Submitted:
01 May 2023
Posted:
02 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Description of the study
2.1. Objectives: Evaluate
2.2. Design, setting and methods
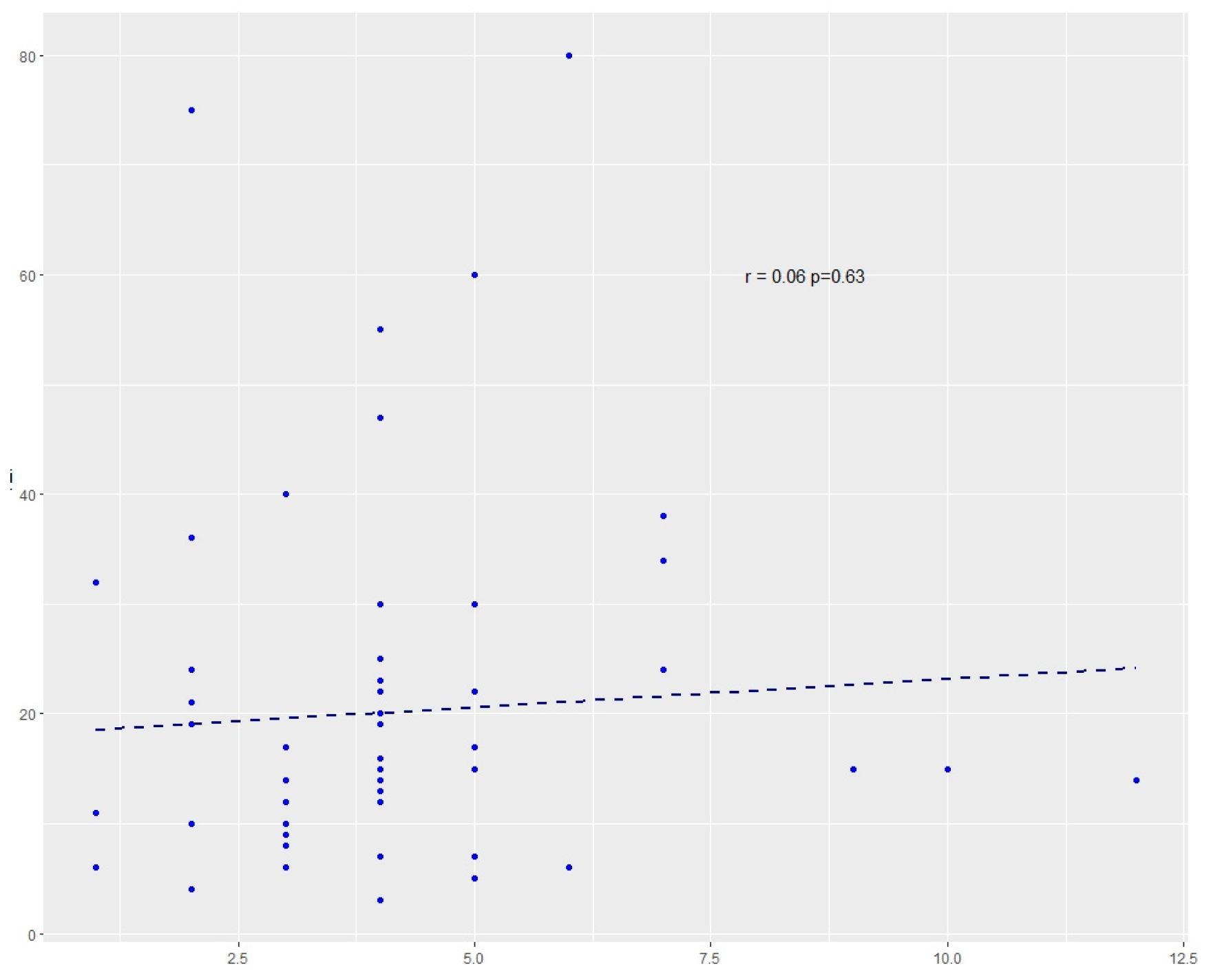
2.3. Results
| Overall (N=97) | |
|---|---|
| GA | |
| Mean (SD) | 28.56 (2.73) |
| Median (Q1, Q3) | 29.00 (27.00, 30.00) |
| Min - Max | 23.00 - 35.00 |
| Non missing N | 97 |
| Birth Weight | |
| Mean (SD) | 1091.48 (287.93) |
| Median (Q1, Q3) | 1110.00 (870.00, 1320.00) |
| Min - Max | 520.00 - 1495.00 |
| Non missing N | 97 |
| Year | |
| 2017 | 24 (24.7%) |
| 2018 | 24 (24.7%) |
| 2019 | 24 (24.7%) |
| 2020 | 8 (8.2%) |
| 2021 | 17 (17.5%) |
| Non missing N | 97 |
| Single/Twin | |
| T | 36 (37.5%) |
| S | 60 (62.5%) |
| Non missing N | 96 |
| Missing N | 1 |
| Delivery | |
| V | 14 (14.6%) |
| CS | 82 (85.4%) |
| Non missing N | 96 |
| Missing N | 1 |
| MA | |
| Mean (SD) | 32.62 (6.11) |
| Median (Q1, Q3) | 33.00 (28.50, 37.50) |
| Min - Max | 19.00 - 46.00 |
| Non missing N | 95 |
| Missing N | 2 |
| DM ml | |
| Mean (SD) | 4015.88 (3840.05) |
| Median (Q1, Q3) | 2900.00 (500.00, 7100.00) |
| Min - Max | 12.00 - 12200.00 |
| Non missing N | 68 |
| Missing N | 29 |
| Start MEF | |
| Mean (SD) | 1.78 (1.03) |
| Median (Q1, Q3) | 2.00 (1.00, 2.00) |
| Min - Max | 1.00 - 7.00 |
| Non missing N | 96 |
| Missing N | 1 |
| MOM | |
| NO | 29 (30.5%) |
| YES | 66 (69.5%) |
| Non missing N | 95 |
| Missing N | 2 |
| Start MOM | |
| Mean (SD) | 4.11 (1.93) |
| Median (Q1, Q3) | 4.00 (3.00, 5.00) |
| Min - Max | 1.00 - 12.00 |
| Non missing N | 65 |
| Missing N | 32 |
| FEF | |
| Mean (SD) | 18.53 (14.30) |
| Median (Q1, Q3) | 15.00 (9.00, 22.00) |
| Min - Max | 3.00 - 80.00 |
| Non missing N | 86 |
| Missing N | 11 |
| Days of hospitalization | |
| Mean (SD) | 62.14 (25.76) |
| Median (Q1, Q3) | 58.00 (45.25, 76.00) |
| Min - Max | 22.00 - 161.00 |
| Non missing N | 94 |
| Missing N | 3 |
| Weight at discharge | |
| Mean (SD) | 2538.55 (529.59) |
| Median (Q1, Q3) | 2390.00 (2157.50, 2802.50) |
| Min - Max | 1781.00 - 4960.00 |
| Non missing N | 96 |
| Missing N | 1 |
| Feeding at discharge | |
