Introduction
Mangrove ecosystems have characteristics that make them functionally and structurally unique. These areas are responsible for long-term geomorphological processes and continuous interactions with adjacent ecosystems (Alongi, 2002). The dynamic of these ecosystems is affected by abiotic and biotic factors, such as tidal flow, salinity, and nutrient cycling (Lugo and Snedaker, 1974). Nutrient cycling is intrinsically related to the benthic fauna (e.g. mollusks and crabs), especially the herbivorous and detritivores species (Kristensen and Alongi, 2006). When it comes to the crabs, the burrowing habitat is essential for sediment topography and biogeochemistry by modifying particle size distribution, drainage, redox conditions, organic matter, and nutrient availability (Botto and Iribarne, 2000).
Neohelice granulata (Dana, 1851), previously known as Chasmagnathus granulatus (Sakai et al., 2006), is a semiterrestrial species, occurring in salt marshes and mangroves between Patagonia and Rio de Janeiro, southeast Brazil (Melo, 1996). It is considered a key species in the mangrove ecosystem due to its influence on substrate quality (Botto and Iribarne, 2000). During the low tide periods, their opened burrows remain full of water and act as passive traps of sediment and detritus that increase substrate penetrability and stability (Botto et al., 2006; Mendez-Casariego et al., 2011). Regarding their high abundance and ecological role in the mangrove forests, N. granulata has been recognized as a model for biochemical, physiological, and ecological research (Spivak, 2010).
Despite N. granulata being a dominant species at higher latitudes, it was included in the checklist of threatened fauna in Rio Grande do Sul state (South Brazil) (Marques et al., 2002). Regarding this, the study of N. granulata biology can be useful for establishing management plans to preserve their natural populations (Hutchinson, 1981). With this knowledge, it is possible to infer the birth and mortality rates and, consequently, the ecological stability of the populations (Kennelly and Watkins, 1994). Some features of the biology of N. granulata are known in the southeast (Greagati and Negreiros-Fransozo, 2007, 2009) and south Brazil (Ruffino et al., 1994; Barutot et al., 2009) and in Argentina (Ituarte et al., 2004; Bas et al., 2005; César et al., 2005; Barcelos et al., 2007). According to Barutot et al. (2009), as N. granulata shows a wide geographic range, is important to compare the biology from distinct populations in order to better understand the life history of this species.
In general, crabs’ life histories can show a great intraspecific variation. For example, aspects such as growth, life expectancy, size of maturity, and fecundity are closely related to a latitudinal gradient, since temperature variation affects animal metabolic rates (Hines, 1989). However, population differences are also observed between geographically close populations as a response to habitat conditions and ecological interactions (Vogt, 2011).
This study detailed the biology of N. granulata, located at the northern limit of their distribution. Considering the geographical range of this species distribution, data from the present study are compared with the literature in order to discuss the effect of the latitudinal variation on their population dynamics. Finally, the present study aims to contribute useful information regarding the future management concerning N. granulata conservation.
Material and Methods
Study Area
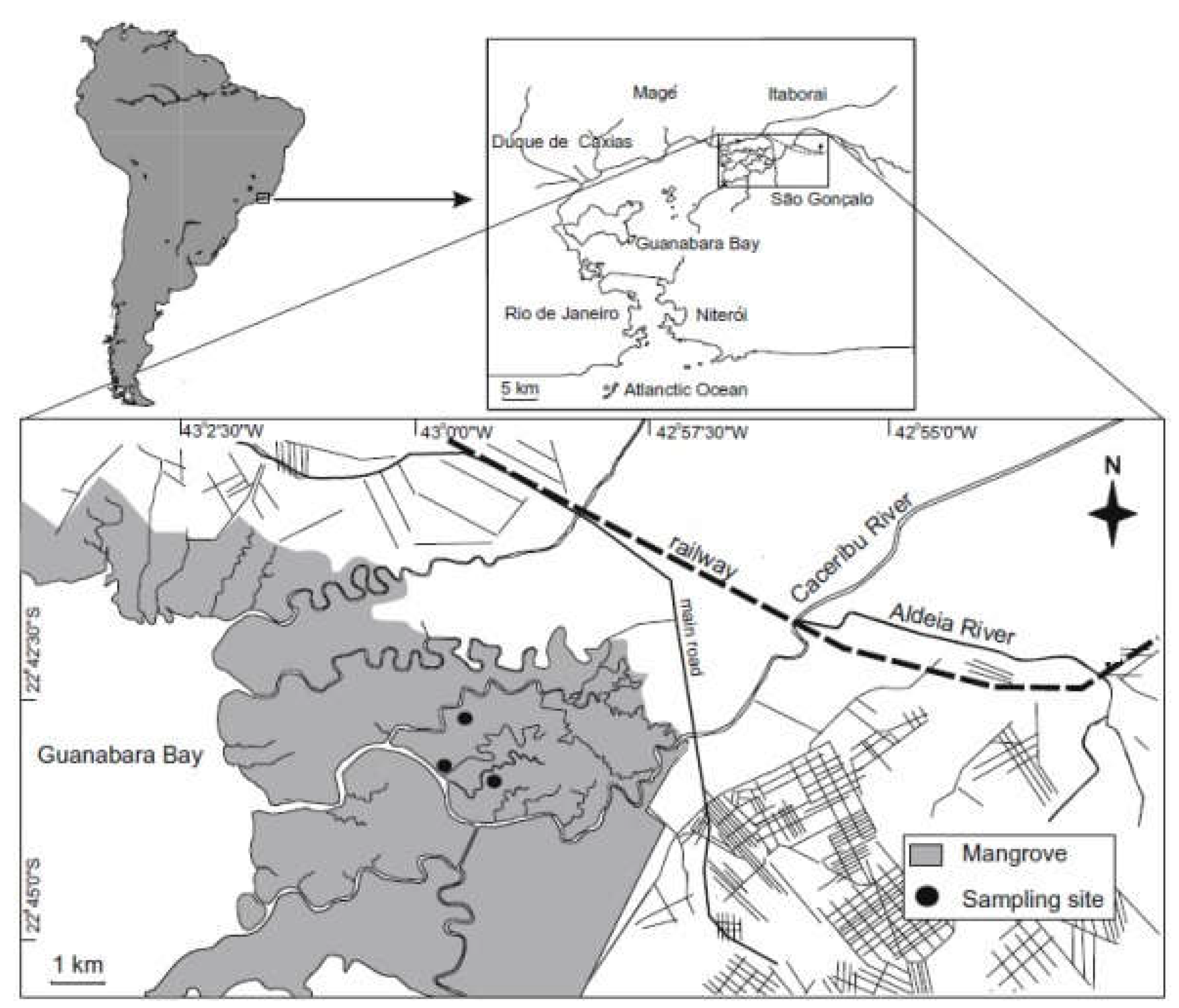
The Marine Protected Area of Guapimirim (APA de Guapimirim) is located in Guanabara Bay, in the metropolitan area of Rio de Janeiro, Southeast Brazil. Almost 50% of its area (61.8 km2) corresponds to the last continuous strip of mangroves in Guanabara Bay, in different conservation and regeneration status (Pires, 1986). Due to its localization, this mangrove area is especially vulnerable to anthropic action, particularly to urban expansion, deforestation, and domestic and industrial pollution. The existence of an environmental protection area ensures the maintenance of natural conditions, enabling the mangrove recuperation and allowing the permanence and survival of the local population that lives off its natural resources (IBAMA, 2001).
