Submitted:
02 May 2023
Posted:
03 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Edible films and coatings
1.2. Pistacia vera L resin and essential oil
1.3. Scope and Purpose of the Study
2. Materials and Methods
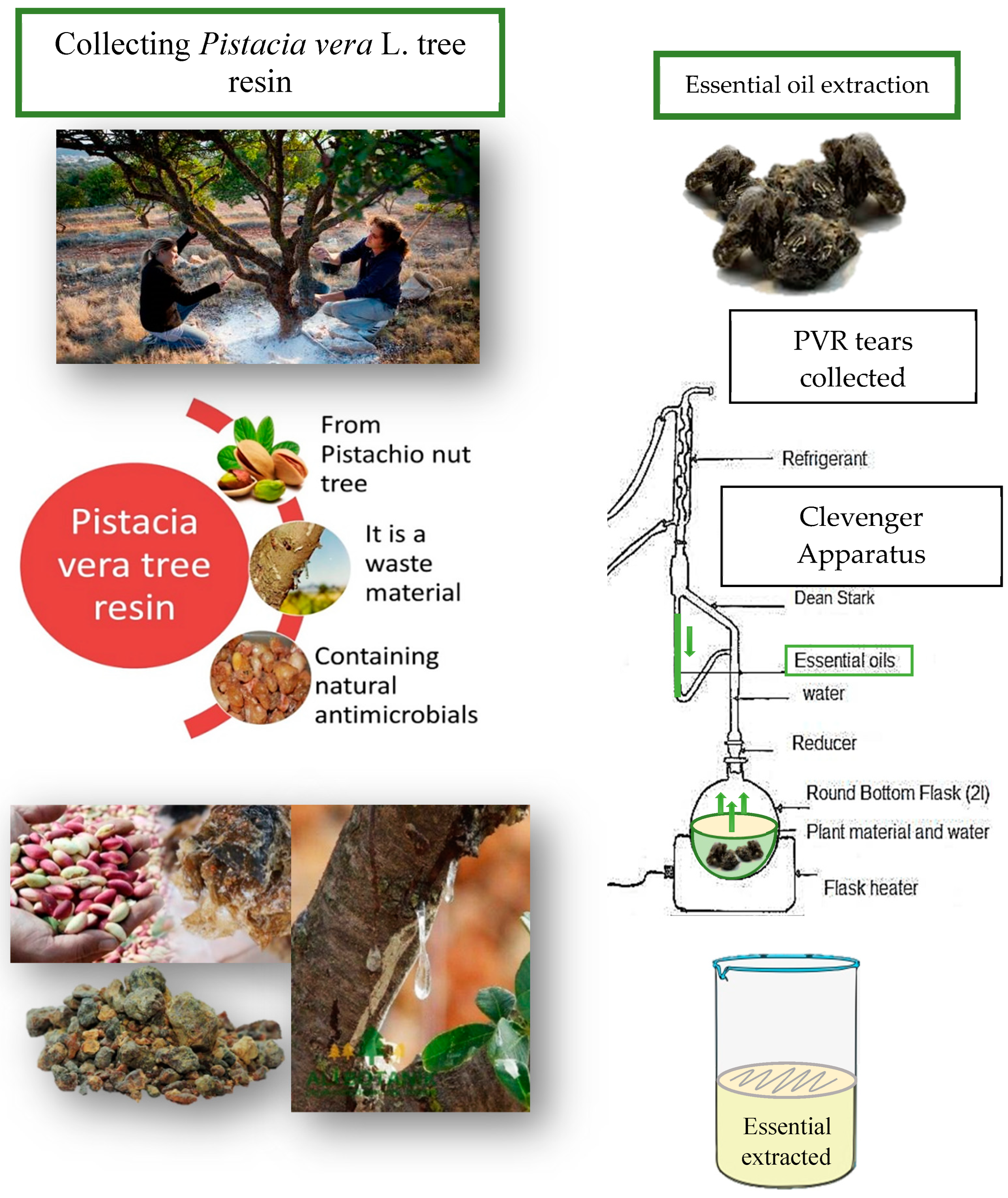
2.1. Obtaining Pistacia vera L. tree resin and essential oil extraction
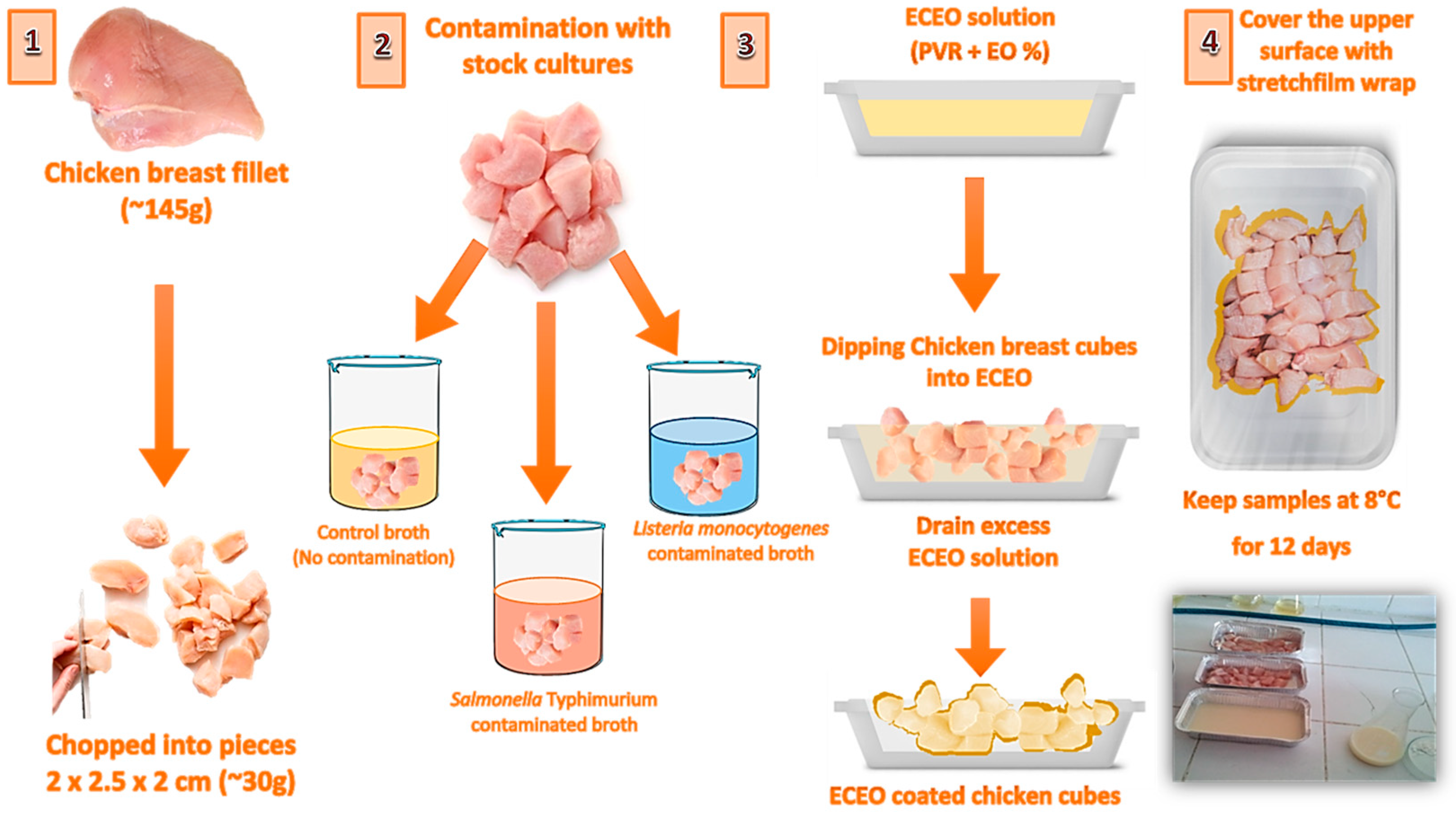
2.2. Preparation of bacterial strains and contamination of chicken breast fillets
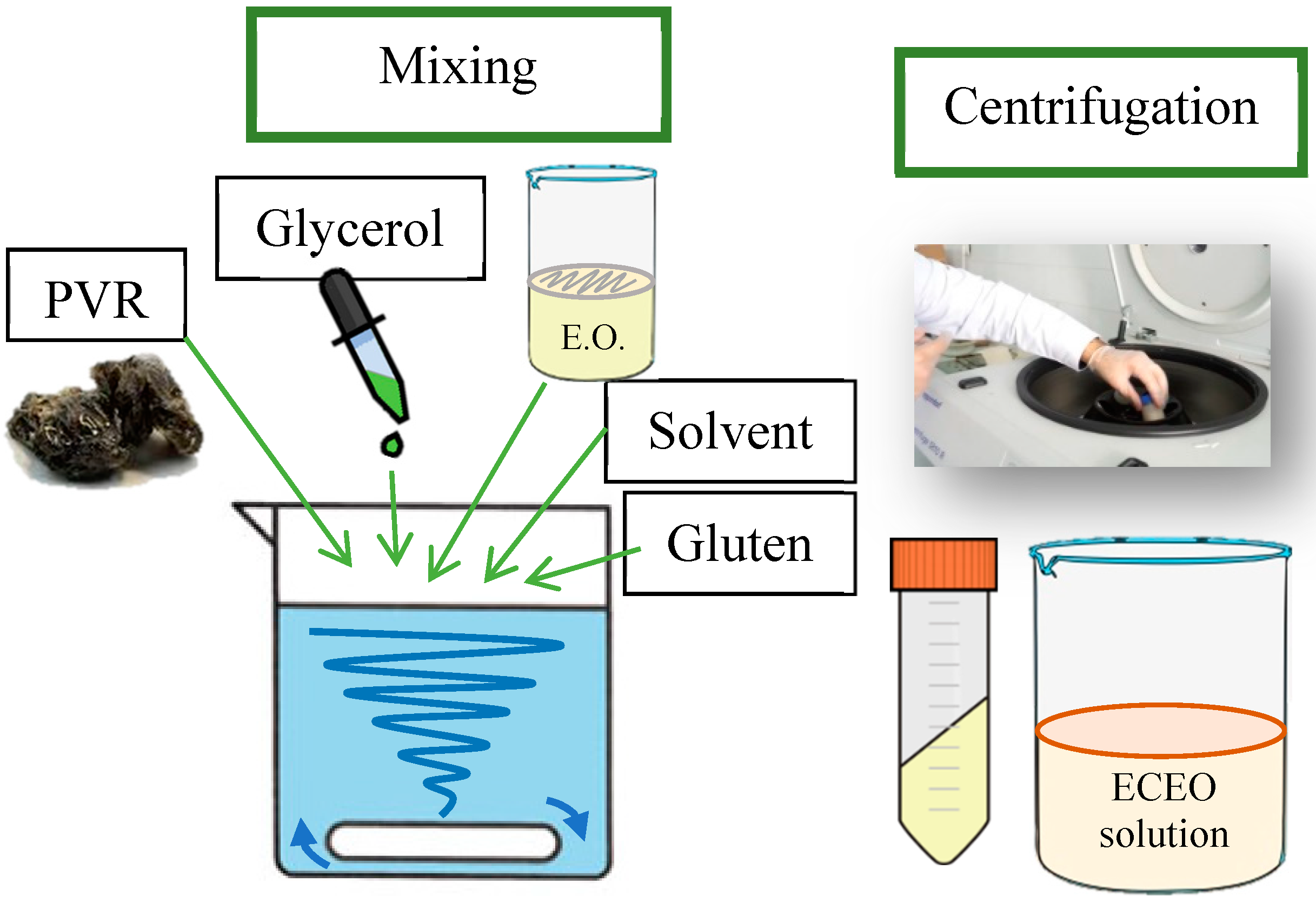
2.3. Preparation of edible coatings and application on chicken breast fillets
| Type of CBF | Edible coating application |
