1. Introduction
Aedes (Ae.) aegypti mosquitoes are the main vector species of dengue viruses worldwide. The incidence of this disease has increased dramatically worldwide over the past decades. The number of dengue fever cases reported to the World Health Organization (WHO) has increased by more than eightfold over the previous twenty years, from 505,430 cases in 2000 to over 2.4 million cases in 2010 and 5.2 million cases in 2019 [
1].
Ae. aegypti is present in Benin and proliferating globally due to the development of trade, rapid urbanization, and high frequency of international travel [
2]. Between 2010 and 2019, dengue fever cases have been diagnosed in Benin, resulting in at least one death [
2].
In the absence of effective vaccines and available treatments, vector control remains the main strategy for dengue virus prevention. Vector control relies on the use of insecticides, such as pyrethroids, for house spraying and personal protection [
3]. As a result of the strong selection pressures exerted by the use of insecticides in agricultural practices and for malaria control, insecticide resistance among
Ae. aegypti populations is common and widespread worldwide. According to the WHO, insecticide resistance is defined as “the ability of mosquitoes to survive exposure to a standard dose of insecticide; this ability may result from physiological or behavioural adaptation”. Two of the main mechanisms underlying insecticide resistance in mosquitoes are alterations in the insecticide target site, including knock-down resistance (
kdr) mutations in the voltage-gated sodium channel (
vgsc) gene and increased metabolic activity [
4,
5]. Metabolic resistance to pyrethroids is mediated by glutathione
S-transferases (GSTs), esterases and mixed-function oxidases (MFOs) [
6,
7,
8].
Kdr mutations have been studied in
Ae. aegypti extensively; these mutations confer cross-resistance to pyrethroids and DDT by altering the structure of the
vgsc, thus decreasing the affinity of target insecticides to bind [
9]. Several
kdr mutations have been identified in
Ae. aegypti populations worldwide, including V1016I, V410L, S989P, I1011V, V1016G, I1011M and F1534C [
9,
10,
11]. In Africa, F1534C, V1016I, V410L and S989P have been associated with pyrethroid resistance in
Ae. aegypti [
12,
13,
14,
15,
16].
