1. Introduction
Medicinal plants have been widely handled in traditional medicine for several centuries for the treatment of many health-related ailments [
1]. Hence, in low-income countries such as Burkina Faso, medicinal plants are used with significant results to treat many pathologies,
e.g., tumors, boils, or chronic wounds, especially in villages where modern health cares are totally inaccessible.
Cymbopogon schoenanthus (L.) Spreng (
C. schoenanthus) is a herbal plant of Southern Asia and Northen Africa, with fragrant foliage, suggesting leafs are enriched in essential oils (EO). This herbaceous plant is well known and used in Burkina Faso in traditional medicine, as well as in other African and Asian countries. Chemical characterization and
in vitro analyses of EO of
C. schoenanthus from various geographic area pointed out specific activities. EO extracted from the Sudanese plant has shown a high antiproliferative activity against human breast carcinoma and human colon adenocarcinoma cell lines [
2]. EO extracted from the Saudi Arabian plant has strong protective effects against
Escherichia coli,
Staphylococcus aureus,
Methicillin-susceptible S. aureus and
Klebsiella pneumoniae [
1]. The authors identified eight main components such as piperitone (14.6%), cyclohexanemethanol (11.6%), β-elemene (11.6%), α-eudesmol (11.5%), elemol (10.8%), β-eudesmol (8.5%), 2-naphthalenemethanol (7.1%) and γ-eudesmol (4.2%) [
1]. EO of
C. schoenanthus from Brazil presents an efficient anthelmintic activity, since the developmental of trichostrongylids obtained from naturally infected sheep is blocked
in vitro [
3]. Extracts from the Algerian plants shows strong antioxidant activities [
4,
5]. So far, only one study has been performed on EO of
C. schoenanthus from Burkina Faso [
6]. Sawadogo et al. focused their work on the putative antifungal activities. The authors studied the inhibition of both mycelial growth of
Aspergillus parasiticus and
Aspergillus flavus, and activity of aflatoxins B2 and G1.
Because EO of leafs of C. schoenanthus from Burkina Faso have been poorly studied so far, we have aimed to evaluate the chemical composition of this EO to investigate its biological activities on the survival characteristics of LNCaP and HeLa cells, respectively derived from prostate and cervical cancers. Effects on the cell cycle and migration were also investigated in LNCaP cells.
2. Results and discussion
2.1. Chemical composition of the essential oil
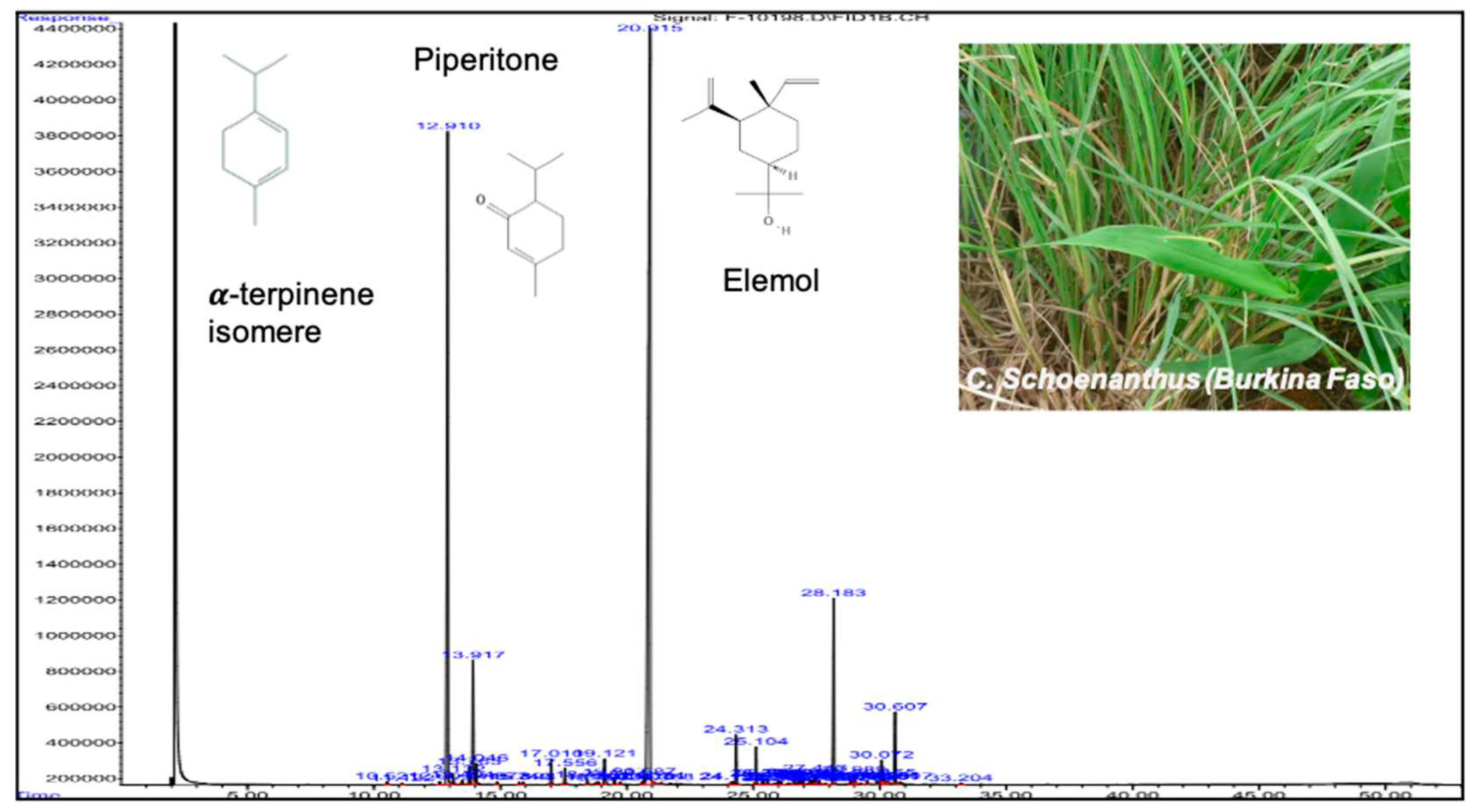
Gas chromatography coupled with a flame ionization detector and mass spectrometry (
Figure 1) identified 37 compounds within the EO of C. schoenanthus, for a percentage of 98.46% (
Table 1).
