1. Introduction
The highly complex etiology of obesity and its dynamic, encompassing genetic, physiologic, environmental, psychological, social, economic, and even political factors interacting in several ways promote and aggravate the obesity pandemic [
1,
2,
3]. For this reason, it is hard to treat obesity effectively [
4]. It is well known that the excess of adipose tissue, particularly the ectopic fat depots, are implicated in more than 200 complications of obesity and negatively impact the health of individuals affected [
5].
The high and rising prevalence of obesity along with the associated health impacts represent a real challenge for the health and policy authorities, since it can be economically prohibitive to offer treatment access to all people in need [
6,
7,
8]. Additionally, obesity is not necessarily synonymous with health risks, as the metabolically healthy obese (MHO) phenotype and also the fat-but-fit paradigm have also been documented [
9,
10].
In fact, there is consistent evidence about the prevalence of MHO with some studies presenting very high rates of that phenotype, like one with Brazilian women in which the prevalence of MHO was around 70% considering the HOMA-IR and the NCEP-ATPIII criteria for metabolic syndrome [
6]. Although, the prevalence of this phenotype can vary between ≈15% and ≈30% depending on the definition of Metabolic Syndrome (MetS), using the criteria of meeting 0 or 0–1 MetS components, respectively [
10]. Studies have also revealed that the MHO is an unstable state, since an important part of the MHO subjects evolved to the unhealthy phenotype within some years [
9,
11]. The necessity to find better ways to diagnose people with obesity with the use of risk stratification categories is important to determine who is in high risk and in need of intervention strategies. The traditional criteria diagnostic of MetS requires the presence of three or more from the 5 components, including greater waist circumference (WC), dyslipidemia with high triglycerides (TG), low high-density lipoprotein cholesterol (HDL-C) levels, elevated blood pressure (BP) and impaired fasting plasma glucose (FPG) [
12]. Because of its dichotomous or binary nature, some authors have proposed one option in which a continuous metabolic syndrome risk sore can be used. This approach has the advantage of preserving the statistical power which is decreased when dichotomizing continuous variables. It also allows to measure in a more precise way the MetS risk along a continuum [
12,
13,
14].
The one-size-fits-all approach should be avoided when programs to treat obesity are offered. The identification of subgroups whose risk profile is distinct is important to improve clinical practice [
7,
10]. It is also essential to make feasible offering access to treatment programs to those whose health risks are higher [
3]. Hence, the main objective of this study was to assess the prevalence of traditional and recently developed risk factors assessing tools like the triglyceride glucose (TyG) index and related indexes, the continuous metabolic severity scores (MetSs) and the atherogenic index of plasma (AIP), related to the different obesity categories in a sample of Brazilian adults.
2. Materials and Methods
This descriptive study of cross-sectional design was carried out with 404 adults of both sexes, aged between 18 and 50 years, with obesity (Body Mass Index - BMI ≥30 kg/m
2). Participants were selected to take part in the research project during the years of 2018, 2019, 2020 (first semester) and 2022 (second semester), in the Multidisciplinary Treatment of Obesity Program (MTOP), coordinated by the Multidisciplinary Obesity Studies Nucleus (NEMO) of the State University of Maringá (UEM) and Regional University Hospital of Maringá (HUM). Detailed description of the study can be found elsewhere [
15]. In brief, eligible participants were invited to take part voluntarily in the study through dissemination in the local media (TV, radio, newspaper) and social networks (website and institutional email, Facebook). The interested participants took part in a pre-inclusion phase (Cardiometabolic Risk Assessment; CAR, divided into two steps) to confirm their eligibility to be involved in the study. In step 1, the conditions of eligibility were verified (age over 18 and under 50 and BMI over 30kg/m
2) and 774 people answered an anamnesis which included socioeconomic and health data. They had also evaluated: body mass, height, BMI, waist circumference (WC), and body composition by bioimpedance. Along with that their blood pressure (BP) and basal heart rate (HR) was measured. Finally, the physical fitness tests including the sit and reach for flexibility, the 30 seconds sit and stand for lower limbs resistance, the plank strength test for abdominal static resistance, and the six minutes’ walk test (6MW) for cardiorespiratory fitness were applied.
After that process, a total of 404 people met the inclusion criteria, and were therefore considered eligible to participate in step 2, which included carrying out laboratory tests to verify the profile of cardiometabolic risk through the fasting measures of blood glucose, insulinemia, glycated hemoglobin, total cholesterol, HDL-c, LDL-c, VLDL-c, triglycerides and ultrasensitive C-reactive protein.
In order to determine the dosages of all those biochemistry variables, standard procedures were applied by specialized professionals from a private laboratory with quality control and an ISO certification.
Beyond the single parameters mentioned above, other surrogate measures of Insulin Resistance (IR) were determined by the Homeostasis Model Assessment (HOMA-IR) calculated as follows: HOMA-IR = (insulin × glucose)/22.5
[16,17]. The evaluation of homeostasis to verify the beta cells of the pancreas was determined by the calculation (Homa-Beta): 20 x Insulin (iu/ml) ÷ (Glycemia - 3.5), and the cut-off reference values were between 167 to 175 [
18].
Triglyceride glucose (TyG) index calculated as ln [fasting triglycerides (mg/dl)×fasting plasma glucose (mg/dl)/2 ]) [
19]. We also calculated the product of triglyceride (TG) and fasting plasma glucose (FPG), the TyG index and also the TyG related to the adiposity status obtained by the equation (TyG/body mass index) and the TyG related to visceral adiposity by the ratio TyG by waist circumference [
20]. It was also used for the risk assessment the atherogenic index of plasma (AIP), which is defined as the logarithm of plasma triglycerides to HDL-c ratio [
21].
