Submitted:
27 April 2023
Posted:
03 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Research status of epididymis in recent years
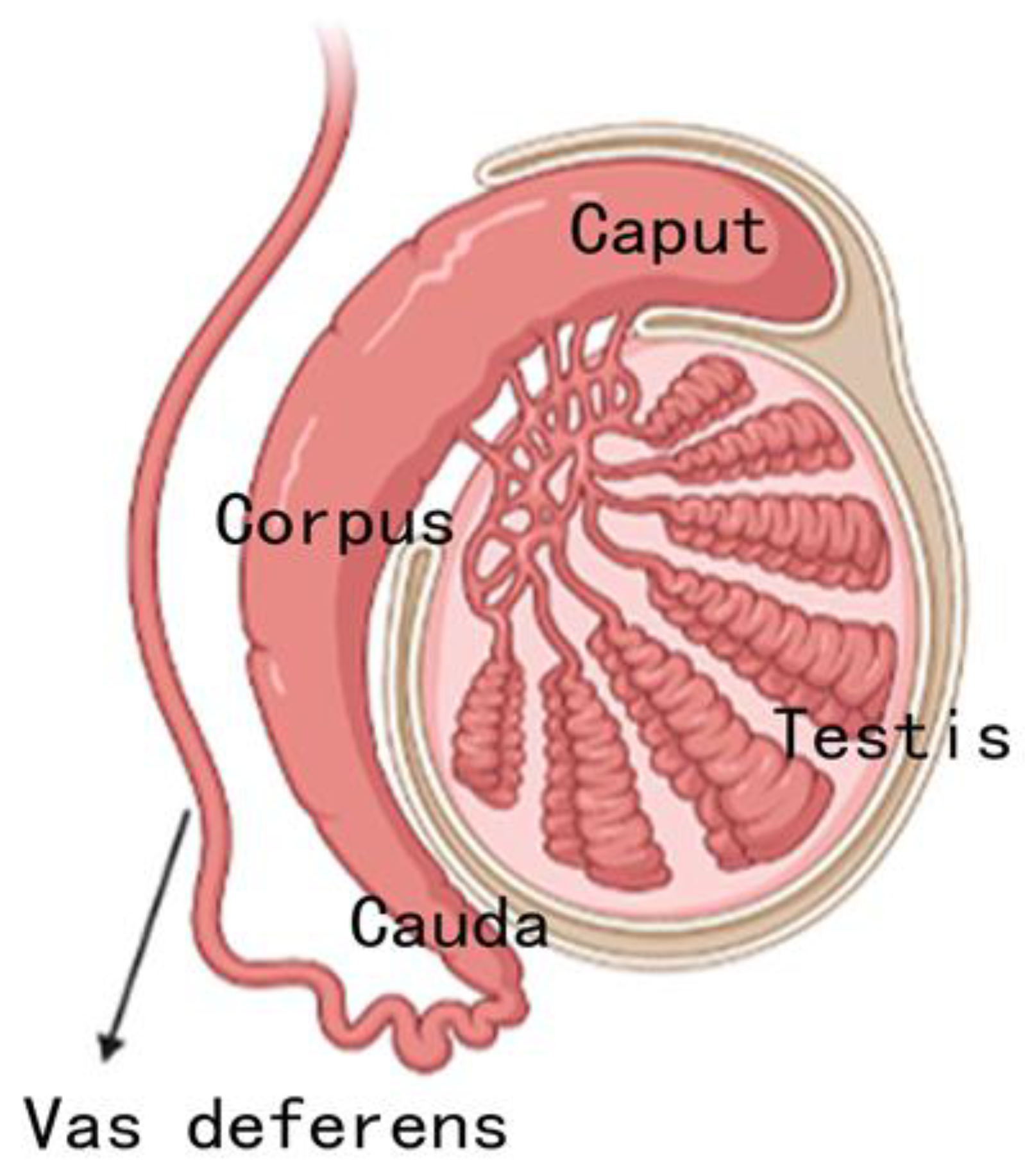
3. Regional division and differential function
4. Different cell types in different epididymis regions
4.1. Epididymis epithelial and cell types
4.2. Principal cells
4.3. Basal cells
4.4. Other cells
5. Different genes in different epididymis regions
5.1. Specially expressed genes and gene families
5.2. Epididymal distribution and function of the Defb family
5.3. Epididymal distribution and function of the Lcn family
5.4. Other gene expression conditions in epididymis
6. Gene expression varies at different epididymal positions and in different cell types
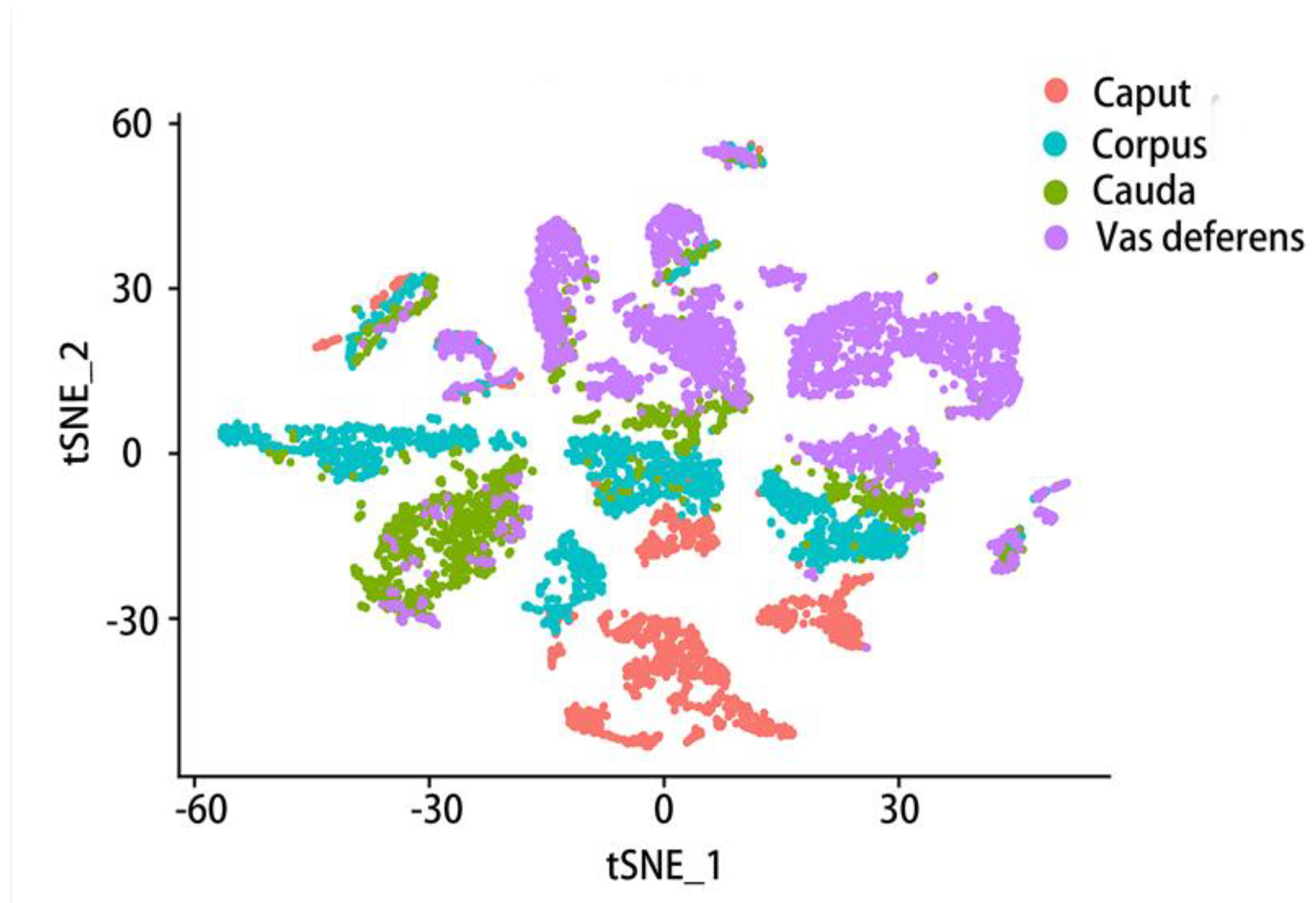
6.1. Comparison of gene expression in different parts of the epididymis
6.2. Comparison of gene expression in different cell types of epididymis
7. Epididymal spermatozoa and epididymis fluid
8. Dysfunction of epididymis and reproductive health
9. Discussion
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Hox | homeobox |
| OCLN | Occludin |
| PGE2 | ProstaglandinE2 |
| LCN | lipocalin |
| DEFB | defensin, beta |
| DICER1 | dicer1, ribonuclease III |
| mERABP | mouse epididymal retinoic acid binding protein |
| mEP17 | mouse epididymal protein of 17 kDa |
| CD52 | CD52 molecule |
| CRISP | cysteine rich secretory protein |
| AQPs | aquaporins |
| SPINK | serine peptidase inhibitor Kazal type |
| t-SNE | t-distributed stochastic neighbor embedding |
| DEGs | differentially expressed genes |
| IVF | in vitro fertilization |
| AI | artificial insemination |
| LM | light microscopy |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
References
- Cosentino, M.J.; Cockett, A.T.K. Review article: Structure and function of the epididymis. Urol. Res. 1986, 14, 229–240. [Google Scholar] [CrossRef] [PubMed]
- LANZ, V. and Neuhaeuser, G. Morphometric analysis of the human epididymis. Zeitschrift fur Anatomie und Entwicklungsgeschichte 1964, 124, 126–152. [Google Scholar] [CrossRef]
- Turner, T.T.; Gleavy, J.L.; Harris, J.M. Fluid Movement in the Lumen of the Rat Epididymis: Effect of Vasectomy and Subsequent Vasovasostomy. J. Androl. 1990, 11, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.X.; Temple-Smith, P.; Wreford, N.G. Postnatal differentiation and development of the rat epididymis: A stereological study. Anat. Rec. 1994, 238, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Stoltenberg, M.; Therkildsen, P.; Andreasen, A.; Jensen, K.B.; Juhl, S.; Ernst, E.; Danscher, G. Computer-assisted visualization of the rat epididymis: a methodological study based on paraffin sections autometallographically stained for zinc ions. Histochem. J. 1998, 30, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.; Renfree, M.B. Wolffian Duct Development. Sex. Dev. 2014, 8, 273–280. [Google Scholar] [CrossRef]
- Branford, W.W. , Benson, G.V., Ma, L., Maas, R.L. and Potter, S.S. Characterization of Hoxa-10/Hoxa-11 transheterozygotes reveals functional redundancy and regulatory interactions. Developmental Biology 2000, 224, 373–387. [Google Scholar] [CrossRef]
- Snyder, E.M.; Small, C.L.; Bomgardner, D.; Xu, B.; Evanoff, R.; Griswold, M.D.; Hinton, B.T. Gene expression in the efferent ducts, epididymis, and vas deferens during embryonic development of the mouse. Dev. Dyn. 2010, 239, 2479–2491. [Google Scholar] [CrossRef]
- Westfalewicz, B.; Dietrich, M.; Mostek, A.; Partyka, A.; Bielas, W.; Niżański, W.; Ciereszko, A. Identification and functional analysis of bull (Bos taurus) cauda epididymal fluid proteome. J. Dairy Sci. 2017, 100, 6707–6719. [Google Scholar] [CrossRef]
- Rowley, M.J.; Teshima, F.; Heller, C.G. Duration of Transit of Spermatozoa through the Human Male Ductular System. Fertil. Steril. 1970, 21, 390–396. [Google Scholar] [CrossRef]
- Acott, T.S.; Carr, D.W. Inhibition of Bovine Spermatozoa by Caudal Epididymal Fluid: II. Interaction of pH and a Quiescence Factor 1. Biol. Reprod. 1984, 30, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Discutient Application to the Indurated Epididymis. Atlanta Med Surg J, 1859. 4(8): p. 515.
