1. Introduction
The
Candida haemulonii complex is classically formed by
C. haemulonii sensu stricto,
C. duobushaemulonii and
C. haemulonii var.
vulnera, and the globally concerning fungus
C. auris is phylogenetically associated with this fungal complex [
1]. Molecular methods are required for the correct identification of these emerging opportunistic yeasts [
1]. Both the
C. haemulonii species complex and
C. auris are well known for their intrinsic multidrug-resistance profile to clinically available antifungal agents, particularly azoles and polyenes, which challenges the clinicians to achieve successful treatments. This scenario is worsened by the medical condition of the affected individuals, who generally have a compromised immunological system and/or severe underlying diseases, such as diabetes, pulmonary, renal and peripheral vascular diseases [
2].
Although species belonging to the
C. haemulonii complex have been reported in clinical cases around the world, they are still considered rare causative agents of human infections; however, some countries such as India, Korea, Kuwait, and Brazil [
2] have experienced an increase in the incidence of these fungi in recent years.
C. auris is considered a serious global health threat by the Centers for Disease Control and Prevention (CDC, USA), mainly due to its capacity to cause outbreaks in healthcare settings. To our knowledge, the ability to cause outbreaks is the major difference between
C. auris and the species comprising the
C. haemulonii complex, which can be attributed to the ability of
C. auris to resist to the commonly used disinfectants, persistence on medical devices and hospital surfaces for weeks, besides its ability to colonize axillae, nares, and groin of patients [
3]. Both the
C. haemulonii species complex and
C. auris can cause superficial to deep-seated infections and produce a wide range of virulence attributes [
2].
Cellular aggregation is a phenomenon that has been described in
C. auris, and isolates can be classified as either aggregative or non-aggregative. However, the understanding of this aggregation phenotype is still being studied [4-6]. According to Borman and coworkers [
4], aggregative strains of
C. auris exhibit large aggregates (many cells attached to one another) that cannot be disrupted by vortex mixing or detergents. The aggregative phenotype has been associated with colonizing isolates, while non-aggregative phenotype was mainly associated with isolates recovered from candidemia cases [
5]. The comparison of virulence between aggregative and non-aggregative phenotypes is still controversial. In this sense, Borman and coworkers [
4] demonstrated that aggregative
C. auris isolates exhibited less virulence compared to non-aggregative ones in the
Galleria mellonella larvae model. Conversely,
C. auris aggregative isolates formed more robust biofilms than non-aggregative isolates [
5]. On the other hand, Carvajal and coworkers [
6] did not observe a clear relationship between the phenotype of aggregation and virulence of
C. auris isolates, including in vivo infection of
G. mellonella larvae.
The aggregative phenotype of
C. auris has been proposed to be a consequence of a defect in cellular division and failure to release daughter cells after the budding event [
4]. However, a recent study reported a new form of aggregation in
C. auris resulting from increased adherence between adjacent cells, likely caused by genomic amplification of the cell wall adhesin
ALS4, which plays a role in virulence in
G. mellonella larvae as well as adhesion to both biotic and abiotic surfaces [
7]. Regarding the
C. haemulonii complex, the aggregative phenotype has not been previously described, but our research group demonstrated that clinical isolates of the
C. haemulonii complex exhibit a tendency to form aggregates [
8,
9]. However, very little is effectively known about this intriguing biological event. Therefore, in this work, we aimed to study the aggregation phenomenon in clinical isolates belonging to the
C. haemulonii species complex and
C. auris, as well as to investigate the effects of chemical and physical factors on their aggregation capability.
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
A total of 18 clinical isolates of
C. haemulonii species complex and
C. auris were used in the present study. The isolates of the
C. haemulonii complex were recovered from patients from Brazilian hospitals between 2005 and 2013, which were identified by molecular approaches as
C. haemulonii (
n = 5; LIP
Ch2 recovered from sole of the foot, LIP
Ch3 from toe nail, LIP
Ch4 from finger nail, LIP
Ch7 from toe nail and LIP
Ch12 from blood),
C. duobushaemulonii (
n = 4; LIP
Ch1 from finger nail, LIP
Ch6 from toe nail, LIP
Ch8 from blood and LIP
Ch10 from bronchoalveolar lavage), and
C. haemulonii var.
vulnera (
n = 3; LIP
Ch5 from toe nail, LIP
Ch9 from urine and LIP
Ch11 from blood) [
10].
C. auris isolates were recovered from patients from Colombian hospitals between 2005 and 2016 (
n = 6; Ca386 was recovered from biopsy of bone tissue, Ca432 from secretion of craniotomy, Ca485 from eye discharge, Ca446 and Ca885 from blood and Ca881 from cerebrospinal fluid) [
11]. Fungal cells were cultured in Sabouraud-dextrose broth (SDB) at 37°C for 48 h and then used in all experiments. The yeast cells were quantified using a Neubauer chamber.
2.2. Aggregation Kinetic Assay
The aggregation assay was performed using a standard method previously described [
12,
13]. In this sense, yeasts grown in SDB were washed twice in sterile phosphate-buffered saline (PBS, pH 7.2) and then fungal suspensions containing 10
8 yeasts/mL were prepared in microcentrifuge tubes (Eppendorf, Hamburg, Germany), vortexed for 30 sec and then transferred by pipetting into plastic cuvettes (1 mL/cuvette). The fungal suspensions were incubated at 37°C without agitation for 30-, 60-, 90- and 120-min. Aggregation was quantified as a percentage reduction in the optical density (OD) and calculated as ([OD
0 – OD
f]/OD
0) × 100, where OD
0 is the OD value at the start of the experiment and OD
f is the value after the different incubation time periods. All measurements were done at 530 nm using PBS without fungal cells as blank. The percentage of aggregation was calculated and was used for classification of aggregation as follows: high (more than 40%), intermediate (30-40%) and low aggregation (less than 30%) [
14]. One isolate of each species was selected for the subsequent experiments.
2.3. Light Microscope Imaging
Fungi (108 yeasts/mL) were incubated in PBS (pH 7.2) at 37°C for 2 h to allow cell aggregation. Afterwards, the systems were gently mixed by pipetting and an aliquot of 10 μL of each cell suspension was transferred to a glass slide, covered with a coverslip, and observed using a fluorescence microscope to obtain differential interference contrast images (DIC; Zeiss Fluorescence Microscope – Axio Imager D2 [Zeiss, Alemanha]). Aliquots (10 μL) of the cell suspensions taken before incubation were used as control of negative aggregation for comparison purposes (time 0 h).
