Submitted:
03 May 2023
Posted:
04 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
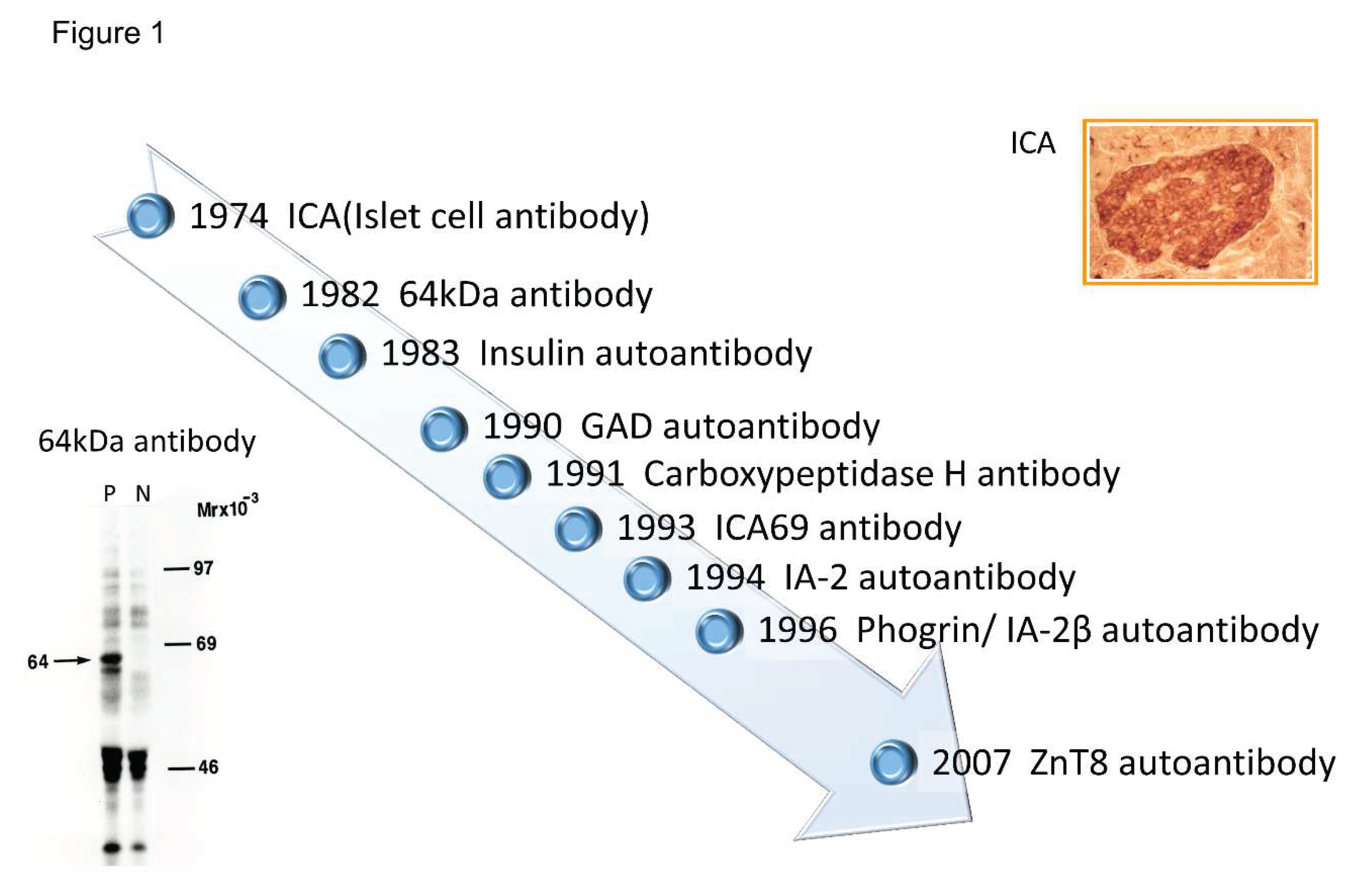
2. History of Anti-Islet Autoantibody Discovery
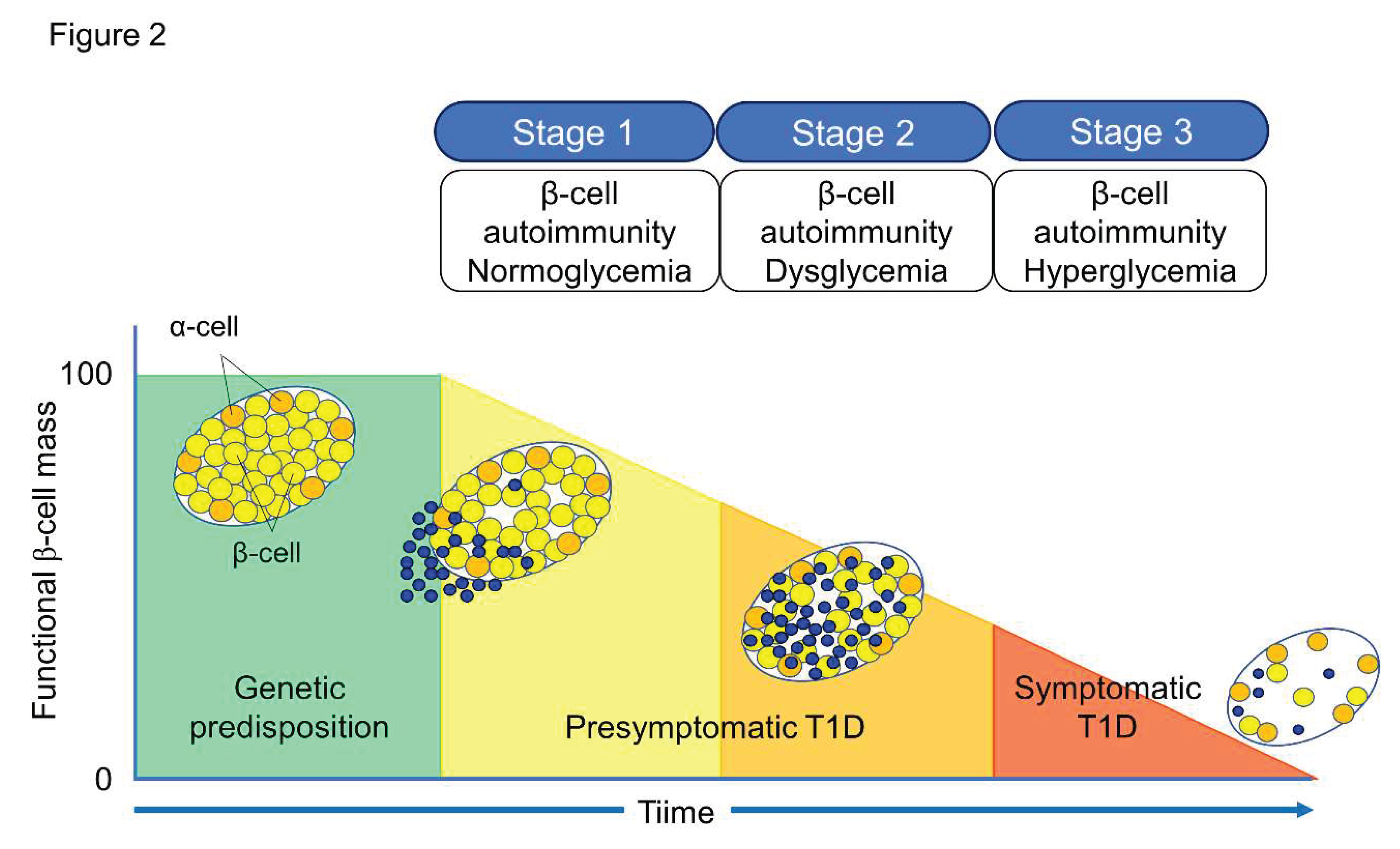
3. Significance of Anti-Islet Autoantibodies in the Pathophysiology of T1D
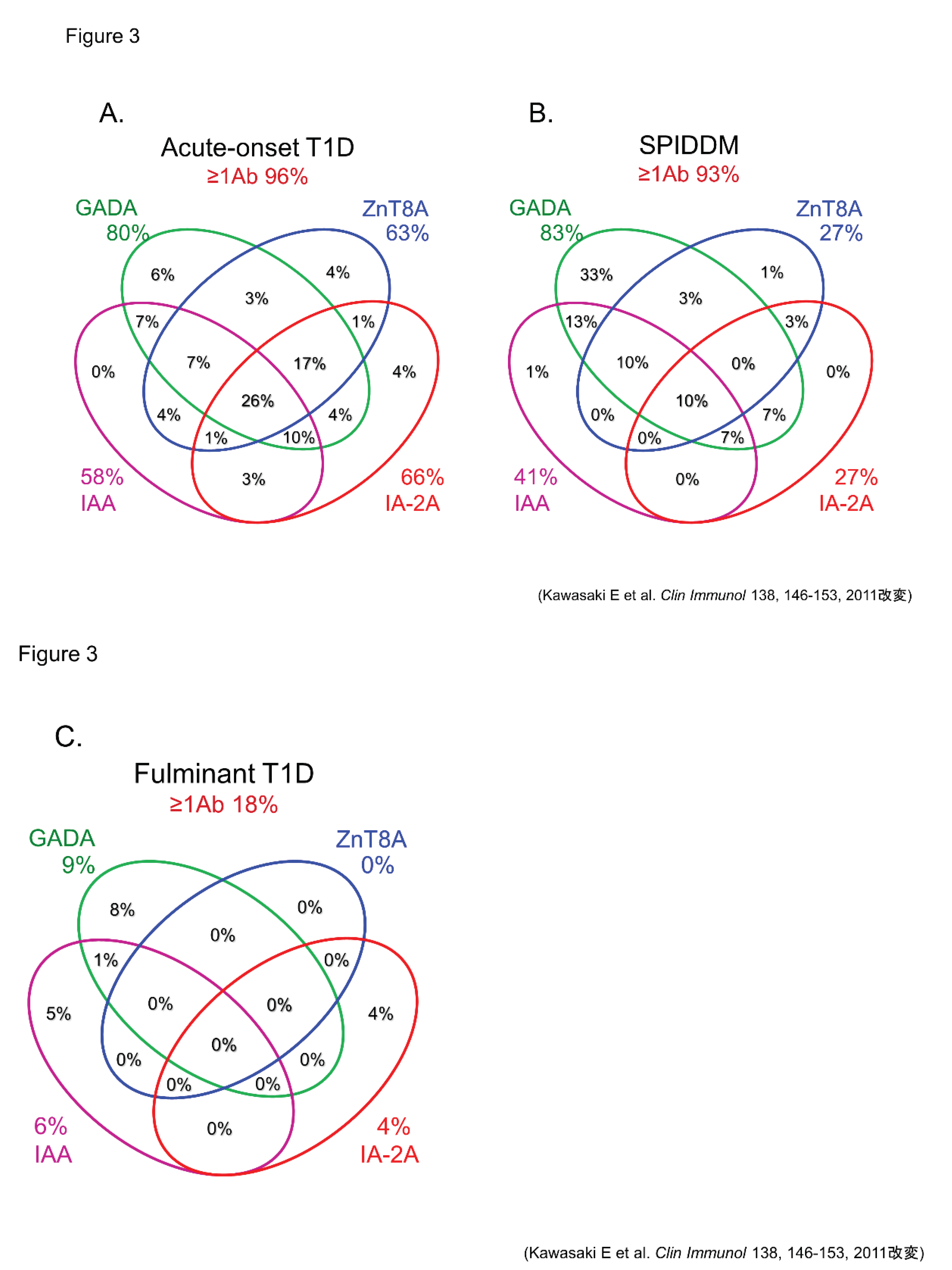
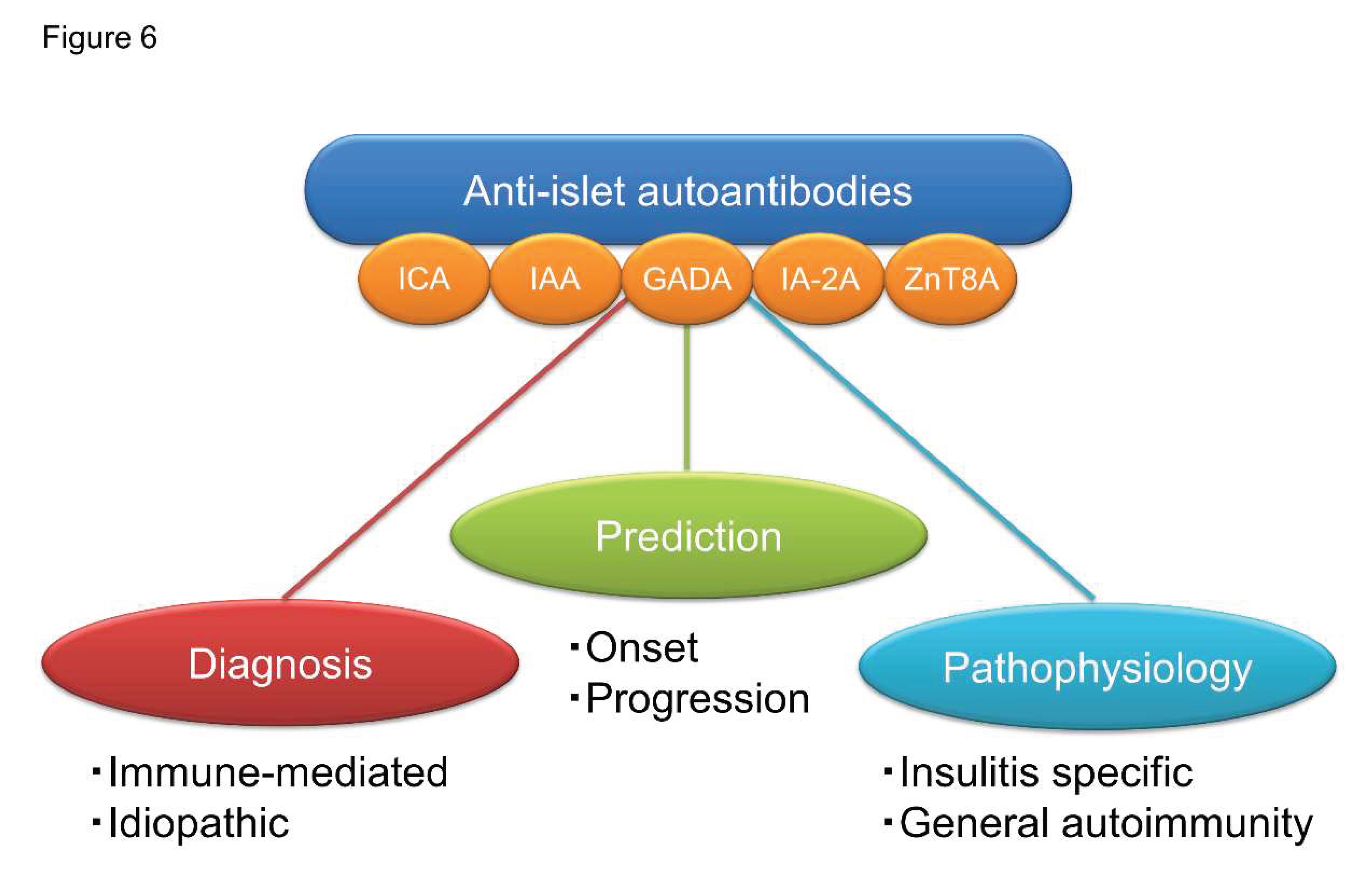
4. Role of Anti-Islet Autoantibodies in the Diagnosis of T1D
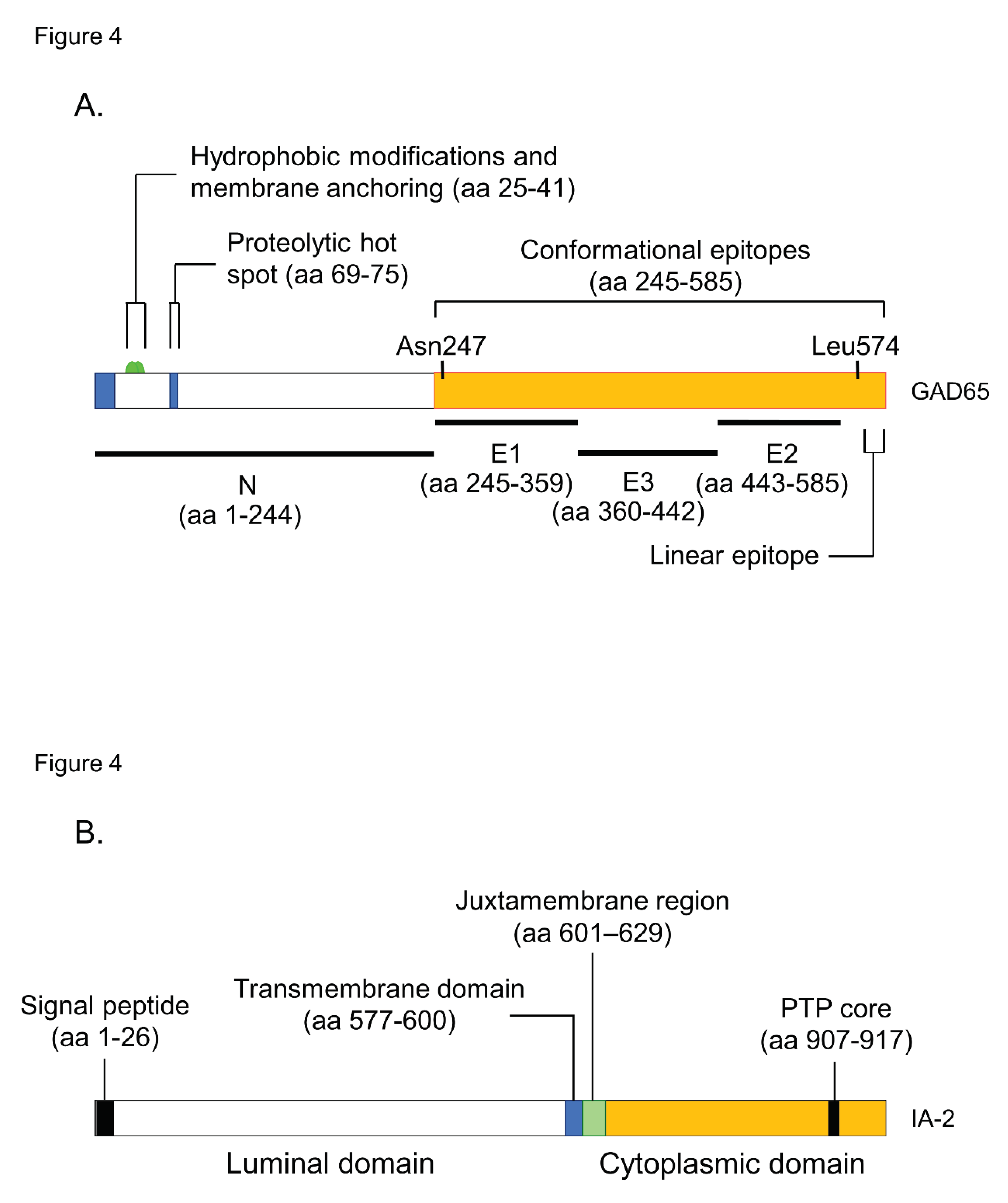
5. Epitopes for Anti-Islet Autoantibodies and Their Clinical Relevance
- 1)
- Insulin autoantibodies
- 2)
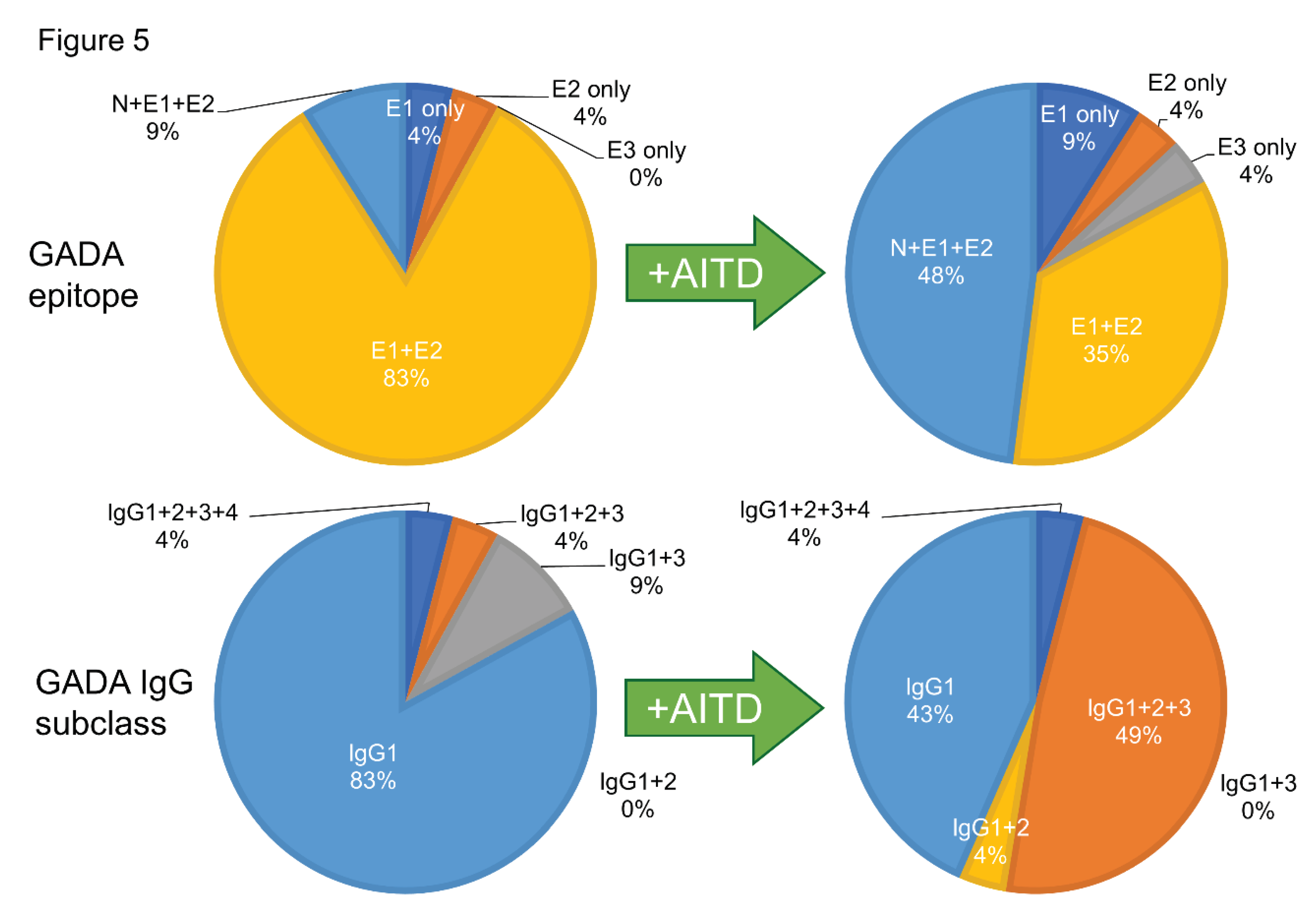
- GAD autoantibodies
- 3)
- IA-2 autoantibodies
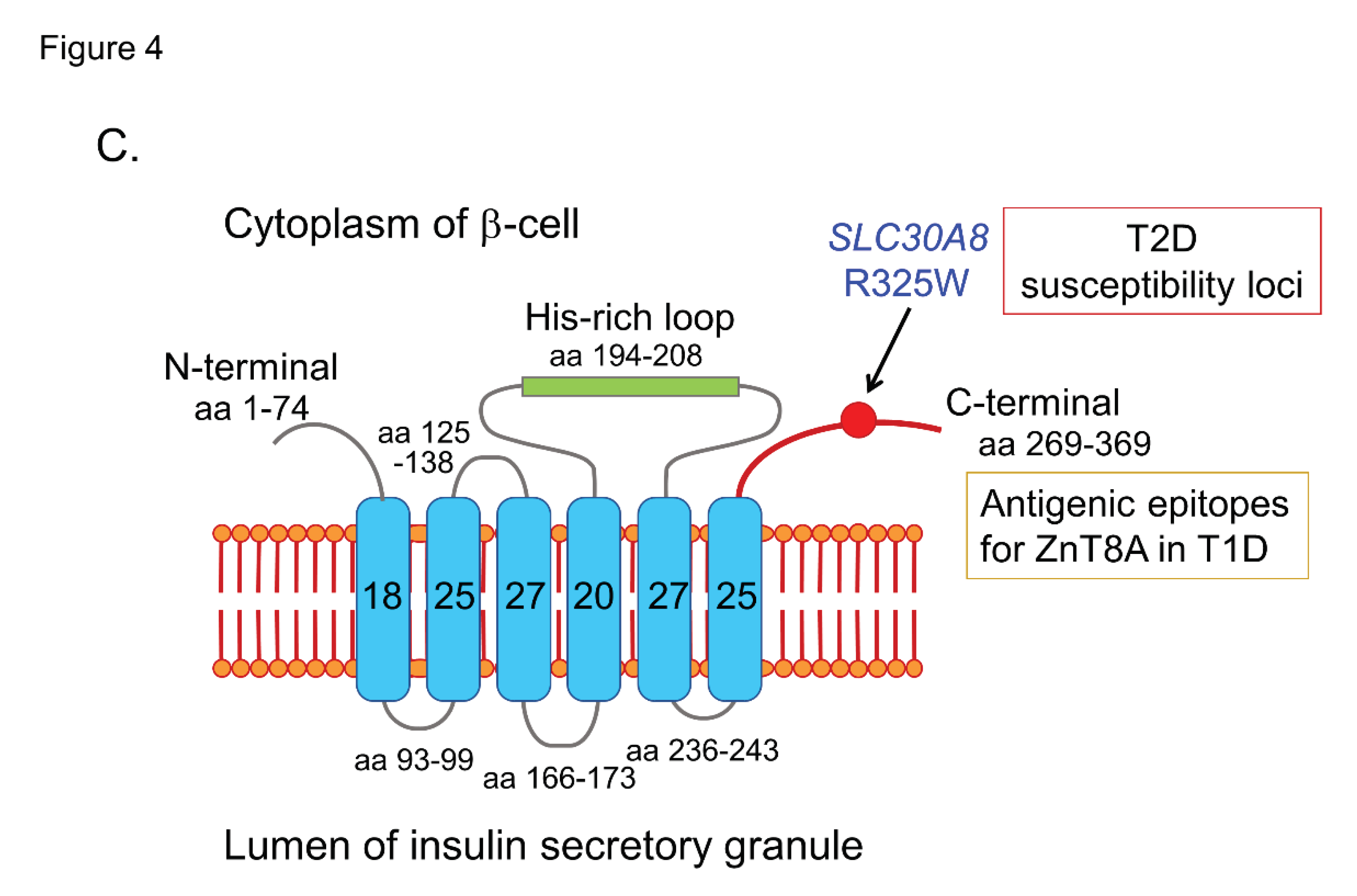
- 4)
- ZnT8 autoantibodies
6. Prediction of Future Insulin Deficiency in Patients with SPIDDM (LADA)
7. Type 1 Diabetes and Associated Autoimmune Diseases
8. Recent Advances in Anti-Islet Autoantibody Assay
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| ADA | American Diabetes Association |
| AITD | autoimmune thyroid disease |
| APS3v | autoimmune polyendocrine syndrome type 3 variant |
| ECL | electrochemiluminescence |
| ELISA | enzyme-linked immunosorbent assay |
| GAD | glutamic acid decarboxylase |
| IA-2 | tyrosine phosphatase-like protein IA-2 |
| IAA | insulin autoantibodies |
| ICA | islet cell antibodies |
| JDRF | Juvenile Diabetes Research Foundation |
| JM | juxta-membrane |
| LADA | latent-autoimmune diabetes in adults |
| MHC | major histocompatibility complex |
| PTP | protein tyrosine phosphatase |
| RBA | radio-ligand binding assay |
| RIA | radioimmunoassay |
| SPIDDM | slowly-progressive type 1 diabetes |
| T1D | type 1 diabetes |
| T2D | type 2 diabetes |
| UKPDS | United Kingdom Prospective Diabetes Study |
| ZnT8 | zinc transporter 8 |