The main compounds were piperitone (49.9%), isomeric alpha terpinene (24.02%), elemol (5.79%) and limonene (4.31%), representing almost 84% of the total composition. Monoterpene ketones, such as piperitone, represent up to 50% of the compounds. The levels of piperitone, a compound generally used for the production of synthetic menthol and thymol, is in accordance with the other studies with 59.1% [
2] and 59.8% [
6]. Interestingly, other authors have described qualitatively and quantitatively different compositions for EO extracted from C. schoenanthus. Hashim et al. [
1] described that piperitone, cyclohexanemethanol, β-elemene, α-eudesmol and elemol were equally distributed in the EO. On the other side, Katiki et al. showed that the major compound quantified by gas chromatography was geraniol (62.5%) [
3]. We have hypothesized that these differences were due to the collection area and/or season.
2.2. Antoxidant potential of the essential oil
The need of antioxidant compounds is important as oxidation is a process strongly involved in many pathologies. Inhibitory activity of DPPH and ABTS+
. radicals proportionally reflects the antioxidant activity. Because, EO of C. schoenanthus was enriched in putative antioxidant molecules, inhibitions of the DPPH and ABTS
+. radicals were measured. Concentrations necessary to reach 50% of the inhibition (IC
50) were calculated as 1730 ±80 μg/mL and 2890 ± 26.9 µg/mL for DPPH and ABTS
+., respectively (
Table 2).
Compared to the Trolox used as a positive control, EO of C. schoenanthus is 900 and 300-fold less efficient on DPPH and ABTS+
. radicals, respectively, which let us to conclude that this EO has a really poor antioxidant activity. Interestingly, EO of C. schoenanthus from Sudan has also a weak antioxidant activity [
2], conversely to the extracts collected from Algeria [
7] and South Tunisia [
8]. The main differences between our EO and those described were the amount of cis- and trans-pmeth-2-en-1-ols (0.81 and 0.55% for our study, vs. 23 and 14%, respectively) in the Algerian EO [
7], and the amount of limonene (above 11% vs. 4% for the Burkina Faso) and α-terpineol (above 7% vs. 1% for the Burkina Faso) for the Tunisian EO [
8].
2.3. Cytotoxic activity of the essential oil
The imperfections of drugs currently used in therapy and the increasing problem of drug resistance have forced a search for new substances with therapeutic potential. Throughout history, numerous organisms have been rich sources of biologically active compounds [
9]. To investigate whether C. schoenanthus could be such a source, effects of EO of C. schoenanthus were evaluated on LNCaP (derived from metastatic prostate cancer) and HeLa (derived from cervical cancer) cell viability.
