Submitted:
20 April 2023
Posted:
02 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Endothelial Dysfunction Due to Diabetes and/or Insulin Resistance
3. A Significance of Endothelial Dysfunction for Development of HF in Patients with Type 2 Diabetes
3.1. Patients with Type 2 Diabetes Are Likely to Develop HF?
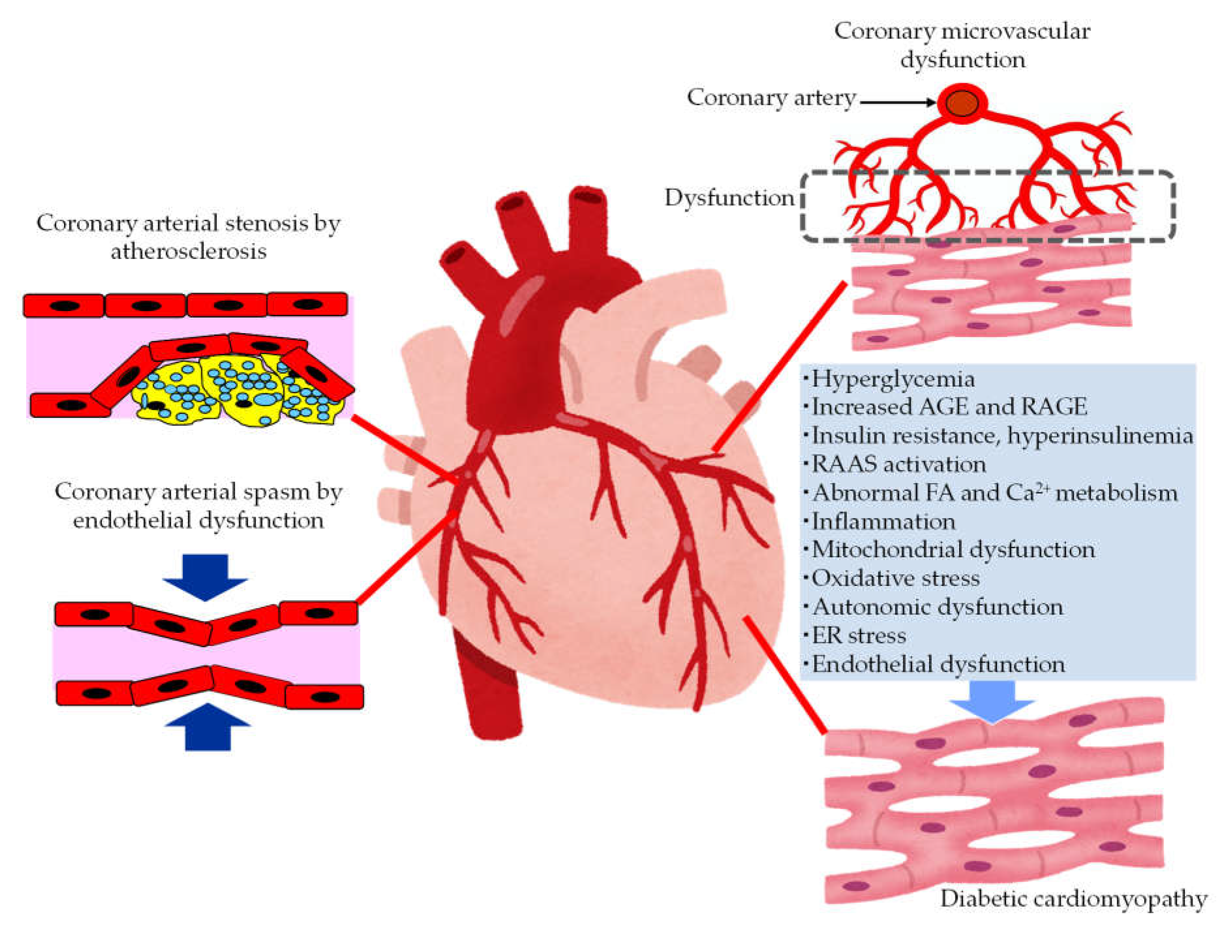
3.2. The Pathological Conditions Leading to the Development of HF in Patients with Type 2 Diabetes
3.2.1. The Mechanisms Leading to Coronary Artery Disease (CAD) in Patients with Type 2 Diabetes
3.2.2. The Mechanisms Leading Coronary Microvascular Dysfunction (CMD) in Patients with Type 2 Diabetes
3.2.3. The Mechanisms Leading to Diabetic Cardiomyopathy (DCM) in Patients with Type 2 Diabetes
3.2.4. A Significance of Endothelial Dysfunction for Development of Pathogenic Conditions for HF in Patients with Type 2 Diabetes
4. A Significance of Endothelial Dysfunction for Development of CKD in Patients with Type 2 Diabetes
5. The Effects of SGLT2i on Endothelial Dysfunction
5.1. The Effects of SGLT2i on Vascular Function Tests
5.2. The Effects of SGLT2i on Factors-Associated with Endothelial Dysfunction
6. The Effects of SGLT2i on Causative Pathological Conditions Leading to HF in Patients with Type 2 Diabetes
6.1. The Effects of SGLT2i on CAD
6.2. The Effects of SGLT2i on CMD
6.2. The Beneficial Effects of SGLT2i on DCM
7. The Effects of SGLT2i on CKD
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H. Sodium-glucose cotransporter 2 inhibitors and death and heart failure in type 2 diabetes. Ann. Transl. Med. 2017, 5, 470. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Cherney, D.Z.I.; Zinman, B.; Inzucchi, S.E.; Koitka-Weber, A.; Mattheus, M.; von Eynatten, M.; Wanner, C. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet. Diabetes. Endocrinol. 2017, 5, 610–621. [Google Scholar] [CrossRef]
- Yilmaz, M.I.; Saglam, M.; Caglar, K.; Cakir, E.; Sonmez, A.; Ozgurtas, T.; Aydin, A.; Eyileten, T.; et al. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am. J. Kidney. Dis. 2006, 47, 42–50. [Google Scholar] [CrossRef]
- Yang, Z.; Ming, X.F. Recent advances in understanding endothelial dysfunction in atherosclerosis. Clin. Med. Res. 2006, 4, 53–65. [Google Scholar] [CrossRef]
- Bauer, V.; Sotnikova, R. Nitric oxide - the endothelium-derived relaxing factor and its role in endothelial functions. Gen. Physiol. Biophys. 2010, 29, 319–340. [Google Scholar] [CrossRef]
- Stankevicius, E.; Kevelaitis, E.; Vainorius, E.; Simonsen, U. Role of nitric oxide and other endothelium-derived factors. Medicina (Kaunas). 2003, 39, 333–341. [Google Scholar]
- Morigi, M.; Angioletti, S.; Imberti, B.; Donadelli, R.; Micheletti, G.; Figliuzzi, M.; Remuzzi, A.; Zoja, C.; Remuzzi, G. Leukocyte–endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-κB-dependent fashion. J. Clin. Invest. 1998, 101, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.B.; Hu, Y.S.; Medcalf, R.L.; Simpson, R.W.; Dear, A.E. Thiazolidinediones inhibit TNFalpha induction of PAI-1 independent of PPARgamma activation. Biochem. Biophys. Res. Commun. 2005, 334, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A.; Madan, B.; Singhal, V.; Ghosh, B. Isoliquiritigenin inhibits IκB kinase activity and ROS generation to block TNF-alpha induced expression of cell adhesion molecules on human endothelial cells. Biochem. Pharmacol. 2007, 73, 1602–1612. [Google Scholar] [CrossRef]