| FM | 51 (52.6%) |
| MOM | 35 (36.1%) |
| MOM+FM | 11 (11.3%) |
| Non missing N | 97 |
| Average daily weight increment | |
| Mean (SD) | 24.02 (7.22) |
| Median (Q1, Q3) | 23.00 (19.67, 26.85) |
| Min - Max | 12.20 - 71.30 |
| Non missing N | 92 |
| Missing N | 5 |
| Year | 2017 | 2018 | 2019 | 2020 | 2021 | p |
|---|---|---|---|---|---|---|
| n° | 24 | 24 | 24 | 8 | 17 | |
| GA (mean (SD)) | 27.29 (2.37) | 28.58 (2.89) | 29.00 (2.59) | 29.25 (3.28) | 29.35 (2.57) | 0.097 |
| Weight (mean (SD)) | 1030.00 (316.32) | 1085.46 (281.31) | 1139.29 (298.99) | 1052.50 (243.35) | 1137.65 (270.64) | 0.680 |
| Sing_Twins = S (%) | 17 (70.83) | 16 (66.67) | 15 (65.22) | 5 ( 62.50) | 7 ( 41.18) | 0.372 |
| CS (%) | 18 (78.26) | 21 (87.50) | 18 (75.00) | 8 (100.00) | 17 (100.00) | 0.117 |
| Maternal age (mean (SD)) | 31.86 (6.15) | 32.38 (6.98) | 31.04 (5.71) | 33.75 (4.13) | 35.65 (5.48) | 0.168 |
| MOM =YES (%) | 16 (66.67) | 15 (62.50) | 21 (91.30) | 1 ( 14.29) | 13 ( 76.47) | 0.003 |
| Start MOM (mean (SD)) | 4.44 (2.16) | 4.47 (2.53) | 4.00 (1.63) | 4.00 (0.00) | 3.30 (1.16) | 0.602 |
| DM ml (mean (SD)) | 3211.25 (3324.12) | 4599.29 (4045.35) | 3200.57 (3510.58) | 9883.33 (2116.99) | 2125.00 (1935.94) | <0.001 |
| Start MEF (mean (SD)) | 1.52 (0.73) | 2.04 (1.40) | 1.67 (0.48) | 2.38 (1.92) | 1.65 (0.61) | 0.180 |
| FEF (mean (SD)) | 22.77 (21.76) | 15.58 (7.37) | 16.22 (10.72) | 20.88 (14.95) | 19.89 (12.75) | 0.433 |
| Length of stay(mean (SD)) | 65.71 (30.32) | 68.54 (28.76) | 60.58 (22.10) | 59.50 (19.34) | 52.12 (21.49) | 0.331 |
| Average daily weight increment (mean (SD)) | 21.08 (3.44) | 22.27 (4.46) | 23.83 (5.72) | 21.55 (3.95) | 31.36 (11.25) | <0.001 |
| NO | YES | p | |
|---|---|---|---|
| n° | 29 | 66 | |
| GA (mean (SD)) | 29.28 (2.95) | 28.23 (2.57) | 0.084 |
| Weight (mean (SD)) | 1169.24 (291.62) | 1059.64 (281.78) | 0.087 |
| Sing_Twins = S (%) | 14 (50.00) | 44 (66.67) | 0.198 |
| CS (%) | 24 (82.76) | 56 (86.15) | 0.910 |
| Maternal Age (mean (SD)) | 32.00 (7.19) | 32.83 (5.68) | 0.553 |
| Start MOM (mean (SD)) | 3.00 (2.83) | 4.07 (1.84) | 0.429 |
