1. Introduction
Advanced high-grade serous ovarian carcinoma is the most common and deadliest subtype of ovarian cancer in women [
1]. Among Brazilian women, ovarian cancer is the eighth most common type of cancer, with 7,310 new cases estimated in 2022, and, as in the rest of the world, presents a worrying proximity between incidence and death rates per 100,000 inhabitants [
2].
Despite advances in therapy, recurrence is common. Loss of sensitivity to platinum, the main drug used for treatment, correlates with poor prognosis. In the last 10 years, there has been an increase in survival with therapies including anti-VEGF (vascular endothelial growth factor) drugs (bevacizumab) [
3] and PARP (poly (ADP-ribose) polymerase 1) enzyme inhibitors (olaparib, rucaparib, veliparib, and niraparib) [
4,
5,
6]. However, maximal cytoreduction (debulking) remains the biggest prognostic factor for increased survival in all stages of ovarian cancer [
7]. Moreover, the high degree of molecular heterogeneity and expression of various neoantigens [
8,
9] hinder the progress of ovarian cancer therapy.
Therefore, immunotherapy has emerged as an alternative treatment. This class of drug has been studied and validated for other types of cancer, such as melanoma and lung cancer [
10,
11,
12,
13,
14,
15].
The results of the first studies on ovarian cancer with immunotherapies alone and/or in combination with conventional drugs are beginning to emerge [
9,
16,
17,
18]; however, the identification of patients who can benefit from this treatment needs to be better established.
To the best of our knowledge, there has been no validation of biomarkers as prognostic, predictive of response, or an indication for immunotherapy for high-grade serous ovarian cancer. Biomarkers such as PD-1 (programmed cell death 1 or PDCD1), PD-L1 (programmed cell death 1 ligand 1 or CD274 molecule), and CD8, in addition to microsatellite instability (MSI), have been recognized as useful markers for indicating immunotherapy in other types of cancer [
19,
20].
MMR (mismatch repair) corresponds to a family of genes related to functional loss and DNA repair:
MLH1 (mutL homolog 1),
MSH2 (mutS homolog 2),
MSH6 (mutS homolog 6),
PMS2 (post meiotic segregation increased 2) and
MLH3 (mutL homolog 3) [
21]. Mutations, including inherited or somatic deletions in both alleles of these genes, lead to impaired genomic repair mechanisms during DNA replication in the S phase of the cell cycle. This confers a high propensity for the accumulation of mutations in cells. This genetic failure can be detected by investigating MSI [
22]. Microsatellites are small regions of the genome in which nucleotides are sequentially repeated. In these regions, replication errors are more easily recognized because the repetitions favor the occurrence of point mutations as well as small deletions and insertions near the repeat sequences. MSI analysis can be performed either by polymerase chain reaction (PCR) or, indirectly, by immunohistochemistry.
In the tumor microenvironment, the binding between PD-L1 on tumor cells and PD-1 on T lymphocytes allows the tumor to escape the immune response by inactivating cytotoxic CD8+ T cells. Thus, blocking this binding using specific antibodies to PD-L1 or PD-1 reverses this condition, and the T cell lyses the neoplastic cell because it now identifies it as foreign [
23].
The premise for the use of immunotherapy in epithelial ovarian cancer is the high expression of PD-L1 in tumor cells, greater than or equal to 1% [
17]. MSI is found in approximately 10% of ovarian cancer cases, particularly the endometrioid and mucinous subtypes [
20]. The prognosis of sporadic tumors exhibiting MSI is favorable. These tumors respond better to immunotherapy [
24,
25,
26] and the presence of MSI is considered a marker and predictor of response to immunotherapy [
19,
20].
The p53 protein is encoded by the tumor suppressor gene
TP53 (tumor protein p53) and regulates the expression of various genes in response to different types of cellular stress, inducing cell cycle arrest, apoptosis, cellular senescence, DNA repair, and changes in metabolism. The expression of p53 is altered in high-grade carcinomas, appearing either completely negative or diffusely positive and overexpressed. In the latter case, the protein is mutated [
27]. Mutations in
TP53 define the characteristics of high-grade serous carcinomas and occur in 96% of these cases [
28,
29].
To optimize this therapy, especially for platinum-resistant and refractory tumors, which lead to death in approximately 1 year, and platinum-sensitive tumors, which relapse in 95% of cases, it is necessary to better understand the markers of immunotherapy and predictors of response, especially in the most common subtype, serous epithelial ovarian tumors.
The objective of this study was to analyze, by immunohistochemistry, the expression of PD-1, PD-L1, CD8, MLH1, MSH2, MSH6, PMS2, and p53 in paraffin-embedded tumor samples from patients with advanced serous ovarian carcinoma and to correlate these expression profiles with the clinicopathological data of the patients.
2. Materials and Methods
2.1. Patients and samples
The samples included in this retrospective study were obtained from the pathology services of two hospitals in Curitiba, PR, Brazil, with the approval of their respective research ethics committees. These tumor samples were obtained during surgical procedures from 2009 to 2020 to remove primary ovarian tumors from patients who had not undergone neoadjuvant chemotherapy.
