1. Introduction
Iron deficiency (ID) is a common micronutrient deficiency, affecting approximately 30% of the world's population [
1]. Iron deficiency anemia (IDA) occurs when iron metabolism is disrupted and, therefore, cannot fulfill necessary physiological needs or participate in essential biological processes [
1,
2]. Hepcidin, a peptide hormone produced in the liver, is of growing interest in research because it is the key regulator of iron homeostasis [
3,
4]. It controls iron release into blood based on the body’s needs and is modulated mainly by body iron stores, erythropoiesis, and inflammation [
5,
6]. Elevated hepcidin levels are linked to reduced intestinal absorption and iron release from the tissues and macrophages, resulting in low circulating serum iron [
7]. People with true IDA without underlying conditions will exhibit reduced hepcidin concentrations to facilitate iron release into the blood for hemoglobin synthesis. Inflammation and infection can increase hepcidin synthesis, accrued body iron stores, and reduce plasma iron pool [
8,
9,
10,
11,
12,
13].
Many diet components have been shown to reduce inflammation, including foods rich in lycopene and polyphenols [
14]. Green tea, abundant in polyphenols, is a popular and widely accessible beverage consumed by much of the general population. Polyphenols are bioactive secondary plant metabolites in fruits and vegetables that contribute to their color, flavor, and pharmacological activities [
15]. Those present in green tea are called catechins, including epicatechin, epicatechin-3-gallate, epigallocatechin, and epigallocatechin-3-gallate (EGCG). EGCG is the major catechin in tea, accounting for 50-70% of catechins in green tea, is well-researched for its health benefits [
16]. It is best known for its antioxidant and anti-inflammatory properties [
17]. As an antioxidant, EGCG has also been shown to increase cell viability by decreasing reactive oxygen species [
18,
19,
20]. Importantly, Kim
et al. [
21] found that EGCG was effective in preventing IL-8 production, which in turn, reduced the degree of inflammatory response. However, no studies to date have reported improved iron status by reducing inflammation. Given its anti-inflammatory properties, we hypothesized that EGCG would reduce inflammation and thus improve iron status. In the present study, we used lipopolysaccharide (LPS) to induced animal model [
22] to study the relationship between obesity-induced inflammation and iron status.
Although the relationship between inflammation and iron status is clear, only a few studies examined these conditions simultaneously. Understanding the relationship betwasween chronic inflammation and iron status is essential when managing global health issues such as IDA and obesity. Thus, the objectives of this study were to (1) determine if LPS-induced inflammation will affect iron status and (2) if EGCG supplementation will suppress LPS-induced inflammation to maintain iron status.
2. Materials and Methods
2.1. Animals Diets and Study Design
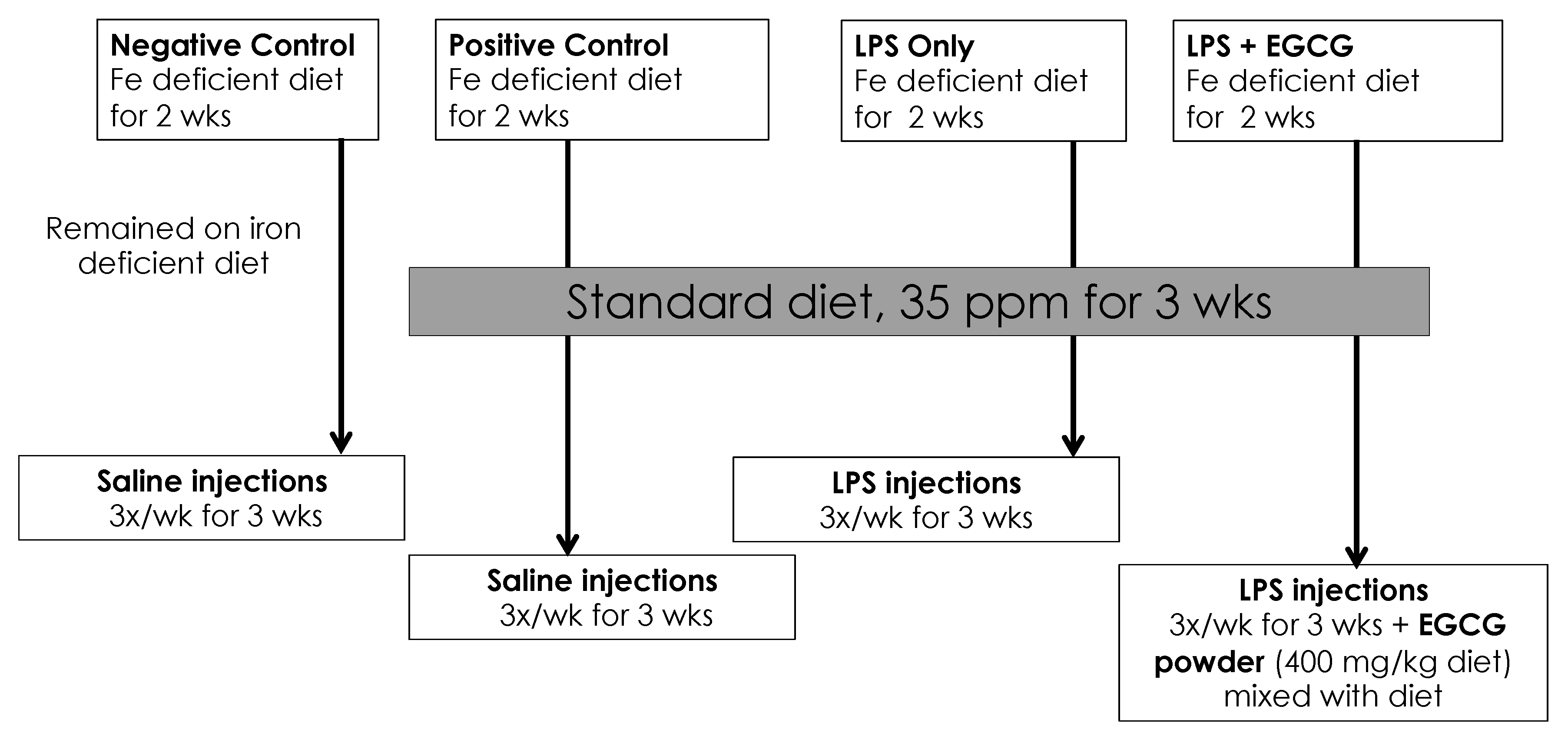
