Submitted:
01 May 2023
Posted:
02 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methodology
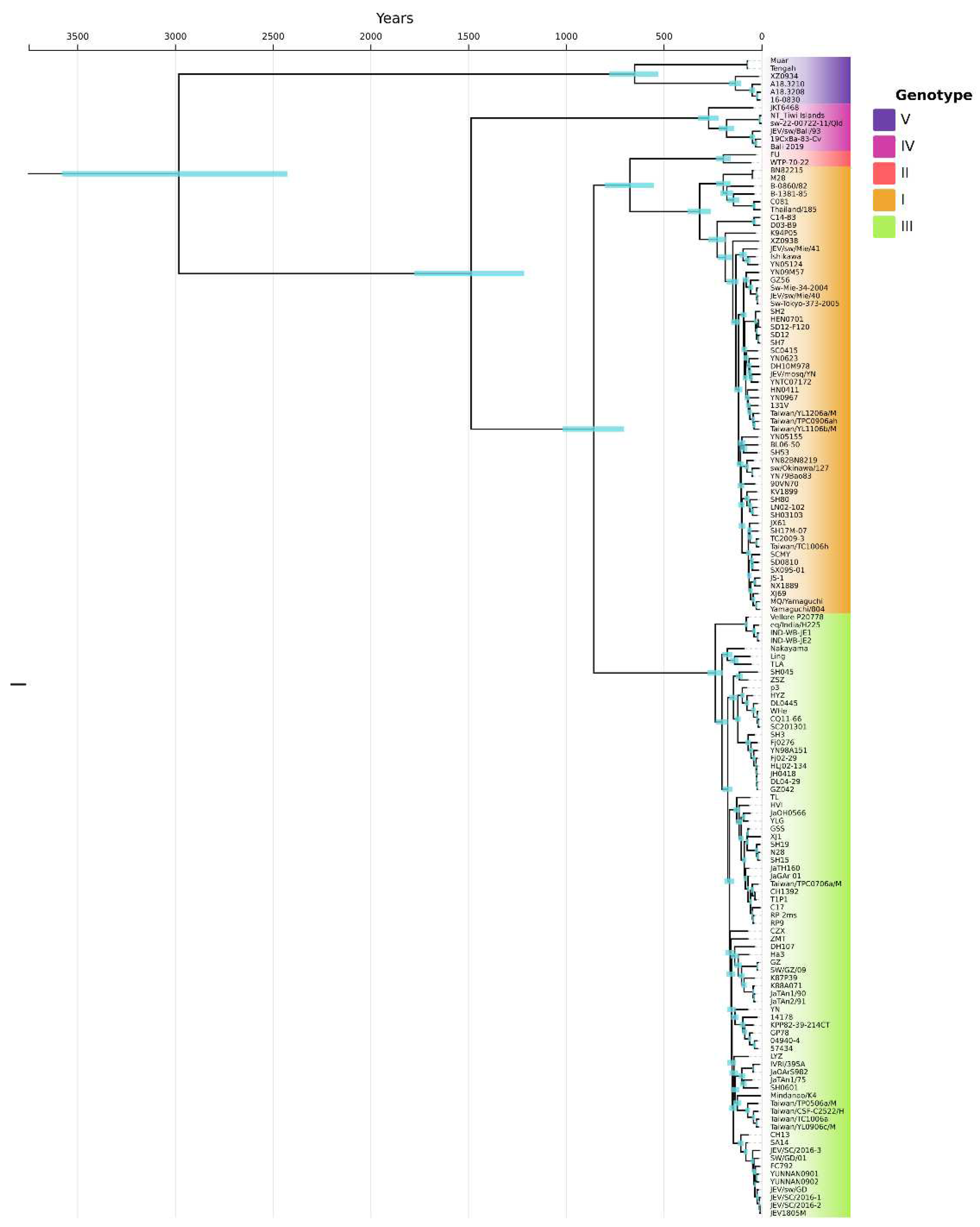
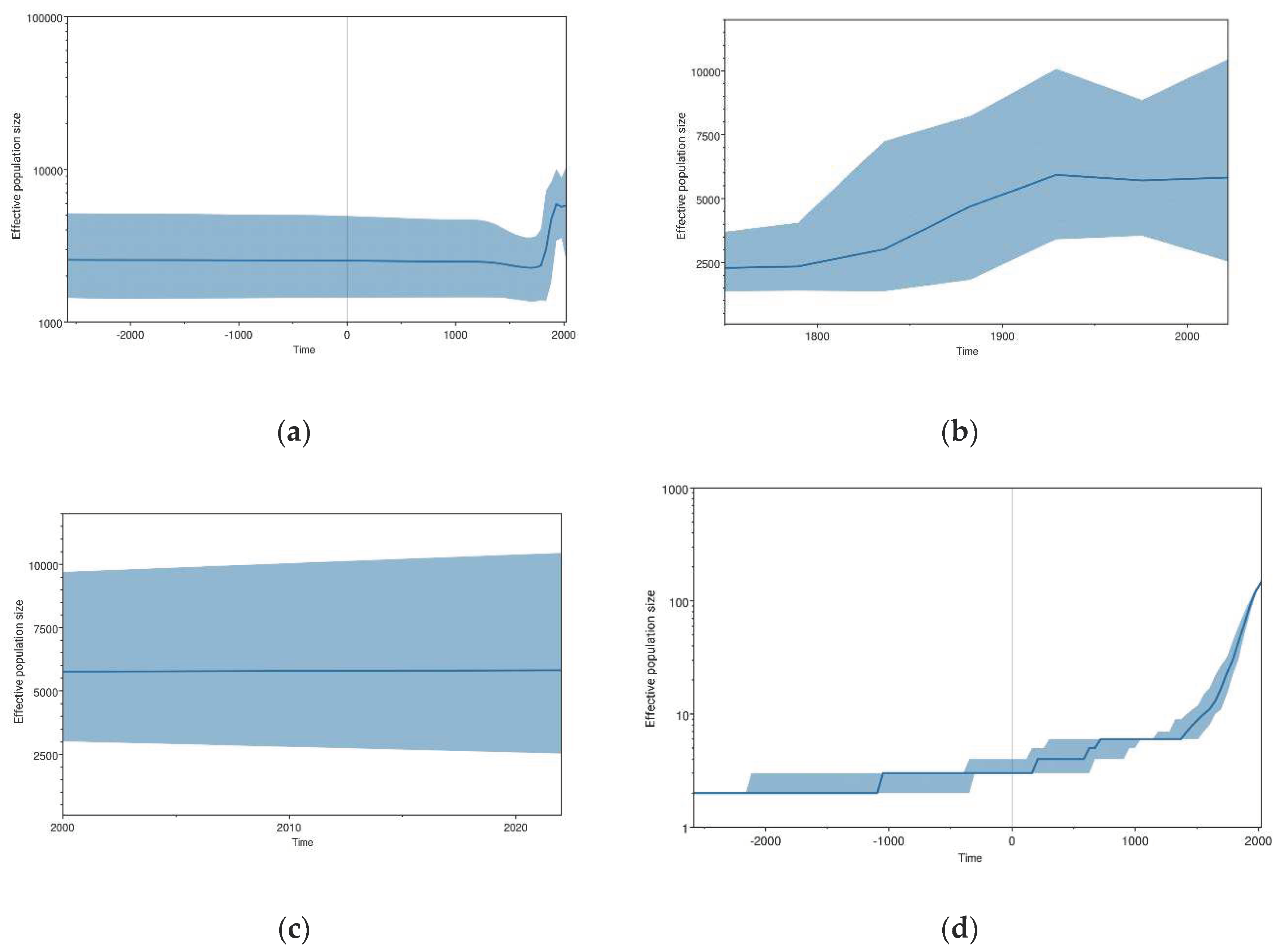
3. Results
4. Discussion
Supplementary Materials
Funding
Conflicts of Interest
Ethical Statement
References
- Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bulletin of the World Health Organization. 2011;89:766-74.