- Bryant, T. Case of Torsion of the Spermatic Cord, with Strangulation of the Epididymis and Testicle in an Incompletely Descended Organ. J. R. Soc. Med. -75. [CrossRef]
- Rockwell, F.W. Case of Removal of Both Testicles for Recurrent Carcinoma of the Epididymis. Annals of Surgery 1888, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Some Diseases of the Male Genital System: VIII. Tuberculous Disease of the Testis and Epididymis. Hospital (Lond 1886), 1908. 43(1120): p. 526.
- Griffiths, J. Observations on the Appendix of the Testicle, and on the Cysts of the Epididymis, the Vasa Efferentia, and the Rete Testis. . 1893, 28, 107–116. [Google Scholar] [PubMed]
- Watson, J.H. Some Observations on the Origin and Nature of the so-called Hydatids of Morgagni found in Men and Women, with Especial Reference to the Fate of the Müllerian Duct in the Epididymis. . 1902, 36, 147–61. [Google Scholar] [PubMed]
- Goglia, G. [Histochemical research on the ductus epididymis of the horse]. . 1954, 30, 1151–4. [Google Scholar] [PubMed]
- Goglia, G.; Magli, G. [Histochemical research on secretory processes of coni vasculosi and epididymis canal in the guinea pig]. . 1957, 33, 418–21. [Google Scholar]
- Montagna, W. SOME CYTOCHEMICAL OBSERVATIONS ON HUMAN TESTES AND EPIDIDYMIDES. Ann. New York Acad. Sci. 1952, 55, 629–642. [Google Scholar] [CrossRef]
- Allen, J.M.; Slater, J.J. A CYTOCHEMICAL STUDY OF GOLGI ASSOCIATED THIAMINE PYROPHOSPHATASE IN THE EPIDIDYMIS OF THE MOUSE. J. Histochem. Cytochem. 1961, 9, 418–423. [Google Scholar] [CrossRef]
- Allen, J.M. THE HISTOCHEMISTRY OF GLUCOSE-6-PHOSPHATASE IN THE EPIDIDYMIS OF THE MOUSE. J. Histochem. Cytochem. 1961, 9, 681–689. [Google Scholar] [CrossRef]
- Birnbaum, D.; Hall, T.; Lee, R. The Zinc Content of Rat Sperm Cells from Ejaculate, Vas, Epididymis and Testis..CONFERENCE NAME, LOCATION OF CONFERENCE, COUNTRYDATE OF CONFERENCE; pp. 321–324.
- Gray, C.P. , Biorn, C. L. and Drinker, H.R. Tumors of the epididymis. The Journal of Urology 1961, 86, 620–624. [Google Scholar]
- Bresler, V.M. [Tumors of the interstitial tissue of the epididymis in mice induced by diethylstibesterol]. . 1960, 28–34. [Google Scholar]
- Bresler, V.M. Tumors of the interstitial tissue of the epididymis in mice induced by diethylstibesterol. Voprosy onkologii 1960, 6, 28–34. [Google Scholar]
- Naide, Y. , [Epididymitis]. Ryoikibetsu Shokogun Shirizu, 1999(25 Pt 3): p. 237-40.
- Jonté, G.; Holstein, A. On the Morphology of the Transitional Zones from the Rete Testis into the Ductuli Efferentes and from the Ductuli Efferentes into the Ductus Epididymidis. Investigations on the Human Testis and Epididymis. Andrologia 2009, 19, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Fouchécourt, S.; Métayer, S.; Locatelli, A.; Dacheux, F.; Dacheux, J.-L. Stallion Epididymal Fluid Proteome: Qualitative and Quantitative Characterization; Secretion and Dynamic Changes of Major Proteins1. Biol. Reprod. 2000, 62, 1790–1803. [Google Scholar] [CrossRef] [PubMed]
- Jury, J.A. , Perry, A.C. and Hall, L. Identification, sequence analysis and expression of transcripts encoding a putative metalloproteinase, eMDC II, in human and macaque epididymis. MHR: Basic science of reproductive medicine 1999, 5, 1127–1134. [Google Scholar] [PubMed]
- Salisbury, G.W.; Graves, C.N.; Nakabayashi, N.T.; Cragle, R.G. OBSERVATIONS ON THE AEROBIC METABOLISM OF BULL AND GOAT EPIDIDYMAL SPERMATOZOA. Reproduction 1963, 6, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, G.W.; Graves, C.N. SUBSTRATE-FREE EPIDIDYMAL-LIKE BOVINE SPERMATOZOA. Reproduction 1963, 6, 351–359. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, Y.; Sun, C.; Ge, S.; Tan, Y.; Shen, H.; Yang, P. A Multi-Omics Study of Human Testis and Epididymis. Molecules 2021, 26, 3345. [Google Scholar] [CrossRef]
- Dacheux, J.-L.; Belleannée, C.; Jones, R.; Labas, V.; Belghazi, M.; Guyonnet, B.; Druart, X.; Gatti, J.L.; Dacheux, F. Mammalian epididymal proteome. Mol. Cell. Endocrinol. 2009, 306, 45–50. [Google Scholar] [CrossRef]
- Wong, J.; Damdimopoulos, A.; Damdimopoulou, P.; Gasperoni, J.G.; Tran, S.C.; Grommen, S.V.; De Groef, B.; Dworkin, S. Transcriptome analysis of the epididymis from Plag1 deficient mice suggests dysregulation of sperm maturation and extracellular matrix genes. Dev. Dyn. 2020, 249, 1500–1513. [Google Scholar] [CrossRef]
- Lye, R.; Hinton, B.T. Technologies for the study of epididymal-specific genes. Mol. Cell. Endocrinol. 2004, 216, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Légaré, C.; Sullivan, R. Differential gene expression profiles of human efferent ducts and proximal epididymis. Andrology 2019, 8, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Domeniconi, R.F. , Souza, A.C.F., Xu, B., Washington, A.M. and Hinton, B.T. Is the epididymis a series of organs placed side by side? Biology of reproduction 2016, 95, 10–1. [Google Scholar] [CrossRef] [PubMed]
- Eddy, E.M. , Washburn, T.F., Bunch, D.O., Goulding, E.H., Gladen, B.C., Lubahn, D.B. and Korach, K.S. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology 1996, 137, 4796–4805. [Google Scholar] [CrossRef]
- Wu, C.; Wang, C.; Zhai, B.; Zhao, Y.; Zhao, Z.; Yuan, Z.; Fu, X.; Zhang, M. Study on the region-specific expression of epididymis mRNA in the rams. PLOS ONE 2021, 16, e0245933. [Google Scholar] [CrossRef]
- Jervis, K.M.; Robaire, B. Effects of caloric restriction on gene expression along the epididymis of the Brown Norway rat during aging. Exp. Gerontol. 2003, 38, 549–560. [Google Scholar] [CrossRef]
- Rinaldi, V.D. , Donnard, E., Gellatly, K., Rasmussen, M., Kucukural, A., Yukselen, O., Garber, M., Sharma, U. and Rando, O.J. An atlas of cell types in the mouse epididymis and vas deferens. elife 2020, 9, e55474. [Google Scholar] [CrossRef]
- Dharmat, R. , Kim, S., Li, Y. and Chen, R. Single-cell capture, RNA-seq, and transcriptome analysis from the neural retina. Retinal Development: Methods and Protocols 2020, 2092, 159–186. [Google Scholar]
- Lang, X. , Adjei, M., Wang, C., Chen, X., Li, C., Wang, P., Pan, M., Li, K., Shahzad, K. and Zhao, W. RNA-Seq reveals the functional specificity of epididymal caput, corpus, and cauda genes of cattleyak. Animal Science Journal 2022, 93, e13732. [Google Scholar] [CrossRef]
- Ikawa, M. , Nakanishi, T., Yamada, S., Wada, I., Kominami, K., Tanaka, H., Nozaki, M., Nishimune, Y. and Okabe, M. Calmegin is required for fertilin α/β heterodimerization and sperm fertility. Developmental biology 2001, 240, 254–261. [Google Scholar] [CrossRef]