| Length of stay (mean (SD)) | 57.34 (27.31) | 64.59 (25.00) | 0.213 |
| Average daily weight increment (mean (SD)) | 23.38 (4.44) | 24.43 (8.26) | 0.527 |
| Start MEF (mean (SD)) | 1.90 (1.18) | 1.72 (0.98) | 0.457 |
| FEF (mean (SD)) | 14.88 (7.30) | 19.53 (15.73) | 0.162 |
| FM vs MOM vs MOM+FM | FM vs MOM | |
|---|---|---|
| p | p | |
| GA (mean (SD)) | 0.101 | 0.032 |
| Weight (mean (SD)) | 0.182 | 0.065 |
| Sing_Twins = S (%) | 0.584 | 0.460 |
| CS (%) | 0.323 | 0.538 |
| Maternal Age (mean (SD)) | 0.048 | 0.059 |
| Start MOM (mean (SD)) | 0.032 | 0.009 |
| Length of stay(mean (SD)) | 0.622 | 0.733 |
| Average daily weight increment(mean (SD)) | 0.845 | 0.562 |
| Start MEF (mean (SD)) | 0.719 | 0.670 |
| FEF (mean (SD)) | 0.225 | 0.620 |
3. Discussion
4. Conclusions and relevance
Abbreviations
| MOM | Mother’s Own Milk |
| EMOM | Exclusive Mother’s Own Milk |
| MMOM | Mixed Mother’s Own Milk |
| NMOM | No Mother’s Own Milk |
| HM | Human Milk |
| DM | Donor Milk |
| FM | Formula Milk |
| HMB | Human Milk Bank |
| BF | Breastfeeding |
| EBF | Exclusive Breastfeeding |
| EF | Enteral Feeding |
| MEF | Minimal Enteral Feeding |
| FEF | Full Enteral Feeding |
| VLBW | Very Low Birth Weight |
| ELBW | Estremely Low Birth Weight |
| NEC | Necrotizing Enterocolitis |
References
- Cao, G.; Liu, J.; Liu, M. Global, Regional, and National Incidence and Mortality of Neonatal Preterm Birth, 1990-2019. JAMA Pediatr. 2022, 176, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Walani, S.R. Global burden of preterm birth. Int. J. Gynecol. Obstet. 2020, 150, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Zeitlin, J.; Szamotulska, K.; Drewniak, N.; Mohangoo, A.; Chalmers, J.; Sakkeus, L.; Irgens, L.; Gatt, M.; Gissler, M.; Blondel, B.; et al. Preterm birth time trends in Europe: a study of 19 countries. BJOG: Int. J. Obstet. Gynaecol. 2013, 120, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Causes of newborn mortality and morbidity in the European Region. Ac-cessed June 26, 2020. https://www.euro.who.int/en/health-topics/Life-stages/maternal-and-newborn-health/causes-of-newborn-mortality-and-morbidity-inthe-european-region.