The emergence and re-emergence of dengue fever epidemics requires effective vector control responses, including monitoring of vector population insecticide susceptibility. To date, there is a considerable paucity of insecticide resistance information for Ae. aegypti populations in Benin.
2. Materials and Methods
2.1. Study sites and mosquito sampling
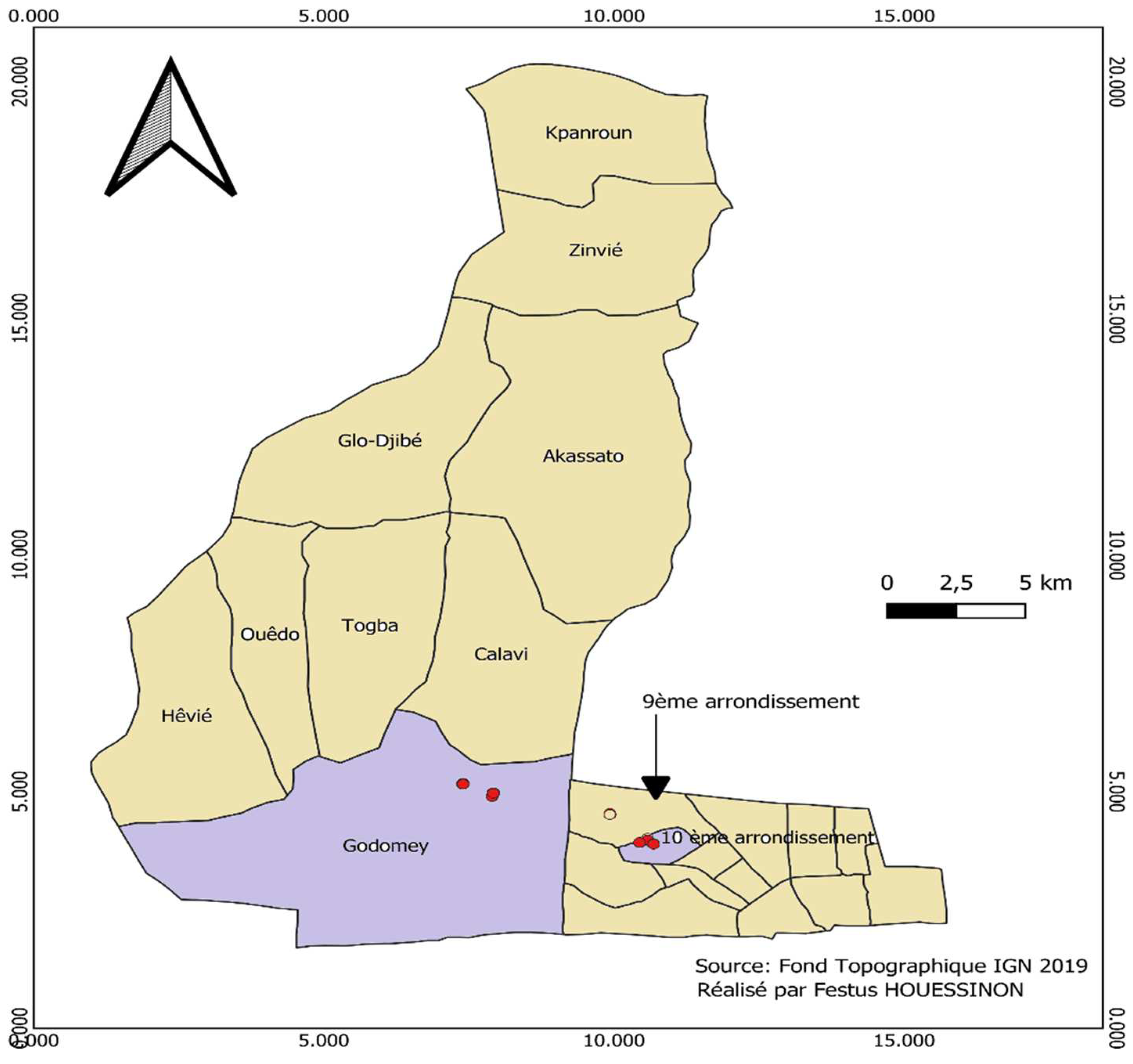
Adult
Ae. aegypti mosquitoes were reared from eggs collected over a 2-week period in the 10
ème arrondissement of Cotonou and in Godomey-Togoudo, Abomey-Calavi from 31st December 2021 (
Figure 1). This collection period coincided with the dry season, which is a period characterized by the scarcity of
Aedes breeding sites. During this sampling period a total of 73 gravid
Aedes traps were set in the 10
ème arrondissement of Cotonou and 74 in Godomey-Togoudo. The traps used are black plastic pots that can contain half a liter of water, in which wooden egg-laying supports have been immersed. The traps were hung on the box or wall with a nail and a wire. The eggs carriers were removed from each trap after 7 days, eggs were counted and then hatched according to standard insectary rearing procedures for
Aedes species. Larval hatching rate was measured by visually counting the number of larvae per bowl using a ladle. Adult emergence rate was measured by an aspirator.
2.2. Insecticide resistance bioassays
Insecticide resistance profiles for
Ae. aegypti field populations were measured using Centers for Disease Control and Prevention (CDC) bottle bioassays [
17]. Two-to-five-day old female
Aedes mosquitoes were exposed to the diagnostic doses of deltamethrin (10 µg/bottle) and permethrin (10 µg/bottle) and bendiocarb (12.5 µg/bottle) in Wheaton 250 ml bottles, alongside an acetone-treated control bottle. In each bioassay, 10-25 unfed mosquitoes were introduced into each bottle and knock-down was scored every 15 minutes until all were dead, or up to two hours.
2.3. Aedes morphological identification
Adult
Aedes spp. tested in insecticide resistance bioassays were identified using Fontenille’s taxonomic keys [
18] by visualisation under a dissecting microscope.
Ae. aegypti and
Ae. albopictus can be recognised by their characteristic white stripes on their legs. Then the thorax can be used to differentiate the two species;
Ae. aegypti has two thin white median lines with a lyre pattern, whereas
Ae. albopictus has only one distinct white central line.