- Sobel, B.E.; Woodcock-Mitchell, J.; Schneider, D.J.; Holt, R.E.; Marutsuka, K.; Gold, H. Increased plasminogen activator inhibitor type 1 in coronary artery atherectomy specimens from type 2 diabetic compared with nondiabetic patients: a potential factor predisposing to thrombosis and its persistence. Circulation. 1998, 97, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.J.; Mao, S.L.; Taylor, K.L.; Kanjanabuch, T.; Guan, Y.; Zhang, Y.; Brown, N.; et al. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004, 53, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Balletshofer, B.M.; Rittig, K.; Enderle, M.D.; Volk, A.; Maerker, E.; Jacob, S.; Matthaei, S.; Rett, K.; Häring, H.U. Endothelial dysfunction is detectable in young normotensive first-degree relatives of subjects with type 2 diabetes in association with insulin resistance. Circulation. 2000, 101, 1780–1784. [Google Scholar] [CrossRef]
- Zeng, G.; Quon, M.J. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J. Clin. Invest. 1996, 98, 894–898. [Google Scholar] [CrossRef]
- Zeng, G.; Nystrom, F.H.; Ravichandran, L.V.; Cong, L.N.; Kirby, M.; Mostowski, H.; Quon, M.J. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000, 101, 1539–1545. [Google Scholar] [CrossRef]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of nitric oxidesynthesis in chronic renal failure. Lancet. 1992, 339, 572–575. [Google Scholar]
- Achan, V.; Broadhead, M.; Malaki, M.; Whitley, G.; Leiper, J.; MacAllister, R.; Vallance, P. Asymmetric dimethy-larginine causes hypertension and cardiac dysfunction inhumans and is actively metabolized by dimethylargininedimethylaminohydrolase. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Kielstein, J.T.; Impraim, B.; Simmel, S.; Bode-Böger, S.M.; Tsikas, D.; Frölich, J.C.; Hoeper, M.M.; Haller, H.; Fliser, D. Cardiovascular effectsof systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation. 2004, 109, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Sydow, K.; Mondon, C.E.; Cooke, J.P. Insulin resistance: potential role of the endogenous nitric oxide synthase inhibitor ADMA. Vasc. Med. 2005, 10 (Suppl 1), S35–S43. [Google Scholar] [CrossRef]
- Madden, J.A. Role of the vascular endothelium and plaque in acute ischemic stroke. Neurology. 2012, 79 (13 Suppl 1), S58–S62. [Google Scholar] [CrossRef]
- Toda, N.; Nakanishi-Toda, M. How mental stress affects endothelial function. Pflugers. Arch. 2011, 462. 779-794.
- Huhtinen, K.; O'Byrne, J.; Lindquist, P.J.; Contreras, J.A.; Alexson, S.E. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J. Biol. Chem. 2002, 277, 3424–3432. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Schiekofer, S.; Schwaninger, M.; Andrassy, M.; Humpert, P.M.; Chen, J.; Hong, M.; et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001, 50, 2792–2808. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Nazarewicz, R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid. Redox. Signal. 2013, 19, 1085–1094. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, C.; Zhang, G.; Tao, J. Endothelial progenitor cells in cardiovascular diseases. Aging. Med (Milton). 2018, 1, 204–208. [Google Scholar] [CrossRef]
- Chopra, H.; Hung, M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into endothelial progenitor cells: origin, classification, potentials, and prospects. Stem. Cells. Int. 2018, 2018, 9847015. [Google Scholar] [CrossRef]
- Rigato, M.; Avogaro, A.; Fadini, G.P. Levels of circulating progenitor cells, cardiovascular outcomes and death: a meta-analysis of prospective observational studies. Circ. Res. 2016, 118, 1930–1939. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Narula, J.; Vasan, R.S. Risk factors for heart failure. Med. Clin. North. Am. 2004, 88, 1145–1172. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, V.H.; Billaudelle, A.M.; Schulz, A.; Keller, K.; Hahad, O.; Tröbs, S.O.; Koeck, T.; et al. Disturbed Glucose Metabolism and Left Ventricular Geometry in the General Population. J. Clin. Med. 2021, 10, 3851. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.; Sarkar, P.; Naskar, S.; Biswas, U.; Islam, S.K.S. Asian J Med Sci. 2021;12:96.