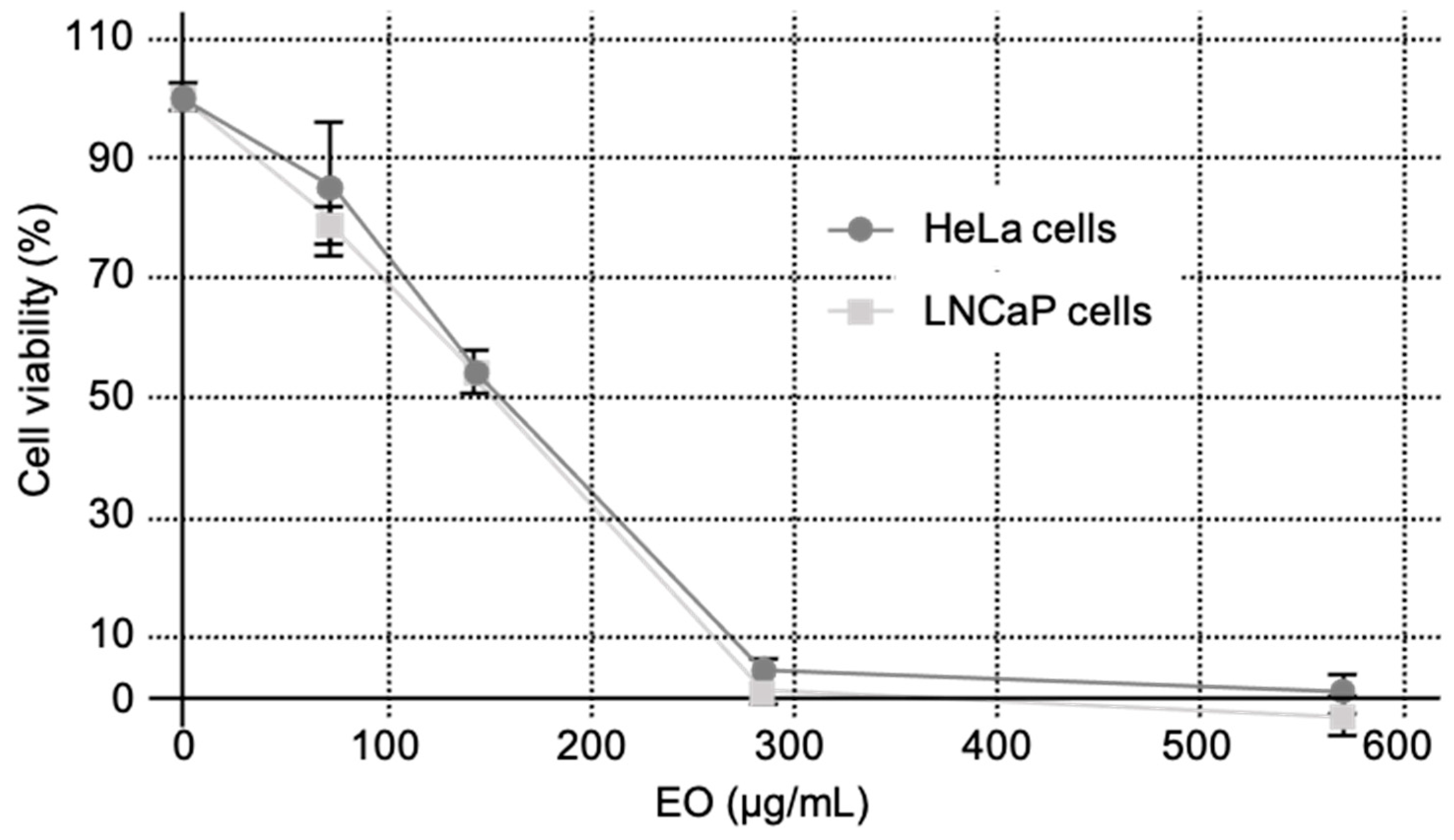
As shown in
Figure 2, EO from C. schoenanthus decreased the cell survival with reasonable IC
50s of 135.53 ± 5.27 μg/mL and 146.17 ± 11.83 μg/mL on LNCaP and HeLa cells, respectively (
Table 3), compared to the positive control cisplatine (3.2 ± 0.5 and 5.2 ± 0.8 μg/mL on the cells, respectively, p<0.001).
Mohamed Abdoul-Latif et al. have shown that EO of C. schoenanthus from Djibouti have a more significant cytotoxic effects [
10]. It should be noted that piperitone was totally absent in their EO, while
3-isopropenyl-5-methyl-1-cyclohexene and D-limonene were the main compound. Conversely, Hakkim et al. pointed that piperitone present at 39% in EO from Oman has a strong cytotoxic activity in triple negative breast cancer and cervical cancer cell lines [
11]. Piperitone representing almost 50 of the EO could be responsible of the results we obtained on LNCaP and HeLa cells. We have also associated the cytotoxic activity to the high amount of terpinenes, known to have anticancer properties [
12]. Monoterpene ketones (50.1%) and monoterpene hydrocarbons (29.73%) could also explain this activity. However, compared to cisplatin, used as control (
Table 3), EO of C. schoenanthus is less efficient on LNCaP (135 vs. 3 µg/mL) and HeLa (146 vs. 5 µg/mL) cells. Altogether, It should thus be interesting to test the cytotoxic activity of piperitone, the major compound identified in our study, alone or in combination with the other compounds.