2.4. Effects of Chemicals on Aggregation
In order to investigate the influence of chemical factors on the aggregation ability of the clinical isolates of
C. haemulonii species complex and
C. auris, fungi suspensions were prepared as described above and then incubated for 2 h to allow aggregation in the following conditions: (i) PBS adjusted to pH 4.5, 7.2 and 8.5 [
15]; (ii) 50 μg/mL of proteinase K (Invitrogen, Carlsbad, USA); (iii) 0.25% trypsin (Nova Biotecnologia, São Paulo, Brazil) [
7]; (iv) 0.05% to 0.25% sodium dodecyl sulfate (SDS); (v) 0.5% to 2% β-mercaptoethanol; (vi) 0.5 mM to 5 mM ethylenediamine tetraacetic acid tetrasodium salt (EDTA; Sigma-Aldrich) [
16]. DIC images were obtained as described above after incubation of the clinical isolates with the chemicals that affected their aggregation capability.
2.5. Effects of Temperature on Aggregation
To evaluate the influence of temperature on the aggregation capability of the isolates of the C. haemulonii species complex and C. auris, fungal cells were prepared as described above and then incubated for 2 h to allow aggregation at either 28°C or 37°C.
2.6. Statistics
All experiments were performed in triplicate, in three independent experimental sets. The results were analyzed statistically by Student's t-test (in the comparisons between two groups) and by the Analysis of Variance One-Way ANOVA followed by Dunnett’s multiple comparison test (in comparisons between three or more groups). All analyzes were performed using the program GraphPad Prism8. In all analyses, P values of 0.05 or less were considered statistically significant.
3. Results and Discussion
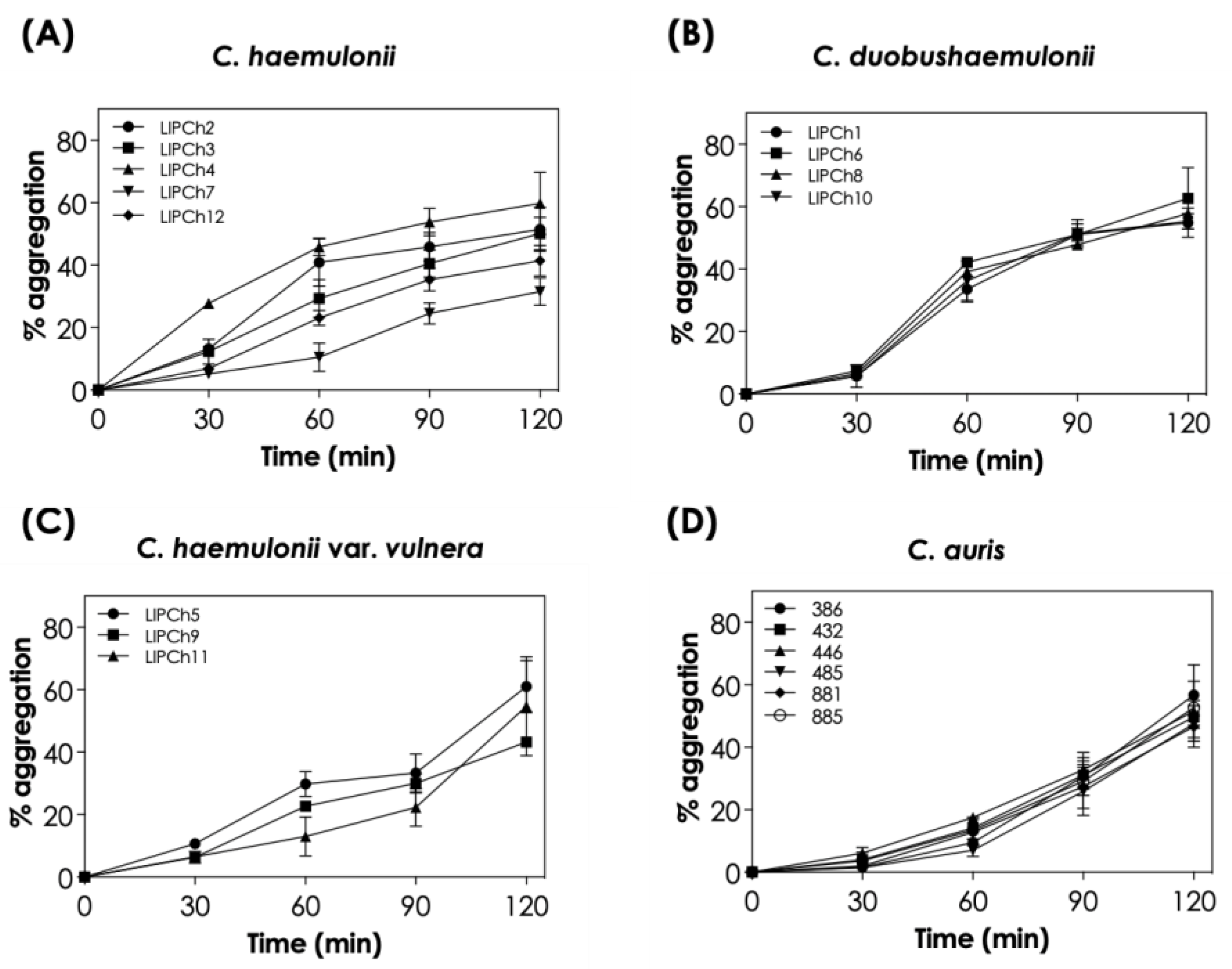
3.1. Aggregation is a Time-Dependent Event
The auto-aggregation capability of the 18 clinical isolates from the
C. haemulonii species complex and
C. auris studied herein was observed to be a time-dependent event (
Figure 1). When analyzing each species individually, we observed that
C. auris isolates had a very similar percentage of aggregation at each time point, with mean aggregation ranging from 3.1 ± 1.8% after 30 min to 50.8 ± 3.7% after 120 min of incubation. Similar results were observed for
C. duobushaemulonii isolates, with mean percentage of aggregation varying from 6.3 ± 0.8% after 30 min to 57.7 ± 3.6% after 120 min. On the other hand,
C. haemulonii and
C. haemulonii var.
vulnera isolates exhibited a more variable profile among the different isolates. In this sense, the mean aggregation of
C. haemulonii isolates varied from 13.1 ± 8.9% after 30 min to 46.9 ± 10.8% after 120 min, while the mean percentage of aggregation of
C. haemulonii var.
vulnera isolates were 7.9 ± 2.4% and 54.3 ± 11.4% after 30 and 120 min of incubation, respectively.
Supporting our observations, Tomici et al. [
15] also demonstrated that the auto-aggregation percentage in isolates of
C. albicans,
C. krusei,
C. glabrata and
Saccharomyces boulardii increases over time. Cellular aggregation is a well-known phenomenon in the microbial field, described in both bacteria and fungi, not only in natural environments but in mammalian hosts as well. For instance, nitrogen-fixing bacteria such as
Azospirillum,
Klebsiella and
Azotobacter can aggregate and flocculate, which contributes to their dispersal and survival in soil [
16]. Microorganisms can exhibit the ability of auto-aggregation, characterized by the aggregation between cells of the same microbial strain, or coaggregation, characterized by the aggregation between different microbial strains/species or even interkingdom interactions [
17]. The formation of dental caries, for example, is highly mediated by coaggregation process and, for this reason, several coaggregation studies have focused on microorganisms of the human oral cavity, such as
Streptococcus salivarius and
Candida albicans, among others [
17,
18]. Additionally, the coaggregation of
Lactobacillus with potential intestinal pathogens, such as
Escherichia coli,
Klebsiella spp. as well as some
Candida species, as an anti-infection mechanism, has also been investigated by research groups [
15,
19].