Thus, a convenience sample of 28 patients with advanced serous ovarian carcinoma at clinical stage III or IV (EC III or IV) was retrospectively analyzed. These patients presented with paraffin blocks suitable for the proposed study and available data in medical records. Twelve patients were excluded as they did not meet these criteria.
Data from the following clinical variables of the patients and pathological variables of the samples was recorded and analyzed: diagnosis, age at diagnosis, body mass index (BMI), Eastern Cooperative Oncology Group (ECOG) performance status [
30], TNM staging which includes the extent of the tumor (T), extent of spread to the lymph nodes (N), and presence of metastasis (M) [
31], history of previous cancer, presence of mutations in the BRCA1 and BRCA2 genes, associated comorbidities, recurrence, site of recurrence, recurrence-free survival, and overall survival. Survival data was updated in November 2021.
2.2. Histological analysis
The anatomopathological patterns of the samples were reviewed by experienced pathologist (L.de N.) on slides stained with hematoxylin and eosin (Harris Hematoxylin: NewProv, Cod. PA203, Paraná, BR; Eosin: BIOTEC Reagentes Analíticos, Cod. 4371, Paraná, BR).
2.3. Immunohistochemical tests
Immunohistochemical analysis was used to determine the expression of PD-1, PD-L1, CD8, MLH1, MSH2, MSH6, PMS2, and p53 in the paraffin-embedded tumor samples.
2.4. PD-1, PD-L1, CD8, and p53
The immunohistochemical assay used a protocol of incubation of specific primary antibodies for PD-1 (J116/14-9989-82; Thermo Fisher; dilution 1:100), PD-L1 (PA5-28115; Thermo Fisher; dilution 1:200), CD8 (SP16; Thermo Fisher; dilution 1:100), and p53 (D07/BSB5844; BioSB; dilution 1:200), in a humid chamber at a temperature between 2 and 8 °C, overnight. The secondary polymer, mouse- and rabbit-specific HRP/DAB IHC Detection Kit (Micro-polymer, ab236466 Abcam, Cambridge, UK) was applied to the test material for 25 min at room temperature. The technique was revealed by the addition of a 2,3-diaminobenzidine and hydrogen peroxide substrate complex for sufficient time for it to develop a brown color, followed by counterstaining with Harris hematoxylin.
The positive controls for these reactions were determined by the immunopositivity of tissue samples known to be reactive to the antibodies in the test. These samples were placed on slides along with the studied samples. Lymph node samples for CD8 and breast carcinoma samples for p53 were analyzed together with the reactions of the tested samples. The negative control consisted of omission of the primary antibody from the reaction.
2.5. MLH1, MSH2, MSH6 e PMS2
The immunohistochemical assay consisted of a protocol of incubation of primary antibodies specific for MLH1 (ES05; Dako; dilution 1:200), MSH2 (FE11; Dako; ready to use), MSH6 (EP49; Dako; ready to use) and PMS2 (EP51; Dako; ready to use) in a humid chamber at a temperature between 2 and 8 °C, overnight. A secondary polymer (Dako EnVisionTM FLEX/HRP, Santa Clara, CA, USA) was applied to the test material for 30 min at room temperature. The technique was revealed by the addition of the complex 2, 3, diaminobenzidine and hydrogen peroxide substrate for sufficient time for the development of a brown color, followed by counterstaining with Harris hematoxylin.
The positive controls for these reactions were determined based on the reactivity of human colon tissue samples known to be reactive to the tested antibodies. These samples were then allocated to slides together with the studied samples. The negative control consisted of omission of the primary antibody from the reaction.
2.6. Tissue immunoexpression analysis
2.6.1. PD-L1
Immunolabeled slides with specific antibodies against PD-L1 were scanned using an Axio Scan.Z1 slide scanner (Zeiss, Jena, Germany). Thirty high-magnification field images, 40X, were generated using the ZEN Blue Edition software (Zeiss).
The analyses were performed blindly using images obtained from random sample regions without interference from an observer. In each image, the areas of immunopositivity were measured using Image Pro-Plus software version 4.5 (Media Cybernetics, Rockville, MD, USA) using a semi-automated color segmentation method, in which the PD-L1 immunopositive area was delimited and quantified.
Next, the value of the immunopositive area, expressed in square micrometers (μm2), was divided by the total tumor area, and transformed into a percentage value. Finally, arithmetic mean values of the images were calculated for each patient.
2.6.2. CD8 e PD-1
Immunostained slides with specific antibodies against PD-1 and CD8 were scanned using an Axio Scan.Z1 slide scanner (Zeiss, Jena, Germany). The digitized files were visualized using ZEN Blue Edition software (Zeiss, Jena, Germany) and a rectangle with an area of 1 mm2 was selected and positioned over the hotspot regions (areas with a high density of lymphocytes); thereafter, the lymphocytes were counted for each immunostained antibody.
2.6.3. MLH1, MSH2, MSH6, and PMS2
Slides immunostained with anti-MLH-1, MSH-2, MSH-6, and PMS-2 antibodies were analyzed using a BX40® microscope (Olympus, Tokyo, Japan) at 40X magnification and paired as follows: MSH2+MSH6 and MLH1+PMS2. Each sample that comprised a case was analyzed. The presence of at least one positive area was classified as positive, and the absence of any positive area was classified as negative for the antibody in question. Patients were defined as MSI carriers when at least one of the four MMR proteins (MLH1, MSH2, MSH6, or PMS2) tested negative.