- Nishimura, H.; Kim, E.; Nakanishi, T.; Baba, T. Possible Function of the ADAM1a/ADAM2 Fertilin Complex in the Appearance of ADAM3 on the Sperm Surface. J. Biol. Chem. 2004, 279, 34957–34962. [Google Scholar] [CrossRef] [PubMed]
- Cho, C. , O'Dell Bunch, D., Faure, J.E., Goulding, E.H., Eddy, E.M., Primakoff, P. and Myles, D.G.,. Fertilization defects in sperm from mice lacking fertilin β. Science 1998, 281, 1857–1859. [Google Scholar] [CrossRef] [PubMed]
- Klinefelter, G.R.; Amann, R.P.; Hammerstedt, R.H. Culture of Principal Cells From the Rat Caput Epididymidis1. Biol. Reprod. 1982, 26, 885–901. [Google Scholar] [CrossRef]
- Lee, G.Y.; A Kenny, P.; Lee, E.H.; Bissell, M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 2007, 4, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bunick, D.; Bahr, J.; Klinefelter, G.; Hess, R. Isolation and culture of epithelial cells from rat ductuli efferentes and initial segment epididymidis. Tissue Cell 1998, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, J.; St-Pierre, N.; Viger, R.S.; Hermo, L.; Cyr, D.G. Characterization of a Novel Rat Epididymal Cell Line to Study Epididymal Function. Endocrinology 2005, 146, 4710–4720. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Suzuki, K.; Matusik, R.J.; Obinata, M.; Orgebin-Crist, M.-C. Immortalized epididymal cell lines from transgenic mice overexpressing temperature-sensitive simian virus 40 large T-antigen gene. . 2002, 23, 854–69. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.; Ong, I.M.; Stanic, A.K. Single-cell technologies in reproductive immunology. Am. J. Reprod. Immunol. 2019, 82, e13157–e13157. [Google Scholar] [CrossRef]
- Shi, J.; Fok, K.L.; Dai, P.; Qiao, F.; Zhang, M.; Liu, H.; Sang, M.; Ye, M.; Liu, Y.; Zhou, Y.; et al. Spatio-temporal landscape of mouse epididymal cells and specific mitochondria-rich segments defined by large-scale single-cell RNA-seq. Cell Discov. 2021, 7, 1–15. [Google Scholar] [CrossRef]
- Ståhl, P.L.; Salmén, F.; Vickovic, S.; Lundmark, A.; Navarro, J.F.; Magnusson, J.; Giacomello, S.; Asp, M.; Westholm, J.O.; Huss, M.; et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 2016, 353, 78–82. [Google Scholar] [CrossRef]
- Johnston, D.S.; Jelinsky, S.A.; Bang, H.J.; DiCandeloro, P.; Wilson, E.; Kopf, G.S.; Turner, T.T. The Mouse Epididymal Transcriptome: Transcriptional Profiling of Segmental Gene Expression in the Epididymis1. Biol. Reprod. 2005, 73, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, M.G.; Visconti, P.E. Molecular changes and signaling events occurring in spermatozoa during epididymal maturation. Andrology 2017, 5, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Jervis, K.M.; Robaire, B. Dynamic Changes in Gene Expression along the Rat Epididymis1. Biol. Reprod. 2001, 65, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, T.R.; Griswold, M.D. Androgen-Regulated Genes in the Murine Epididymis1. Biol. Reprod. 2004, 71, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhai, X.; Ma, Y.; Zhang, T.; Wang, Z.; Chong, T. Anatomic characteristics of epididymis based on histology, proteomic, and 3D reconstruction. Andrology 2020, 8, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Dacheux, J.; Belghazi, M.; Lanson, Y.; Dacheux, F. Human epididymal secretome and proteome. Mol. Cell. Endocrinol. 2006, 250, 36–42. [Google Scholar] [CrossRef] [PubMed]
- I Adamali, H.; Somani, I.H.; Huang, J.Q.; Mahuran, D.; A Gravel, R.; Trasler, J.M.; Hermo, L. I. Abnormalities in cells of the testis, efferent ducts, and epididymis in juvenile and adult mice with beta-hexosaminidase A and B deficiency. . 1999, 20, 779–802. [Google Scholar]
- Jelinsky, S.A.; Turner, T.T.; Bang, H.J.; Finger, J.N.; Solarz, M.K.; Wilson, E.; Brown, E.L.; Kopf, G.S.; Johnston, D.S. The Rat Epididymal Transcriptome: Comparison of Segmental Gene Expression in the Rat and Mouse Epididymides1. Biol. Reprod. 2007, 76, 561–570. [Google Scholar] [CrossRef]
- Harris, A.; Browne, J.; Leir, S.-H.; Eggener, S. Region-specific microRNA signatures in the human epididymis. Asian J. Androl. 2018, 20, 539. [Google Scholar] [CrossRef]
- Browne, J.A.; Yang, R.; Leir, S.-H.; Eggener, S.E.; Harris, A. Expression profiles of human epididymis epithelial cells reveal the functional diversity of caput, corpus and cauda regions. Mol. Hum. Reprod. 2015, 22, 69–82. [Google Scholar] [CrossRef]
- Sipilä, P.; Pujianto, D.A.; Shariatmadari, R.; Nikkilä, J.; Lehtoranta, M.; Huhtaniemi, I.T.; Poutanen, M. Differential Endocrine Regulation of Genes Enriched in Initial Segment and Distal Caput of the Mouse Epididymis as Revealed by Genome-Wide Expression Profiling1. Biol. Reprod. 2006, 75, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Hamzeh, M.; Robaire, B. Identification of Early Response Genes and Pathway Activated by Androgens in the Initial Segment and Caput Regions of the Regressed Rat Epididymis. Endocrinology 2010, 151, 4504–4514. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.P.B.; Lopes, T.N.; da Silva, A.F.T.; Santi, L.; Beys-Da-Silva, W.O.; Yates, J.R.; Bustamante-Filho, I.C. Changes in porcine cauda epididymal fluid proteome by disrupting the HPT axis: Unveiling potential mechanisms of male infertility. Mol. Reprod. Dev. 2020, 87, 952–965. [Google Scholar] [CrossRef] [PubMed]
- Kunkitti, P.; Bergqvist, A.-S.; Sjunnesson, Y.; Axnér, E. The ability of feline spermatozoa in different epididymal regions to undergo capacitation and acrosome reaction. Anim. Reprod. Sci. 2015, 161, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Kunkitti, P.; Axnér, E.; Bergqvist, A.-S.; Sjunnesson, Y. In vitro fertilization using frozen-thawed feline epididymal spermatozoa from corpus and cauda regions. Theriogenology 2016, 86, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Kunkitti, P.; Bergqvist, A.-S.; Sjunnesson, Y.; Johannisson, A.; Axnér, E. The tolerance of feline corpus and cauda spermatozoa to cryostress. Theriogenology 2015, 85, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Esponda, P.; Bedford, J.M. The influence of body temperature and castration on the protein composition of fluid in the rat cauda epididymidis. Reproduction 1986, 78, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Regalado, F.; Esponda, P.; Nieto, A. Temperature and androgens regulate the biosynthesis of secretory proteins from rabbit cauda epididymidis. Mol. Reprod. Dev. 1993, 36, 448–453. [Google Scholar] [CrossRef]
- Wong, P.Y.D.; Au, C.L.; Bedford, J.M. Biology of the Scrotum. II. Suppression by Abdominal Temperature of Transepithelial Ion and Water Transport in the Cauda Epididymidis. Biol. Reprod. 1982, 26, 683–689. [Google Scholar] [CrossRef]