2.4. Measurement of detoxification enzyme activity
Biochemical tests were performed to quantify the activity of families of detoxification enzymes, including non-specific esterases (α and β esterases), mixed-function oxidases (MFOs), glutathione S-transferases (GSTs) and total proteins in 3-5-day old female Aedes mosquitoes; a total of 80 non-insecticide exposed mosquitoes were tested per study site.
Individual mosquitoes were ground in 200 µl of distilled water using sterile pestles, after which the lysate was centrifuged at 14,000 rpm for two minutes. For non-specific esterases, per mosquito, 10 µl of lysate was added to two wells (technical duplicate) of a 96-well ELISA plate, followed by 90 µl of 1% Triton Phosphate Saline (TBS) buffer was added. The plate was then incubated at 25 °C for 10 minutes, after which 100 µl of a solution of 500 µl α-naphthyl acetate or β-naphthyl acetate + 2.5 ml 1X Triton PBS buffer (pH 6.5) + 7 ml H2O, was added to each well and the plate was again incubated at 25 °C for 30 minutes. Finally, 100 µl of a solution of 8 mg Fast Garnett Salt dissolved in 12 ml distilled water, was added to each well and the plate was incubated at 25 °C for 10 minutes. Plate absorbance values were read on an ELx808 spectrophotometer at 550 nm. Esterase activity of each mosquito was expressed as µmol α- or β-naphthol produced/min/mg protein and calculated according to: α- or β-naphthol in µmol per ml/amount of protein in mg per ml/30.
For MFOs, 20 µl of mosquito lysate was added to two wells (technical duplicate) of a 96-well ELISA plate, followed by 80 µl of 0.0625M Potassium Phosphate Buffer (KHPO4; pH 7.2), then 200 µl of a solution of 10 mg of 3,3,5,5-tetramethylbenzidine or TMBZ in 4 ml ethanol, and 15 µl of sodium acetate buffer. After adding 25 µl of 3% hydrogen peroxide, the plate was incubated for 30 minutes, and plate absorbance values were read on an ELx808 spectrophotometer at 630 nm. Oxidase activity of each mosquito was expressed as nmol P450/mg protein and calculated according to: nmol P450/2*amount of protein in mg in 10 µl of lysate.
For GSTs, 10 µl of mosquito lysate was added to two wells (technical duplicate) of a 96-well ELISA plate, followed by 200 µl of a solution of 60 mg of reduced glutathione (GSH), 20 ml of 0.1M Sodium Phosphate Buffer (pH 6.5) and 13 mg of CDNB (1-chloro-2,4-dinitrobenzene) dissolved in 1 ml of methanol. Plate absorbance values were read kinetically on an ELx808 spectrophotometer at 340 nm for 5 minutes. GST activity of each mosquito was expressed as nmol GSH conjugate/min/mg of protein and calculated according to: (MilliDO*0.21)/(5.76*1000)/(amount of protein in mg in 10 µl of lysate.
For total protein, 10 µl of mosquito lysate was added to two wells (technical duplicate) of a 96-well ELISA plate, followed by 290 µl of a solution of Coomassie Plus Protein Assay diluted by half in distilled water. Plate absorbance values were read on an ELx808 spectrophotometer at 590 nm after incubating the plate for 5 minutes at 25 °C.
2.5. Ae. aegypti kdr genotyping
After grinding each mosquito in 200μl of 2% CTAB, it is then placed in a water bath or heating block at 65 °C for 05 minutes. Thereafter 200μL of chloroform is added and centrifuged at 12,000 rpm for 05 minutes at room temperature after mixing by inversion at least 10 times. The supernatant collected in well-labeled tubes is mixed with 200μl of isopropanol and centrifuged at 12,000 rpm at room temperature for 10 minutes. The isopropanol is drained and centrifuged for 05 minutes at 12,000 rpm after adding 200μl of 70% ethanol. After emptying the ethanol the resulting DNA pellet is dried for 05 minutes at speed-va or half a day on the bench. We add 140μl of sterile H2O to the DNA pellets using a new cone for each tube which is left hanging on the bench overnight or half a day.