All procedures followed the requirements of Resolution 466/2012 of the National Health Council for research involving human beings which is made based on the principles of the Helsinki Declaration. Participants read and signed the Term of Free and Informed Consent agreeing to voluntarily participate in the research. The research was previously approved by the Permanent Committee of Ethics in Research of the State University of Maringá (Record nº 2,655,268).
The researchers involved in the assessments were all trained and followed standard procedures measuring anthropometric variables with the proper tools like height with a wall stadiometer (Sanny®), waist circumference (WC) with a flexible anthropometric tape (Medical Starrett-SN-4010 model, Sanny®) and body weight with a bioimpedance electric device (model InBody 520, Biospace®). Blood pressure was measured using an automatic arm monitor (model HEM-7113, Omron®). Blood collection and analysis for measuring blood glucose, high-density lipoprotein (HDL-c) and triglycerides was performed by qualified professionals in a private clinical analysis laboratory with quality certification between 7:00-9:00am with patients observing fasting for at least 8 hours.
To calculate the BMI, we used the formula: weight (kg) / [height (m) x height (m)] and the classification was based on the cutoff points of the World Health Organization (WHO, 2011). The WC measurement rating was also based on the WHO cutoff points, namely: WC >94 cm for men and >80 cm for women indicating increased risk of metabolic complications; whereas WC >102 cm for men and >88 cm for women indicated substantially increased risk of metabolic complications [
22].
Blood pressure was classified according to the 7th Brazilian Guideline on Hypertension, as follows: normotension; systolic blood pressure (SBP) and diastolic blood pressure (DBP) ≤120/80 mmHg; prehypertension: SBP between 121 and 139 and/or DBP between 81 and 89 mmHg and hypertension SBP ≥140 mmHg and/or DBP ≥90 mmHg [
23].
Classification of fasting blood glucose followed the criteria of the Guidelines of the Brazilian Society of Diabetes 2017-2018, as follows: normoglycemia: fasting blood glucose <100 mg/dL; pre-diabetes (or increased risk for diabetes mellitus): ≥100 to <126 mg/dL; and established diabetes: ≥126mg/dL (Oliveira, Montenegro Junior and Vencio 2017). The lipid profile was classified according to the 2017 Brazilian Guideline for Dyslipidemia and Atherosclerosis Prevention, as follows: high level of fasting triglycerides: ≥150 (mg/dL); low fasting HDL-c level <40 mg/dL for men and <50 mg/dL for women [
24].
For data analysis, normality was verified using the Kolgomorov-Smirnov test. The average (
) and standard deviation (SD) were used as descriptive statistics. To compare variables according to gender, unpaired t-tests were used. To compare the variables according to age group and level of obesity, a one-way ANOVA was used, with Bonferroni correction for multiple comparations, and post-hoc tests were used to indicate between which groups there were differences. To correlate the variables, Pearson correlation coefficient was used. Analyzes were performed using the Statistical Package for Social Sciences (SPSS)® version 20.0 [
25]. A significance level of p<0.05 was adopted for all analyses.
3. Results
The participants of this study were 404 adults with obesity (
Table 1), 85 men (21%) and 319 women (79%), aged between 18 and 50 years (mean±SD: 36.6±8.8 years). The BMI of the sample ranged from 31.3 to 77.2 kg/m
2 (mean±SD: 42.5±6.7). According to cutoff points of WHO, 76 (18.8%) had grade 1 obesity, 141 (34.9%) had grade 2 obesity, 160 (39.6%) had grade 3 obesity and 27 (6.7%) had obesity grade IV or super obesity. Considering the degree of obesity, significant differences (p<0.05) were observed in all anthropometric/body composition variables, except height and in the relation lean mass/fat mass. At the same time, the classes/degree of obesity also presented significant differences related to the hemodynamic and physical fitness variables with DBP being higher in the group with obesity class IV and heart rate (HR) being higher in the group with obesity class III compared to class I (p<0.001). There were also significant differences in the total distance at the 6 minutes walk test with the group with obesity class IV presenting the lowest distance (p<0.001). Flexibility was also lower in the groups obesity class III and IV compared to class I and II (p<0.001).
The biochemistry parameters indicated significant differences in glucose, insulin, HOMA-IR, Homa-beta, and hs-PCR among the class III and IV compared to class I and II with the higher BMI classes presenting the unhealthier results (p<0.01). However, the markers of dyslipidemia and the HbA1c levels did not show significant differences. Lastly, related to the index or ratios applied to identify alterations related to insulin resistance (IR) or dyslipidemia, significant differences were observed in the percentile of the continuous metabolic syndrome score related to BMI, with the higher classes of obesity presenting the more severe score risks of metabolic syndrome (p<0.001). The same pattern is observed in the MetS-WC and Percentile of MetS-WC. The TYG parameters also have that pattern with the groups with obesity classes III and IV presenting the higher risks (p<0.001).
When we look to the data stratified by sex (
Table 2 and
Table 3), we noticed that among males (
Table 2) there were significant differences among BMI groups except for age, height and fat percentage in the group of anthropometric and body composition variables, with higher values corresponding to the higher classes of BMI, with the only exception to the lean/fat ratio which has lower values in the higher BMI groups. Considering the male results for hemodynamic and physical fitness, the only variables presenting significant differences were heart rate (bpm), with higher values in the obesity class IV and lowers values to the distance in the six minutes’ walk test and in the sit and reach (flexibility) test for the higher BMI groups. With the biochemistry parameters, we observed significant differences only with the high sensitive C-reactive protein (hsCRP), with higher values in the class IV BMI and in total cholesterol, LDL-c and non-HDL-c with, contrary to what was expected, lower values in the class IV BMI. Whereas in the last group of variables (biochemistry index or ratios), the significant differences were observed for MetS - Z BMI, and its respective percentile, and also for the MetS – Z WC and its percentile, always with higher values, indicative of the severity of the MetS in the class IV BMI group. There were also significant differences in the TYG-BMI and TYG-WC following the same pattern for the highest groups of BMI (classes III and IV).