The APA de Guapimirim hydrographic bay is the second major draining area in Guanabara Bay, corresponding to 20.7% of this hydrographic zone. It is about 60 km long and flows into APA de Guapi-mirim, on the East side of Guanabara Bay (Negreiros et al., 2002). The samples were taken in the fringe and neighborhoods of the Caceribu River, one of the most important rivers in APA de Guapimirim.
Fieldwork
Samples were taken monthly, between January and December (2010), in the intertidal zone of the Caceribu River´s mangrove (
Figure 1). One collector captured manually the crabs on the surface or in the interior of the substratum, for 25 minutes in the low tide (capture per unit of effort – CPUE). The specimens were placed in plastic bags and kept in a cooler for transportation.
Laboratory Procedures
In the laboratory, crabs were sexed and the following biometric measures were taken using a vernier caliper (0.01 mm): carapace width (CW), right and left cheliped propodus length (RQL, LQL), abdomen width (AW) for females, and gonopod length (GL) for males. Ovigerous females had their individual fecundity estimated through the volumetric method (Díaz et al., 1983). These females had their egg mass (with the pleopods) removed and immersed for a few seconds in a sodium hypochlorite (70%) solution for egg dissociation and then quickly washed in tap water. After that, the eggs were taken into a becker, supplementing the volume to 40 mL. After homogenization, five sub-samples of 1 mL each were taken (with reposition) and the eggs were counted using a stereoscopic microscope, a mechanical counter with four digits, and a petri dish with a grid. The average fecundity of the five sub-samples was performed to estimate individual fecundity. The embryonic stage of the eggs in each ovigerous female was determined according to the following features: Stage I – freshly extruded egg mass sponge, a large quantity of yolk filing all egg volume; Stage II – a large quantity of yolk, marginal areas of the eggs without yolk fill; Stage III – compound eyes of the larvae are differentiated, the embryo occupies 1/3 of the volume of the egg and the segmentation is not visible; Stage IV – the larvae is totally formed, the segmentation is well visible.
Data Analysis
Individuals were distributed in twenty-eight CW classes of 1 mm amplitude each, ranging from 11 to 39 mm. The sex ratio significance was tested monthly using the Chi-Square test (α = 0.05) as a function of size (CW). The size at maturity was estimated through the discontinuity (breakpoint - BP) of the relationships CWxAW for females and CWxGL for males using the software REGRANS (Pezzuto, 1993). These biometrics measures (AW and GL) were chosen because they represent sexual characters that present allometric growth when crabs attain maturity (Hartnoll, 1974). The linear regression [egg number (EN) = a + b (CW)] and Pearson´s correlation index (r) were employed to verify the correlation between fecundity and CW. Analyses of relative growth were performed to describe the growth ratio of the reference measure (CW) and the dependent variables (AW, RQL, LQL, and GL) through the logarithmic form (log y = log a + b log CW) of the power function y = abCW (Hartnoll, 1978). The Student´s T-test (α = 0.05) was used to test the significant deviation of the allometric constant (b) from 1, determining the isometric or allometric condition between CW and the dependent variables.
The breeding season and recruitment were estimated by the monthly frequency of ovigerous females and juveniles, respectively. Monthly precipitation (mm3) and mean temperature (0C) were obtained from the nearest meteorologic station monitored by INMET (Instituto Nacional de Metereologia do Brasil) in order to detect a possible influence of these parameters on the N. granulata reproduction and recruitment.
The von Bertalanffy growth function (Ricker, 1975) was adjusted through the CW frequency distribution to describe the growth of crabs: Lt = L∞ (1-e-(k (t - t0 )), where Lt is the length at time t, t0 is the age at zero length, L∞ is the maximum size when the individual attains the maximum age estimated and K is the curving parameter that represents the growth rate. The parameters were calculated using Munro’s plot (Munro, 1982): K = (ti + 1 - ti) - ln (L∞ - Li) - ln (L∞ - Li + 1), where Li and Li + 1 are the lengths at times ti and ti + 1, respectively. Munro’s phi prima (ᶲ) (Pauly and Munro, 1984) was calculated to compare the growth performance between males and females: ᶲ = ln K + 2*ln L∞. Length-converted catch curves (Sparre and Venema, 1997) were used to calculate total mortality rate (Z) based on monthly CW sampling data. The annual finite mortality rate (A) was estimated through the equation A = 1 - eZ. Maximum longevity (T(max)) was calculated by the 95% asymptotic size estimate (Taylor, 1958): T(max) = 2.996/K.