- Foldesy, R.G.; Bedford, J.M. Biology of the Scrotum. I. Temperature and Androgen as Determinants of the Sperm Storage Capacity of the Rat Cauda Epididymidis. Biol. Reprod. 1982, 26, 673–682. [Google Scholar] [CrossRef]
- Bedford, J.M. Human Spermatozoa and Temperature: The Elephant in the Room. Biol. Reprod. 2015, 93, 97. [Google Scholar] [CrossRef] [PubMed]
- Arrighi, S. Are the basal cells of the mammalian epididymis still an enigma? Reproduction, Fertility and Development 2014, 26, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.L.; Flickinger, C.J. Development of cell types and of regional differences in the postnatal rat epididymis. Am. J. Anat. 1979, 154, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, J.; Gregory, M.; Pinel, L.; Cyr, D.G. Differential gene expression and hallmarks of stemness in epithelial cells of the developing rat epididymis. Cell Tissue Res. 2022, 389, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.S. Isolation and Cell Culture of the Epithelial Cells of Cauda Epididymidis of the Bull1. Biol. Reprod. 1985, 33, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Bongso, A. and Trounson, A. Andrology: Evaluation of motility, fertilizing ability and embryonic development of murine epididymal sperm after coculture with epididymal epithelium. Human reproduction 1996, 11, 1451–1456. [Google Scholar] [CrossRef]
- Moore, H.D.M.; Hartman, T.D. In-vitro development of the fertilizing ability of hamster epididymal spermatozoa after co-culture with epithelium from the proximal cauda epididymidis. Reproduction 1986, 78, 347–352. [Google Scholar] [CrossRef]
- Orgebin-Crist, M.C. , Jonas-Davies, J., Storey, P. and Olson, G.E. Effect of d-valine and cytosine arabinoside on [3 H] thymidine incorporation in rat and rabbit epididymal epithelial cell cultures. In vitro 1984, 20, 45–52. [Google Scholar] [CrossRef]
- Castellón, E.; Huidobro, C. Androgen regulation of glycosidase secretion in epithelial cell cultures from human epididymis. Hum. Reprod. 1999, 14, 1522–1527. [Google Scholar] [CrossRef]
- Higgins, S.J. , Young, P. and Cunha, G.R. Induction of functional cytodifferentiation in the epithelium of tissue recombinants: II. Instructive induction of Wolffian duct epithelia by neonatal seminal vesicle mesenchyme. Development 1989, 106, 235–250. [Google Scholar] [CrossRef]
- De Gendt, K.; Verhoeven, G. Tissue- and cell-specific functions of the androgen receptor revealed through conditional knockout models in mice. Mol. Cell. Endocrinol. 2012, 352, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Hinton, B.T.; Palladino, M.A. Epididymal epithelium: Its contribution to the formation of a luminal fluid microenvironment. Microsc. Res. Tech. 1995, 30, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.-L.; Li, S.; Huang, J.-H.; Yang, D.-L.; Zhang, G.; Chen, S.-L.; Ruan, Y.-C.; Ye, K.-N.; Cheng, C.H.K.; Zhou, W.-L. Sodium Coupled Bicarbonate Influx Regulates Intracellular and Apical pH in Cultured Rat Caput Epididymal Epithelium. PLOS ONE 2011, 6, e22283. [Google Scholar] [CrossRef] [PubMed]
- Cornwall, G.A. New insights into epididymal biology and function. Hum. Reprod. Updat. 2008, 15, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Shum, W.W.C.; Da Silva, N.; Brown, D.; Breton, S. Regulation of luminal acidification in the male reproductive tract via cell–cell crosstalk. J. Exp. Biol. 2009, 212, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Leir, S.-H.; Yin, S.; Kerschner, J.L.; Cosme, W.; Harris, A. An atlas of human proximal epididymis reveals cell-specific functions and distinct roles for CFTR. Life Sci. Alliance 2020, 3, e202000744. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A. , Shur, B.D. and Hess, R.A. Estrogen, efferent ductules, and the epididymis. Biology of reproduction 2011, 84, 207–217. [Google Scholar] [CrossRef]
- Hermo, L. , Oka, R. and Morales, C.R. Secretion and endocytosis in the male reproductive tract: a role in sperm maturation. International review of cytology 1994, 154, 105–189. [Google Scholar]
- Sullivan, R.; Saez, F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction 2013, 146, R21–R35. [Google Scholar] [CrossRef]
- Cyr, D.G.; Robaire, B.; Hermo, L. Structure and turnover of junctional complexes between principal cells of the rat epididymis. Microsc. Res. Tech. 1995, 30, 54–66. [Google Scholar] [CrossRef]
- Cyr, D.G.; Gregory, M.; Dubé. ; Dufresne, J.; Chan, P.T.K.; Hermo, L. Orchestration of occludins, claudins, catenins and cadherins as players involved in maintenance of the blood-epididymal barrier in animals and humans. Asian J. Androl. 2007, 9, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Y.; Zhang, B.L.; Gao, D.Y.; Li, Q.; Xu, X.Y.; Shum, W. Epididymal epithelial degeneration and lipid metabolism impairment account for male infertility in occludin knockout mice. Front. Endocrinol. 2022, 13, 1069319. [Google Scholar] [CrossRef] [PubMed]
- Arrighi, S.; Romanello, M.G.; Domeneghini, C. Morphological examination of epididymal epithelium in the mule (E. hinnus) in comparison with parental species (E. asinus and E. caballus).. 1991, 6, 325–37. [Google Scholar]
- Schön, J.; Blottner, S. Seasonal variations in the epididymis of the roe deer (Capreolus capreolus). Anim. Reprod. Sci. 2009, 111, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Nashan, D.; Sorg, C.; Oberpenning, F.; Schulze, H.; Nieschlag, E.; Cooper, T.G. Basal Cells of the Human Epididymis—Antigenic and Ultrastructural Similarities to Tissue-Fixed Macrophages1. Biol. Reprod. 1994, 50, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Seiler, P.; Wenzel, I.; Wagenfeld, A.; Yeung, C.H.; Nieschlag, E.; Cooper, T.G. The appearance of basal cells in the developing murine epididymis and their temporal expression of macrophage antigens. Int. J. Androl. 1998, 21, 217–226. [Google Scholar] [CrossRef]
- Shum, W.W.C.; Da Silva, N.; McKee, M.; Smith, P.J.; Brown, D.; Breton, S. Transepithelial Projections from Basal Cells Are Luminal Sensors in Pseudostratified Epithelia. Cell 2008, 135, 1108–1117. [Google Scholar] [CrossRef]
- Mou, H.; Vinarsky, V.; Tata, P.R.; Brazauskas, K.; Choi, S.; Crooke, A.K.; Zhang, B.; Solomon, G.M.; Turner, B.; Bihler, H.; et al. Dual SMAD Signaling Inhibition Enables Long-Term Expansion of Diverse Epithelial Basal Cells. Cell Stem Cell 2016, 19, 217–231. [Google Scholar] [CrossRef]
- Mandon, M.; Hermo, L.; Cyr, D.G. Isolated Rat Epididymal Basal Cells Share Common Properties with Adult Stem Cells1. Biol. Reprod. 2015, 93, 115. [Google Scholar] [CrossRef]
- Cyr, D.G.; Pinel, L. Emerging organoid models to study the epididymis in male reproductive toxicology. Reprod. Toxicol. 2022, 112, 88–99. [Google Scholar] [CrossRef]
