1. Introduction
Cereal crops naturally have very low grain zinc (Zn) and iron (Fe) concentrations, and growing them on potentially plant available Zn- and Fe-deficient soils further affects yield as well as grain Zn and Fe concentrations [
1]. Studies show that about half of the cereal-cultivated soils globally are deficient in plant-available Zn [
2], particularly in acidic soils, and high rainfall areas of the tropics. In spite of high total concentration of Fe in tropical soils, high level oxidation and fixation significantly affect its plant availability [
3].
Crop genotypes breeding for resistance to Zn- and Fe-deficiency is a realistic and long-term solution to overcome Zn- and Fe-deficiency in soils [
4]. However, breeding genotypes perhaps take substantial time [
5] as well as relatively higher investment as compared to agronomic biofortification [
6].
Fertilization of Zn and Fe is a common practice to help to combat Zn and Fe deficiencies as a short term strategy [
3,
7]. Crop Zn and Fe deficiencies are most frequently amended by agronomic biofortification through soil application of Zn and Fe fertilizers [
8]. Zinc sulfate (ZnSO
4) and ferrous sulfate (FeSO
4) are used extensively as a source of Zn and Fe fertilizer, because of their higher solubility in water and existence in both crystalline and granular forms [
9].
Crop selection in agronomic biofortification plays a critical role in its effectiveness. Identification and improvement of traditional or native crops that are highly adaptive to local climate and efficiently withstand biotic and abiotic stresses is crucial. Finger millet (
Eleusine coracana L.) represents one of the critical plant genetic resources for the agriculture and food security of populations inhabiting arid, infertile and marginal lands [
10]. In the semiarid tropics of Eastern Africa, it is the major staple food for millions of resource poor people and plays an important nutritional and economic role [
11]. Finger millet is adaptable to adverse agro-ecological conditions with minimal inputs (fertilizer, pesticides, and herbicides) like low moisture stress and disease tolerance, productive on marginal land where other crops cannot perform and tolerance to acidic soil [
12,
13]. In Ethiopia, finger millet is the sixth important crops after tef, wheat, maize, sorghum, and barley. It was produced on 480,343 hectare of land, from which ~1.2M tons were obtained at the national level per year [
14]. Nationwide ~1.55M households are directly engaged in finger millet production and the production is increasing by 300 % in the previous 20 years [
14].
Previous reports on the impact of agronomic biofortification of Zn and Fe, genotype as well as slope position on indigenous crops like finger millet and teff in tropical smallholding farming systems is lacking. However, a report from Ethiopia indicated that wheat yield was more strongly influenced by slope positions than either the nutrient sources or rates, thus, site specific fertilizer treatment is strongly recommended [
15]. Therefore, this paper reports the effect of basal application of Zn and Fe fertilizer on grain yield and yield attributes of three finger millet varieties at different locations and slope positions.
2. Material and method
2.1. Field experiment
The agronomic biofortification trials with Zn and Fe micronutrients were carried out at Gojjam (11º 41′ 54′’N 37º 29′ 79′’E foot slope and 11º 40′ 23′’N 37º 30′ 29′’E hill slope) and Arsi Negelle (7º 19′ 38′’N 38
º 38′ 54′’E foot slope and 7º 18′ 43′’N 38º 39′ 57′’E hill slope) areas at farmers land. According to the classification of agro-ecological zonation of Ethiopia, both sites are characterized as sub-humid midlands located between 1500-2300 m.a.s.l. and receive an average annual rainfall 800-1200 mm [
16]. The experiment was laid out in randomized complete block design (RCBD) (Supplementary Material 1) with factorial concept with 4 replications consisted of 15 treatment combinations involving 3 finger millet varieties ( Dagi-01 black in colour, Urji white in colour and Meba brown in colour) and 5 levels of fertilizer application (
Table 1).
2.2. Agronomic management
The plot size was 4m x 4m, with gangway between plots being 1m2 while distance between block and the border were 0.5m each. The experiment was repeated for two seasons but only at Arsi Negelle (due to Covid-19 pandemic travel restriction) and different farms were used in each year, sowed between mid-June - mid July and harvested in November. Planting was done by hand drilling at a seed rate of 7 kg h-1. Each experimental plot had ten rows with 40 cm inter-row spacing. NPKS, ZnSO47H2O & FeSO47H2O were applied at planting and urea was applied after 45 days at first weeding. Each plots were weeded at least six times using human lobar and no pesticide or herbicides applied. Plant samples for data collection were tagged right after 100% seed germination.
2.3. Soil sample collection
Soil samples were taken from a 60 m
2 circular plot in the experimental field. Five sub-sample sites were located, the first at the centre. Two sub-sample points were selected at locations on a line through the plot centre along the crop rows, and two on a line orthogonal to the first through the plot centre. The ‘long’ axis of the sample array (with sample locations at 5.64 and 4.89 m) was oriented in the direction of crop rows with the ‘short axis’ (with sample locations at 3.99 and 2.82 m) perpendicular to the crop rows. A single soil sub-sample was collected at each of the five sub-sample points with a Dutch auger with a flight of length 150 mm and diameter 50 mm. Any plant material adhering to the auger was carefully removed, and the five sub-samples stored in a single bag [
17].
2.4. Soil mineral analysis
Soil sample digestion were performed following Aqua -Regia digestion for extraction of trace element method (ISO 11466) (ISO, 1995). CRM Wageningen -WEPAL ISE-850 (Calcareous soil) was used as certified laboratory reference material and % mineral recovery were calculated. Blanks were also analysed at the same time. A three-step sequential extraction scheme for the fractionation of sulphur was followed. The detailed procedure for soil sample collection, mineral analysis and three-step sequential extraction is reported elsewhere [
17]. Soil mineral concentration of each experimental site are presented in
Table 2. Calcium, potassium, boron, sulphur and iron content of soil samples were significantly different among the two locations and slope positions. The recovery for all minerals is between the acceptable ranges (85 – 120%).
2.5. Agronomic data collection
The plant height, total tiller number, productive tiller number, finger length of the longest spike and number of fingers per main ear were measured at maturity stage (International Board for Plant Genetic Resources [
18]. Grain yield at 12% average moisture and biomass at 18% average moisture were taken (from the eight central rows) on a plot basis and then converted to hectare basis.
2.6. Data Analysis
Data collected for all agronomic quantitative characters were subjected to analysis of variance (ANOVA) using R software version 3.3.2. The major descriptive statistics such as mean, range and standard deviation of each trait for the study varieties, fertilizer levels, study location and slope positions were computed. Slope, fertilizer level and variety were treated as fixed effects whereas season, block within farm, farm within location and location were treated as random effects. Yield and biomass data were transformed to natural logarithms as the dispersion of the random effects on the original scale appears to increase with the fitted value.