Results
Population Structure
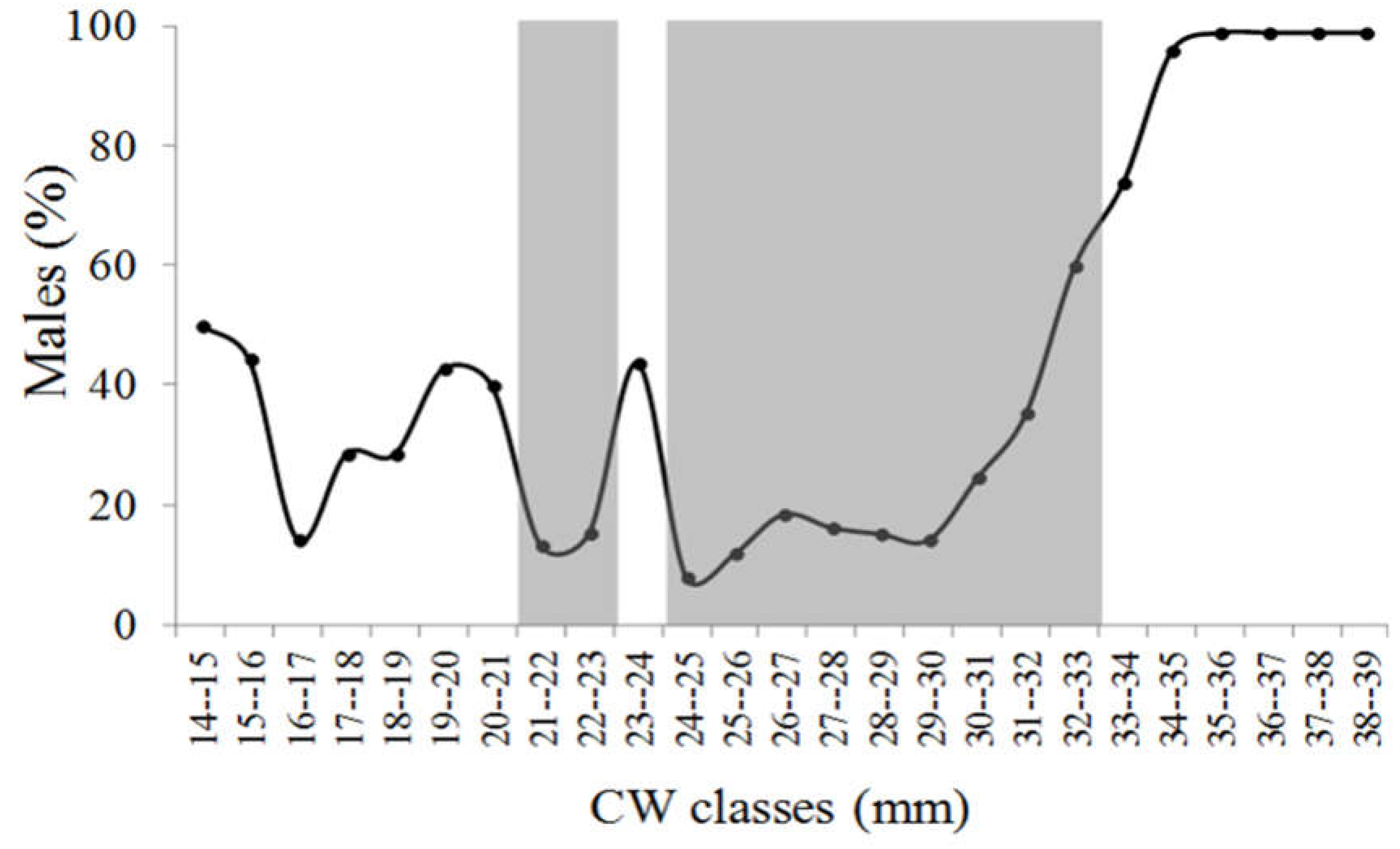
Sampling resulted in 551 crabs, 164 males, and 387 females (169 ovigerous) during a year of fieldwork. The overall sex ratio (M: F) was female-biased in all months (p < 0.05). The sex ratio as a function of size (CW) was female-biased (p < 0.05) in the intermediary size classes (
Figure 2). The CW of males ranged from 11.11 to 38.03 mm (mean ± SD = 29.14 ± 5.66), with higher frequency in the 30-31 and 32-33 mm CW classes. In females, CW ranged from 12.21 to 33.87 mm (27.32 ± 4.03), with higher frequency in the 29-30 mm CW class. CW was significantly higher in males.
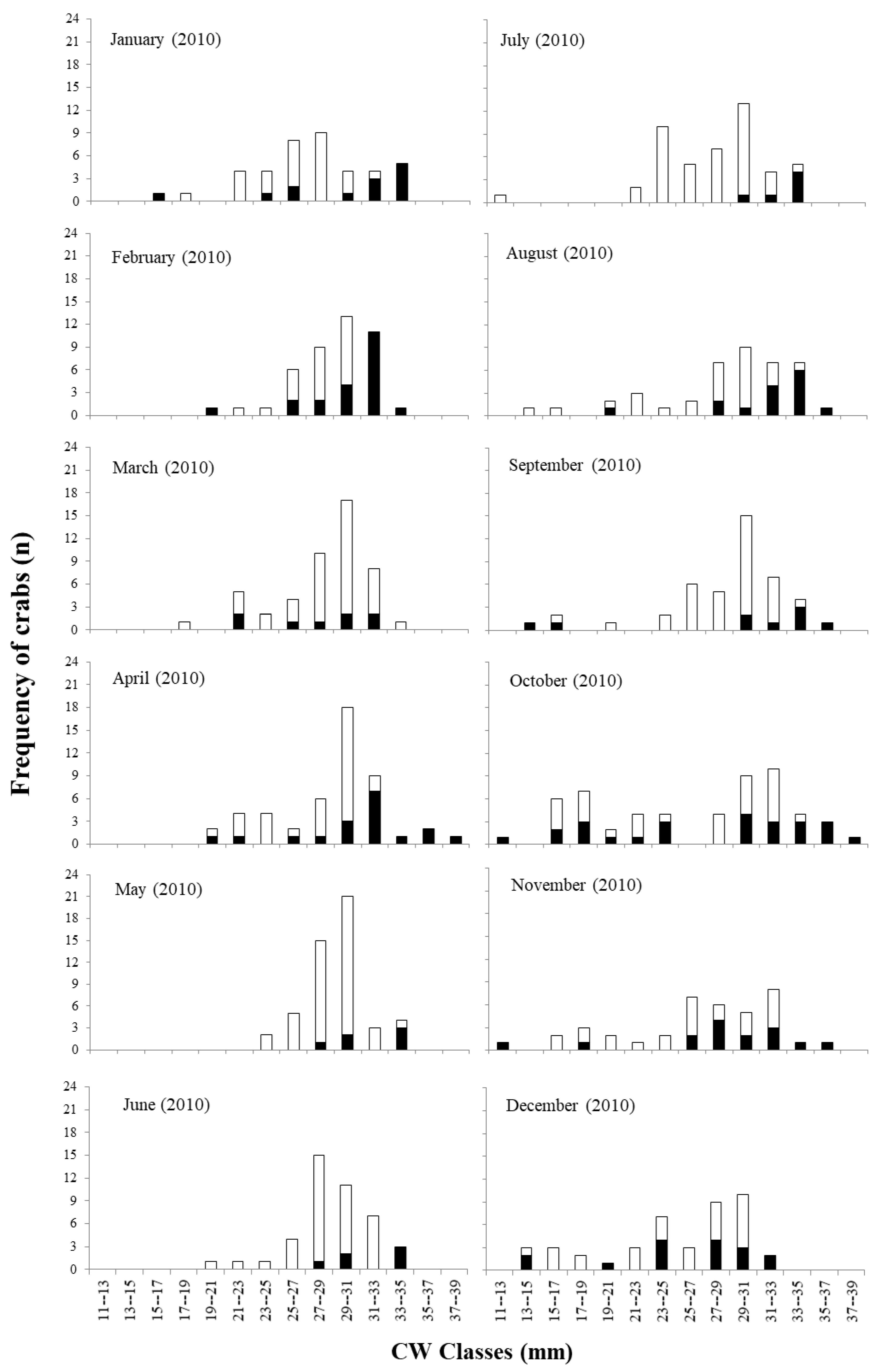
Figure 3 presents the monthly frequency of crabs into CW size classes.
Relative Growth
The relative growth analyses showed that the relationships between CWxAW and CWxGL changed from the juvenile to the adult phase. For females, the relationship CWxAW showed positive allometry in juveniles and negative allometry in adult specimens. For males, the relationship CWxGL showed positive allometry in juveniles and isometry in adults. The remaining relationships did not change from the juvenile to the adult phase (Table 1).