Our animal study was approved by the Institutional Animal Care and Use Committee at Iowa State University and was performed according to the Iowa State University Laboratory Animal Resources Guidelines. Male Sprague–Dawley rats (n = 32) were obtained at 21 days of age (Charles River, Chicago, IL, USA). After 3 days of acclimation on a standard rat chow, rats were randomly assigned to one of four groups (n = 8): negative control, positive control, treatment group 1 (LPS only), and treatment group 2 (LPS + EGCG). All rats were placed on a powdered iron-deficient diet for two weeks at the start of the study: AIN-76A modified diet containing 2-6 ppm Fe, 20% casein; 0.3% DL-Methionine; 55% sucrose; 15% corn starch; 5% corn oil, 3.5% mineral mix (iron deficient); 1% vitamin mix; 0.2% choline bitartrate. After two weeks, the positive control and treatment groups were placed on a powdered iron repletion diet, while the negative control remained on the iron-deficient diet. The iron repletion diet contained 35 ppm/kg Fe added as FeSO4, 20% casein; 0.3% DL-Methionine; 55% sucrose; 15% corn starch; 5% corn oil, 3.5% mineral mix; 0.02% ferrous sulfate; 1% vitamin mix; 0.2% choline bitartrate. Both diets were purchased from Envigo -Teklnad (Indianapolis, IN, USA) and stored at 4°C until needed. The green tea extract powder contained 50% of total polyphenols as EGCG and was kindly provided commercial supplier. It was mixed thoroughly (600 mg EGCG/kg diet) with the iron-sufficient diet just before use. Rats were kept under controlled conditions with a daily 12 h light: dark cycle. Food and water were provided ad lib. The detailed study design is shown in Figure 1.
Figure 1.
Study Design. LPS: lipopolysaccharide; EGCG: epigallocatechin-3-gallate; LPS injections given three times a week for 3 wks intraperitoneally = 0.5mg/Kg body weight.
Figure 1.
Study Design. LPS: lipopolysaccharide; EGCG: epigallocatechin-3-gallate; LPS injections given three times a week for 3 wks intraperitoneally = 0.5mg/Kg body weight.
Both negative and positive control groups received saline (Sigma Aldrich, St. Louis, MO, USA) via intraperitoneal injections (0.5 mL/kg BW) 3 times per week, and both treatment groups received lipopolysaccharide (LPS) injections (0.5 mg/kg BW), intraperitoneally. The LPS was derived from Escherichia coli 055:B5 and was obtained from Sigma Aldrich (St. Louis, MO, USA). It was dissolved in phosphate-buffered saline and stored at 4°C until use.
2.2. Growth Assessment and Tissue Collection
Body weight and food intake were measured daily. After 3 wks, all the rats were anesthetized by injecting ketamine (90 mg/kg) /xylazine (10mg/kg) intraperitoneally. After collecting blood, tissues (liver and spleen,) were harvested, weighed, and frozen immediately. The collected blood was used to measure iron status (hemoglobin, hematocrit, ferritin, and serum iron) and inflammation markers (CRP, IL6, and serum amyloid A [SAA)]. The tissues samples were used to measure total iron concentrations.
2.3. Iron Status Indicators
Whole blood was used immediately to measure hemoglobin (hemocue Hb 201+) concentrations and hematocrit. Serum iron was determined by a commercial kit based on the total iron binding capacity and serum iron assay kit (Abcam, Waltham, MA, USA). Tissue iron content was measured to assess iron stores using a standard ferrozine assay used in a previous study [
23]. Briefly, livers were homogenized in water and subjected to trichloroacetic acid (TCA) protein precipitation at 65°C for 20 h. Nonheme iron assay was determined calorimetrically using ferrozine in thioglycolic acid by measuring the absorbance using a microplate reader (BioTek Instruments, Winooski, VT, USA) to assess soluble iron and calculating tissue iron content based on the weight of the tissue used. The same process was used to measure spleen iron content.