The global threat posed by
C. auris depends in part on its described aggregative phenotype [
4]. The impact of this phenotype on fungal cells and virulence is still being investigated and studies are somehow contradictory, but it has been shown to influence biofilm formation, fungal virulence and antifungal susceptibility, including tolerance to clinical concentrations of sodium hypochlorite, with some isolates persisting alive after 14 days of treatment [
20].
All clinical isolates of the C. haemulonii species complex and C. auris that underwent testing demonstrated high aggregation after 2 h of incubation, according to the criteria established in this study (i.e., aggregation > 40%), with the exception of one C. haemulonii isolate (LIPCh7), which showed intermediate aggregation. The following isolates were chosen for further experiments: LIPCh4 from C. haemulonii, LIPCh6 from C. duobushaemulonii, LIPCh5 from C. haemulonii var. vulnera, and Ca386 from C. auris.
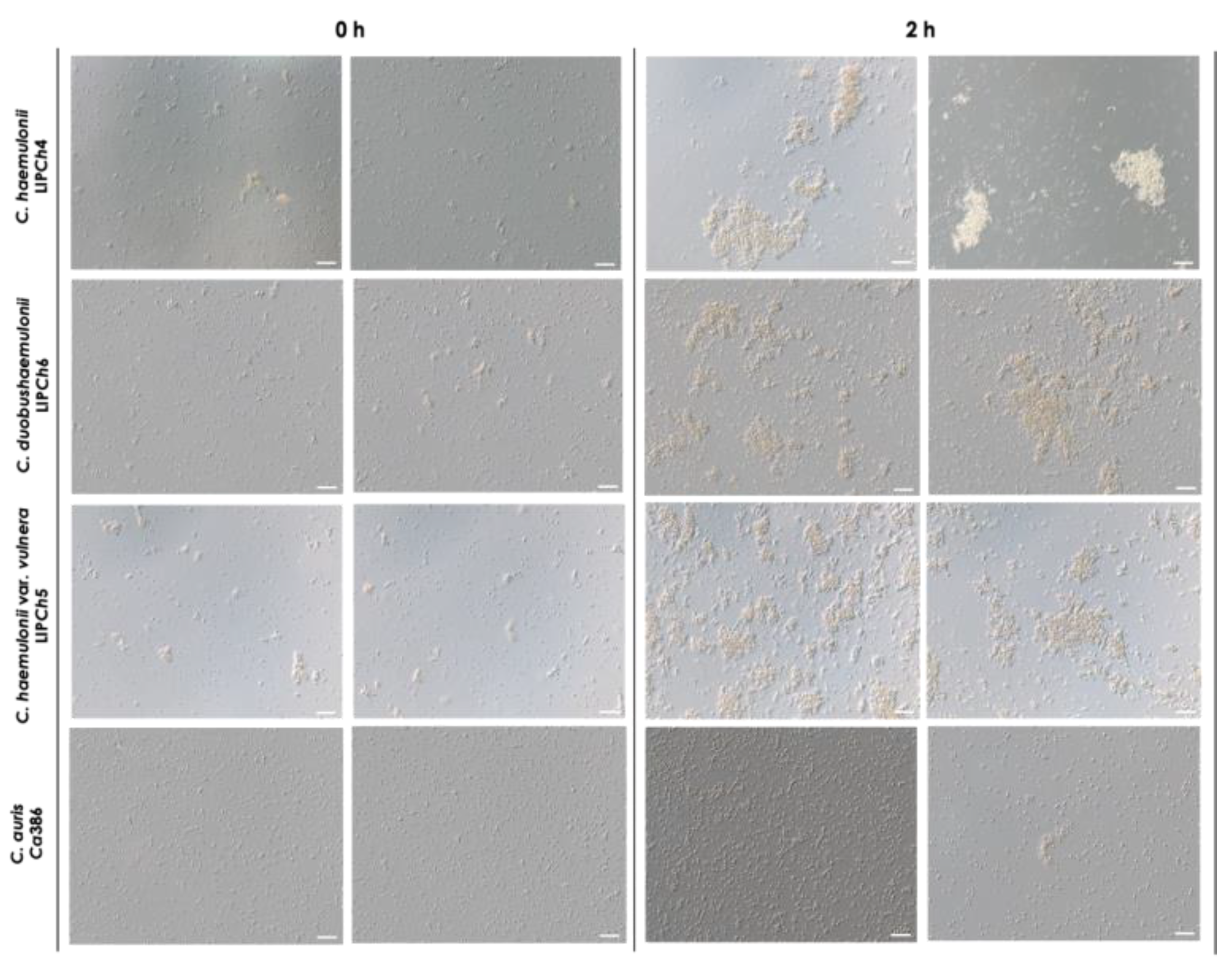
3.2. Morphological Analysis of Cellular Aggregation
The clinical isolates of the four
Candida species selected for this study were analyzed both before and after a 2-h incubation at 37°C in PBS at pH 7.2. Interestingly, we observed that the members of the
C. haemulonii species complex exhibited clusters of cells even after vigorous vortex mixing, which represents the time 0 h of the experiment. These aggregates became noticeably larger after the incubation period, indicating the occurrence of significant cell-to-cell interactions. In contrast,
C. auris also displayed clusters of cells at time 0, but these were considerably smaller than those observed in the
C. haemulonii complex isolates, and remained similarly sized after incubation. However, the number of aggregates and the amount of cells per aggregate visibly increased, but these were clearly smaller in comparison with the
C. haemulonii complex isolates (
Figure 2).
