Submitted:
03 May 2023
Posted:
04 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Green Chemistry: monomers and polymers from renewable resources
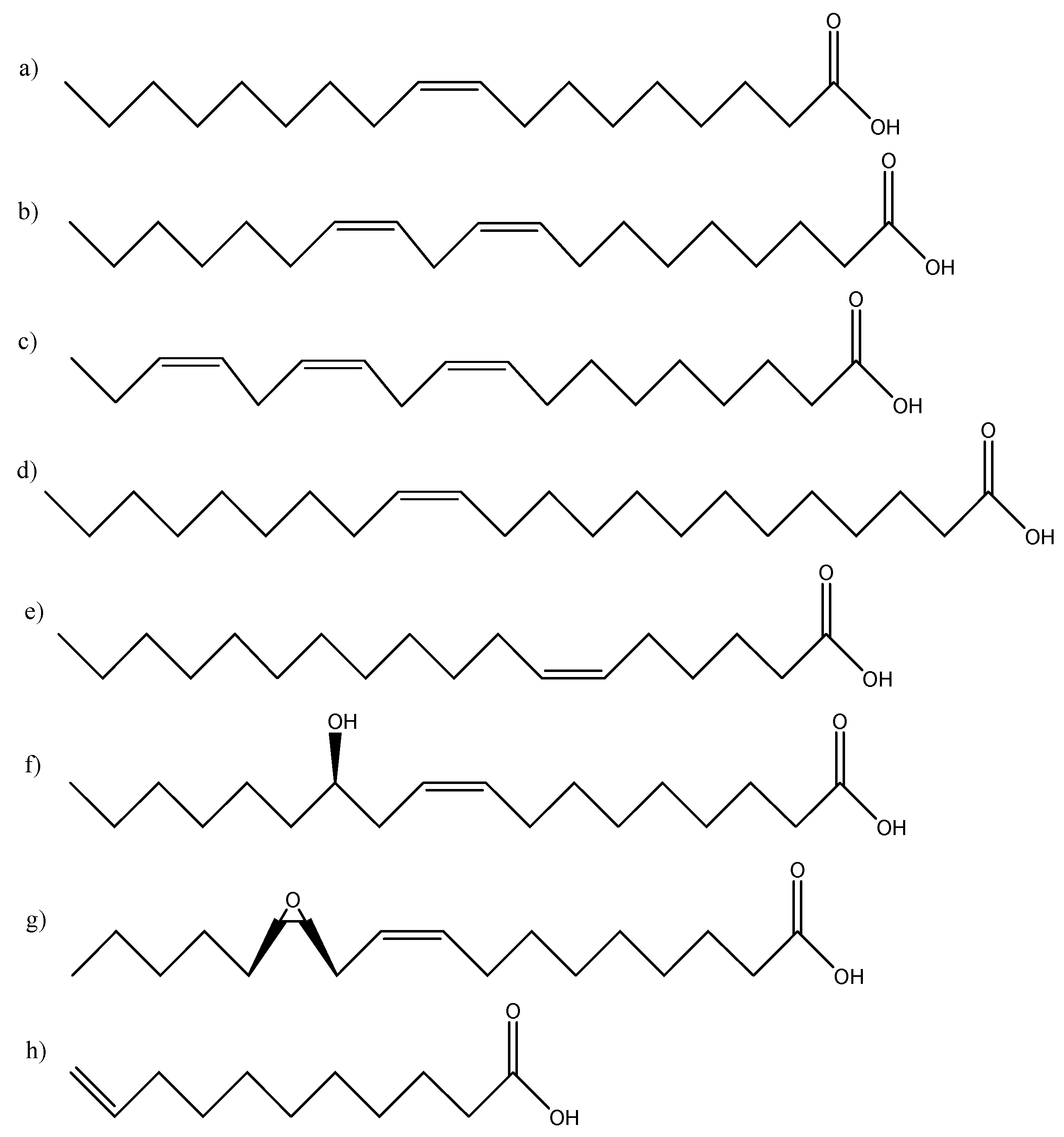
3. Synthesis of monomers from vegetable oils
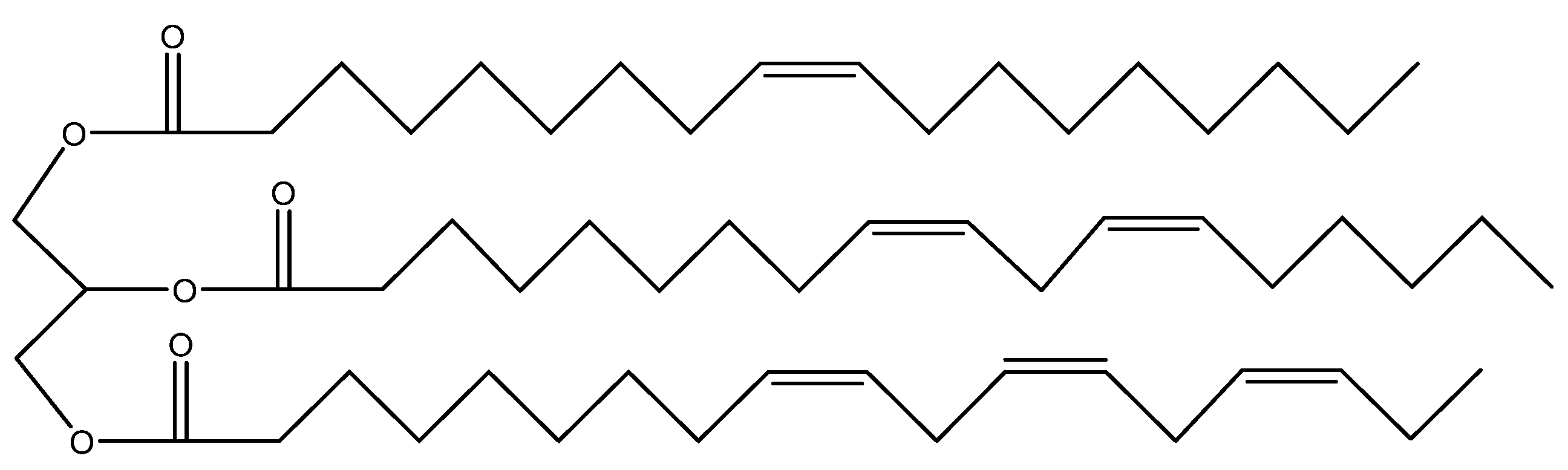
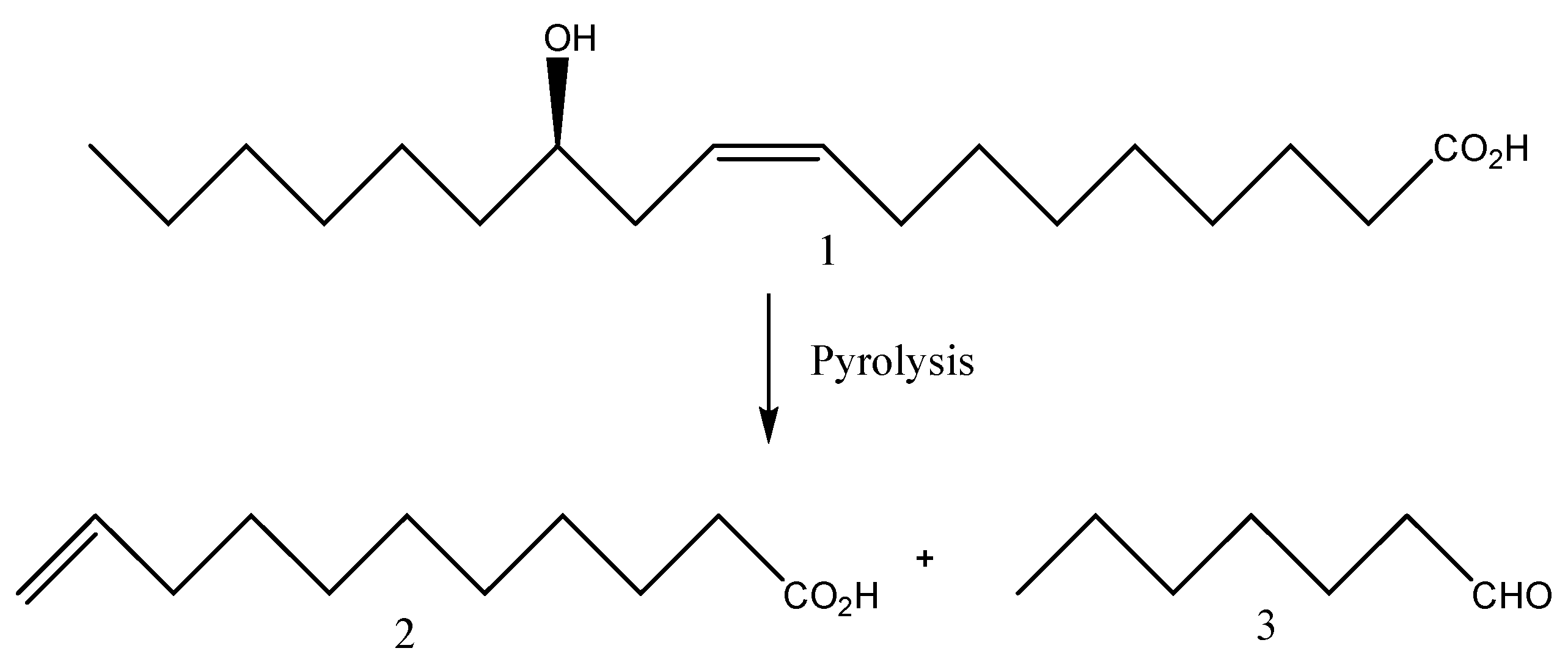
4. Castor oil as renewable raw material
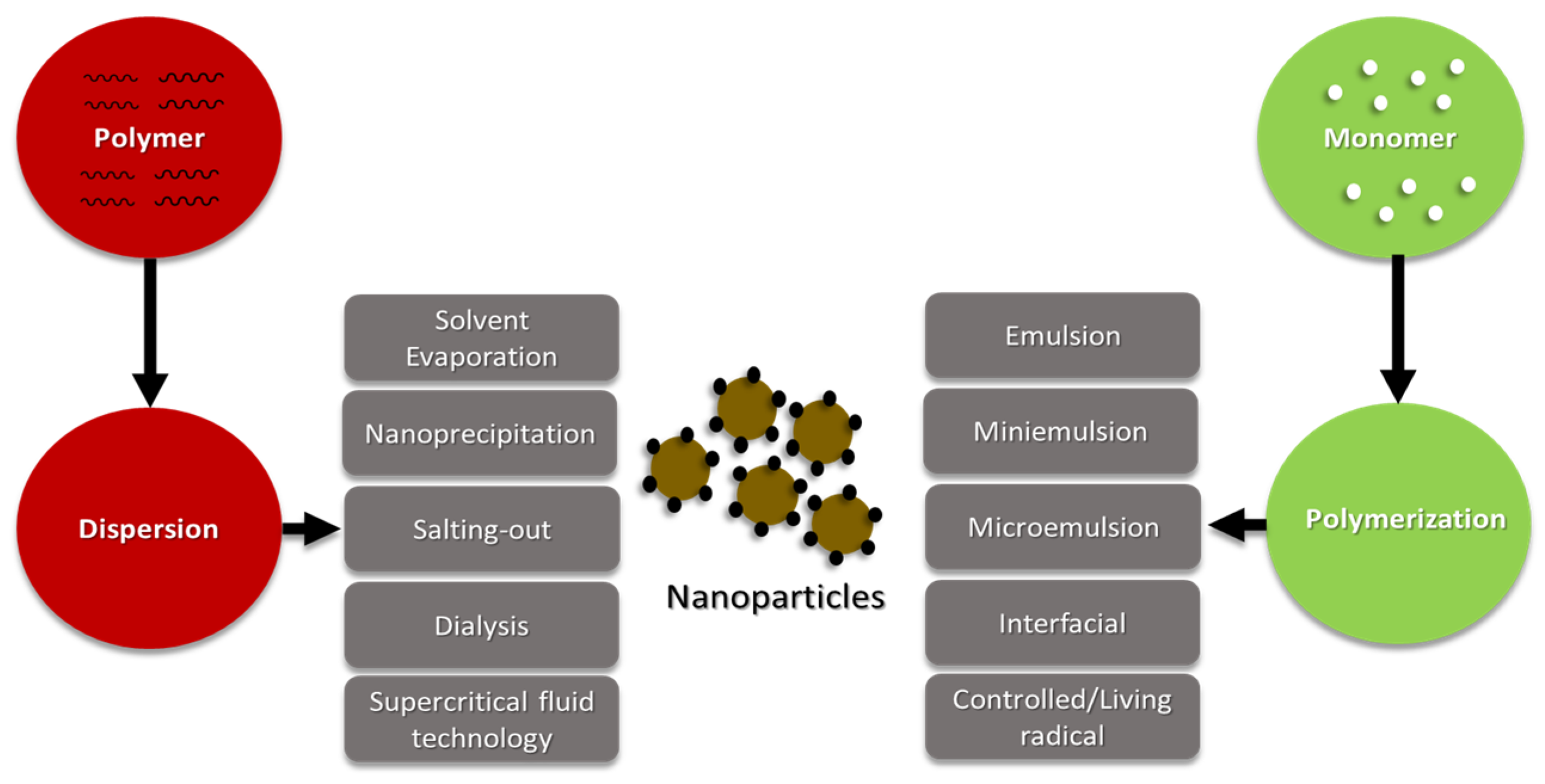
5. Polymeric nanoparticles and some production techniques
5.1. Solvent evaporation technique
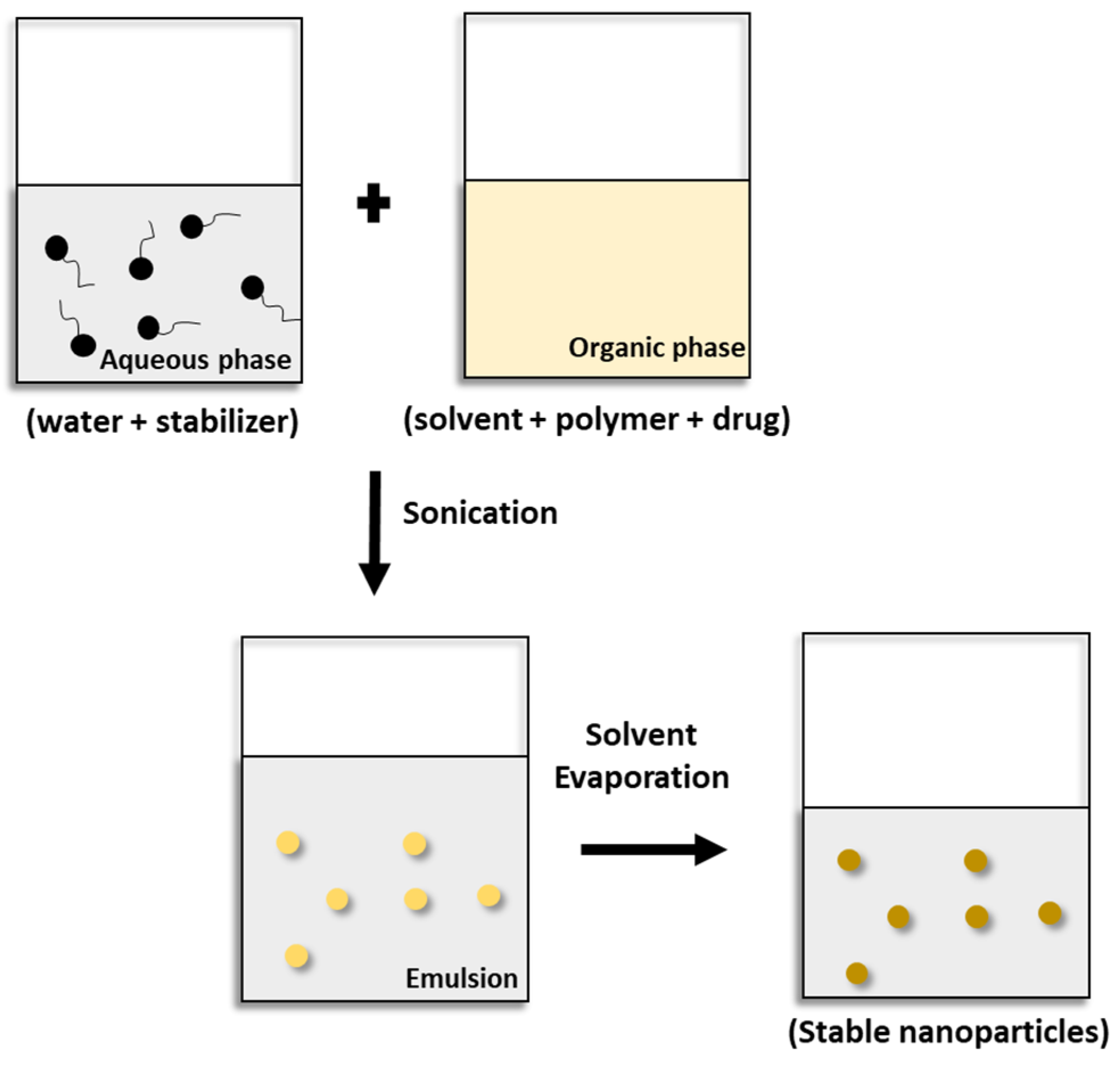
5.2. Miniemulsion polymerization
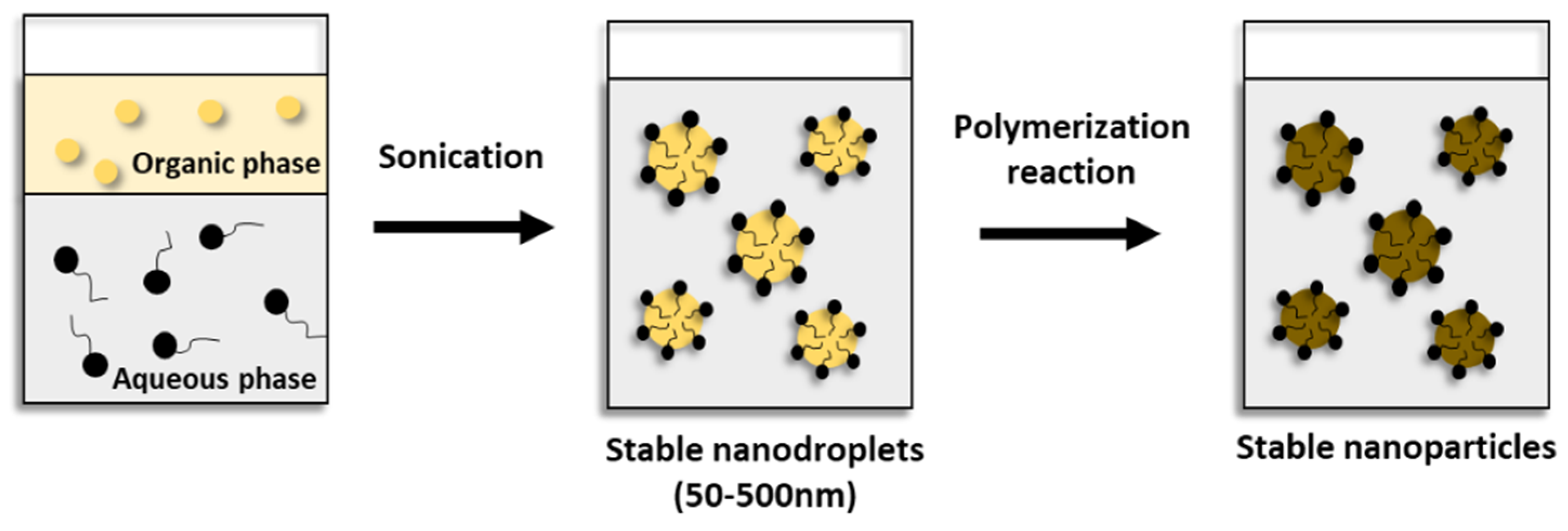
6. Thiol-ene polymerization for nanoparticles production

7. Application of polymeric nanoparticles in cancer therapy
| Polymeric nanoparticles | Oncology APIs | Nanoparticles production | Biological study | Ref. |
| Polyhydroxyalkanoates (PHAs) nanoparticles | Ellipticine | Emulsification/Solvent evaporation | in vitro | [61,62] |
| Cisplatin | Emulsification/Solvent evaporation | in vitro | [63] | |
| Thymoquinone | Emulsification/Solvent evaporation | in vitro | [64] | |
| Paclitaxel | Double emulsification/Solvent evaporation | in vitro | [65] | |
| 5-Fluorouracil | Double emulsification/Solvent evaporation | in vitro | [66] | |
| Etoposide | Solvent evaporation | in vitro | [67] | |
| Doxorubicin | Double emulsification/Solvent evaporation | in vitro | [68] | |
| Rhodamine B isothiocyanate (RBITC) | Emulsification/Solvent evaporation | in vitro | [69] | |
| Cyclodextrin (CDs) nanoparticles | Docetaxel | Nanoprecipitation | in vitro | [70] |
| Camptothecin | Nanoprecipitation | in vitro | [71] | |
| Acyclovir | Nanoprecipitation | in vitro | [72] | |
| Paclitaxel | Emulsification/Solvent evaporation method | in vivo | [73] | |
| Poly(thioether-ester) nanoparticles | Zinc phthalocyanine | Thiol-ene miniemulsion polymerization | in vitro | [74] |
| Full-spectrum cannabis extract | Thiol-ene miniemulsion and Emulsification/Solvent evaporation | in vitro | [75] | |
| 4-nitrochalcone | Thiol-ene miniemulsion polymerization | in vitro | [76] | |
| Polymeric nanoparticles | Oncology APIs | Nanoparticles production | Biological study | References |
| Poly-(lactic-co-glycolic acid) (PLGA) nanoparticles | Paclitaxel | Emulsification and Nanopracipitation | Pre clinical (mice) | [77] |
| Topotecan–tamoxifen | Double emulsification/Solvent evaporation | in vitro | [78] | |
| Lupeol | Emulsification/Solvent evaporation | in vitro | [79] | |
| Gemcitabine | Emulsification/Solvent evaporation | in vitro | [80] | |
| 9-nitro-camptothecin | Nanoprecipitation | in vitro | [81] | |
| Paclitaxel, Doxorubicin | Double emulsification/Solvent evaporation | in vitro | [82] | |
| Paclitaxel | Nanoprecipitation | in vitro | [83] | |