- Rørth, R.; Jhund, P.S.; Mogensen, U.M.; Kristensen, S.L.; Petrie, M.C.; Køber, L.; McMurray, J.J.V. Risk of Incident Heart Failure in Patients With Diabetes and Asymptomatic Left Ventricular Systolic Dysfunction. Diabetes. Care. 2018, 41, 1285–1291. [Google Scholar] [CrossRef]
- MacDonald, M.R.; Petrie, M.C.; Varyani, F.; Ostergren, J.; Michelson, E.L.; Young, J.B.; Solomon, S.D.; et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur. Heart. J. 2008, 29, 1377–1385. [Google Scholar] [CrossRef]
- Boonman-de Winter, L.J.; Rutten, F.H.; Cramer, M.J.; Landman, M.J.; Liem, A.H.; Rutten, G.E.; Hoes, A.W. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia. 2012, 55, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Suwaidi, J.A.; Hamasaki, S.; Higano, S.T.; Nishimura, R.A.; Holmes, D.R. Jr; Lerman, A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000, 101, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Kugiyama, K.; Yasue, H.; Okumura, K.; Ogawa, H.; Fujimoto, K.; Nakao, K.; Yoshimura, M.; Motoyama, T.; Inobe, Y.; Kawano, H. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation. 1996, 94, 266–271. [Google Scholar] [CrossRef]
- Nakayama, M.; Yasue, H.; Yoshimura, M.; Shimasaki, Y.; Kugiyama, K.; Ogawa, H.; Motoyama, T.; et al. T-786-->C mutation in the 5'-flanking region of the endothelial nitric oxide synthase gene is associated with coronary spasm. Circulation. 1999, 99, 2864–2870. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Vetrano, E.; Loffredo, G.; Rinaldi, L.; Catalini, C.; et al. Coronary Microvascular Dysfunction in Diabetes Mellitus: Pathogenetic Mechanisms and Potential Therapeutic Options. Biomedicines. 2022, 10, 2274. [Google Scholar] [CrossRef]
- Marwick TH, Ritchie R, Shaw JE, Kaye D. Implications of Underlying Mechanisms for the Recognition and Management of Diabetic Cardiomyopathy. J Am Coll Cardiol. 2018 Jan 23;71(3):339-351.
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Dillmann, W.H. Diabetic Cardiomyopathy. Circ. Res. 2019, 124, 1160–1162. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Givertz, M.M.; Aguilar, D.; Allen, L.A.; Chan, M.; Desai, A.S.; Deswal, A.; et al. Type 2 Diabetes Mellitus and Heart Failure: A Scientific Statement From the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019, 140, e294–e324. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Y.; Li, S.; Lv, J. Endothelial Dysfunction and Diabetic Cardiomyopathy. Front. Endocrinol (Lausanne). 2022, 13, 851941. [Google Scholar] [CrossRef] [PubMed]
- Persson, F.; Rossing, P.; Hovind, P.; Stehouwer, C.D.; Schalkwijk, C.G.; Tarnow, L.; Parving, H.H. Endothelial dysfunction and inflammation predict development of diabetic nephropathy in the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (IRMA 2) study. Scand. J. Clin. Lab. Invest. 2008, 68, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Clausen, P.; Jensen, J.S.; Jensen, G.; Borch-Johnsen, K.; Feldt-Rasmussen, B. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation. 2001, 103, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Sydow, K.; T. Münzel, T. ADMA and oxidative stress. Atherosclerosis. 2003, 4 (Suppl), 41–51. [Google Scholar] [CrossRef] [PubMed]
- Ghiadoni, L.; Cupisti, A.; Huang, Y.; Mattei, P.; Cardinal, H.; Favilla, S.; Rindi, P.; Barsotti, G.; Taddei, S.; Salvetti, A. Endothelial dysfunction and oxidative stress in chronic renal failure. J. Nephrol. 2004, 17, 512–519. [Google Scholar]