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46, S19–S40. [Google Scholar] [CrossRef]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 2020, 17, 150–161. [Google Scholar] [CrossRef]
- Karvonen, M.; Viik-Kajander, M.; Moltchanova, E.; Libman, I.; LaPorte, R.; Tuomilehto, J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. . Diabetes Care 2000, 23, 1516–1526. [Google Scholar] [CrossRef]
- Kawasaki, E.; Matsuura, N.; Eguchi, K. Type 1 diabetes in Japan. Diabetologia 2006, 49, 828–836. [Google Scholar] [CrossRef]
- Bottazzo, G.F.; Florin-Christensen, A.; Doniach, D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 1974, 2, 1279–1283. [Google Scholar] [CrossRef]
- Baekkeskov, S.; Nielsen, J.H.; Marner, B.; Bilde, T.; Ludvigsson, J.; Lernmark, A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature 1982, 298, 167–169. [Google Scholar] [CrossRef]
- Palmer, J.P.; Asplin, C.M.; Clemons, P.; Lyen, K.; Tatpati, O.; Raghu, P.K.; Paquette, T.L. Insulin Antibodies in Insulin-Dependent Diabetics Before Insulin Treatment. Science 1983, 222, 1337–1339. [Google Scholar] [CrossRef]
- Baekkeskov, S.; Aanstoot, H.-J.; Christgai, S.; Reetz, A.; Solimena, M.; Cascalho, M.; Folli, F.; Richter-Olesen, H.; Camilli, P.-D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 1990, 347, 151–156. [Google Scholar] [CrossRef]
- Lan, M.S.; Lu, J.; Goto, Y.; Notkins, A.L. Molecular cloning and identification of a receptor- type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 1994, 13, 505–14. [Google Scholar] [CrossRef]
- Wenzlau, J.M.; Juhl, K.; Yu, L.; Moua, O.; Sarkar, S.A.; Gottlieb, P.; Rewers, M.; Eisenbarth, G.S.; Jensen, J.; Davidson, H.W.; et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 17040–17045. [Google Scholar] [CrossRef]