2.4. Inflammatory Markers
CRP was determined by a commercial kit based on the Rat CRP SimpleStep ELISA kit (Abcam, Waltham, MA, USA). This assay employs an affinity tag labeled capture antibody and a reporter conjugated detector antibody, which immunocaptures the sample analyte in solution. This entire complex (capture antibody/analyte/detector antibody) is, in turn, immobilized via immunoaffinity of an anti-tag antibody coating the well. TMD solution was added to the sample wells and catalyzed by HRP, and the blue color intensity was read at 450 nm using a microplate reader. IL-6 concentrations were measured using a commercial kit based on the Rat IL-6 ELISA kit (Millipore, St. Louis, MO, USA) in which the detection antibody was a biotinylated rat IL-6 antibody incubated with HRP + Streptavidin to determine results. SAA (an acute phase protein and biomarker of inflammation) was measured using a commercial kit using sandwich ELISA (MyBioSource, San Diego, CA, USA). This kit used the pre-coated anti-rat SAA monoclonal antibody, and the detection antibody was a biotinylated polyclonal antibody.
2.5. Statistical Analysis
Data were analyzed using GraphPad Prism version 9 (La Jolla, CA, USA). Data are expressed as mean ± SEM. Statistical differences among the groups were determined using one-way ANOVA with Tukey multiple comparisons test. Differences were considered significant at P<0.05.
3. Results
The average weight at the beginning of the study was 61 g, then increased to 269 g at the end (Table 1). There were no statistical differences among the groups when weight was compared each week (data not shown). The average daily food intake was 10.0 ± 0.6 g, with no statistical differences among groups.
Table 1.
Final body weights and average daily food intake1.
Table 1.
Final body weights and average daily food intake1.
| Treatment Group |
Final weight (g) |
Daily Food Intake (g) |
| Positive Control |
279 ± 4 |
10.98 ± 0.4 |
| Negative Control |
259 ± 6 |
9.48 ± 0.3 |
| LPS |
272 ± 5 |
9.94 ± 0.7 |
| LPS + EGCG |
264 ± 9 |
9.61 ± 0.9 |
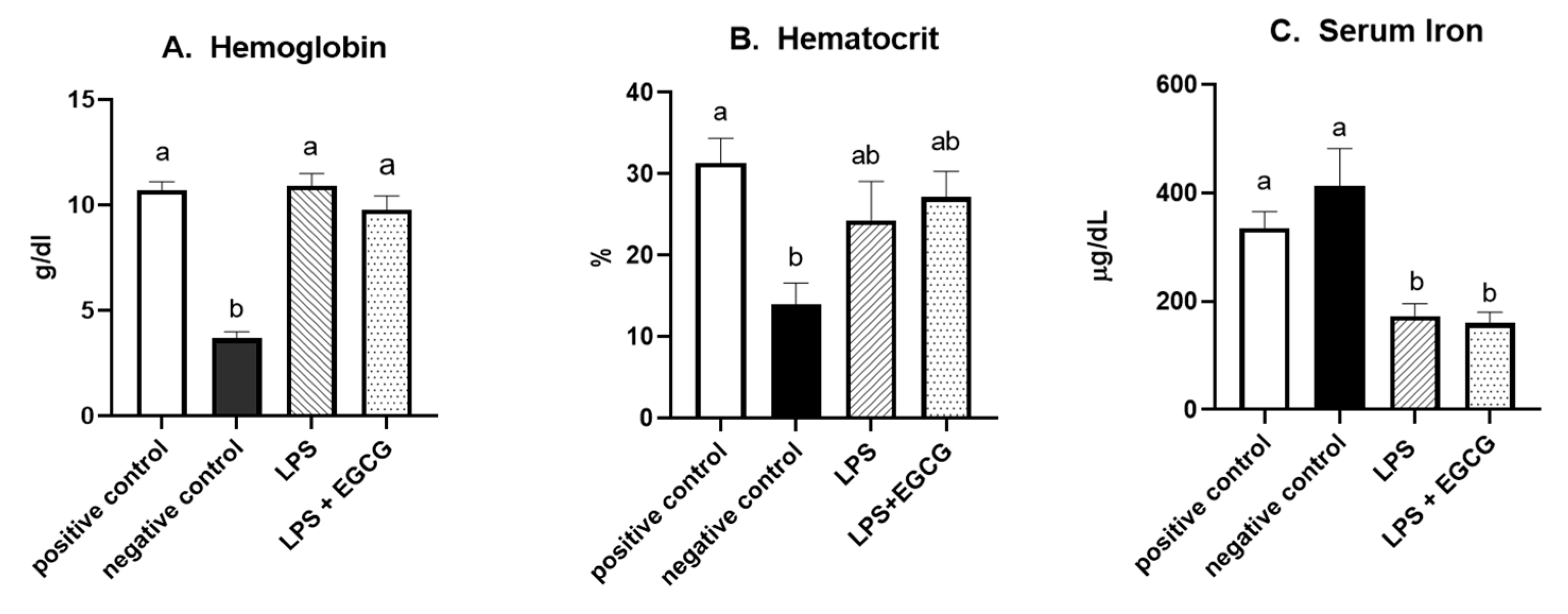
As expected, hemoglobin concentration in the negative control group was lower (p<0.0001) than in the positive control. In both LPS treatment groups (LPS, 10.9 ± 0.6 g/dL; LPS + EGCG, 9.8 ± 0.7 g/dL), hemoglobin concentrations were significantly higher than the negative control group (3.7 ± 0.3 g/dL) but no statistical differences compared to positive control (10.7+ 0.4). We observed the same trend with hematocrit values. The negative control (13.9 ± 2.9%) was significantly (p<0.005) lower than the positive control (31.4 ± 3.3%). The values in LPS (24.3 ± 5.2%) and LPS + EGCG (27.2 ± 3.4%) were not significantly different from each other or with the positive or negative control groups. Overall, EGCG treatment had no effect on hemoglobin and hematocrit—as noted by comparable estimates between LPS and LPS+ EGCG groups (Figure 2 A and B).