- Yalcin-Ozuysal. ; Fiche, M.; Guitierrez, M.; Wagner, K.-U.; Raffoul, W.; Brisken, C. Antagonistic roles of Notch and p63 in controlling mammary epithelial cell fates. Cell Death Differ. 2010, 17, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Acebron, S.P.; Herbst, J.; Hatiboglu, G.; Niehrs, C. Post-transcriptional Wnt Signaling Governs Epididymal Sperm Maturation. Cell 2015, 163, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Boyer, A.; Yeh, J.R.; Zhang, X.; Paquet, M.; Gaudin, A.; Nagano, M.C.; Boerboom, D. CTNNB1 Signaling in Sertoli Cells Downregulates Spermatogonial Stem Cell Activity via WNT4. PLOS ONE 2012, 7, e29764. [Google Scholar] [CrossRef] [PubMed]
- Girardet, L.; Cyr, D.G.; Belleannée, C. Arl13b controls basal cell stemness properties and Hedgehog signaling in the mouse epididymis. Cell. Mol. Life Sci. 2022, 79, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Zhang, L.; Yang, L.; Yang, P.; Ullah, S.; Zhang, Q.; Chen, Q. Ultrastructure of epididymal epithelium and its interaction with the sperm in the soft-shelled turtle Pelodiscus sinensis. Micron 2013, 54-55, 65–74. [Google Scholar] [CrossRef]
- Robaire, B.; Viger, R.S. Regulation of Epididymal Epithelial Cell Functions1. Biol. Reprod. 1995, 52, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.A.M.E.S. , Garrett, S.H. and Garrett, J.E. Differential patterns of regulated gene expression in the adult rat epididymis. Annals of the New York Academy of Sciences 1991, 637, 384–398. [Google Scholar] [CrossRef]
- Hermo, L.; Dworkin, J.; Oko, R. Role of epithelial clear cells of the rat epididymis in the disposal of the contents of cytoplasmic droplets detached from spermatozoa. Am. J. Anat. 1988, 183, 107–124. [Google Scholar] [CrossRef]
- Hermo, L.; Oko, R.; Robaire, B. Epithelial cells of the epididymis show regional variations with respect to the secretion or endocytosis of immobilin as revealed by light and electron microscope immunocytochemistry. Anat. Rec. 1992, 232, 202–220. [Google Scholar] [CrossRef]
- Hamilton, D.W.; Olson, G.E.; Cooper, T.G. Regional variation in the surface morphology of the epithelium of the rat ductuli efferentes, ductus epididymidis and vas deferens. Anat. Rec. 1977, 188, 13–27. [Google Scholar] [CrossRef]
- Moore, H.D.M.; Bedford, J.M. Short-term effects of androgen withdrawal on the structure of different epithelial cells in the rat epididymis. Anat. Rec. 1979, 193, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Belleannée, C.; Da Silva, N.; Shum, W.W.; Brown, D.; Breton, S.; Roy, J.W.; Hill, E.; Ruan, Y.C.; Vedovelli, L.; Păunescu, T.G.; et al. Role of purinergic signaling pathways in V-ATPase recruitment to apical membrane of acidifying epididymal clear cells. Am. J. Physiol. Physiol. 2010, 298, C817–C830. [Google Scholar] [CrossRef]
- Kujala, M.; Hihnala, S.; Tienari, J.; Kaunisto, K.; Hästbacka, J.; Holmberg, C.; Kere, J.; Höglund, P. Expression of ion transport-associated proteins in human efferent and epididymal ducts. Reproduction 2007, 133, 775–784. [Google Scholar] [CrossRef]
- Da Silva, N.; Shum, W.W.C.; Breton, S. Regulation of vacuolar proton pumping ATPase-dependent luminal acidification in the epididymis. Asian J. Androl. 2007, 9, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.-H.; Leung, G.P.; Leung, M.C.; Shum, W.W.; Zhou, W.-L.; Wong, P.Y. Cell–cell Interaction Underlies Formation of Fluid in the Male Reproductive Tract of the Rat. J. Gen. Physiol. 2005, 125, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R. , Legare, C., Thabet, M. and Thimon, V. Gene expression in the epididymis of normal and vasectomized men: what can we learn about human sperm maturation? Journal of andrology 2011, 32, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.S.; Turner, T.T.; Finger, J.N.; Owtscharuk, T.L.; Kopf, G.S.; Jelinsky, S.A. Identification of epididymis-specific transcripts in the mouse and rat by transcriptional profiling. Asian J. Androl. 2007, 9, 522–527. [Google Scholar] [CrossRef]
- Xie, S.; Zhu, Y.; Ma, L.; Lu, Y.; Zhou, J.; Gui, Y.; Cao, L. Genome-wide profiling of gene expression in the epididymis of alpha-chlorohydrin-induced infertile rats using an oligonucleotide microarray. Reprod. Biol. Endocrinol. 2010, 8, 37–37. [Google Scholar] [CrossRef]
- Krutskikh, A.; Poliandri, A.; Dacheux, J.L.; Poutanen, M.; Huhtaniemi, I.; Cabrera-Sharp, V. Epididymal protein Rnase10 is required for post-testicular sperm maturation and male fertility. FASEB J. 2012, 26, 4198–4209. [Google Scholar] [CrossRef]
- Devor, E.J.; Moffat-Wilson, K.A.; Galbraith, J.J. LOC 390443 (RNase 9) on Chromosome 14q11.2 Is Related to the RNase A Superfamily and Contains a Unique Amino-Terminal Preproteinlike Sequence. Hum. Biol. 2004, 76, 921–935. [Google Scholar] [CrossRef]
- Castella, S.; Fouchécourt, S.; Teixeira-Gomes, A.P.; Vinh, J.; Belghazi, M.; Dacheux, F.; Dacheux, J.-L. Identification of a Member of a New RNase A Family Specifically Secreted by Epididymal Caput Epithelium1. Biol. Reprod. 2004, 70, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.A. , Cai, Y., Sang, Y., Blecha, F. and Zhang, G. Cross-species analysis of the mammalian β-defensin gene family: presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiological genomics 2005, 23, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Semple, C.A. , Gautier, P., Taylor, K. and Dorin, J.R. The changing of the guard: Molecular diversity and rapid evolution of β-defensins. Molecular diversity 2006, 10, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Semple, C.A. , Gautier, P., Taylor, K. and Dorin, J.R. The changing of the guard: Molecular diversity and rapid evolution of β-defensins. Molecular diversity 2006, 10, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Pazgier, M. , Hoover, D.M., Yang, D., Lu, W. and Lubkowski, J. Human β-defensins. Cellular and Molecular Life Sciences CMLS 2006, 63, 1294–1313. [Google Scholar] [CrossRef] [PubMed]
- Schutte, B.C. , Mitros, J.P., Bartlett, J.A., Walters, J.D., Jia, H.P., Welsh, M.J., Casavant, T.L. and McCray Jr, P.B. Discovery of five conserved β-defensin gene clusters using a computational search strategy. Proceedings of the National Academy of Sciences 2002, 99, 2129–2133. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Ouchi, Y. Antimicrobial peptide defensin: Identification of novel isoforms and the characterization of their physiological roles and their significance in the pathogenesis of diseases. Proc. Jpn. Acad. Ser. B 2012, 88, 152–166. [Google Scholar] [CrossRef]