- Boquien, C.-Y. Human Milk: An Ideal Food for Nutrition of Preterm Newborn. Front. Pediatr. 2018, 6, 295. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E., Di Nicola, P., Giuliani, F., Peila, C., Cester, E., Vassia, C., Pirra, A., Tonetto, P., & Coscia, A. 2012. Benefits of human milk in preterm infant feeding. Journal of Pediatric and Neonatal Individualized Medicine, 1(1), 19-24. [CrossRef]
- Sankar, M.J.; Sinha, B.; Chowdhury, R.; Bhandari, N.; Taneja, S.; Martines, J.; Bahl, R. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta-analysis. Acta Paediatr. 2015, 104, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; Franca, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Moro, G.E.; Arslanoglu, S.; Bertino, E.; Corvaglia, L.; Montirosso, R.; Picaud, J.-C.; Polberger, S.; Schanler, R.J.; Steel, C.; van Goudoever, J.; et al. XII. Human Milk in Feeding Premature Infants. J. Pediatr. Gastroenterol. Nutr. 2015, 61, S16–S19. [Google Scholar] [CrossRef]
- WHO Guidelines on optimal feeding of low birth-weight infants in low- and middle-income countries. https://www.who.int/maternal_child_adolescent/documents/infant_feeding_low_bw/en/.
- Ericson, J.; Flacking, R.; Hellström-Westas, L.; Eriksson, M. Changes in the prevalence of breast feeding in preterm infants discharged from neonatal units: a register study over 10 years. BMJ Open 2016, 6, e012900. [Google Scholar] [CrossRef]
- Johnson, T.J.; Patel, A.L.; Schoeny, M.E.; Meier, P.P. Cost Savings of Mother’s Own Milk for Very Low Birth Weight Infants in the Neonatal Intensive Care Unit. PharmacoEconomics - Open 2022, 6, 451–460. [Google Scholar] [CrossRef]
- Patel, A.L.; Johnson, T.J.; Robin, B.; Bigger, H.R.; Buchanan, A.; Christian, E.; Nandhan, V.; Shroff, A.; Schoeny, M.; Engstrom, J.L.; et al. Influence of own mother's milk on bronchopulmonary dysplasia and costs. Arch. Dis. Child. - Fetal Neonatal Ed. 2016, 102, F256–F261. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.P. More evidence: Mothers’ own milk is personalized medicine for very low birthweight infants. Cell Rep. Med. 2022, 3, 100710. [Google Scholar] [CrossRef] [PubMed]
- Lechner, B.E.; Vohr, B.R. Neurodevelopmental Outcomes of Preterm Infants Fed Human Milk: A Systematic Review. Clin. Perinatol. 2017, 44, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Thoene, M.; Anderson-Berry, A. Early Enteral Feeding in Preterm Infants: A Narrative Review of the Nutritional, Metabolic, and Developmental Benefits. Nutrients 2021, 13, 2289. [Google Scholar] [CrossRef] [PubMed]
- Twilhaar, E.S.; Wade, R.M.; de Kieviet, J.F.; van Goudoever, J.B.; van Elburg, R.M.; Oosterlaan, J. Cognitive Outcomes of Children Born Extremely or Very Preterm Since the 1990s and Associated Risk Factors: A Meta-analysis and Meta-regression. JAMA Pediatr. 2018, 172, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yu, Z.; Li, Q.; Zhou, J.; Yin, X.; Ma, Y.; Yin, Y.; Jiang, S.; Zhu, R.; Wu, Y.; et al. Dose-dependent effect of human milk on Bronchopulmonary dysplasia in very low birth weight infants. BMC Pediatr. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mitha, A.; Piedvache, A.; Khoshnood, B.; Fresson, J.; Glorieux, I.; Roué, J.; Blondel, B.; Durox, M.; Burguet, A.; Ancel, P.; et al. The impact of neonatal unit policies on breast milk feeding at discharge of moderate preterm infants: The EPIPAGE-2 cohort study. Matern. Child Nutr. 2019, 15, e12875. [Google Scholar] [CrossRef] [PubMed]
- Heller, N.; Rüdiger, M.; Hoffmeister, V.; Mense, L. Mother’s Own Milk Feeding in Preterm Newborns Admitted to the Neonatal Intensive Care Unit or Special-Care Nursery: Obstacles, Interventions, Risk Calculation. Int. J. Environ. Res. Public Heal. 2021, 18, 4140. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Severo, M.; Zeitlin, J.; Barros, H.; (, O.B.O.T.P.E. The Type of Feeding at Discharge of Very Preterm Infants: Neonatal Intensive Care Units Policies and Practices Make a Difference. Breastfeed. Med. 2018, 13, 50–59. [Google Scholar] [CrossRef]
- Daglas, Maria; Sidiropoulou, Charikleia; Galanis, Petros; Bilali, Angeliki; Antoniou, Evangelia; Iatrakis, Georgios Maternal and Neonatal Factors Associated with Successful Breastfeeding in Preterm Infants. International Journal of Caring Sciences January– April 2020 Volume 13 | Issue 1| Page 152 www.internationaljournalofcaringsciences.org.
- Iliodromiti, Z., Papamichail, D., Ekizoglou, Ch., Nteka, E., Mavrika, P., Zografaki, E., Koutentakis, K., Zidropoulos, S., Stavrou, D., Panagiotopoulos, T., Antoniadou-Koumatou, I. (2018). National Study to estimate the frequency and determinants of Breastfeeding in Greece. Athens: Institute of Child Health.