Comparing the BMI groups among the women (
Table 3), we can see very similar results to those described above (
Table 1 and
Table 2). In the anthropometric and body composition variables, only age and the ratio lean to fat mass didn’t show significant differences. Whereas all the other variables presented higher values for the classes III and IV of BMI. Considering the group of hemodynamic and physical fitness variables, there were significant differences among the BMI groups for heart rate, with the class I obesity presenting the lowest values. For the distance in the six minutes’ walk test, the sit and stand (dynamic) test and for the sit and reach (flexibility) test, we found that the higher BMI groups presented the lowest values. For the biochemistry parameters, there were significant differences in insulin, HOMA-IR, Homa-Beta, and hsCRP, all with higher values corresponding to the classes III and IV of BMI. The VLDL-c presented significant differences between the BMI class I against class II. Finally, for the biochemistry index or ratios, the significant differences were observed for MetS - Z BMI percentile and for the MetS – Z WC and its percentile, always with higher values, indicative of the severity of the MS in the class IV BMI group. There were also significant differences in the TYG 1 and 2 and TYG-BMI and TYG-WC following the same pattern with the highest values for groups of BMI (classes III and IV).
In Table 4, the participants are grouped according to their age group. They were split in two groups, the young adults (18 and 39 years old) and the middle age group with participants aged 40 to 50 years old. These groups were compared according to the same categories presented above with the BMI stratification. The differences here (Table 4) were observed in height, weight, BMI, LBM, FM, WC, abdominal circumference and hip circumference, all with higher values for the younger group. The other groups of variables presented different patterns, with the younger group having lower values for SBP and lower glucose levels. They also have total cholesterol, HDL-c, LDL-c, non-HDL-c, HbA1c, TyG1,2, and TyG-BMI lower than the middle age group. Whereas their HOMA-Beta was higher than the middle age group.
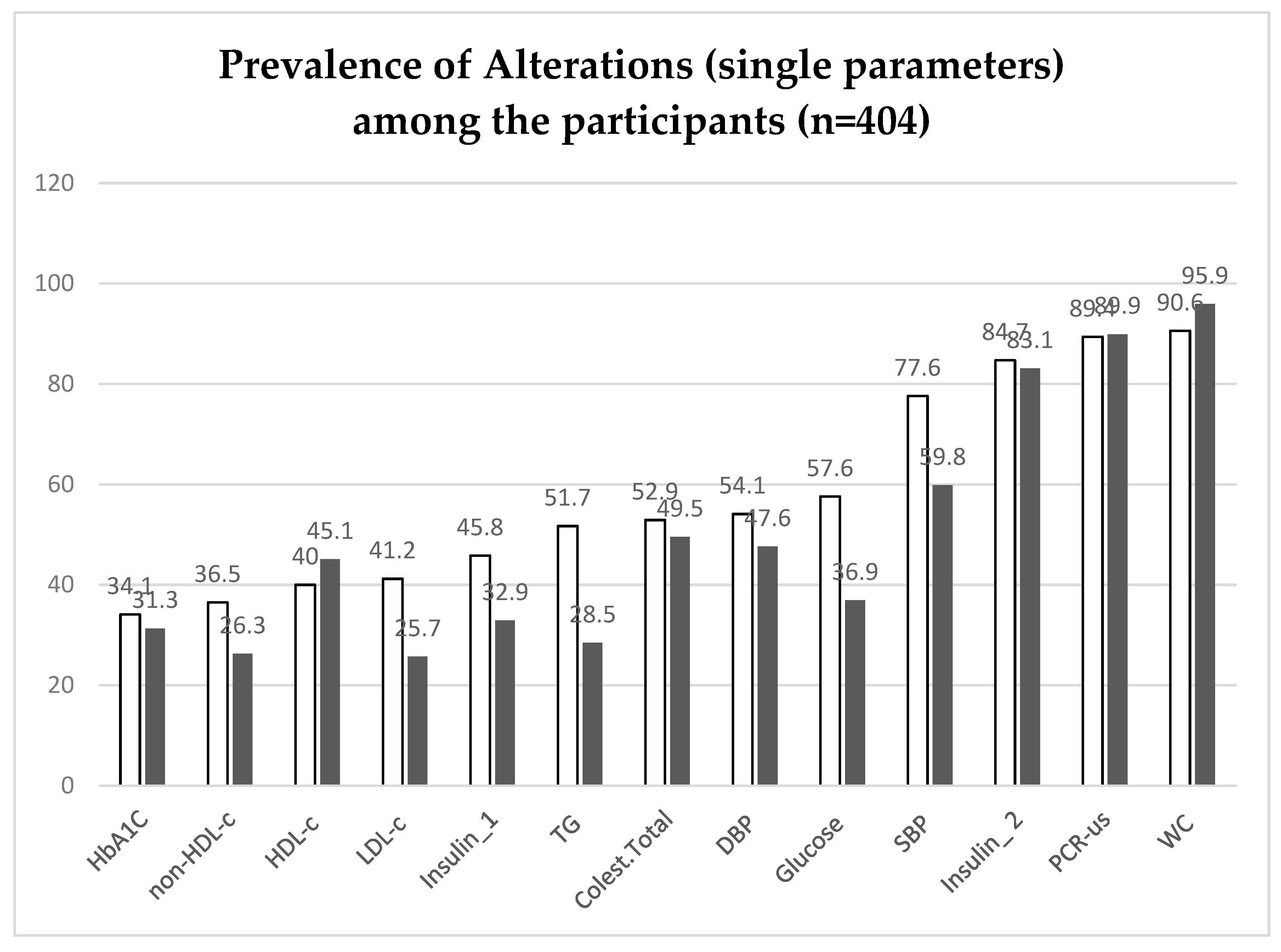
Table 5 and
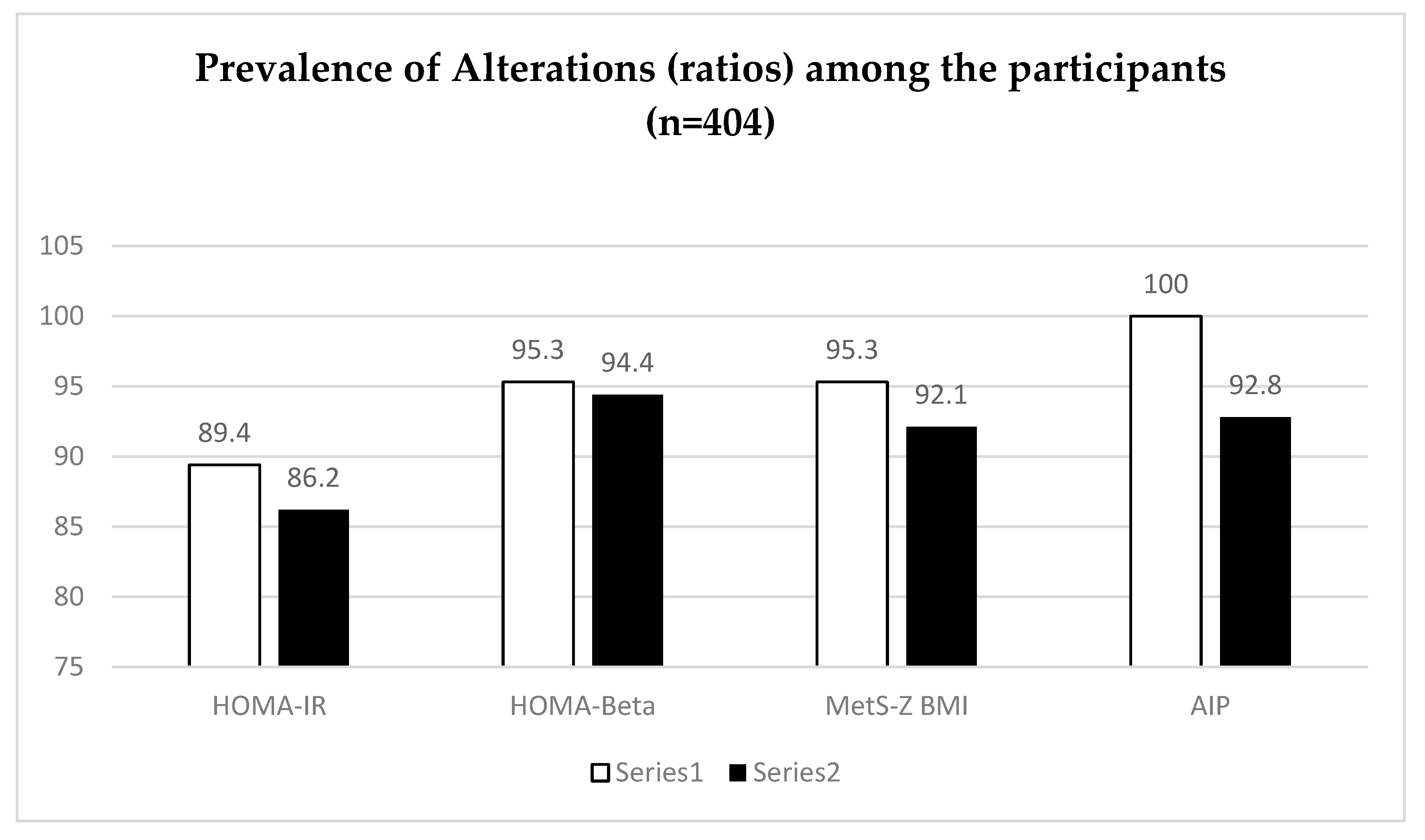
Figure 1 and
Figure 2 present the prevalence of single biochemical or anthropometric parameters and also indexes or ratios used to the assessment of metabolic risks. The lower prevalences were observed for HbA1c, with 34.1% of man and 31.3% of women showing elevated values (above of the cutoff point of 5.7%) for that parameter [
26]. On the other hand, the index whose prevalence was higher was the AIP with 100% of man and 92.8% of women presenting values above the cutoff point of 0.1–0.24 as moderate risk, and >0.24 as high-risk occurrence of cardiovascular disease proposed by [
27].