| Cisplatin | Emulsification/Solvent evaporation | in vitro | [84] | |
| Paclitaxel/superparamagnetic iron oxide | Emulsification/Solvent evaporation | in vitro | [85] | |
| Tamoxifen, Quercetin | Emulsification/Solvent evaporation | in vitro | [86] | |
| Docetaxel | Nanoprecipitation | in vitro | [87] | |
| Δ9 -Tetrahidrocannabinol | Nanoprecipitation | in vitro | [88] | |
| Doxorubicin | Solvent displacement | in vitro | [89] | |
| Paclitaxel | Nanoprecipitation | Pre clinical | [90] | |
| Bicalutamide | Nanoprecipitation | in vitro | [91] | |
| siRNA, Paclitaxel | Emulsification/Solvent evaporation | in vitro | [92] | |
| Paclitaxel, Doxorubicin | Double emulsification/Solvent evaporation | in vivo | [93] | |
| Methotrexate | Emulsification and diffusion | in vivo | [94] | |
| Cisplatin | Nanoprecipitation | Pre clinical | [95] | |
| Polymeric nanoparticles | Oncology APIs | Nanoparticles production | Biological study | References |
| Poly-(lactic-co-glycolic acid) (PLGA) nanoparticles |
Doxorubicin | Solvent displacement | in vitro | [96] |
| Paclitaxel | Nanoprecipitation | Pre clinical (mice) | [97] | |
| Curcumin | Nanoprecipitation | in vivo | [98] | |
| PE38KDL | Double emulsification/Solvent evaporation | Pre clinical (mice) | [99] | |
| Paclitaxel and magnetic fluid | Emulsification/Solvent evaporation | in vitro | [100] | |
| Gemcitabine | Double emulsification/Solvent evaporation | in vitro | [101] | |
| Paclitaxel | Emulsification/ Precipitation | in vitro | [102] | |
| Capecitabine | Emulsification/Solvent evaporation | in vitro | [103] | |
| SN-38 | Emulsification/Solvent evaporation | in vitro | [104] | |
| BSA | Double emulsification/Solvent evaporation | in vitro | [105] | |
| Chitosan nanoparticles | Quercetin | Coordination reaction | in vitro | [106] |
| Curcumin | Ionic gelation method | in vitro | [107] | |
| Metformin | Ionic gelation method | in vitro and in vivo | [108] | |
| Chlorin e6 | Nonsolvent-aided counterion complexation | in vitro | [109] | |
| Adriamycin | Dialysis method | in vitro and in vivo | [110] | |
| Polycaprolactone (PCL) nanoparticles | Docetaxel | Emulsification/Solvent evaporation | in vitro | [111] |
| Thalidomide | Dialysis method | in vitro and in vivo | [112] | |
| Docetaxel | Nanoprecipitation technique | in vitro and in vivo | [113] | |
| Dihydroartemisinin | Self-assembly method | in vitro and in vivo | [114] | |
| Oxymatrine | pH gradient method | in vitro | [115] | |
| Polymeric nanoparticles | Oncology APIs | Nanoparticles production | Biological study | References |
| Polycaprolactone (PCL) nanoparticles | Paclitaxel and curcumin | Self-assembly method | in vitro and in vivo | [116] |
| Flutamide | Nanoprecipitation method | - | [117] | |