3. Result
3.1. Yield and Biomass effects
Finger millet yield and biomass per hectare for each fertilizer treatment, variety, location and slope position are presented in
Figure 1,
Figure 2,
Figure 3 and
Figure 4. While the total and productive tiller numbers, number of fingers per main ear, plant height and finger lengths (cm) for each fertilizer treatment, variety, location and slope are presented in
Table 3 and
Table 4. Yield and biomass showed wide variation ranging from 94 to 3828 kg and from 6.25 to 242.97 quintals per hectare, respectively. Maximum yield of the NPKS at recommended rate in current experiment (3594 kg ha
-1) is much higher than the national average [
14] and estimated potential finger millet yields (Mulatu & Kebede, 1994) 2504 kg and 3000 kg ha
-1, respectively. Finger number per main ear, total tiller number and productive tiller number ranges between 1-14, 1-16 and 1-14, respectively. Plant height and finger length ranges between 4-120 and 3-17 cm, respectively.
Significant response from Meba variety to the combined FeSO
47H
2O and ZnSO
47H
2O fertilizer application at Gojjam hill slope was exhibited where average yield was increased by 51.6% (
Table 5). Diga-01 variety responded significantly to FeSO
47H
2O fertilizers at Arsi Negelle hill slope position in which 18.3% average yield enhancement was recorded. A significant response was observed from Urji variety to ZnSO
47H
2O fertilizers at Gojjam hill slope position where 27.6% average yield increase was observed. Irrespective of locations, slope position and variety, grain yield was enhanced by 20% due to soil application of FeSO
47H
2O fertilizer.
Strong evidence (p<0.001) exhibited for fertilizer effect on finger millet yield irrespective of location, slope and variety (
Table 5). Similarly, variety shows strong evidence (p<0.001) of effect on yield over location, slope and fertilizer. Moderate evidence (p<0.05) has been seen for a slope position effect on finger millet yield irrespective of location, fertilizer application and variety(
Table 5). However, there is no evidence for the effect on yield due to interaction of fertilizer and variety (
Table 5).
Fertilizer effect shows strong evidence (p<0.001) on finger millet biomass irrespective of location, slope and variety (
Table 6). Some evidence (p<0.05) has been seen for a slope position effect on biomass irrespective of location, fertilizer and variety (
Table 6). Similarly, slight variety effect (p<0.05) on biomass irrespective of location, slope and fertilizer is observed. However, interaction of fertilizer and variety exhibited no effect on biomass (
Table 6).
Varieties differed significantly for both yield (p<0.001) and biomass (p<0.05) of finger millet showing the average result of Diga-01 > Meba > Urji. Similarly, fertilizer treatment significantly (p<0.001) affects both yield and biomass of finger millet showing the average result of T5 > T1 > T3 > T2 > T4 and T3 > T1 > T2 > T5 >T4, respectively.
Strong evidence (p<0.001) seen for fertilizer effect on finger millet plant height as well as finger length irrespective of location, slope and variety. Variety shows strong evidence (p<0.001) for effect on finger length and some effect on plant height (p<0.05). Slope position shows weak evidence (p<0.1) for effect on plant height.
4. Discussion
The present study reports finger millet genotypic response to Zn and Fe agronomic biofortification, location and slope position towards yield and biomass. Irrespective of genotype, locations and slope position, grain yield was enhanced by 20% due to soil application of FeSO
4 fertilizer. However, different finger millet genotypes responds differently for both fertilizer treatment and location in respect to yield and yield traits. This suggests finger millet genotypes differ in their ability to remobilization and retranslocation of Zn and Fe deposited which plays a critical role in better partitioning of carbohydrates from leaf to reproductive parts affecting the yield and yield attributes. Therefore, the current finding should be taken into consideration in evaluating cereal genotypes for their response to agronomic biofortification. To our knowledge, there is no available previous data on finger millet for Zn, Fe, or combined fertiliser, genotype, location, and slope position effect on grain yield and yield traits. Thus, this experiment is the first in its kind to report the triple impact of Zn and Fe agronomic biofortification, genotype, and environment (location and slope position) on grain yield and biomass of finger millet. However, previous study shows that different finger millet genotypes responded differently to phosphorus fertilizer as well as locations in Kenya (Wafula
et al., 2016), to NPK fertilizaton in India [
19], and to location in Ethiopia [
20]. On the other hand, wheat and rice genotypes responded differently to Zn fertilization in Turkey [
8] and to Zn fertilization as well as climate in India [
21], respectively.
4.1. Finger millet genotypic response to Zn fertiliser towards yield affected by location and slope position
The present study indicates that Urji variety responds significantly to ZnSO
4 fertilizers at Gojjam hill slope position where 27.6% yield increase was observed. The possible reason behind the fact that finger millet responds well at Gojjam hill slope position to Zn fertilization is that the significantly higher soil sulphur concentration (
Table 1), since sulphur is reported to enhances the solubility of Zn and its uptake by the plant [
22]. On the other hand application of ZnSO
4 fertilizers significantly increases the total and productive tiller number for Urji genotype at the hill slope position (
Table 4) and this might also play a major role in yield increase. There is no available previous data that explores the impact of Zn fertilization on finger millet grain yield. However, previous researches on wheat, rice, maize, sorghum etc, explores the effect of Zn fertilization on grain yield. Study from India shows 14.2% yield increase as a result of application of Zn fertilization [
23]. Similarly, Phattarakul et al. [
24] reported an increase of 10% crop yield in their experiment conducted in China and India. Another experiment from India indicates 23.5% increase in grain yield by applying Zn fertilization [
25]. Increase in yield up to 33% was observed as a result of application of Zn fertiliser [
21]. Narwal et al. [
26] also found 5% yield enhancement by applying Zn fertiliser. Overwhelming evidence from all-over the world witnessed application of Zn fertilizer improves crops yield [
1,
2,
8,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42]. The positive response of yield in cereal towards Zn fertilization in both current and previous studies is possibly due to the enhancement of plant available Zn for uptake [
8] which in turn helps the plant for better protein breakdown and enzyme activation resulted in higher vegetative growth and yield increase [
25]. However, one studies from Thailand and Turkey shows little or insignificant effect of Zn fertilizer application on yield [
24]. The irresponsiveness of rice to Zn fertilization in previous study possibly due to the absence of sulphur fertilization since sulphur reported to enhances the solubility of Zn and its uptake by spring wheat [
22]. In addition to that the lower soil Zn concentration was reported (ranging from 0.5 to 6.5 mg kg
-1 in previous study whereas 85 to 105 mg kg
-1 in present study). The other possibility might be due to different crop genotypes responded differently to Zn fertilizer treatment, climate and environment (location and slope position) as it is witnessed from the current as well as previous studies [
8,
20,
21,
43].
