and temperature averages of 551 mm and 15.8 °C, respectively , ii) dry summer (average temperature of 24°C), iii) rainy and moderately cold winters (average temperature of 7°C) (Fazzini et al. 2013).
Starting from the map generate in previous study in which we analysed the RPW distribution during the period July 2007- July 2013 (Cinnirella et al. 2020), through aerial photos of Google Earth time series, Google Street view time series and subsequent field surveys, all the Phoenix canariensis palm trees in San Benedetto del Tronto municipality were recollected at the end of 2020 year and reclassified in terms of contagious (health, dead-infested).
Based on the interviews with municipal employees and analysis of the municipal administration’s acts (
www.comunesbt.it), what was done in this area against the RPW in the period 2013-2020 was reconstructed. It was therefore possible on the one hand, to classify the palms in terms of treatment (treated, untreated) and on the other hand, to estimate the quantities and type of pesticides used.
2.2. Geo-statistical analysis
To analyze the recent distribution of the RPW (palms dead or infested) with respect to the chemical treatments carried out (palms treated or untreated), the new collected data were entered into a previous GIS (ESRI® ArcGIS© 10.1 (ESRI 2012)).
The Hot spot analysis (Getis-Ord Gi*) was performed to highlight statistically significant areas of clustering (infestation; chemical treatment), based on a new output shape file that reports a z-score, a p-value and a confidence level of aggregation or dispersion bin field (Gi_Bin) for each feature (high z-score and small p-value: spatial cluster with high values; low negative z-score and small p-value: spatial clustering with low values; z-score near zero: no apparent spatial clustering).
In our case, we performed two Hot spot analyses, one for the infested palms (dead or attached) and another one for the treated palms.
For this reason, the input shape file was modified by adding short numerical attribute fields, based on the classification of all the palms in terms of the contagious event (1, infested or dead palm; 0, healthy palm) and chemical treatment (1, treated palm; 0, no treated palm).
Through overlapping of the resulting maps (the two generated shapefiles displayed by the Gi_Bin values), it was possible to verify if a possible data cluster of health palms is superimposed on a possible data cluster of treated palms.
For this purpose, we generate a map in which polygons of a net (20 mt x 20 mt) report by different colour, the information about “healthy and treated” and “healthy and not treated” palms; in this context we also generated an index based on the overlapping percentage.
Finally, to support this evaluation, statistical analysis was performed for PRW infections on the considered palm trees. The risk factor affecting RPW occurrence was determined using the Chi-square test of independence between the status of each palm (infested or dead; health) and a different treatment (chemical treatment yes or not) (Diakou et al. 2016). The Chi-square testing was securely performed as his assumptions were respected: no more than 20% of the cells can have an expected frequency less than five (in mean 1845.75), no cell can have an expected frequency less than one and the sample size was big enough (Whitlock & Schluter 2015; Roscoe & Byars 1971). In our study the null hypothesis for this test stated that the infection's occurrence does not depend on the chemicals treatment (treated vs untreated).
2.3. In Silico Prediction of insecticide Toxicity
IMI and CPF were investigated using an integrative systems toxicological model to predict biological effects at the human, bird, amphibian, fish and insect system level. We used existing data from the Search Tool for Interactions of Chemicals (STITCH v5.0) database containing the interactions between chemicals and proteins to develop a network-based predictive model of the selected pesticides with targets. This approach has also been widely accepted to study chemical-gene interactions (Iskar et al. 2010; Chakraborty 2014). STITCH acts as a probabilistic network, by collecting interactions from multiple sources, including experimental evidence, databases, and published data. The overall confidence score ranges from 0 to 1, where a value of 0.15 is considered as low confidence, 0.4 as medium confidence, and a score equal to or higher than 0.7 is regarded as high confidence. To obtain a reliable set of interactions, we removed all interactions with a confidence score lower than 0.7 and pesticides connections with uncharacterized protein. IMI and CPF were annotated with a canonical SMILES code retrieved from PubChem (C1CN(C(=N1)N[N+](=O)[O-])CC2=CN=C(C=C2)Cl; CCOP(=S)(OCC)OC1=NC(=C (C=C1Cl)Cl)Cl, respectively). In order to clarify the relationship between the selected pesticides and protein and their involvement in signaling pathways, we represented data using InterPro database, GO enrichment and KEGG pathway enrichment analyses provided by STITCH.
3. Results and discussions
3.1. RPW occurrence
Table 1 shows the classification of the palms according to the state (healthy / dead-infested) and the type of treatment (chemical treatment yes or no). Since at the beginning of the infestation (2007) more than half of the palms have died and of these, just over 60% after 2013.
From the second half of 2013, the Municipality has systematically treated (4 treatments per palm per year) by chemicals (
Table 2), palms placed on the Sea-road, “Dei Mille” road, city centre and urban parks (1627 palms).
At the end of 2020, about 90% (89.73) of treated palms and about 35% (34.42) of untreated palms were not infested by the RPW. It should be noted that the latter percentage drops to 16.5% if untreated palms placed in nurseries, gardens of villas or hotels (places where there is interest and economic availability) are not included in the calculation.