2.6.4. p53
Slides immunostained for p53 were analyzed under a BX40 microscope (Olympus, Tokyo, Japan) at 40X magnification (
Figure 2). The expression of p53 was classified as overexpression (mutated) or wild-type (non-mutated) in the samples studied.
2.7. Statistical analysis
Quantitative variables are described as means and standard deviations. Categorical variables are described as frequencies and percentages. The correlation between two quantitative variables was evaluated using Spearman’s correlation coefficient. The Student’s t-test for independent samples or the non-parametric Mann-Whitney U test were used to compare two groups defined by dichotomous categorical variables with respect to quantitative variables. The association between two categorical variables was analyzed using Fisher's exact test. The Cox Regression model and log-rank test were used to analyze factors associated with recurrence-free and overall survival. The normality of continuous quantitative variables was evaluated using the Shapiro-Wilk test. Statistical significance was set at a p value of less than 0.05. Data was analyzed using Stata/SE software version 14.1 (StataCorp LP, USA).
3. Results
All 28 patients were Caucasian and had an average age of 61.3 ± 14 years at diagnosis. The youngest and oldest patient were 34 years old and 90 years old, respectively. Patients were generally observed to be overweight, with an average BMI of 25.9 ± 5.8 (
Table 1).
Most of the patients (n=21) had high-grade serous epithelial carcinoma. Eight patients had stage IV disease at diagnosis and 19 had stage III disease. Seven patients reported a history of cancer, three of whom had breast cancer. Most patients did not have mutations in BRCA1 (11/18) or BRCA2 (12/18), but three had both germline and somatic mutations in BRCA1 and BRCA2.
These three patients were sensitive to platinum and had wild-type p53 (different from the majority of cases in the study). One patient was assigned to the MSI-unstable group. All three patients had lower PD-1 expression than the average seen in the studied group. Similarly, for PD-L1, two patients had below average expression and only one had a CD8 count within the median value. The CD8/PD1 ratios of these three patients were above the study's average.
Most patients (17) underwent cytoreduction rather than surgical biopsy. The most used chemotherapy regimen (21 patients) was a combination of carboplatin and paclitaxel, and 23 patients were sensitive to platinum. The median overall survival was 50.8 months, and the median progression-free survival was 17.2 months. Fifteen patients relapsed and six died during the study period (
Table 2).
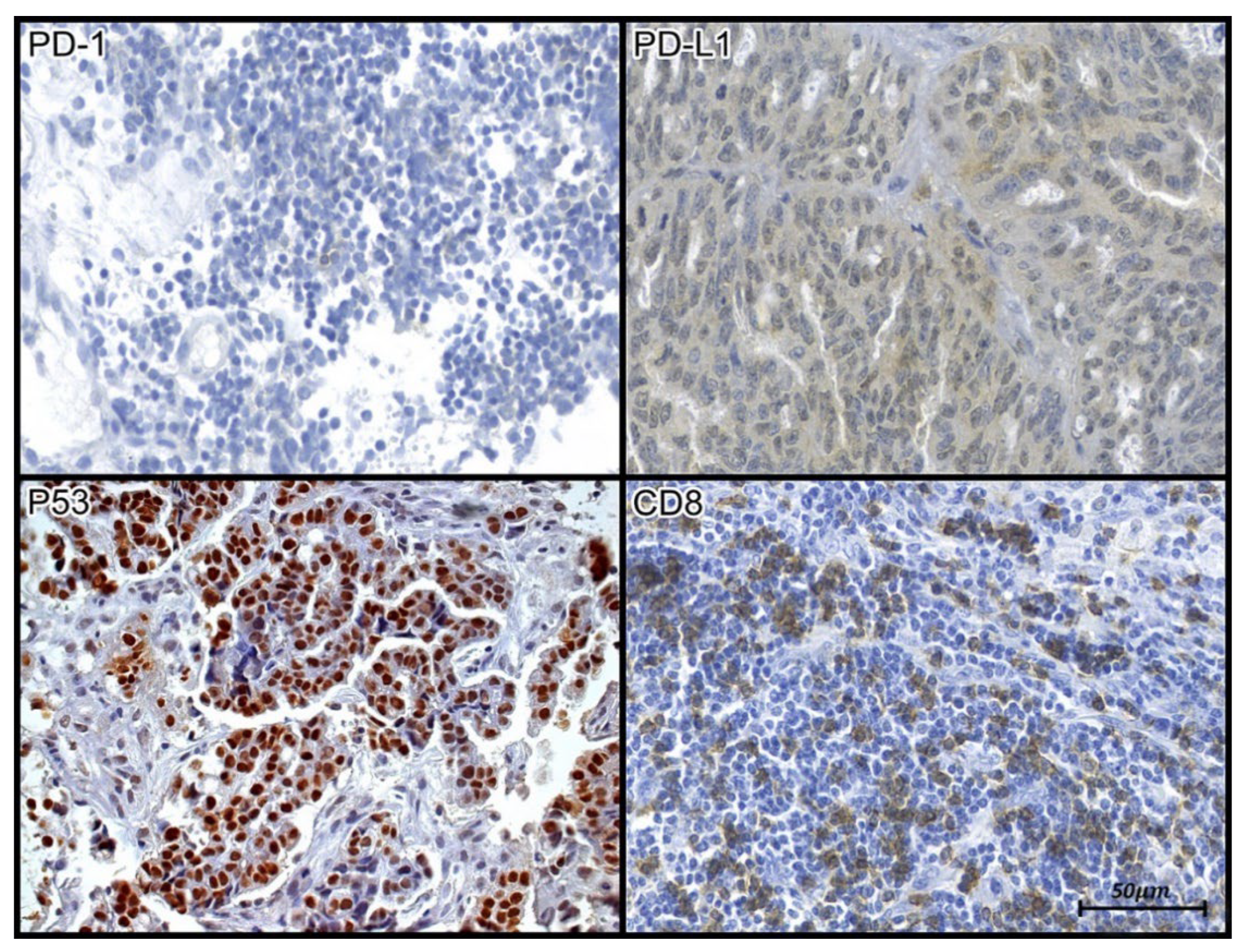
Figure 1.