2.4. Effects of essential oil on migration and cycle of LNCaP cells
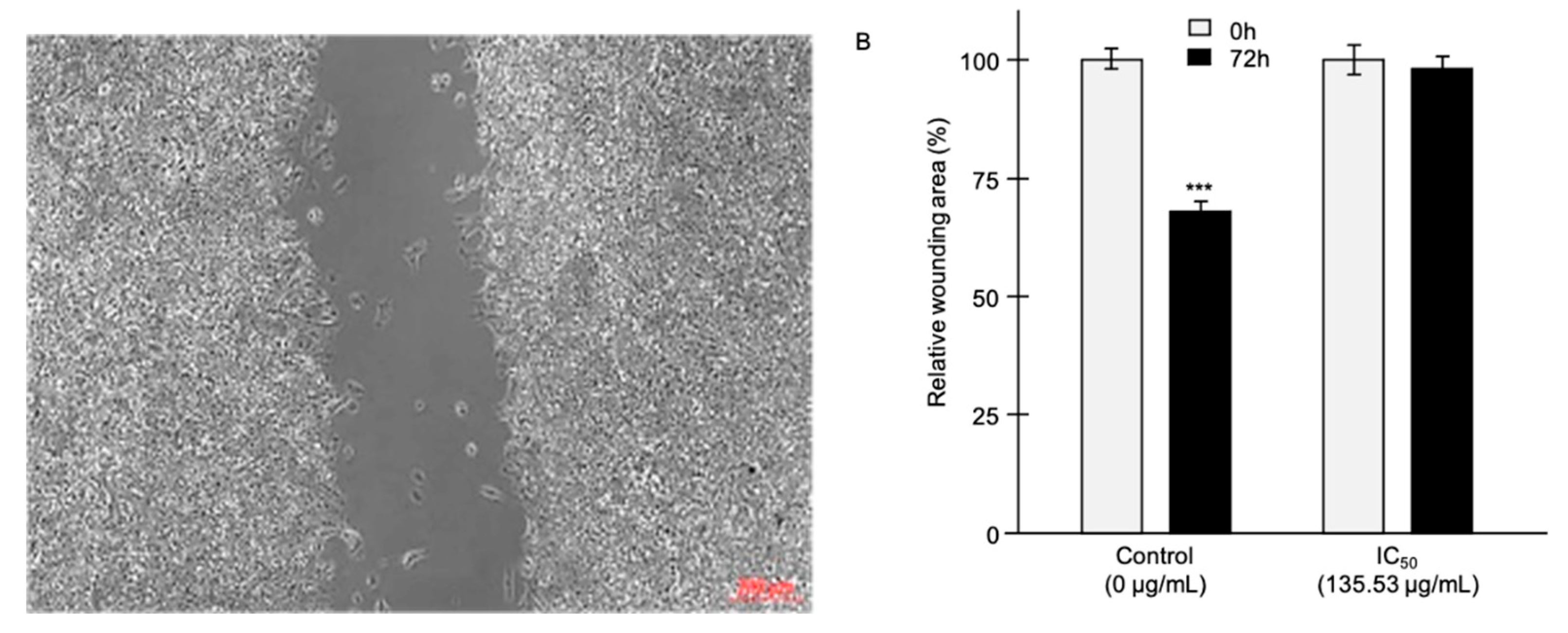
Killing cells and blocking their migration are important pharmacological targets in the clinical management of tumors [
13,
14]. As cell migration is a hallmark in tumor development, we performed scratch cell assays to measure the effects of the tested EO on the migration of the LNCaP cells (
Figure 3). While, the wounded area was decreased by 32% (p<0.001) after 72h, EO used at the IC
50 blocked the cell migration.