- Kielstein, J.T.; Böger, R.H.; Bode-Böger, S.M.; Frölich, J.C.; Haller, H.; Ritz, E.; Fliser, D. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J. Am. Soc. Nephrol. 2002, 13, 170–176. [Google Scholar] [CrossRef]
- Baylis, C. Arginine, arginine analogs and nitric oxide production in chronic kidney disease. Nat. Clin. Pract. Nephrol. 2006, 2, 209–220. [Google Scholar] [CrossRef]
- Ueda, S.; Yamagishi, S.; Matsumoto, Y.; Kaida, Y.; Fujimi-Hayashida, A.; Koike, K.; Tanaka, H.; Fukami, K.; Okuda, S. Involvement of asymmetric dimethylarginine (ADMA) in glomerular capillary loss and sclerosis in a rat model of chronic kidney disease (CKD). Life. Sci. 2009, 84, 853–856. [Google Scholar] [CrossRef]
- Tojo, A.; Welch, W.J.; Bremer, V.; Kimoto, M.; Kimura, K.; Omata, M.; Ogawa, T.; Vallance, P.; Wilcox, C.S. Colocalization of demethylating enzymes and NOS and functional effects of methylarginines in rat kidney. Kidney. Int. 1997, 52, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.; van Hell, A.J.; Visser, M.; Nijveldt, R.J.; van Leeuwen, P.A.; Teerlink, T. Role of the human erythrocyte in generation and storage of asymmetric dimethylarginine. Am. J. Physiol. Heart. Circ. Physiol. 2012, 302, H1762–H1770. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Nakamura, T.; Sato, E.; Kawagoe, Y.; Hikichi, Y.; Ueda, Y.; Node, K. Renovascular protective effects of erythropoietin in patients with chronic kidney disease. Intern. Med. 2011, 50, 1929–1934. [Google Scholar] [CrossRef] [PubMed]
- Fliser, D. Perspectives in renal disease progression: the endothelium as a treatment target in chronic kidney disease. J. Nephrol. 2010, 23, 369–376. [Google Scholar]
- Shi, L.; Zhao, C.; Wang, H.; Lei, T.; Liu, S.; Cao, J.; Lu, Z. Dimethylarginine Dimethylaminohydrolase 1 Deficiency Induces the Epithelial to Mesenchymal Transition in Renal Proximal Tubular Epithelial Cells and Exacerbates Kidney Damage in Aged and Diabetic Mice. Antioxid. Redox. Signal. 2017, 27, 1347–1360. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N. Roles of Nrf2 in Protecting the Kidney from Oxidative Damage. Int. J. Mol. Sci. 2020, 21, 2951. [Google Scholar] [CrossRef]
- Greenland, P.; Alpert, J.S.; Beller, G.A.; Benjamin, E.J.; Budoff, M.J.; Fayad, Z.A.; Foster, E.; et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2010, 122, e584–e636. [Google Scholar]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cifkova, R.; Cosentino, F.; De Carlo, M.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015, 241, 507–532. [Google Scholar]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Iwamoto, A.; Kajikawa, M.; et al. Endothelial dysfunction, increased arterial stiffness, and cardiovascular risk prediction in patients with coronary artery disease: FMD-J (Flow-Mediated Dilation Japan) Study A. J. Am. Heart. Assoc. 2018, 7, e008588. [Google Scholar] [CrossRef]
- Shigiyama, F.; Kumashiro, N.; Miyagi, M.; Ikehara, K.; Kanda, E.; Uchino, H.; Hirose, T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc. Diabetol. 2017, 16, 84. [Google Scholar] [CrossRef]
- Solini, A.; Giannini, L.; Seghieri, M.; Vitolo, E.; Taddei, S.; Ghiadoni, L.; Bruno, R.M. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc. Diabetol. 2017, 16, 138. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.J.; Sanchez, M.J.; Sanchez, R.A. Diabetic patients with essential hypertension treated with amlodipine: blood pressure and arterial stiffness effects of canagliflozin or perindopril. J. Hypertens. 