4.2. Finger millet genotypic response to Fe fertiliser towards yield affected by location and slope position
The present study indicates that Diga-01 variety respond significantly to FeSO
4 fertilizer application at Arsi Negelle hill slope position in which 18.3% average yield enhancement was recorded. The possible reason behind the fact that finger millet responds well at Arsi Negelle hill slope position to Fe fertilization is that the significantly higher soil potassium concentration (
Table 1), since it is reported that potassium seems to have a very specific role in the plant for maximum utilization of Fe [
44,
45]. On the other hand application of FeSO
4 fertilizers significantly increases the total and productive tiller number for Diga-01genotype at the hill slope position (
Table 3) and this might also play a major role in yield increase. Even though no data is available for Fe fertilization impact on finger millet grain yield, few previous experiments on wheat, barley and oat investigate the effect of Fe fertilization on grain yield and discussed with the current result. Agronomic biofortification of Fe fertilizer is less well studied as compared to Zn fertilizer. For instance a study from India indicated the enhancement of 13 % yield due to application of Fe fertilization [
26]. The positive response of yield towards Fe fertilization in both current and previous study is possibly due to the enhancement of plant available/soluble Fe for uptake [
8] which in turn helps the plant for better chlorophyll synthesis, protein and carbohydrate metabolism, and enzyme activation resulted in better vegetative growth and yield increase [
46]. However, studies from Turkey and Canada on Fe biofortifiction shows no yield improvement [
27,
47]. This might be due to three possible reasons: the first reason could be, in general, different crops and varieties responded differently to mineral fertilization as well as location [
1,
20,
21]. The other possibility is that when applied to calcareous soils, Fe rapidly converted into unavailable forms and the poor mobility of Fe in phloem makes Fe fertilization impact limited or unsuccessful [
2,
41]. It is also possibly due to the graminaceoues species release phytosiderophores (Fe-mobilizing compounds) to solubilize and absorb Fe from calcareous soils with low Fe concentration, thus, they can maintain adequate plant growth by satisfy Fe demand without requirement for Fe fertilization [
48,
49,
50].
5. Conclusion
Finger millet genotype greatly influences the response to agronomic biofortification of Zn and Fe fertilizer in present study, which indicates the varied yield and yield traits performance of the varieties across environments (location and slope position). Which revealing the vitality of experimenting finger millet varieties response to Zn and Fe fertilizer at different environments (location and slope positions) prior to scale up for mass production. The soil application of 20 kg FeSO47H2O per hectare along with recommended rate of NPKS could be a finest agronomic biofortification strategy to enhance yield of all genotypes in the study areas and area with similar agro-ecologies. Moreover, soil application of 20 kg ZnSO47H2O and combined 20 kg FeSO47H2O and 25 kg ZnSO47H2O on Urji and Meba, respectively at Gojjam hill slope and area with similar agro-ecologies could be a premium agronomic biofortification strategy to improve finger millet grain yield. Future studies as well as development programs on agronomic biofortification should consider environmental (location and slope position) effect beside the main fertilizer effect, which is a gap in current knowledge.
Author Contributions
DT: DG, EJ, RL, TA, and MB: conceptualization. DT: data collection, supervision, and original draft preparation. DT and TA: experimental design. DT and EB: soil sample preparation and analysis. DT, DG, and RL. Statistical analysis. DT, DG, EJ, MRL, EB, LW, TA, and MB: writing - review and editing.
Funding
This work was also supported, in part, by the Bill & Melinda Gates Foundation [INV-009129]. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. Funding was also provided by USAID: Feed the future. The funders had no role in the design, execution, analyses or interpretation of the data.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Amhara and Oromia regional states agriculture bureau, Melkasa and Bako Agricultural Research Centres, data collectors, supervisors and participating farmers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cakmak, I.; Pfeiffer, W.H.; McClafferty, B. (a). Biofortification of durum wheat with zinc and iron. Cereal chem. 2010, 87, 10–20. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- García-Bañuelos, M.L.; Sida-Arreola, J.P.; Sánchez, E. Biofortification-promising approach to increasing the content of iron and zinc in staple food crops. J. Elem. 2014, 19, 865–888. [Google Scholar]
- Graham, R.D.; Welch, R.M. Breeding for staple food crops with high micronutrient density; Internationall Food Policy Research Institute: Washington, DC, USA, 1996; Volume 3, pp. 14–16. [Google Scholar]
- Ma, G.; Jin, Y.; Li, Y.; Zhai, F.; Kok, F.J.; Jacobsen, E.; Yang, X. Iron and zinc deficiencies in China: what is a feasible and cost-effective strategy? Public Health Nutr. 2008, 11, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.J.; Nestel, P.; Meenakshi, J.V.; Qaim, M.; Sachdev, H.P.S.; Bhutta, Z.A. Plant breeding to control zinc deficiency in India: how cost-effective is biofortification? Public Health Nutr. 2007, 10, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Velu, G.; Ortiz-Monasterio, I.; Cakmak, I.; Hao, Y.; Singh, R.Á. Biofortification strategies to increase grain zinc and iron concentrations in wheat. J. Cereal Sci. 2014, 59, 365–372. [Google Scholar] [CrossRef]
- Yilmaz, A.; Ekiz, H.; Torun, B.; Gultekin, I.; Karanlik, S.; Bagci, S.A.; Cakmak, I. Effect of different zinc application methods on grain yield and zinc concentration in wheat cultivars grown on zinc-deficient calcareous soils. J. Plant Nutr. 1997, 20, 461–471. [Google Scholar] [CrossRef]
- Mortvedt, J.J.; Gilkes, R.J. Zinc fertilisers. In Zinc in soil and plants; Robson, A.D., Ed.; Springer: Dordrecht, Netherlands, 1993; pp. 33–44. [Google Scholar]
- Gupta, S.M.; Arora, S.; Mirza, N.; Pande, A.; Lata, C.; Puranik, S.; Kumar, J.; Kumar, A. Finger millet: a “certain” crop for an “uncertain” future and a solution to food insecurity and hidden hunger under stressful environments. Front. Plant Sci. 2017, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Lata, C. Advances in Omics for Enhancing Abiotic Stress Tolerance in Millets. Proc Indian Natn Sci Acad 2015, 81, 397–417. [Google Scholar]
- Gull, A.; Jan, R.; Nayik, G.A.; Prasad, K.; Kumar, P. Significance of finger millet in nutrition, health and value added products: a review. Magnesium, 2014, 130, 1601–1608. [Google Scholar]
- Upadhyaya, H.D.; Gowda, C.L.L.; Reddy, V.G. Morphological diversity in finger millet germplasm introduced from Southern and Eastern Africa. Journal of SAT Agricultural Research 2007, 3, 1–3. [Google Scholar]
