Submitted:
03 May 2023
Posted:
04 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Fish Oils Extraction
3.3. Oils Purification
3.4. Fatty Acids Analysis
3.5. FTIR Spectra Measurement
3.6. Chemometrics Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pasini, F.; Gómez-Caravaca, A.M.; Blasco, T.; Cvejić, J.; Caboni, M.F.; Verardo, V. Assessment of Lipid Quality in Commercial Omega-3 Supplements Sold in the French Market. Biomolecules 2022; 12, 1361. [CrossRef]
- Innes, J.K.; Calder, P.C. Marine omega-3 (N-3) fatty acids for cardiovascular health: An update for 2020. Int. J. Mol. Sci. 2020, 21, 1–21. [CrossRef]
- Mozaffari, H.; Daneshzad, E.; Larijani, B.; Bellissimo, N.; Azadbakht, L. Dietary intake of fish, n-3 polyunsaturated fatty acids, and risk of inflammatory bowel disease: a systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2020, 59, 1-17. [CrossRef]
- Giacobbe, J.; Benoiton, B.; Zunszain, P.; Pariante, C.M.; Borsini, A. The Anti-Inflammatory Role of Omega-3 Polyunsaturated Fatty Acids Metabolites in Pre-Clinical Models of Psychiatric, Neurodegenerative, and Neurological Disorders. Front Psychiatry 2020, 11, 1-13. [CrossRef]
- Alinafiah, S.M.; Azlan, A.; Ismail, A.; Rashid, N.K.M.A. Method development and validation for omega-3 fatty acids (DHA and EPA) in fish using gas chromatography with flame ionization detection (GC-FID). Molecules 2021, 26, 6592. [CrossRef]
- Yi, T.; Li, S.M.; Fan, J.Y.; Fan, L.L.; Zhang, Z.F.; Luo, P.; Zhang, X.J.; Wang, J.G.; Zhu, L.; Zhao, Z.Z. Chen HB. Comparative analysis of EPA and DHA in fish oil nutritional capsules by GC-MS. Lipids Health Dis. 2014, 13, 1–6. [CrossRef]
- Kiełbasa, A.; Buszewski, B.; Gadzała-Kopciuch, R. A novel non-derivatization HPLC/UV method for the determination of some n-3 free fatty acids in breast milk matrix. Microchem. 2022, 181, 107789. [CrossRef]
- Viswanathan, S.; Verma, P.R.P.; Ganesan, M.; Manivannan, J. A novel liquid chromatography/tandem mass spectrometry (LC–MS/MS) based bioanalytical method for quantification of ethyl esters of Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA) and its application in pharmacokinetic study. J. Pharm. Biomed. Anal. 2017, 141, 250–261. [CrossRef]
- Rohman, A.; Putri, A.R.; Irnawati; Windarsih, A.; Nisa, K.; Lestari, L.A. The employment of analytical techniques and chemometrics for authentication of fish oils: A review. Food Control 2021, 124, 107864. [CrossRef]