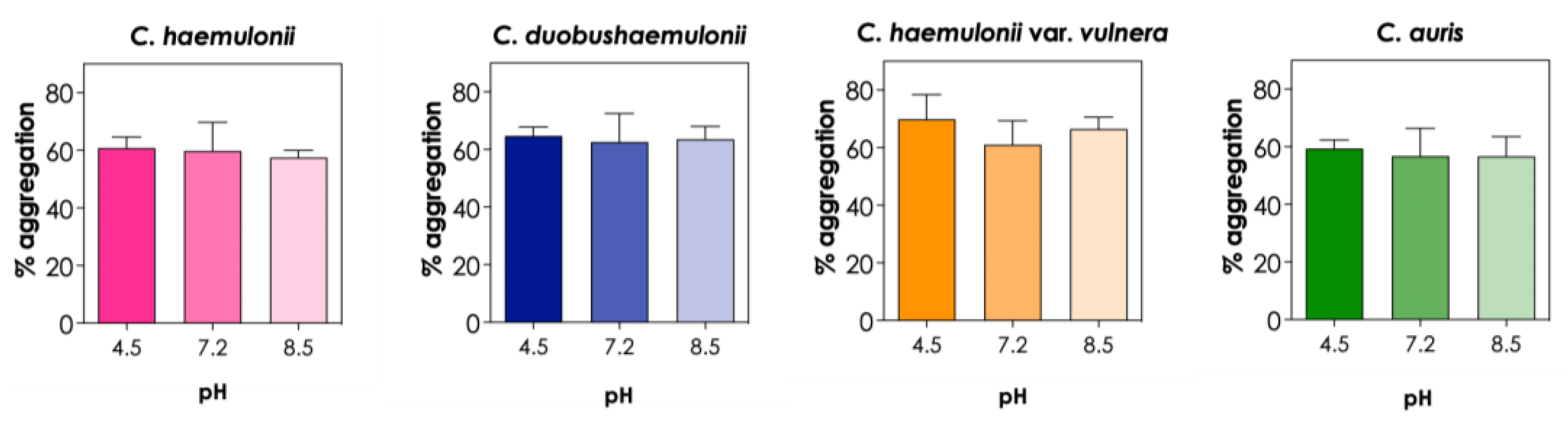
3.3. Effects of Chemical Factors on Aggregation
The adaptation of opportunistic pathogens to different pH values favors their survival in the hostile environment of the human body, for example, facing basic pH in the mouth, acidic pH in the stomach and neutral pH in the large intestine. In order to investigate the potential involvement of charged groups in the aggregation of
C. haemulonii complex and
C. auris isolates, we tested their ability to aggregate at different pH values. Since pH values ranging from 4.5 to 8.5 are relevant for biological systems, we evaluated the impact of PBS adjusted at three different pHs (4.5, 7.2 and 8.5) on the aggregation capability of the isolates and observed that none of the species tested were affected by pH variation under the conditions used in our study (
Figure 3). On the other hand, Tomicic et al. [
15] reported that the auto-aggregation of
C. albicans,
C. krusei,
C. glabrata and
S. boulardii varied depending on pH values after 5 h of incubation, with the highest auto-aggregation observed at acidic pH (pH 4.5) and the lowest at basic pH (pH 8.5); however, after 24 h of incubation, no differences were observed in the auto-aggregation ability of these different
Candida species.
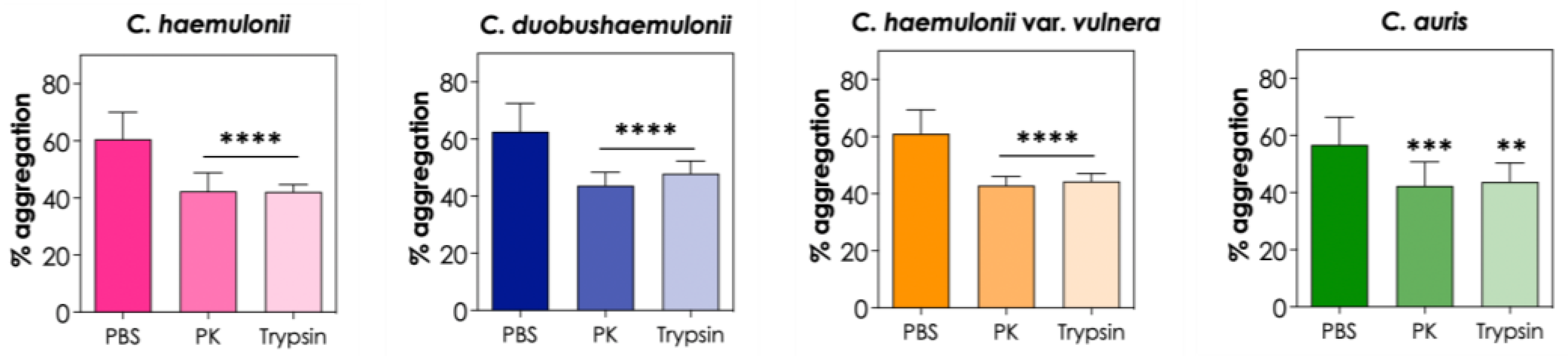
Subsequently, the fungi were treated with two broad-spectrum proteases, proteinase K and trypsin, to investigate whether surface proteins, such as adhesins, play a role in the aggregation process of
C. haemulonii species complex and
C. auris. Our results showed that both proteinase K and trypsin led to a significant reduction in the percentage of aggregation (
P <0.05), indicating that surface proteins are indeed important in cell-cell interactions that lead to aggregation in these emerging
Candida species (
Figure 4).
In this context, Bing and colleagues [
7] demonstrated that treatment of
C. auris with proteinase K and trypsin led to the separation of cell clumps into individual yeast cells in an isolate that did not exhibit the typical aggregative phenotype; however, the enzymes were not able to disrupt the aggregates of a typical aggregative isolate of
C. auris. Furthermore, quantitative transcriptional expression assays demonstrated that the relative expression levels of
ALS4 gene in the typical aggregative isolate were comparable to a non-aggregative strain of
C. auris, whereas the isolate whose aggregates were disrupted by the action of proteinase K and trypsin exhibited 400 times higher relative expression levels of the
ALS4 gene [
7]. Therefore, those authors suggested the existence of two aggregative phenotypes in
C. auris: the typical aggregative phenotype resulting from a defect in cell division and release of the budding daughter cells, and the new aggregative phenotype caused by the expansion of the
ALS4 gene adhesin [
7]. Additionally, the authors demonstrated that the
C. auris isolate with the new aggregative phenotype developed more robust biofilms on both polystyrene and silicone surfaces compared to the typical aggregative isolate and non-aggregative isolates of