Cancer cells could also be characterized by a strong activity of division [
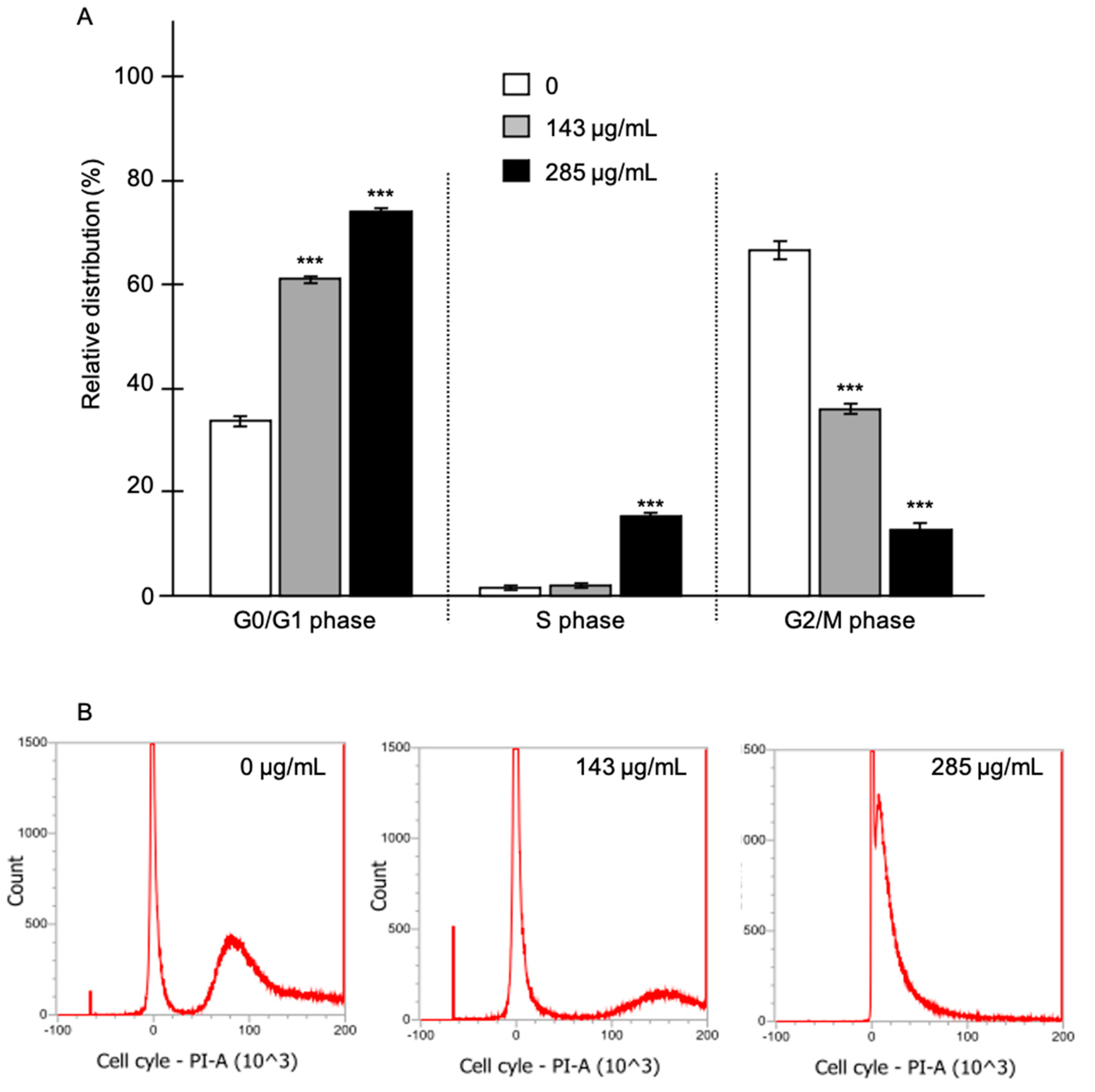
15]. Whether EO from C. schoenanthus could modify the cell cycle of LNCaP cells was also investigated. Our experiments enlightened a significant activity of EO on the distribution of the various phases of the cell cycle (
Figure 4). An increased accumulation of cells in the subG1 phase was observed together with a 2-fold increase in the G0/G1 (from 35% to 70%, for 0 and 285 μg/mL of EO, respectively) and S (from 2% to 13%, for 0 and 285 μg/mL of EO) phases. In parallel, a significant decrease in G2/M phase was shown in cells trated by 285 μg/mL of EO compared to the control cells (from 63% to 17%, p<0.01). It could thus be suggested that EO of C. Schoenanthus reduces the cell proliferation. Whether an early apoptosis is induced needs to be demonstrated.
3. Materials and Methods
3.1. Plant material and Essential Oil (EO) extraction
Leaves of
C. schoenanthus were collected at the National Institute of Applied Sciences and Technologies (IRSAT) in Ouagadougou, Burkina Faso (GPS location: 12°25’29,5’’N 1°29’14,3’’W). Identification and authentication were performed by Dr. A. SEREME (IRSAT / CNRST, Ouagadougou, Burkina Faso). A specimen was deposited in the herbarium of the Laboratory of Biology and Plant Ecology of University Joseph KI-ZERBO, publicly accessible under ID: 15936 and sample number 03 of September 2019. Fresh leaves of
C. schoenanthus (1 Kg) were submitted to hydrodistillation using an alembic / Clevenger-type apparatus for 3h as described previously [
16]. EO was stored in airtight containers in a refrigerator at 4
°C until GC-FID and GC/MS analyses and biological tests. EO was therefore diluted in hexane (1/30, v/v) for GC/FID analysis.
3.2. Chemical composition
Gas chromatography–flame ionization detector (GC/FID) analysis - Composition of EO was performed as previously mentioned [
17]. Briefly, gas chromatography of hexane-diluted EO was performed on an Agilent gas chromatograph Model 6890 (Agilent, Palo Alto, Ca), equipped with a DB5 MS column (30 m x 0.25 mm, 0.25 µm film thickness). Hyrdogene was used as carrier gas (flow 1.0 mL/min). Oven temperature program was 50
oC (5 min) to 300
oC with an increasing temperature of 5
oC/min. The sample (1 mL) was injected in split mode (1:60), injector and detector temperatures being respectively at 280 and 300
oC [
16].