Tissue immunoexpression of PD-1, PD-L1, p53, and CD8 in samples of high-grade serous carcinoma. (PD1 and CD8) The evaluation of tissue immunoexpression of CD-8 and PD-1 was performed from the nuclear count (hotspots) of labeled lymphocytes, as evidenced by their brown staining; (p53) Tissue immunoexpression of p53 was evaluated through immunohistochemical expression of an anti-p53 antibody. Positive staining was determined from the nuclear count of immunolabeled cells, as evidenced by the brown staining of the antigen-antibody reaction.; (PD-L1) Evaluation of the tissue expression of PD-L1 was performed through morphometric analysis, as evidenced by the areas observed by brown staining. Photomicrograph at 40X magnification.
Figure 1.
Tissue immunoexpression of PD-1, PD-L1, p53, and CD8 in samples of high-grade serous carcinoma. (PD1 and CD8) The evaluation of tissue immunoexpression of CD-8 and PD-1 was performed from the nuclear count (hotspots) of labeled lymphocytes, as evidenced by their brown staining; (p53) Tissue immunoexpression of p53 was evaluated through immunohistochemical expression of an anti-p53 antibody. Positive staining was determined from the nuclear count of immunolabeled cells, as evidenced by the brown staining of the antigen-antibody reaction.; (PD-L1) Evaluation of the tissue expression of PD-L1 was performed through morphometric analysis, as evidenced by the areas observed by brown staining. Photomicrograph at 40X magnification.
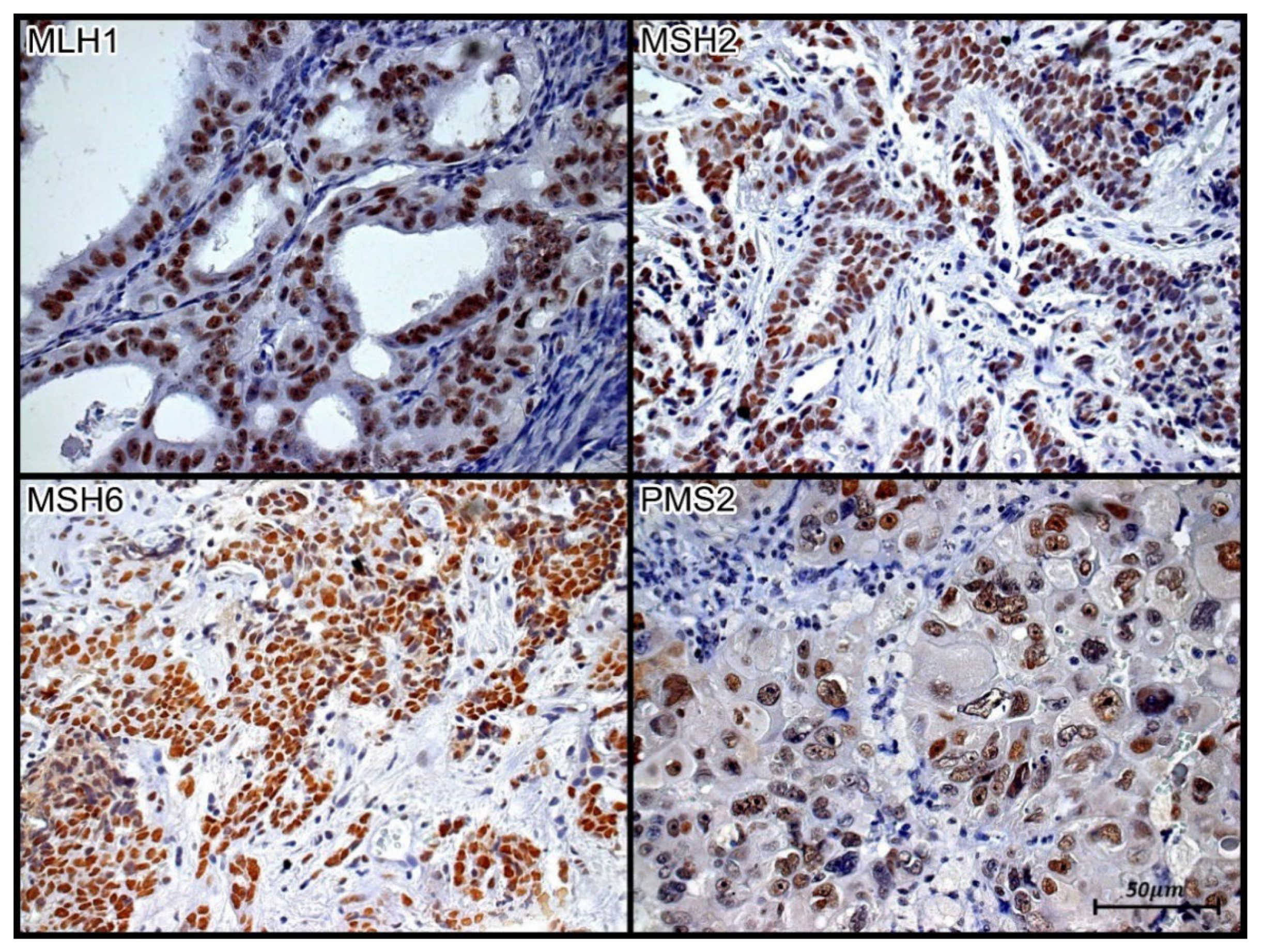
Figure 2.