| 5-fluorouracil | Double emulsion technique | in vitro | [118] | |
| Silibinin | Solvent displacement process | in vitro and in vivo | [119] | |
| Cellulose Nanoparticles | Doxorubicin | Self-assembly method | in vitro and in vivo | [120] |
| 5-Fluorouracil | co-precipitation method | in vitro | [121] | |
| Coumarin and curcumin | oil in water emulsion technique | in vitro | [122] |
8. Oncology Active Pharmaceutical Ingredients (APIs)
| Oncology (APIs) | Kinds of Cancer | Biological study | References |
|---|---|---|---|
| Quercetin | Breast, lung, liver, colon cancers, intestine | in vitro and in vivo | [128,129,130,131] |
| Bevacizumab | Colorectal, glibastoma | in vitro and vitro | [132,133,134,135] |
| Catharanthus roseus extract | Breast, cervical, liver | in vitro | [136,137,138] |
| Irinotecan | Colorectal, colon, gastric | in vitro | [139,140,141] |
| Isolated cannabinoids or full-spectrum cannabis extract | Melanoma, glioma, ovarian, leukemia, adenocarcinoma, lung | in vitro, in ovo and in vivo | [75,88,142,143] |
| Olaparib | Prostate, pancreatic, breast, ovarian | in vitro and vitro | [144,145,146] |
| Podophyllum extract | Carcinoma, breast | in vitro | [147,148,149] |
| Temozolomide | Glioma, gliobastoma, lung | in vitro and vitro | [150,151,152] |
| Vemurafenib | Resistent melanoma | in vitro and vitro | [153,154] |
| Zinc phthalocyanine | Breast, liver, carcinoma, cervical adenocarcinoma | in vitro and in vivo | [74,155,156] |
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Montero De Espinosa, L.; Meier, M.A.R. Plant Oils: The Perfect Renewable Resource for Polymer Science?! Eur. Polym. J. 2011, 47, 837–852. [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable Polymers from Renewable Resources. Nature 2016, 540, 354–362. [CrossRef]
- Llevot, A.; Meier, M.A.R. Renewability-a Principle of Utmost Importance! Green Chem. 2016, 18, 4800–4803. [CrossRef]
- Lowe, A.B. Thiol-Ene “Click” Reactions and Recent Applications in Polymer and Materials Synthesis: A First Update. Polym. Chem. 2014, 5, 4820–4870. [CrossRef]
- Machado, F.; Lima, E.L.; Pinto, J.C. A Review on Suspension Polymerization Processes. Polimeros 2007, 17, 166–179. [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. [CrossRef]
- Nguyen, K.T. Targeted Nanoparticles for Cancer Therapy:Promises and Challenges. J. Nanomedicine Nanotechnol. 2011, 2. [CrossRef]
- Piccolo, M.; Menale, C.; Crispi, S. Combined Anticancer Therapies: An Overview of the Latest Applications. Anticancer. Agents Med. Chem. 2015, 15, 408–422. [CrossRef]
- Vaccaro, L. Green Chemistry. Beilstein J. Org. Chem. 2016, 12, 2763–2765. [CrossRef]
- Britannica Green Chemistry Available online: https://www.britannica.com/science/green-chemistry (accessed on 19 May 2020).