- Singh, I.; Juneja, P.; Kaur, B.; Kumar, P. Pharmaceutical Applications of Chemometric Techniques. ISRN Anal. Chem. 2013, 2013, 1–13. [CrossRef]
- Yu, X.; Du, S.; van de Voort, F.R.; Yue, T.; Li, Z. Automated and simultaneous determination of free fatty acids and peroxide values in edible oils by FTIR spectroscopy using spectral reconstitution. Anal. Sci. 2009, 25, 627–632. [CrossRef]
- Tarhan, İ.; Ismail, A.A.; Kara, H. Quantitative determination of free fatty acids in extra virgin olive oils by multivariate methods and Fourier transform infrared spectroscopy considering different absorption modes. Int. J. Food Prop. 2017, 20, S790–S797. [CrossRef]
- Aryee, A.N.A.; van de Voort, F.R.; Simpson, B.K. FTIR determination of free fatty acids in fish oils intended for biodiesel production. Process Biochem. 2009, 44, 401–405. [CrossRef]
- Rohman, A.; Man, Y.B.C. Application of Fourier Transform Infrared Spectroscopy for Authentication of Functional Food Oils. Appl. Spectroscy Rev. 2012, 47, 1–13. [CrossRef]
- Putri, A.R.; Rohman, A.; Riyanto, S. Authentication of patin (Pangasius micronemus) fish oil adulterated with palm oil using ftir spectroscopy combined with chemometrics. Int. J. Appl. Pharm. 2019, 11, 195–199. [CrossRef]
- Rohman, A.; Che Man, Y.B. Analysis of cod-liver oil adulteration using fourier transform infrared (FTIR) spectroscopy. J. Am. Oil Chem. Soc. 2009, 86. [CrossRef]
- Kaya-Celiker, H.; Mallikarjunan, P.K.; Kaaya, A. Mid-infrared spectroscopy for discrimination and classification of Aspergillus spp. contamination in peanuts. Food Control 2015, 52, 103–111. [CrossRef]
- Albayrak, M.; Demirkaya-Miloglu, F.; Senol, O.; Polatdemir, E. Design, optimization, and validation of chemometrics-assisted spectrophotometric methods for simultaneous determination of etodolac and thiocolchicoside in pharmaceuticals. J. Anal. Sci. Technol. 2019, 10, 2-8. [CrossRef]
- Eticha, T.; Kahsay, G.; Asefa, F.; Hailu, T.; Gebretsadik, H.; Gebretsadikan, T.; Thangabalan, B. Chemometric-Assisted Spectrophotometric Method for the Simultaneous Determination of Ciprofloxacin and Doxycycline Hyclate in Pharmaceutical Formulations. J. Anal. Methods Chem. 2018, 9538435. [CrossRef]
- Rohman, A.; Riyanto, S.; Sasi, A.M.; Yusof, F.M. The use of FTIR spectroscopy in combination with chemometrics for the authentication of red fruit (Pandanus conoideus Lam) oil from sunflower and palm oils. Food Bioscience 2014, 7, 64–70. [CrossRef]