Contamination of pathogens | Microbial count |
|---|---|---|---|
| C1 | Uncoated CBF | No contamination | TBC |
| C2 | Coating CBF with EC | ||
| C2(0.5)*, C2(1), C2(1.5), C2(2) | Coating CBF with ECEO | ||
| UCLM | Uncoated CBF | L. monocytogenes contamination | L. monocytogenes count |
| CLM | Coating CBF with EC | ||
| CLM(0.5), CLM(1) CLM(1.5), CLM(2) | Coating CBF with ECEO | ||
| UCST | Uncoated CST | S. Typhimurium contamination | S. Typhimurium count |
| CST | Coating CBF with EC | ||
| CST0.5), CST(1), CST(1.5), CST(2) | Coating CBF with ECEO | ||
| *Numbers on the subscripts indicate essential oil (EO) concentration in the EC, EC: edible coating, ECEO: edible coating containing EO. | |||
2.4. Microbiological analysis
2.5. Sensory Analysis
| Name of the sample | Treatment | Sensory analysis |
|---|---|---|
| UCR | Uncoated raw CBF |
Appearance, Smell, Texture, General acceptance, and Taste (for only grilled samples) |
| CR | Coating raw CBF with ECEO (containing 2% EO) | |
| UCG | Uncoated CBF grilled for 5 min | |
| CG | Coating CBF with ECEO (2%) + grilled for 5 min |
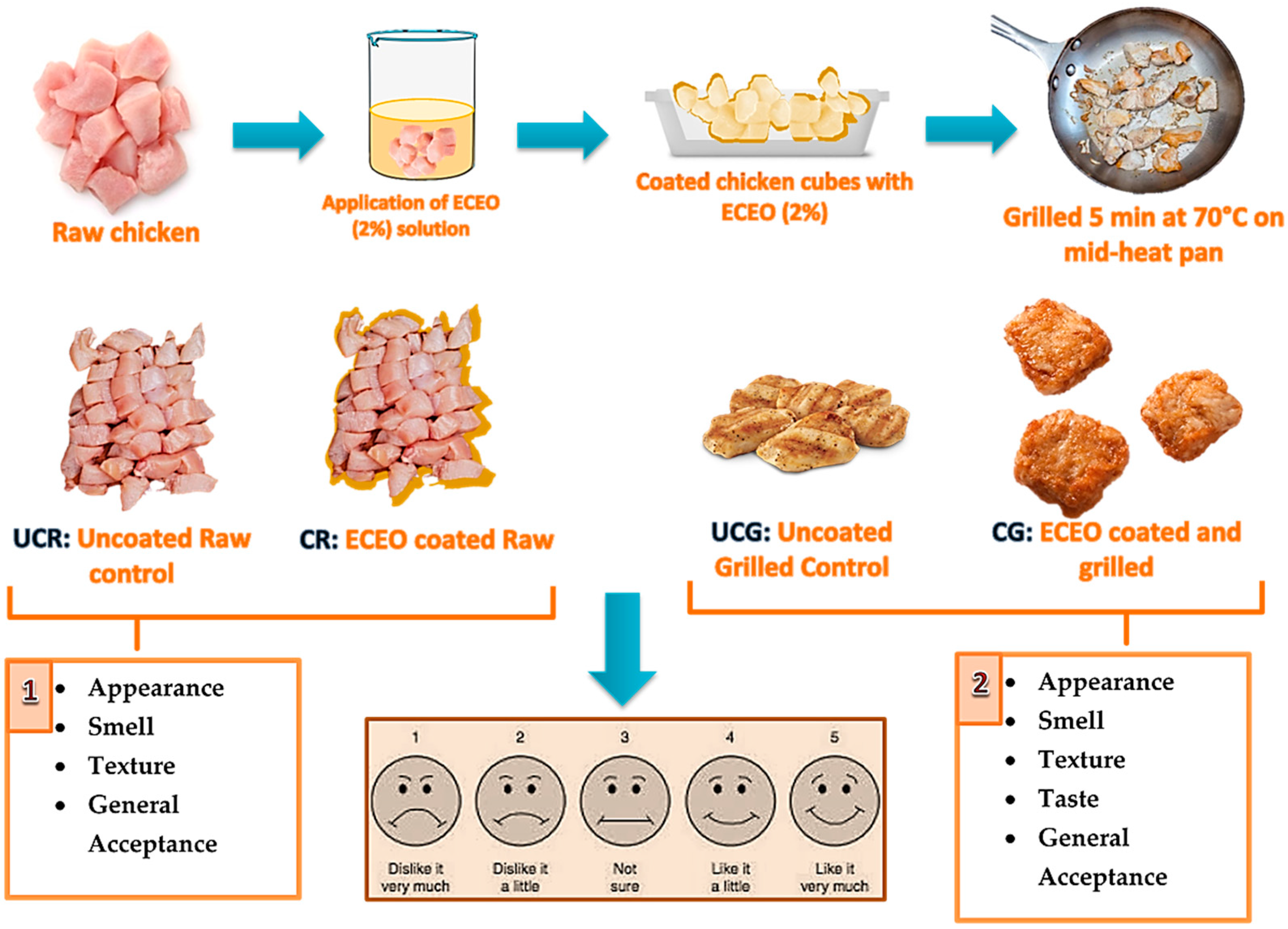
2.6. Statistical Analysis
3. Results and Discussion
3.1. Antimicrobial effects of PVR edible coating on chicken breast fillets