C. auris, which formed weaker biofilms [
7]. Based on our findings, the clinical isolate of
C. auris used in this study fits this newly described aggregative phenotype.
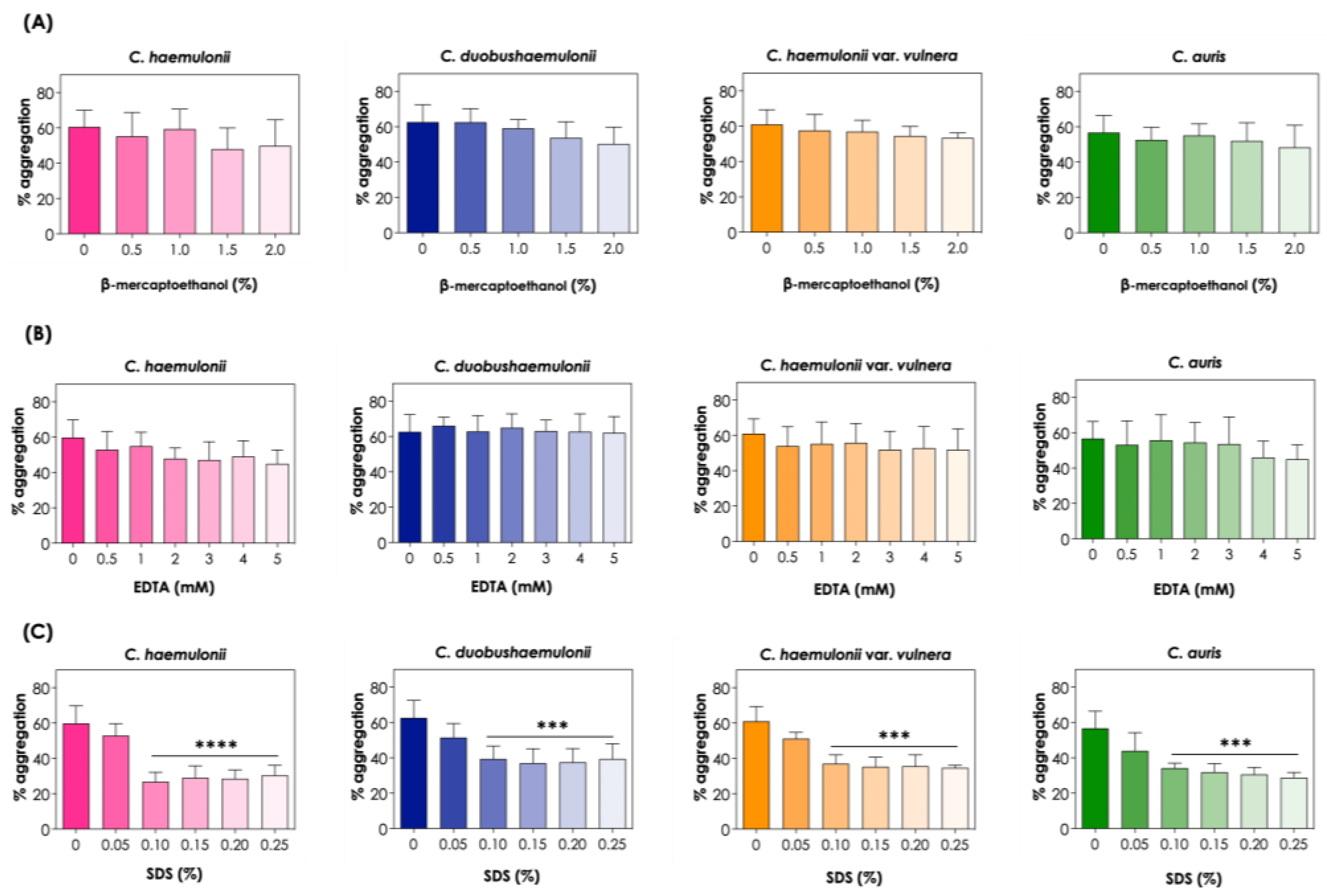
In order to investigate the role of proteins in aggregation and other features related to cell adhesion, we utilized chemicals, such as the protein-denaturing agent β-mercaptoethanol, the chelator agent EDTA and the anionic detergent SDS, to assess cell-to-cell interactions. In this sense, treatments with 0.5 to 2% β-mercaptoethanol and 0.5 to 5 mM EDTA had no effect on the aggregation ability of the clinical isolates tested, indicating that disulfide bonds and divalent cations did not appear to mediate aggregation in the
C. haemulonii species complex and
C. auris (
Figure 5A and 5B). Conversely, treatment with SDS significantly reduced the aggregation ability of all clinical isolates studied, at concentrations varying from 0.10 to 0.25%, which suggests that hydrophobic interactions may play a role in cell-to-cell aggregation of isolates of the
C. haemulonii complex and
C. auris (
Figure 5C).
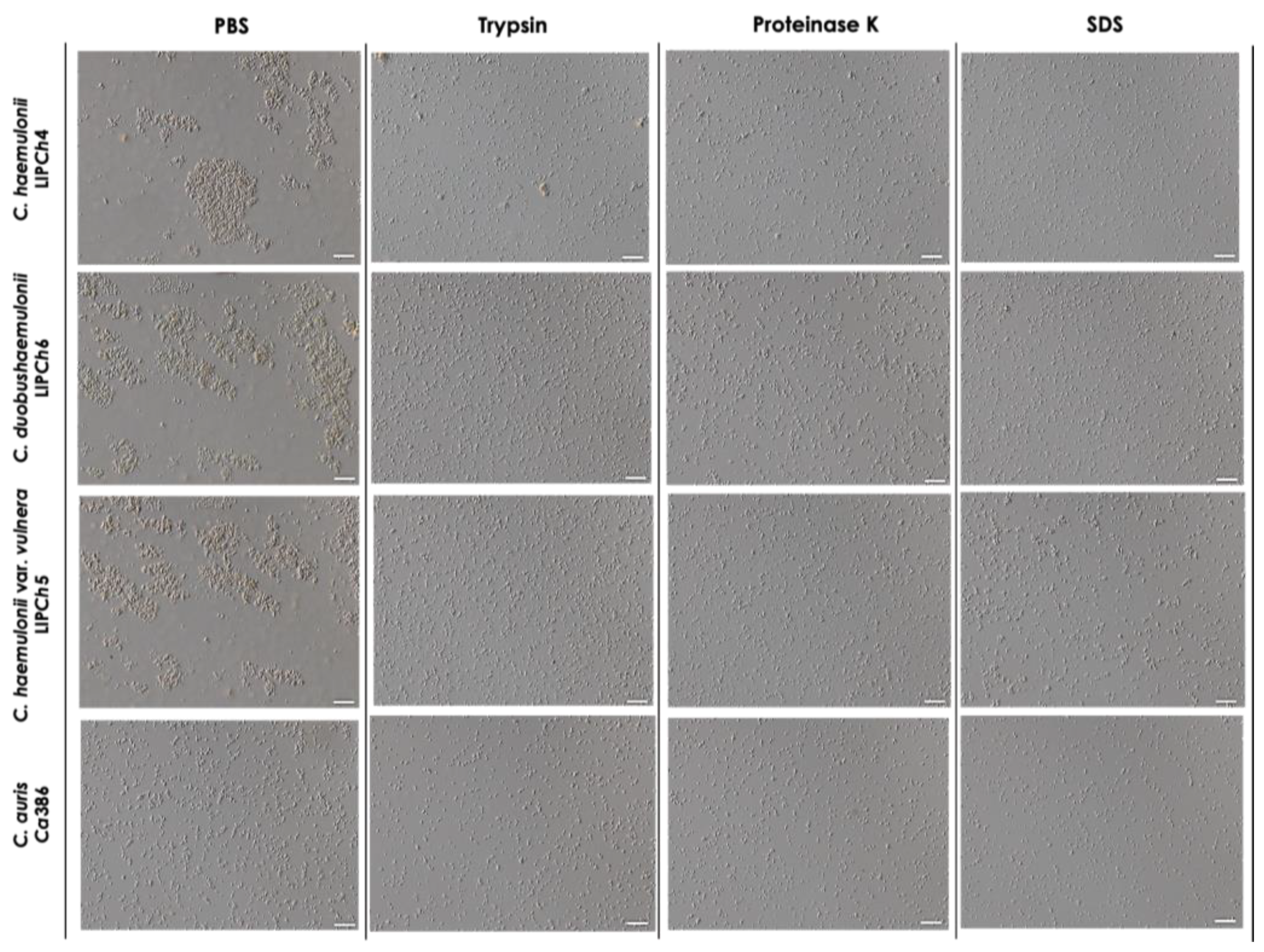
Microscopic analyses confirmed the spectrometric results, revealing that the incubation of the clinical isolates of the four species tested with trypsin, proteinase K and SDS drastically reduced their aggregation ability. As a result, the aggregates observed were considerably smaller than those observed in PBS systems (
Figure 6).