Gas chromatography–mass spectrometry (GC/MS) analysis - Mass spectrometry analyses of EO were performed on an Agilent gas chromatograph Model 7890 coupled to an Agilent MS model 5975, equipped with a DB5 MS column (20 m x 0.20 mm, 0.20 mm film thickness), programming from 50°C (5 min) to 300°C at 8°C/min, 5 min hold, as described [
17]. Briefly, helium was used as carrier gas (average flow of 1.0 mL/min). Oven temperature program was from 50
oC (3.2 min) to 300
oC at 8
oC/min, 5 min post run at 300
oC. Sample (1µL) was injected in split mode (1:150), injector and detector temperature being at 250
oC and 280
oC, respectively [
16]. The MS worked in electron impact mode at 70eV; electron multiplier, 1500 V; ion source temperature, 230
oC. Mass spectra data were acquired in the scan mode in m/z range 33-450 [
16].
Identification of components - The main compounds in the EO of
C. schoenanthus were identified by comparison of their retention indices with those of the literature, determined in relation to a homologous series of n-alkanes (C8–C32) under the same operating conditions [
17]. In replace of standard compounds, we performed retention indices and comparisons with the NIST library [
18] or literature [
19]. Component relative percentages were calculated based on GC peak areas without using correction factors [
16,
20]. The major identified compounds are indicated on
Figure 1.
3.3. Cell culture
LNCaP cells, derived from prostate cancer (ATCC # CRL-1740), or Hela cells, derived from cervical cancer (ATCC # CCL-2), were used. These cell lines were available in the GReD Institute (Université Clermont Auvergne, France) [
21]. Cells were cultured and maintained at 37°C in a chamber moistened with 5% CO
2 in 75 cm
2 flasks in RPMI-1640 or DMEM medium (Invitrogen, Oslo, Norway) supplemented with 10% fetal calf serum (FCS, Biowest, Nuaillé, France), 1% penicillin and 1% streptomycin (Invitrogen).
3.4. Antioxidant activity
DPPH radical scavenging assay - As was already described [
17], each 100μL of serial dilutions EO of
C. schoenanthus starting at 3.8 mg/mL was mixed with 100μL of DPPH (30 mg/L in methanol) and incubated 30 min in darkness at room temperature. The absorbance was read at 517 nm against a blank (mixture without EO). Trolox (Sigma-Aldrich, L’Ile d’Abeau, France) was used as positive control. The radical scavenging activity was calculated according to the formula (absorbance blank-absorbance sample)/absorbance blank x 100. The concentration of extract able of scavenging 50% of the DPPH radicals was then determined graphically and expressed as μg of EO / μg of DPPH [
21].
ABTS
+. radical cation decolorization assay - The spectrophotometric analysis of ABTS
+. scavenging activity was performed using 96-well plates with 50μL of ethanolic solution of EO of
C. schoenanthus at an initial concentration of 3.8 mg/mL, added to 200μL of freshly prepared ABTS
+. solution, as previously described [
17,
21]. Briefly, ABTS
+. solution was prepared by dissolving 10 mg of ABTS in 2.6 mL of distilled water in which 1.7212 mg of potassium persulfate was added. ABTS was then incubated at room temperature for 12h and diluted with ethanol in order to obtain an absorbance of 0.70±0.02 to 734 nm. Trolox (Trolox, Sigma-Aldrich) was used as a positive control. The 96-well plates were then incubated in the dark at room temperature for 15 min and the absorbance read at 734 nm. The activity of EO of
C. schoenanthus on the radical cation ABTS
+. was expressed in micromoles Trolox equivalent per gram of EO (µmol TE g
-1) [
21].
3.5. Measurement of cell survival
3[4,5-dimethylthiazol-2-yl]-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich) was used to measure the mitochondrial activity, which reflects the number of viable cells [
16,
17]. Briefly, 12500 cells were seeded for 24 h in 96-well plates. EO of
C. schoenanthus was added at various concentrations for 72 h. The number of living cells is directly proportional to the intensity of the violet color measured quantitatively by spectrophotometry using a microplate reader spectrophotometer Thermo Fisher Scientific SN 1510-02948 at 570 nm. Three independent experiments were performed in octuplicate for each cell line.
3.6. Measurement of cell survival
The effects of EO of C. schoenanthus on the migration of LNCaP and HeLa cells were evaluated by in vitro wound healing assay. Cells were seeded in 6-well plates at a rate of 35x104 cells per well in a final volume of 2 ml of complete medium for 24 h. A scratch was performed on the cell monolayer with a sterile micropipette tip. Detached cells were then removed with phosphate buffered saline 1X (Life Technologies). Cells were next grown in medium containing EO of C. schoenanthus at the concentration corresponding to the IC50. Multiple images were then taken for 72 hours by Zeiss AxioObserver 7 inverted microscope equipped with a Colibri 7 LED source, which made possible to determine the effect of the EO on LNCaP cells migration.