- Tschan, M.J.L.; Brulé, E.; Haquette, P.; Thomas, C.M. Synthesis of Biodegradable Polymers from Renewable Resources. Polym. Chem. 2012, 3, 836–851. [CrossRef]
- Belgacem, M.; Gandini, A. Monomers, Polymers and Composites from Renewable Resources; 2008; ISBN 9780080453163.
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant Oil Renewable Resources as Green Alternatives in Polymer Science. Chem. Soc. Rev. 2007, 36, 1788–1802. [CrossRef]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.R.; Metzger, J.O.; Schäfer, H.J. Oils and Fats as Renewable Raw Materials in Chemistry. Angew. Chemie Int. Ed. 2011, 50, 3854–3871. [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-Oil-Based Polymers as Future Polymeric Biomaterials. Acta Biomater. 2014, 10, 1692–1704. [CrossRef]
- Türünç, O.; Meier, M.A.R. Fatty Acid Derived Monomers and Related Polymers via Thiol-Ene (Click) Additions. Macromol. Rapid Commun. 2010, 31, 1822–1826. [CrossRef]
- Del Rio, E.; Lligadas, G.; Ronda, J.C.; Gali??, M.; Meier, M.A.R.; C??diz, V. Polyurethanes from Polyols Obtained by ADMET Polymerization of a Castor Oil-Based Diene: Characterization and Shape Memory Properties. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 518–525. [CrossRef]
- Mutlu, H.; Meier, M.A.R. Castor Oil as a Renewable Resource for the Chemical Industry. Eur. J. Lipid Sci. Technol. 2010, 112, 10–30. [CrossRef]
- Firdaus, M.; Meier, M.A.R.; Biermann, U.; Metzger, J.O. Renewable Co-Polymers Derived from Castor Oil and Limonene. Eur. J. Lipid Sci. Technol. 2014, 116, 31–36. [CrossRef]
- Kreye, O.; Tóth, T.; Meier, M.A.R. Poly-α,β-Unsaturated Aldehydes Derived from Castor Oil via ADMET Polymerization. Eur. J. Lipid Sci. Technol. 2011, 113, 31–38. [CrossRef]
- Mensah, M.B.; Awudza, J.A.M.; O’Brien, P. Castor Oil: A Suitable Green Source of Capping Agent for Nanoparticle Syntheses and Facile Surface Functionalization. R. Soc. Open Sci. 2018, 5. [CrossRef]
- Rajalakshmi, P.; Marie, J.M.; Maria Xavier, A.J. Castor Oil-Derived Monomer Ricinoleic Acid Based Biodegradable Unsaturated Polyesters. Polym. Degrad. Stab. 2019, 170, 109016. [CrossRef]
- Laurentino, L.S.; Medeiros, A.M.M.S.; Machado, F.; Costa, C.; Araújo, P.H.H.; Sayer, C. Synthesis of a Biobased Monomer Derived from Castor Oil and Copolymerization in Aqueous Medium. Chem. Eng. Res. Des. 2018, 137, 213–220. [CrossRef]
- Cardoso, P.B.; Machado, T.O.; Feuser, P.E.; Sayer, C.; Meier, M.A.R.; Araújo, P.H.H. Biocompatible Polymeric Nanoparticles From Castor Oil Derivatives via Thiol-Ene Miniemulsion Polymerization. Eur. J. Lipid Sci. Technol. 2018, 120, 1–8. [CrossRef]
- Machado, T.O.; Cardoso, P.B.; Feuser, P.E.; Sayer, C.; Araújo, P.H.H. Thiol-Ene Miniemulsion Polymerization of a Biobased Monomer for Biomedical Applications. Colloids Surfaces B Biointerfaces 2017, 159, 509–517. [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric Nanoparticles: Promising Platform for Drug Delivery. Int. J. Pharm. 2017, 528, 675–691. [CrossRef]
- Chauhan, N.; Chawla, S.; Pundir, C.S.; Jain, U. An Electrochemical Sensor for Detection of Neurotransmitter-Acetylcholine Using Metal Nanoparticles, 2D Material and Conducting Polymer Modified Electrode. Biosens. Bioelectron. 2017, 89, 377–383. [CrossRef]
- Zhang, J.; Chen, H.; Zhou, T.; Wang, L.; Gao, D.; Zhang, X.; Liu, Y.; Wu, C.; Yuan, Z. A PIID-DTBT Based Semi-Conducting Polymer Dots with Broad and Strong Optical Absorption in the Visible-Light Region: Highly Effective Contrast Agents for Multiscale and Multi-Spectral Photoacoustic Imaging. Nano Res. 2017, 10, 64–76. [CrossRef]
- Klepac, D.; Kostková, H.; Petrova, S.; Chytil, P.; Etrych, T.; Kereïche, S.; Raška, I.; Weitz, D.A.; Filippov, S.K. Interaction of Spin-Labeled HPMA-Based Nanoparticles with Human Blood Plasma Proteins-the Introduction of Protein-Corona-Free Polymer Nanomedicine. Nanoscale 2018, 10, 6194–6204. [CrossRef]
- Talianov, P.; Fatkhutdinova, L.I.; Timin, A.S.; Milichko, V.A.; Zyuzin, M. V. Adaptive Nanoparticle-Polymer Complexes as Optical Elements: Design and Application in Nanophotonics and Nanomedicine. Laser Photonics Rev. 2021, 15, 1–28. [CrossRef]
- Khan, M.M.; Madni, A.; Filipczak, N.; Pan, J.; Rehman, M.; Rai, N.; Attia, S.A.; Torchilin, V.P. Folate Targeted Lipid Chitosan Hybrid Nanoparticles for Enhanced Anti-Tumor Efficacy. Nanomedicine Nanotechnology, Biol. Med. 2020, 28, 102228. [CrossRef]
- Nagavarma, B.V.N.; Yadav, H.K.S.; Ayaz, A.; Vasudha, L.S.; Shivakumar, H.G. Different Techniques for Preparation of Polymeric Nanoparticles- A Review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23.
- Santos, P.C.M. dos; Feuser, P.E.; Cardoso, P.B.; Steiner, B.T.; Córneo, E. da S.; Scussel, R.; Viegas, A. da C.; Machado-de-Ávila, R.A.; Sayer, C.; Araújo, P.H.H. de Evaluation of in Vitro Cytotoxicity of Superparamagnetic Poly(Thioether-Ester) Nanoparticles on Erythrocytes, Non-Tumor (NIH3T3), Tumor (HeLa) Cells and Hyperthermia Studies. J. Biomater. Sci. Polym. Ed. 2019. [CrossRef]
- Zhang, Z.; Grijpma, D.W.; Feijen, J. Poly(Trimethylene Carbonate) and Monomethoxy Poly(Ethylene Glycol)-Block-Poly(Trimethylene Carbonate) Nanoparticles for the Controlled Release of Dexamethasone. J. Control. Release 2006, 111, 263–270. [CrossRef]
- Sheikh, F.A.; Barakat, N.A.M.; Kanjwal, M.A.; Aryal, S.; Khil, M.S.; Kim, H.Y. Novel Self-Assembled Amphiphilic Poly(ε-Caprolactone)-Grafted- Poly(Vinyl Alcohol) Nanoparticles: Hydrophobic and Hydrophilic Drugs Carrier Nanoparticles. J. Mater. Sci. Mater. Med. 2009, 20, 821–831. [CrossRef]
- Mishima, K. Biodegradable Particle Formation for Drug and Gene Delivery Using Supercritical Fluid and Dense Gas. Adv. Drug Deliv. Rev. 2008, 60, 411–432. [CrossRef]
- Ahlin Grabnar, P.; Kristl, J. The Manufacturing Techniques of Drug-Loaded Polymeric Nanoparticles from Preformed Polymers. J. Microencapsul. 2011, 28, 323–335. [CrossRef]
- Gurny R, Peppas NA, Harrington DD, B.G. Development of Biodegradable and Injectable Latices for Controlled Release of Potent Drugs. Drug Dev Ind Pharm 1981, 7, 1–25. [CrossRef]
- Masood, F. Polymeric Nanoparticles for Targeted Drug Delivery System for Cancer Therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [CrossRef]
- Quintanar-Guerrero, D.; Allémann, E.; Fessi, H.; Doelker, E. Preparation Techniques and Mechanisms of Formation of Biodegradable Nanoparticles from Preformed Polymers. Drug Dev. Ind. Pharm. 1998, 24, 1113–1128. [CrossRef]
- Bagherzadeh-Khajehmarjan, E.; Nikniazi, A.; Olyaeefar, B.; Ahmadi-kandjani, S.; Nunzi, J.-M. Morphology Enhancement of Self-Assembled CH3NH3PbI3 Nanoparticles through Customized Solvent Evaporation Temperatures. J. Cryst. Growth 2023, 601, 126970. [CrossRef]
- Ma, W.; Lopez, G.; Ameduri, B.; Takahara, A. Fluoropolymer Nanoparticles Prepared Using Trifluoropropene Telomer Based Fluorosurfactants. Langmuir 2020, 36, 1754–1760. [CrossRef]
- Niyom, Y.; Crespy, D.; Flood, A.E. Compatibility between Drugs and Polymer in Nanoparticles Produced by the Miniemulsion-Solvent Evaporation Technique. Macromol. Mater. Eng. 2021, 306, 1–7. [CrossRef]
- Antonietti, M.; Landfester, K. Polyreactions in Miniemulsions. Prog. Polym. Sci. 2002, 27, 689–757. [CrossRef]
- McClements, D.J. Nanoemulsions versus Microemulsions: Terminology, Differences, and Similarities. Soft Matter 2012, 8, 1719–1729. [CrossRef]
- UGELSTAD, J.; EL-AASSER, MS.; VANDERHOFF, J. Emulsion Polymeriza-Tion: Initiation of Polymerization in Monomer Droplets. J Polym SciPolym Lett 1973, 11, 503–2013.