- Karvonen, M.; Viik-Kajander, M.; Moltchanova, E.; Libman, I.; LaPorte, R.; Tuomilehto, J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000, 23, 1516–1526. [Google Scholar] [CrossRef]
- Ziegler, A.G.; Rewers, M.; Simell, O.; Simell, T.; Lempainen, J.; Steck, A.; Winkler, C.; Ilonen, J.; Veijola, R.; Knip, M.; et al. Seroconversion to Multiple Islet Autoantibodies and Risk of Progression to Diabetes in Children. JAMA 2013, 309, 2473–2479. [Google Scholar] [CrossRef]
- Krischer, J.P.; the Type 1 Diabetes TrialNet Study Group. The use of intermediate endpoints in the design of type 1 diabetes prevention trials. Diabetologia 2013, 56, 1919–1924. [Google Scholar] [CrossRef]
- Verge, C.F.; Gianani, R.; Kawasaki, E.; Yu, L.; Pietropaolo, M.; Jackson, R.A.; Chase, H.P.; Eisenbarth, G.S. Prediction of type 1 diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 1996, 45, 926–933. [Google Scholar] [CrossRef]
- Yu, L.; Rewers, M.; Gianani, R.; Kawasaki, E.; Zhang, Y.; Verge, C.; Chase, P.; Klingensmith, G.; Erlich, H.; Norris, J.; et al. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J. Clin. Endocrinol. Metab. 1996, 81, 4264–4267. [Google Scholar] [CrossRef]
- Nakamura, K.; Kawasaki, E.; Abiru, N.; Jo, O.; Fukushima, K.; Satoh, T.; Kuriya, G.; Kobayashi, M.; Kuwahara, H.; Yamasaki, H.; et al. Trajectories of anti-islet autoantibodies before development of type 1 diabetes in interferon-treated hepatitis C patients. Case reports and a literature review. Endocr. J. 2010, 57, 947–951. [Google Scholar] [CrossRef]
- Kawasaki, E.; Yu, L.; Rewers, M.J.; Hutton, J.C.; Eisenbarth, G.S. Definition of multiple ICA512/phogrin autoantibody epitopes and detection of intramolecular epitope spreading in relatives of patients with type 1 diabetes. Diabetes 1998, 47, 733–742. [Google Scholar] [CrossRef]