Tissue immunoexpression of MLH1, MSH2, MSH6, and PMS2 in high-grade serous ovarian carcinoma. Brown nuclear staining represents tissue immunopositivity for the immunohistochemical reaction with the monoclonal antibodies anti-MLH-1, anti-MSH2, anti-MSH6, and anti-PMS2, demonstrating the presence of the respective proteins in the nuclei of the papillary serous ovarian carcinoma cells. Immunolabeling of at least one cell was considered positive in the evaluated case. Photomicrograph at 40X magnification.
Figure 2.
Tissue immunoexpression of MLH1, MSH2, MSH6, and PMS2 in high-grade serous ovarian carcinoma. Brown nuclear staining represents tissue immunopositivity for the immunohistochemical reaction with the monoclonal antibodies anti-MLH-1, anti-MSH2, anti-MSH6, and anti-PMS2, demonstrating the presence of the respective proteins in the nuclei of the papillary serous ovarian carcinoma cells. Immunolabeling of at least one cell was considered positive in the evaluated case. Photomicrograph at 40X magnification.
The expression profiles of PD-1 and PD-L1 did not correlate with each other (r=0.10, p=0.642), nor were their profiles, as well as those of p53, associated with the grade and clinical stage of serous ovarian carcinoma (p>0.05).
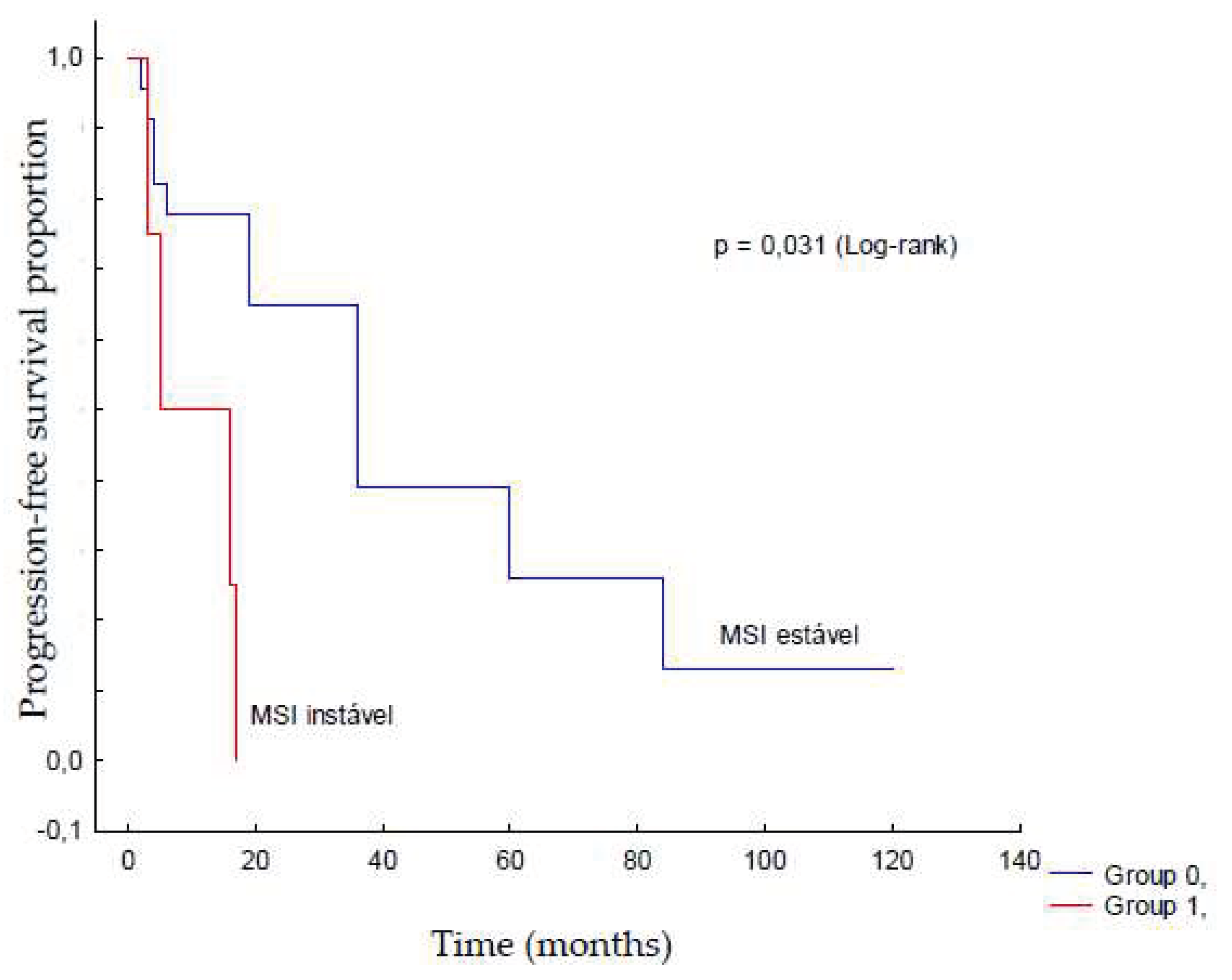
The MSI status was compared with the expression of PD-1, PD-L1, and CD8/PD-1 ratio. There was a trend towards a higher CD8/PD-1 ratio in patients with stable MSI (p=0.049) (
Table 4), although only four patients showed instability. MSI status was not associated with serous ovarian carcinoma grade, clinical stage, or platinum sensitivity (p>0.05); however, it did seem to affect recurrence-free survival (
Table 5;
Figure 3). Furthermore, there was no significant correlation of the studied variables with death in this sample.
4. Discussion
The prognosis of advanced ovarian cancer is poor, with approximately only 20% of patients surviving 5 years after diagnosis [
32]. The standard therapy of complete cytoreduction combined with chemotherapy has not been sufficient for more advanced stages as most patients develop platinum resistance within 16-18 months of treatment [
33]. The data from this study showed a progression-free survival of 17.2 months.
A previous study found that an age of more than 60 years old was an independent factor associated with worse survival [
32], and most patients (68%) in this study were diagnosed at more than 60 years of age. Additionally, clinical stage was an independent prognostic factor for shorter survival. Ovarian cancer is usually diagnosed at stages III and IV, with approximately 30% of patients presenting with metastasis at diagnosis (stage IV), indicating inoperability and poor prognosis.
TP53 expression is an important prognostic indicator of malignant neoplasms. Epithelial ovarian cancer is mutated in 40-80% of cases. In a previous study of 105 patients with ovarian cancer, mutations were found in approximately 57% of cases [