- Asua, J.M. Miniemulsion Polymerisation. Prog. Polym. Sci 2002, 27, 1283–1346. [CrossRef]
- Fonseca, L.B.; Nele, M.; Volpato, N.M.; Seiceira, R.C.; Pinto, J.C. Production of PMMA Nanoparticles Loaded with Praziquantel Through “In Situ” Miniemulsion Polymerization. Macromol. React. Eng. 2013, 7, 54–63. [CrossRef]
- Landfester, K. Synthesis of Colloidal Particles in Miniemulsions. Annu. Rev. Mater. Res. 2006, 36, 231–279. [CrossRef]
- Schork, F.J.; Poehlein, G.W.; Wang, S.; Reimers, J.; Rodrigues, J.; Samer, C. Miniemulsion Polymerization. Colloids Surfaces A Physicochem. Eng. Asp. 1999, 153, 39–45. [CrossRef]
- Machado, T.O.; Sayer, C.; Araujo, P.H.H. Thiol-Ene Polymerisation: A Promising Technique to Obtain Novel Biomaterials. Eur. Polym. J. 2017, 86, 200–215. [CrossRef]
- Türünç, O.; Meier, M.A.R. A Novel Polymerization Approach via Thiol-Yne Addition. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1689–1695. [CrossRef]
- Lowe, A.B. Thiol-Ene “Click” Reactions and Recent Applications in Polymer and Materials Synthesis. Polym. Chem. 2010, 1, 17–36. [CrossRef]
- Lluch, C.; Ronda, J.C.; Galiá, M.; Lligadas, G.; Cádiz, V. Rapid Approach to Biobased Telechelics through Two One-Pot Thiol-Ene Click Reactions. Biomacromolecules 2010, 11, 1646–1653. [CrossRef]
- Hu, Y.; Deng, M.; Yang, H.; Chen, L.; Xiao, C.; Zhuang, X.; Chen, X. Multi-Responsive Core-Crosslinked Poly (Thiolether Ester) Micelles for Smart Drug Delivery. Polymer (Guildf). 2017, 110, 235–241. [CrossRef]
- Chen, C.K.; Law, W.C.; Aalinkeel, R.; Yu, Y.; Nair, B.; Wu, J.; Mahajan, S.; Reynolds, J.L.; Li, Y.; Lai, C.K.; et al. Biodegradable Cationic Polymeric Nanocapsules for Overcoming Multidrug Resistance and Enabling Drug-Gene Co-Delivery to Cancer Cells. Nanoscale 2014, 6, 1567–1572. [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-Ene Click Chemistry. Angew. Chemie - Int. Ed. 2010, 49, 1540–1573. [CrossRef]
- Vandenbergh, J.; Peeters, M.; Kretschmer, T.; Wagner, P.; Junkers, T. Cross-Linked Degradable Poly(β-Thioester) Networks via Amine-Catalyzed Thiol-Ene Click Polymerization. Polymer (Guildf). 2014, 55, 3525–3532. [CrossRef]
- Vandenbergh, J.; Ranieri, K.; Junkers, T. Synthesis of (Bio)-Degradable Poly( β-Thioester)s via Amine Catalyzed Thiol-Ene Click Polymerization. Macromol. Chem. Phys. 2012, 213, 2611–2617. [CrossRef]
- Vivek, R.; Thangam, R.; Nipunbabu, V.; Rejeeth, C.; Sivasubramanian, S.; Gunasekaran, P.; Muthuchelian, K.; Kannan, S. Multifunctional HER2-Antibody Conjugated Polymeric Nanocarrier-Based Drug Delivery System for Multi-Drug-Resistant Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2014, 6, 6469–6480. [CrossRef]
- F, M.P.C.T.Y. Encapsulation of Ellipticine in Poly- ( 3-Hydroxybutyrate-Co-3- Hydroxyvalerate ) Based Nanoparticles and Its in Vitro Application. Mater. Sci. Eng. 2013, 33, 1054–106. [CrossRef]
- Masood, F. et al. Synthesis of Poly-(3-Hydroxybutyrate-Co-12 Mol % 3-Hydroxyvalerate) by Bacillus Cereus FB11: Its Characterization and Application as a Drug Carrier. J. Mater. Sci. Mater. Med. 2013, 24, 1927–37. [CrossRef]
- Shah, M., Ullah, N., Choi, M. H., Kim, M. O. & Yoon, S.C. Amorphous Amphiphilic P(3HV-Co-4HB)-b-MPEG Block Copolymer Synthesized from Bacterial Copolyester via Melt Transesterification: Nanoparticle Preparation, Cisplatin-Loading for Cancer Therapy and in Vitro Evaluation. Eur. J. Pharm. Biopharm. 2012, 80, 518–527. [CrossRef]
- Shah, M., Imran, M., Hwan, M., Ok, M. & Chul, S. Amphiphilic PHA – MPEG Copolymeric Nanocontainers for Drug Delivery: Preparation , Characterization and in Vitro Evaluation. Int. J. Pharm. 2010, 400, 165–175. [CrossRef]
- Vilos, C. et al. Paclitaxel-PHBV Nanoparticles and Their Toxicity to Endometrial and Primary Ovarian Cancer Cells. Biomaterials 2013, 34, 4098–4108. [CrossRef]
- Lu, X. Y., Zhang, Y. & Wang, L. Preparation and in Vitro Drug-Release Behavior of 5- Fluorouracil-Loaded Poly(Hydroxybutyrateco-Hydroxyhexanoate) Nanoparticles and Microparticles. J. Appl. Polym. Sci. 2010, 116, 2944–2950. [CrossRef]
- Kılıçay, E. et al. Preparation and Characterization of Poly(3-Hydroxybutyrate-Co-3- Hydroxyhexanoate) (PHBHHX) Based Nanoparticles for Targeted Cancer Therapy. Eur. J. Pharm. Sci. 2011, 44, 310–20. [CrossRef]
- Chan, Z.; Zhao, L.; Dong, Y.; Zhang, X.; Lin, J.; Chen, Z. Folate-Mediated Poly(3-Hydroxybutyrate-Co-3-Hydroxyoctanoate) Nanoparticles for Targeting Drug Delivery. Eur. J. Pharm. Biopharm. 2010, 76, 10–16. [CrossRef]
- Yao, Y. et al. A Specific Drug Targeting System Based on Polyhydroxyalkanoate Granule Binding Protein PhaP Fused with Targeted Cell Ligands. Biomaterials 2008, 29, 4823–4830. [CrossRef]
- Varan, C. & Bilensoy, E. Development of Implantable Hydroxypropyl-β-Cyclodextrin Coated Polycaprolactone Nanoparticles for the Controlled Delivery of Docetaxel to Solid Tumors. J. Incl. Phenom. Macrocycl. Chem. 2014, 1–7. [CrossRef]
- Cirpanli, Y., Bilensoy, E., Lale Doğan, a & Caliş, S. Comparative Evaluation of Polymeric and Amphiphilic Cyclodextrin Nanoparticles for Effective Camptothecin Delivery. Eur. J. Pharm. Biopharm. 2009, 73, 82–9. [CrossRef]
- Perret, F., Duffour, M., Chevalier, Y. & Parrot-Lopez, H. Design, Synthesis, and in Vitro Evaluation of New Amphiphilic Cyclodextrin-Based Nanoparticles for the Incorporation and Controlled Release of Acyclovir. Eur. J. Pharm. Biopharm 2013, 83, 25–32. [CrossRef]
- Miao, Q. et al. Construction of Hydroxypropyl-β-Cyclodextrin Copolymer Nanoparticles and Targeting Delivery of Paclitaxel. J. Nanoparticle Res 2012, 14, 1043. [CrossRef]
- Freire, N.F.; Feuser, P.E.; da Silva Abel, J.; Machado-de-Ávila, R.A.; Lopes Fialho, R.; Cabral Albuquerque, E.; Sayer, C.; Hermes de Araújo, P.H. Zinc Phthalocyanine Encapsulation via Thiol-Ene Miniemulsion Polymerization and in Vitro Photoxicity Studies. Int. J. Polym. Mater. Polym. Biomater. 2020, 0, 1–10. [CrossRef]
- Freire, N.; Emílio, P.; Maria, E.; Ambel, T.; Cordani, M.; Zielinski, A.F.; Sayer, C.; Pieri, E. De; Avila, R.A.M.-; Henrique, P.; et al. Colloids and Surfaces A : Physicochemical and Engineering Aspects Preparation and Characterization of Full-Spectrum Cannabis Extract Loaded Poly ( Thioether-Ester ) Nanoparticles : In Vitro Evaluation of Their Antitumoral Efficacy. 2023, 658. [CrossRef]