- Kawasaki, E.; Yasui, J.-I.; Tsurumaru, M.; Takashima, H.; Ikeoka, T.; Mori, F.; Akazawa, S.; Ueki, I.; Kobayashi, M.; Kuwahara, H.; et al. Sequential elevation of autoantibodies to thyroglobulin and glutamic acid decarboxylase in type 1 diabetes. World J. Diabetes 2013, 4, 227–230. [Google Scholar] [CrossRef]
- Kawasaki, E.; Nakamura, K.; Kuriya, G.; Satoh, T.; Kobayashi, M.; Kuwahara, H.; Abiru, N.; Yamasaki, H.; Matsuura, N.; Miura, J.; et al. Differences in the humoral autoreactivity to zinc transporter 8 between childhood- and adult-onset type 1 diabetes in Japanese patients. Clin. Immunol. 2011, 138, 146–153. [Google Scholar] [CrossRef]
- Achenbach, P.; Lampasona, V.; Landherr, U.; Koczwara, K.; Krause, S.; Grallert, H.; Winkler, C.; Pflüger, M.; Illig, T.; Bonifacio, E.; et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 2009, 52, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Wenzlau, J.M.; Frisch, L.M.; Hutton, J.C.; Fain, P.R.; Davidson, H.W. Changes in Zinc Transporter 8 Autoantibodies Following Type 1 Diabetes Onset: The Type 1 Diabetes Genetics Consortium Autoantibody Workshop. Diabetes Care 2015, 38, S14–S20. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, I.; Weets, I.; Asanghanwa, M.; Ruige, J.; Van Gaal, L.; Mathieu, C.; Keymeulen, B.; Lampasona, V.; Wenzlau, J.M.; Hutton, J.C.; et al. Contribution of Antibodies Against IA-2β and Zinc Transporter 8 to Classification of Diabetes Diagnosed Under 40 Years of Age. Diabetes Care 2011, 34, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Castaño, L.; Ziegler, A.G.; Ziegler, R.; Shoelson, S.; Eisenbarth, G.S. Characterization of insulin autoantibodies in relatives of patients with type I diabetes. Diabetes 1993, 42, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Padoa, C.J.; Crowther, N.J.; Thomas, J.W.; Hall, T.R.; Bekris, L.M.; Torn, C.; Landin-Olsson, M.; Ortqvist, E.; Palmer, J.P.; Lernmark, A.; et al. Epitope analysis of insulin autoantibodies using recombinant Fab. Clin. Exp. Immunol. 