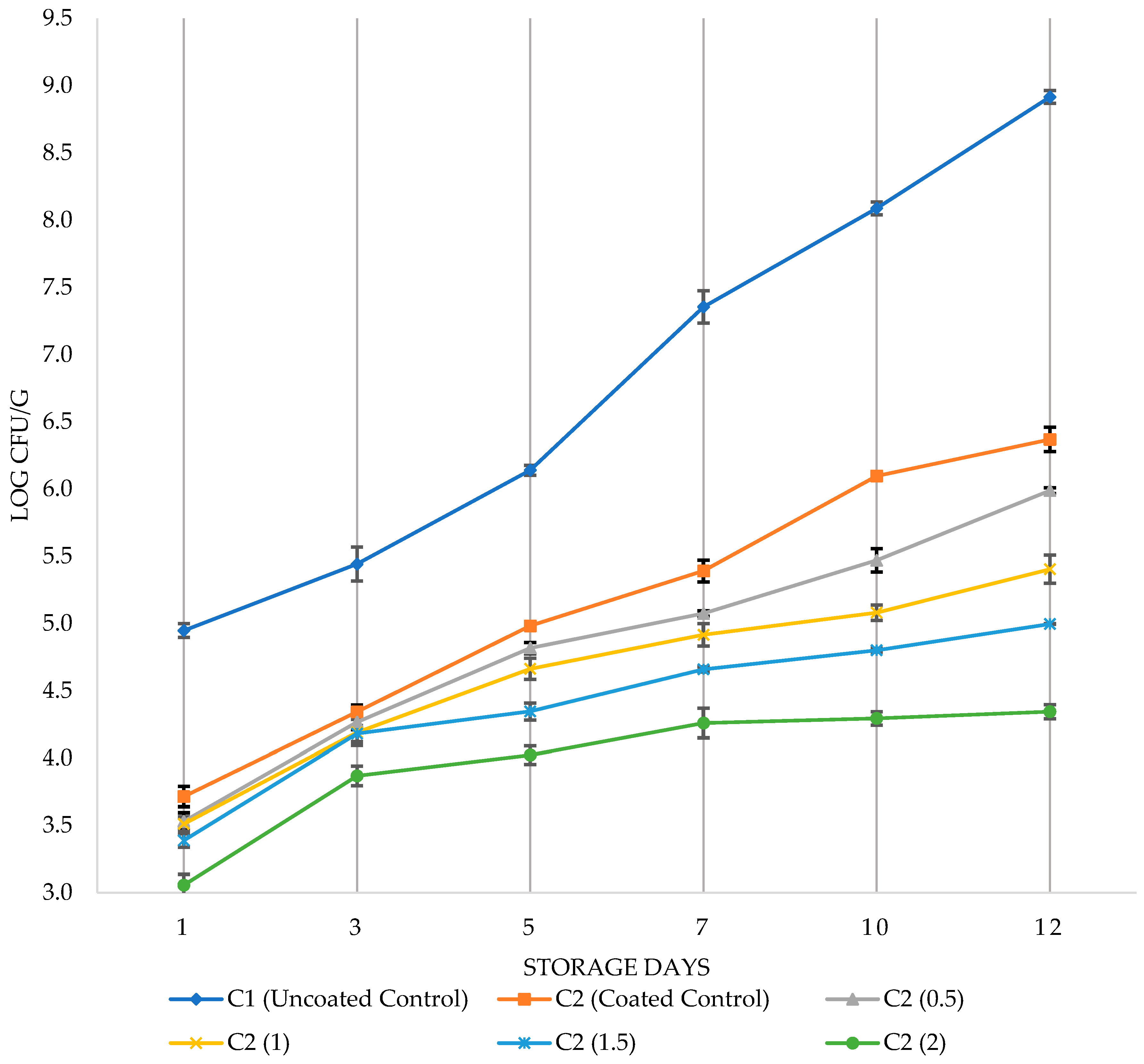
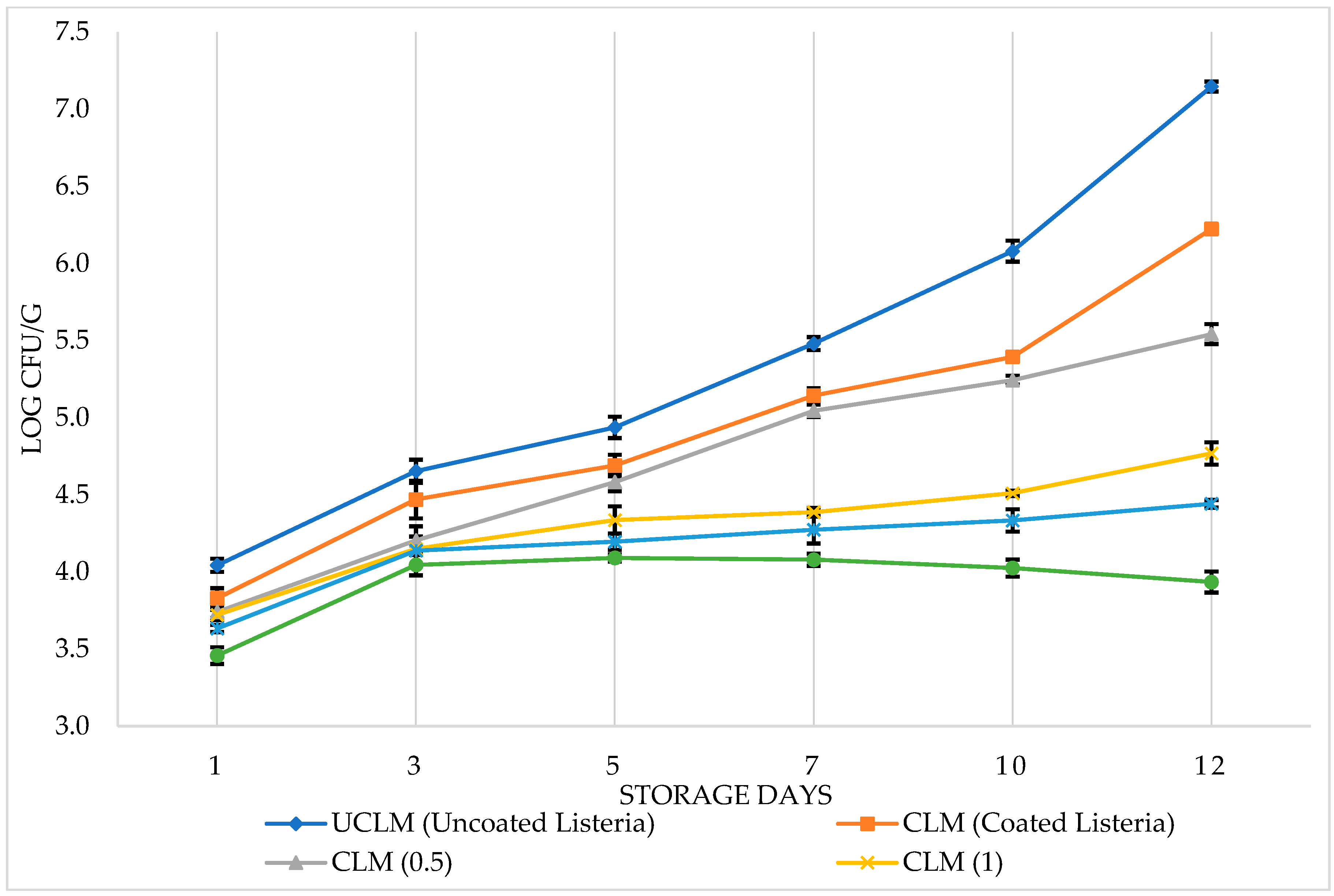
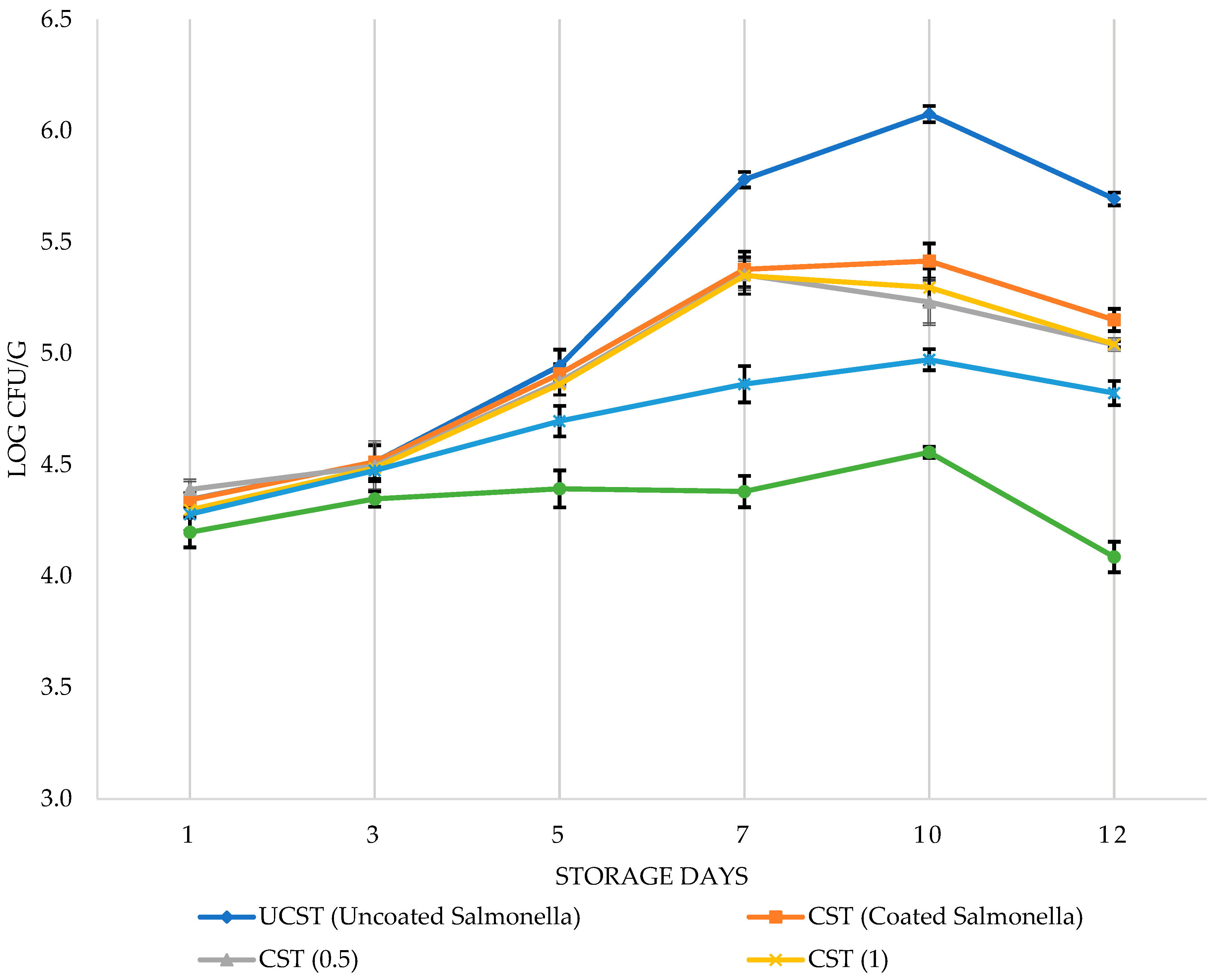
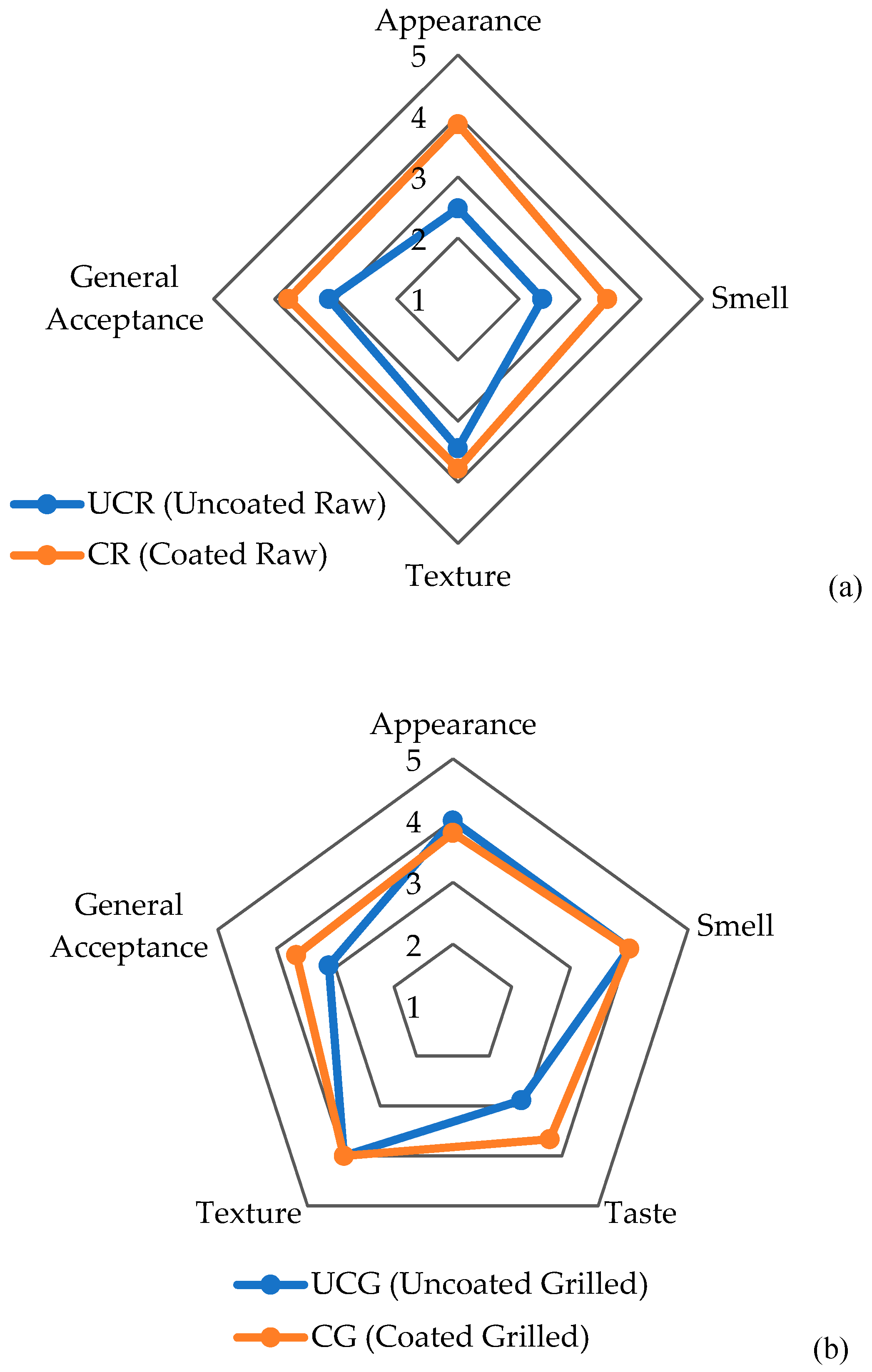
3.2. Sensory analysis of chicken breast fillets with PVR edible coating
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Additional notes
References
- Sánchez-Ortega, I.; García-Almendárez, B.E.; Santos-López, E.M.; Amaro-Reyes, A.; Barboza-Corona, J.E.; Regalado, C. Antimicrobial Edible Films and Coatings for Meat and Meat Products Preservation. Sci. World J. 2014, 2014, 248935. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pan, I.; Carrión-Granda, X.; Maté, J.I. Antimicrobial efficiency of edible coatings on the preservation of chicken breast fillets. Food Control 2014, 36, 69–75. [Google Scholar] [CrossRef]
- Garavito, J.; Moncayo-Martínez, D.; Castellanos, D.A. Evaluation of antimicrobial coatings on preservation and shelf life of fresh chicken breast fillets under cold storage. Foods 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.L.A.; Frizzo, A.; Benito, C.E.; da Silva Júnior, R.C.; Alvares, L.K.; Pinto, A.N.; Tellini, C.; de Oliveira Monteschio, J.; Fernandes, J.I.M. Effect of an antimicrobial photoinactivation approach based on a blend of curcumin and Origanum essential oils on the quality attributes of chilled chicken breast. LWT 2023, 176, 114484. [Google Scholar] [CrossRef]
- Tack, D.M.; Marder, E.P.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Hurd, S.; Scallan, E.; Lathrop, S.; Muse, A.; Ryan, P.; et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food — Foodborne Diseases Active Surveillance Network, 10 U.S. sites, 2015–2018. Am. J. Transplant. 2019, 19, 1859–1863. [Google Scholar] [CrossRef]
- ECDC ECDC Surveillance Atlas of infectious disease Available online: https://atlas.ecdc.europa.eu/public/index.aspx.