- Feuser, Paulo Emílio ; Matos dos Santos, Paula Christina ; Cordeiro, Arthur Poester ; Stefanes, Natália Marceli ; Walter, Laura Otto ; Maioral, Mariana Franzoni ; Santos-Silva, Maria Cláudia ; Hermes de Araújo, Pedro Henrique ; Sayer, C. Antineoplastic Activity of Free 4-Nitrochalcone and Encapsulated in Poly(Thioether-Ester) Nanoparticles Obtained by Thiol-Ene Polymerization in Two Human Leukemia Cell Lines (Jurkat and K562). J. Drug Deliv. Sci. Technol. 2022, 67, 102924. [CrossRef]
- Danhier, F.; Lecouturier, N.; Vroman, B.; Jerome, C.; Marchand-Brynaert, J.; Feron, O.; Préat, V. Paclitaxel-Loaded PEGylated PLGA-Based Nanoparticles: In Vitro and in Vivo Evaluation. J. Control. Release 2009, 133, 11–17. [CrossRef]
- Khuroo, T. et al. Topotecan–Tamoxifen Duple PLGA Polymeric Nanoparticles: Investigation of in Vitro, in Vivo and Cellular Uptake Potential. Int. J. Pharm. 2014, 473, 384–394. [CrossRef]
- Cháirez-Ramírez, M.H. et al. Morphological and Release Characterization of Nanoparticles Formulated with Poly (Dl-Lactide-Co-Glycolide) (PLGA) and Lupeol: In Vitro Permeability and Modulator Effect on NF-ΚB in Caco-2 Cell System Stimulated with TNF-α. Food Chem. Toxicol. 2015. [CrossRef]
- Jaidev, L. R., Krishnan, U. M. & Sethuraman, S. Gemcitabine Loaded Biodegradable PLGA Nanospheres for in Vitro Pancreatic Cancer Therapy. Mater. Sci. Eng. C. Mater. Biol. Appl. 2015, 47, 40–7. [CrossRef]
- Derakhshandeh, K., Erfan, M. & Dadashzadeh, S. Encapsulation of 9-Nitrocamptothecin, a Novel Anticancer Drug, in Biodegradable Nanoparticles: Factorial Design, Characterization and Release Kinetics. Eur. J. Pharm. Biopharm. 2007, 66, 34–41. [CrossRef]
- Wang, H. et al. Enhanced Anti-Tumor Efficacy by Co-Delivery of Doxorubicin and Paclitaxel with Amphiphilic Methoxy PEG-PLGA Copolymer Nanoparticles. Biomaterials 2011, 32, 8281– 90. [CrossRef]
- Le Broc-Ryckewaert, D. et al. Development of Innovative Paclitaxel-Loaded Small PLGA Nanoparticles: Study of Their Antiproliferative Activity and Their Molecular Interactions on Prostatic Cancer Cells. Int. J. Pharm. 2013, 454, 712–9. [CrossRef]
- Mattheolabakis, G., Taoufik, E., Haralambous, S., Roberts, M. L. & Avgoustakis, K. In Vivo Investigation of Tolerance and Antitumor Activity of Cisplatin-Loaded PLGA-MPEG Nanoparticles. Eur. J. Pharm. Biopharm. 2009, 71, 190–5. [CrossRef]
- Schleich, N. et al. Dual Anticancer Drug/Superparamagnetic Iron Oxide-Loaded PLGAbased Nanoparticles for Cancer Therapy and Magnetic Resonance Imaging. Int. J. Pharm. 2013, 447, 94–101. [CrossRef]
- Jain, A. K., Thanki, K. & Jain, S. Co-Encapsulation of Tamoxifen and Quercetin in Polymeric Nanoparticles: Implications on Oral Bioavailability, Antitumor Efficacy, and Drug-Induced Toxicity. Mol. Pharm. 2013, 10, 3459–74. [CrossRef]
- Chan, J.M. et al. PLGA-Lecithin-PEG Core-Shell Nanoparticles for Controlled Drug Delivery. Biomaterials 2009, 30, 1627–1634. [CrossRef]
- Martín-Banderas, L.; Muñoz-Rubio, I.; Prados, J.; Álvarez-Fuentes, J.; Calderón-Montaño, J.M.; López-Lázaro, M.; Arias, J.L.; Leiva, M.C.; Holgado, M.A.; Fernández-Arévalo, M. In Vitro and in Vivo Evaluation of Delta9-Tetrahidrocannabinol/PLGA Nanoparticles for Cancer Chemotherapy. Int. J. Pharm. 2015, 487, 205–212. [CrossRef]
- Chittasupho, C. et al. ICAM-1 Targeting of Doxorubicin-Loaded PLGA Nanoparticles to Lung Epithelial Cells. Eur. J. Pharm. Sci. 2009, 37, 141–150. [CrossRef]
- Liang, C. et al. Improved Therapeutic Effect of Folate-Decorated PLGA-PEG Nanoparticles for Endometrial Carcinoma. Bioorganic Med. Chem. 2011, 19, 4057–4066. [CrossRef]
- Dhas, N. L., Ige, P. P. & Kudarha, R.R. Design, Optimization and in-Vitro Study of Folic Acid Conjugated-Chitosan Functionalized PLGA Nanoparticle for Delivery of Bicalutamide in Prostate Cancer. Powder Technol. 2015, 283, 234–245. [CrossRef]
- Su, W.-C., Su, W.-P., Cheng, S.& Y. PLGA Nanoparticles Codeliver Paclitaxel and Stat3 SiRNA to Overcome Cellular Resistance in Lung Cancer Cells. Int. J. Nanomedicine 2012. [CrossRef]
- Cui, Y., Xu, Q., Chow, P. K.-H., Wang, D. & Wang, C.-H. Transferrin-Conjugated Magnetic Silica PLGA Nanoparticles Loaded with Doxorubicin and Paclitaxel for Brain Glioma Treatment. Biomaterials 2013, 34, 8511–20. [CrossRef]
- Jain, A. et al. Surface Engineered Polymeric Nanocarriers Mediate the Delivery of Transferrin-Methotrexate Conjugates for an Improved Understanding of Brain Cancer. Acta Biomater. 2015, 24, 140–51. [CrossRef]
- Dhar, S., Gu, F. X., Langer, R., Farokhzad, O. C. & Lippard, S.J. Targeted Delivery of Cisplatin to Prostate Cancer Cells by Aptamer Functionalized Pt(IV) Prodrug-PLGA-PEG Nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 7356–17361. [CrossRef]
- Chittasupho, C., Lirdprapamongkol, K., Kewsuwan, P. & Sarisuta, N. Targeted Delivery of Doxorubicin to A549 Lung Cancer Cells by CXCR4 Antagonist Conjugated PLGA Nanoparticles. Eur. J. Pharm. Biopharm. 2014. [CrossRef]
- Danhier, F. et al. Targeting of Tumor Endothelium by RGD-Grafted PLGA-Nanoparticles. Methods Enzym. 2012, 508, 157–175. [CrossRef]
- Li, L. et al. Epithelial Cell Adhesion Molecule Aptamer Functionalized PLGA-Lecithincurcumin- PEG Nanoparticles for Targeted Drug Delivery to Human Colorectal Adenocarcinoma Cells. Int. J. Nanomedicine 2014, 9, 1083–96. [CrossRef]
- Chen, H. et al. Preparation and Characterization of PE38KDEL-Loaded Anti-HER2 Nanoparticles for Targeted Cancer Therapy. J. Control. Release 2008, 128, 209–216. [CrossRef]
- Aravind, A. et al. Aptamer Conjugated Paclitaxel and Magnetic Fluid Loaded Fluorescently Tagged PLGA Nanoparticles for Targeted Cancer Therapy. J. Magn. Magn. Mater. 2013, 344, 116– 123. [CrossRef]
- Aggarwal, S., Yadav, S. & Gupta, S. EGFR Targeted PLGA Nanoparticles Using Gemcitabine for Treatment of Pancreatic Cancer. J. Biomed. Nanotechnol. 2011, 7, 137–138. [CrossRef]
- Narayanan, S. et al. Sequential Release of Epigallocatechin Gallate and Paclitaxel from PLGA-Casein Core/Shell Nanoparticles Sensitizes Drug-Resistant Breast Cancer Cells. Nanomedicine Nanotechnology, Biol. Med. 2015, 11, 1399–1406. [CrossRef]
- Wei, K., Peng, X. & Zou, F. Folate-Decorated PEG-PLGA Nanoparticles with Silica Shells for Capecitabine Controlled and Targeted Delivery. Int. J. Pharm. 2014, 464, 225–233. [CrossRef]
- VANGARA, K. K., LIU, J. L. & PALAKURTHI, S. Hyaluronic Acid-Decorated PLGAPEG Nanoparticles for Targeted Delivery of SN-38 to Ovarian Cancer. Anticancer Res. 2013, 33, 2425–2434.