Table 1.
Neohelice granulata. Relative growth of carapace width (CW) and the dependent variables (CW: carapace width; AW: abdomen width; RCL: right cheliped length; LCL: left cheliped length; GL: gonopod length; n: sample size; r: correlation coefficient; R2: determination coefficient; a: intercept axis; b: allometric constant; I.C.: confidence interval; t: Student’s T-test result; AL: allometry; *: significant difference (p < 0.05); +: positive allometry; -: negative allometry; and 0: isometry).
Table 1.
Neohelice granulata. Relative growth of carapace width (CW) and the dependent variables (CW: carapace width; AW: abdomen width; RCL: right cheliped length; LCL: left cheliped length; GL: gonopod length; n: sample size; r: correlation coefficient; R2: determination coefficient; a: intercept axis; b: allometric constant; I.C.: confidence interval; t: Student’s T-test result; AL: allometry; *: significant difference (p < 0.05); +: positive allometry; -: negative allometry; and 0: isometry).
| |
|
Regression |
n |
r |
R2
|
a |
b (C.I. 95%) |
t(α=0.05) |
AL |
| Females |
Juvenile |
CW/AW |
26 |
0.903 |
0.811 |
-0.781 |
1.426 (1.19 - 1.65) |
3.80* |
+ |
| Adult |
CW/AW |
530 |
0.336 |
0.11 |
0.554 |
0.889 (0.86 - 0.91) |
-9.50* |
- |
| Males |
Juvenile |
CW/GL |
26 |
0.956 |
0.914 |
-0.715 |
1.868 (1.73 - 2.00) |
14.46* |
+ |
| CW/RQL |
26 |
0.963 |
0.927 |
-0.307 |
1.11 (1.10 - 1.31) |
12.06* |
+ |
| CW/LQL |
26 |
0.974 |
0.865 |
-0.238 |
1.21 (1.17 - 1.32) |
11.3 |
+ |
| Adult |
CW/GL |
396 |
0.916 |
0.839 |
-0.473 |
0.876 (0.82 - 0.94) |
1.96 |
0 |
| CW/RQL |
132 |
0.969 |
0.939 |
-0.39 |
1.09 (0.95 - 1.21) |
-1.83* |
+ |
| CW/LQL |
138 |
0.875 |
0.833 |
-0.362 |
1.12 (1.01 - 1.26) |
-1.64* |
+ |
Breeding Season and Recruitment
A total of 169 ovigerous females were observed between February and November, with the highest relative frequency in May. The CW of ovigerous females ranged from 19.01 to 33.87 mm (mean ± SD = 28.97 ± 2.5), with higher frequency observed in the 29-30 CW class. The juvenile individuals were observed all over the year but with a higher frequency between October and December. Juveniles were found in all months (with the exception of May) with a higher frequency from October to December, probably due to the high reproductive activity observed in the previous months (Table 2). Linear regression pointed out a negative relation between ovigerous females’ (OF) frequency and temperature (T) (OF = 229.3 – 7.86T; r = - 0.80; p < 0.05). Precipitation was not related to ovigerous females and juvenile frequencies.
Table 2.
Neohelice granulata. Relative frequency of ovigerous females and juveniles, mean temperature, and total precipitation over the sampling period (2010). Data on temperature and precipitation were provided by INMET (Instituto Nacional de Metereologia do Brasil).
Table 2.
Neohelice granulata. Relative frequency of ovigerous females and juveniles, mean temperature, and total precipitation over the sampling period (2010). Data on temperature and precipitation were provided by INMET (Instituto Nacional de Metereologia do Brasil).
| Month |
Jan |
Feb |
Mar |
Apr |
May |
Jun |
Jul |
Ago |
Sep |
Oct |
Nov |
Dec |
Total
Precipitation (mm3) |
287 |
87 |
325 |
280 |
45 |
42 |
79 |
20 |
36 |
118 |
67 |
291 |
Mean
Temperature (oC) |
30 |
30 |
27 |
26 |
24 |
21 |
23 |
22 |
23 |
24 |
25 |
27 |
Ovigerous
Females (%) |
0 |
2.3 |
12.5 |
18.7 |
85 |
73 |
34 |
51 |
54 |
24 |
18 |
0 |
| Juveniles (%) |
4.7 |
2.3 |
6.25 |
4.16 |
0 |
2.04 |
2.12 |
7.3 |
6.8 |
30.9 |
15 |
25 |
Size of Sexual Maturity
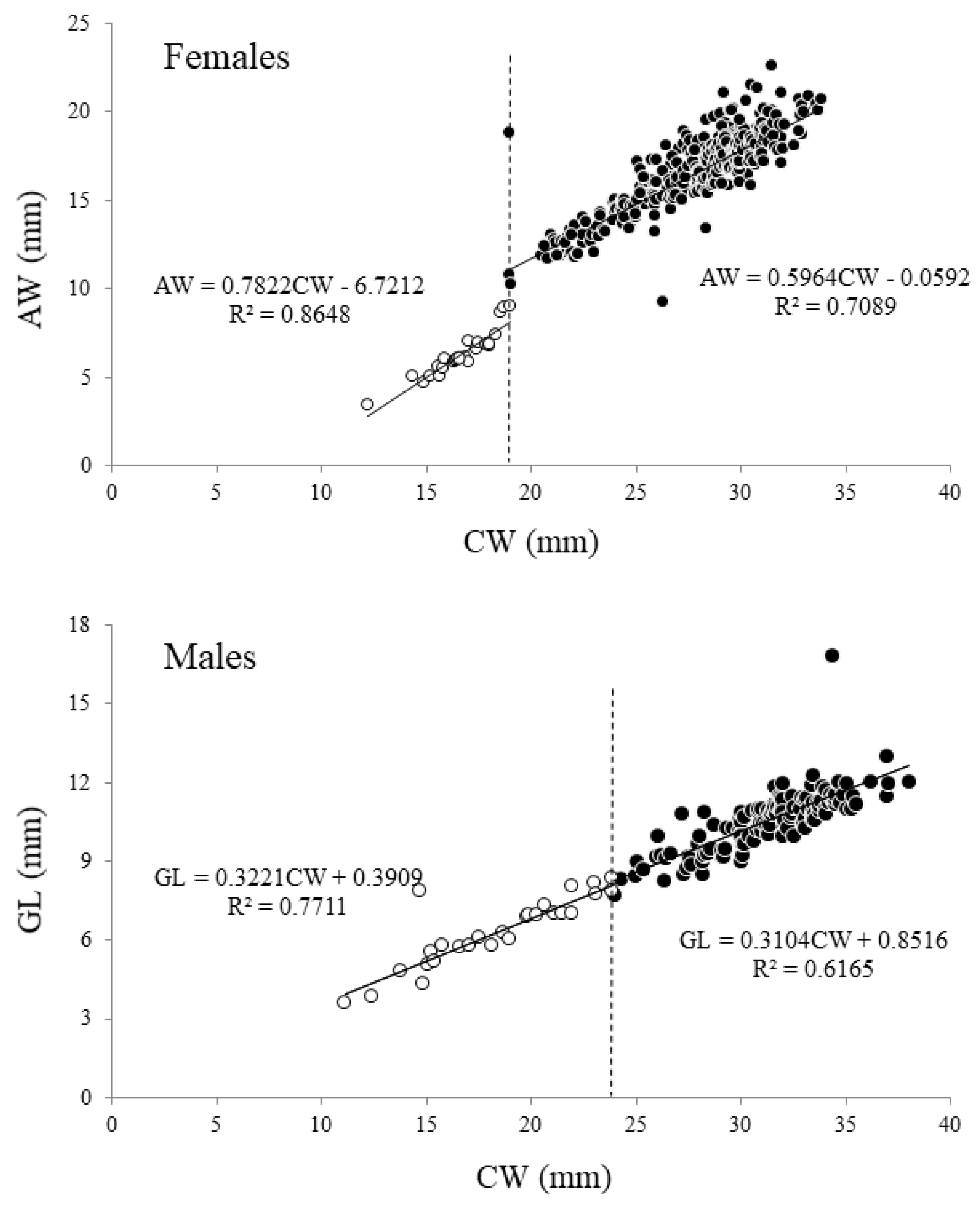
The size of sexual maturity was lower in females (
Figure 3). The breakpoint in the relationship between CW and AW [(SSR): F (3.03) = 67.3] resulted in the size of maturity of 19.0 mm (CW) for females. The breakpoint between CWxGL [(SSR): F (3.01) = 44.5] was estimated at 23.9 mm (CW) for males.