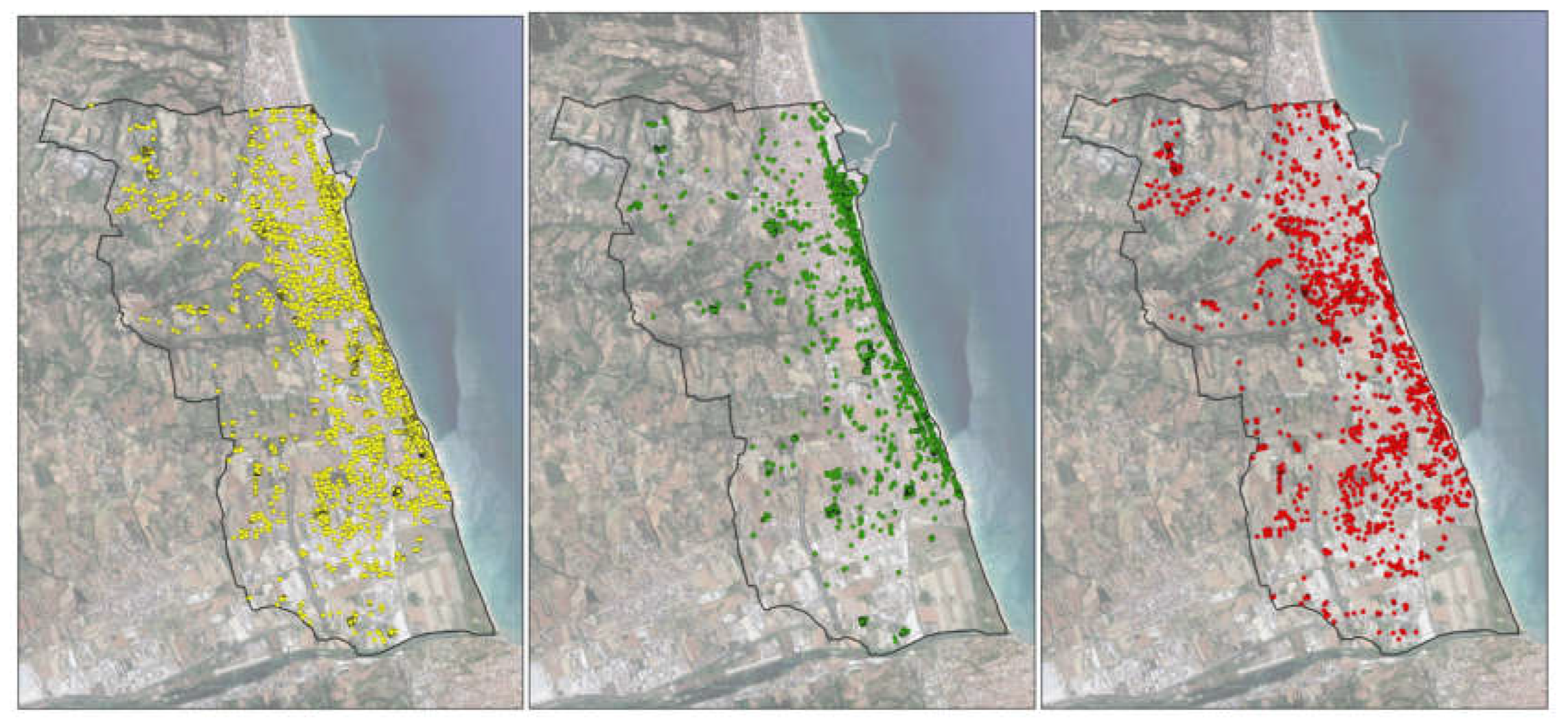
Figure 2 shows how the evolutionary effects of the RPW distribution affected the entire study area.
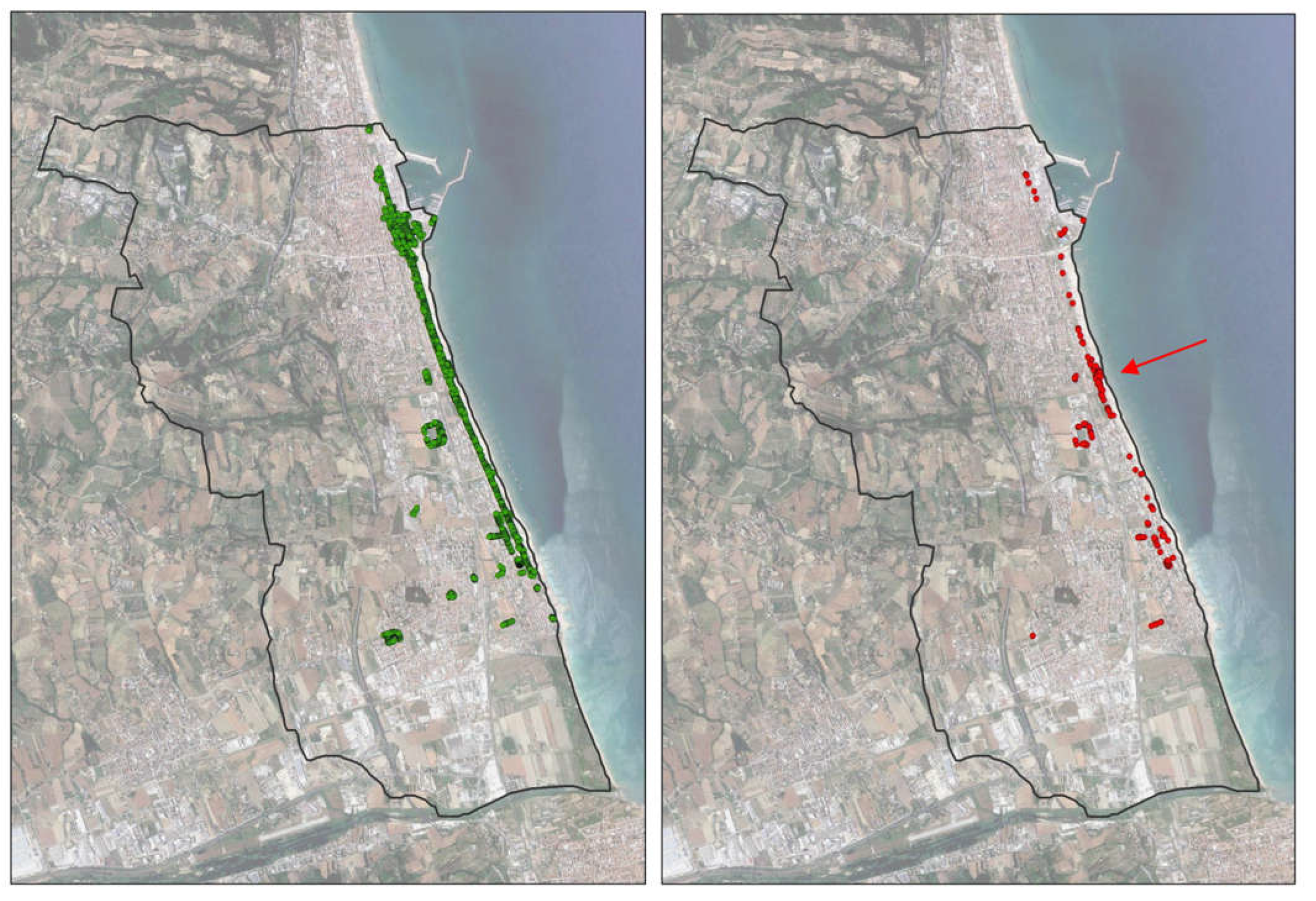
Considering the dead-infested palms among the treated ones (
Figure 3) it is possible to notice a central area in which the concentration of the infested is higher compared to the remaining areas.
Here the direct management of the Municipality (application of the treatments by municipal employees) is interrupted and that entrusted to an external company begins; the interviews revealed that in this area there were often misunderstandings between the two parties which led to the skipping of treatments and therefore, the possibility of the RPW to attack.