An allele-specific PCR (AS-PCR) was used to detect the presence of S989P, V1016G and F1534C kdr mutations. Each individual mosquito was tested by AS-PCR twice, the first PCR used a primer specific to the susceptible wild-type and the second PCR used a primer specific to the mutant. The primers used for the genotyping were: S989PF: 5′AATGATATTAACAAAATTGCGC3′ and S989PR: 5′GCACGCCTCTAATATTGATGC; V1016GF: 5′GCCACCGTAGTGATAGGAAATC3′ and V1016GVal-R: 5′CGGGTTAAGTTTCGTTTAGTAGC3′; F1534CF: 5′GGAGAACTACACGTGGGAGAAC3′ and F1534CR:5′CGCCACTGAAATTGAGAATAGC3′.
Each reaction was performed in a final volume of 25 µl containing 2.5 µl of 10X buffer, 1.5 µl of MgCl2, 1 µl of dNTPs, 2.5 µl of forward primer, 2.5 µl of reverse primer, 2.5 µl of the susceptible or mutant primer, 0.2 µl of Taq polymerase, 6.4 µl pf H2O and 6 µl of gDNA. PCR reaction conditions were: one cycle at 94 °C for 3 minutes, then 35 cycles of 94 °C for 30 seconds, 60 °C (for F1534C and V1016G) or 62 °C (for S989P) for 30 seconds and 72 °C for 1 minute, followed by one cycle of 72 °C for 7 minutes.
PCR products were separated by gel electrophoresis in a 2% agarose gel, stained with ethidium bromide. PCR amplicon sizes for the detection of kdr mutations were 240 bp (S989P), 284 bp (F1534C) or 348 bp (V1016G). Non-allele specific external primers produced bands of 594 bp (S989P), 517 bp (F1534C) or 592 bp (V1016G).
2.6. Data analysis
Global Positioning System (GPS) coordinates of the gravid Aedes traps were recorded using the OSM Tracker for Android application. Insecticide susceptibility test results were recorded and analysed using Microsoft Excel 2016. Biochemical assay data were recorded and analysed using GraphPad Prism 5 software. All statistical analyses were conducted in Stata/SE 17.0, including Pearson’s Chi-squared test to investigate deviations from Hardy-Weinberg equilibrium.
3. Results
3.1. Mosquito sampling
A total of 147 gravid Aedes traps were used in the two study areas, of which 142 were positive (i.e., female Aedes oviposited in them); yielding an attractiveness rate of 97%. The total number of eggs obtained was 15,844; 8846 eggs in the 10ème arrondissement of Cotonou and 6998 eggs in Godomey-Togoudo.
Of 8846 eggs obtained in the 10ème arrondissement of Cotonou, 3154 hatched (35%) and 1240 adult Aedes mosquitoes emerged (39%). Of 6998 eggs obtained in Godomey-Togoudo, 4918 eggs hatched (70%) and 2486 adult Aedes mosquitoes emerged (50%).
Of the 3726 total mosquitoes that emerged, 3723 were Ae. aegypti (>99%), while 3 were Ae. albopictus (<1%). One of the 3 Ae.albopictus comes from the 10ème arrondissement of Cotonou and the others from Godomey-Togoudo.
3.2. Insecticide resistance profiles
High resistance to permethrin and deltamethrin was evident in both populations of Ae. aegypti. Mosquito mortality was 85.7% and 82.7%, following exposure to the diagnostic dose of deltamethrin or permethrin after 30 mins, respectively, in Godomey-Togoudo. Similarly, in the 10ème arrondissement of Cotonou, mortality was 88% with both deltamethrin and permethrin. By comparison, complete susceptibility to bendiocarb was observed in both vector populations.