- Central Statistical Agency (CSA). Central statistical agency agricultural sample survey. Report on Area and Production of Major Crop. Statistical Bulletin 2021, 590, 1. [Google Scholar]
- Amede, T.; Gashaw, T.; Legesse, G.; Tamene, L.; Mekonen, K.; Thorne, P.; Schultz, S. Landscape positions dictating crop fertilizer responses in wheat-based farming systems of East African Highlands. Renew. Agric. Food Syst. 2022, 37, 4–16. [Google Scholar] [CrossRef]
- Gorfu, D.; Ahmed, E. Crops and agro-ecological zones of Ethiopia. Ethiopian Institute of Agricultural Research 2012. [Google Scholar]
- Gashu, D.; Nalivata, P.C.; Amede, T.; Ander, E.L.; Bailey, E.H.; Botoman, L.; Chagumaira, C.; Gameda, S.; Haefele, S.M.; Hailu, K.; Joy, E.J.M. The nutritional quality of cereals varies geospatially in Ethiopia and Malawi. Nature 2021, 594, 71–76. [Google Scholar] [CrossRef]
- International Board for Plant Genetic Resources. Descriptors for finger millet (Eleusine coracana (L.) Gaertn); International Board for Plant Genetic Resources: Rome, Italy, 1985; pp. 20–35. [Google Scholar]
- Nevse, G.P.; Chavan, L.S.; Jagtap, D.N. Performance of Finger millet (Eleusine coracana [L.] Gaertn) to age of seedlings, FYM and fertilizer levels. J Indian Soc. Coastal agric. Res. 2013, 31, 64–70. [Google Scholar]
- Simion, T.; Markos, S.; Samuel, T. Evaluation of finger millet (Eleusine coracana (L). Gaertn.) varieties for grain yield in lowland areas of southern Ethiopia. Cogent food agric. 2020, 6, 1788895. [Google Scholar] [CrossRef]
- Saha, S.; Chakraborty, M.; Padhan, D.; Saha, B.; Murmu, S.; Batabyal, K.; Seth, A.; Hazra, G.C.; Mandal, B.; Bell, R.W. Agronomic biofortification of zinc in rice: Influence of cultivars and zinc application methods on grain yield and zinc bioavailability. Field Crops Res. 2017, 210, 52–60. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Q. Interaction effect of zinc and elemental sulfur on their uptake by spring wheat. J. Plant Nutr. 2005, 28, 639–649. [Google Scholar] [CrossRef]
- Pal, V.; Singh, G.; Dhaliwal, S.S. Agronomic biofortification of chickpea with zinc and iron through application of zinc and urea. Commun Soil Sci Plant Anal 2019, 50, 1864–1877. [Google Scholar] [CrossRef]
- Phattarakul, N.; Rerkasem, B.; Li, L.J.; Wu, L.H.; Zou, C.Q.; Ram, H.; Sohu, V.S.; Kang, B.S.; Surek, H.; Kalayci, M.; Yazici, A. Biofortification of rice grain with zinc through zinc fertilization in different countries. Plant Soil 2012, 361, 131–41. [Google Scholar] [CrossRef]
- Hussain, S.T.; Bhat, M.A.; Hussain, A.; Dar, S.A.; Dar, S.H.; Ganai, M.A.; Telli, N.A. Zinc fertilization for improving grain yield, zinc concentration and uptake in different rice genotypes. J. pharmacogn. phytochem. 2018, 7, 287–291. [Google Scholar]
- Narwal, R.P.; Malik, R.S.; Dahiya, R.R. Addressing variations in status of a few nutritionally important micronutrients in wheat crop. In 19th World Congress of Soil Science, Soil Solutions for a Changing World 2010, 3, pp. 1–6. [Google Scholar]
- Aciksoz, S.B.; Yazici, A.; Ozturk, L.; Cakmak, I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil 2011, 349, 215–225. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of fertilizers with zinc: An excellent investment for humanity and crop production in India. J Trace Elem Med Biol 2009, 23, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kalayci, M.; Kaya, Y.; Torun, A.A.; Aydin, N.; Wang, Y.; Arisoy, Z.; Erdem, H.A.; Yazici, A.; Gokmen, O.; Ozturk, L. Biofortification and localization of zinc in wheat grain. J. Agric. Food Chem. 2010, 58, 9092–9102. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I. HarvestPlus zinc fertilizer project: HarvestZinc. Better Crops 2012, 96, 17–19. [Google Scholar]
- Haileselassie, B.; Stomph, T.J.; Hoffland, E. Teff (Eragrostis tef) production constraints on Vertisols in Ethiopia: farmers’ perceptions and evaluation of low soil zinc as yield-limiting factor. Soil Sci. Plant Nutr. 2011, 57, 587–596. [Google Scholar] [CrossRef]
- Jat, S.L.; Shivay, Y.S.; Parihar, C.M. Dual purpose summer legumes and zinc fertilization for improving productivity and zinc utilization in aromatic hybrid rice (Oryza sativa). indian j Agron 2011, 56, 328–333. [Google Scholar]
- Kumar, N.; Salakinkop, S.R. Agronomic biofortification of maize with zinc and iron micronutrients. Mod. concepts dev. agron. 2018, 1, 1–4. [Google Scholar]
- Mishra, J.S.; Hariprasanna, K.; Rao, S.S.; Patil, J.V. Biofortification of post-rainy sorghum (Sorghum bicolor) with zinc and iron through fertilization strategy. Indian J. Agric. Sci. 2015, 85, 721–724. [Google Scholar] [CrossRef]
- Pooniya, V.; Shivay, Y.S. Summer green-manuring crops and zinc fertilization on productivity and economics of basmati rice (Oryza sativa L.). Arch. Agron. Soil Sci. 2012, 58, 593–616. [Google Scholar] [CrossRef]
- Prasad, S.K.; Singh, M.K.; Singh, R.E.N.U. Effect of nitrogen and zinc fertilizer on pearl millet (Pennisetum glaucum) under agri-horti system of eastern Uttar Pradesh. Significance 2014, 400, 1–5. [Google Scholar]
- Saleem, I.; Javid, S.; Bibi, F.; Ehsan, S.; Niaz, A.; Ahmad, Z.A. Biofortification of maize grain with zinc and iron by using fertilizing approach. J. agric. ecol 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Shivay, Y.S.; Kumar, D.; Prasad, R. Effect of zinc-enriched urea on productivity, zinc uptake and efficiency of an aromatic rice–wheat cropping system. Nutr. Cycling Agroecosyst. 2008, 81, 229–243. [Google Scholar] [CrossRef]
- Shivay, Y.S.; Prasad, R.; Rahal, A. Relative efficiency of zinc oxide and zinc sulphate-enriched urea for spring wheat. Nutr. Cycling Agroecosyst. 2008, 82, 259–264. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, L.H.; Wang, M.Y. Iron and zinc biofortification in polished rice and accumulation in rice plant (Oryza sativa L.) as affected by nitrogen fertilization. Acta Agric. Scand. - B Soil Plant Sci. 2008, 58, 267–272. [Google Scholar]
- Zhang, Y.; Shi, R.; Rezaul, K.M.; Zhang, F.; Zou, C. Iron and zinc concentrations in grain and flour of winter wheat as affected by foliar application. J. Agric. Food Chem. 2010, 58, 12268–12274. [Google Scholar] [CrossRef]
- Zou, C.Q.; Zhang, Y.Q.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.Z.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.H.; Sohu, V.; Hassan, M.U. Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 2012, 361, 119–130. [Google Scholar] [CrossRef]
- Wafula, W.N.; Korir, K.N.; Ojulong, H.F.; Siambi, M.; Gweyi-Onyango, J.P. (2016). Finger millet (Eleusine coracana L.) grain yield and yield components as influenced by phosphorus application and variety in Western Kenya. Trop. Plant Res. 2016, 3, 673–680. [Google Scholar] [CrossRef]
- Oertli, J.J.; Opoku, A.A. Interaction of potassium in the availability and uptake of iron from ferric hydroxide. Soil Sci Soc Am J. 1974, 38, 451–454. [Google Scholar] [CrossRef]
- Wu, L.B.; Holtkamp, F.; Wairich, A.; Frei, M. Potassium ion channel gene OsAKT1 affects iron translocation in rice plants exposed to iron toxicity. Front. Plant Sci. 2019, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Mahler, R.L. Nutrients plants require for growth. Iniversity of Idaho. College of Agricultural and Life Science. CIS, 1124; 2004.