- Mirghani, M.E.S.; Che Man, Y.B.; Jinap, S.; Baharin, B.S.; Bakar, J. Rapid method for determining malondialdehyde as secondary oxidation product in palm olein system by Fourier transform infrared spectroscopy. Phytochem. Anal. 2002, 13, 195–201. [CrossRef]
- Hermanto, S.; Sumarlin, L.O.; Fatimah, W. Differentiation of Bovine and Porcine Gelatin Based on Spectroscopic and Electrophoretic Analysis. J. Food Pharm. Sci. 2013, 1, 68-73. [CrossRef]
- Lorensia, A.; Budiono, R.; Suryadinata, R.V.; Tiarasari, N. Quantitative determination of EPA and DHA in fish oil capsules for cardiovascular disease therapy in Indonesia by GC-MS. J. Public health Res. 2021; 10. [CrossRef]
- Brotas, M.S.C.; Carvalho, G.A.; Pereira, P.A.P. Determination, through derivatization and GC-MS analysis, of omega-3 and omega-6 fatty acids in fish oil capsules sold in Salvador, Bahia. J. Braz. Chem. Soc. 2020, 31, 447–455. [CrossRef]
- Lv, J.; Wang, C.; Zhang, X.; Lv, Z.; Yu, M. 1H NMR Quantification of DHA and EPA in Fish Oil. J. Ocean Univ. China 2020, 19, 1193–1197. [CrossRef]
- Dais, P.; Misiak, M.; Hatzakis, E. Analysis of marine dietary supplements using NMR spectroscopy. Anal. Methods 2015, 7, 5226–5238. [CrossRef]
- Williamson, K.; Hatzakis, E. NMR Spectroscopy as a Robust Tool for the Rapid Evaluation of the Lipid Profile of Fish Oil Supplements. J. Vis. Exp. 2017, 2017, 55547. [CrossRef]
- Serafim, V.; Tiugan, D.A.; Andreescu, N.; Mihailescu, A.; Paul, C.; Velea, I.; Puiu, M.; Niculescu, M.D. Development and Validation of a LC–MS/MS-Based Assay for Quantification of Free and Total Omega 3 and 6 Fatty Acids from Human Plasma. Molecules 2019, 24. [CrossRef]
- Kotani, A.; Watanabe, M.; Yamamoto, K.; Kusu, F.; Hakamata, H. Determination of eicosapentaenoic, docosahexaenoic, and arachidonic acids in human plasma by high-performance liquid chromatography with electrochemical detection. Anal. Sci. 2016, 32, 1011–1014. [CrossRef]
- Rincón-Cervera, M.Á.; Villarreal-Rubio, M.B.; Valenzuela, R.; Valenzuela, A. Comparison of fatty acid profiles of dried and raw by-products from cultured and wild fishes. Eur. J. Lipid Sci. Technol. 2017, 119, 1600516. [CrossRef]
- Quero-Jiménez, P.C.; Felipe, L.A.A.; Prieto García, J.O.; Rodríguez, M.E.J.; De La Torre, L.J.B.; Montenegro, O.N.; Molina, R.R. Local Cuban bentonite clay as potential low-cost adsorbent for shark liver oil pool purification. J. Pharm. Pharmacogn. Res. 2021, 9, 525–536. [CrossRef]