- Schuh AJ, Ward MJ, Brown AJL, Barrett AD. Phylogeography of Japanese encephalitis virus: genotype is associated with climate. PLoS neglected tropical diseases. 2013;7(8):e2411. [CrossRef]
- Hameed M, Liu K, Anwar MN, Wahaab A, Safdar A, Di D, et al. The emerged genotype I of Japanese encephalitis virus shows an infectivity similar to genotype III in Culex pipiens mosquitoes from China. PLoS neglected tropical diseases. 2019;13(9):e0007716. Epub 2019/09/27. PubMed PMID: 31557156; PubMed Central PMCID: PMCPMC6762057. [CrossRef]
- Hameed M, Wahaab A, Nawaz M, Khan S, Nazir J, Liu K, et al. Potential Role of Birds in Japanese Encephalitis Virus Zoonotic Transmission and Genotype Shift. Viruses. 2021;13(3). Epub 20210224. PubMed PMID: 33668224; PubMed Central PMCID: PMCPMC7996159. [CrossRef]
- Gould E, Solomon T. Pathogenic flaviviruses. The Lancet. 2008;371(9611):500-9. [CrossRef]
- Misra UK, Kalita J. Overview: japanese encephalitis. Progress in neurobiology. 2010;91(2):108-20. [CrossRef]
- Heffelfinger JD, Li X, Batmunkh N, Grabovac V, Diorditsa S, Liyanage JB, et al. Japanese Encephalitis Surveillance and Immunization - Asia and Western Pacific Regions, 2016. MMWR Morbidity and mortality weekly report. 2017;66(22):579-83. Epub 2017/06/09. PubMed PMID: 28594790; PubMed Central PMCID: PMCPMC5720240. [CrossRef]
- Gao X, Liu H, Li M, Fu S, Liang G. Insights into the evolutionary history of Japanese encephalitis virus (JEV) based on whole-genome sequences comprising the five genotypes. Virol J. 2015;12:43. Epub 2015/04/18. PubMed PMID: 25884184; PubMed Central PMCID: PMCPMC4369081. [CrossRef]
- Xu G, Gao T, Wang Z, Zhang J, Cui B, Shen X, et al. Re-Emerged Genotype IV of Japanese Encephalitis Virus Is the Youngest Virus in Evolution. Viruses. 2023;15(3). Epub 20230224. PubMed PMID: 36992335; PubMed Central PMCID: PMCPMC10054483. [CrossRef]
- Zhang H, Rehman MU, Li K, Luo H, Lan Y, Nabi F, et al. Epidemiologic Survey of Japanese Encephalitis Virus Infection, Tibet, China, 2015. Emerging infectious diseases. 2017;23(6):1023-4. Epub 2017/05/19. PubMed PMID: 28518046; PubMed Central PMCID: PMCPMC5443422. [CrossRef]
- Zhang H, Luo H, Ur Rehman M, Nabi F, Li K, Lan Y, et al. Evidence of JEV in Culex tritaeniorhynchus and pigs from high altitude regions of Tibet, China. Journal of vector borne diseases. 2017;54(1):69-73. Epub 2017/03/30. PubMed PMID: 28352048.