Figure 2.
Effect of EGCG on hemoglobin concentrations (A), hematocrit (B), and serum iron (C). Data are presented as means ± SEM, n = 8 per treatment group, and means with different letters are significantly different (p<0.05) based on ANOVA with Tukey's multiple comparison test for each measure. n = 7 in the positive control group for hematocrit due to insufficient blood, and n = 7 in both experimental groups due to unexpected rodent deaths early in the study.
Figure 2.
Effect of EGCG on hemoglobin concentrations (A), hematocrit (B), and serum iron (C). Data are presented as means ± SEM, n = 8 per treatment group, and means with different letters are significantly different (p<0.05) based on ANOVA with Tukey's multiple comparison test for each measure. n = 7 in the positive control group for hematocrit due to insufficient blood, and n = 7 in both experimental groups due to unexpected rodent deaths early in the study.
Interestingly, feeding an iron-deficient diet did not significantly reduce serum iron concentrations in the negative control. However, inducing inflammation with LPS significantly reduced serum iron compared to positive control. Compared to the positive control (335.8 ± 32.1 µg/dL), serum iron concentrations (mean + SEM) were 50% lower in the LPS only (172.5 ± 25.1 µg/dL) (p=0.02) and the LPS+EGCG (159.80 ± 22.34 µg/dL) (p=0.01) groups. No difference in serum iron concentration between the LPS only and the LPS + EGCG groups suggests that EGCG had no effect on serum iron concentrations (Figure 2C).
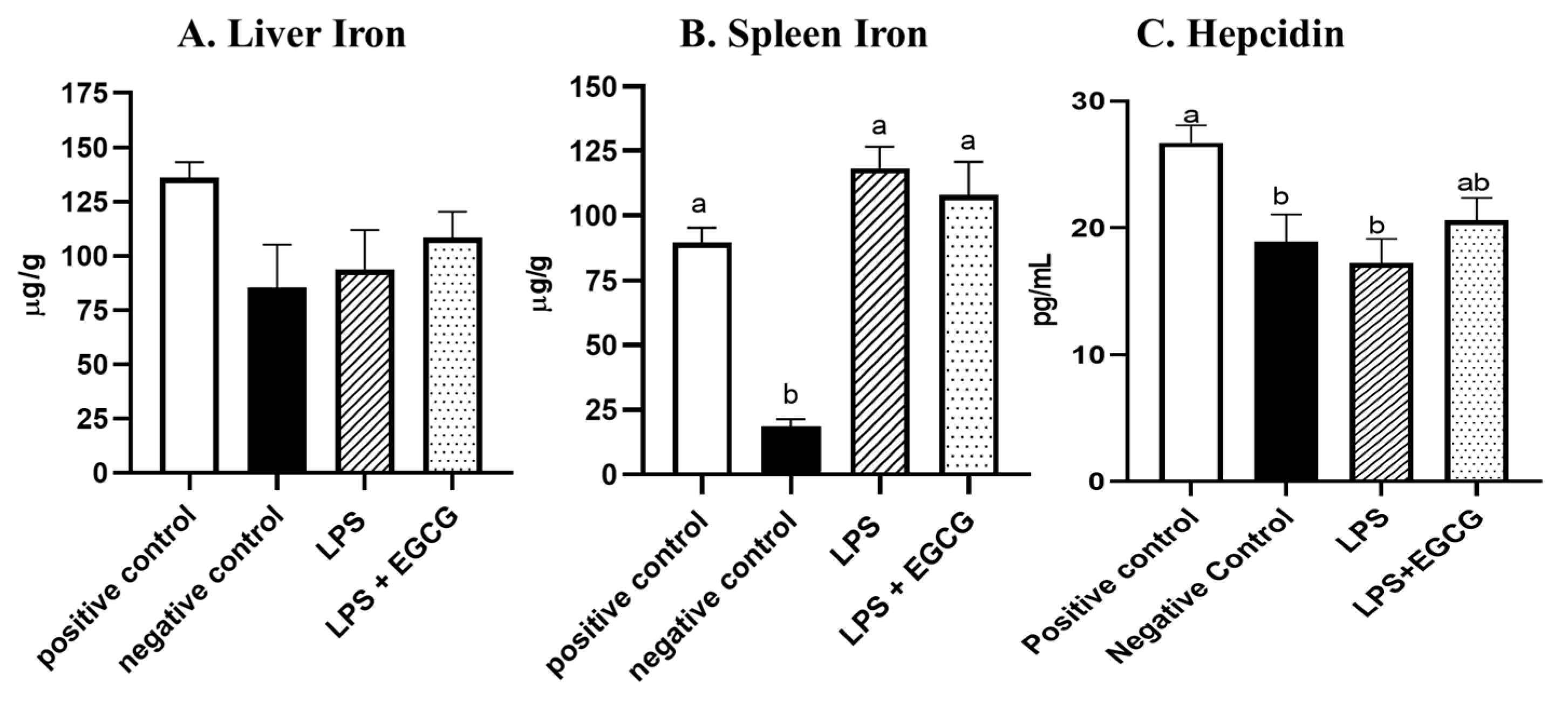
Though there were no significant differences in liver iron concentrations (Figure 3A) among the groups, spleen iron concentrations were significantly lower in the negative control (18.6 ± 2.8 µg/g) (p<0.001) compared to the positive control group (89.6 ± 5.7 µg/g). Both LPS treatment groups had significantly higher iron content than the negative control, but no significant