3.3. Expression of detoxification enzymes
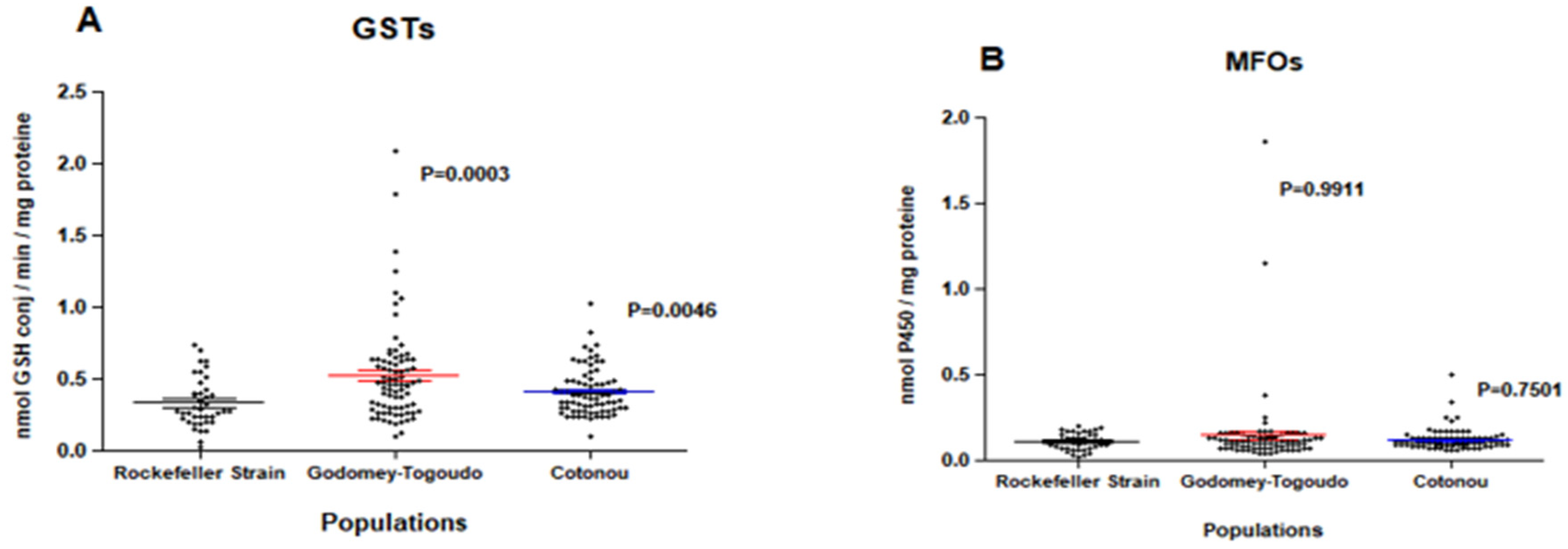
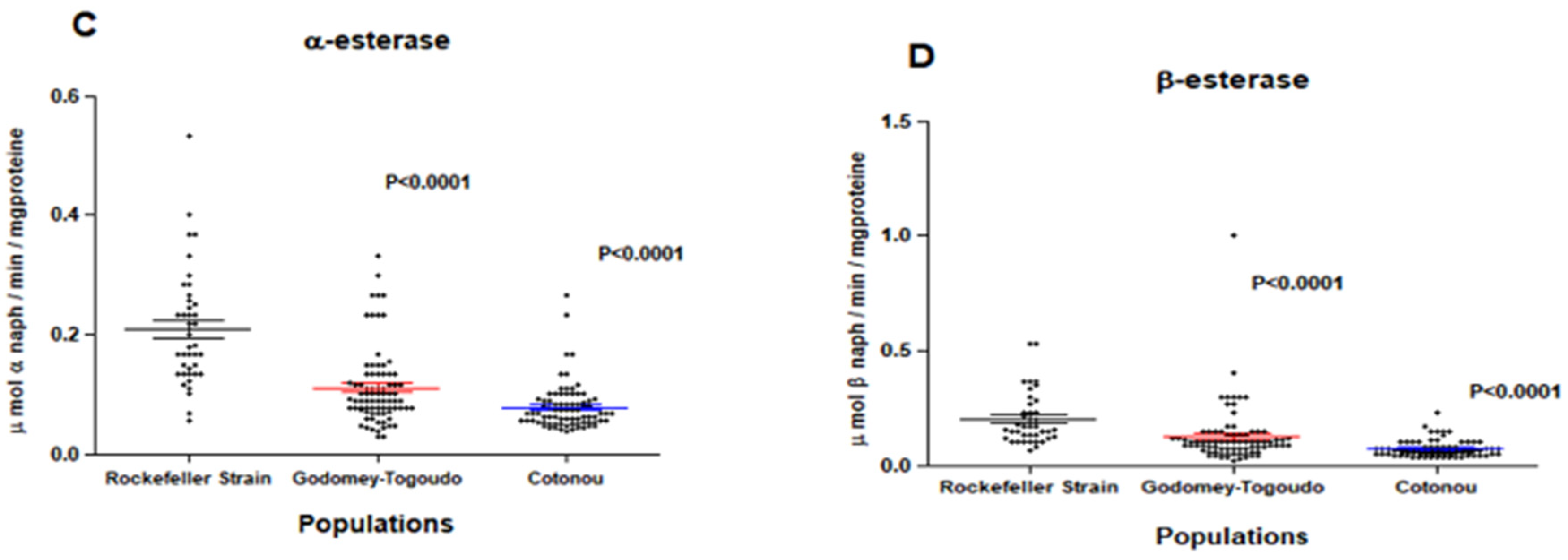
Field insecticide-resistant
Ae. aegypti populations had significantly higher median levels of GSTs compared to the insecticide-susceptible Rockefeller reference strain, with
p = 0.0003 in Godomey-Togoudo and
p = 0.0046 in the 10
ème arrondissement of Cotonou (
Figure 2A). By comparison, field
Ae. aegypti did not differ in their expression levels of MFOs, compared to the susceptible strain (
Figure 2B) and had significantly lower median levels of non-specific esterases (α and β esterases) (
Figure 2C,D.)
3.4. Kdr mutation screening
Three
kdr mutations (S989P, V1016G and F1534C) were identified in 82.9% (262/316) pyrethroid-resistant
Ae. aegypti from both study sites (
Table 1). S989P ranged in frequency from 0.63 to 0.78 and was under significant selection in Godomey-Togoudo (χ2 = 6.89;
p = 0.0087; Table 2). F1534C was present in the highest frequencies (0.64-0.75), with significant deviations from Hardy-Weinberg equilibrium in both study sites (χ2 = 6.21;
p = 0.013 and χ2 = 14.27;
p = 0.00016, in the 10
ème arrondissement of Cotonou and Godomey-Togoudo, respectively). V1016G ranged in frequency from 0.55 to 0.69, with no evidence for ongoing selection in either site (
Table 1). We identified 13 insecticide-resistance
Ae. aegypti with the simultaneous presence of all three
kdr mutations.
4. Discussion
To deploy appropriate control strategies targeting arbovirus vectors, it is crucial to understand the distribution of key mosquito species, their bionomics and insecticide resistance profiles. Study findings report Ae. aegypti in the 10ème arrondissement of Cotonou