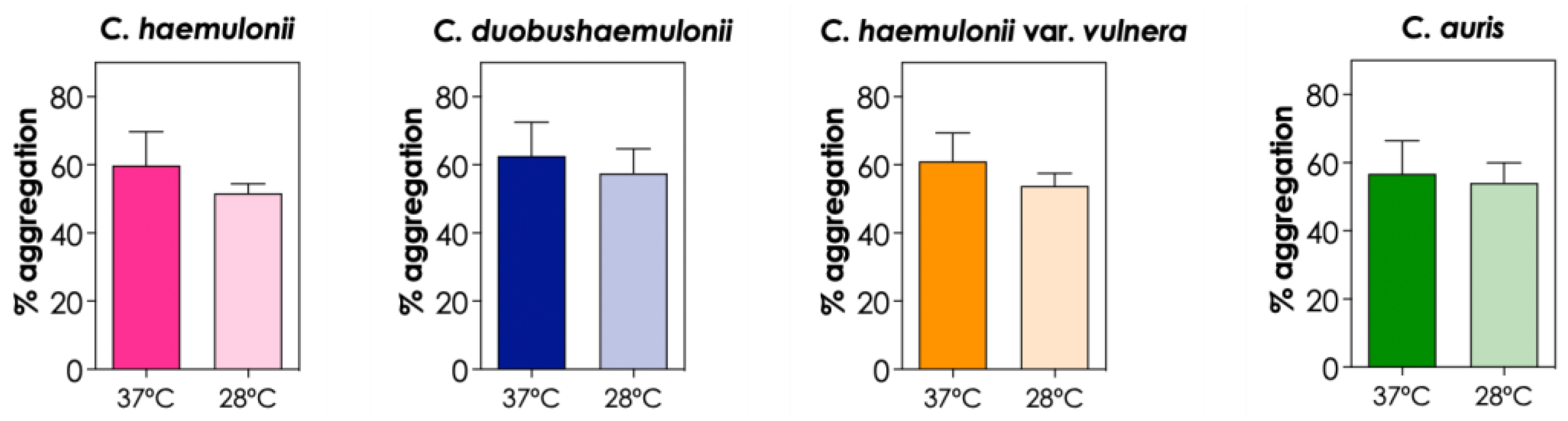
3.3. Effects of Temperature on Aggregation
All experiments were conducted at 37°C to mimic the normal temperature of the human body. However, we also evaluated the aggregation ability of the isolates at room temperature (28°C). The results indicated that the clinical isolates of
C. haemulonii complex and
C. auris tended to form fewer aggregates at room temperature, but no significant differences were observed (P>0.05;
Figure 7). Tomicic et al. [
7] reported that
C. krusei and
C. glabrata isolates exhibited a higher percentage of auto-aggregation at 37°C compared to the same process at 28°C and 42°C; conversely, for
C. albicans, a higher percentage of auto-aggregation was observed at 42°C.
5. Conclusions
This study collectively demonstrated the ability of the C. haemulonii species complex and C. auris to form cell aggregates in a typically time-dependent manner. The presence of proteinase K, trypsin and SDS significantly impacted the auto-aggregation process, suggesting that surface proteins and hydrophobic interactions play a crucial role in mediating the cell-to-cell adhesion of these Candida species. However, further studies are necessary to better understand this issue.
Author Contributions
Conceptualization, L.S.R., M.H.B., and A.L.S.S.; methodology, L.S.R., M.H.B., and A.L.S.S.; formal analysis, L.S.R., M.H.B., and A.L.S.S.; investigation, L.S.R., M.H.B., and A.L.S.S.; resources, M.H.B., and A.L.S.S.; writing—original draft preparation, L.S.R.; writing—review and editing, L.S.R., C.M.P.G., M.H.B., and A.L.S.S.; funding acquisition, M.H.B. and A.L.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Brazilian Agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa no Estado do Rio de Janeiro (FAPERJ) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – Financial code 001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Denise Rocha de Souza (UFRJ) for technical assistance and Jefferson Bomfim Silva Cypriano (Unimicro, UFRJ) for microscopic images.
Conflicts of Interest
“The authors declare no conflict of interest.”.