The chemicals employed in this period are pesticides usually used in open field agriculture, whose ministerial authorizations for use in urban environments changed over the years (
Table 2).
3.2. Geo-statistical analysis
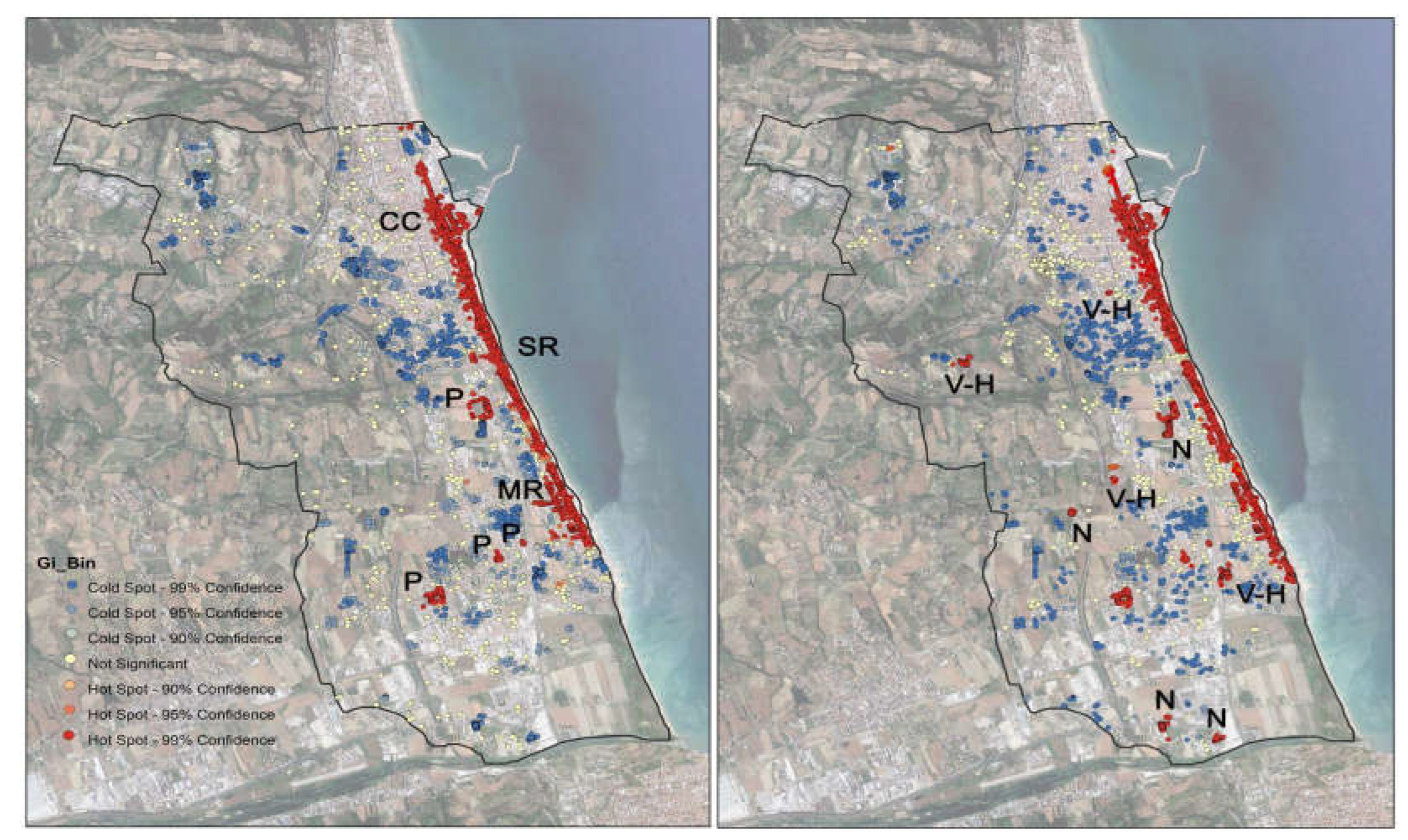
Comparing the hot spot map for treated palms with that of infested palms, it has been observed a very strong overlap (
Figure 4). This means that where the palms were treated, the healthy palms concentration is high. Only little red areas (little hot spot clusters) are placed outside the areas interested by Municipality’s treatments (
Figure 4 dx).