2019, 37, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Miura, S. Effects of Sodium-Glucose Cotransporter 2 Inhibitor on Vascular Endothelial and Diastolic Function in Heart Failure With Preserved Ejection Fraction- Novel Prospective Cohort Study. Circ. Rep. 2019, 1, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Sezai, A.; Sekino, H.; Unosawa, S.; Taoka, M.; Osaka, S.; Tanaka, M. Canagliflozin for Japanese patients with chronic heart failure and type II diabetes. Cardiovasc. Diabetol. 2019, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Tochiya, M.; Makino, H.; Tamanaha, T.; Matsuo, M.; Hishida, A.; Koezuka, R.; Ohata, Y. Effect of tofogliflozin on cardiac and vascular endothelial function in patients with type 2 diabetes and heart diseases: A pilot study. J. Diabetes. Investig. 2020, 11, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Uzu, K.; Hashimoto, N.; Onishi, T.; Takaya, T.; Shimane, A.; Taniguchi, Y.; Yasaka, Y.; Ohara, T.; Kawai, H. Empagliflozin's Ameliorating Effect on Plasma Triglycerides: Association with Endothelial Function Recovery in Diabetic Patients with Coronary Artery Disease. J. Atheroscler. Thromb. 2020, 27, 644–656. [Google Scholar] [CrossRef]
- Correale, M.; Mazzeo, P.; Mallardi, A.; Leopizzi, A.; Tricarico, L.; Fortunato, M.; Magnesa, M.; et al. Switch to SGLT2 Inhibitors and Improved Endothelial Function in Diabetic Patients with Chronic Heart Failure. Cardiovasc. Drugs. Ther. 2022, 36, 1157–1164. [Google Scholar] [CrossRef]
- Batzias, K.; Antonopoulos, A.S.; Oikonomou, E.; Siasos, G.; Bletsa, E.; Stampouloglou, P.K.; Mistakidi, C.V.; et al. Effects of Newer Antidiabetic Drugs on Endothelial Function and Arterial Stiffness: A Systematic Review and Meta-Analysis. J. Diabetes. Res. 2018, 2018, 1232583. [Google Scholar] [CrossRef]
- Patoulias, D.; Papadopoulos, C.; Kassimis, G.; Vassilikos, V.; Karagiannis, A.; Doumas, M. Meta-Analysis Addressing the Effect of Sodium-Glucose Cotransporter 2 Inhibitors on Flow-Mediated Dilation in Patients With Type 2 Diabetes Mellitus. Am. J. Cardiol. 2022, 165, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Oelze, M.; Kröller-Schön, S.; Welschof, P.; Jansen, T.; Hausding, M.; Mikhed, Y.; Stamm, P.; et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS. One. 2014, 9, e112394. [Google Scholar] [CrossRef]
- Salim, H.M.; Fukuda, D.; Yagi, S.; Soeki, T.; Shimabukuro, M.; Sata, M. Glycemic Control with Ipragliflozin, a Novel Selective SGLT2 Inhibitor, Ameliorated Endothelial Dysfunction in Streptozotocin-Induced Diabetic Mouse. Front. Cardiovasc. Med. 2016, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, T.; Spizzo, I.; Liu, H.; Hu, Y.; Simpson, R.W.; Widdop, R.E.; Dear, A.E. Dapagliflozin attenuates human vascular endothelial cell activation and induces vasorelaxation: A potential mechanism for inhibition of atherogenesis. Diab. Vasc. Dis. Res. 2018, 15, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wang, L.X.; Dong, S.S.; Hong, Y.; Zhou, X.H.; Zheng, W.W.; Zheng, C. Phlorizin Exerts Direct Protective Effects on Palmitic Acid (PA)-Induced Endothelial Dysfunction by Activating the PI3K/AKT/eNOS Signaling Pathway and Increasing the Levels of Nitric Oxide (NO). Med. Sci. Monit. Basic. Res. 2018, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Adingupu, D.D.; Göpel, S.O.; Grönros, J.; Behrendt, M.; Sotak, M.; Miliotis, T.; Dahlqvist, U.; Gan, L.M.; Jönsson-Rylander, A.C. SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob-/- mice. Cardiovasc. Diabetol. 2019, 18, 16. [Google Scholar] [CrossRef]