- Kocbek, P., Obermajer, N., Cegnar, M., Kos, J. & Kristl, J. Targeting Cancer Cells Using PLGA Nanoparticles Surface Modified with Monoclonal Antibody. J. Control. Release 2007, 120, 18–26. [CrossRef]
- Bhartiya, P.; Chawla, R.; Dutta, P.K. PH-Responsive Charge-Convertible N-Succinyl Chitosan-Quercetin Coordination Polymer Nanoparticles for Effective NIR Photothermal Cancer Therapy. Macromol. Chem. Phys. 2022, 223, 1–12. [CrossRef]
- Gogoi, P.; Dutta, A.; Ramteke, A.; Maji, T.K. Preparation, Characterization and Cytotoxic Applications of Curcumin-(±) α-Lipoic Acid Coloaded Phosphorylated Chitosan Nanoparticles in MDA MB 231 Breast Cancer Cell Line. Polym. Adv. Technol. 2020, 31, 2827–2841. [CrossRef]
- Snima, K.S.; Jayakumar, R.; Lakshmanan, V.K. In Vitro and in Vivo Biological Evaluation of O-Carboxymethyl Chitosan Encapsulated Metformin Nanoparticles for Pancreatic Cancer Therapy. Pharm. Res. 2014, 31, 3361–3370. [CrossRef]
- Ding, Y.F.; Li, S.; Liang, L.; Huang, Q.; Yuwen, L.; Yang, W.; Wang, R.; Wang, L.H. Highly Biocompatible Chlorin E6-Loaded Chitosan Nanoparticles for Improved Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 9980–9987. [CrossRef]
- Kou, C.H.; Han, J.; Han, X.L.; Zhuang, H.J.; Zhao, Z.M. Preparation and Characterization of the Adriamycin-Loaded Amphiphilic Chitosan Nanoparticles and Their Application in the Treatment of Liver Cancer. Oncol. Lett. 2017, 14, 7833–7841. [CrossRef]
- Ma, Y.; Zheng, Y.; Zeng, X.; Jiang, L.; Chen, H.; Liu, R.; Huang, L.; Mei, L. Novel Docetaxel-Loaded Nanoparticles Based on PCL-Tween 80 Copolymer for Cancer Treatment. Int. J. Nanomedicine 2011, 6, 2679–2688. [CrossRef]
- Chen, L.X.; Ni, X.L.; Zhang, H.; Wu, M.; Liu, J.; Xu, S.; Yang, L.L.; Fu, S.Z.; Wu, J. Preparation, Characterization, in Vitro and in Vivo Anti-Tumor Effect of Thalidomide Nanoparticles on Lung Cancer. Int. J. Nanomedicine 2018, 13, 2463–2476. [CrossRef]
- Raspantini, G.L.; Luiz, M.T.; Abriata, J.P.; Eloy, J. de O.; Vaidergorn, M.M.; Emery, F. da S.; Marchetti, J.M. PCL-TPGS Polymeric Nanoparticles for Docetaxel Delivery to Prostate Cancer: Development, Physicochemical and Biological Characterization. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 627, 127144. [CrossRef]
- Lu, Y.; Wen, Q.; Luo, J.; Xiong, K.; Wu, Z.X.; Wang, B.Q.; Chen, Y.; Yang, B.; Fu, S.Z. Self-Assembled Dihydroartemisinin Nanoparticles as a Platform for Cervical Cancer Chemotherapy. Drug Deliv. 2020, 27, 876–887. [CrossRef]
- Liu, X.; Li, J.; Huang, L.; Yang, J.; Wang, Y.; Yang, M.; Tang, M.; Qiu, T. Preparation and Evaluation of MPEG-PCL Polymeric Nanoparticles Against Gastric Cancer. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 1162–1168. [CrossRef]
- Xiong, K.; Zhang, Y.; Wen, Q.; Luo, J.; Lu, Y.; Wu, Z.X.; Wang, B.Q.; Chen, Y.; Zhao, L.; Fu, S.Z. Co-Delivery of Paclitaxel and Curcumin by Biodegradable Polymeric Nanoparticles for Breast Cancer Chemotherapy. Int. J. Pharm. 2020, 589, 119875. [CrossRef]
- Rao, S. Venkateswara ; Kumar, S.S. MPEG-PCL Nanoparticles as New Carriers for Delivery of a Prostae Cancer Drug Fluamide. Res. J. Pharm. Technol. 2021, 14, 3657–3661. [CrossRef]
- Badran, M.M.; Mady, M.M.; Ghannam, M.M.; Shakeel, F. Preparation and Characterization of Polymeric Nanoparticles Surface Modified with Chitosan for Target Treatment of Colorectal Cancer. Int. J. Biol. Macromol. 2017, 95, 643–649. [CrossRef]
- Patel, P.; Raval, M.; Manvar, A.; Airao, V.; Bhatt, V.; Shah, P. Lung Cancer Targeting Efficiency of Silibinin Loaded Poly Caprolactone /Pluronic F68 Inhalable Nanoparticles: In Vitro and In Vivo Study; 2022; Vol. 17; ISBN 1111111111.
- Li, M.; Tang, Z.; Lin, J.; Zhang, Y.; Lv, S.; Song, W.; Huang, Y.; Chen, X. Synergistic Antitumor Effects of Doxorubicin-Loaded Carboxymethyl Cellulose Nanoparticle in Combination with Endostar for Effective Treatment of Non-Small-Cell Lung Cancer. Adv. Healthc. Mater. 2014, 3, 1877–1888. [CrossRef]
- Yusefi, M.; Lee-Kiun, M.S.; Shameli, K.; Teow, S.Y.; Ali, R.R.; Siew, K.K.; Chan, H.Y.; Wong, M.M.T.; Lim, W.L.; Kuča, K. 5-Fluorouracil Loaded Magnetic Cellulose Bionanocomposites for Potential Colorectal Cancer Treatment. Carbohydr. Polym. 2021, 273. [CrossRef]
- Asabuwa Ngwabebhoh, F.; Ilkar Erdagi, S.; Yildiz, U. Pickering Emulsions Stabilized Nanocellulosic-Based Nanoparticles for Coumarin and Curcumin Nanoencapsulations: In Vitro Release, Anticancer and Antimicrobial Activities. Carbohydr. Polym. 2018, 201, 317–328. [CrossRef]
- Han, L.; Ren, Y.; Long, L.; Zhong, Y.; Shen, C.; Pu, P.; Yuan, X.; Kang, C. Inhibition of C6 Glioma in Vivo by Combination Chemotherapy of Implantation of Polymer Wafer and Intracarotid Perfusion of Transferrin-Decorated Nanoparticles. Oncol. Rep. 2012, 27, 121–128. [CrossRef]
- Feuser, P.E.; Bubniak, L.D.S.; Bodack, C.D.N.; Valério, A.; Silva, M.C.D.S.; Ricci, E.; Sayer, C.; De Araújo, P.H.H. In Vitro Cytotoxicity of Poly(Methyl Methacrylate) Nanoparticles and Nanocapsules Obtained by Miniemulsion Polymerization for Drug Delivery Application. J. Nanosci. Nanotechnol. 2016, 16, 7669–7676. [CrossRef]
- World Health Organization Definition of Active Pharmaceutical Ingredient. World Heal. Organ. 2011, 1–4.
- Montané, X.; Bajek, A.; Roszkowski, K.; Montornés, J.M.; Giamberini, M.; Roszkowski, S.; Kowalczyk, O.; Garcia-Valls, R.; Tylkowski, B. Encapsulation for Cancer Therapy. Molecules 2020, 25, 1–25. [CrossRef]
- Kifle, Z.D.; Tadele, M.; Alemu, E.; Gedamu, T.; Ayele, A.G. A Recent Development of New Therapeutic Agents and Novel Drug Targets for Cancer Treatment. SAGE Open Med. 2021, 9, 205031212110670. [CrossRef]
- Wadhwa, K.; Kadian, V.; Puri, V.; Bhardwaj, B.Y.; Sharma, A.; Pahwa, R.; Rao, R.; Gupta, M.; Singh, I. New Insights into Quercetin Nanoformulations for Topical Delivery. Phytomedicine Plus 2022, 2, 100257. [CrossRef]
- Lawson, M.K. Improvement of Therapeutic Value of Quercetin with Chitosan Nanoparticle Delivery Systems and Potential Applications Quercetin Is a Member of a Group of Related Compounds Kno Secondary Metabolite in Plants . It Is Believed , along with Caroten Properties . 2023.