- Dong, D.; Ru, X.; Huang, X.; Sang, T.; Li, S.; Wang, Y.; Feng, Q. A prospective cohort study on lactation status and breastfeeding challenges in mothers giving birth to preterm infants. Int. Breastfeed. J. 2022, 17, 1–13. [Google Scholar] [CrossRef]
- USA Boundy EO, Perrine CG, Nelson JM, Hamner HC. Disparities in hospital reported breast Milk use in neonatal intensive care units - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(48):1313–7. [CrossRef]
- Kalluri, N.S.; Burnham, L.A.; Lopera, A.M.; Stickney, D.M.; Combs, G.L.; Levesque, B.M.; Philipp, B.L.; Parker, M.G. A Quality Improvement Project to Increase Mother’s Milk Use in an Inner-City NICU. Pediatr. Qual. Saf. 2019, 4, e204. [Google Scholar] [CrossRef] [PubMed]
- Dinesh Dharel, MD1,2, Nalini Singhal, MD1, Christel Wood, LC1, Zenon Cieslak, MD3, Fabiana Bacchi-ni, MSc4, Prakesh S. Shah, MD5, Xiang Y. Ye, MSc5, and Belal Alshaikh, MD, MSc, MSCE1, on behalf of the Canadian Neonatal Network (CNN) and Canadian Preterm Birth Network (CPTBN) Investigators. Rates and Determinants of Mother’s Own Milk Feeding in Infants Born Very Preterm Pediatr 2021;236:21-7).
- Silva, M.D.B.; Oliveira, R.d.V.C.d.; Alves, D.d.S.B.; Melo, E.C.P. Predicting risk of early discontinuation of exclusive breastfeeding at a Brazilian referral hospital for high-risk neonates and infants: a decision-tree analysis. Int. Breastfeed. J. 2021, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Quitadamo, P. A. (2020). Correspondence: the donation of human milk during the COVID-19 pandemic. Journal of Pediatric and Neonatal Individualized Medicine (JPNIM), 10(1), e100131. [CrossRef]
- Meier, P.P. Prioritizing High-Dose Long Exposure to Mothers' Own Milk During the Neonatal Intensive Care Unit Hospitalization. Breastfeed. Med. 2019, 14, S20–20. [Google Scholar] [CrossRef]
- Modi, M.; Ramji, S.; Jain, A.; Kumar, P.; Gupta, N. Early Aggressive Enteral Feeding in Neonates Weighing 750–1250 Grams: A Randomized Controlled Trial. Indian Pediatr. 2019, 56, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Montealegre-Pomar, A.d.P.; Bertolotto-Cepeda, A.M.; Romero-Marquez, Y.; -Ramírez, K.J.M. Effectiveness and Safety of Fast Enteral Advancement in Preterm Infants Between 1000 and 2000 g of Birth Weight. J. Parenter. Enter. Nutr. 2020, 45, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Raban, S.; Santhakumaran, S.; Keraan, Q.; Joolay, Y.; Uthaya, S.; Horn, A.; Modi, N.; Harrison, M. A randomised controlled trial of high vs low volume initiation and rapid vs slow advancement of milk feeds in infants with birthweights ≤ 1000 g in a resource-limited setting. Ann. Trop. Paediatr. 2016, 36, 288–295. [Google Scholar] [CrossRef]
- Abbott, J.; Berrington, J.; Bowler, U.; Boyle, E.; Dorling, J.; Embleton, N.; Juszczak, E.; Leaf, A.; Linsell, L.; et al.; The Sift Investigators Group The Speed of Increasing milk Feeds: a randomised controlled trial. BMC Pediatr. 2017, 17, 1–6. [Google Scholar] [CrossRef]
- Ahmed, F.; Dey, S.K.; Shahidullah, M.; A Mannan, M.; Raj, A.Y.; Sharmin, S. Early Versus Delayed Enteral Feeding for Achieving Full Feeding in Preterm Growth-Restricted Infants: A Randomized Clinical Trial. 2020, 29, 638–645. [Google Scholar]
- Nangia, S.; Vadivel, V.; Thukral, A.; Saili, A. Early Total Enteral Feeding versus Conventional Enteral Feeding in Stable Very-Low-Birth-Weight Infants: A Randomised Controlled Trial. Neonatology 2019, 115, 256–262. [Google Scholar] [CrossRef]
- de Waard, M.; Li, Y.; Zhu, Y.; Ayede, A.I.; Berrington, J.; Bloomfield, F.H.; Busari, O.O.; Cormack, B.E.; Embleton, N.D.; van Goudoever, J.B.; et al. Time to Full Enteral Feeding for Very Low-Birth-Weight Infants Varies Markedly Among Hospitals Worldwide But May Not Be Associated With Incidence of Necrotizing Enterocolitis: The NEOMUNE-NeoNutriNet Cohort Study. J. Parenter. Enter. Nutr. 2018, 43, 658–667. [Google Scholar] [CrossRef]