- Gupta, U.C. Iron status of crops in Prince Edward Island and effect of soil pH on plant iron concentration. Can. J. Soil Sci. 1991, 71, 197–202. [Google Scholar] [CrossRef]
- Marschner, H.; Römheld, V.; Kissel, M. Different strategies in higher plants in mobilization and uptake of iron. J. Plant Nutr. 1986, 9, 695–713. [Google Scholar] [CrossRef]
- Römheld, V. The role of phytosiderophores in acquisition of iron and other micronutrients in graminaceous species: An ecological approach. Plant Soil, 1991, 130, 127–134. [Google Scholar] [CrossRef]
- Takagi, S.I.; Kamei, S.; Yu, M.H. Efficiency of iron extraction from soil by mugineic acid family phytosiderophores. J. Plant Nutr. 1988, 11, 643–651. [Google Scholar] [CrossRef]
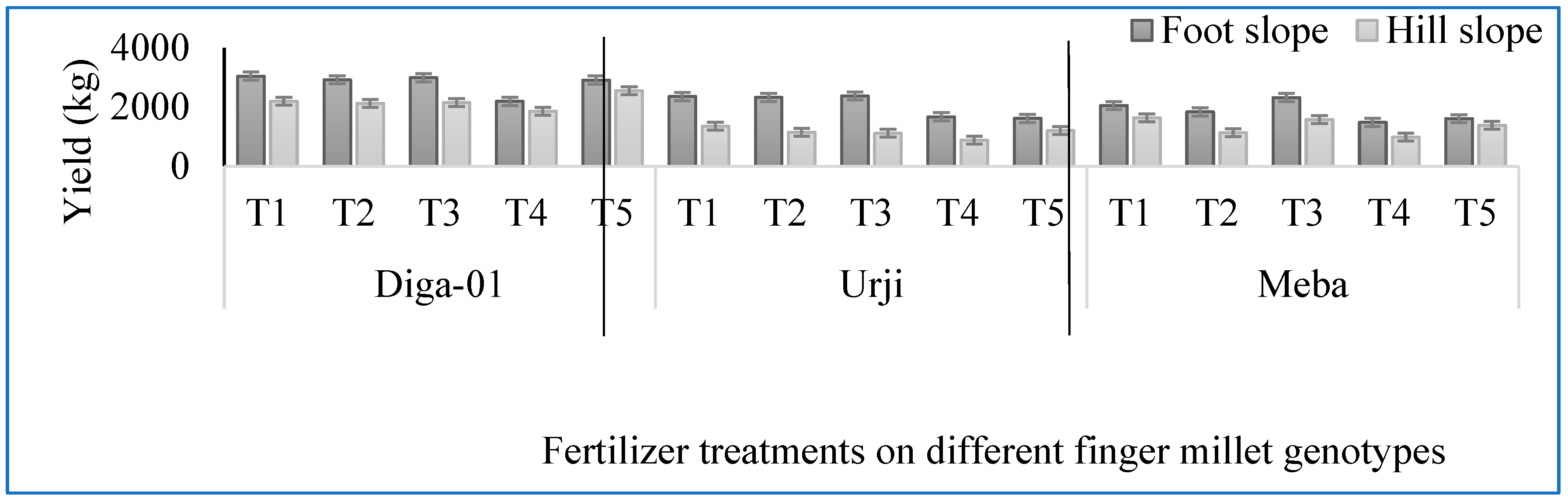
Figure 1.
Effect of fertilizer treatment on yield (kg) of finger millet genotypes at Arsi Negelle as affected by slope position. T1: 25 kg ZnSO47H2O, 20 kg FeSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T2: 25 kg ZnSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T3: 131 kg NPS, 60 kg K, 54 kg urea ha-1; T4: 30% of T3; T5: 20 kg FeSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1.
Figure 1.
Effect of fertilizer treatment on yield (kg) of finger millet genotypes at Arsi Negelle as affected by slope position. T1: 25 kg ZnSO47H2O, 20 kg FeSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T2: 25 kg ZnSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T3: 131 kg NPS, 60 kg K, 54 kg urea ha-1; T4: 30% of T3; T5: 20 kg FeSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1.
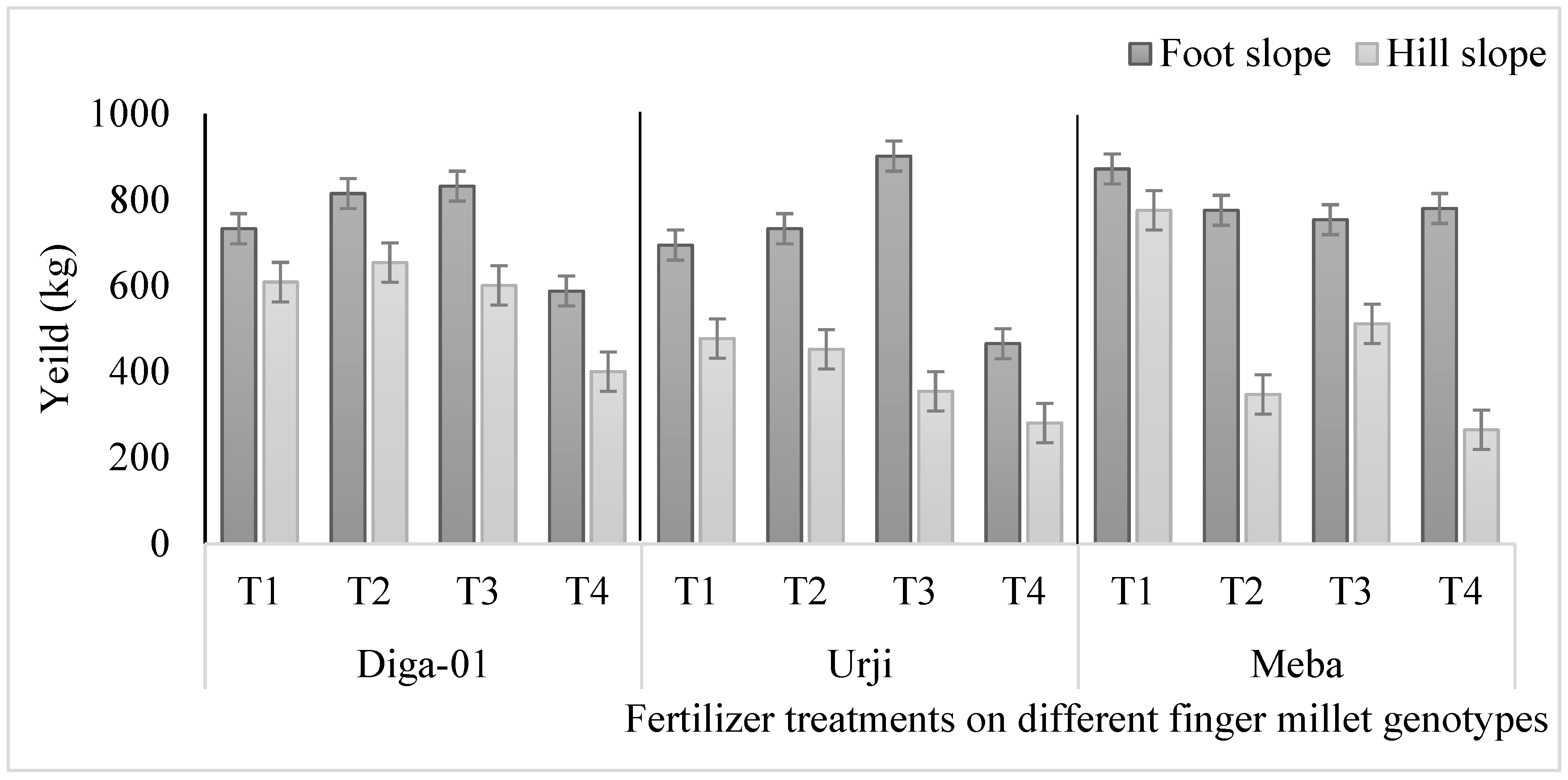
Figure 2.