and Godomey-Togoudo during the dry season, which might be explained by climate change, transport development and increasing urbanisation. Both populations of Ae. aegypti were characterised by high pyrethroid resistance (to deltamethrin and permethrin), but complete susceptibility to bendiocarb. Pyrethroid resistance in these populations is not expected, given that insecticide treatment specifically targeting Ae. aegypti in Benin is rare; rather it may have been driven by several alternate factors. It is possible that insecticides used to control other vector species, e.g., long-lasting insecticidal nets (LLINs), indoor residual spraying (IRS) or household mosquito repellents used to target Anopheles vectors of malaria, may have exerted indirect selection pressure on Ae. aegypti for the evolution of pyrethroid resistance. Furthermore, water contamination caused by pesticides used for agricultural practices may have also contributed to the development of resistance.
Regarding insecticide resistance mechanisms, Ae. aegypti populations were characterised by overexpression of GSTs and a slight, but non-significant, increase in the activity of MFOs. It is likely that overexpression of both types of metabolic enzymes could confer pyrethroid resistance in these populations. Further investigation is warranted of molecular mechanisms involving the GSTe2 gene, which may be contributing to pyrethroid resistance in Ae. aegypti in Benin. By comparison, under expression of non-specific esterases (α and β esterases) observed in these same populations may explain the strong sensitivity to bendiocarb; therefore, this insecticide has the potential to be used in both areas to suppress Ae. aegypti.