2005, 140, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.R.; Thomas, J.W.; Padoa, C.J.; Torn, C.; Landin-Olsson, M.; Ortqvist, E.; Hampe, C.S. Longitudinal epitope analysis of insulin-binding antibodies in type 1 diabetes. Clin. Exp. Immunol. 2006, 146, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Tree, T.I.; Morgenthaler, N.G.; Duhindan, N.; Hicks, K.E.; Madec, A.M.; Scherbaum, W.A.; Banga, J.P. Two amino acids in glutamic acid decarboxylase act in concert for maintenance of conformational determinants recognised by type 1 diabetic autoantibodies. Diabetologia 2000, 43, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.; Yano, M.; Abiru, N.; Akazawa, S.; Nagataki, S. Detection of recombinant GAD65 and GAD67 antibodies using a simple radioimmunoassay. Diabetes Res. Clin. Pr. 1996, 32, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, B.; Hoke, D.E.; Langendorf, C.G.; Buckle, A.M.; Rowley, M.J. An Analysis of the Cross-Reactivity of Autoantibodies to GAD65 and GAD67 in Diabetes. PLOS ONE 2011, 6, e18411. [Google Scholar] [CrossRef]
- Daw, K.; Powers, A.C. Two distinct glutamic acid decarboxylase auto-antibody specificities in IDDM target different epitopes. Diabetes 1995, 44, 216–220. [Google Scholar] [CrossRef]
- Hampe, C.S.; Hammerle, L.P.; Bekris, L.; Örtqvist, E.; Kockum, I.; Rolandsson, O.; Landin-Olsson, M.; Törn, C.; Persson, B.; Lernmark, A. Recognition of Glutamic Acid Decarboxylase (GAD) by Autoantibodies from Different GAD Antibody-Positive Phenotypes1. J. Clin. Endocrinol. Metab. 2000, 85, 4671–4679. [Google Scholar] [CrossRef]