Fecundity
Individual fecundity ranged from 336 to 82,592 eggs/brood (20,419.36 ± 12,627.12).
Table 3 shows the fecundity found in each stage of egg development, which had a decrease from stage I to IV. Females with eggs in the last stage of development were found throughout the breeding season, with higher frequency (> 80%) in April, May, and November. The linear regression between CW and fecundity was significant (egg number = 37.07CW - 586.0; r = 0.5; p < 0.01).
Growth and Mortality
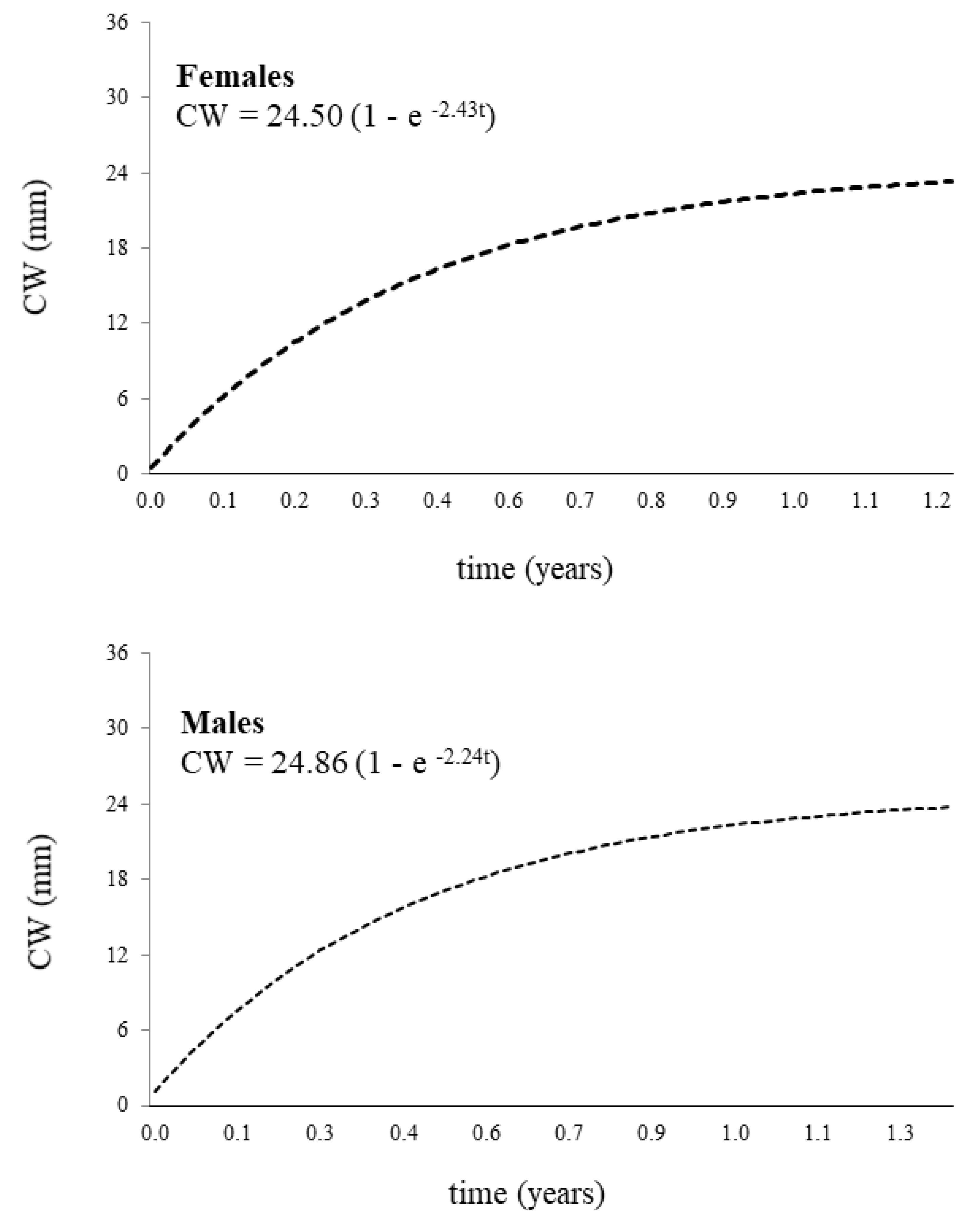
The growth rate was slightly higher in females (K = 2.43) than in males (K = 2.24) and very similar growth performances were found (ᶲ = 3.14 for males and 3.16 for females). However, different seasonal oscillations of growth were detected. The winter- point was estimated in May (C < 0.9) for males and in March (C < 0.5) for females. The asymptotic size (CW∞) was estimated at 24.86 mm for males and 24.50 mm for females (
Figure 4).