References
- Gade, L.; Muñoz, J.F.; Sheth, M.; Wagner, D.; Berkow, E.L.; Forsberg, K.; Jackson, B.R.; Ramos-Castro, R.; Escandón, P.; Dolande, M.; et al. Understanding the emergence of multidrug-resistant Candida: using Whole-Genome Sequencing to describe the population structure of Candida haemulonii species complex. Front Genet 2020, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gaviria, M.; Martínez-Álvarez, J.A.; Chávez-Santiago, J.O.; Mora-Montes, H.M. Candida haemulonii complex and Candida auris: biology, virulence factors, immune response, and multidrug resistance. Infection and drug resistance 2023, 16, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Sood, P. On the emergence, spread and resistance of Candida auris: host, pathogen and environmental tipping points. J. Med. Microbiol. 2021, 70. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative pathogenicity of United Kingdom isolates of the emerging pathogen Candida auris and other key pathogenic Candida Species. mSphere 2016, 1. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaur, M.; Chakrabarti, A.; Shankarnarayan, S.A.; Rudramurthy, S.M. Biofilm formation by Candida auris isolated from colonising sites and candidemia cases. Mycoses 2019, 62, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, S.K.; Alvarado, M.; Rodríguez, Y.M.; Parra-Giraldo, C.M.; Varón, C.; Morales-López, S.E.; Rodríguez, J.Y.; Gómez, B.L.; Escandón, P. Pathogenicity assessment of Colombian strains of Candida auris in the Galleria mellonella invertebrate model. J Fungi (Basel) 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Bing, J.; Guan, Z.; Zheng, T.; Zhang, Z.; Fan, S.; Ennis, C.L.; Nobile, C.J.; Huang, G. Clinical isolates of Candida auris with enhanced adherence and biofilm formation due to genomic amplification of ALS4. PLoS Pathog. 2023, 19, e1011239. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.S.; Oliveira, S.S.C.; Silva, L.N.; Granato, M.Q.; Gonçalves, D.S.; Frases, S.; Seabra, S.H.; Macedo, A.J.; Kneipp, L.F.; Branquinha, M.H.; et al. Surface, adhesiveness and virulence aspects of Candida haemulonii species complex. Med. Mycol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.S.; Oliveira, S.S.C.; Souto, X.M.; Branquinha, M.H.; Santos, A.L.S. Planktonic growth and biofilm formation profiles in Candida haemulonii species complex. Med. Mycol. 2017, 55, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.S.; Figueiredo-Carvalho, M.H.; Barbedo, L.S.; Ziccardi, M.; Chaves, A.L.; Zancope-Oliveira, R.M.; Pinto, M.R.; Sgarbi, D.B.; Dornelas-Ribeiro, M.; Branquinha, M.H.; et al. Candida haemulonii complex: species identification and antifungal susceptibility profiles of clinical isolates from Brazil. J. Antimicrob. Chemother. 2015, 70, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.E.; Ramirez, L.M.; Dias, L.D.S.; Rivas, L.A.; Ramos, L.S.; Santos, A.L.S.; Taborda, C.P.; Parra-Giraldo, C.M. Pathogenicity levels of Colombian strains of Candida auris and Brazilian strains of Candida haemulonii species complex in both murine and Galleria mellonella experimental models. J Fungi (Basel) 2020, 6. [Google Scholar] [CrossRef]
- Das, T.; Krom, B.P.; van der Mei, H.C.; Busscher, H.J.; Sharma, P.K. DNA-mediated bacterial aggregation is dictated by acid–base interactions. Soft Matter 2011, 7, 2927–2935. [Google Scholar] [CrossRef]
- Liu, H.H.; Yang, Y.R.; Shen, X.C.; Zhang, Z.L.; Shen, P.; Xie, Z.X. Role of DNA in bacterial aggregation. Curr. Microbiol. 2008, 57, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Arzmi, M.H.; Dashper, S.; Catmull, D.; Cirillo, N.; Reynolds, E.C.; McCullough, M. Coaggregation of Candida albicans, Actinomyces naeslundii and Streptococcus mutans is Candida albicans strain dependent. FEMS Yeast Res. 2015, 15, fov038. [Google Scholar] [CrossRef] [PubMed]
- Tomičić, R.; Tomičić, Z.; Raspor, P. Influence of culture conditions on co-aggregation of probiotic yeast Saccharomyces boulardii with Candida spp. and their auto-aggregation. Folia Microbiol. (Praha) 2022, 67, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Burdman, S.; Jurkevitch, E.; Schwartsburd, B.; Hampel, M.; Okon, Y. Aggregation in Azospirillum brasilense: effects of chemical and physical factors and involvement of extracellular components. Microbiology 1998, 144 ( Pt 7) Pt 7, 1989–1999. [Google Scholar] [CrossRef]
- Shen, S.; Samaranayake, L.P.; Yip, H.K. Coaggregation profiles of the microflora from root surface caries lesions. Arch. Oral Biol. 2005, 50, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Rismayuddin, N.A.R.; Mat Yassim, A.S.; Ahmad, H.; Abdul Wahab, R.; Dashper, S.; Arzmi, M.H. Streptococcus salivarius K12 inhibits Candida albicans aggregation, biofilm formation and dimorphism. Biofouling 2021, 37, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Burdman, S.; Jurkevitch, E.; Soria-Díaz, M.E.; Serrano, A.M.; Okon, Y. Extracellular polysaccharide composition of Azospirillum brasilense and its relation with cell aggregation. FEMS Microbiol. Lett. 2000, 189, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Short, B.; Brown, J.; Delaney, C.; Sherry, L.; Williams, C.; Ramage, G.; Kean, R. Candida auris exhibits resilient biofilm characteristics in vitro: implications for environmental persistence. J. Hosp. Infect. 2019, 103, 92–96. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Aggregation capability of the clinical isolates comprising the C. haemulonii species complex and C. auris. The aggregation was evaluated by the reduction in the optical density (at 530 nm) of fungal suspensions in PBS (pH 7.2) containing the fungi (108 yeasts/mL) after 30, 60, 90 and 120 min of incubation at 37°C in inert conditions. The results were expressed as percentage of aggregation for each clinical isolate of C. haemulonii (A), C. duobushaemulonii (B), C. haemulonii var. vulnera (C) and C. auris (D). Values represent the mean ± standard deviation of three independent experiments.
Figure 1.
Aggregation capability of the clinical isolates comprising the C. haemulonii species complex and C. auris. The aggregation was evaluated by the reduction in the optical density (at 530 nm) of fungal suspensions in PBS (pH 7.2) containing the fungi (108 yeasts/mL) after 30, 60, 90 and 120 min of incubation at 37°C in inert conditions. The results were expressed as percentage of aggregation for each clinical isolate of C. haemulonii (A), C. duobushaemulonii (B), C. haemulonii var. vulnera (C) and C. auris (D). Values represent the mean ± standard deviation of three independent experiments.
Figure 2.
Aggregation of the clinical isolates comprising the C. haemulonii species complex and C. auris. The fungi (108 yeasts/mL) were incubated at 37°C for 2 h in PBS (pH 7.2), gently homogenized by pipetting and observed using a light microscope. DIC images represent the isolate LIPCh4 from C. haemulonii, LIPCh6 from C. duobushaemulonii, LIPCh5 from C. haemulonii var. vulnera and Ca386 from C. auris before (0 h) and after incubation (2 h). Images were obtained at ×20 magnification. Bars = 50 μm.
Figure 2.
Aggregation of the clinical isolates comprising the C. haemulonii species complex and C. auris. The fungi (108 yeasts/mL) were incubated at 37°C for 2 h in PBS (pH 7.2), gently homogenized by pipetting and observed using a light microscope. DIC images represent the isolate LIPCh4 from C. haemulonii, LIPCh6 from C. duobushaemulonii, LIPCh5 from C. haemulonii var. vulnera and Ca386 from C. auris before (0 h) and after incubation (2 h). Images were obtained at ×20 magnification. Bars = 50 μm.
Figure 3.