- Kawasaki, E.; Abiru, N.; Ide, A.; Sun, F.; Fukushima, T.; Takahashi, R.; Kuwahara, H.; Fujita, N.; Kita, A.; Oshima, K.; et al. Epitope analysis of GAD65 autoantibodies in Japanese patients with autoimmune diabetes. Ann. New York Acad. Sci. 2003, 1005, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, M.; Banga, J.P.; Madec, A.M.; Binder, K.A.; Strebelow, M.; Rjasanowski, I.; Wassmuth, R.; Gilliam, L.K.; Luo, D.; Hampe, C.S. Dynamic changes of GAD65 autoantibody epitope specificities in individuals at risk of developing type 1 diabetes. Diabetologia 2005, 48, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Rabin, D.U.; Pleasic, S.M.; Palmer-Crocker, R.; Shapiro, J.A. Cloning and expression of IDDM- specific human autoantigens. Diabetes 1992, 41, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.; Hutton, J.C.; Eisenbarth, G.S. Molecular Cloning and Characterization of the Human Transmembrane Protein Tyrosine Phosphatase Homologue, Phogrin, an Autoantigen of Type 1 Diabetes. Biochem. Biophys. Res. Commun. 1996, 227, 440–447. [Google Scholar] [CrossRef]
- Harashima, S.-I.; Clark, A.; Christie, M.R.; Notkins, A.L. The dense core transmembrane vesicle protein IA-2 is a regulator of vesicle number and insulin secretion. Proc. Natl. Acad. Sci. 2005, 102, 8704–8709. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Hirai, H.; Zhang, G.; Zhang, M.; Takahashi, N.; Kasai, H.; Satin, L.S.; Leapman, R.D.; Notkins, A.L. Deletion of Ia-2 and/or Ia-2β in mice decreases insulin secretion by reducing the number of dense core vesicles. Diabetologia 2011, 54, 2347–2357. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.; Yu, L.; Gianani, R.; Verge, C.F.; Babu, S.; Bonifacio, E.; Eisenbarth, G.S. Evaluation of islet cell antigen (ICA) 512/IA-2 autoantibody radioassays using overlapping ICA512/IA-2 constructs. J Clin Endocrinol Metab. 1997, 82, 375–380. [Google Scholar] [CrossRef]