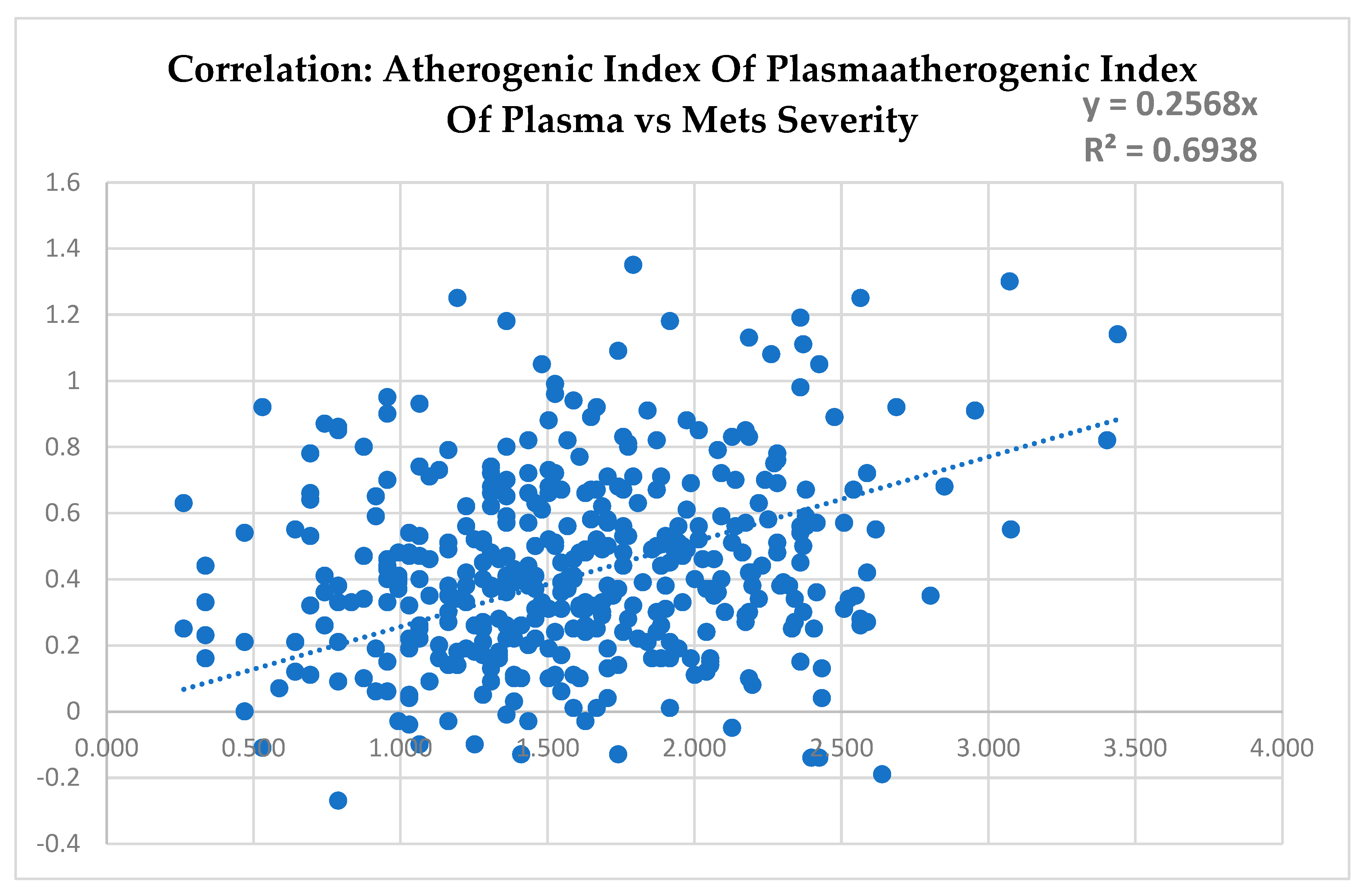
It can be noted in
Figure 3 that the score of severity of metabolic syndrome (MetSs) and the atherogenic index of plasma (AIP) presented a significant correlation with a coefficient of determination (R
2) of 69%, indicating that the two index are strongly correlated.
4. Discussion
The main goal of this study was to verify the risk profile of participants in one multiprofessional treatment program of obesity (MTPO) offered regularly by the Maringa State University to different age groups since 2005. The adults involved in that program had a real need of professional care to treat their obesity and comorbidities. In Maringa, there is no public funded program offering that kind of treatment to this public. Therefore, this population needs to volunteer as research subjects to get access to that model of assistance. This is the first, and maybe the most important data to present. This reality is not different from the one observed in most of the countries around the world which also don’t have enough MTPO to assist their population [
8].
The second important information provided by this study is the high prevalence of metabolic risk found in this sample as even 100% of the male participants were classified as having moderate or high risk of occurrence of cardiovascular disease according to the AIP. The atherogenic lipoprotein profile of plasma has been recognized as a substantial risk factor for atherosclerosis. That is due to the significance of triglycerides in atherosclerotic and cardiovascular disease. In addition to individual serum cholesterol levels, the atherogenic index of plasma (AIP) has been suggested as a marker of plasma atherogenicity based on the evidence of its positive association with lipoprotein particle size, cholesterol esterification rates, and remnant lipoproteinemia [
28].
The AIP was found to be one of the strongest markers in predicting the cardiovascular disease (CVD) risk. There is also evidence indicating that AIP is associated with other CVD risk factors. Thus, MTPO which promotes modification of lifestyle, is strongly recommended and the monitoring of parameters like AIP can be used to assess the efficacy or effectiveness of that kind of intervention program [
29].
It is worth to explain that the AIP is defined as the logarithm of triglycerides to high-density lipoprotein cholesterol (HDL-C) ratio. Thus, it is a strong predictor of future cardiovascular disease. AIP was directly and independently associated with arterial stiffness and it is also known to inversely correlate with LDL particle size. Besides of that it can be readily calculated from the routine lipid profiles [
21].