- Silva, F.; Domingues, F.C.; Nerín, C. Trends in microbial control techniques for poultry products. https://doi.org/10.1080/10408398.2016.1206845 2017, 58, 591–609. [CrossRef]
- Fernández-Pan, I.; Mendoza, M.; Maté, J.I. Whey protein isolate edible films with essential oils incorporated to improve the microbial quality of poultry. J. Sci. Food Agric. 2013, 93, 2986–2994. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Santaescolástica, C.; Munekata, P.E.S.; Feng, X.; Liu, Y.; Bastianello Campagnol, P.C.; Lorenzo, J.M. Active edible coatings and films with Mediterranean herbs to improve food shelf-life. Crit. Rev. Food Sci. Nutr. 2022, 62, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, Z.; Aminzare, M.; Hassanzad Azar, H.; Rostamizadeh, K. Effect of corn starch coating incorporated with nanoemulsion of Zataria multiflora essential oil fortified with cinnamaldehyde on microbial quality of fresh chicken meat and fate of inoculated Listeria monocytogenes. J. Food Sci. Technol. 2021, 58, 2677–2687. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Krochta, J.M. Edible Packaging Materials. Annu. Rev. Food Sci. Technol. 2010, 1, 415–448. [Google Scholar] [CrossRef]
- Bharti, S.K.; Pathak, V.; Alam, T.; Arya, A.; Singh, V.K.; Verma, A.K.; Rajkumar, V. Materialization of novel composite bio-based active edible film functionalized with essential oils on antimicrobial and antioxidative aspect of chicken nuggets during extended storage. J. Food Sci. 2020, 85, 2857–2865. [Google Scholar] [CrossRef]
- Debeaufort, F.; Quezada-Gallo, J.-A.; Voilley, A. Edible Films and Coatings: Tomorrow’s Packagings: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Bourtoom, T. Review Article Edible films and coatings : characteristics and properties. Int. Food Res. J. 2008, 15, 237–248. [Google Scholar]
- Cagri, A.; Ustunol, Z.; Ryser, E.T. Inhibition of three pathogens on bologna and summer sausage using antimicrobial edible films. J. Food Sci. 2002, 67, 2317–2324. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Guilbert, S.; Gontard, N.; Gorris, L.G.M. Prolongation of the Shelf-life of Perishable Food Products using Biodegradable Films and Coatings. LWT - Food Sci. Technol. 1996, 29, 10–17. [Google Scholar] [CrossRef]
- Han, J.H. Edible Films and Coatings; Elsevier Ltd, 2014; ISBN 9780123946010.
- Cagri, A.; Ustunol, Z.; Ryser, E.T. Antimicrobial Edible Films and Coatings. J. Food Prot. 2004, 67, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Khaledian, Y.; Pajohi-Alamoti, M.; Bazargani-Gilani, B. Development of cellulose nanofibers coating incorporated with ginger essential oil and citric acid to extend the shelf life of ready-to-cook barbecue chicken. J. Food Process. Preserv. 2019, 43, 1–13. [Google Scholar] [CrossRef]
- Khezrian, A.; Shahbazi, Y. Application of nanocompostie chitosan and carboxymethyl cellulose films containing natural preservative compounds in minced camel’s meat. Int. J. Biol. Macromol. 2018, 106, 1146–1158. [Google Scholar] [CrossRef]
- Sallak, N.; Motallebi Moghanjoughi, A.; Ataee, M.; Anvar, S.A.A.; Golestan, L. Evaluation of the effect of corn starch film composed of Ag-TiO₂ nanocomposites and Satureja khuzestanica essential oil on the shelf-life of chicken fillet. Food Heal. 2022, 5, 20–29. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, A.; Wang, W.; Ye, R.; Liu, Y.; Xiao, J.; Wang, K. Characterisation of microemulsion nanofilms based on Tilapia fish skin gelatine and ZnO nanoparticles incorporated with ginger essential oil: meat packaging application. Int. J. Food Sci. Technol. 2017, 52, 1670–1679. [Google Scholar] [CrossRef]
- Ghalem, B.R.; Mohamed, B. Antimicrobial activity evaluation of the oleoresin oil of Pistacia vera L. African J. Plant Sci. 2010, 4, 300–303. [Google Scholar]
- Digrak, M.; Alma, M.H.; Ilçim, A.; Sen, S. Antibacterial and Antifungal Effects of Various Commercial Plant Extracts. Pharm. Biol. 1999, 37, 216–220. [Google Scholar] [CrossRef]
- Digrak, M.; Alma, M.H.; Ilçim, A. Antibacterial and Antifungal Activities of Turkish Medicinal Plants. Pharm. Biol. 2001, 39, 346–350. [Google Scholar] [CrossRef]
- Alma, M.H.; Nitz, S.; Kollmannsberger, H.; Digrak, M.; Efe, F.T.; Yilmaz, N. Chemical composition and antimicrobial activity of the essential oils from the gum of Turkish Pistachio (Pistacia vera L.). J. Agric. Food Chem. 2004, 52, 3911–3914. [Google Scholar] [CrossRef] [PubMed]
- Erkmen, O. Bacterial inactivation mechanism of SC-CD and TEO combinations in watermelon and melon juices. Food Sci. Technol. 2021, 42, e62520. [Google Scholar] [CrossRef]
- Tornuk, F.; Ozturk, I.; Sagdic, O.; Yilmaz, A.; Erkmen, O. Application of Predictive Inactivation Models to Evaluate Survival of Staphylococcus aureus in Fresh-Cut Apples Treated with Different Plant Hydrosols. http://dx.doi.org/10.1080/10942912.2011.650340 2013, 17, 587–598. [CrossRef]
- Erkmen, O. Practice 10. Counting of mesophilic and thermophilic spore formers. In Microbiological Analysis of Foods and Food Processing Environments; Elsevier: London, 2021; pp. 77–90. ISBN 978-0-323-91651-6. [Google Scholar]