Effect of fertilizer treatment on yield (kg) of finger millet genotypes at Gojjam as affected by slope position. T1: 25 kg ZnSO47H2O, 20 kg FeSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T2: 25 kg ZnSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T3: 131 kg NPS, 60 kg K, 54 kg urea ha-1; T4: 30% of T3.
Figure 2.
Effect of fertilizer treatment on yield (kg) of finger millet genotypes at Gojjam as affected by slope position. T1: 25 kg ZnSO47H2O, 20 kg FeSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T2: 25 kg ZnSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T3: 131 kg NPS, 60 kg K, 54 kg urea ha-1; T4: 30% of T3.
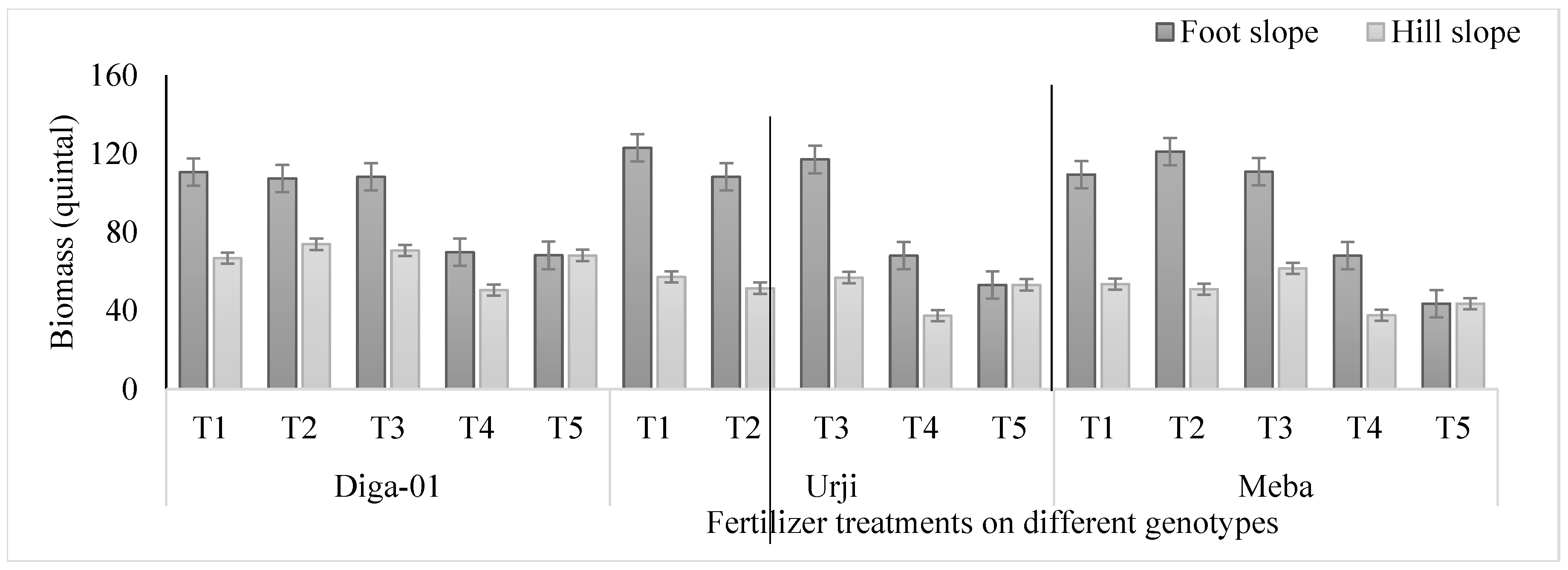
Figure 3.
Effect of fertilizer on biomass (quintal) of finger millet genotypes at Arsi Negelle as affected by slope position. T1: 25 kg ZnSO47H2O, 20 kg FeSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T2: 25 kg ZnSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T3: 131 kg NPS, 60 kg K, 54 kg urea ha-1; T4: 30% of T3; T5: 20 kg FeSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1.
Figure 3.
Effect of fertilizer on biomass (quintal) of finger millet genotypes at Arsi Negelle as affected by slope position. T1: 25 kg ZnSO47H2O, 20 kg FeSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T2: 25 kg ZnSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T3: 131 kg NPS, 60 kg K, 54 kg urea ha-1; T4: 30% of T3; T5: 20 kg FeSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1.
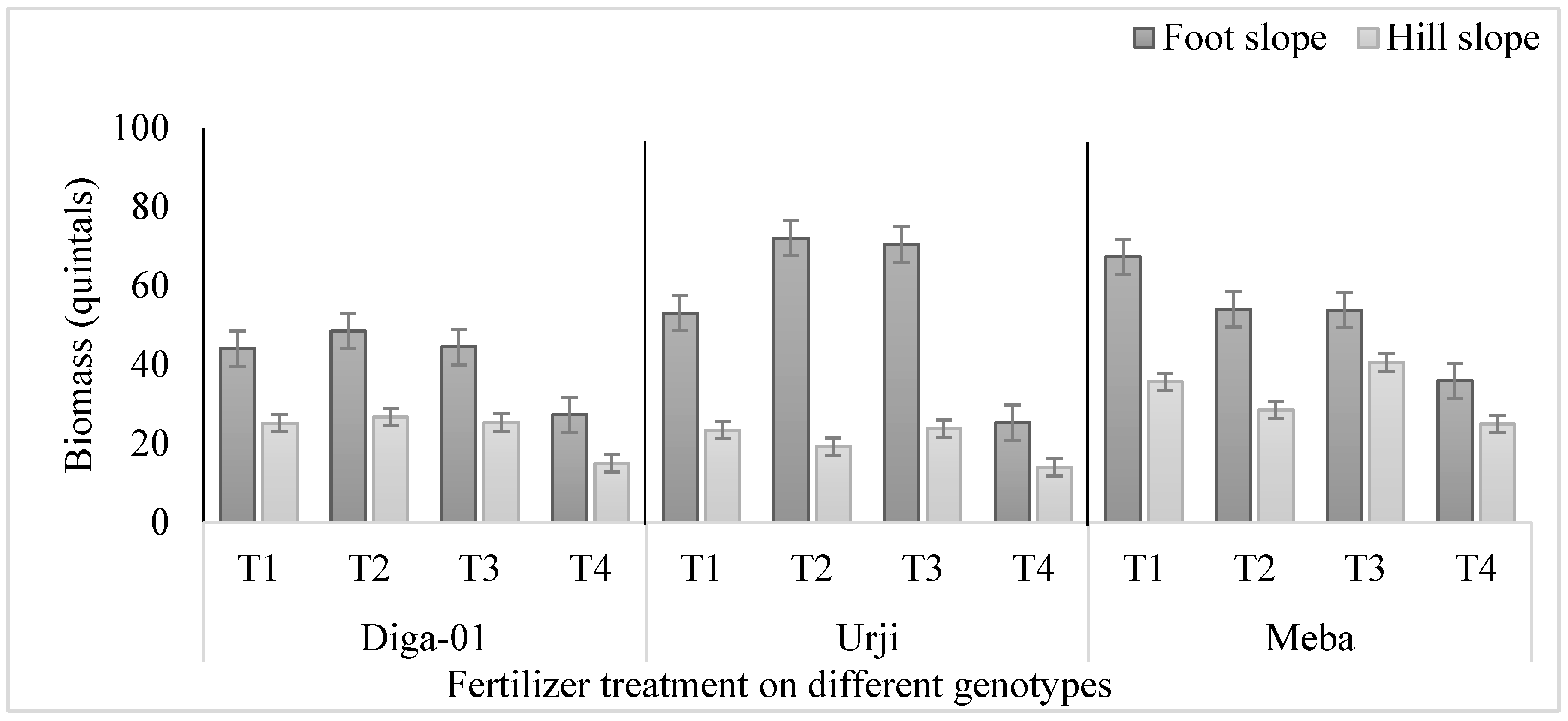
Figure 4.