- Uthman, L.; Homayr, A.; Juni, R.P.; Spin, E.L.; Kerindongo, R.; Boomsma, M.; Hollmann, M.W.; et al. Empagliflozin and Dapagliflozin Reduce ROS Generation and Restore NO Bioavailability in Tumor Necrosis Factor α-Stimulated Human Coronary Arterial Endothelial Cells. Cell. Physiol. Biochem. 2019, 53, 865–886. [Google Scholar] [PubMed]
- Herat, L.Y.; Magno, A.L.; Rudnicka, C.; Hricova, J.; Carnagarin, R.; Ward, N.C.; Arcambal, A.; et al. SGLT2 Inhibitor-Induced Sympathoinhibition: A Novel Mechanism for Cardiorenal Protection. JACC. Basic. Transl. Sci. 2020, 5, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Mone, P.; Varzideh, F.; Jankauskas, S.S.; Pansini, A.; Lombardi, A.; Frullone, S.; Santulli, G. SGLT2 Inhibition via Empagliflozin Improves Endothelial Function and Reduces Mitochondrial Oxidative Stress: Insights From Frail Hypertensive and Diabetic Patients. Hypertension. 2022, 79, 1633–1643. [Google Scholar] [CrossRef]
- Campeau, M.A.; Leask, R.L. Empagliflozin mitigates endothelial inflammation and attenuates endoplasmic reticulum stress signaling caused by sustained glycocalyx disruption. Sci. Rep. 2022, 12, 12681. [Google Scholar] [CrossRef]
- Kawade, S.; Ogiso, K.; Shayo, S.C.; Obo, T.; Arimura, A.; Hashiguchi, H.; Deguchi, T.; Nishio, Y. Luseogliflozin and caloric intake restriction increase superoxide dismutase 2 expression, promote antioxidative effects, and attenuate aortic endothelial dysfunction in diet-induced obese mice. J. Diabetes. Investig. 2023, 14, 548–559. [Google Scholar] [CrossRef]
- Ye, G.; Wang, S.; Peng, D. Effects of SGLT2 Inhibitor on Ischemic Events Stemming From Atherosclerotic Coronary Diseases: A Systematic Review and Meta-analysis With Trial Sequential Analysis of Randomized Controlled Trials. J. Cardiovasc. Pharmacol. 2021, 77, 787–795. [Google Scholar] [CrossRef]
- Eraikhuemen, N.; Leung, S.; Warren, S.B.; Lazaridis, D.; Smith, C.H.; Kearson, M.L.; Marcellus, V. Effects of the Sodium-Glucose Cotransporter Inhibitors on Cardiovascular Death and All-Cause Mortality: A Systematic Review and Meta-analysis of Randomized Placebo-Controlled Clinical Trials. Am. J. Cardiovasc. Drugs. 2023, 23, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, J.; Chen, S.; Xu, X.; Guo, D.; He, Y.; Huang, Z.; et al. Sodium Glucose Cotransporter Type 2 Inhibitors Improve Cardiorenal Outcome of Patients With Coronary Artery Disease: A Meta-Analysis. Front Endocrinol (Lausanne). 2022, 13, 850836. [Google Scholar] [CrossRef]
- Tu, Y.; Li, Q.; Zhou, Y.; Ye, Z.; Wu, C.; Xie, E.; Li, Y.; et al. Empagliflozin inhibits coronary microvascular dysfunction and reduces cardiac pericyte loss in db/db mice. Front. Cardiovasc. Med. 2022, 9, 995216. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, W. The Sodium-Glucose Co-Transporter 2 Inhibitor, Empagliflozin, Protects against Diabetic Cardiomyopathy by Inhibition of the Endoplasmic Reticulum Stress Pathway. Cell. Physiol. Biochem. 2017, 41, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Li, T.; Wang, Y.; Chang, Y.; Cheng, Y.; Lu, Y.; Liu, X.; et al. Empagliflozin prevents cardiomyopathy via sGC-cGMP-PKG pathway in type 2 diabetes mice. Clin. Sci (Lond). 2019, 133, 1705–1720. [Google Scholar] [CrossRef] [PubMed]
- Arow, M.; Waldman, M.; Yadin, D.; Nudelman, V.; Shainberg, A.; Abraham, N.G.; Freimark, D.; et al. Sodium-glucose cotransporter 2 inhibitor Dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc. Diabetol. 2020, 19, 7. [Google Scholar] [CrossRef]
- Moellmann, J.; Klinkhammer, B.M.; Droste, P.; Kappel, B.; Haj-Yehia, E.; Maxeiner, S.; Artati, A.; et al. Empagliflozin improves left ventricular diastolic function of db/db mice. Biochim. Biophys. Acta. Mol. Basis. Dis. 2020, 1866, 165807. [Google Scholar] [CrossRef] [PubMed]