differences were observed between LPS (118.4 ± 8.3 µg/g)) and LPS+ EGCG (108.1 ± 12.8 µg/g) (Figure 3B). As expected, hepcidin concentrations in the negative control (p<0.05) and LPS only groups (p=0.01) were significantly lower than in the positive control. It appears feeding rats with EGCG had no effect on hepcidin concentrations, as there was no difference between the two treatment groups (Figure 3C).
Figure 3.
Effects of EGCG and inflammation on liver (A) and spleen iron concentrations (B) and hepcidin concentrations (C). Data are presented as mean ± SEM, n = 8 per treatment group( n = 7 in both experimental groups due to unexpected rodent deaths early in the study). Means with different letters indicate statistical difference (p<0.05) according to Tukey’s multiple comparison test.
Figure 3.
Effects of EGCG and inflammation on liver (A) and spleen iron concentrations (B) and hepcidin concentrations (C). Data are presented as mean ± SEM, n = 8 per treatment group( n = 7 in both experimental groups due to unexpected rodent deaths early in the study). Means with different letters indicate statistical difference (p<0.05) according to Tukey’s multiple comparison test.
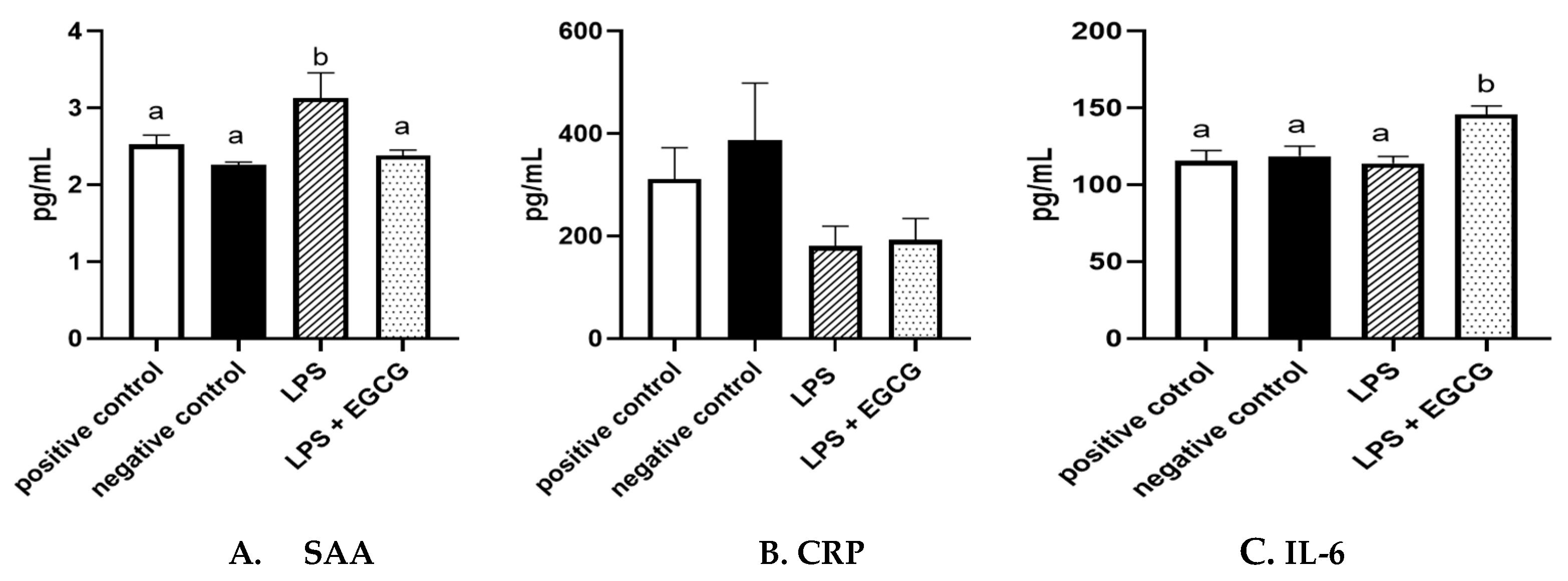
When observing inflammatory markers, SAA concentrations were significantly higher in LPS only group (3.1 ± 0.4 ng/mL) (p=0.01) compared to the negative control group (2.3 ± 0.03 ng/mL) (Figure 4A). Feeding EGCG significantly (p<0.05) lowered SAA concentrations to a level similar to the negative and positive control groups suggesting reduced inflammation. Surprisingly, no significant differences were observed in CRP concentrations among the groups (Figure 4B). IL-6 concentrations were significantly higher in the LPS+EGCG group than in all the groups. Unexpectedly, the LPS +EGCG group had significantly (146.1 ± 6.0 pg/mL p=0.01) higher IL-6 concentrations than LPS alone (114.33 ± 4.78 pg/mL) group. (Figure 4C).