The estimations of life span and mortality were similar between males and females. The maximum longevity was estimated at 1.33 years (16 months) in males and 1.23 (15 months) in females. The total mortality (Z) was estimated at 3.71 and 3.41 for males and females, respectively. The annual finite mortality rate (A) was estimated at 0.96 and 0.97 for males and females, respectively
Discussion
In this study, the sex ratio was female-biased, as already noticed by other populations from Argentina as Salado River (Botto and Irigoyen, 1979), Mar Chiquita Lagoon (Spivak et al., 1996) and Samborombón Bay (Cesar et al., 2007). In contrast, Bas et al. (2005) and Angeletti and Cervellini (2015) found a male-biased sex ratio throughout the year in Santo Antonio Bay and Bahia Blanca Estuary (South Argentina), respectively. The sex ratio as a function of the size was female-biased in the intermediary size classes. The dominance of males in the largest size classes is a common pattern for N. granulata as other studies have demonstrated (Gregati and Negreiros-Fransozo, 2007; Angeletti and Cervellini, 2015). One reasonable explanation for this could be the difference in energy investment after reaching maturity as usually mature females apply more energy for reproduction, presenting a decrease in growth rates (Hartnoll, 1982). However, growth rates in this study were similar between males and females. Furthermore, since mortality rates were also similar between males and females, a hypothesis of differential mortality in large-sized females seems unreasonable. According to Botto and Irigoyen (1979) and Spivak et al. (1996), N. granulata presents a variation in the spatial distribution related to sex and age. Maybe, this differential distribution can bring additional constraints to crabs collection being necessary more accurate sample design to include crabs of all ages of males and females.
In general, the relative growth of N. granulata presented in this study followed the predictions for Brachyuran’s growth (Hartnoll, 1978). Also, the relative growth analyses here described were comparable to those presented by Gragati and Negreiros-Fransozo (2007) who studied another N. granulata subtropical population (Paraty, RJ, Brazil). The only exception was the relationship between CWxAW in adult females, which showed negative allometry in this study while the previous authors found isometry. However, in the present study, the changing of growth in the transition phase from juveniles to adults was observed in organs that are related to sexual maturity, namely the abdomen (females) and gonopods (males). Therefore, we conclude that morphometric analysis in this study was robust to estimate the size of maturity of males and females.
The estimated size of sexual maturity in this study was higher for males than for females. This result seems to follow a general pattern for this species when we analyze the literature data, with the exception of the study of López et al. (1997) and Luppi et al. (2004), where males presented a higher size of maturity than females (Tab. 4). In the last study, which was implemented in the laboratory, the estimations of size at maturity were very similar between males and females. The size at sexual maturity in the present study was higher than the values found by other studies in southern locations, contradicting the latitudinal pattern proposed by Sastry (1983). This author claims that populations inhabiting tropical habitats show smaller size at maturity than populations in higher latitudes but our data showed the opposite. Indeed, Barutot et al. (2009) studied two N. granulata populations at the mesoscale in Patos Lagoon, southeast Brazil, and found different estimations of size at maturity between them. These results suggest that mesoscale factors can be more deterministic to the size of maturity of N. granulata than its latitudinal variation. The breeding season of N. granulata presented in this study and the literature also reinforces the hypothesis that mesoscale factors exert a main role to shape the reproduction traits of this specie.
Generally, brachyuran’s populations from tropical areas have enlarged breeding seasons, mainly during the warmest months, while populations from higher latitudes show more restricted reproductive periods (Sastry, 1983; Adiyodi, 1988). The breeding season of N. granulata follows this pattern of longer breeding seasons in populations from subtropical regions, including this study and Gregati & Negreiros-Fransozo (2009), southeast Brazil. However, this prediction is not totally clear when we analyze populations over a latitudinal range (Tab. 4), where it can vary in extension and season showing no compliance with latitudinal assumptions. In this study, the breeding season of N. granulata was more intense during the dry season when a higher frequency of ovigerous females was observed. Despite the frequency of ovigerous females and precipitation not being related, the precipitation seems to exert an influence on reproduction once breeding decreases in the months of the rainy season (September to March) (Tab. 1). Long-term studies have observed that the breeding season changes between two consecutive years, suggesting that the temperature is correlated with the start and duration of the breeding season (Ituarte et al., 2004; Ituarte et al., 2006). Bas et al. (2005) raised the possibility that the reproductive period should be related to the temperature range seen among seasons. The difference between the extreme temperatures found during winter and summer affects the reproductive process in populations from higher latitudes. In this study, temperature was negatively related to ovigerous females’ frequency, which lead to the hypothesis that higher temperatures affect negatively N. granulata reproduction being a limiting factor to this species distribution to the north. However, further studies are necessary to confirm this statement. On the other hand, Spivak et al. (2016) compared two populations in Southern Argentina, distant 190 km and, therefore, submitted to a resembling temperature range, which showed consistent differences in reproductive aspects, including breeding season.
The literature review about the reproduction of
N. granulata is presented in
Table 4, where data from the size at sexual maturity, breeding season, and fecundity is shown along a latitudinal gradient from the southernmost (Bas et al., 2005) to the northernmost (this study). There is no clear pattern related to the latitude to clarify the analyzed reproductive aspects of
N. granulata. In general, all of these studies were focused on the species’ features while biotic and abiotic interactions can exert a central role in
N. granulata reproduction. In order to contribute with a broader understanding of the reproduction of this species, further approaches should be addressed to explain the variations in the size at maturity and breeding season of
N. granulata.
The average fecundity in this study was related to the female’s size as already reported by Luppi et al. (1997) and César et al. (2007). This may be due to the fact that the body cavity space restricts the yolk accumulation, being a limiting factor in the brood size (Hines, 1982). The decrease in the number of eggs along the embryonic development stages observed in this study may be a response to abiotic variations, pollution, egg predation, and infection by parasites or microbes (Balsundaran and Pandian, 1982; Ruffino et al., 1994; Luppi et al., 1997). Fecundity can vary largely between populations as a result of different life conditions (Ruffino et al., 1994; Silva et al., 2008; Barutot et al., 2009).
Table 4 presents estimations of
N. granulata fecundity from different regions and shows that the reproductive efforts of this specie can vary significantly. According to Hines (1982), the reproductive effort exhibits significant intraspecific variation with respect to limited resources, molting stage, tidal height, and latitude (especially in large distribution species). However, despite
N. granulata presenting a large distribution range, the fecundity varies independently to the latitude. The higher fecundities of this species were reported in Jabaquara Beach, a location near to the northern limit of the
N. granulata distribution, and in Saborombóm Bay, near to the southern limit of its distribution.
The parameters of growth in the present study were very similar between males and females, being comparable with the results found by Barcelos et al. (2007) concerning growth rates (K ~ 2) in Peixe Lagoon, south Brazil. However, a study developed in the laboratory by Luppi et al. (2004) found contrasting results in which the authors estimated a very lower growth rate (K ~ 0.5). The differences in growth rates from these studies resulted in different life span estimations, where the formers noticed a longevity of around one year (this study) and two years (Barcelos et al. 2007) while Luppi et al. (2004) estimated a maximum life span of three years. The dissimilar estimations of growth parameters in the same species can be a consequence of the applied methodology. It is known that different methodologies or misinterpretations of models can produce contrasting results on the estimations of growth in crabs and many studies present unreliable growth estimations (Costa et al. 2014). Beyond the difficulty to predict the growth of crabs, environmental factors aren’t taken into account in most studies, preventing a broader understanding of the results. In the case of
U. cordatus, another conspicuous species in Atlantic coastal wetlands, mesoscale habitat differences in the rainfall regime and salinity fluctuations can influence the growth of this species through the amount of energy expended by the osmoregulatory mechanisms (Koch, 1999). The growth of crabs can also be indirectly affected by the productivity and composition of the wetlands due to the influence on the availability and quality of food (Diele and Koch 2010). The anthropogenic impacts in coastal wetlands can also affect the growth rates and life span (Costa & Soares-Gomes, 2015).