- Irnawati, I.; Riyanto, S.; Martono, S.; Rohman, A. The employment of FTIR spectroscopy and chemometrics for the classification and prediction of antioxidant activities of pumpkin seed oils from different origins. J. Appl. Pharm. Sci. 2021, 11, 100–107. [CrossRef]
- Irnawati, I.; Riyanto, S.; Martono, S.; Windarsih, A.; Rohman, A. Physicochemical properties and antioxidant activities of pumpkin seed oil as affected by different origins and extraction methods. J. Appl. Pharm. Sci. 2022, 12, 115–122. [CrossRef]
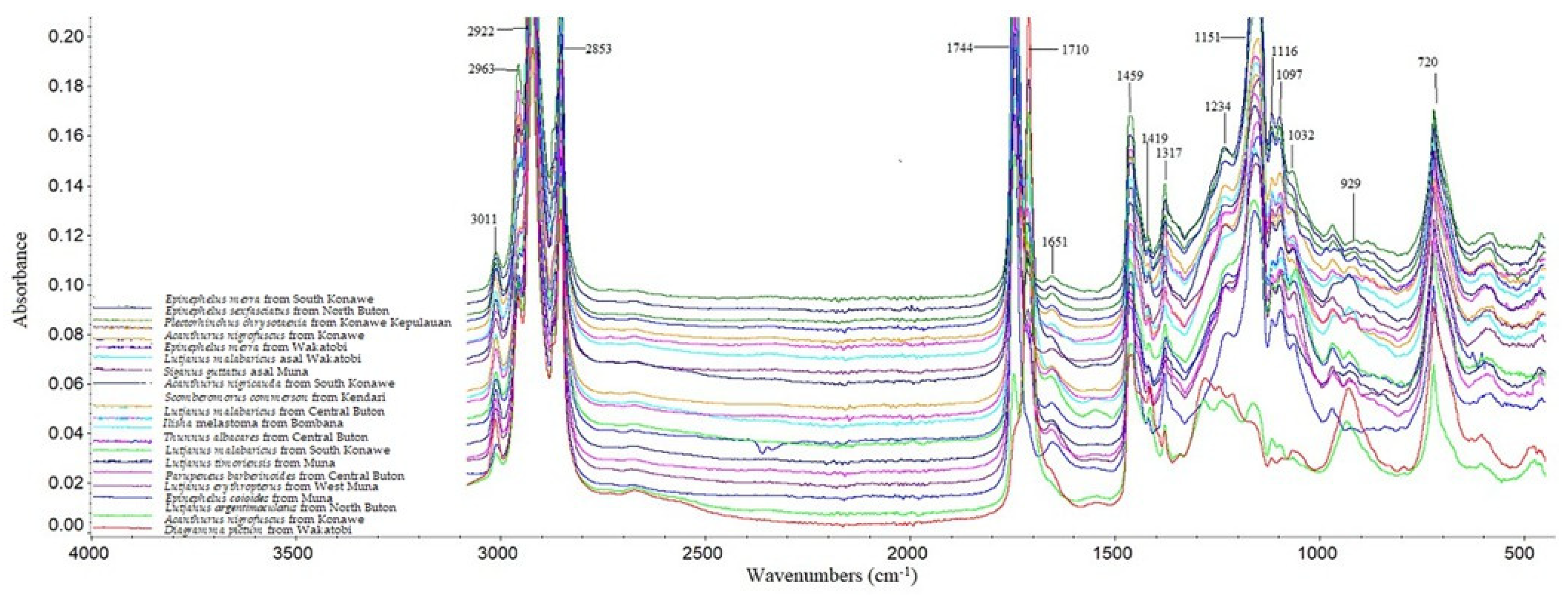
| No. | Samples | Eicosapentaenoate (%) |
Docosahexaenoate (%) |
Eicosatrienoate (%) |
Linolenate (%) |
|---|---|---|---|---|---|
| 1. | Lutjanus malabaricus from South Konawe | 1.6 | 11.5 | 0.4 | 0.5 |
| 2. | Epinephelus merra from South Konawe | 2.0 | 6.6 | 0.6 | 0.5 |
| 3. | Acanthurus nigricauda from South Konawe | 1.0 | 6.0 | 0.3 | 0.2 |
| 4. | Lutjanus timoriensis from Muna | 2.0 | 5.4 | 0.9 | 0.3 |
| 5. | Siganus guttatus asal Muna | 1.9 | 13.3 | 0.1 | 0.8 |
| 6. | Lutjanus erythropterus from West Muna | 2.4 | 15.8 | 0.1 | 0.2 |
| 7. | Epinephelus sexfasciatus from North Buton | 1.0 | 6.1 | 0.1 | 0.4 |
| 8 | Lutjanus argentimaculatus from North Buton | 1.0 | 4.8 | 0.1 | 1.0 |
| 9 | Lutjanus malabaricus from Central Buton | 2.7 | 18.8 | 0.2 | 0.4 |
| 10 | Parupeneus barberinoides from Central Buton | 2.0 | 14.0 | 0.2 | 0.5 |
| 11 | Thunnus albacares from Central Buton | 1.9 | 12.3 | 0.3 | 0.4 |
| 12 | Ilisha melastoma from Bombana | 1.4 | 5.2 | 0.6 | 0.7 |
| 13 | Plectorhinchus chrysotaenia from Konawe Kepulauan | 0.5 | 16.0 | 4.1 | 0.5 |
| 14 | Acanthurus xanthopterus from Konawe | 0.8 | 2.6 | 1.0 | 0.4 |
| 15 | Acanthurus nigrofuscus from Konawe | 2.6 | 4.1 | 4.9 | 1.2 |
| 16 | Scomberomorus commerson from Kendari | 2.1 | 16.6 | 0.1 | 0.5 |
| 17 | Epinephelus merra from Wakatobi | 2.0 | 17.3 | 0.4 | 0.3 |
| 18 | Epinephelus coioides from Muna | 0.1 | 7.2 | 0.3 | 0.4 |
| 19 | Lutjanus malabaricus asal Wakatobi | 1.7 | 13.6 | 0.5 | 0.3 |
| 20 | Diagramma pictum from Wakatobi | 2.1 | 26.5 | 0.2 | 0.3 |
| Multivariate Calibrations | Wavenumbers (cm-1) |
Spectra | Calibration | Validation | ||
|---|---|---|---|---|---|---|
| R2 | RMSEC | R2 | RMSEP | |||
| PLS | 4000 – 600 | Normal | 0.8736 | 2.97 | 0.8605 | 3.23 |
| First derivative | 0.9699 | 1.49 | 0.9476 | 1.96 | ||
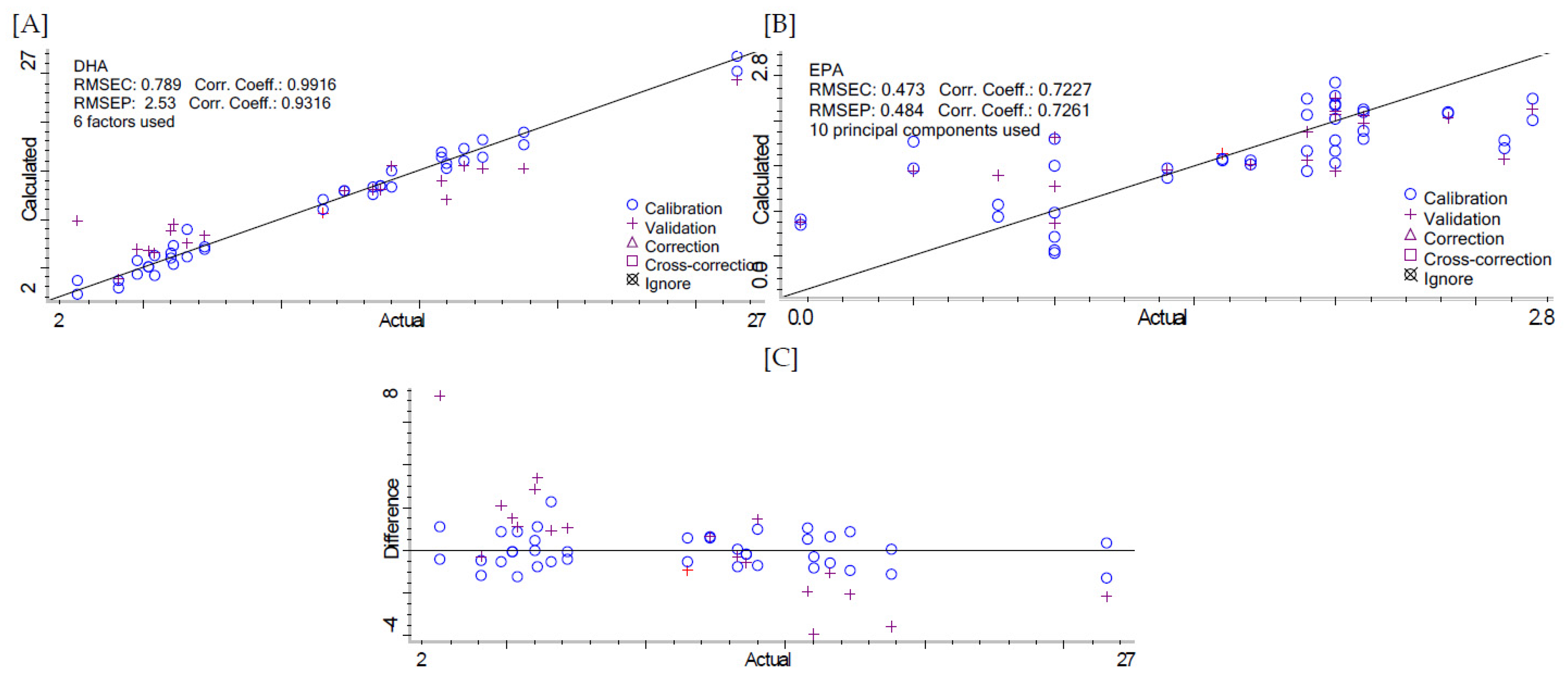
| Second Derivative | 0.9916 | 0.789 | 0.9316 | 2.53 | ||
| 1800-600 | Normal | 0.8785 | 2.92 | 0.8723 | 3.06 | |
| First derivative | 0.4930 | 5.32 | 0.4141 | 5.58 | ||
| Second Derivative | 0.5092 | 5.26 | 0.4135 | 5.59 | ||