Figure 4.
Effects of EGCG on inflammation markers. Serum Amyloid A (SAA) (A), C-reactive protein (CRP) (B). IL-6 concentrations (C), Data are presented as means ± SEM, n = 8 per treatment group (n = 7 in both experimental groups due to unexpected rodent deaths early in the study). Means with different letters are significantly different (p<0.05) based on ANOVA with Tukey's multiple comparison test for each measure.
Figure 4.
Effects of EGCG on inflammation markers. Serum Amyloid A (SAA) (A), C-reactive protein (CRP) (B). IL-6 concentrations (C), Data are presented as means ± SEM, n = 8 per treatment group (n = 7 in both experimental groups due to unexpected rodent deaths early in the study). Means with different letters are significantly different (p<0.05) based on ANOVA with Tukey's multiple comparison test for each measure.
4. Discussion
Previous studies have shown a relationship between inflammatory markers and IDA [
8,
24], but no studies to date propose improved iron status by reducing inflammation through dietary components. In the present study, serum iron was reduced in response to inflammation induced by LPS, but no effect was found by feeding EGCG. However, the elevated SAA concentrations with LPS were significantly reduced by EGCG, suggesting its anti-inflammatory property.
Contrary to our hypothesis, in the present study, there were no significant differences in either experimental group when comparing hemoglobin concentrations and hematocrit to the positive control. We expected a similar trend in the negative control and LPS only group; however, only the negative control showed a significant difference compared to the positive control. The low hemoglobin concentrations in the negative control indicate that the rats were indeed iron deficient, thus providing knowledge for future studies to allow more focus on reducing chronic inflammation rather than inducing iron deficiency.
We also anticipated significant differences among liver iron concentrations, but no such findings were observed. Based on the reduction of serum iron following LPS-induced inflammation (based on SAA concentrations), we also expected to see high iron stores in the liver, but this was not observed. However, the LPS only group showed a significant increase in spleen iron concentrations compared to the negative control. This could indicate "iron trapping," which is driven by hepcidin. Increased hepcidin levels will lead to an increase in endocytosis and the breakdown of ferroportin, thus keeping iron trapped within cells. This reduces the natural flow of iron from hepatocytes, enterocytes, and macrophages resulting in reduced serum iron concentration [
25]. Compared to the positive control, serum iron concentrations were 50% lower in the LPS only and the LPS+EGCG groups. This indicates that EGCG did not improve serum iron, although some reduction in inflammation was found in terms of SAA concentration. However, the lower serum iron in the LPS only and LPS + EGCG groups demonstrates iron trapping theory in inflammation. The low serum iron was likely in response to inflammation, as it is consistent with previous studies that focused on inflammatory biomarkers [
26,
27].
Higher concentrations of SAA in the LPS only group but not in the LPS + EGCG group support our hypothesis. This data point alone indicates that the EGCG did reduce inflammation. The IL-6 data contradicts this, as concentrations were elevated in the LPS + EGCG group but not in the LPS only group or negative control. However, IL-6 concentrations are elevated in LPS + EGCG, which might be an anti-inflammatory response to ECCG as IL-6 also has extensive anti-inflammatory functions as a myokine [
27]. We also expected to see differences in CRP across groups, but no significant differences were observed. Overall, inflammatory markers as a whole were inconclusive. We chose an intermittent bolus of LPS injection based on a previous study showing that 0.5 mg of LPS per kg of body weight produced chronic systemic inflammation with a low risk of fatality [
22]. However, it is important to note that two rodents, one from each treatment group, died following one and two LPS injections, respectively. We suspect these deaths may be attributed to the LPS dosage used in the study. We can only speculate two rats died due to the LPS dosage and/or administration.