There is great interest in the development of new and comprehensive lipid index, like the atherogenic index of plasma, which might reflect the balance between atherogenic and anti-atherogenic factors. Recently, AIP has been shown to be a strong marker for predicting the risk of CAD and the value of AIP was positively associated with waist circumference and BMI and inversely associated with physical activity [
30].
Another recent study found evidence to propose that AIP can be considered as a novel and better biomarker for obesity since subjects in the higher quartiles of AIP had all a significantly increased risk of obesity compared with those in the lowest quartile in a Chinese population [
31]. Interestingly, the mean value for the group with obesity in that study was 0.13, whereas in our study that mean was 0.44 or more than three times higher. We also identified a similar fact comparing the results from our study to another applying the AIP in a Chinese population in which the average AIP was 0.17 to the group with coronary artery disease and 0.12 in the control group [
30].
Therefore, results like these can be very important to balance the idea of metabolic health obese (MHO) which can mislead and postpone some more the intervention program that a higher number of individuals need. Besides, even when the MHO phenotype is correctly identified there is still a chance of reversing it over time, since the MHO is an instable state with an important part of the subjects evolving to the unhealthy phenotype within some years [
9,
11].
It is important to highlight too that even simple measures like the waist circumference can be very useful as a clinical tool, since that was the single parameter that diagnosed more than 90% in this study, regardless of gender. That inexpensive variable, alone or combined with more complex models, can be very useful for the risk stratification. But it is also important to express that increased WC is undeniably an excellent marker of cardiometabolic status in individuals with normal weight or overweight, but it is less useful in individuals with higher BMI [
3].
In that context it becomes relevant to remind that the abdominally obese males increases the risk of cardiovascular disease 20-fold over the course of 5 years and that reinforce the necessity of protocols including simple measures like the WC as important tools to identify high risk individuals [
7,
32]. The same authors have also demonstrated that the excess fat occurs predominantly due to subcutaneous or visceral abdominal fat and are closely related to insulin resistance (IR) or diabetes.
The data presented in our study corroborated that with prevalence of HOMA-IR above the cutoff point over 80% in both genders with men presenting the higher prevalence (89.4%) with really close numbers from the women (86.2%). This is an impressive number since insulin resistance is one of the main factors associated with cardiovascular diseases. It is also important because in Brazil that diagnosis test is not included in the routine exams and therefore, an important proportion of persons with pre-diabetes or diabetes are not identified at the proper time [
33].
It is also important to bring information about the HOMA-Beta utility since it is proposed as a measure of the functionality of the pancreatic beta cells. It has a cutoff point of 167 to 175 and it is interpreted as lower values indicating better beta cells functioning. Based on that cutoff point it was shown that a very high proportion of the studied population exposed to higher risk of long term damage of the beta cells functioning and, consequently, higher risks of the development of DM2 [
18].
Another single parameter which shows very higher prevalence was the hsCRP with number close to 90% above the cutoff point. That reinforce the proinflammatory state promoted by the excess of adipose tissue which is common among people with obesity. This data is align with another study in which the hsCRP was the most important biomarker associated with the cardiovascular risk [
34]. We have also a systematic review in which the hsCRP was used to stratify risks in several chronic non communicable diseases and it was recognized as the most promisor of the seric biomarkers to be employed in these conditions to assess the clinical status and evolution [
35].
Despite the overall importance of the traditional way to diagnose the metabolic syndrome (MetS) there are difficulties related to the utility of this information for the present or future (follow up) use, due to its nature binary. That has been recognized and other ways to use it has been presented, like the continuous metabolic syndrome risk score (cMSy) as a more appropriate and valid alternative for epidemiological and clinical studies [
12,
13,
36]. We applied the concept of the MetS severity score or MetSs to assess as a continuous variable with the mean is to represent one score of risk, or severity of MetS. This score can be interpreted as a Z-score (mean 0, SD=1), with higher scores corresponding to a higher risk of MetS [
14,
36]. These authors have been reveling racial/ethnic discrepancies using the traditional MetS criteria. They have also found that some racial groups with diabetes had a low prevalence of MetS but they have high MetS severity scores with is a contradictory result. The same group also have found that the MetS severity score correlated with risk of future type 2 diabetes and CVD [
37]. More importantly, maybe is the fact that their study showed no meaningful differences between the MetS-Z- WC and MetS-Z-BMI scores in their associations with future CHD and T2DM, indicating the potential utility for clinical use of MetS-Z-BMI.
That kind of tool has great potential to monitor prospectively high-risk patients since it has revealed that compared with individuals with a change in score smaller than (<0), participants with a change greater than half a point (>0.5) had a hazard ratio (HR) of 2.66 for incident diabetes (p<0.001). Our data show an increasing mean of both MetS-Z- WC and MetS-Z-BMI scores among the BMI classes as the higher BMI classes presenting significative higher averages, especially related to MetS-Z- WC.
Overall, these results made it clear that adults with obesity have higher risks to chronic non communicable diseases. They also have shown that different criteria, even the simple ones like WC can be very important tools to be used in the assessment of the population and they can have their diagnose improved combining results with more sophisticated parameters like those ratios and indexes presented in this study.
5. Conclusions
Finally, it is important to recognize that this study has some limitations like the cross-sectional design of it which does not allow conclusions about causality, therefore these findings should be confirmed in follow-up studies. On the other hand, it made it possible to verify that this population can be facing very challenging situations reflected by the high risks profile presented that have been ignored probably because of the fact that obesity has been recognized as a neglected disease around the world.