| 1800-900 | Normal | 0.9330 | 2.20 | 0.9298 | 2.46 | |
| First derivative | 0.4691 | 5.40 | 0.3845 | 5.67 | ||
| Second Derivative | 0.4865 | 5.34 | 0.3967 | 5.64 | ||
| 2997-2806 and 1800-900 | Normal | 0.4709 | 5.39 | 0.3777 | 5.68 | |
| First derivative | 0.4836 | 5.35 | 0.3901 | 5.65 | ||
| Second Derivative | 0.4905 | 5.33 | 0.3951 | 5.64 | ||
| PCR | 4000 – 600 | Normal | 0.8946 | 2.73 | 0.8813 | 3.12 |
| First derivative | 0.7851 | 3.79 | 0.7756 | 3.86 | ||
| Second Derivative | 0.8070 | 3.61 | 0.7493 | 4.13 | ||
| 1800-600 | Normal | 0.9055 | 2.59 | 0.8961 | 2.93 | |
| First derivative | 0.8697 | 3.02 | 0.8699 | 3.02 | ||
| Second Derivative | 0.7617 | 3.96 | 0.6472 | 4.68 | ||
| 1800-900 | Normal | 0.8261 | 3.44 | 0.8191 | 3.59 | |
| First derivative | 0.8569 | 3.15 | 0.8337 | 3.38 | ||
| Second Derivative | 0.7555 | 4.00 | 0.6319 | 4.76 | ||
| 2997-2806 and 1800-900 | Normal | 0.8640 | 3.08 | 0.8602 | 3.19 | |
| First derivative | 0.7598 | 4.97 | 0.7260 | 4.21 | ||
| Second Derivative | 0.7241 | 4.21 | 0.6073 | 4.88 | ||
| Multivariate Calibrations | Wavenumbers (cm-1) | Spectra | Calibration | Validation | ||
|---|---|---|---|---|---|---|
| R2 | RMSEC | R2 | RMSEP | |||
| PLS | 4000 – 600 | Normal | 0.4812 | 0.600 | 0.4435 | 0.619 |
| First derivative | 0.3076 | 0.651 | 0.3871 | 0.640 | ||
| Second Derivative | 0.4474 | 0.612 | 0.5079 | 0.603 | ||
| 1800-600 | Normal | 0.2479 | 0.663 | 0.3567 | 0.652 | |
| First derivative | 0.2022 | 0.670 | 0.1889 | 0.672 | ||
| Second Derivative | 0.2123 | 0.669 | 0.1850 | 0.673 | ||
| 1800-900 | Normal | 0.2567 | 0.661 | 0.3658 | 0.650 | |
| First derivative | 0.1874 | 0.672 | 0.1733 | 0.674 | ||
| Second Derivative | 0.1808 | 0.673 | 0.1615 | 0.676 | ||
| 2997-2806 and 1800-900 | Normal | 0.3099 | 0.651 | 0.3487 | 0.644 | |
| First derivative | 0.2843 | 0.656 | 0.3600 | 0.646 | ||
| Second Derivative | 0.2978 | 0.653 | 0.3621 | 0.645 | ||
| PCR | 4000 – 600 | Normal | 0.6644 | 0.512 | 0.5303 | 0.656 |
| First derivative | 0.6818 | 0.501 | 0.6271` | 0.543 | ||
| Second Derivative | 0.7227 | 0.473 | 0.7261 | 0.484 | ||
| 1800-600 | Normal | 0.6537 | 0.518 | 0.6135 | 0.553 | |
| First derivative | 0.6563 | 0.516 | 0.6014 | 0.557 | ||
| Second Derivative | 0.7033 | 0.487 | 0.6939 | 0.506 | ||
| 1800-900 | Normal | 0.6637 | 0.512 | 0.5948 | 0.575 | |
| First derivative | 0.6546 | 0.517 | 0.5957 | 0.561 | ||
| Second Derivative | 0.6891 | 0.496 | 0.6990 | 0.496 | ||