The inconclusive inflammatory biomarkers could also be due, in part, to the short half-life of CRP, which is approximately 19 hours [
28]. As an acute-phase protein, it has been reported that the plasma concentration of CRP deviates by at least 25% during inflammatory conditions [
29]. Importantly, when there is no longer a stimulus, CRP values reportedly decrease over 18–20 hours [
30]. Thus, the timing of the blood draws in relation to the final dose of LPS may have been too large a time gap to interpret our results for inflammatory biomarkers. We believe this to be the case with hepcidin, as it is also a transient hormone with a reported half-life of 2.3 minutes [
31]. Thus, a blood draw closer to the time of LPS injection may have produced the expected hepcidin results. Importantly, previous studies suggest inflammatory regulators take precedence over iron stores: iron-deficient mice injected with LPS up-regulated hepcidin expression [
32], while iron-loaded mice with experimentally induced anemia down-regulated hepcidin expression [
33]. These contradictory assessment factors caused by inflammation could also explain the insignificant results observed in the liver iron.
The rationale behind using LPS was to induce inflammation similar to that of obesity, and it has been reported that inflammation is linked to increased adiposity [
34]. IDA cannot be differentiated from the more prominent anemia of inflammation because inflammation confounds the measurement of iron status [
8]. Stoffel
et al. [
35] evaluated different iron and inflammatory biomarkers in normal-weight vs. obese women. They found higher levels of central adiposity correlated with elevated CRP, α-1 glycoprotein, serum hepcidin, total iron-binding capacity, and lower serum iron-to-hepcidin ratio and transferrin saturation [
35]. Similar results were reported by our group when normal-weight and obese subjects were compared to obese subjects [
8]. The CRP values in the obese group were 8 times higher than in normal-weight women, and most of the normal-weight subjects were within the normal range, while those of the obese group were elevated [
8].
There were many strengths and limitations to this research. Measuring inflammatory markers such as CRP and hepcidin at a more optimal time could provide a clearer picture of whether inflammation was present due to LPS treatment. Despite this limitation, the findings of this study are important because we measured a range of physiological factors that affect iron status and have opened the door to determining more ways to treat anemia and chronic inflammation. Future studies could include a different LPS and EGCG administration regimen and dosage. However, it should be noted that in the present study, we took a conservative route for each regimen to minimize stress on the rodents. Most likely, the results would have been different with chronic inflammation induced by obesity from acute inflammation induced by LPS.
5. Conclusions
Serum iron was reduced in response to inflammation induced by LPS, but EGCG did not normalize concentrations. On the contrary, with higher SAA concentration with LPS, hepcidin was lower in that group. However, the relationship between serum iron and inflammation is complex and multi-faceted. It is difficult to discern whether the results were caused by LPS-induced inflammation, the antioxidant effects of EGCG, or a combination of both. SAA results suggest that EGCG reduced inflammation induced by LPS, but future studies are needed to address the interaction between the reduction of inflammation and improving iron status.
Author Contributions
Conceptualization, MJ, SA, and MBR; methodology, MJ, SA, KS, and MBR; formal analysis, MJ, SA, and MBR; investigation, MJ, SA, and MBR; resources, MBR; writing—original draft preparation, MJ, and SA.; writing—review and editing, KS and MBR; supervision, MBR; funding acquisition, MBR. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding, and the APC was paid by the Doris A. Adams Endowed Chair for MBR at the College of Human Sciences, Iowa State University.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Iowa State University (protocol code IACUC-21-039; approved on 12-Apr-2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO, Micronutrient deficiencies. Available online: https://apps.who.int/nutrition/topics/ida/en/ (accessed on 14 January 2022).
- 2022, World Health Organization.
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. The Role of Hepcidin in Iron Metabolism. Acta Haematol. 2009, 122, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to Tango: Regulation of Mammalian Iron Metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C.; Pagani, A. Advances in understanding iron metabolism and its crosstalk with erythropoiesis. Br. J. Haematol. 2018, 182, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.V.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Aguree, S.; Reddy, M.B. Inflammatory Markers and Hepcidin are Elevated but Serum Iron is Lower in Obese Women of Reproductive Age. Nutrients 2021, 13, 217. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Zeder, C.; Muthayya, S.; Winichagoon, P.; Chaouki, N.; Aeberli, I.; Hurrell, R.F. Adiposity in women and children from transition countries predicts decreased iron absorption, iron deficiency and a reduced response to iron fortification. Int. J. Obes. 2008, 32, 1098–1104. [Google Scholar] [CrossRef]
- Aeberli, I., R.F. Hurrell, and M.B. Zimmermann, Overweight children have higher circulating hepcidin concentrations and lower iron status but have dietary iron intakes and bioavailability comparable with normal weight children. Int J Obes (Lond), 2009. 33(10): p. 1111-7.