- Aure WE, Sayama Y, Saito-Obata M, Salazar NP, Malbas FF, Jr., Galang HO, et al. Japanese encephalitis virus genotype III from mosquitoes in Tarlac, Philippines. IJID Reg. 2022;4:59-65. Epub 20220516. PubMed PMID: 36093364; PubMed Central PMCID: PMCPMC9453045. [CrossRef]
- Howard-Jones AR, Pham D, Jeoffreys N, Eden JS, Hueston L, Kesson AM, et al. Emerging Genotype IV Japanese Encephalitis Virus Outbreak in New South Wales, Australia. Viruses. 2022;14(9). Epub 20220824. PubMed PMID: 36146660; PubMed Central PMCID: PMCPMC9505215. [CrossRef]
- Waller C, Tiemensma M, Currie BJ, Williams DT, Baird RW, Krause VL. Japanese Encephalitis in Australia - A Sentinel Case. N Engl J Med. 2022;387(7):661-2. PubMed PMID: 36070717. [CrossRef]
- Liu W, Fu S, Ma X, Chen X, Wu D, Zhou L, et al. An outbreak of Japanese encephalitis caused by genotype Ib Japanese encephalitis virus in China, 2018: A laboratory and field investigation. PLoS neglected tropical diseases. 2020;14(5):e0008312. Epub 2020/05/27. PubMed PMID: 32453787; PubMed Central PMCID: PMCPMC7274457. [CrossRef]
- Fang Y, Li XS, Zhang W, Xue JB, Wang JZ, Yin SQ, et al. Molecular epidemiology of mosquito-borne viruses at the China-Myanmar border: discovery of a potential epidemic focus of Japanese encephalitis. Infectious diseases of poverty. 2021;10(1):57. Epub 2021/04/28. PubMed PMID: 33902684; PubMed Central PMCID: PMCPMC8073957. [CrossRef]
- Hameed M, Wahaab A, Shan T, Wang X, Khan S, Di D, et al. A Metagenomic Analysis of Mosquito Virome Collected From Different Animal Farms at Yunnan-Myanmar Border of China. Frontiers in microbiology. 2020;11:591478. Epub 2021/02/26. PubMed PMID: 33628201; PubMed Central PMCID: PMCPMC7898981. [CrossRef]
- Hameed M, Khan S, Xu J, Zhang J, Wang X, Di D, et al. Detection of Japanese encephalitis virus in mosquitoes from Xinjiang during next-generation sequencing arboviral surveillance. Transbound Emerg Dis. 2021;68(2):467-76. Epub 20200815. PubMed PMID: 32614516. [CrossRef]
- Sanborn MA, Wuertz KM, Heung-Chul K, Yang Y, Li T, Pollett SD, et al. Identification of Japanese Encephalitis Virus Genotype V and Other Mosquito-borne Viruses in Camp Humphreys, Republic of Korea, using Metagenomic Analysis. bioRxiv. 2021.
- Mackenzie JS, Williams DT, van den Hurk AF, Smith DW, Currie BJ. Japanese Encephalitis Virus: The Emergence of Genotype IV in Australia and Its Potential Endemicity. Viruses. 2022;14(11). Epub 20221109. PubMed PMID: 36366578; PubMed Central PMCID: PMCPMC9698845. [CrossRef]
- van den Hurk AF, Skinner E, Ritchie SA, Mackenzie JS. The Emergence of Japanese Encephalitis Virus in Australia in 2022: Existing Knowledge of Mosquito Vectors. Viruses. 2022;14(6). Epub 20220602. PubMed PMID: 35746679; PubMed Central PMCID: PMCPMC9231386. [CrossRef]
- Hall TA, editor BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic acids symposium series; 1999: [London]: Information Retrieval Ltd., c1979-c2000.
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods. 2017;14(6):587-9. [CrossRef]
- Bouckaert R, Heled J, Kuhnert D, Vaughan T, Wu CH, Xie D, et al. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS computational biology. 2014;10(4):e1003537. Epub 2014/04/12. PubMed PMID: 24722319; PubMed Central PMCID: PMCPMC3985171. [CrossRef]
- Drummond AJ, Nicholls GK, Rodrigo AG, Solomon W. Estimating mutation parameters, population history and genealogy simultaneously from temporally spaced sequence data. Genetics. 2002;161(3):1307-20. Epub 2002/07/24. PubMed PMID: 12136032; PubMed Central PMCID: PMCPMC1462188.
- Hasegawa M, Kishino H, Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. Journal of molecular evolution. 1985;22(2):160-74. Epub 1985/01/01. PubMed PMID: 3934395. [CrossRef]
- Drummond AJ, Rambaut A, Shapiro B, Pybus OG. Bayesian coalescent inference of past population dynamics from molecular sequences. Molecular biology and evolution. 2005;22(5):1185-92. Epub 2005/02/11. PubMed PMID: 15703244. [CrossRef]
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic biology. 2018;67(5):901-4. [CrossRef]
- Lewis L, Taylor HG, et al. Japanese B encephalitis; clinical observations in an outbreak on Okinawa Shima. Archives of neurology and psychiatry. 1947;57(4):430-63. Epub 1947/04/01. PubMed PMID: 20297184.