- Kumar, D.; Gautam, A.; Kundu, P.P. Synthesis of PH-Sensitive Grafted Psyllium: Encapsulation of Quercetin for Colon Cancer Treatment. J. Appl. Polym. Sci. 2022, 139, 1–16. [CrossRef]
- Chen, L.C.; Chen, Y.C.; Su, C.Y.; Hong, C.S.; Ho, H.O.; Sheu, M.T. Development and Characterization of Self-Assembling Lecithin-Based Mixed Polymeric Micelles Containing Quercetin in Cancer Treatment and an in Vivo Pharmacokinetic Study. Int. J. Nanomedicine 2016, 11, 1557–1566. [CrossRef]
- Luis de Redín, I.; Expósito, F.; Agüeros, M.; Collantes, M.; Peñuelas, I.; Allemandi, D.; Llabot, J.M.; Calvo, A.; Irache, J.M. In Vivo Efficacy of Bevacizumab-Loaded Albumin Nanoparticles in the Treatment of Colorectal Cancer. Drug Deliv. Transl. Res. 2020, 10, 635–645. [CrossRef]
- Battaglia, L.; Gallarate, M.; Peira, E.; Chirio, D.; Solazzi, I.; Giordano, S.M.A.; Gigliotti, C.L.; Riganti, C.; Dianzani, C. Bevacizumab Loaded Solid Lipid Nanoparticles Prepared by the Coacervation Technique: Preliminary in Vitro Studies. Nanotechnology 2015, 26. [CrossRef]
- Sousa, F.; Dhaliwal, H.K.; Gattacceca, F.; Sarmento, B.; Amiji, M.M. Enhanced Anti-Angiogenic Effects of Bevacizumab in Glioblastoma Treatment upon Intranasal Administration in Polymeric Nanoparticles. J. Control. Release 2019, 309, 37–47. [CrossRef]
- Di Filippo, Leonardo Delello ; Lobato Duarte, Jonatas ; Hofstätter Azambuja, Juliana ; Isler Mancuso, Rubia ; Tavares Luiz, Marcela ; Hugo Sousa Araújo, Victor ; Delbone Figueiredo, Ingrid ; Barretto-de-Souza, Lucas ; Miguel Sábio, Rafael ; Sasso-CerriDi, M. Glioblastoma Multiforme Targeted Delivery of Docetaxel Using Bevacizumab-Modified Nanostructured Lipid Carriers Impair in Vitro Cell Growth and in Vivo Tumor Progression. Int. J. Pharm. 2022, 618, 121682–121682. [CrossRef]
- Siti, Z.S.; Ahmad, N.H.; Hamid, S. Characterization of PLGA-PEG Catharanthus Roseus Nanoparticles and Assessing Its Anticancer Effects in Her2-Overexpressed Breast Cancer Cells. Pharmacogn. Mag. 2022, 18, 273. [CrossRef]
- Ke, Y.; Al Aboody, M.S.; Alturaiki, W.; Alsagaby, S.A.; Alfaiz, F.A.; Veeraraghavan, V.P.; Mickymaray, S. Photosynthesized Gold Nanoparticles from Catharanthus Roseus Induces Caspase-Mediated Apoptosis in Cervical Cancer Cells (HeLa). Artif. Cells, Nanomedicine Biotechnol. 2019, 47, 1938–1946. [CrossRef]
- Azhar, Nur Asna ; Ghozali, Siti Zulaikha ; Abu Bakar, Siti Aishah ; Lim, Vuanghao ; Ahmad, N.H. Suppressing Growth, Migration, and Invasion of Human Hepatocellular Carcinoma HepG2 Cells by Catharanthus Roseus-silver Nanoparticles. Toxicol. Vitr. 2020, 67, 104910. [CrossRef]
- Liu, Y.; Zhang, H.; Cui, H.; Zhang, F.; Zhao, L.; Liu, Y.; Meng, Q. Combined and Targeted Drugs Delivery System for Colorectal Cancer Treatment: Conatumumab Decorated, Reactive Oxygen Species Sensitive Irinotecan Prodrug and Quercetin Co-Loaded Nanostructured Lipid Carriers. Drug Deliv. 2022, 29, 342–350. [CrossRef]
- Liu, X.; Jiang, J.; Chan, R.; Ji, Y.; Lu, J.; Liao, Y.P.; Okene, M.; Lin, J.; Lin, P.; Chang, C.H.; et al. Improved Efficacy and Reduced Toxicity Using a Custom-Designed Irinotecan-Delivering Silicasome for Orthotopic Colon Cancer. ACS Nano 2019, 13, 38–53. [CrossRef]
- Hong, J.; Feng, Z. Synergic Fabrication of Combination Therapy of Irinotecan and 5-Fluorouracil Encapsulated Polymeric Nanoparticles for the Treatment of Gastric Cancer Therapy. Process Biochem. 2021, 106, 191–198. [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I.; Cohen, M.; Delie, F.; Bastida-Ruiz, D.; Yart, L.; Martin-Sabroso, C.; Fernández-Carballido, A. PLGA Nanoparticles for the Intraperitoneal Administration of CBD in the Treatment of Ovarian Cancer: In Vitro and in Ovo Assessment. Pharmaceutics 2020, 12, 1–19. [CrossRef]
- De La Ossa, D.H.P.; Gil-Alegre, M.E.; Ligresti, A.; Aberturas, M.D.R.; Molpeceres, J.; Torres, A.I.; Di Marzo, V. Preparation and Characterization of Delta9- Tetrahydrocannabinol-Loaded Biodegradable Polymeric Microparticles and Their Antitumoral Efficacy on Cancer Cell Lines. J. Drug Target. 2013, 21, 710–718. [CrossRef]
- Tangutoori, Shifalika ; Korideck, Houari ; Makrigiorgos, Mike ; Cormack, Robert ; Sridhar, S. A Novel Nano-Formulation for Systemic Administration of PARPi-Olaparib (Nano-Olaparib) for Radiosensitization, Chemosensitization, and Combinatorial Therapy in Prostate Cancer. Mol. Cancer Ther. 2013, 12, A81–A81. [CrossRef]
- Zhang, Shentao ; Li, Erjing ; Liu, Zhao ; Shang, Haitao ; Chen, Yichi ; Jing, H. Anoparticle-Based Olaparib Delivery Enhances Its Effect, and Improves Drug Sensitivity to Cisplatin in Triple Negative Breast Cancer. J. Drug Deliv. Sci. Technol. 2022, 76, 103731. [CrossRef]
- Anwer, M.K.; Ali, E.A.; Iqbal, M.; Ahmed, M.M.; Aldawsari, M.F.; Saqr, A. Al; Alalaiwe, A.; Soliman, G.A. Development of Chitosan-Coated PLGA-Based Nanoparticles for Improved Oral Olaparib Delivery: In Vitro Characterization, and In Vivo Pharmacokinetic Studies. Processes 2022, 10. [CrossRef]
- Jeyaraj, M.; Rajesh, M.; Arun, R.; MubarakAli, D.; Sathishkumar, G.; Sivanandhan, G.; Dev, G.K.; Manickavasagam, M.; Premkumar, K.; Thajuddin, N.; et al. An Investigation on the Cytotoxicity and Caspase-Mediated Apoptotic Effect of Biologically Synthesized Silver Nanoparticles Using Podophyllum Hexandrum on Human Cervical Carcinoma Cells. Colloids Surfaces B Biointerfaces 2013, 102, 708–717. [CrossRef]
- Kumbhar, P.S.; Sakate, A.M.; Patil, O.B.; Manjappa, A.S.; Disouza, J.I. Podophyllotoxin-Polyacrylic Acid Conjugate Micelles: Improved Anticancer Efficacy against Multidrug-Resistant Breast Cancer. J. Egypt. Natl. Canc. Inst. 2020, 32, 1–8. [CrossRef]
- Li, Y.; Chen, M.; Yao, B.; Lu, X.; Zhang, X.; He, P.; Vasilatos, S.N.; Ren, X.; Bian, W.; Yao, C. Transferrin Receptor-Targeted Redox/PH-Sensitive Podophyllotoxin Prodrug Micelles for Multidrug-Resistant Breast Cancer Therapy. J. Mater. Chem. B 2019, 7, 5814–5824. [CrossRef]
- Zhang, P.; Tang, M.; Huang, Q.; Zhao, G.; Huang, N.; Zhang, X.; Tan, Y.; Cheng, Y. Combination of 3-Methyladenine Therapy and Asn-Gly-Arg (NGR)-Modified Mesoporous Silica Nanoparticles Loaded with Temozolomide for Glioma Therapy in Vitro. Biochem. Biophys. Res. Commun. 2019, 509, 549–556. [CrossRef]
- Fang, C.; Wang, K.; Stephen, Z.R.; Mu, Q.; Kievit, F.M.; Chiu, D.T.; Press, O.W.; Zhang, M. Temozolomide Nanoparticles for Targeted Glioblastoma Therapy. ACS Appl. Mater. Interfaces 2015, 7, 6674–6682. [CrossRef]
- Li, Kaidi ; Liang, Naixin ; Yang, Huaxia ; Liu, Hongsheng ; Li, S. Temozolomide Encapsulated and Folic Acid Decorated Chitosan Nanoparticles for Lung Tumor Targeting: Improving Therapeutic Efficacy Both in Vitro and in Vivo. Oncotarget 2017, 8, 111318–111332.
- Almajidi, Y.Q.; Maraie, N.K.; Raauf, A.M.R. Modified Solid in Oil Nanodispersion Containing Vemurafenib-Lipid Complex-in Vitro/in Vivo Study. F1000Research 2022, 11, 1–22. [CrossRef]
- Fu, Y.; Saraswat, A.; Wei, Z.; Agrawal, M.Y.; Dukhande, V. V.; Reznik, S.E.; Patel, K. Development of Dual Arv-825 and Nintedanib-Loaded Pegylated Nano-Liposomes for Synergistic Efficacy in Vemurafnib-Resistant Melanoma. Pharmaceutics 2021, 13. [CrossRef]
- Xia, L.; Kong, X.; Liu, X.; Tu, L.; Zhang, Y.; Chang, Y.; Liu, K.; Shen, D.; Zhao, H.; Zhang, H. An Upconversion Nanoparticle - Zinc Phthalocyanine Based Nanophotosensitizer for Photodynamic Therapy. Biomaterials 2014, 35, 4146–4156. [CrossRef]
- Yurt, F.; Ocakoglu, K.; Ince, M.; Colak, S.G.; Er, O.; Soylu, H.M.; Gunduz, C.; Biray Avci, C.; Caliskan Kurt, C. Photodynamic Therapy and Nuclear Imaging Activities of Zinc Phthalocyanine-Integrated TiO 2 Nanoparticles in Breast and Cervical Tumors. Chem. Biol. Drug Des. 2018, 91, 789–796. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