3.7. Flow cytometry analysis
LNCaP cells (3x105) were seeded in 6-well dishes and treated with EO after 24 hours at the dose of 0 µg/mL (control; DMSO, 0.001), 143 µg/mL and 285 µg/mL respectively for 72 hours at 37°C. After the treatment, cells were harvested with trypsine, centrifuged and fixed with paraformaldehyde (4%) for 15 min at room temperature and then washed with PBS. 106 cells were prepared in suspension, centrifuged and the supernatant removed. Then, 0.2 ml of FxCycle ™ PI/RNase staining solution (Invitrogen, Oregon, USA) was added to each tube and mixed. The samples were incubated for 30 min at room temperature, protected from light, and then analyzed by FACS using excitation at 488 nm and the emissions were collected using a 585/42 bandpass filter.
3.8. Statistical analysis
The data were analyzed by analysis of variance followed by the Tukey multiple comparison test. The analyses were performed using XLSTAT 7.1 software. P < 0.05 was used as a criterion for statistical significance.
5. Conclusion
Our work demonstrates for the time the effects of EO of C. Schoenanthus from Burkina Faso on LNCaP and HeLa cells. The chemical composition points out the richness of EO in piperitone and terpenes. While the antioxidant capacity of the EO is quite low, cytotoxic activities on LNCaP and HeLa cells are significant. Besides, EO also prevents the migration of LNCaP cells and leads to the arrest of their cell cycle in the G2/M phase. Altogether, this work constitutes a scientific basis for the use of EO of C. schoenanthus from Burkina Faso for the traditional management of tumors.
Author Contributions
BB and JML designed the research; BB and LLC performed chemical synthesis and analysis; the experiments and analyzed the data. BB, LLC, FD, JS, JBN and JML wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by The World Academy of Sciences (TWAS) for the advancement of science in developing according to references No. 18-073 RG/BIO/AF/AC_I – FR3240303631; and Plan National de Recherche sur les Perturbateurs Endocriniens (13-MRES-PNRPE-1-CVS043).
Data Availability Statement
Datasets generated during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Sample Availability
Samples of the compounds analyzed in this study are available from the authors.
References
- Hashim, G.M.; Almasaudi, S.B.; Azhar, E.; Al Jaouni, S.K.; Harakeh, S. Biological Activity of Cymbopogon Schoenanthus Essential Oil. Saudi J. Biol. Sci. 2017, 24, 1458–1464. [CrossRef]
- Yagi, S.; Mohammed, A.B.A.; Tzanova, T.; Schohn, H.; Abdelgadir, H.; Stefanucci, A.; Mollica, A.; Zengin, G. Chemical Profile, Antiproliferative, Antioxidant, and Enzyme Inhibition Activities and Docking Studies of Cymbopogon Schoenanthus (L.) Spreng. and Cymbopogon Nervatus (Hochst.) Chiov. from Sudan. J. Food Biochem. 2020, 44, e13107. [CrossRef]
- Katiki, L.M.; Chagas, A.C.S.; Bizzo, H.R.; Ferreira, J.F.S.; Amarante, A.F.T. Anthelmintic Activity of Cymbopogon Martinii, Cymbopogon Schoenanthus and Mentha Piperita Essential Oils Evaluated in Four Different in Vitro Tests. Vet. Parasitol. 2011, 183, 103–108. [CrossRef]
- Aous, W.; Benchabane, O.; Outaleb, T.; Hazzit, M.; Mouhouche, F.; Yekkour, A.; Baaliouamer, A. Essential Oils of Cymbopogon Schoenanthus (L.) Spreng. from Algerian Sahara: Chemical Variability, Antioxidant, Antimicrobial and Insecticidal Properties. J. Essent. Oil Res. 2019, 31, 562–572. [CrossRef]
- Malti, C.E.W.; El Haci, I.A.; Hassani, F.; Paoli, M.; Gibernau, M.; Tomi, F.; Casanova, J.; Bekhechi, C. Composition, Chemical Variability and Biological Activity of Cymbopogon Schoenanthus Essential Oil from Central Algeria. Chem. Biodivers. 2020, 17, e2000138. [CrossRef]
- Sawadogo, I.; Paré, A.; Kaboré, D.; Montet, D.; Durand, N.; Bouajila, J.; Zida, E.P.; Sawadogo-Lingani, H.; Nikiéma, P.A.; Nebié, R.H.C.; et al. Antifungal and Antiaflatoxinogenic Effects of Cymbopogon Citratus, Cymbopogon Nardus, and Cymbopogon Schoenanthus Essential Oils Alone and in Combination. J. Fungi 2022, 8, 117. [CrossRef]