Biomass (quintal) of finger millet genotypes at Gojjam as affected by zinc and iron fertilization. T1: 25 kg ZnSO47H2O, 20 kg FeSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T2: 25 kg ZnSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T3: 131 kg NPS, 60 kg K, 54 kg urea ha-1; T4: 30% of T3.
Figure 4.
Biomass (quintal) of finger millet genotypes at Gojjam as affected by zinc and iron fertilization. T1: 25 kg ZnSO47H2O, 20 kg FeSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T2: 25 kg ZnSO47H2O, 131 kg NPS, 60 kg K, 54 kg urea ha-1; T3: 131 kg NPS, 60 kg K, 54 kg urea ha-1; T4: 30% of T3.
Table 1.
Elemental application of nutrients in kg per hectare.
Table 1.
Elemental application of nutrients in kg per hectare.
| Treatments |
Zn |
Fe |
N |
P |
S |
K |
| T1 |
5.5 |
4 |
32.1 |
3.59 |
15.89 |
31.2 |
| T2 |
5.5 |
- |
32.1 |
3.59 |
7.64 |
31.2 |
| T3 |
- |
- |
32.1 |
3.59 |
5.24 |
31.2 |
| T4 |
- |
- |
9.63 |
1.1 |
1.57 |
9.36 |
| T5 |
- |
4 |
32.1 |
3.59 |
13.49 |
31.2 |
Table 2.
Mineral concentrations (mg/kg) of soil from the finger millet agronomic biofortification experimental sites in Ethiopia.
Table 2.
Mineral concentrations (mg/kg) of soil from the finger millet agronomic biofortification experimental sites in Ethiopia.
| Location |
Slope |
B |
Mg |
P |
S |
K |
Ca |
Fe |
Zn |
Arsi-
Negelle |
Foot |
3.9±0.79A
|
2052±47 |
2061±49 |
122.7±0.4 |
3227±185A
|
4662±481A
|
26918±1149A
|
89±7 |
| Hill |
3.0±0.70B
|
1728±240 |
1725±240 |
105.8±7.3 |
2729±448B
|
4050±918B
|
23952±1804B
|
105±10 |
| Mean |
3.4±0.95a
|
1890±259a
|
1893±264a
|
114.3±10.3a
|
2978±464a
|
4356±870 a
|
25435± 2320a
|
97±13a
|
| Gojjam |
Foot |
1.0±0.37C
|
1731±131 |
1731±131 |
136.6±6C
|
934±33C
|
1185±149C
|
107973±3372C
|
81±4 |
| Hill |
0.1±0.08D
|
1597±167 |
1597±167 |
207.1±42.8D
|
859±85D
|
1668±430D
|
124304±5913D
|
104±11 |
| Mean |
0.55±0.5b
|
1664±180a
|
1664±180a
|
171.8±47.3b
|
897±82b
|
1426±440b
|
116138±10383b
|
92±16a
|
Table 3.
Effect of fertilizer treatment on yield traits of finger millet genotypes at Arsi Negelle as affected by slope postition.
Table 3.
Effect of fertilizer treatment on yield traits of finger millet genotypes at Arsi Negelle as affected by slope postition.
| |
Foot slope |
Hill slope |
| Variety |
Fertilizer |
Total tiller number |
Productive tiller number |
Finger no/ main ear |
Plant Height (cm) |
Finger Length (cm) |
Total tiller number |
Productive tiller number |
Finger no/ main ear |
Plant Height (cm) |
Finger Length (cm) |
| Diga-01 |
T1 |
4.58±1.88 |
4.4±1.9 |
6.0±1.4 |
68.6±14.2 |
8.0±1.6 |
4.8±1.7 |
4.5±1.7 |
5.8±1.8 |
54.3±8.8 |
7.3±1.3 |
| T2 |
4.54±1.49 |
4.1±1.3 |
6.5+2 |
71.8±21.6 |
7.6±1.5 |
5.1±1.5 |
4.7±1.7 |
5.8±1.4 |
56.9±7.6 |
7.4±1.3 |
| T3 |
4.46±1.72 |
4.2±1.6 |
6.4±1.7 |
70.8±16.2 |
8.1±1.7 |
5.0±1.9 |
4.7±1.7 |
5.4±1.4 |
57.2±7.7 |
7.3±1.3 |
| T4 |
5.63±2.22 |
5.4±2.2 |
6.3±1.6 |
57.2±12 |
7.6±1.3 |
4.5±1.4 |
4.2±1.2 |
5.2±1.1 |
49.9±7.3 |
7.6±1.3 |
| T5 |
5.5±1.9 |
5.0±1.6 |
6.0±2.4 |
58.1±8.8 |
8.0±1.5 |
5.2±2 |
4.8±1.9 |
4.4±0.8 |
54.4±7.6 |
7.2±1.4 |
| Urji |
T1 |
4.38±1.58 |
3.9±1.6 |
7.1±2.2 |
64.6±16.4 |
7.7±1.4 |
4.9±1.9 |
4.1±1.5 |
7.3±1.8 |
52.1±7.2 |
7.9±1 |
| T2 |
4.86±1.74 |
4.3±1.5 |
7.9±1.8 |
65.6±18.2 |
8.3±1.5 |
4.9±2.3 |
4.3±1.7 |
7.4±1.8 |
51.8±8.3 |
7.6±1.4 |
| T3 |
4.81±1.96 |
4.3±2 |
7.6±1.6 |
64.8±13.4 |
7.9±1.7 |
5.4±2 |
4.5±1.4 |
7.2±1.9 |
53.0±8.3 |
7.4±1.3 |
| T4 |
5.6±2.9 |
5.2±2.8 |
7.8±1.5 |
56.2±13.7 |
8.3±1.7 |
5.6±2.6 |
4.9±2.5 |
7.0±1.6 |
47.1±6.8 |
6.6±1.2 |
| T5 |
5.0±2.2 |
4.1±1.9 |
6.7±1.3 |
49.0±9.7 |
7.5±2 |
5.4±3 |
4±1.8 |
6.8±1.8 |
48.4±7.9 |
6.8±1.7 |
| Meba |
T1 |
4.94±2.2 |
4.1±1.6 |
5.8±1.3 |
63.5±12.8 |
5.8±1.1 |
5.3±2 |
4.5±1.6 |
5.7±1.7 |
56.0±7.3 |
5.4±1.1 |
| T2 |
5.35±2.8 |
4.5±1.6 |
6.1±1.6 |
65.7±11 |
6.2±1.3 |
5.2±1.9 |
4.3±1.6 |
5.6±1.2 |
53.6±9 |
5.7±0.9 |
| T3 |
5.06±1.66 |
4.5±1.5 |
6.2±1.1 |
64.0±17.1 |
5.8±1.1 |
5.2±1.9 |
4.1±1.6 |
5.6±1.2 |
53.3±8.6 |
5.6±1.1 |
| T4 |
5.0 ±2.2 |
4.7±2.1 |
6.0±1.4 |
56.8±12.7 |
5.7±1.1 |
5.9 ±2.4 |
4.6±1.9 |
5.9±1.4 |
47.0±8.9 |
5.2±0.8 |
| T5 |
4.7±1.4 |
4.0±1 |
5.6±1.3 |
50.4±6.9 |
5.3±0.8 |
7.2±2 |
5.8±2.1 |
5.9±1.2 |
44.8±12.5 |
5.4±0.9 |
Table 4.