Author Contributions
Conceptualization, G.W.N., J.P.C. and N.N.J.; methodology, G.W.N., C.F.C., C.A.M.F., E.C.A.G., R.T.U., K.O., F.M.F.G. and N.N.J.; software, G.W.N.; validation, G.W.N, C.P.C. and N.N.J.; formal analysis, G.W.N., J.P.C., and N.N.J; investigation, G.W.N., C.F.C., C.A.M.F. E.C.A.G., R.T.U., K.O., F.M.F.G. and N.N.J.; resources, G.W.N., J.P.C. and N.N.J.; data curation, G.W.N, C.P.C. and N.N.J.; writing—original draft preparation, , G.W.N., J.P.C. and N.N.J; writing—review and editing, G.W.N., J.P.C. and N.N.J.; visualization, J.P.C and N.N.J.; supervision, J.P.C and N.N.J.; project administration, G.W.N. and N.N.J.; funding acquisition, N.N.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Effectiveness of a multidisciplinary program in the assessment of cardiometabolic risk factors and treatment of abdominal obesity in two municipalities in northwestern Paraná, funded by the Araucaria Foundation and the Ministry of Health through the public notice “CP 01/2016 Research Program for the Unified Health System : Shared Management in Health - PPSUS 2015 Edition Araucaria Foundation-PR / SESA-PR /CNPq / MS-Decit and Coordination for the Improvement of Higher Education Personnel - CAPES Brazil for the Sandwich Doctorate Scholarship Abroad.
Acknowledgments
The Coordination for the Improvement of Higher Education Personnel - CAPES Brazil for the Sandwich Doctorate Scholarship Abroad and the Healthy Active Living and Obesity Research Group - HALO, Children's Hospital of Eastern Ontario Research Institute - CHEO Ottawa Canada for the opportunity to carry out a doctoral internship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- L. J. Aronne, D. S. Nelinson, and J. L. Lillo, “Obesity as a disease state: A new paradigm for diagnosis and treatment,” Clin Cornerstone, vol. 9, no. 4, pp. 9–29, 2009. [CrossRef]
- David W. Haslam, A. M. Sharma, and C. W. le Roux, Controversies in Obesity. 2014. [CrossRef]
- B. Halpern et al., “Proposal of an obesity classification based on weight history: An official document by the Brazilian Society of Endocrinology and Metabolism (SBEM) and the Brazilian Society for the Study of Obesity and Metabolic Syndrome (ABESO),” Arch Endocrinol Metab, vol. 66, no. 2, 2022. [CrossRef]
- S. Kahan, “Overweight and Obesity Management Strategies,” no. May, pp. 186–196, 2016.
- C. W. le Roux and H. Alruwaili, “Treating obesity as a disease,” Academia Letters, no. July, pp. 1–6, 2021. [CrossRef]
- M. Scorsatto, G. Rosa, A. de C. Pimentel, R. R. Luiz, and G. M. M. De Oliveira, “Is it possible to easily identify metabolically healthy obese women?,” Arq Bras Cardiol, vol. 111, no. 5, pp. 733–737, 2018. [CrossRef]
- C. Scarsella and J.-P. Després, “Tratamiento de la obesidad: necesidad de centrar la atención en los pacientes de alto riesgo caracterizados por la obesidad abdominal,” Cad Saude Publica, vol. 19, no. suppl 1, pp. S7–S19, 2003. [CrossRef]
- World Obesity Federation, “Obesity: missing the 2025 global targets,” no. March, pp. 1–242, 2020.
- de Castro Pimentel, M. Scorsatto, G. M. Moraes de Oliveira, G. Rosa, and R. R. Luiz, “Characterization of metabolically healthy obese Brazilians and cardiovascular risk prediction,” Nutrition, vol. 31, no. 6, pp. 827–833, 2015. [CrossRef]
- F. B. Ortega, C. J. Lavie, and S. N. Blair, “Obesity and cardiovascular disease,” Circ Res, vol. 118, no. 11, pp. 1752–1770, 2016. [CrossRef]
- T. Dippe Jr. and R. Julio Cerci, “Obesity: A Risk Marker or an Independent Risk Factor for Coronary Artery Disease?,” International Journal of Cardiovascular Sciences, vol. 33, no. 1, pp. 55–56, 2020. [CrossRef]
- G. D. Kang, L. Guo, Z. R. Guo, X. S. Hu, M. Wu, and H. T. Yang, “Continuous metabolic syndrome risk score for predicting cardiovascular disease in the Chinese population,” Asia Pac J Clin Nutr, vol. 21, no. 1, pp. 88–96, 2012. [CrossRef]
- K. Wijndaele et al., “A continuous metabolic syndrome risk score: Utility for epidemiological analyses [6],” Diabetes Care, vol. 29, no. 10, p. 2329, 2006. [CrossRef]
- M. D. Deboer and M. J. Gurka, “Clinical utility of metabolic syndrome severity scores: Considerations for practitioners,” Diabetes Metab Syndr Obes, vol. 10, pp. 65–72, 2017. [CrossRef]
- R. H. Bim et al., “Revista Brasileira de Obesidade, Nutrição e Emagrecimento PREVALÊNCIA DE FATORES DE RISCO CARDIOMETABÓLICO EM ADULTOS COM OBESIDADE,” vol. 2, pp. 1270–1282, 2022.