By interpretation of aerial and terranean photos (Google Earth, Google Maps, Google Street) and field surveys we verified the nature of this little hot spot; they correspond to palm nurseries or villas/ farmhouses/hotels’ gardens.
This fact is best appreciated in the overlap map between treated healthy palms and untreated healthy palms (
Figure 5). The overlapping degree index, generated based on the percentage of number of squares superimposed between the two derived hot spot maps (fishnet 20 x 20 mt), is equal to 84.92%. This percentage grows up to 95% not considering the squares relating to the nurseries and villas/hotels gardens.
Finally, to support these spatial results by a statistical test, the Chi square test was calculated based on the RPW occurrence on “Treated” and “No treated” category palms (starting values in
Table 1). We perform two X2 tests, one considering all “No treated” palms and another one subtracting those placed in nurseries or hotels/villas/farmhouses’ gardens. With 1 as the degree of freedom (contingency table 2 x 2), the resulting values were X2= 358.82 (p-value= 2.2-16) and X2= 441.84 (p-value= 2.2-16), respectively. Therefore, the null hypothesis of independence was rejected mining that the RPW occurrence depends on whether a given palm has been treated or not.
3.3. Computational approach to predict Imidacloprid- and Chlorpyrifos -protein interaction networks
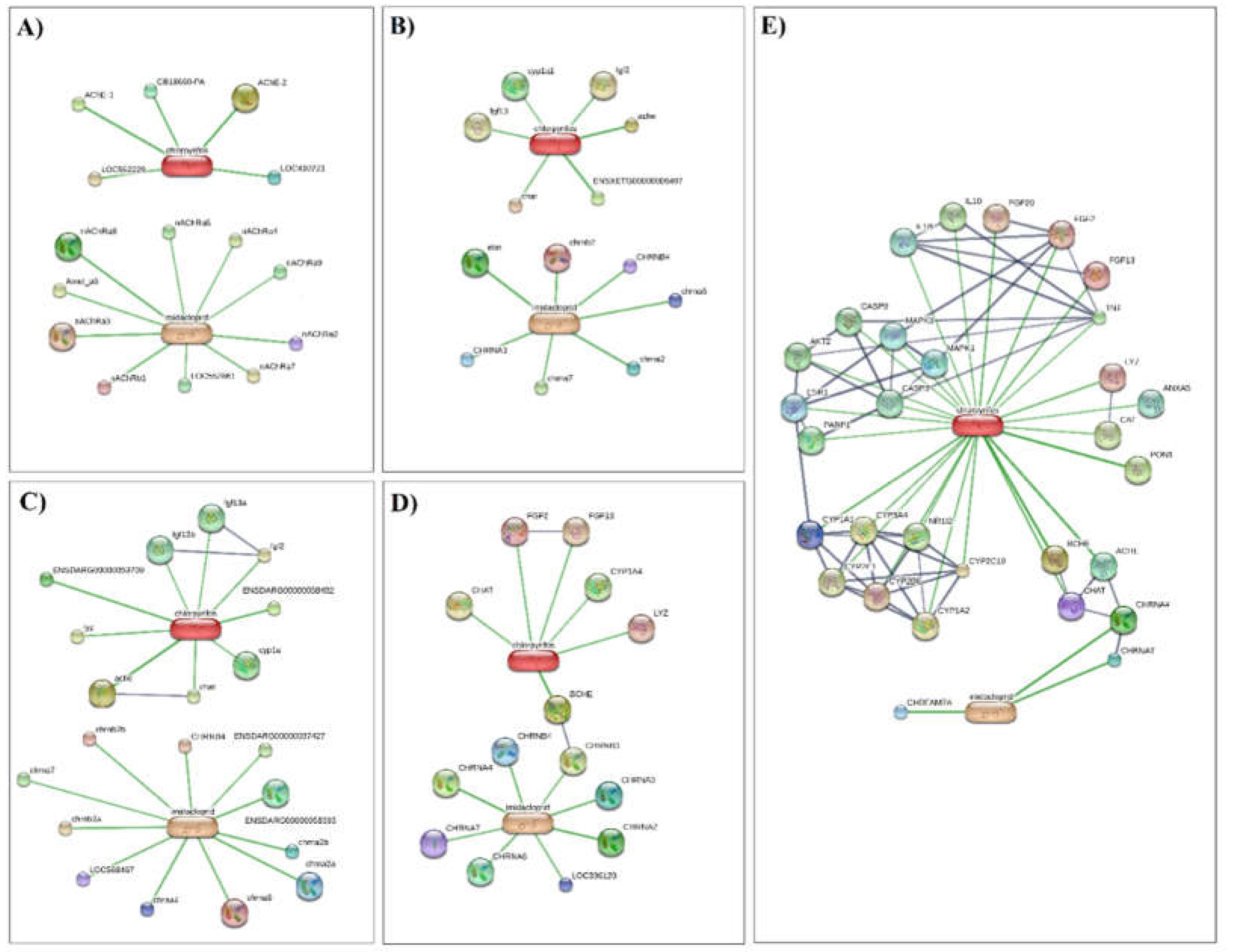
We built a chemical and biological interaction network to investigate the molecular pathways associated to the effects of IMI and CPF on five model organisms (i.e. Apis mellifera, Gallus gallus, Danio rerio, Xenopus silurana, Homo sapiens). Using a confidence score > 0.7 (high confidence score according to STITCH), we identified four main metabolic processes associated with proteins interacting with both chemicals (
Table 3).