- Gao X, Nasci R, Liang G. The neglected arboviral infections in mainland China. PLoS neglected tropical diseases. 2010;4(4):e624. [CrossRef]
- Yu Y. Phenotypic and genotypic characteristics of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and their stabilities. Vaccine. 2010;28(21):3635-41. [CrossRef]
- Pan XL, Liu H, Wang HY, Fu SH, Liu HZ, Zhang HL, et al. Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J Virol. 2011;85(19):9847-53. Epub 2011/06/24. PubMed PMID: 21697481; PubMed Central PMCID: PMCPMC3196406. [CrossRef]
- Huang YS, Higgs S, Vanlandingham DL. Emergence and re-emergence of mosquito-borne arboviruses. Curr Opin Virol. 2019;34:104-9. Epub 20190208. PubMed PMID: 30743191. [CrossRef]
- Peinado RDS, Eberle RJ, Arni RK, Coronado MA. A Review of Omics Studies on Arboviruses: Alphavirus, Orthobunyavirus and Phlebovirus. Viruses. 2022;14(10). Epub 20221005. PubMed PMID: 36298749; PubMed Central PMCID: PMCPMC9607206. [CrossRef]
- Duchêne S, Di Giallonardo F, Holmes EC. Substitution Model Adequacy and Assessing the Reliability of Estimates of Virus Evolutionary Rates and Time Scales. Mol Biol Evol. 2016;33(1):255-67. Epub 20150928. PubMed PMID: 26416981. [CrossRef]
- Añez G, Grinev A, Chancey C, Ball C, Akolkar N, Land KJ, et al. Evolutionary dynamics of West Nile virus in the United States, 1999-2011: phylogeny, selection pressure and evolutionary time-scale analysis. PLoS Negl Trop Dis. 2013;7(5):e2245. Epub 20130530. PubMed PMID: 23738027; PubMed Central PMCID: PMCPMC3667762. [CrossRef]
- Egyed L, Rónai Z, Dán Á. Hungarian tick-borne encephalitis viruses isolated from a 0.5-ha focus are closely related to Finnish strains. Ticks Tick Borne Dis. 2018;9(5):1064-8. Epub 20180407. PubMed PMID: 29655579. [CrossRef]
- Bryant JE, Holmes EC, Barrett AD. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 2007;3(5):e75. PubMed PMID: 17511518; PubMed Central PMCID: PMCPMC1868956. [CrossRef]
- Garjito TA, Widiarti, Anggraeni YM, Alfiah S, Tunggul Satoto TB, Farchanny A, et al. Japanese encephalitis in Indonesia: An update on epidemiology and transmission ecology. Acta Trop. 2018;187:240-7. Epub 20180815. PubMed PMID: 30118700. [CrossRef]
- Solomon T, Ni H, Beasley DW, Ekkelenkamp M, Cardosa MJ, Barrett AD. Origin and evolution of Japanese encephalitis virus in southeast Asia. J Virol. 2003;77(5):3091-8. PubMed PMID: 12584335; PubMed Central PMCID: PMCPMC149749. [CrossRef]
- Xiao C, Li C, Di D, Cappelle J, Liu L, Wang X, et al. Differential replication efficiencies between Japanese encephalitis virus genotype I and III in avian cultured cells and young domestic ducklings. PLoS neglected tropical diseases. 2018;12(12):e0007046. [CrossRef]
- Palm EC, Newman SH, Prosser DJ, Xiao X, Ze L, Batbayar N, et al. Mapping migratory flyways in Asia using dynamic Brownian bridge movement models. Movement ecology. 2015;3(1):3. Epub 2015/02/25. PubMed PMID: 25709838; PubMed Central PMCID: PMCPMC4337761. [CrossRef]
- Bae W, Kim JH, Kim J, Lee J, Hwang ES. Changes of epidemiological characteristics of Japanese encephalitis viral infection and birds as a potential viral transmitter in Korea. Journal of Korean medical science. 2018;33(9). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