- Ribeiro, C.M. , Silva, E.J., Hinton, B.T. and Avellar, M.C.W. β-defensins and the epididymis: contrasting influences of prenatal, postnatal, and adult scenarios. Asian journal of andrology 2016, 18, 323. [Google Scholar]
- Hu, S.-G.; Zou, M.; Yao, G.-X.; Ma, W.-B.; Zhu, Q.-L.; Li, X.-Q.; Chen, Z.-J.; Sun, Y. Androgenic regulation of beta-defensins in the mouse epididymis. Reprod. Biol. Endocrinol. 2014, 12, 76–76. [Google Scholar] [CrossRef]
- Jalkanen, J.; Huhtaniemi, I.; Poutanen, M. Discovery and characterization of new epididymis-specific beta-defensins in mice. Biochim. et Biophys. Acta (BBA) - Gene Struct. Expr. 2005, 1730, 22–30. [Google Scholar] [CrossRef]
- Pujianto, D.A.; Muliawati, D.; Rizki, M.D.; Parisudha, A.; Hardiyanto, L. Mouse defensin beta 20 (Defb20) is expressed specifically in the caput region of the epididymis and regulated by androgen and testicular factors. Reprod. Biol. 2020, 20, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Ni, M.; Xie, S.; Zhang, Y.; Zhang, C.; Ni, Z.; Chu, C.; Wu, L.; Zhou, Y.; Zhang, Y. DICER1 regulates antibacterial function of epididymis by modulating transcription of β-defensins. J. Mol. Cell Biol. 2018, 11, 408–420. [Google Scholar] [CrossRef]
- Radhakrishnan, Y. , Hamil, K.G., Yenugu, S., Young, S.L., French, F.S. and Hall, S.H. Identification, characterization, and evolution of a primate β-defensin gene cluster. Genes & Immunity 2005, 6, 203–210. [Google Scholar]
- Rodríguez-Jiménez, F.J. , Krause, A., Schulz, S., Forssmann, W.G., Conejo-Garcia, J.R., Schreeb, R. and Motzkus, D. Distribution of new human β-defensin genes clustered on chromosome 20 in functionally different segments of epididymis. Genomics 2003, 81, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. , Diao, H., Ni, Z., Hu, S., Yu, H. and Zhang, Y. The epididymis-specific antimicrobial peptide β-defensin 15 is required for sperm motility and male fertility in the rat (Rattus norvegicus). Cellular and Molecular Life Sciences 2011, 68, 697–708. [Google Scholar] [CrossRef]
- Björkgren, I.; Alvarez, L.; Blank, N.; Balbach, M.; Turunen, H.; Laajala, T.D.; Toivanen, J.; Krutskikh, A.; Wahlberg, N.; Huhtaniemi, I.; et al. Targeted inactivation of the mouse epididymal beta-defensin 41 alters sperm flagellar beat pattern and zona pellucida binding. Mol. Cell. Endocrinol. 2016, 427, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Dubé, E.; Hermo, L.; Chan, P.T.; Cyr, D.G. Alterations in Gene Expression in the Caput Epididymides of Nonobstructive Azoospermic Men1. Biol. Reprod. 2008, 78, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Tollner, T.L.; Venners, S.A.; Hollox, E.J.; Yudin, A.I.; Liu, X.; Tang, G.; Xing, H.; Kays, R.J.; Lau, T.; Overstreet, J.W.; et al. A Common Mutation in the Defensin DEFB126 Causes Impaired Sperm Function and Subfertility. Sci. Transl. Med. 2011, 3, 92ra65–92ra65. [Google Scholar] [CrossRef]
- Aram, R.; Chan, P.T.K.; Cyr, D.G. Beta-defensin126 is correlated with sperm motility in fertile and infertile men†. Biol. Reprod. 2019, 102, 92–101. [Google Scholar] [CrossRef]
- Tollner, T.L.; Yudin, A.I.; Treece, C.A.; Overstreet, J.W.; Cherr, G.N. Macaque sperm coating protein DEFB126 facilitates sperm penetration of cervical mucus. Hum. Reprod. 2008, 23, 2523–2534. [Google Scholar] [CrossRef]
- Charkoftaki, G. , Wang, Y., McAndrews, M., Bruford, E.A., Thompson, D.C., Vasiliou, V. and Nebert, D.W. Update on the human and mouse lipocalin (LCN) gene family, including evidence the mouse Mup cluster is result of an “evolutionary bloom”. Human genomics 2019, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hamil, K.G.; Liu, Q.; Sivashanmugam, P.; Anbalagan, M.; Yenugu, S.; Soundararajan, R.; Grossman, G.; Rao, A.; E Birse, C.; Ruben, S.M.; et al. LCN6, a novel human epididymal lipocalin. Reprod. Biol. Endocrinol. 2003, 1, 112–112. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kaur, S.; Guha, S.; Batra, S.K. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta BBA Rev. Cancer 2012, 1826, 129–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. and Rui, L. Lipocalin 13 regulation of glucose and lipid metabolism in obesity. Vitamins & Hormones 2013, 91, 369–383. [Google Scholar]
- Åkerstrom, B.; Flower, D.R.; Salier, J.-P. Lipocalins: unity in diversity. Biochim. et Biophys. Acta (BBA) - Protein Struct. Mol. Enzym. 2000, 1482, 1–8. [Google Scholar] [CrossRef]
- Fouchécourt, S.; Lareyre, J.-J.; Chaurand, P.; DaGue, B.B.; Suzuki, K.; Ong, D.E.; Olson, G.E.; Matusik, R.J.; Caprioli, R.M.; Orgebin-Crist, M.-C. Identification, Immunolocalization, Regulation, and Postnatal Development of the Lipocalin EP17 (Epididymal Protein of 17 Kilodaltons) in the Mouse and Rat Epididymis. Endocrinology 2003, 144, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Lareyre, J.-J.; Sánchez, D.; Gutierrez, G.; Araki, Y.; Matusik, R.J.; Orgebin-Crist, M.-C. Molecular evolution of epididymal lipocalin genes localized on mouse chromosome 2. Gene 2004, 339, 49–59. [Google Scholar] [CrossRef]
- Chu, S.-T.; Lee, Y.-C.; Nein, K.-M.; Chen, Y.-H. Expression, immunolocalization and sperm-association of a protein derived from 24p3 gene in mouse epididymis. Mol. Reprod. Dev. 2000, 57, 26–36. [Google Scholar] [CrossRef]
- Garrett, S.H.; Garrett, J.E.; Douglass, J. In situ histochemical analysis of region-specific gene expression in the adult rat epididymis. Mol. Reprod. Dev. 1991, 30, 1–17. [Google Scholar] [CrossRef]
- Rankin, T.L.; Tsuruta, K.J.; Holland, M.K.; Griswold, M.D.; Orgebin-Crist, M.-C. Isolation, Immunolocalization, and Sperm-Association of Three Proteins of 18, 25, and 29 Kilodaltons Secreted by the Mouse Epididymis1. Biol. Reprod. 1992, 46, 747–766. [Google Scholar] [CrossRef]
- Ong, D.E.; Newcomer, M.E.; Lareyre, J.-J.; Orgebin-Crist, M.-C. Epididymal retinoic acid-binding protein. Biochim. et Biophys. Acta (BBA) - Protein Struct. Mol. Enzym. 2000, 1482, 209–217. [Google Scholar] [CrossRef]
- Tone, M.; Nolan, K.F.; Walsh, L.A.; Tone, Y.; Thompson, S.A.; Waldmann, H. Structure and chromosomal location of mouse and human CD52 genes. Biochim. et Biophys. Acta (BBA) - Gene Struct. Expr. 1999, 1446, 334–340. [Google Scholar] [CrossRef]
- Pujianto, D.A.; Permatasari, S. Mouse CD52 is predominantly expressed in the cauda epididymis, regulated by androgen and lumicrine factors. J. Hum. Reprod. Sci. 2021, 14, 350–355. [Google Scholar] [CrossRef]