- Erkmen, O. Practice 15. Isolation and counting of Salmonella. In Microbiological Analysis of Foods and Food Processing Environments; Elsevier: London, 2021; pp. 151–168. ISBN 978-0-323-91651-6. [Google Scholar]
- Erkmen, O. Practice 16. Isolation and counting of Listeria monocytogenes. In Microbiological Analysis of Foods and Food Processing Environments; Elsevier: London, 2021; pp. 169–180. ISBN 978-0-323-91651-6. [Google Scholar]
- Economou, T.; Pournis, N.; Ntzimani, A.; Savvaidis, I.N. Nisin–EDTA treatments and modified atmosphere packaging to increase fresh chicken meat shelf-life. Food Chem. 2009, 114, 1470–1476. [Google Scholar] [CrossRef]
- Bazargani-Gilani, B.; Aliakbarlu, J.; Tajik, H. Effect of pomegranate juice dipping and chitosan coating enriched with Zataria multiflora Boiss essential oil on the shelf-life of chicken meat during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2015, 29, 280–287. [Google Scholar] [CrossRef]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef]
- Bolumar, T.; Andersen, M.L.; Orlien, V. Antioxidant active packaging for chicken meat processed by high pressure treatment. Food Chem. 2011, 129, 1406–1412. [Google Scholar] [CrossRef]
- Soni, A.; Kandeepan, G.; Mendiratta, S.K.; Shukla, V.; Kumar, A. Development and characterization of essential oils incorporated carrageenan based edible film for packaging of chicken patties. Nutr. Food Sci. 2016, 46, 82–95. [Google Scholar] [CrossRef]
- Shekarforoush, S.S.; Basiri, S.; Ebrahimnejad, H.; Hosseinzadeh, S. Effect of chitosan on spoilage bacteria, Escherichia coli and Listeria monocytogenes in cured chicken meat. Int. J. Biol. Macromol. 2015, 76, 303–309. [Google Scholar] [CrossRef]
- Patsias, A.; Badeka, A. V.; Savvaidis, I.N.; Kontominas, M.G. Combined effect of freeze chilling and MAP on quality parameters of raw chicken fillets. Food Microbiol. 2008, 25, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Senter, S.D.; Arnold, J.W.; Chew, V. APC values and volatile compounds formed in commercially processed, raw chicken parts during storage at 4 and 13°C and under simulated temperature abuse conditions. J. Sci. Food Agric. 2000, 80, 1559–1564. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Hagenmaier, R.D.; Bai, J.; Edition, S. Edible Coatings and Films to Improve Food Quality SECOND EDITION; Second.; CRC Press, 2012; ISBN 9781138198937.
- Ala, M.A.; Shahbazi, Y. The effects of novel bioactive carboxymethyl cellulose coatings on food-borne pathogenic bacteria and shelf life extension of fresh and sauced chicken breast fillets. Lwt 2019, 111, 602–611. [Google Scholar] [CrossRef]
- Khezerlou, A.; Zolfaghari, H.; Banihashemi, S.A.; Forghani, S.; Ehsani, A. Plant gums as the functional compounds for edible films and coatings in the food industry: A review. Polym. Adv. Technol. 2021, 32, 2306–2326. [Google Scholar] [CrossRef]
- Matiacevich, S.; Acevedo, N.; López, D. Characterization of Edible Active Coating Based on Alginate-Thyme Oil-Propionic Acid for the Preservation of Fresh Chicken Breast Fillets. J. Food Process. Preserv. 2015, 39, 2792–2801. [Google Scholar] [CrossRef]
- Raeisi, M.; Tabaraei, A.; Hashemi, M.; Behnampour, N. Effect of sodium alginate coating incorporated with nisin, Cinnamomum zeylanicum, and rosemary essential oils on microbial quality of chicken meat and fate of Listeria monocytogenes during refrigeration. Int. J. Food Microbiol. 2016, 238, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Aykut, B.; Osman, E. Antimicrobial activity of a novel biodegradable edible film produced from Pistacia vera resin and Origanum vulgare essential oil. Res. J. Biotechnol. 2017, 12, 15–21. [Google Scholar]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil. Coatings 2018, Vol. 8, Page 177 2018, 8, 177. [Google Scholar] [CrossRef]
- Janes, M.E.; Kooshesh, S.; Johnson, M.G. Control of Listeria monocytogenes on the surface of refrigerated, ready-to-eat chicken coated with edible zein film coatings containing nisin and/or calcium propionate. J. Food Sci. 2002, 67, 2754–2757. [Google Scholar] [CrossRef]
- İncili, G.K.; Akgöl, M.; Aydemir, M.E.; Alan, S.; Mutlu, M.; İlhak, O.İ.; Öksüztepe, G. Fate of Listeria monocytogenes and Salmonella Typhimurium in homemade marinade and on marinated chicken drumsticks, wings, and breast meat. Lwt 2020, 134. [Google Scholar] [CrossRef]
- Goswami, N.; Han, J.H.; Holley, R.A. Effectiveness of antimicrobial starch coating containing thyme oil against Salmonella, Listeria, Campylobacter, and Pseudomonas on chicken breast meat. Food Sci. Biotechnol. 2009, 18, 425–431. [Google Scholar]
- Burt, S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Panahi, Z.; Mohsenzadeh, M. Sodium alginate edible coating containing Ferulago angulata (Schlecht.) Boiss essential oil, nisin, and NaCl: Its impact on microbial, chemical, and sensorial properties of refrigerated chicken breast. Int. J. Food Microbiol. 2022, 380, 109883. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Farshidi, M.; Ehsani, A. Effects of lactoperoxidase system-alginate coating on chemical, microbial, and sensory properties of chicken breast fillets during cold storage. J. Food Saf. 2018, 38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