- Trang, N.N.; Chung, C.C.; Lee, T.W.; Cheng, W.L.; Kao, Y.H.; Huang, S.Y.; Lee, T.I.; Chen, Y.J. Empagliflozin and Liraglutide Differentially Modulate Cardiac Metabolism in Diabetic Cardiomyopathy in Rats. Int. J. Mol. Sci. 2021, 22, 1177. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, M.; Suo, M.; Liu, D.; Wang, X.; Liu, M.; Pan, J.; Jin, T.; An, F. Dapagliflozin alleviates cardiac fibrosis through suppressing EndMT and fibroblast activation via AMPKα/TGF-β/Smad signalling in type 2 diabetic rats. J. Cell. Mol. Med. 2021, 25, 7642–7659. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Liu, H.; Chen, Y.; Li, P.; Liu, L.; Li, J.; et al. Empagliflozin Ameliorates Diabetic Cardiomyopathy via Attenuating Oxidative Stress and Improving Mitochondrial Function. Oxid. Med. Cell. Longev. 2022, 2022, 1122494. [Google Scholar] [CrossRef]
- Xi, Y.; Chen, D.; Dong, Z.; Zhang, J.; Lam, H.; He, J.; Du, K.; Chen, C.; Guo, J.; Xiao, J. Multi-omics insights into potential mechanism of SGLT2 inhibitors cardiovascular benefit in diabetic cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 999254. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Shi, H.; Xiong, L.; Wang, P.; Shi, Y. Canagliflozin mitigates ferroptosis and improves myocardial oxidative stress in mice with diabetic cardiomyopathy. Front. Endocrinol (Lausanne). 2022, 13, 1011669. [Google Scholar] [CrossRef] [PubMed]
- Kadosaka, T.; Watanabe, M.; Natsui, H.; Koizumi, T.; Nakao, M.; Koya, T.; Hagiwara, H.; et al. Empagliflozin attenuates arrhythmogenesis in diabetic cardiomyopathy by normalizing intracellular Ca2+ handling in ventricular cardiomyocytes. Am. J. Physiol. Heart. Circ. Physiol. 2023, 324, H341–H354. [Google Scholar] [CrossRef] [PubMed]
- Madonna, R.; Moscato, S.; Cufaro, M.C.; Pieragostino, D.; Mattii, L.; Del Boccio, P.; Ghelardoni, S.; Zucchi, R.; De Caterina, R. Empagliflozin inhibits excessive autophagy through the AMPK/GSK3β signaling pathway in diabetic cardiomyopathy. Cardiovasc. Res. 2023, cvad009. [Google Scholar]
- Croteau, D.; Baka, T.; Young, S.; He, H.; Chambers, J.M.; Qin, F.; Panagia, M.; et al. SGLT2 inhibitor ertugliflozin decreases elevated intracellular sodium, and improves energetics and contractile function in diabetic cardiomyopathy. Biomed. Pharmacother. 2023, 160, 114310. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Lang, J.; Xia, P.; Zhao, X.; Wang, L.; Yu, Y.; Chen, L. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibition on renal function and albuminuria in patients with type 2 diabetes: a systematic review and meta-analysis. PeerJ. 2017, 5, e3405. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Hakoshima, M.; Adachi, H.; Katsuyama, H. Multi-Organ Protective Effects of Sodium Glucose Cotransporter 2 Inhibitors. Int. J. Mol. Sci. 2021, 22, 4416. [Google Scholar] [CrossRef] [PubMed]
- Koguchi, A.; Adachi, H.; Yanai, H. The Application of Sodium-Glucose Cotransporter 2 Inhibitors to Chronic Kidney Disease Stage 4. J. Clin. Med. Res. 2017, 9, 1029–1031. [Google Scholar] [CrossRef]
- Yanai, H. Dipeptidyl Peptidase-4 Inhibitor versus Sodium-Glucose Cotransporter-2 Inhibitor in the Management of Type 2 Diabetes. J. Endocrinol. Metab. 2019, 9, 117–119. [Google Scholar] [CrossRef]
- Sano, M.; Takei, M.; Shiraishi, Y.; Suzuki, Y. Increased Hematocrit during Sodium-Glucose Cotransporter 2 Inhibitor Therapy Indicates Recovery of Tubulointerstitial Function in Diabetic Kidneys. J. Clin. Med. Res. 2016, 8, 844–847. [Google Scholar] [CrossRef]