Table 5 shows the maximum sizes available in literature along the distribution area of
N. granulata. Despite this study in the northern limit of this species distribution (lower latitude) reported the smallest sizes in literature, data from
Table 5 shows that the crab’s sizes did not follow the latitudinal gradient pattern of size relative to temperature, where the smaller individuals are found on lower latitudes (Atkinson 1994), and these differences may be attributed to mesoscale factors such as wetland productivity, salinity and habitat quality as discussed above.
The population biology of N. granulata has been extensively studied, especially in the Southern portion of its distribution, namely in Southern Brazil and Argentina. Data from this study indicate that mesoscale factors are the main drivers to determine some features of the biology of N. granulata, such as size at maturity, breeding season, growth, and life span. Considering the relevant ecological role of N. granulata in bioturbation and the increasing degradation of their habitats along the southwest Atlantic coast, further studies addressing the conservation status and potential use of this crab as an indicator of the environmental state of coastal wetlands (mangroves and salt marshes) is recommended.
References
- Adiyodi RG 1988. Reproduction and development. In: Burggren, W. W., & McMahon, B. R. (Eds). Biology of the land crabs. Cambridge University Press.
- Alongi DM 2002. Present state and future of the world's mangrove forests. Environmental conservation, 29(3): 331-349. [CrossRef]
- Angeletti and Cervellini PM 2015. Population structure of the burrowing crab Neohelice granulata (Brachyura, Varunidae) in a southwestern Atlantic salt marsh. Latin American Journal of Aquatic Research, 43 (3): 539-547. [CrossRef]
- Balsundaran C and Pandian TJ 1982. Egg loss during incubation in Macrobrachium nobilii (Henderson & Mathias). Journal of Experimental Marine Biology and Ecology, 59(2,3): 289-299.
- Barcelos DF; Castigliono DS; Barutot R and Santos S 2007. Crescimento de Chasmagnathus granulatus (Crustacea, Decapoda, Varunidae) na Lagoa do Peixe, Rio Grande do Sul, Brasil. Iheringia, 97 (3): 263-267.
- Barutot RA; D’Incao F and Fonseca DB 2009. Reproductive biology of Neohelice granulata (Decapoda: Varunidae) in two salt marshes of the estuarine region of the Lagoa dos Patos Lagoon, southern Brazil. Journal of the Marine Biological Association of the United Kingdom, 89 (4): 769-774. [CrossRef]
- Bas C; Luppi T and Spivak E 2005. Population structure of the South American Estuarine crab, Chasmagnathus granulatus (Brachyura: Varunidae) near the southern limit of its geographical distribution: comparison with northern populations. Hidrobiologia, 537: 217-228. [CrossRef]
- Botto F and Iribarne O 2000. Contrasting effects of two burrowing crabs (Chasmagnathus granulata and Uca uruguayensis) on sediment composition and transport in estuarine environments. Estuarine, Coastal and Shelf Science, 51: 141–151. [CrossRef]
- Botto F; Iribarne O; Guitierrez J; Bava J and Gagliardini A 2006. The ecological importance of passive deposition of meiofauna and organic matter into burrows of the SW Atlantic burrowing crab Chasmagnathus granulatus. Marine Ecology Progress Series, 312: 201-2010.
- Botto JL and Irigoyen HR 1979. Bioecologia de la comunidad del cangrejal. I. Contribucion al conocimiento biologico del cangrejo de estuario, Chasmagnathus granulata Dana (Crustacea, Decapoda, Grapsidae) en la desembocadura del rio Salado, Provincia de Buenos Aires. Seminario Latinoamericano de Ecologia Bentonica y Sedimentologia de la Plataforma Continental del Atlantico Sur. UNESCO, Montevideo: 161–169.
- César II; Armendáriz LC and Becerra RV 2007. Fecundity of Uca uruguayensis and Chasmagnathus granulatus (Decapoda: Brachyura) from the “Refugio de Vida Silvestre” Bahía Samborombón, Argentina. Brazilian Journal of Biology, 67 (4): 749-753. [CrossRef]
- Costa TMM; Pitombo FB and Soares-Gomes A 2014. The population biology of the exploited crab Ucides cordatus (Linnaeus, 1763) in a southeastern Atlantic Coast mangrove area, Brazil. Invertebrate Reproduction & Development, 58(4): 259-268. [CrossRef]
- Costa TMM and Soares-Gomes A 2015. Secondary production of the fiddler crab Uca rapax from mangrove areas under anthropogenic eutrophication in the Western Atlantic, Brazil. Marine Pollution Bulletin, 101: 533-538.
- Conde JE and Bevilacqua M 1983. A volumetric method for estimating fecundity in Decapoda. Marine Ecology Progress Series, 10: 206-210. [CrossRef]
- Diele K and Koch V 2010. Growth and mortality of the exploited mangrove crab Ucides cordatus (Ucididae) in N-Brazil. Journal of Experimental Marine Biology and Ecology, 395: 171-180. [CrossRef]
- Feller IC, Lovelock CE, Berger U, McKee KL, Joye SB, Ball MC. 2010. Annual Review of Marine Science, 2: 395-417.
- Gregati RA and Negreiros-Fransozo ML 2007. Relative growth and morphological sexual maturity of Chasmagnathus granulatus (Crustacea, Varunidae) from a mangrove area in southeastern Brazilian coast. Iheringia, 97(3): 268-272. [CrossRef]
- Gregati RA and Negreiros-Fransozo ML 2009. Population biology of the burrowing crab Neohelice granulata, (Crustacea: Decapoda: Varunidae) from a tropical mangrove in Brazil. Zoologia, 26 (1): 31-37. [CrossRef]
- Hartnoll RG 1974. Variation in growth patterns between some secondary sexual characters in crabs (Decapoda: Brachyura). Crustaceana, 27: 131-136. [CrossRef]
- Hartnoll RG 1978. The determination of relative growth in Crustacea. Crustaceana, 34 (3): 281-293. [CrossRef]
- Hartnoll RG 1982. Growth, p.III-196.1n: L.G. ABELE (Ed.). The Biology of Crustacea. 2. Embryology, morphology and genetics. New York, Academic Press New York, 440p.