Effect of fertilizer treatment on yield traits of finger millet genotypes at Gojjam as affected by slope position.
Table 4.
Effect of fertilizer treatment on yield traits of finger millet genotypes at Gojjam as affected by slope position.
| |
Foot slope |
Hill slope |
| Variety |
Fertilizer |
Total tiller number |
Productive tiller number |
Finger no/ main ear |
Plant Height (cm) |
Finger Length (cm) |
Total tiller number |
Productive tiller number |
Finger no/ main ear |
Plant Height (cm) |
Finger Length (cm) |
| Diga-01 |
T1 |
1.35±0.6 |
1.31±0.6 |
6.1±1.5 |
65.1±17.1 |
9.8±1.6 |
1.56+0.7 |
1.45+0.7 |
5.7±1 |
46.5±6 |
9.2±1.1 |
| T2 |
1.41±0.8 |
1.34±0.8 |
5.3±1.2 |
61.3±12.9 |
9.6±1.9 |
1.83±0.9 |
1.77±0.9 |
5.2±0.8 |
44.2±7 |
9.5±1.9 |
| T3 |
1.38±0.6 |
1.31±0.6 |
5.8±1.2 |
66.2±9.1 |
9.8±1.5 |
1.45±0.7 |
1.39±0.7 |
5.2±1.2 |
44.0±6.9 |
8.8±1.1 |
| T4 |
1.36±0.4 |
1.29±0.4 |
5.1±0.9 |
57.4±10.8 |
9.7±1.9 |
1.44±0.6 |
1.35±0.6 |
4.8±1 |
42.3±10.4 |
7.7±1.4 |
| Urji |
T1 |
1.29±0.5 |
1.22±0.5 |
7.3±2.1 |
66.0±15.2 |
9.9±2.1 |
1.77±0.8 |
1.67±0.8 |
7.4±1.1 |
48.4±7.2 |
9.9±1.7 |
| T2 |
1.38±0.6 |
1.27±0.6 |
8.1±1.8 |
77.4±19.1 |
10.4±1.7 |
2.2±1.8 |
1.98±1.8 |
7.2±0.9 |
49.9±5 |
10.1±1.4 |
| T3 |
1.41±0.8 |
1.35±0.8 |
7.6±1.7 |
70.5±13.5 |
10.0±1.9 |
1.56±0.8 |
1.45±0.8 |
6.6±1.7 |
47.5±8.1 |
9.5±1.1 |
| T4 |
1.34±0.5 |
1.28±0.5 |
6.1±1.9 |
52.4±18.2 |
9.0±1.8 |
1.9±1.2 |
1.85±1.2 |
6.2±1.8 |
42.4±9.6 |
8.8±1.3 |
| Meba |
T1 |
1.24±0.5 |
1.17±0.5 |
4.8±0.8 |
70.3±7.9 |
7.6±1.4 |
1.74±1 |
1.67±1 |
4.7±0.7 |
60.1±7.6 |
7.5±1.6 |
| T2 |
1.33±0.6 |
1.25±0.6 |
4.9±1 |
69.0±11.7 |
8.2±1.4 |
1.55±0.6 |
1.45±0.6 |
4.7±1.2 |
52.0±9.9 |
6.7±2.2 |
| T3 |
1.42±0.7 |
1.37±0.7 |
4.8±1 |
70.4±11.1 |
7.5±1.5 |
1.5±0.9 |
1.5±0.9 |
5.0±1.2 |
57.3±9.2 |
7.4±2 |
| T4 |
1.44±0.7 |
1.38±0.7 |
4.7±1.5 |
60.3±11 |
7.2±1.3 |
1.8±1.2 |
1.8±1.2 |
4.2±1.1 |
51.3±9.2 |
6.9±1.6 |
Table 5.
Effect of fertilizer treatment, genotype and slope position on finger millet yield.
Table 5.
Effect of fertilizer treatment, genotype and slope position on finger millet yield.
| |
Sum Sq |
Mean Sq |
NumDF |
DenDF |
F value |
Pr(>F) |
| Slope position |
0.787 |
0.787 |
1 |
3.005 |
9.0260 |
0.05734 # |
| Fertilizer |
7.2168 |
1.8042 |
4 |
274.966 |
20.6909 |
6.382e-15 *** |
| Genotype |
10.3013 |
5.1506 |
2 |
274.491 |
59.0686 |
< 2.2e-16 *** |
| Fertilizer:genotype |
0.8588 |
0.1073 |
8 |
275.993 |
1.2311 |
0.2807 |
Table 6.
Effect of fertilizer treatment, genotype and slope position on finger millet biomass.
Table 6.
Effect of fertilizer treatment, genotype and slope position on finger millet biomass.
| |
Sum Sq |
Mean Sq |
NumDF |
DenDF |
F value |
Pr(>F) |
| Slope position |
1.1258 |
1.1258 |
1 |
3.004 |
13.5885 |
0.03453 * |
| Fertilizer |
13.8891 |
3.4723 |
4 |
274.516 |
41.9113 |
< 2e-16 *** |
| Genotype |
0.5367 |
0.2684 |
2 |
274.036 |
3.2392 |
0.0407 * |
| Fertilizer:genotype |
0.7399 |
0.0925 |
8 |
274.873 |
1.1163 |
0.3522 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).