- Geloneze et al., “HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS),” Arquivos Brasileiros de Endocrinologia & Metabologia, vol. 53, no. 2, pp. 281–287, 2009. [CrossRef]
- M. de Fátima Haueisen Sander Diniz et al., “Homeostasis model assessment of insulin resistance (HOMA-IR) and metabolic syndrome at baseline of a multicentric Brazilian cohort: ELSA-Brasil study,” Cad Saude Publica, vol. 36, no. 8, 2020. [CrossRef]
- C. J. Vasques, L. E. F. P. L. Rosado, R. de C. G. Alfenas, and B. Geloneze, “Análise crítica do uso dos índices do Homeostasis Model Assessment (HOMA) na avaliação da resistência à insulina e capacidade funcional das células-beta pancreáticas,” Arquivos Brasileiros de Endocrinologia & Metabologia, vol. 52, no. 1, pp. 32–39, 2008. [CrossRef]
- J. Lim, J. Kim, S. H. Koo, and G. C. Kwon, “Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007-2010 Korean national health and nutrition examination survey,” PLoS One, vol. 14, no. 3, pp. 1–11, 2019. [CrossRef]
- L. K. Er et al., “Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for insulin resistance in nondiabetic individuals,” PLoS One, vol. 11, no. 3, pp. 1–12, 2016. [CrossRef]
- M. K. Choudhary et al., “Atherogenic index of plasma is related to arterial stiffness but not to blood pressure in normotensive and never-treated hypertensive subjects,” Blood Press, vol. 28, no. 3, pp. 157–167, 2019. [CrossRef]
- W. World Health Organization, “WHO | Waist Circumference and Waist–Hip Ratio. Report of a WHO Expert Consultation. Geneva, 8-11 December 2008.,” no. December, pp. 8–11, 2011, [Online]. Available: http://www.who.int.
- Malachias MVB, Plavnik FL, Machado CA, Malta D, Scala LCN, and Fuchs S., “7a Diretriz Brasileira de Hipertensão Arterial: Capítulo 1 - Conceituação, Epidemiologia e Prevenção Primária,” Arq Bras Cardiol, vol. 107, no. 3, pp. 1–6, 2016, [Online]. [CrossRef]
- “FALUDI, A. A. et al. Atualização da Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose – 2017. Arquivos Brasileiros de Cardiologia, v. 109, n. 2, supl. 1, p. 1-76, 2017”. [CrossRef]
- Andy Field, “Discovering Statistics using SPSS Statistics,” SAGE Publications, vol. 66, p. 822, 2009, [Online]. Available: http://www.amazon.com/Discovering-Statistics-using-IBM-SPSS/dp/1446249182.
- J. P. de O. Egídio, M. C. Foss-Freitas, R. M. M. Junior, and S. Vencio, Diretrizes da Sociedade Brasileira de Diabetes 2017-2018. 2017. [CrossRef]
- K. A. Mcauley et al., “Diagnosing insulin resistance in the general population,” Diabetes Care, vol. 24, no. 3, pp. 460–464, 2001. [CrossRef]
- K. B. Won et al., “Atherogenic index of plasma and the risk of rapid progression of coronary atherosclerosis beyond traditional risk factors,” Atherosclerosis, vol. 324, no. December 2020, pp. 46–51, 2021. [CrossRef]
- M. S. Bo, W. L. Cheah, S. Lwin, T. Moe Nwe, T. T. Win, and M. Aung, “Understanding the Relationship between Atherogenic Index of Plasma and Cardiovascular Disease Risk Factors among Staff of an University in Malaysia,” J Nutr Metab, vol. 2018, no. 2015, 2018. [CrossRef]
- G. Cai, G. Shi, S. Xue, and W. Lu, “The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population,” Medicine (United States), vol. 96, no. 37, pp. 1–6, 2017. [CrossRef]
- X. Zhu et al., “Atherogenic index of plasma is a novel and better biomarker associated with obesity: A population-based cross-sectional study in China,” Lipids Health Dis, vol. 17, no. 1, pp. 1–6, 2018. [CrossRef]
- M. Eickemberg et al., “Indicators of abdominal adiposity and carotid intima-media thickness: Results from the longitudinal study of adult health (ELSA-Brazil),” Arq Bras Cardiol, vol. 112, no. 3, pp. 220–227, 2019. [CrossRef]
- A. de Carvalho, P. C. de A. Fonseca, J. B. Barbosa, S. P. Machado, A. M. Dos Santos, and A. A. Da Moura Silva, “The association between cardiovascular risk factors and anthropometric obesity indicators in university students in São Luís in the state of Maranhão, Brazil,” Ciencia e Saude Coletiva, vol. 20, no. 2, pp. 479–490, 2015. [CrossRef]
- S. M. Junqueira, L. J. M. Romêo Filho, and C. de L. C. Junqueira, “Evaluation of the degree of vascular inflammation in patients with metabolic syndrome,” Arq Bras Cardiol, vol. 93, no. 4, pp. 360–6, 353–9, 2009. [CrossRef]
- G. Westphal, S. B. S. Baruki, T. A. de Mori, M. I. de L. Montebello, and E. M. Pazzianotto-Forti, “Effects of Individualized Functional Training on the Physical Fitness of Women with Obesity,” Lecturas: Educación Física y Deportes, vol. 25, no. 268, pp. 61–75, 2020. [CrossRef]
- M. J. Gurka, C. L. Lilly, M. N. Oliver, and M. D. Deboer, “An Examination of Sex and Racial/Ethnic Differences in the Metabolic Syndrome among Adults: A Confirmatory Factor Analysis and a Resulting Continuous Severity Score,” Metabolism, vol. 63, no. 2, pp. 218–225, 2014. [CrossRef]
- M. J. Gurka, S. L. Filipp, S. K. Musani, M. Sims, and M. D. DeBoer, “Use of BMI as Marker of Adiposity in a Metabolic Syndrome Severity Score: Derivation and Validation in Predicting Long- term Disease Outcomes,” Metabolism, vol. 83, no. 1, pp. 68–74, 2018. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).