- Mujica-Coopman, M.F.; Brito, A.; de Romaña, D.L.; Pizarro, F.; Olivares, M. Body mass index, iron absorption and iron status in childbearing age women. J. Trace Elements Med. Biol. 2015, 30, 215–219. [Google Scholar] [CrossRef]
- Laftah, A.H.; Ramesh, B.; Simpson, R.J.; Solanky, N.; Bahram, S.; Schümann, K.; Debnam, E.S.; Srai, S.K.S. Effect of hepcidin on intestinal iron absorption in mice. Blood 2004, 103, 3940–3944. [Google Scholar] [CrossRef]
- Cepeda-Lopez, A.C.; Aeberli, I.; Zimmermann, M.B. Does Obesity Increase Risk for Iron Deficiency? A Review of the Literature and the Potential Mechanisms. Int. J. Vitam. Nutr. Res. 2010, 80, 263–270. [Google Scholar] [CrossRef]
- Ghavipour, M.; Saedisomeolia, A.; Djalali, M.; Sotoudeh, G.; Eshraghyan, M.R.; Moghadam, A.M.; Wood, L.G. Tomato juice consumption reduces systemic inflammation in overweight and obese females. Br. J. Nutr. 2013, 109, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.C; Andujar, I.; Rios, J.L. Anti-inflammatory agents from plants: Progress and potential. Curr. Med. Chem. 2012, 19, 2088–2103. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green Tea Extracts Epigallocatechin-3-gallate for Different Treatments. BioMed Res. Int. 2017, 2017, 5615647. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, M.; Lu, F.; Luo, N.; He, Z.-P.; Yang, H. Involvement of α7 nAChR Signaling Cascade in Epigallocatechin Gallate Suppression of β-Amyloid-Induced Apoptotic Cortical Neuronal Insults. Mol. Neurobiol. 2013, 49, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea Catechins and Polyphenols: Health Effects, Metabolism, and Antioxidant Functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; Higdon, J.V. Antioxidant Activity of Tea Polyphenols In Vivo: Evidence from Animal Studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef]
- Kim, I.-B.; Kim, D.-Y.; Lee, S.-J.; Sun, M.-J.; Lee, M.-S.; Li, H.; Cho, J.-J.; Park, C.-S. Inhibition of IL-8 Production by Green Tea Polyphenols in Human Nasal Fibroblasts and A549 Epithelial Cells. Biol. Pharm. Bull. 2006, 29, 1120–1125. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Mokhtarrudin, N.; Fadel, A.; Albujja, M.H.K. Induction of Chronic Subclinical Systemic Inflammation in Sprague–Dawley Rats Stimulated by Intermittent Bolus Injection of Lipopolysaccharide. Arch. Immunol. et Ther. Exp. 2019, 67, 385–400. [Google Scholar] [CrossRef]
- Swain, J.H.; Reddy, M.B.; Tabatabai, L.B. Histidine Content of Low-Molecular-Weight Beef Proteins Influences Nonheme Iron Bioavailability in Caco-2 Cells. J. Nutr. 2002, 132, 245–251. [Google Scholar] [CrossRef]
- McClung, J.P.; Karl, J.P. Iron deficiency and obesity: the contribution of inflammation and diminished iron absorption. Nutr. Rev. 2009, 67, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Takasawa, K. Impact of Inflammation on Ferritin, Hepcidin and the Management of Iron Deficiency Anemia in Chronic Kidney Disease. Nutrients 2018, 10, 1173. [Google Scholar] [CrossRef] [PubMed]
- Suchdev, P.S.; Williams, A.M.; Mei, Z.; Flores-Ayala, R.; Pasricha, S.-R.; Rogers, L.M.; Namaste, S.M. Assessment of iron status in settings of inflammation: challenges and potential approaches. Am. J. Clin. Nutr. 2017, 106 (Suppl 6), 1626S–1633S. [Google Scholar] [CrossRef] [PubMed]
- Nara, H.; Watanabe, R. Anti-Inflammatory Effect of Muscle-Derived Interleukin-6 and Its Involvement in Lipid Metabolism. Int. J. Mol. Sci. 2021, 22, 9889. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: a critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-Phase Proteins and Other Systemic Responses to Inflammation. New Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Ridker, P.M. Clinical Application of C-Reactive Protein for Cardiovascular Disease Detection and Prevention. Circ. 2003, 107, 363–369. [Google Scholar] [CrossRef]
- Xiao, J.J.; Krzyzanski, W.; Wang, Y.-M.; Li, H.; Rose, M.J.; Ma, M.; Wu, Y.; Hinkle, B.; Perez-Ruixo, J.J. Pharmacokinetics of Anti-hepcidin Monoclonal Antibody Ab 12B9m and Hepcidin in Cynomolgus Monkeys. AAPS J. 2010, 12, 646–657. [Google Scholar] [CrossRef]
- Constante, M.; Jiang, W.; Wang, D.; Raymond, V.-A.; Bilodeau, M.; Santos, M.M. Distinct requirements for Hfe in basal and induced hepcidin levels in iron overload and inflammation. Am. J. Physiol. Liver Physiol. 2006, 291, G229–G237. [Google Scholar] [CrossRef]
- Nicolas, G. , et al., The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest, 2002. 110(7): p. 1037-44.
- Welsh, P.; Polisecki, E.; Robertson, M.; Jahn, S.; Buckley, B.M.; de Craen, A.J.M.; Ford, I.; Jukema, J.W.; Macfarlane, P.W.; Packard, C.J.; et al. Unraveling the Directional Link between Adiposity and Inflammation: A Bidirectional Mendelian Randomization Approach. J. Clin. Endocrinol. Metab. 2010, 95, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, N.U.; El-Mallah, C.; Herter-Aeberli, I.; Bissani, N.; Wehbe, N.; Obeid, O.; Zimmermann, M.B. The effect of central obesity on inflammation, hepcidin, and iron metabolism in young women. Int. J. Obes. 2020, 44, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).