34]. Alterations in p53 levels are associated with poorly differentiated disease, platinum resistance, early relapse, and decreased overall and disease-free survival [
34]. Immunohistochemical analysis revealed that 85% of the cases overexpressed p53, indicating a mutation. Moreover, a few studies have quantitatively correlated p53 expression with survival in ovarian cancer [
35].
The frequency of MSI in ovarian cancer varies from 2-20% [
36,
37]. Most MSI cases are clear cell and endometrioid carcinoma types [
38]. Notwithstanding that, in this study, 14.8% of patients had MSI; they were high-grade serous adenocarcinoma cases.
Yamashita et al. [
39], in a review of 136 cases, found that 4.4% of MSI cases included endometrioid, mucinous, and clear cell carcinoma subtypes. High-grade serous carcinoma had only two MSI cases out of the 67 studied. In line with Yamashita, there was no significant correlation between MSI and clinical stage or expression of the immune markers, PD-1, PD-L1 or CD8 in this analysis.
PD-L1 expression in tumor cells is the main strategy for immune evasion in cancer. Higher PD-L1 expression is associated with lower overall survival [
16]. This is due to a reduction in T-lymphocyte infiltration into the tumor, suggesting that PD-L1 expression promotes an immunosuppressive microenvironment by inhibiting lymphocyte infiltration. Hamanishi et al. [
16] were the first to describe PD-L1 expression in ovarian cancer and found expression in 88% of the tumor cells. The authors demonstrated an inverse relationship between PD-L1 expression and the number of CD8+ lymphocytes. Women with high PD-L1 expression have worse overall and progression-free survival [
40].
In this study, all patients had low percentages of CD8/PD-1 ratios (median of 11%), similar to the immunosuppressive microenvironment described in the literature. Furthermore, patients with stable MSI had higher CD8/PD-1 ratios.
The PD-1/PD-L1 pathway is an immune escape phenomenon in tumors that has not been validated as a biomarker for ovarian cancer yet. Currently, there are no predictive or prognostic biomarkers to determine which patients would benefit from ovarian cancer immunotherapy.
The isolated expression of PD-L1 cannot be used as a potential indicator of the benefits of PD-1/PD-L1 inhibitors in the absence of TILs (tumor-infiltrating lymphocytes) [
41]. Zhang et al. [
42] analyzed 174 patients and showed that the presence of TILs was associated with increased overall survival in ovarian cancer compared with a cohort without TILs. This data is consistent with those of other studies summarized in a meta-analysis of 1815 patients [
43]. This positive prognostic effect can be attributed to the subgroup of intratumoral CD8+ T cells.