- Maas, C.; Franz, A.R.; von Krogh, S.; Arand, J.; Poets, C.F. Growth and morbidity of extremely preterm infants after early full enteral nutrition. Arch. Dis. Child. - Fetal Neonatal Ed. 2017, 103, F79–F81. [Google Scholar] [CrossRef] [PubMed]
- Leaf, A. Introducing enteral feeds in the high-risk preterm infant. Semin. Fetal Neonatal Med. 2013, 18, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Chitale, R.; Ferguson, K.; Talej, M.; Yang, W.-C.; He, S.; Edmond, K.M.; Smith, E.R. Early Enteral Feeding for Preterm or Low Birth Weight Infants: a Systematic Review and Meta-analysis. PEDIATRICS 2022, 150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, X.; Zhu, J.; Jiang, C.; Yu, Z.; Su, A. Effect of First Mother's Own Milk Feeding Time on the Risk of Moderate and Severe Bronchopulmonary Dysplasia in Infants With Very Low Birth Weight. Front. Pediatr. 2022, 10, 887028. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.A.; Sullivan, S.; Krueger, C.; Mueller, M. Association of Timing of Initiation of Breastmilk Expression on Milk Volume and Timing of Lactogenesis Stage II Among Mothers of Very Low-Birth-Weight Infants. Breastfeed. Med. 2015, 10, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.G.; Melvin, P.; Graham, D.A.; Gupta, M.; Burnham, L.A.; Lopera, A.M.; Zera, C.A.; Belfort, M.B. Timing of First Milk Expression to Maximize Breastfeeding Continuation Among Mothers of Very Low-Birth-Weight Infants. Obstetrics & Gynecology 2019, 133, 1208–1215. [Google Scholar] [CrossRef]
- Ikonen, R.; Paavilainen, E.; Helminen, M.; Kaunonen, M. Preterm infants’ mothers’ initiation and frequency of breast milk expression and exclusive use of mother's breast milk in neonatal intensive care units. J. Clin. Nurs. 2017, 27, e551–e558. [Google Scholar] [CrossRef]
- Murase, M.; Nommsen-Rivers, L.; Morrow, A.L.; Hatsuno, M.; Mizuno, K.; Taki, M.; Miyazawa, T.; Nakano, Y.; Aizawa, M.; Itabashi, K. Predictors of Low Milk Volume among Mothers Who Delivered Preterm. J. Hum. Lact. 2014, 30, 425–435. [Google Scholar] [CrossRef]
- Daljeet KAK, Geetanjli K, Praveen K. Current practices related to feeding preterm neonates with ex-pressed breast milk: a pilot project. COJ Nurse Health Care. 2018;3(3).
- Alves, E.; Magano, R.; Amorim, M.; Nogueira, C.; Silva, S. Factors Influencing Parent Reports of Facilitators and Barriers to Human Milk Supply in Neonatal Intensive Care Units. J. Hum. Lact. 2016, 32, 695–703. [Google Scholar] [CrossRef]
- Parker, M.G.; Patel, A.L. Using quality improvement to increase human milk use for preterm infants. Semin. Perinatol. 2017, 41, 175–186. [Google Scholar] [CrossRef]
- Spatz, D.L.; Froh, E.B.; Schwarz, J.; Houng, K.; Brewster, I.; Myers, C.; Prince, J.; Olkkola, M. Pump Early, Pump Often: A Continuous Quality Improvement Project. J. Périnat. Educ. 2015, 24, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Takako, H.; Mizue, M.; Izumi, H.; Chie, O.; Harue, T.; Uchida, M.; Spatz, D.L. Improving Human Milk and Breastfeeding Rates in a Perinatal Hospital in Japan: A Quality Improvement Project. Breastfeed. Med. 2020, 15, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Hoban, R.; Bigger, H.; Schoeny, M.; Engstrom, J.; Meier, P.; Patel, A.L. Milk Volume at 2 Weeks Predicts Mother's Own Milk Feeding at Neonatal Intensive Care Unit Discharge for Very Low Birthweight Infants. Breastfeed. Med. 2018, 13, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bigger, H.R.; Fogg, L.J.; Patel, A.; Johnson, T.; Engstrom, J.L.; Meier, P.P. Quality indicators for human milk use in very low-birthweight infants: are we measuring what we should be measuring? J. Perinatol. 2014, 34, 287–291. [Google Scholar] [CrossRef] [PubMed]
- WHO. Indicators for assessing breastfeeding practices; 2021.