Several mutations at nine different loci in
Ae. aegypti have been identified, which are implicated in reduced insecticide susceptibility. Of these, F1534C, S989P and V1016I are widely reported
kdr mutations and have been associated with DDT and pyrethroid resistance [
12,
13,
14,
15]. In this study, AS-PCR genotyping revealed the presence of S989P, F1534C and V1016G mutations in both populations of
Ae. aegypti in Benin; 82.9% of genotyped mosquitoes carried at least one mutation. The frequencies of S989P in both study sites (63% and 78%) were much higher compared to that observed in Nigeria (7%) for the first time in Africa. To our knowledge, this is the first report of V1016G in Africa, and this is the first time all three
kdr mutations have been detected in
Ae. aegypti in Benin. We identified 13 insecticide-resistance
Ae. aegypti with the simultaneous presence of all three
kdr mutations. The co-occurrence of two or three
kdr mutations has been previously reported in China, Nigeria and Malaysia, which results in highly intense pyrethroid resistance [
11,
15,
20].
In this study the detection of multiple kdr mutations and overexpressed detoxification enzymes in the major dengue virus vector Ae. aegypti is concerning and cautions against the use of pyrethroids in arbovirus control programmes in Benin. In this part of West Africa, additional surveillance activities are needed to further investigate other co-occurring molecular and metabolic insecticide resistance mechanisms, as well as to assess the susceptibility of these Ae. aegypti populations to other pyrethroids (e.g., alpha-cypermethrin, cyfluthrin or lambda-cyhalothrin), with our without synergists (e.g., piperonyl butoxide), or classes of insecticides (e.g., organophosphates, neonicotinoids, pyrroles etc.).
5. Conclusions
This study reports resistance to deltamethrin and permethrin in Ae. aegypti populations, collected from the 10ème arrondissement of Cotonou and Godomey-Togoudo in Abomey-Calavi. Molecular and metabolic mechanisms associated with pyrethroid resistance included kdr mutations: F1534C, S989P and V1016G, and significant overexpression of certain detoxification enzymes. To our knowledge, this is the first report of V1016G in Africa, and this is the first time all three kdr mutations have been detected in Ae. aegypti in Benin, suggesting alternative vector tools may be required for arbovirus control in this part of West Africa. Study results highlight the importance of strengthening and scaling-up surveillance activities to respond to the growing global threat of insecticide resistance to the control of vector-borne diseases.
Author Contributions
Conceptualization: T.F.T., R.O. and M.A.; data collection, T.F.T., R.O., S.D.Z.Z., G.A., G.I. and F.H.; formal analysis, T.F.T., R.O., S.D.Z.Z., G.A., A.S. and L.A.M.; mobilization of funding, T.F.T., G.A., S.D.Z.Z., FH., G.I. and R.O.; methodology, T.F.T., R.O., M.A., L.A.M. and M.A.; project administration, T.F.T. and R.O.; original draft preparation formal, T.F.T., R.O., L.A.M. and B.A.; supervision, T.F.T., R.O. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly funded by Researchers in the Entomological Research Center of Cotonou.
Informed Consent Statement
Mosquito collections did not directly involve humans, but their living environments and permissions were requested and obtained prior to the placement of the mosquito traps.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The findings and conclusions in this manuscript are those of the author(s). We acknowledge support received from the management of the Entomological Research Center of Cotonou and its staff in the field and laboratory work.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
References
- https://www.who.int/fr/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- Padonou GG, Ossè R, Salako AS, Aikpon R, Sovi A, Kpanou C, Sgbaohan H Akadiri Y, Lamine B and Akogbeto CM. Entomological assessment of the risk of dengue outbreak in Abomey-Calavi Commune, Benin. Tropical Medicine and Health 2020:48:20.
- Esu E, Lenhart A, Smith L, Horstick O. Effectiveness of peridomestic space spraying with insecticide on dengue transmission; systematic review. Trop Med Int Heal. 2010;15(5):619–31. [CrossRef]
- Liu N. Résistance aux insecticides chez les moustiques: impact, mécanismes et directions de recherche. Ann Rev Entomol. 2015;60:537–59.
- Brogdon WG, McAllister JC. Résistance aux insecticides et lutte antivectorielle. Urgence Infect Dis. 1998;4:605–13.
- Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–91. [CrossRef]
- Flores AE, Abeldaño W, Fernandez I, Badii MH, Loaiza H, Ponce G, et al. Elevated α-esterase levels associated with permethrin tolerance in Aedes aegypti (L.) from Baja California, México. Pestic Biochem Phys. 2005;82:66–78.