A limitation of this study was the small sample size (some blocks were lost because of poor material quality). Nevertheless, the statistically significant demonstration related to the association between recurrence and MSI status should be considered in the context of the sample size in this study (n=4). Moreover, it should also be noted that the samples were exclusively serous carcinomas, without other histologies, as reported in previous studies. However, we cannot exclude the possibility that a larger sample size may yield different results.
Furthermore, making inferences based solely on the results presented here, that the presence of MSI impacts recurrence-free survival, may seem ambitious. According to robust data from the literature, malignant tumors with unstable MSI respond better to immunotherapy [
44].
Given tumor heterogeneity, the selection of patients is essential for the effective use of immunotherapy in high-grade epithelial cancer. This selection can also promote cost optimization in cancer treatment by providing specific therapies to selected patients.
5. Conclusions
The clinical and histopathological characteristics of the studied samples corroborated data from the literature. In the present study, samples with MSI stability showed a higher proportion of CD8/PD-1 cases, and patients with this condition had less recurrence. In contrast, there was an association between recurrence and MSI instability (p=0.03).
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Pontificia Universiade Católica do Paraná (CAAE: 64575817.7.0000.0020), Hospital de Clínicas do Paraná (CAAE: 64575817.7.3001.0096), and Hospital Erasto Gaertner (CAAE: 64575817.7.3002.0098).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Globocan. Available online: https://gco.iarc.fr/; The Global Cancer Observatory, 2020.
- INCA- Instituto Nacional do Câncer 2020. Available online: www.inca.gov.br.
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, M.R.; Fotopoulou, C.; Blagden, S.; Gabra, H. The next steps in improving the outcomes of advanced ovarian cancer. Womens Health (Lond). 2015, 11, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Mouw, K.W.; Goldberg, M.S.; Konstantinopoulos, P.A.; D’Andrea, A.D. DNA damage and repair biomarkers of immunotherapy response. Cancer Discov. 2017, 7, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, A.; Suszczyk, D.; Okla, K.; Barczyński, B.; Kotarski, J.; Wertel, I. Immunotherapies based on PD-1/PD-L1 pathway inhibitors in ovarian cancer treatment. Clin Exp Immunol. 2019, 195, 334–344. [Google Scholar] [CrossRef]
- Holloway, R.W.; Gupta, S.; Stavitzski, N. M.; Zhu, X.; Takimoto, E. L.; Gubbi, A.; Bigsby, G. E.; Brudie, L. A.; Kendrick, J. E.; Ahmad, S. Sentinel Lymph Node Mapping with Staging Lymphadenectomy for Patients with Endometrial Cancer Increases the Detection of Metastasis. Gynecol. Oncol. 2016, 141, 206–210. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non–Small-Cell Lung Cancer. N Engl J Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.M.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia- Carbonero, R.; Benavides, M.; Gibbs, P.; de la Fouchardiere, C.; Rivera, F.; Elez, E.; Bendell, J.; Le, D.T.; Yoshino, T.; Van Cutsem, E.; Yang, P.; Farooqui, M.Z.H.; Marinello, P.; Diaz, L.A. Jr; KEYNOTE-177 Investigators. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020 Dec 3;383(23):2207-2218. [CrossRef]
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015, 33, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lang, J. Programmed death-1 pathway blockade produces a synergistic antitumor effect: comined application in ovarian cancer. J Gynecol Oncol. 2017, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Pietzner, K.; Nasser, S.; Alavi, S.; Darb-Esfahani, S.; Passler, M.; Muallem, M.Z.; Sehouli, J. Checkpoint-inhibition in ovarian cancer: rising star or just a dream? J Gynecol Oncol. 2018, 29, e93. [Google Scholar] [CrossRef] [PubMed]
- Gatalica, Z.; Vranic, S.; Xiu, J.; Swensen, J.; Reddy, S. High microsatellite instability (MSI-H) colorectal carcinoma: a brief review of predictive biomarkers in the era of personalized medicine. Fam Cancer. 2016, 15, 405–412. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the Phase II KEYNOTE-158 study. J Clin Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Sehgal, R.; Sheahan, K.; O'Connell, P.R.; Hanly, A.M.; Martin, S.T.; Winter, D.C. Lynch syndrome: an updated review. Genes (Basel). 2014 Jun 27;5(3):497-507. [CrossRef]
- Richman, S. Deficient mismatch repair: read all about it (Review) [Review]. Int J Oncol. 2015, 47, 1189–1202. [Google Scholar] [CrossRef]
- Blank, C.; Mackensen, A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007 May;56(5):739-45. [CrossRef]
- Popat, S.; Hubner, R.; Houlston, R.S. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005 Jan 20;23(3):609-18. [CrossRef]
- Colle, R.; Cohen, R.; Cochereau, D.; Duval, A.; Lascols, O.; Lopez-Trabada, D.; Afchain, P.; Trouilloud, I.; Parc, Y.; Lefevre, J.H.; Fléjou, J.F.; Svrcek, M.; André, T. Immunotherapy and patients treated for cancer with microsatellite instability. Bull Cancer. 2017 Jan;104(1):42-51. [CrossRef]
- Chang, L.; Chang, M.; Chang, H.M.; Chang, F. Microsatellite Instability: A Predictive Biomarker for Cancer Immunotherapy. Appl Immunohistochem Mol Morphol. 2018 Feb;26(2):e15-e21. [CrossRef]
- Skilling, J.S.; Sood, A.; Niemann, T.; Lager, D.J.; Buller, R.E. An abundance of p53 null mutations in ovarian carcinoma. Oncogene. 1996, 13, 117–123. [Google Scholar]
- Chiesa-Vottero, A.G.; Malpica, A.; Deavers, M.T.; Broaddus, R.; Nuovo, G.J.; Silva, E.G. Immunohistochemical overexpression of p16 and p53 in uterine serous carcinoma and ovarian high-grade serous carcinoma. Int J Gynecol Pathol. 2007 Jul;26(3):328-33. [CrossRef]
- Ahmed, A.A.; Etemadmoghadam, D.; Temple, J.; Lynch, A.G.; Riad, M.; Sharma, R.; Stewart, C.; Fereday, S.; Caldas, C.; Defazio, A.; Bowtell, D.; Brenton, J.D. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. 2010 May;221(1):49-56. [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
-
American Joint Committee on Cancer AJCC. Available online: www.uicc.org, 2021.