Effects of different pH values on the aggregation capability of clinical isolates of the C. haemulonii species complex and C. auris. The aggregation was evaluated by the reduction in the optical density (at 530 nm) of fungal suspensions in PBS (pH adjusted for 4.5, 7.2 and 8.5) containing the fungi (108 yeasts/mL) after 2 h of incubation at 37°C. The results were expressed as percentage of aggregation of isolate LIPCh4 from C. haemulonii, LIPCh6 from C. duobushaemulonii, LIPCh5 from C. haemulonii var. vulnera and Ca386 from C. auris. Values represent the mean ± standard deviation of three independent experiments.
Figure 3.
Effects of different pH values on the aggregation capability of clinical isolates of the C. haemulonii species complex and C. auris. The aggregation was evaluated by the reduction in the optical density (at 530 nm) of fungal suspensions in PBS (pH adjusted for 4.5, 7.2 and 8.5) containing the fungi (108 yeasts/mL) after 2 h of incubation at 37°C. The results were expressed as percentage of aggregation of isolate LIPCh4 from C. haemulonii, LIPCh6 from C. duobushaemulonii, LIPCh5 from C. haemulonii var. vulnera and Ca386 from C. auris. Values represent the mean ± standard deviation of three independent experiments.
Figure 4.
Effects of proteinase K (PK) and trypsin on the aggregation capability of the clinical isolates of the C. haemulonii species complex and C. auris. The aggregation was evaluated by the reduction in the optical density (at 530 nm) of fungal suspensions in PBS containing the fungi (108 yeasts/mL) and PK (50 μg/mL) and trypsin (0.25%) after 2 h of incubation at 37°C. The results were expressed as percentage of aggregation of isolate LIPCh4 from C. haemulonii, LIPCh6 from C. duobushaemulonii, LIPCh5 from C. haemulonii var. vulnera and Ca386 from C. auris. Values represent the mean ± standard deviation of three independent experiments. The asterisks mean the following: (****) P <0.0001; (***) P <0.001; (**) P <0.01.
Figure 4.
Effects of proteinase K (PK) and trypsin on the aggregation capability of the clinical isolates of the C. haemulonii species complex and C. auris. The aggregation was evaluated by the reduction in the optical density (at 530 nm) of fungal suspensions in PBS containing the fungi (108 yeasts/mL) and PK (50 μg/mL) and trypsin (0.25%) after 2 h of incubation at 37°C. The results were expressed as percentage of aggregation of isolate LIPCh4 from C. haemulonii, LIPCh6 from C. duobushaemulonii, LIPCh5 from C. haemulonii var. vulnera and Ca386 from C. auris. Values represent the mean ± standard deviation of three independent experiments. The asterisks mean the following: (****) P <0.0001; (***) P <0.001; (**) P <0.01.
Figure 5.
Effects of β-mercaptoethanol (A), EDTA (B) and SDS (C) on the aggregation capability of the clinical isolates of the C. haemulonii species complex and C. auris. The aggregation was evaluated by the reduction in the optical density (at 530 nm) of fungal suspensions (108 yeasts/mL) after 2 h of incubation at 37°C in PBS (pH 7.2) containing different concentrations of β-mercaptoethanol (0.5 to 2%), EDTA (0.5 to 5 mM) and SDS (0.05 to 0.25%). The results were expressed as percentage of aggregation of isolate LIPCh4 from C. haemulonii, LIPCh6 from C. duobushaemulonii, LIPCh5 from C. haemulonii var. vulnera and Ca386 from C. auris. Values represent the mean ± standard deviation of three independent experiments. The asterisks mean the following: (****) P <0.0001; (***) P <0.001.
Figure 5.
Effects of β-mercaptoethanol (A), EDTA (B) and SDS (C) on the aggregation capability of the clinical isolates of the C. haemulonii species complex and C. auris. The aggregation was evaluated by the reduction in the optical density (at 530 nm) of fungal suspensions (108 yeasts/mL) after 2 h of incubation at 37°C in PBS (pH 7.2) containing different concentrations of β-mercaptoethanol (0.5 to 2%), EDTA (0.5 to 5 mM) and SDS (0.05 to 0.25%). The results were expressed as percentage of aggregation of isolate LIPCh4 from C. haemulonii, LIPCh6 from C. duobushaemulonii, LIPCh5 from C. haemulonii var. vulnera and Ca386 from C. auris. Values represent the mean ± standard deviation of three independent experiments. The asterisks mean the following: (****) P <0.0001; (***) P <0.001.
Figure 6.
Effects of trypsin, proteinase K and SDS on the aggregation capability of the clinical isolates belonging to the C. haemulonii species complex and C. auris. The fungi (108 yeasts/mL) were incubated under inert conditions at 37°C for 2 h in PBS (pH 7.2), trypsin (0.25%), proteinase K (50 μg/mL) and SDS (0.25%), and gently homogenized by pipetting and observed using a light microscope. DIC images represent the isolate LIPCh4 from C. haemulonii, LIPCh6 from C. duobushaemulonii, LIPCh5 from C. haemulonii var. vulnera and Ca386 from C. auris after incubation. Images were obtained at ×20 magnification. Bars = 50 μm.
Figure 6.
Effects of trypsin, proteinase K and SDS on the aggregation capability of the clinical isolates belonging to the C. haemulonii species complex and C. auris. The fungi (108 yeasts/mL) were incubated under inert conditions at 37°C for 2 h in PBS (pH 7.2), trypsin (0.25%), proteinase K (50 μg/mL) and SDS (0.25%), and gently homogenized by pipetting and observed using a light microscope. DIC images represent the isolate LIPCh4 from C. haemulonii, LIPCh6 from C. duobushaemulonii, LIPCh5 from C. haemulonii var. vulnera and Ca386 from C. auris after incubation. Images were obtained at ×20 magnification. Bars = 50 μm.
Figure 7.
Effects of temperature on the aggregation capability of the clinical isolates of the C. haemulonii species complex and C. auris. The aggregation was evaluated by the reduction in the optical density (at 530 nm) of fungal suspensions (108 yeasts/mL) after 2 h of incubation in PBS (pH 7.2) at 37°C (human body temperature) and 28°C (room temperature). The results were expressed as percentage of aggregation of isolate LIPCh4 from C. haemulonii, LIPCh6 from C. duobushaemulonii, LIPCh5 from C. haemulonii var. vulnera and Ca386 from C. auris. Values represent the mean ± standard deviation of three independent experiments.
Figure 7.
Effects of temperature on the aggregation capability of the clinical isolates of the C. haemulonii species complex and C. auris. The aggregation was evaluated by the reduction in the optical density (at 530 nm) of fungal suspensions (108 yeasts/mL) after 2 h of incubation in PBS (pH 7.2) at 37°C (human body temperature) and 28°C (room temperature). The results were expressed as percentage of aggregation of isolate LIPCh4 from C. haemulonii, LIPCh6 from C. duobushaemulonii, LIPCh5 from C. haemulonii var. vulnera and Ca386 from C. auris. Values represent the mean ± standard deviation of three independent experiments.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).