- Yanai, H.; Katsuyama, H. A Possible Mechanism for Renoprotective Effect of Sodium-Glucose Cotransporter 2 Inhibitor: Elevation of Erythropoietin Production. J. Clin. Med. Res. 2017, 9, 178–179. [Google Scholar] [CrossRef]
- Toba, H.; Sawai, N.; Morishita, M.; Murata, S.; Yoshida, M.; Nakashima, K.; Morita, Y.; Kobara, M.; Nakata, T. Chronic treatment with recombinant human erythropoietin exerts renoprotective effects beyond hematopoiesis in streptozotocin-induced diabetic rat. Eur. J. Pharmacol. 2009, 612, 106–114. [Google Scholar] [CrossRef]
- Ruester, C.; Franke, S.; Bondeva, T.; Wolf, G. Erythropoietin protects podocytes from damage by advanced glycation end-products. Nephron Exp. Nephrol. 2011, 117, e21–30. [Google Scholar] [CrossRef]
- Loeffler, I.; Ruster, C.; Franke, S.; Liebisch, M.; Wolf, G. Erythropoietin ameliorates podocyte injury in advanced diabetic nephropathy in the db/db mouse. Am. J. Physiol. Renal. Physiol. 2013, 305, F911–918. [Google Scholar] [CrossRef]
- Vallon, V.; Mühlbauer, B.; Osswald, H. Adenosine and kidney function. Physiol. Rev. 2006, 86, 901–940. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Richter, K.; Blantz, R.C.; Thomson, S.; Osswald, H. Glomerular hyperfiltration in experimental diabetes mellitus: Potential role of tubular reabsorption. J. Am. Soc. Nephrol. 1999, 10, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.C.; Rieg, T.; Miracle, C.; Mansoury, H.; Whaley, J.; Vallon, V.; Singh, P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R75–R83. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Lang, J.; Xia, P.; Zhao, X.; Wang, L.; Yu, Y.; Chen, L. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibition on renal function and albuminuria in patients with type 2 diabetes: a systematic review and meta-analysis. PeerJ. 2017, 5, e3405. [Google Scholar] [CrossRef]
- Toyama, T.; Neuen, B.L.; Jun, M.; Ohkuma, T.; Neal, B.; Jardine, M.J.; Heerspink, H.L.; et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes. Obes. Metab. 2019, 21, 1237–1250. [Google Scholar] [CrossRef]
- Salah, H.M.; Al'Aref, S.J.; Khan, M.S.; Al-Hawwas, M.; Vallurupalli, S.; Mehta, J.L.; Mounsey, J.P.; et al. Effect of sodium-glucose cotransporter 2 inhibitors on cardiovascular and kidney outcomes-Systematic review and meta-analysis of randomized placebo-controlled trials. Am. Heart. J. 2021, 232, 10–22. [Google Scholar] [CrossRef]
- Yamada, T.; Wakabayashi, M.; Bhalla, A.; Chopra, N.; Miyashita, H.; Mikami, T.; Ueyama, H.; et al. Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Cardiovasc. Diabetol. 2021, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, Y.; Tian, Z.; Lian, Y.; Jia, J.; Liu, M.; Li, D. Sodium-glucose cotransporter 2 inhibitors benefit to kidney and cardiovascular outcomes for patients with type 2 diabetes mellitus and chronic kidney disease 3b-4: A systematic review and meta-analysis of randomized clinical trials. Diabetes. Res. Clin. Pract. 2021, 180, 109033. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lv, D.; Zhu, X.; Wei, P.; Gui, Y.; Liu, S.; Zhou, E.; Zheng, M.; Zhou, D.; Zhang, L. Effects of SGLT2 Inhibitors on Renal Outcomes in Patients With Chronic Kidney Disease: A Meta-Analysis. Front. Med (Lausanne). 2021, 8, 728089. [Google Scholar] [CrossRef] [PubMed]
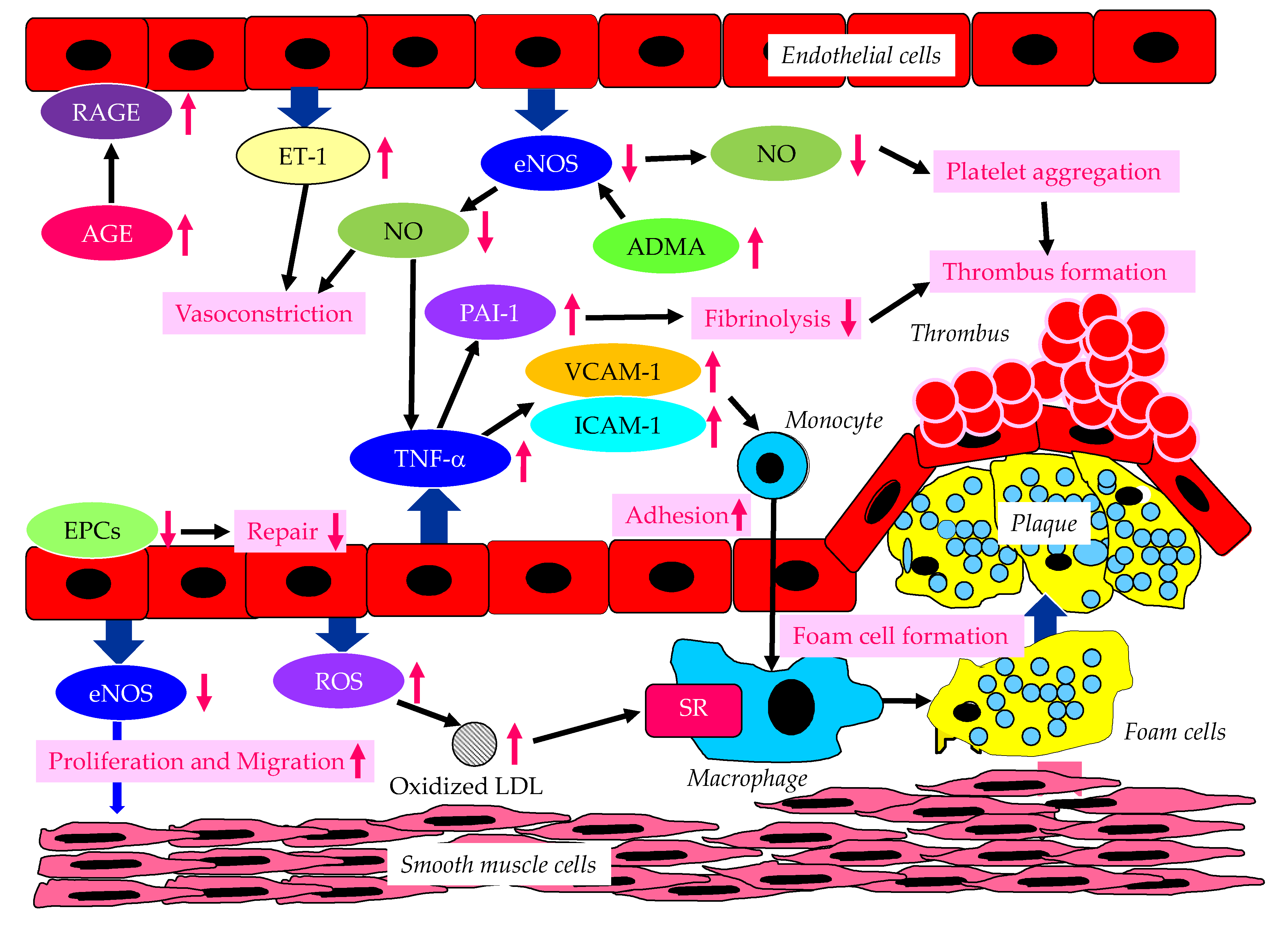
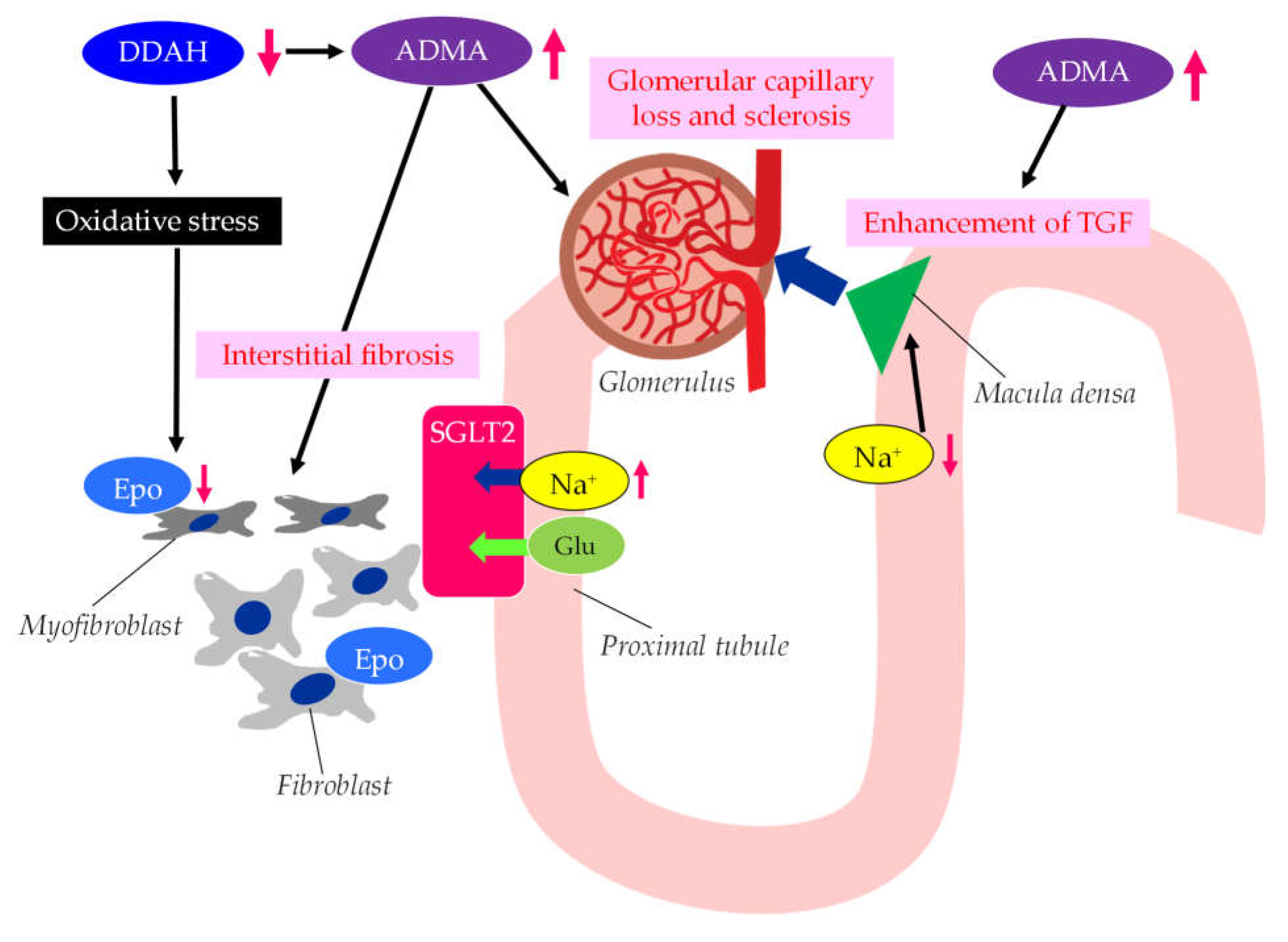
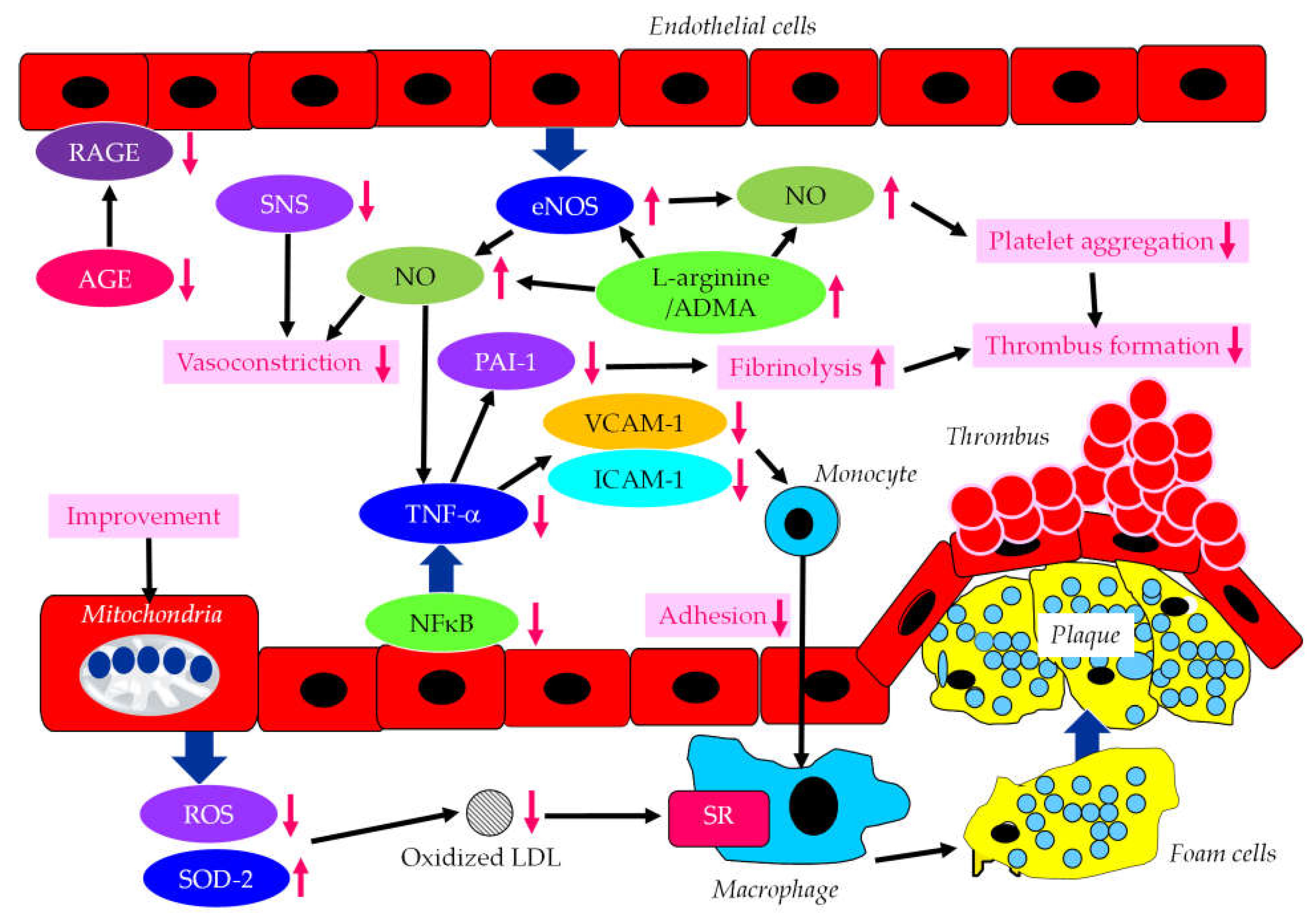
| Beneficial factors by SGLT2i to improve DCM |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