- Alshaikh, B.; Dharel, D.; Yusuf, K.; Singhal, N. Early total enteral feeding in stable preterm infants: a systematic review and meta-analysis. J. Matern. Neonatal Med. 2019, 34, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Oddie, S.J.; Young, L.; McGuire, W. Slow advancement of enteral feed volumes to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst. Rev. 2021, 2021, CD001241. [Google Scholar] [CrossRef] [PubMed]
- Quitadamo, P.A.; Palumbo, G.; Cianti, L.; Lurdo, P.; Gentile, M.A.; Villani, A. The Revolution of Breast Milk: The Multiple Role of Human Milk Banking between Evidence and Experience—A Narrative Review. Int. J. Pediatr. 2021, 2021, 1–11. [Google Scholar] [CrossRef]
- Hair AB. Approach to enteral nutrition in the premature infant. Available at: https://www.uptodate.com/contents/approach-to-enteral-nutrition-in-the-premature-infant.
- Walsh, V.; Brown, J.V.E.; Copperthwaite, B.R.; Oddie, S.J.; McGuire, W. Early full enteral feeding for preterm or low birth weight infants. Cochrane Database Syst. Rev. 2020, 2020. [Google Scholar] [CrossRef]
- Embleton, N.D.; Berrington, J.E.; Dorling, J.; Ewer, A.K.; Juszczak, E.; Kirby, J.A.; Lamb, C.A.; Lanyon, C.V.; McGuire, W.; Probert, C.S.; et al. Mechanisms Affecting the Gut of Preterm Infants in Enteral Feeding Trials. Front. Nutr. 2017, 4, 14. [Google Scholar] [CrossRef]
- Walsh, V.; Brown, J.V.E.; Copperthwaite, B.R.; Oddie, S.J.; McGuire, W. Early full enteral feeding for preterm or low birth weight infants. Cochrane Database Syst. Rev. 2020, 2020. [Google Scholar] [CrossRef]
- Thoene, M.; Anderson-Berry, A. Early Enteral Feeding in Preterm Infants: A Narrative Review of the Nutritional, Metabolic, and Developmental Benefits. Nutrients 2021, 13, 2289. [Google Scholar] [CrossRef] [PubMed]
- Cuestas, E.; Aguilera, B.; Cerutti, M.; Rizzotti, A. Sustained Neonatal Inflammation Is Associated with Poor Growth in Infants Born Very Preterm during the First Year of Life. J. Pediatr. 2019, 205, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Twilhaar, E.S.; Wade, R.M.; de Kieviet, J.F.; van Goudoever, J.B.; van Elburg, R.M.; Oosterlaan, J. Cognitive Outcomes of Children Born Extremely or Very Preterm Since the 1990s and Associated Risk Factors: A Meta-analysis and Meta-regression. JAMA Pediatr. 2018, 172, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Linafelter, A.; Cuna, A.; Liu, C.; Quigley, A.; Truog, W.E.; Sampath, V.; Oschman, A. Extended course of prednisolone in infants with severe bronchopulmonary dysplasia. Early Hum. Dev. 2019, 136, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Leaf, A. Introducing enteral feeds in the high-risk preterm infant. Semin. Fetal Neonatal Med. 2013, 18, 150–154. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Díaz-Rossello, J.L. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst. Rev. 2016, 2017, CD002771. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, B.; Dharel, D.; Yusuf, K.; Singhal, N. Early total enteral feeding in stable preterm infants: a systematic review and meta-analysis. J. Matern. Neonatal Med. 2019, 34, 1479–1486. [Google Scholar] [CrossRef]
- Embleton, N.D. Early nutrition and later outcomes in preterm infants. World Rev. Nutr. Diet 2013, 106, 26–32. [Google Scholar] [CrossRef]
- Parker, M.G.; Greenberg, L.T.; Edwards, E.M.; Ehret, D.; Belfort, M.B.; Horbar, J.D. National Trends in the Provision of Human Milk at Hospital Discharge Among Very Low-Birth-Weight Infants. JAMA Pediatr. 2019, 173, 961–968. [Google Scholar] [CrossRef]
- Davanzo, R.; Monasta, L.; Ronfani, L.; Brovedani, P.; Demarini, S.; Breastfeeding in Neonatal Intensive Care Unit Study Group. Breastfeeding at NICU discharge: A multicenter Italian study. J. Hum. Lact. 2012, 29, 374–380. [Google Scholar] [CrossRef]