| 2997-2806 and 1800-900 | Normal | 0.6692 | 0.509 | 0.5819 | 0.589 | |
| First derivative | 0.6874 | 0.497 | 0.6276 | 0.547 | ||
| Second Derivative | 0.6757 | 0.505 | 0.6849 | 0.502 | ||
| No. | Samples | Previous methods | Ref. | Advantages of the current study |
|---|---|---|---|---|
| 1. | Analysis of EPA and DHA in fish oil capsules | GC-MS (gas chromatography-mass spectrometry) a using capillary column RTX-5SM (60 m x 0.25 mm, layer thickness 0.25 μm) | [23] | No need for sample derivatization, lower in cost, more efficient, and require minimum solvent |
| 2. | Analysis of EPA and DHA in fish oil nutritional capsules | GC-MS using a high-resolution DB-5MS capillary column (thickness: 0.25 μm, length: 30 m, diameter: 0.25 mm) | [6] | No need for sample derivatization, faster, low cost, more efficient, and require minimum solvent |
| 3. | Analysis of EPA and DHA in fish oil capsules | GC-MS using a column of PE-FFAP (nitroterephthalic acid modified polyethylene glycol, PEG bonded) | [24] | No need for sample derivatization, faster, low cost, more efficient, and require minimum solvent |
| 4. | Analysis of EPA and DHA in fish | GC-FID using a high polarity of capillary column (GC HP-88 column (60 m length, 0.25 mm ID, 0.2 μm) | [5] | No need for sample derivatization, faster, low cost, more efficient, and require minimum solvent |
| 5. | Analysis of EPA and DHA in Fish Oils | 1H-NMR (500 MHz) spectrometer | [25] | Minimum sample preparation steps, faster, low cost |
| 6. | Analysis of EPA and DHA in encapsulated marine fish oil supplements | 1H-NMR and 13C-NMR (850 MHz) spectrometer | [26] | Minimum sample preparation steps, faster, low cost |
| 7. | Analysis of EPA and DHA in fish oil supplements | 1H-NMR and 13C-NMR (850 MHz) spectrometer | [27] | Minimum sample preparation steps, faster, low cost |
| 8. | Quantification of total omega 3 and omega 6 in human plasma | LC-MS/MS reverse-phase using a C18 column (Acquity UPLC 100 × 2.1 mm, 1.7 µm BEH C18 column) | [28] | Minimum sample preparation steps, faster, low cost, efficient |
| 9. | Analysis of EPA and DHA in biological samples | LC-MS/MS reverse-phase using a C18 column (50 mm, 4.6 mm, 5 µm) | [8] | Minimum sample preparation steps, faster, low cost, efficient |
| 10. | Analysis of EPA and DHA in human plasma | HPLC-ECD (electrochemical detector) using a Develosil C30-XG-3 | [29] | Minimum sample preparation steps, faster, low cost, efficient |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