- Ernesto, J.I.; Muñoz, M.W.; Battistone, M.A.; Vasen, G.; Martínez-López, P.; Orta, G.; Figueiras-Fierro, D.; De la Vega-Beltran, J.L.; Moreno, I.A.; Guidobaldi, H.A.; et al. CRISP1 as a novel CatSper regulator that modulates sperm motility and orientation during fertilization. J. Cell Biol. 2015, 210, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, V.G.; Maldera, J.A.; Willis, W.D.; Cohen, D.J.; Goulding, E.H.; Gelman, D.M.; Rubinstein, M.; Eddy, E.M.; Cuasnicu, P.S. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Dev. Biol. 2008, 320, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.W.; Battistone, M.A.; Carvajal, G.; Maldera, J.A.; Curci, L.; Torres, P.; Lombardo, D.; Pignataro, O.P.; Da Ros, V.G.; Cuasnicu, P.S.; et al. Influence of the genetic background on the reproductive phenotype of mice lacking Cysteine-Rich Secretory Protein 1 (CRISP1). Biol. Reprod. 2018, 99, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Haendler, B.E.R.N.A.R.D. , Krätzschmar, J., Theuring, F.R.A.N.Z. and Schleuning, W.D. Transcripts for cysteine-rich secretory protein-1 (CRISP-1; DE/AEG) and the novel related CRISP-3 are expressed under androgen control in the mouse salivary gland. Endocrinology 1993, 133, 192–198. [Google Scholar] [CrossRef]
- Jalkanen, J.; Huhtaniemi, I.; Poutanen, M. Mouse Cysteine-Rich Secretory Protein 4 (CRISP4): A Member of the Crisp Family Exclusively Expressed in the Epididymis in an Androgen-Dependent Manner1. Biol. Reprod. 2005, 72, 1268–1274. [Google Scholar] [CrossRef]
- Cohen, D.J.; Maldera, J.A.; Vasen, G.; Ernesto, J.I.; Muñoz, M.W.; Battistone, M.A.; Cuasnicú, P.S. Epididymal Protein CRISP1 Plays Different Roles During the Fertilization Process. J. Androl. 2011, 32, 672–678. [Google Scholar] [CrossRef]
- Carvajal, G.; Brukman, N.G.; Muñoz, M.W.; Battistone, M.A.; Guazzone, V.A.; Ikawa, M.; Haruhiko, M.; Lustig, L.; Breton, S.; Cuasnicu, P.S. Impaired male fertility and abnormal epididymal epithelium differentiation in mice lacking CRISP1 and CRISP4. Sci. Rep. 2018, 8, 17531. [Google Scholar] [CrossRef]
- King, L.S.; Kozono, D.; Agre, P. From structure to disease: the evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004, 5, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.; Silberstein, C.; Beaulieu, V.; Piétrement, C.; Van Hoek, A.N.; Brown, D.; Breton, S. Postnatal Expression of Aquaporins in Epithelial Cells of the Rat Epididymis1. Biol. Reprod. 2006, 74, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Li, Y.; Bi, Z.W.; Yu, H.M.; Li, X.J. Expression and Immunohistochemical Localization of Aquaporin-1 in Male Reproductive Organs of the Mouse. Anat. Histol. Embryol. 2007, 37, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Squillacioti, C.; Mirabella, N.; Liguori, G.; Germano, G.; Pelagalli, A. Aquaporins Are Differentially Regulated in Canine Cryptorchid Efferent Ductules and Epididymis. Animals 2021, 11, 1539. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Lee, B.; Kim, J.; Kim, J.; Hong, S.H.; Kim, D.; Choi, S.; Cho, B.-N.; Cho, C. Expressional and functional analyses of epididymal SPINKs in mice. Gene Expr. Patterns 2019, 31, 18–25. [Google Scholar] [CrossRef]
- Jalkanen, J.; Kotimäki, M.; Huhtaniemi, I.; Poutanen, M. Novel epididymal protease inhibitors with Kazal or WAP family domain. Biochem. Biophys. Res. Commun. 2006, 349, 245–254. [Google Scholar] [CrossRef]
- Ma, L.; Yu, H.; Ni, Z.; Hu, S.; Ma, W.; Chu, C.; Liu, Q.; Zhang, Y. Spink13, an Epididymis-specific Gene of the Kazal-type Serine Protease Inhibitor (SPINK) Family, Is Essential for the Acrosomal Integrity and Male Fertility. J. Biol. Chem. 2013, 288, 10154–10165. [Google Scholar] [CrossRef]
- Mangiola, S.; A Doyle, M.; Papenfuss, A.T. Interfacing Seurat with the R tidy universe. Bioinformatics 2021, 37, 4100–4107. [Google Scholar] [CrossRef]
- Satija, R.; A Farrell, J.; Gennert, D.; Schier, A.F.; Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 2015, 33, 495–502. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Liu, Q.; Li, Y.-M.; Hall, S.H.; French, F.S.; Zhang, Y.-L. Genome-wide profiling of segmental-regulated transcriptomes in human epididymis using oligo microarray. Mol. Cell. Endocrinol. 2006, 250, 169–177. [Google Scholar] [CrossRef]
- Suzuki, K.; Yu, X.; Chaurand, P.; Araki, Y.; Lareyre, J.-J.; Caprioli, R.M.; Orgebin-Crist, M.-C.; Matusik, R.J. Epididymis-specific lipocalin promoters. Asian J. Androl. 2007, 9, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Yamanaka, H.; Taguchi, I.; Shida, K. Free Amino Acids in the Caput and the Cauda Epididymis of Adult Rats. Endocrinol. Jpn. 1976, 23, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Dacheux, J.-L.; Castella, S.; Gatti, J.L.; Dacheux, F. Epididymal cell secretory activities and the role of proteins in boar sperm maturation. Theriogenology 2005, 63, 319–341. [Google Scholar] [CrossRef]
- Axnér, E.; Linde-Forsberg, C.; Einarsson, S. Morphology and motility of spermatozoa from different regions of the epididymal duct in the domestic cat. Theriogenology 1999, 52, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, T.; Wada, M.; Anzai, M.; Hori, T. Artificial Insemination with Frozen Epididymal Sperm in Cats. J. Veter- Med Sci. 2003, 65, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Toyonaga, M.; Sato, Y.; Sasaki, A.; Kaihara, A.; Tsutsui, T. Artificial insemination with cryopreserved sperm from feline epididymides stored at 4 °C. Theriogenology 2011, 76, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H. , Martinez, E.A., Calvete, J.J., Pena Vega, F.J. and Roca, J. Seminal plasma: relevant for fertility? International Journal of Molecular Sciences 2021, 22, 4368. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Kvist, U.; Saravia, F.; Wallgren, M.; Johannissono, A.; Sanz, L.; Pena, F.; Martinez, E.; Roca, J.; Vazquez, J.; et al. The physiological roles of the boar ejaculate. 2020, 18, 1–21. [CrossRef]
- E Lake, P. The male reproductive tract of the fowl. . 1957, 91, 116–29. [Google Scholar]
- Lee, J.Y.; Dada, R.; Sabanegh, E.; Carpi, A.; Agarwal, A. Role of Genetics in Azoospermia. Urology 2011, 77, 598–601. [Google Scholar] [CrossRef]
- Maduro, M.R.; Lamb, D.J. Understanding new genetics of male infertility. . 2002, 168, 2197–205. [Google Scholar] [CrossRef]
- O'Hara, L.; Welsh, M.; Saunders, P.T.; Smith, L.B. Androgen Receptor Expression in the Caput Epididymal Epithelium Is Essential for Development of the Initial Segment and Epididymal Spermatozoa Transit. Endocrinology 2010, 152, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Witkin, S.S. , Jeremias, J., Bongiovanni, A.M. and Munoz, M.G. Immune regulation in the male genital tract. Infectious diseases in obstetrics and gynecology 1996, 4, 131–135. [Google Scholar] [CrossRef]
- Wagenlehner, F.; Pilatz, A.; Linn, T.; Diemer, T.; Schuppe, H.C.; Schagdarsurengin, U.; Hossain, H.; Meinhardt, A.; Ellem, S.; Risbridger, G.; et al. Prostatitis and andrological implications. . 2013, 65. [Google Scholar]