- Hines AH 1982. Allometric constraints and variables of reproductive effort in Brachyuran crabs. Marine Biology, 69: 309-320. [CrossRef]
- Hines AH 1989. Geographic variation in size at maturity in brachyuran crabs. Bulletin of Marine Science, 45 (2): 356-368.
- Hutchinson GE 1981. Introduccíon a la ecologia de poblaciones. Barcelona Blume Editorial, 492p.
- IBAMA 2001. Área de Proteção Ambiental de Guapi-Mirim - Plano de manejo. https://www.icmbio.gov.br/apaguapimirim/planos-de-manejo. Accessed in 12/05/2023.
- Ituarte RB; Spivak ED and Luppi TA 2004. Female reproductive cycle of the Southwestern Atlantic estuarine crab Chasmagnathus granulatus (Brachyura: Grapsoidea: Varunidae). Scientia Marina, 68 (1): 127-137. [CrossRef]
- Ituarte RB ; Bas C ; Luppi TA and Spivak ED 2006. Interpopulational differences in the female reproductive cycle of the southwestern Atlantic estuarine crab Chasmagnathus granulatus Dana, 1851 (Brachyura: Grapsoidea: Varunidae). Scientia Marina, 70(4): 709-718. [CrossRef]
- Kennelly SJ, Watkins D. 1994. Fecundity and reproductive period, and their relationship to catch rates of spanner crabs, Ranina ranina of the East coast of Australia. Journal of Crustacean Biology, 14(1): 146-150. [CrossRef]
- Koch V 1999. Epibenthic Production and Energy Flow in the Caeté Mangrove Estuary, North Brazil. Bremen, University of Bremen, Doctoral dissertation, 77p. [Unpublished].
- Kristensen E and Alongi DM 2006. Control by fiddler crabs (Uca vocans) and plant roots (Avicennia marina) on carbon, iron and sulfur biogeochemistry in mangrove sediment. Limnology and Oceanography, 51: 1557–1571. [CrossRef]
- López LS; Stella VS and Rodríguez EM 1997. Size at onset of sexual maturity in Chasmagnathus granulate (Decapoda, Brachyura). Nauplius, 5(2): 65-75.
- Lugo AE and Snedaker SC 1974. The ecology of mangroves. Annual review of ecology and systematics, 39-64.
- Luppi T; Bas C; Spivak E and Anger K 1997. Fecundity of two grapsid crab species in the laguna mar Chiquita, Argentina. Archive of fishery and marine research, 45: 149-166.
- Luppi TA; Spivak ED; Bas CC and Anger K 2004. Molt and growth of an estuarine crab, Chasmagnathus granulatus (Brachyura: Varunidae), in Mar Chiquita coastal lagoon, Argentina. Journal of Applied Ichthyology, 20: 333-344. [CrossRef]
- Marques ABM; Fontana CS; Vélez E; Bencke GA; Schneider M and Reis RE 2002. Lista das espécies da fauna ameaçadas de extinção no Rio Grande do Sul. Decreto nº41.672 de 11 de junho de 2002.
- Melo GAS 1996. Manual de identificação dos Brachyura (caranguejos e siris) do litoral brasileiro. São Paulo, Plêiade. 604p.
- Mendez-Casariego A; Luppi T; Iribarne O and Daleo P 2011. Increase of organic matter transport between marshes and tidal flats by the burrowing crab Neohelice (Chasmagnathus) granulata Dana in SW Atlantic salt marshes. Journal of Experimental Marine Biology and Ecology, 401: 110-117. [CrossRef]
- Negreiros DHD; Araújo FP and Coreixas MA 2002. Nossos rios. Niterói: Instituto Baía de Guanabara. 34 p.
- Ng P; Guinot D and Davie P 2008. Systema Brachyururum: Part I. An Annotated Checklist of Extant Brachyuran Crabs of the World. The Raffles Bulletin of Zoology, 17:1-286.
- Pezzuto PR 1993. Regrans: a “basic” program for an extensive analysis of relative growth. Atlântica, Rio Grande, 15: 93-105.
- Pinheiro MAA; Santana W; Boos H; Matsunaga AMF and Lianos L. 2016. Avaliação do Caranguejo Neohelice granulata (Dana, 1851) (Decapoda: Varunidae). Cap. 34: p. 459-466. In: Pinheiro, M. & Boos, H. (Org.), Livro Vermelho dos Crustáceos do Brasil: Avaliação 2010-2014. Porto Alegre, RS, Sociedade Brasileira de Carcinologia - SBC, 466 p.
- Pires IO 1986. Mapeamento de manguezais do recôncavo da Baía de Guanabara, RJ, através de técnicas de sensoriamento remoto. Dissertação. Instituto de Pesquisas Espaciais, São José dos Campos, 86 p.
- Ruffino ML; Telles MD and D'Incao F 1994. Reproductive aspects of Chasmagnathus granulata Dana, 1851 (Decapoda, Grapsidae) in the Patos Lagoon Estuary - Brazil. Nauplius, 2: 43-52.
- Sakai K ; Turkay M and Yang SL 2006. Revision of the Helice/Chasmagnathus complex (Crustacea: Decapoda: Brachyura). Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, 565: 1–76.
- Sastry AN 1983. Ecological aspects of reproduction. In: Bliss, D.E. (Eds). The Biology of Crustacea (vol.8) Environmental adaptations, 179-270.
- Shine R 1988. The evolution of large body size in females: a critique on Darwin’s “fecundity advantage” model. The American Naturalist, 131(1): 124-131. [CrossRef]
- Silva PV; Luppi TA; Spivak ED and Anger K 2009. Reproductive traits of an estuarine crab, Neohelice (= Chasmagnathus) granulata (Brachyura: Grapsoidea: Varunidae), in two contrasting habitats. Scientia Marina, 73(1): 117-127. [CrossRef]
- Spivak ED 2010. The crab Neohelice (= Chasmagnathus) granulata: an emergent animal model from emergent countries. Helgoland Marine Research, 64: 149-154. [CrossRef]
- Spivak ED; Bas CC and Luppi TA 2016. Great unexpected differences between two populations of the intertidal carb Neohelice granulata inhabiting close but contrasting habitats (Crustacea: Decapoda: Brachyura). Zoologia, 33 (6): e20160020.
- Spivak ED; Anger K ; Bas CC ; Luppi TA and Ismael D 1996. Size structure, sex ratio, and breeding season in two intertidal grapsid crab species from Mar Chiquita Lagoon, Argentina. Neritica, 10: 7–26.
- Vogt G 2011. Ageing and longevity in the Decapoda (Crustacea): A review. Zoologischer Anzeiger, 251(1): 1-2. [CrossRef]
- Wenner AM 1972. Sex ratio as a function of size in marine Crustacea. American Naturalist, 321-350. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).