- Bisset JA, Marin R, Rodriguez MM, Severson DW, Ricardo Y, French L, Díaz M and Perez O. Insecticide resistance in two Aedes aegypti (Diptera: Culicidae) strains from Costa Rica. J Med Entomol. 2013;50:352–61. [CrossRef]
- Hernandez JR, Longnecker M, Fredregill CL, Debboun M and Pietrantonio PV. (2021) Kdr genotyping (V1016I, F1534C) of the Nav channel of Aedes aegypti (L.) mosquito populations in Harris County (Houston), Texas, USA, after Permanone 31–66 field tests and its influence on probability of survival. PLoS Negl Trop Dis 15(11): e0009833. [CrossRef]
- Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, Raghavendra K, Pinto J, Corbel V, David JP and Weetman D. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS neglected tropical diseases, 11(7), e0005625. [CrossRef]
- Li CX, Kaufman PE, Xue RD, Zhao MH, Wang G, Yan T, Guo XX, Zhang YM, Dong YD, Xing D, Zhang HD and Zhao TY. Relationship between insecticide resistance and kdr mutations in the dengue vector Aedes aegypti in Southern China. Parasit Vectors. 2015 Jun 12;8:325. [CrossRef]
- Kawada H, Higa Y, Futami K, Muranami Y, Kawashima E, Osei JH, Sakyi KY, Dadzie S, de Souza DK and Appawu M. Discovery of point mutations in the voltagegated sodium channel from African Aedes aegypti populations: Potential phylogenetic reasons for gene introgression. PLoS Negl. Trop. Dis. 2016;10:e0004780.
- Sombié A, Saiki E, Yaméogo F, Sakurai T, Shirozu T, Fukumoto S, Sanon A, Weetman D, McCall P, Kanuka H and Badolo A. Fréquences élevées des mutations F1534C et V1016I kdr et association avec la résistance aux pyréthroïdes chez Aedes aegypti de Somgandé (Ouagadougou), Burkina Faso. Trop Med Santé 47,2 (2019).
- Kawada H, Oo SZM, Thaung, SKE, Maung YNM, Thu HM, Thant KZ and Minakawa N. Co-occurrence of point mutations in the voltage-gated sodium channel of pyrethroid-resistant Aedes aegypti populations in Myanmar. PLoS Negl. Trop. Dis. 2014, 8. [CrossRef]
- Agbohun, IK, Idowu ET, Oyeniyi TA, Adeogun AO, Adesalu K, Nwanya O, Okonkwo F, Oladosu Y and Otubanjo O. First Detection and Co-occurrence of kdr (F1534C and S989P) Mutations in Multiple Insecticides Resistant Aedes aegypti in Nigeria. Preprints 2021, 2021070302. [CrossRef]
- Ayres CFJ, Seixas G, Borrego S, Marques C, Monteiro I, Marques CS, Gouveia B, Leal S, Troco AD, Fortes F, Parreira R, Pinto J, Sousa CA. The V410L knockdown resistance mutation occurs in island and continental populations of Aedes aegypti in West and Central Africa. PLoS Negl Trop Dis. 2020 May 8;14(5):e0008216. PMID: 32384079; PMCID: PMC7304628. [CrossRef]
- http://www.cdc.gov/malaria, Guideline for Evaluating Insecticide Resistance in Vectors Using the CDC Bottle Bioassay.
- Fontenille D, Traore-Lamizana M, Diallo M, Thonnon J, Digoutte JP and Zeller HG. (1998). New vectors of Rift Valley fever in West Africa. Emerging infectious diseases, 4(2), 289–293. [CrossRef]
- Zuharah WF, Sufian M. The discovery of a novel knockdown resistance (kdr) mutation A1007G on Aedes aegypti (Diptera: Culicidae) from Malaysia. Sci Rep 11, 5180 (2021). [CrossRef]
- AhbiRami R, Ishak IH, Yahya ZS and Zuharah WF. Knockdown resistance (kdr) in dengue vectors, Aedes aegypti and Aedes albopictus from Malaysia: A post-flood risk assessment. Gen. Mol. Biol. 19(2), gmr18604 (2020).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).