Data indicated that most of the identified proteins belong to the neurotransmitter-gated ion-channel family. The neurotransmitter-gated ion-channels constitute a very important group of transmembrane receptor-ion channel complexes in vertebrates and invertebrates. These receptors open transiently upon binding of specific ligands and govern synaptic neurotransmissions in the nervous system and neuromuscular transmissions (Smart and Paoletti 2012). Overall, our findings are not surprising since they exactly match the insecticidal mode of action of the neonicotinoids (Shimada et al., 2020). Indeed, nicotinic acetylcholine receptors (nAChRs) are the main targets of neonicotinoid insecticides, including IMI. Structural analyses of nAChR have recently increased revealing that the nitro group of IMI plays a pivotal role in the selective action of neonicotinoids with insect nAChR (Ihara et al., 2022). However, recent studies suggest possible adverse impacts of neonicotinoids on non-target organisms including mammals (Burke et al. 2018; Berheim et al. 2019). IMI was found to activate the nAChR signalling in neonatal rats (Kimura-Kuroda et al. 2012) and cultured human neurons at concentrations that can be potentially reached by dietary or accidental exposure (Loser et al. 2021a).
Along with the neurotransmitter-gated ion-channels, high confidence scores were found also for genes involved in neurotransmitter catabolic processes, particularly acetylcholinesterase (AChE). The inhibition of AChE is a well-known mechanism triggered by CPF and it is responsible for neurotoxic effects in insects. However, several studies suggest the potential of CPF in generating harmful effects also in non-target species. For example, exposure to CPF has been associated with neurodevelopmental disorders in rodents and humans (Berg et al. 2022; Slotkin and Seidler 2005). In amphibians, CPF was found to induce subletal toxicity and indirect effects by impacting their ecosystem (Beasley 2020). CPF is also known to have noncholinergic effects such as inhibition of the oxidative metabolism of sex steroids (Hodgson 2012). This is in line with our analysis that identified the cytochrome P450 (CYP) pathway as one of the key targets for CPF in each analyzed vertebrate model. Previous reports have shown that CPF induces reproductive disorders in mammals by affecting gametes (Nardi et al. 2011; Tanvir et al. 2016) probably through oxidative stress mechanisms (Abolaji et al. 2017). In the human model, we found that the cluster associated to metabolic pathways accounts for the largest number of CYPs and also includes genes encoding antioxidant enzymes such as CAT, enzymes involved in the choline pathway such as choline O-acetyltransferase (chat) and butyrylcholinesterase (BCHE), and esterase (PON1). Similarly, two esterase proteins were found involved in biological processes associated with IMI/CPF exposure in Apis millifera. In conclusion, this computational approach was conducted to gain insight into the consequences of prolonged exposure to both IMI and CPF widely used in the chemical control of Rhynchophorus ferrugineus. Our analysis demonstrates that both pesticides have a high toxic potential to all organisms for inducing neurotoxic effects and likely endocrine interference, especially in mammals (
Figure 6).
These effects are closely related to their bioaccumulation capacity that is evident for organochlorines, but it may also concern neonicotinoids. Indeed, IMI can persist for months in some soil conditions and may pose a risk to other organisms in the area. Thus, more research is needed for tracking these pesticides and their metabolites in the study area and for monitoring their long-term effects on non-target species, including humans.
4. Conclusions
Our monitoring, carried out about 7 years from the previous one and about 13 years from the appearance of the RPW in this area, showed how this beetle has heavily influenced the palm tree heritage in the study area, reducing it by more than half.
In recent years, the Municipality, in concert with the Phytosanitary Service of the Marche Region, has actively worked to cope with the advance of this plague, initially implementing large-scale Integrated Pest Management and localised preventive treatments, later on.
Our data show that the fight against the weevil is now concentrated in the areas of municipal relevance (front sea road, parks) and at villas, hotels, farmhouses and nurseries; in the rest of the territory, the large number of died-infested palm trees observed in recent years, clearly highlights the failure of the large-scale Integrated Pest Management with respect to a punctual approach (street, park).
In this perspective, Milosavljević et al. (2019) proposed the inappropriate management planning, poor coordination between stakeholders, and public resistance to implementation of controls, as potential causes of this failure and we substantially agree on this.