- Gianni, M.L.; Bezze, E.N.; Sannino, P.; Baro, M.; Roggero, P.; Muscolo, S.; Plevani, L.; Mosca, F. Maternal views on facilitators of and barriers to breastfeeding preterm infants. BMC Pediatr. 2018, 18, 283. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Teixeira, R.; Fonseca, M.J.; Zeitlin, J.; Barros, H.; Portuguese EPICE (Effective Perinatal Intensive Care in Europe) Network. Prevalence and duration of breast milk feeding in very preterm infants: A 3-year follow-up study and a systematic literature review. Paediatr. Périnat. Epidemiology 2018, 32, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Sokou, R.; Parastatidou, S.; Ioakeimidis, G.; Tavoulari, E.-F.; Makrogianni, A.; Isaakidou, E.; Iacovidou, N.; Konstantinidi, A. Breastfeeding in Neonates Admitted to an NICU: 18-Month Follow-Up. Nutrients 2022, 14, 3841. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; Blondel, B.; Piedvache, A.; Wilson, E.; Bonamy, A.E.; Gortner, L.; Rodrigues, C.; Van Heijst, A.; Draper, E.S.; Cuttini, M.; et al. Low breastfeeding continuation to 6 months for very preterm infants: A E uropean multiregional cohort study. Matern. Child Nutr. 2019, 15, e12657. [Google Scholar] [CrossRef]
- Casey, L.; Fucile, S.; Dow, K.E. Determinants of Successful Direct Breastfeeding at Hospital Discharge in High-Risk Premature Infants. Breastfeed. Med. 2018, 13, 346–351. [Google Scholar] [CrossRef]
- Quitadamo, P. A., Palumbo, G., Gatta, A., Cianti, L., Copetti, M., Gentile, M. A., & Cristalli, P. (2018). How do characteristics of donors and their children influence volume and composition of banked milk?. Journal of Pediatric and Neonatal Individualized Medicine (JPNIM), 7(1), e070121. [CrossRef]
- Gates, A.; Marin, T.; De Leo, G.; Waller, J.L.; Stansfield, B.K. Nutrient composition of preterm mother’s milk and factors that influence nutrient content. Am. J. Clin. Nutr. 2021, 114, 1719–1728. [Google Scholar] [CrossRef]
- Quitadamo, P.A.; Comegna, L.; Palumbo, G.; Copetti, M.; Lurdo, P.; Zambianco, F.; Gentile, M.A.; Villani, A. Feeding Twins with Human Milk and Factors Associated with Its Duration: A Qualitative and Quantitative Study in Southern Italy. Nutrients 2021, 13, 3099. [Google Scholar] [CrossRef]
- Pineda, R.G. Predictors of Breastfeeding and Breastmilk Feeding Among Very Low Birth Weight Infants. Breastfeed. Med. 2011, 6, 15–19. [Google Scholar] [CrossRef]
- Hilditch, C.; Howes, A.; Dempster, N.; Keir, A. What evidence-based strategies have been shown to improve breastfeeding rates in preterm infants? J. Paediatr. Child Heal. 2019, 55, 907–914. [Google Scholar] [CrossRef]
- Zachariassen, G.; Faerk, J.; Grytter, C.; Esberg, B.; Juvonen, P.; Halken, S. Factors associated with successful establishment of breastfeeding in very preterm infants. Acta Paediatr. 2010, 99, 1000–1004. [Google Scholar] [CrossRef]
- Santiago, A.C.T.; da Cunha, L.P.M.; Vieira, N.S.A.; Moreira, L.M.O.; de Oliveira, P.R.; Lyra, P.P.R.; Alves, C.d.A.D. Breastfeeding in children born small for gestational age and future nutritional and metabolic outcomes: a systematic review. J. de Pediatr. 2018, 95, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Bellù, R.; Turoli, D.; De Nisi, G.; Tonetto, P.; Bertino, E. Presence of human milk bank is associated with elevated rate of exclusive breastfeeding in VLBW infants. jpme 2012, 41, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.; Embleton, N.D.; E Jacobs, S.; O'Connell, L.A.F.; A Kuschel, C. Enteral feeding practices in very preterm infants: an international survey. Arch. Dis. Child. - Fetal Neonatal Ed. 2011, 97, F56–F61. [Google Scholar] [CrossRef] [PubMed]
- Shlomai, N.O.; Patt, Y.S.; Wazana, Y.; Ziv-Baran, T.; Strauss, T.; Morag, I. Early Enteral Feeding of the Preterm Infant—Delay until Own Mother’s Breastmilk Becomes Available? (Israel, 2012–2017). Nutrients 2022, 14, 5035. [Google Scholar] [CrossRef]
- Maastrup, R.; Rom, A.L.; Walloee, S.; Sandfeld, H.B.; Kronborg, H. Improved exclusive breastfeeding rates in preterm infants after a neonatal nurse training program focusing on six breastfeeding-supportive clinical practices. PLOS ONE 2021, 16, e0245273. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