- Khadri, A.; Neffati, M.; Smiti, S.; Falé, P.; Lino, A.R.L.; Serralheiro, M.L.M.; Araújo, M.E.M. Antioxidant, Antiacetylcholinesterase and Antimicrobial Activities of Cymbopogon Schoenanthus L. Spreng (Lemon Grass) from Tunisia. LWT - Food Sci. Technol. 2010, 43, 331–336. [CrossRef]
- Khadri, A.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Neffati, M.; Smiti, S.; Araújo, M.E.M. Antioxidant and Antiacetylcholinesterase Activities of Essential Oils from Cymbopogon Schoenanthus L. Spreng. Determination of Chemical Composition by GC–Mass Spectrometry and 13C NMR. Food Chem. 2008, 109, 630–637. [CrossRef]
- Godzieba, M.; Ciesielski, S. Natural DNA Intercalators as Promising Therapeutics for Cancer and Infectious Diseases. Curr. Cancer Drug Targets 2019. [CrossRef]
- Mohamed Abdoul-Latif, F.; Elmi, A.; Merito, A.; Nour, M.; Risler, A.; Ainane, A.; Bignon, J.; Ainane, T. Chemical Analysis of Essential Oils of Cymbopogon Schoenanthus (L.) Spreng. and Nepeta Azurea R.Br. Ex Benth from Djbouti, In-Vitro Cytotoxicity against Cancer Cell Lines and Antibacterial Activities. Appl. Sci. 2022, 12, 8699. [CrossRef]
- Hakkim, F.; Al-Buloshi, M.; Achankunju, J. Growth Inhibitory Effect of Cymbopogan Schoenanthus on Triple Negative Breast Cancer (MDA-MB-231) and Cervical Cancer (HEp-2) Cells: Piperitone and Elemol as an Active Principle. Austin Journal of Medical Oncology 2016, 3, 1027.
- Assmann, C.E.; Cadoná, F.C.; Bonadiman, B. da S.R.; Dornelles, E.B.; Trevisan, G.; Cruz, I.B.M. da Tea Tree Oil Presents in Vitro Antitumor Activity on Breast Cancer Cells without Cytotoxic Effects on Fibroblasts and on Peripheral Blood Mononuclear Cells. Biomed. Pharmacother. Biomedecine Pharmacother. 2018, 103, 1253–1261. [CrossRef]
- Prager, I.; Watzl, C. Mechanisms of Natural Killer Cell-Mediated Cellular Cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [CrossRef]
- Kobelt, D.; Dahlmann, M.; Dumbani, M.; Güllü, N.; Kortüm, B.; Vílchez, M.E.A.; Stein, U.; Walther, W. Small Ones to Fight a Big Problem-Intervention of Cancer Metastasis by Small Molecules. Cancers 2020, 12, E1454. [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell Cycle Control in Cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [CrossRef]
- Bayala, B.; Bassole, I.H.N.; Maqdasy, S.; Baron, S.; Simpore, J.; Lobaccaro, J.-M.A. Cymbopogon Citratus and Cymbopogon Giganteus Essential Oils Have Cytotoxic Effects on Tumor Cell Cultures. Identification of Citral as a New Putative Anti-Proliferative Molecule. Biochimie 2018, 153, 162–170. [CrossRef]
- Bayala, B.; Bassole, I.H.N.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Figueredo, G.; Nikiema, J.-B.; Lobaccaro, J.-M.A.; Simpore, J. Chemical Composition, Antioxidant, Anti-Inflammatory and Anti-Proliferative Activities of Essential Oils of Plants from Burkina Faso. PloS One 2014, 9, e92122. [CrossRef]
- Stein, S.; Mirokhin, D.; Tchekhovskoi, D.; Mallard, G. The NIST Mass Spectral Search Program for the NIST/EPA/NIH Mass Spectra Library, Standard Refer- Ence Data Program of the National Institute of Standards and Technology, Gaithersburg, MD, USA. Addict. Abingdon Engl. 2002.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, Allured Publishing, Carol Stream, IL, USA,. 2007, 804. [CrossRef]
- Bayala, B.; Coulibaly, A.Y.; Djigma, F.W.; Nagalo, B.M.; Baron, S.; Figueredo, G.; Lobaccaro, J.-M.A.; Simpore, J. Chemical Composition, Antioxidant, Anti-Inflammatory and Antiproliferative Activities of the Essential Oil of Cymbopogon Nardus, a Plant Used in Traditional Medicine. Biomol. Concepts 2020, 11, 86–96. [CrossRef]
- Bayala, B.; Nadembega, C.; Guenné, S.; Buñay, J.; Mahoukèdè Zohoncon, T.; Wendkuuni Djigma, F.; Yonli, A.; Baron, S.; Figueredo, G.; A Lobaccaro, J.-M.; et al. Chemical Composition, Antioxidant and Cytotoxic Activities of Hyptis Suaveolens (L.) Poit. Essential Oil on Prostate and Cervical Cancers Cells. Pak. J. Biol. Sci. PJBS 2020, 23, 1184–1192. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).