The geospatial analysis approach used in this work demonstrates that preventive treatments, if well implemented, are very effective in preserving the palms from the RPW attack. This success, however, must deal with the problems related to the use of chemicals, as well as with the economic impact related to their massive usage. Indeed, the systems toxicology approach used herein, highlights potential hazards to non-target species due to repeated applications and long-term insecticide exposure.
We conclude that the multidisciplinary approach adopted in the present study has proved to be very useful to evaluate the many aspects related to the fight against RPW in an urban environment.
In accordance with Sawyer & Casagrande (1983), we believe that the long-term objective should be to elaborate new designs for urban environments that will minimize the negative interactions between people and pests, limiting the use of pesticides in urban areas. In this regard, it would be advisable to promote a diversified and therefore more resilient urban vegetal landscape, with quite many different plant species, as this composition would more successfully weather new possible alien pest invasions.
Author Contributions
Luca Bracchetti: Investigation, Methodology, Data curation, Writing- Original draft, Writing- Reviewing and Editing; Conceptualization. Paolo Cocci: Methodology, investigation, Writing- Original draft. Francesco Alessandro Palermo: Conceptualization, Supervision, Writing - original draft, Writing- Reviewing and Editing.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due they are partly owned by local governments.
Acknowledgments
We would like to thank the Environment Service of the Municipality of San Benedetto del Tronto for the help provided in the reconstruction of the interventions made in its territory against the red palm weevil.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abolaji AO, Ojo M, Afolabi TT, Arowoogun MD, Nwawolor D, Farombi EO (2017) Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem Biol Interact 25; 270: 15-23. [CrossRef]
- Al-Dosary N M N, Al-Dobai S, Faleiro JR (2016) Review on the management of red palm weevil Rhynchophorus ferrugineus Olivier in date palm Phoenix dactylifera L. Emirates Journal of Food and Agriculture 28(1):34–44.
- Al-Shawaf A, Al-Shagag A, Al-Bagshi M, Al-Saroj S, AlBather S, Al-Dandan AM, Faleiro JR. (2013) A quarantine protocol against red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleptera: Curculiondae) in date palm. Journal of Plant Protection Research, 53(4):409–415. [CrossRef]
- Berg EL, Ching TM, Bruun DA et al. (2020) Translational outcomes relevant to neurodevelopmental disorders following early life exposure of rats to chlorpyrifos. J Neurodevelop Disord 12,40. [CrossRef]
- Berheim EH, Jenks JA, Lundgren JG, Michel ES, Grove D, Jensen WF (2019) Effects of neonicotinoid insecticides on physiology and reproductive characteristics of captive female and fawn white-tailed deer. Sci Rep 9, 4534.
- Beasley VR (2020) Direct and Indirect Effects of Environmental Contaminants on Amphibians. Refrence Module in Earth Systems and Environmental Sciences.
- Burke AP, Niibori Y, Terayama H, Ito M, Pidgeon A, Arsenault J, Camarero PR, Cummins CL, Mateo R, Sakabe K, Hampson DR (2018) Mammalian susceptibility to a neonicotinoid insecticide after fetal and early postnatal exposure. Sci Rep 8, 16639.
- Chagnon M, Kreutzweiser D, Mitchell EAD, Morrissey CA, Noome DA, Van der Sluijs JP (2015) Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. 22:119–134.
- Chakraborty S (2014). In silico analysis identifies genes common between five primary gastrointestinal cancer sites with potential clinical applications. Ann Gastroenterol. 27(3):231-236.
- Chihaoui-Meridja S, Harbi A, Abbes K, Chaabane H, La Pergola A, Chermiti B, Suma P (2020) Systematicity, persistence and efficacy of selected insecticides used in endotherapy to control the red palm weevil Rhynchophorus ferrugineus (Olivier, 1790) on Phoenix canariensis. Phytoparasitica 48:75–85. [CrossRef]
- Cinnirella A, Bisci C, Nardi S, Ricci E, Palermo AP, Bracchetti L (2020) Analysis of the spread of Rhynchophorus ferrugineus in an urban area, using GIS techniques: a study case in Central Italy. Urban Ecosystems 23:255–269. [CrossRef]
- Cohen Y, Alchanatis V, Prigojin A, Levi A, Soroker V, Cohen Y (2012) Use of aerial thermal imaging to estimate water status of palm trees. Precis Agric 13:123–140.
- Cox ML (1993) Red palm weevil, Rhynchophorus ferrugineus in Egypt. FAO Plant Protection Bulletin 41(1):30–31.
- Dembilio Ó, Riba JM, Gamón M, Jacas JA (2014) Mobility and efficacy of abamectin and imidacloprid against Rhynchophorus ferrugineus in Phoenix canariensis by different application methods. Pest Management Science, 71(8):1091–1098.
- Diakou A, Kapantaidakis E, Tamvakis A, Vassilios G, Strus N (2016) Dirofilaria infections in dogs in different areas of Greece. Parasites Vectors 9, 508. [CrossRef]
- DOA (Department of Agriculture) (2016) Report on Current Status of Attack of the Red Palm Weevil, Rhynchophorus ferrugineus in Terengganu. Government Press, Malaysia.
- EPA (2000) Toxicology chapter for chlorpyrifos. Washington, DC: U.S. Environmental Protections Agency. Office of Prevention, pesticides and toxic substances http://www.epa.gov/oppsrrd1/op/chlorpyrifos/rev_tox.pdf. April 18, 2000.