- Drakes, M.L.; Mehrotra, S.; Aldulescu, M.; Potkul, R.K.; Liu, Y.; Grisoli, A.; Joyce, C.; O’Brien, T.E.; Stack, M.S.; Stiff, P.J. Stratification of ovarian tumor pathology by expression of programmed cell death-1 (PD-1) and PD-ligand-1 (PD-L1) in ovarian cancer. In J Ovarian Res. 2018, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Cortez, A.J; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother Pharmacol. 2018 Jan;81(1):17-38. [CrossRef]
- Reles, A.; Wen, W.H.; Schmider, A.; Gee, C.; Runnebaum, I.B.; Kilian, U.; Jones, L.A.; El-Naggar, A.; Minguillon, C.; Schönborn, I.; et al. Correlation of p53 mutations with resistance to platinum-based chemotherapy and shortened survival in ovarian cancer. Clin Cancer Res. 2001, 7, 2984–2997. [Google Scholar] [PubMed]
- Geisler, J.P.; Geisler, H.E.; Wiemann, M.C.; Givens, S.S.; Zhou, Z.; Miller, G.A. Quantification of p53 in epithelial ovarian cancer. In. Gynecol Oncol. 1997, 66, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Rambau, P.F.; Duggan, M.A.; Ghatage, P.; Warfa, K.; Steed, H.; Perrier, R.; Kelemen, L.E.; Köbel, M. Significant frequency of MSH2/MSH6 abnormality in ovarian endometrioid carcinoma supports histotype-specific Lynch syndrome screening in ovarian carcinomas. Histopathology. 2016, 69, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Aysal, A.; Karnezis, A.; Medhi, I.; Grenert, J.P.; Zaloudek, C.J.; Rabban, J.T. Ovarian endometrioid adenocarcinoma: incidence and clinical significance of the morphologic and immunohistochemical markers of mismatch repair protein defects and tumor microsatellite instability. Am J Surg Pathol. 2012 Feb;36(2):163-72. [CrossRef]
- Jensen, K.C.; Mariappan, M.R.; Putcha, G.V.; Husain, A.; Chun, N.; Ford, J.M.; Schrijver, I.; Longacre, T.A. Microsatellite Instability and Mismatch Repair Protein Defects inOvarian Epithelial Neoplasms in Patients 50 Years of Age and Younger. Am. J. Surg.Pathol. 2008, 32, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Nakayama, K.; Ishikawa, M.; Ishibashi, T.; Nakamura, K.; Sawada, K.; Yoshimura, Y.; Tatsumi, N.; Kurose, S.; Minamoto, T.; et al. Relationship between microsatellite instability, immune cells infiltration, and expression of immune checkpoint Molecules in ovarian carcinoma: immunotherapeutic strategies for the future. Int J Mol Sci. 2019, 20, 5129. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Hamanishi, J.; Abiko, K.; Matsumura, N.; Baba, T.; Konishi, I. Anti-PD-L1/PD-1 immune therapies in ovarian cancer: basic mechanism and future clinical application. Int J Clin Oncol. 2016, 21, 456–461. [Google Scholar] [CrossRef]
- Patel, S.J.; Sanjana, N.E.; Kishton, R.J.; Eidizadeh, A.; Vodnala, S.K.; Cam, M.; Gartner, J.J.; Jia, L.; Steinberg, S.M.; Yamamoto, T.N.; et al. Identification of essential genes for cancer immunotherapy. Nature. 2017, 548, 537–542. [Google Scholar] [CrossRef]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; Rubin, S.C.; Coukos, G. Intratumoral T cells, recurrence, and Survivalin Epithelial Ovarian Cancer. N Engl J Med. 2003, 348, 203–213. [Google Scholar] [CrossRef]
- Hwang, W.T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).