- Aitken, R.; Smith, T.; Jobling, M.; Baker, M.; De Iuliis, G. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–8. [Google Scholar] [CrossRef] [PubMed]
- Ghyselinck, N.B. , Dufaure, I., Lareyre, J.J., Rigaudiere, N., Mattei, M.G. and Dufaure, J.P. Structural organization and regulation of the gene for the androgen-dependent glutathione peroxidase-like protein specific to the mouse epididymis. Molecular Endocrinology 1993, 7, 258–272. [Google Scholar] [PubMed]
- Drevet, J.R. The antioxidant glutathione peroxidase family and spermatozoa: A complex story. Mol. Cell. Endocrinol. 2006, 250, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Wijayarathna, R.; Hedger, M.P. Activins, follistatin and immunoregulation in the epididymis. Andrology 2019, 7, 703–711. [Google Scholar] [CrossRef]
- Jones, R.C.; Dacheux, J.-L.; Nixon, B.; Ecroyd, H.W. Role of the epididymis in sperm competition. Asian J. Androl. 2007, 9, 493–499. [Google Scholar] [CrossRef]
- Elbashir, S.; Magdi, Y.; Rashed, A.; Henkel, R.; Agarwal, A. Epididymal contribution to male infertility: An overlooked problem. Andrologia 2020, 53, e13721. [Google Scholar] [CrossRef]
- Lemaire, R. [Contributions to the histochemical study of nuclear inclusions in the dog epididymis]. . 1960, 154, 460–3. [Google Scholar]
- Shorluian, P.M. [A case of primary cancer of the epididymis]. . 1960, 25. [Google Scholar]
- Krylov, V.S.; Borovikov, A.M. Microsurgical method of reuniting ductus epididymis. Fertil. Steril. 1984, 41, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Goyal, H.; Vig, M.M. Histochemical activity of alkaline phosphatase and acid phosphatase in the epididymis of mature intact and androgen-deprived bulls. . 1984, 45, 444–50. [Google Scholar] [PubMed]
- BRIZ, M.D. , BONET, S., PINART, B., EGOZCUE, J. and CAMPS, R. Comparative study of boar sperm coming from the caput, corpus, and cauda regions of the epididymis. Journal of andrology 1995, 16, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Setty, B.S.; Jehan, Q. Functional maturation of the epididymis in the rat. Reproduction 1977, 49, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, T.; Weng, X.; Yao, R.; Li, W.; Xie, L.; Yue, X.; Li, F. Antioxidant properties and transcriptome of cauda epididymis with different levels of fertility in Hu lambs. Theriogenology 2022, 182, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ahmed, S.; Ahmed, S.; Yangliu, Y.; Wang, H.; Cai, X. Analysis of long non-coding RNAs in epididymis of cattleyak associated with male infertility. Theriogenology 2020, 160, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Cyr, D.G.; Dufresne, J.; Gregory, M. Cellular junctions in the epididymis, a critical parameter for understanding male reproductive toxicology. Reprod. Toxicol. 2018, 81, 207–219. [Google Scholar] [CrossRef]
- Belleannée, C.; Thimon, V.; Sullivan, R. Region-specific gene expression in the epididymis. Cell Tissue Res. 2012, 349, 717–731. [Google Scholar] [CrossRef]
- Zhao, W.; Solangi, T.H.; Wu, Y.; Yang, X.; Xu, C.; Wang, H.; Zheng, X.; Cai, X.; Zhu, J. Comparative rna-seq analysis of region-specific miRNA expression in the epididymis of cattleyak. Reprod. Domest. Anim. 2021, 56, 555–576. [Google Scholar] [CrossRef]
- Wu, C.; Wang, C.; Zhai, B.; Zhao, Y.; Zhao, Z.; Yuan, Z.; Zhang, M.; Tian, K.; Fu, X. Study of microRNA Expression Profile in Different Regions of Ram Epididymis. Reprod. Domest. Anim. 2021, 56, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Carnett, J.B.; Levi, J.V.; E Pennington, M. The surgical treatment of sterility due to obstruction at the epididymis; together with a study of the morphology of human spermatozoa. . 1902, 15, 2–15. [Google Scholar]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Légaré, C.; Lamontagne-Proulx, J.; Breton, S.; Soulet, D. Revisiting structure/functions of the human epididymis. Andrology 2019, 7, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Abou-Haïla, A.; Fain-Maurel, M.-A. Regional differences of the proximal part of mouse epididymis: Morphological and histochemical characterization. Anat. Rec. 1984, 209, 197–208. [Google Scholar] [CrossRef]
- Kirchhoff, C. The dog as a model to study human epididymal function at a molecular level. Mol. Hum. Reprod. 2002, 8, 695–701. [Google Scholar] [CrossRef]
- Chen, H.; Alves, M.B.R.; Belleannée, C. Contribution of epididymal epithelial cell functions to sperm epigenetic changes and the health of progeny. Hum. Reprod. Updat. 2021, 28, 51–66. [Google Scholar] [CrossRef]
- Robaire, B.; Hamzeh, M. Androgen Action in the Epididymis. J. Androl. 2011, 32, 592–599. [Google Scholar] [CrossRef]
- Turner, T.T. , Bomgardner, D., Jacobs, J.P. and Nguyen, Q.A.T. Association of segmentation of the epididymal interstitium with segmented tubule function in rats and mice. REPRODUCTION-CAMBRIDGE- 2003, 125, 871–878. [Google Scholar] [CrossRef]
- Guyonnet, B.; Marot, G.; Dacheux, J.-L.; Mercat, M.-J.; Schwob, S.; Jaffrézic, F.; Gatti, J.-L. The adult boar testicular and epididymal transcriptomes. BMC Genom. 2009, 10, 369–369. [Google Scholar] [CrossRef]
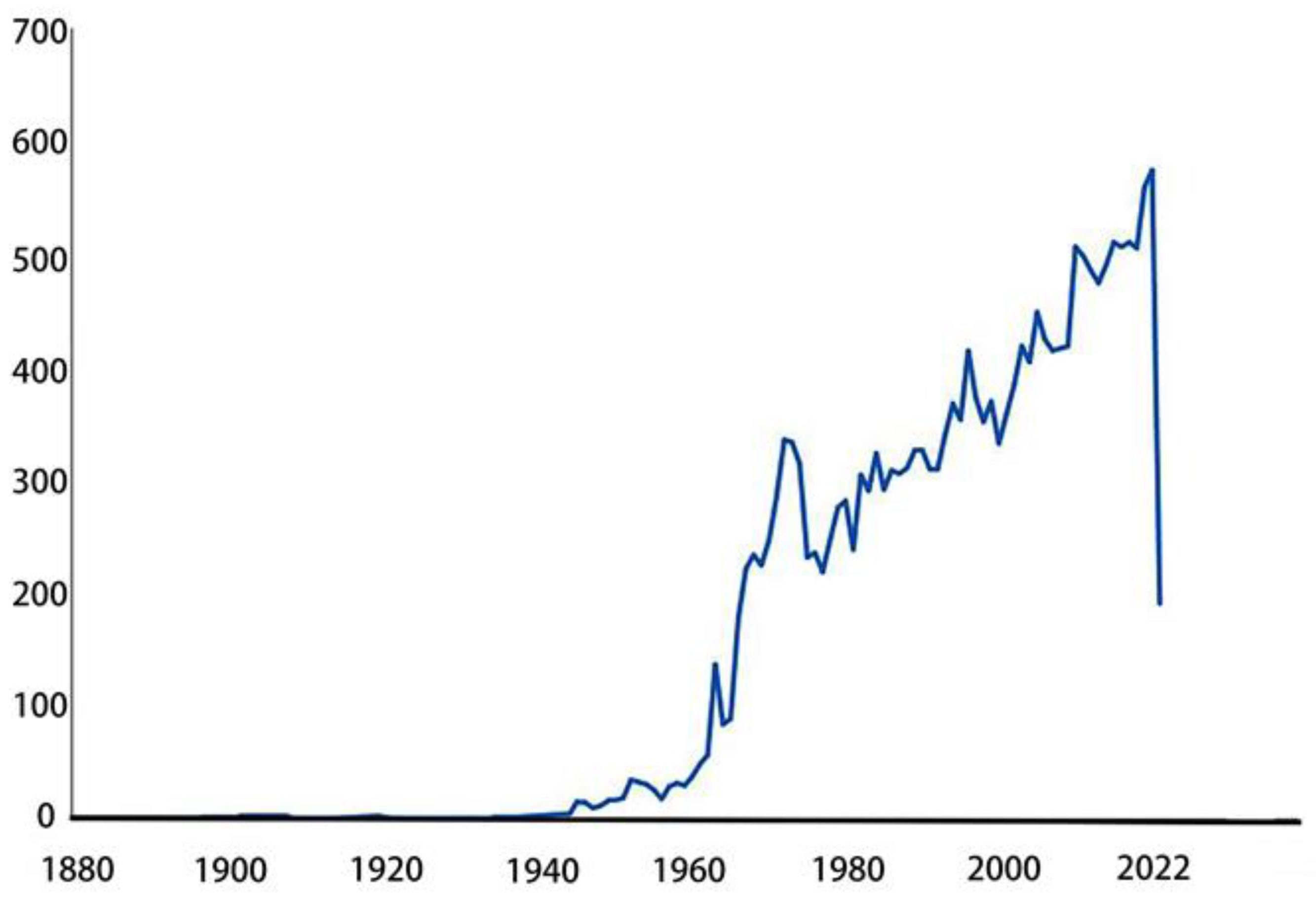
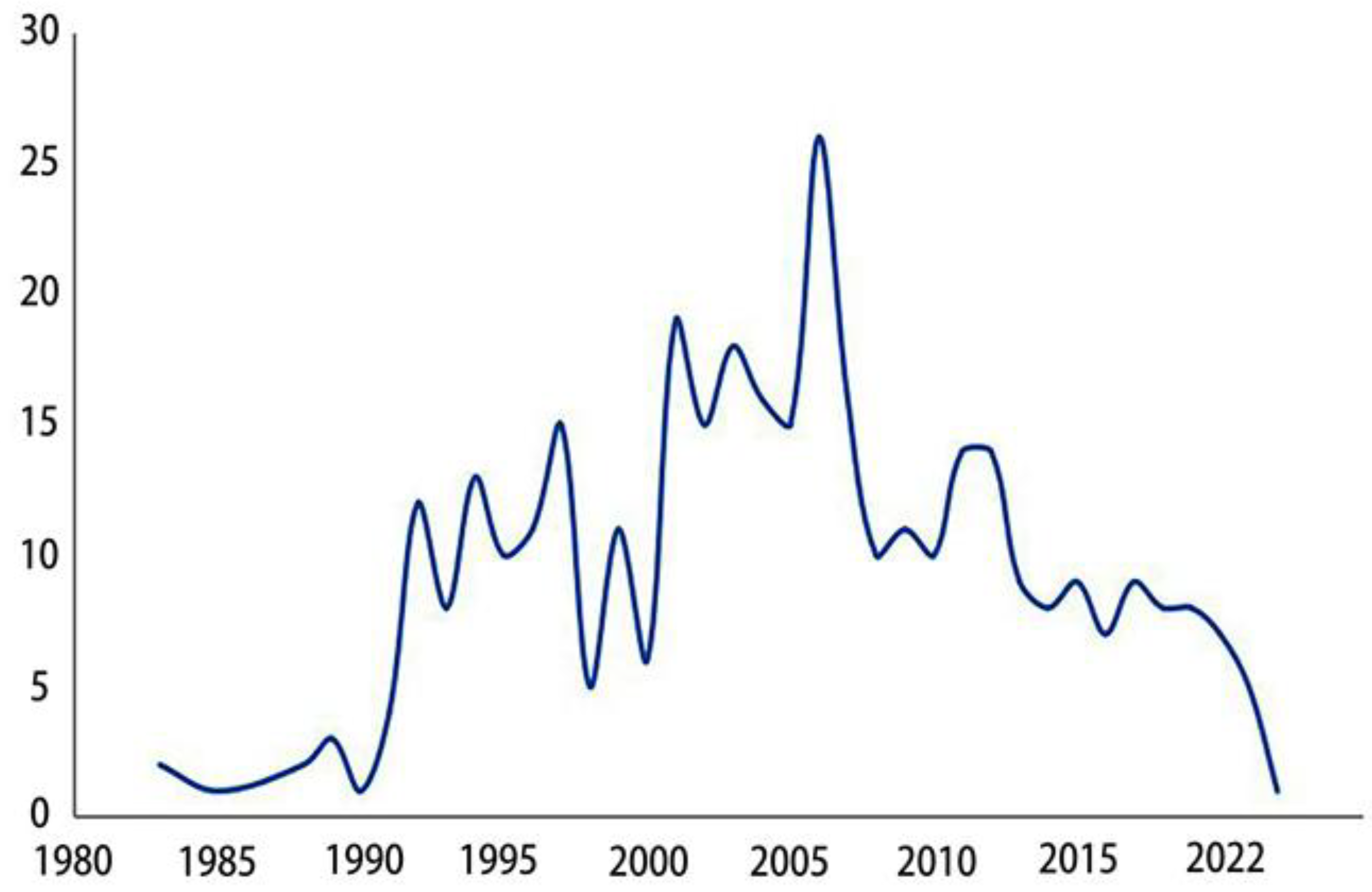
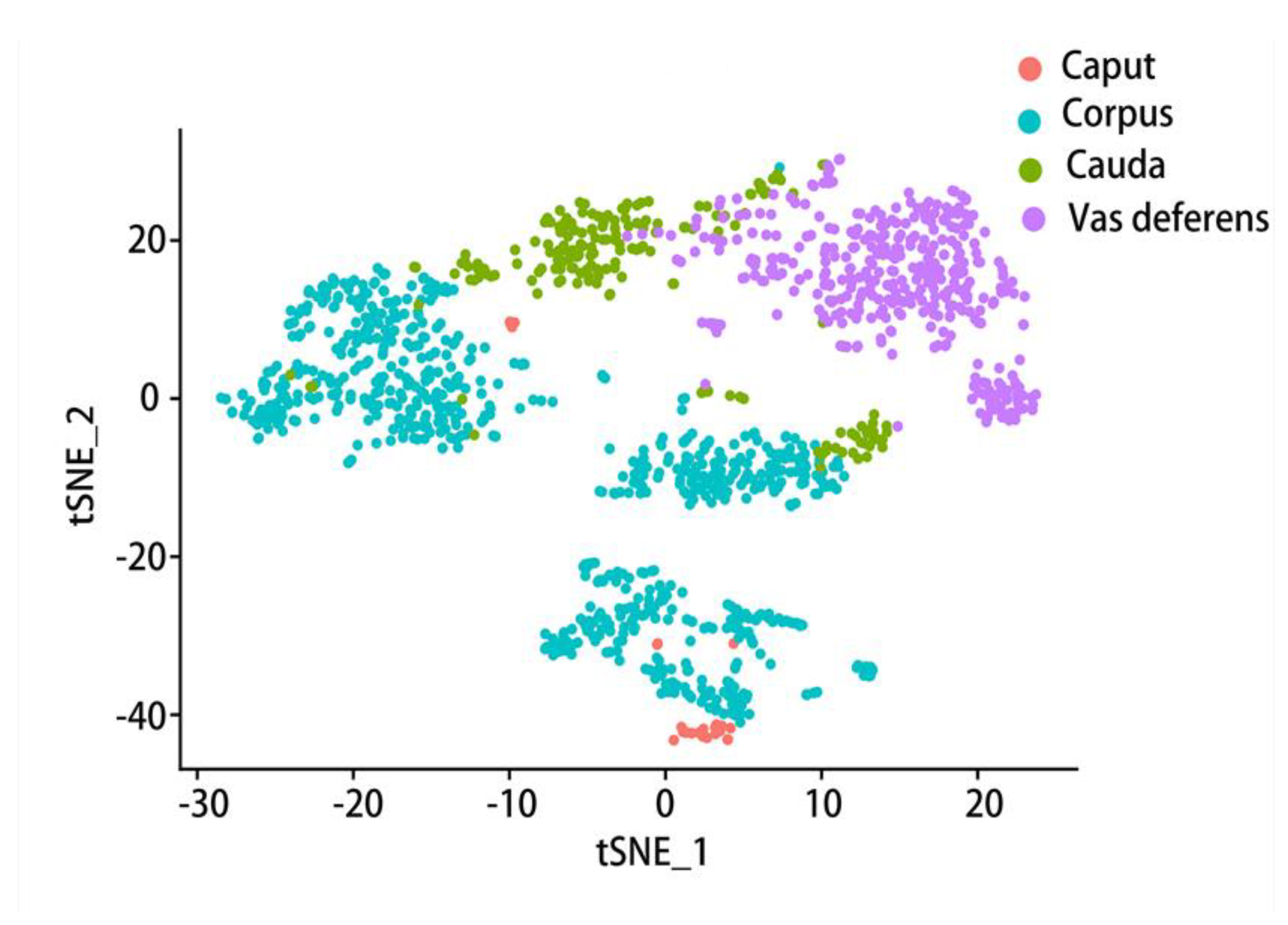
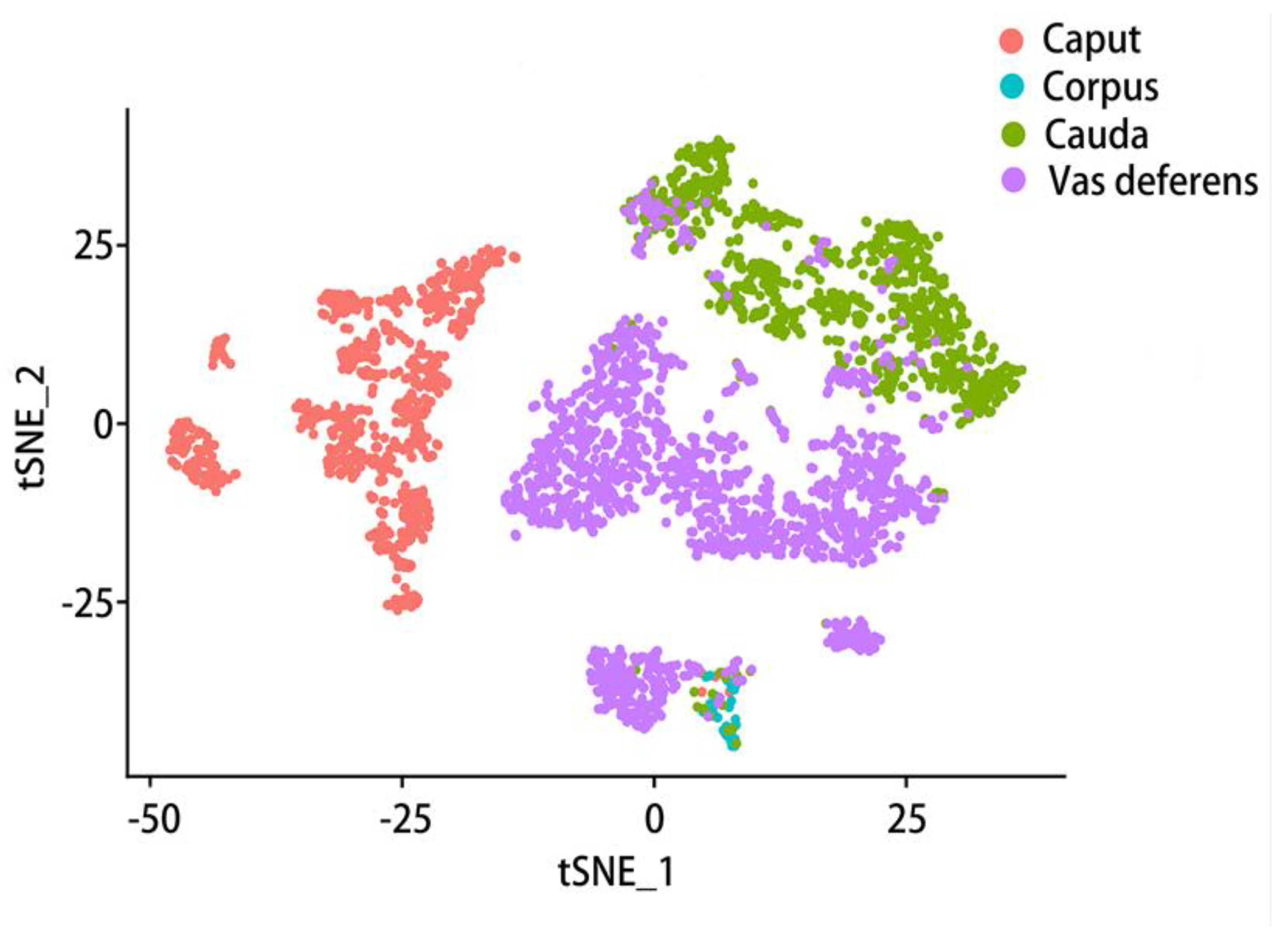
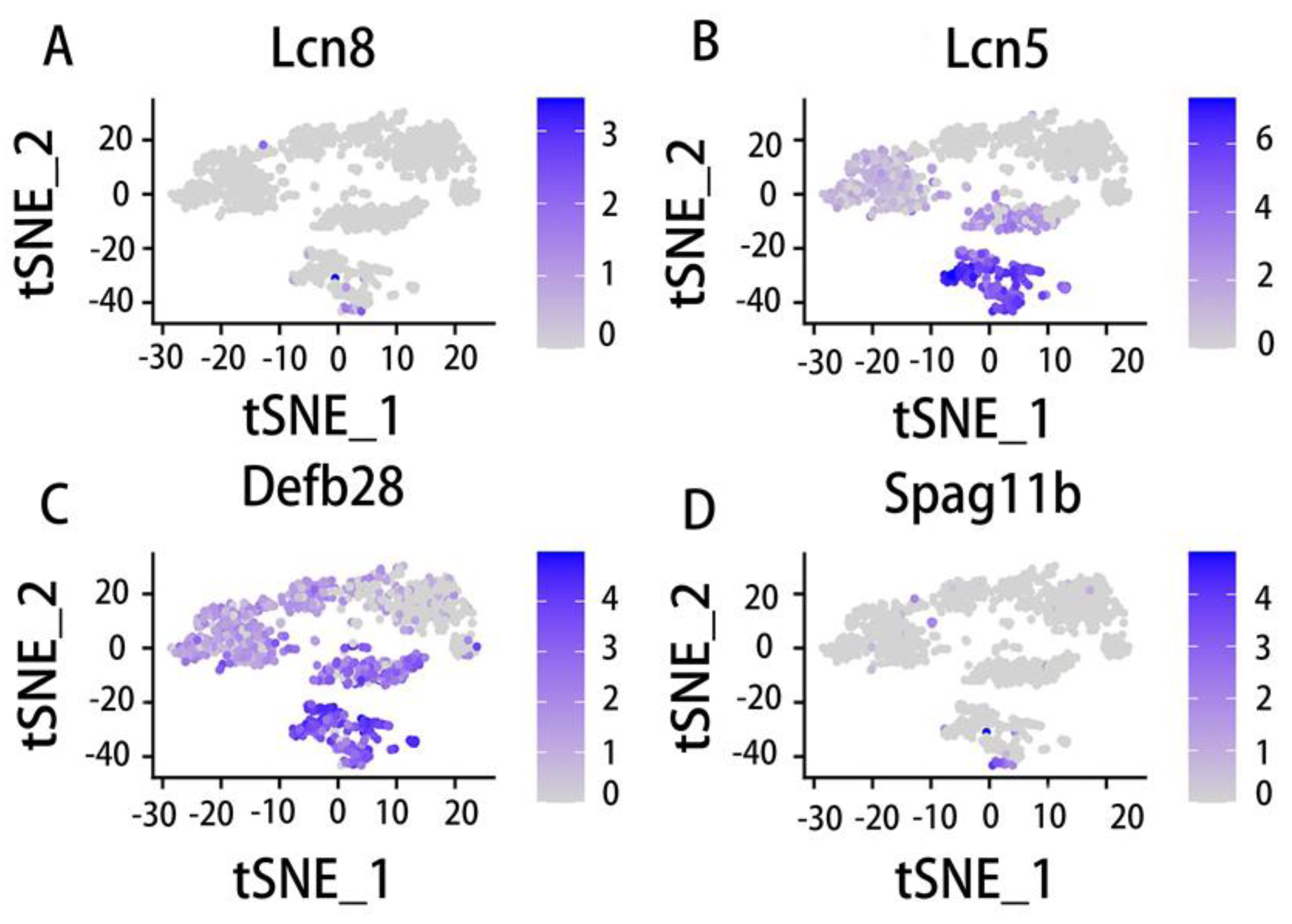
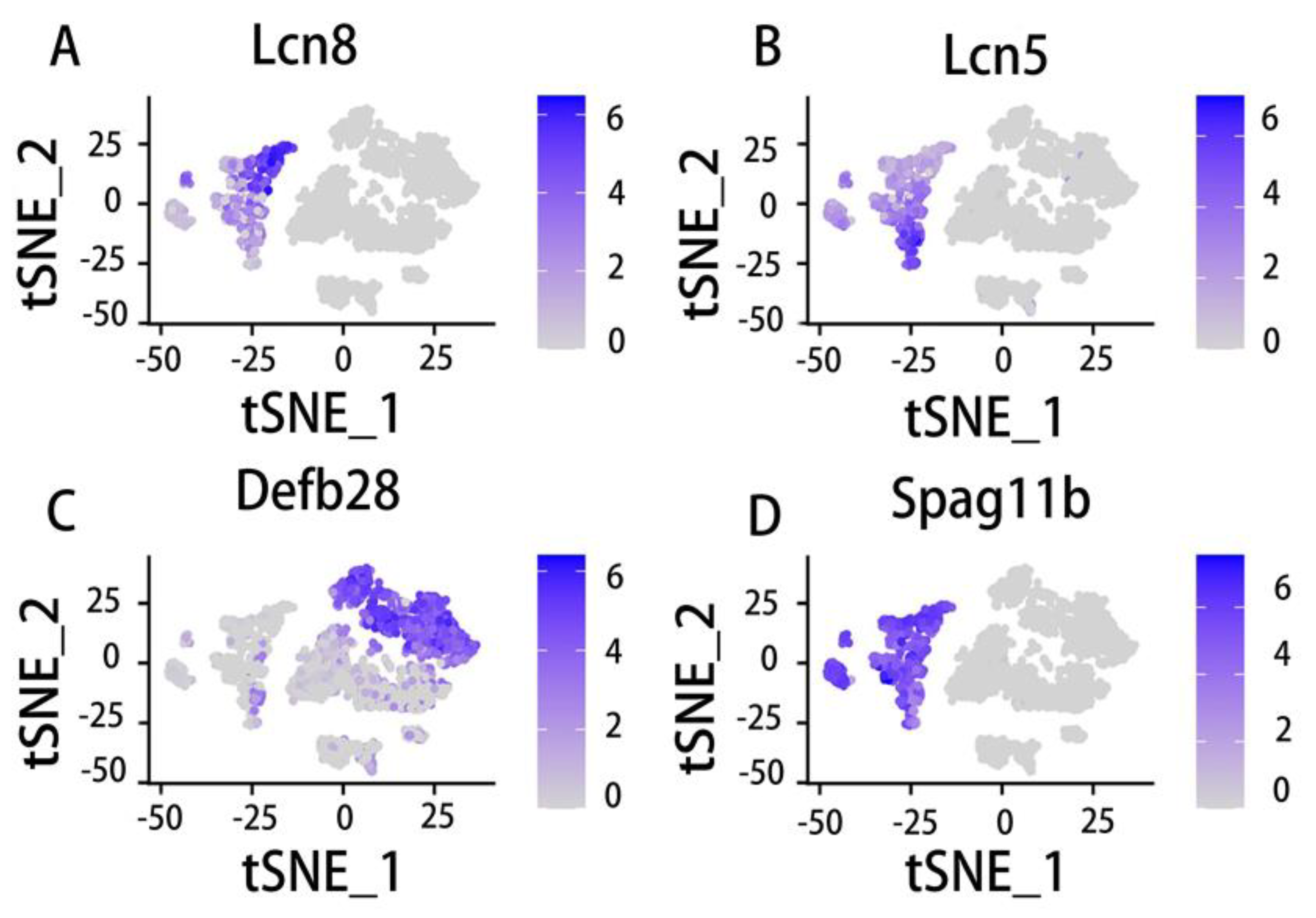
| Annual Range | Representative Article | New Technology | Findings |
|---|---|---|---|
| 1996-2000 | Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility | Gene-knockout | To explore the effect of specific genes on epididymal function |
| 2000-2001 | Stallion epididymal fluid proteome: qualitative and quantitative characterization; secretion and dynamic changes of major proteins | Proteomics | Understand epididymal mRNA expression and protein secretion |
| 2001-2005 | Dynamic changes in gene expression along the rat epididymis | Microarray technology | Obtain differential expression profiles of epididymis multiple genes. |
| 2005-2020 | The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis | Transcriptome sequencing technology | It aims to improve the integrity of epididymal transcriptome by using whole genome array, and provide higher sensitivity by studying fragments in each region |
| 2020-2022 | An atlas of cell types in the mouseepididymis and vas deferens | Single-cell RNA-Seq | A bird's-eye view of the cell composition of epididymis was conducted to determine the new biological characteristics of epididymal cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