- EPPO Reporting Service (1996) Introduction of the Asiatic palm weevil, Rhynchophorus ferrugineus into Spain. 96 / 096, No 05.
- EPPO Reporting Service (1999a) First report of Rhynchophorus ferrugineus in Jordan. 99 / 78, No 05.
- EPPO Reporting Service (1999b). First report of Rhynchophorus ferrugineus in Israel. 99 / 119, No 07.
- EPPO Reporting Service (2004) Rhynchophorus ferrugineus found in Comunidad Valenciana, Spain. 2004 / 60, No 04.
- EPPO Reporting Service (2006a) First report of Rhynchophorus ferrugineus in Italy. 2006 / 001, No 1.
- EPPO Reporting Service (2006b) Details on Rhynchophorus ferrugineus in Sicilia (Italy). 2006 / 028, No 2.
- EPPO Reporting Service (2006c) Rhynchophorus ferrugineus found in Lazio region, Italy. 2006 / 227, No 11.
- EPPO Reporting Service (2007a) First report of Rhynchophorus ferrugineus in Turkey. 2007 / 001, No 1.
- EPPO Reporting Service (2007b) First report of Rhynchophorus ferrugineus in Syria. 2007 / 002, No 1.
- EPPO Reporting Service (2007c) Further reports of Rhynchophorus ferrugineus in Puglia and Sardegna, Italy. 2007 / 003, No 1.
- EPPO Reporting Service (2007d) First report of Rhynchophorus ferrugineus in Cyprus. 2007 / 022, No 2.
- EPPO Reporting Service (2007e) Situation of Rhynchophorus ferrugineus in Spain. 2007 / 023, No 2.
- EPPO Reporting Service (2008a) First report of Rhynchophorus ferrugineus in Portugal. 2008 / 022, No 2.
- EPPO Reporting Service (2008b) Further details on the situation of Rhynchophorus ferrugineus in Puglia and Saregna, Italy. 2008 / 093, No 5.
- EPPO Reporting Service (2009) First report of Rhynchophorus ferrugineus in Albania. 2009 / 207, No 11.
- EPPO Reporting Service (2010a) Situation of Rhynchophorus ferrugineus in Italy in 2009. 2010 / 139, No 8.
- EPPO Reporting Service (2010b) Rhynchophorus ferrugineus occurs in Malta. 2010 / 204, No 11.
- EPPO/OEPP (2013) PQR-EPPO Database on quarantine pets. (http://www.eppo.int).
- Ernest Hodgson (2020) in Pesticide Biotransformation and Disposition.
- ESRI (2012) ArcGIS 10.X. Redlands: Environmental Systems Research Institute ESRI. GIS mapping Software, Solutions, Services, map Apps, and Data. http://www.esri.com/. Accessed may 2021.
- Faleiro JR, Ben Abdallah A, El-Bellaj M, Al Ajlan AM, Oihabi A (2012) Threat of the Red Palm Weevil, Rhynchophorus ferrugineus (Olivier) to Date Palm Plantations in North Africa. Arab. Journal of Plant Protection 30:274-280.
- Fazzini M, Beltrando G, Billi P (2013) Intense rainfalls and flooding problems in the beach resort of San Benedetto del Tronto, Adriatic Sea, Central Italy. Proc. 3°GEOMED 2013, Antalya Turkey, 10–14 june; Ibrahim Athalay and Recept EYE ed. 128–131.
- Ferry M, Gomez S (2012) Red palm weevil, focus on it control. Phytoma, 658:38–41.
- Hogan S, Kelly M, Stark B, Chen Y (2017) Unmanned aerial systems for agriculture and natural resources. Calif. Agric. 71:5–14.
- Gervais JA, Luukinen B, Buhl K, Stone D (2010) “Imidacloprid Technical Fact Sheet.” National Pesticide Information Center, Oregon State University Extension Service.
- Giblin-Davis RM, Faleiro JR, Jacas JA, Peña JE, Vidyasagar PSPV (2013) Biology and management of the red palm weevil, Rhynchophorus ferrugineus. In: Peña JE (ed) Potential invasive pests of agricultural crops. Commonwealth Agricultural Bureau (CAB) International, London, pp 1–34.
- Hodgson E (2012) Chapter 9 Biotransformation of Individual Pesticides: Some Examples. Pesticide Biotransformation and Disposition, Pages 195-208.
- Ihara M (2022) Ligand-gated ion channels as targets of neuroactive insecticides. Biosci Biotechnol Biochem. 86(2):157-164. [CrossRef]
- Iskar M, Campillos M, Kuhn M, Jensen LJ, van Noort V, Bork P (2010) Drug-Induced Regulation of Target Expression. PLOS Computational Biology 6(9):e1000925.
- Johnson JD, Pettis JS (2014) A survey of imidacloprid levels in water sources potentially frequented by honeybees (Apis mellifera) in the Eastern USA. Water Air Soil Pollut. 225,2127.
- Kielbaso JJ, Davidson H, Hart J, Jones A, Kennedy MK (1979) Characteristics of successful systemic chemicals. In Kielbaso JJ et al. (Ed.), Proceedings of symposium on systemic chemical treatment in tree culture (pp. 19–34). East Lansing, MI.
- Lin GLE, Salim JM, Halim MFA, Azmi WA (2017) Entomopathogenic fungi isolated from the soil of Terengganu, Malaysia as potential bio-pesticides against the red palm weevil, Rhynchophorus ferrugineus. J. Sustain. Sci. Manag. 12 (2):71–79.
- Llopis VN, González SV, Jaques JA, Moraga EQ, Vives ÓD, Moya P (2018) Desarrollo de un sistema de atracción-infección para el control del picudo rojo de la (Homoptera: Asterolecaniidae) infesting date palm in northern Sudan. Acta Horticulturae, 882:937–955.
- Loser D, Hinojosa MG, Blum J et al. (2021) Functional alterations by a subgroup of neonicotinoid pesticides in human dopaminergic neurons. Arch Toxicol 95:2081–2107. [CrossRef]
- Masilamany D, Tang B (2013) Pengurusan Bersepadu Kumbang Badak dan Kumbang Jalur Merah – Perosak Utama Tanaman Kelapa. Buletin Teknologi MARDI 5:51-59.
- Masilamany D, Wan Ali WKA, Baki R (2012) Biologi dan Pengurusan Kumbang Jalur Merah - Perosak Invasif Tanaman Kelapa. Bulletin Teknologi MARDI 1:151-157.
- Mazza G, Francardi V, Simoni S, Benvenuti C, Cervo R, Romeno J, Llácer, E, Longo S, Nannelli R, Tarasco E, Federico P (2014) An Overview on the Natural Enemies of Rhynchophorus Palm Weevils, with Focus on R. ferrugineus. Biological Control 77:83-92.
- Milosavljević I, El-Shafie HAF, Faleiro JR, Hoddle CD, Leewis M, Hoddle MS (2019) Palmageddon: the wasting of ornamental palms by invasive palm weevils, Rhynchophorus spp. J. Pest. Sci. 92:143–156. [CrossRef]
- Nardi S, Ricci E, Lozzi R, Marozzi F, Ladurner E, Chiabrando F, Granchelli L, Verdolini E, Isidoro N, Riolo P (2011) OEPP/EPPO Bulletin 41:103–115.
- Peri E, Colazza S, Guarino S, Suma P, Pergola A, Longo S (2013) The red palm weevil in Sicily: The introduction and spread of an invasive alien species. In proceedings of the palm pest mediterranean conference (171-177). Nice, Rrance, 16-18 January 2013. Association Française de protection des Plantes (AFPP).
- Pesaresi S, Biondi E, Casavecchia S (2017) Bioclimates of Italy. Journal of Maps 13 (2):955–960. [CrossRef]
- Rochat D, Chapin E, Ferry M, Avand-Faghih A, Brun L (2006) Le charançon rouge du palmier dans le bassin méditerranéen. Phytoma-La Défense des Végetaux 595:20–24.
- Rogers KH, McMillin S, Olstad KJ, Poppenga RH (2019) Imidacloprid Poisoning of Songbirds Following a Drench Application of Trees in a Residential Neighborhood in California, USA. Environ Toxicol Chem. 38(8):1724-1727.
- Roscoe JT, Byars JA (1971) Sample size restraints commonly imposed on the use of the chi-square statistic. J. Am. Stat. Assoc. 66:755–9.
- Sawyer AJ, Casagrande RA (1983) Urban pest management: A conceptual framework. Urban Ecology 7 (2):145-157. [CrossRef]
- Shimada S, Kamiya M, Shigetou S, Tomiyama K, Komori Y, Magara L, Makoto I, Matsuda K (2020) The mechanism of loop C-neonicotinoid interactions at insect nicotinic acetylcholine receptor α1 subunit predicts resistance emergence in pests. Sci Rep 10, 7529.
- Smart TG, Paoletti P (2012) Synaptic neurotransmitter-gated receptors. Cold Spring Harb Perspect Biol. 4(3):a009662.
- Slotkin TA, Seidler FJ (2005) The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Brain es Dev Brain Res. 158(1-2):115-9.
- Soroker V, Suma P, La Pergola A, Liopis NV, Vacas S, Cohen Y, Alchanatis V, Milonas P, Golomb O, Goldshtein E, El Banna AM, Hetzroni A (2017) Surveillance techniques and detection methods for Rhynchophorus ferrugineus and Paysandisia archon. Handbook of Major Palm Pests. Biology and Management. John Wiley & Sons, Ltd, Chichester.
- Stoner KA, Eitzer BD (2012) Movement of soil-applied imidacloprid and thiamethoxam into nectar and pollen of squash (Cucurbita pepo). PLoS One 7:e39114.
- Ubaid U, Rahman H, Asghar W, Nazir W, Sandhu MA, Ahmed A, Khalid N (2021) A comprehensive review on chlorpyrifos toxicity with special reference to endocrine disruption: Evidence of mechanisms, exposures and mitigation strategies. Sci Total Environ. 755(Pt 2):142649.
- Watts M (2012) Chlorpyrifos as a Possible Global POP Pesticide Action Network North America.
- Whitlock M, Schluter D (2015) The analysis of biological data. Greenwood Village, Colo, Roberts and Co. Publishers.
- Wraight SP, Jackson MA, Kock SL (2001) Production, Stabilization and Formulation of Fungal Biocontrol Agents. In: Butt, T. M., Jackson, C., Magan, N. (eds) Fungi as Biocontrol Agents: Progress, Problems and Potential. Centre for Agriculture and Bioscience International, 253-287.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).