- Acevedo-Calado, M.; James, E.A.; Morran, M.P.; Pietropaolo, S.L.; Ouyang, Q.; Arribas-Layton, D.; Songini, M.; Liguori, M.; Casu, A.; Auchus, R.J.; et al. Identification of Unique Antigenic Determinants in the Amino Terminus of IA-2 (ICA512) in Childhood and Adult Autoimmune Diabetes: New Biomarker Development. Diabetes Care 2017, 40, 561–568. [Google Scholar] [CrossRef]
- Kawasaki, E.; Sera, Y.; Fujita, N.; Yamauchi, M.; Ozaki, M.; Abe, T.; Yamakawa, K.; Uotani, S.; Takino, H.; Yamasaki, H.; Yamaguchi, Y.; Uchigata, Y.; Matsuura, N.; Eguchi, K. Association between IA-2 autoantibody epitope specificities and age of onset in Japanese patients with autoimmune diabetes. J Autoimmun. 2001, 17, 323–331. [Google Scholar] [CrossRef]
- Dwivedi, O.P.; Lehtovirta, M.; Hastoy, B.; Chandra, V.; Krentz, N.A.J.; Kleiner, S.; Jain, D.; Richard, A.-M.; Abaitua, F.; Beer, N.L.; et al. Loss of ZnT8 function protects against diabetes by enhanced insulin secretion. Nat. Genet. 2019, 51, 1596–1606. [Google Scholar] [CrossRef]
- Nicolson, T.J.; Bellomo, E.A.; Wijesekara, N.; Loder, M.K.; Baldwin, J.M.; Gyulkhandanyan, A.V.; Koshkin, V.; Tarasov, A.I.; Carzaniga, R.; Kronenberger, K.; et al. Insulin Storage and Glucose Homeostasis in Mice Null for the Granule Zinc Transporter ZnT8 and Studies of the Type 2 Diabetes–Associated Variants. Diabetes 2009, 58, 2070–2083. [Google Scholar] [CrossRef] [PubMed]
- Sladek, R.; Rocheleau, G.; Rung, J.; Dina, C.; Shen, L.; Serre, D.; Boutin, P.; Vincent, D.; Belisle, A.; Hadjadj, S.; et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007, 445, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Wenzlau, J.M.; Liu, Y.; Yu, L.; Moua, O.; Fowler, K.T.; Rangasamy, S.; Walters, J.; Eisenbarth, G.S.; Davidson, H.W.; Hutton, J.C. A Common Nonsynonymous Single Nucleotide Polymorphism in the SLC30A8 Gene Determines ZnT8 Autoantibody Specificity in Type 1 Diabetes. Diabetes 2008, 57, 2693–2697. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.; Uga, M.; Nakamura, K.; Kuriya, G.; Satoh, T.; Fujishima, K.; Ozaki, M.; Abiru, N.; Yamasaki, H.; Wenzlau, J.M.; Davidson, H.W.; Hutton, J.C.; Eguchi, K. Association between anti- ZnT8 autoantibody specificities and SLC30A8 Arg325Trp variant in Japanese patients with type 1 diabetes. Diabetologia. 2008, 51, 2299–2302. [Google Scholar] [CrossRef] [PubMed]
- Wenzlau, J.M.; Frisch, L.M.; Hutton, J.C.; Davidson, H.W. Mapping of conformationalautoantibody epitopes in ZnT8. Diabetes Metab Res Rev. 2011, 27, 883–886. [Google Scholar] [CrossRef]
- Buzzetti, R.; Tuomi, T.; Mauricio, D.; Pietropaolo, M.; Zhou, Z.; Pozzilli, P.; Leslie, R.D. Management of Latent Autoimmune Diabetes in Adults: A Consensus Statement From an International Expert Panel. Diabetes 2020, 69, 2037–2047. [Google Scholar] [CrossRef]
- Shimada, A.; Kawasaki, E.; Abiru, N.; Awata, T.; Oikawa, Y.; Osawa, H.; Kajio, H.; Ozawa, J.; Takahashi, K.; Chujo, D.; Noso, S.; Fukui, T.; Miura, J.; Yasuda, K.; Yasuda, H.; Imagawa, A.; Ikegami, H. New diagnostic criteria (2023) of slowly progressive type 1 diabetes (SPIDDM) -Report from Committee of type 1 diabetes in Japan Diabetes Society-. J Japan Diab Soc. in press (in Japanese)
- Kasuga, A.; Maruyama, T.; Nakamoto, S.; Ozawa, Y.; Suzuki, Y.; Saruta, T. High-Titer Autoantibodies against Glutamic Acid Decarboxylase Plus Autoantibodies against Insulin and IA-2 Predicts Insulin Requirement in Adult Diabetic Patients. J. Autoimmun. 1999, 12, 131–135. [Google Scholar] [CrossRef]
- Takino, H.; Yamasaki, H.; Abiru, N.; Sera, Y.; Abe, T.; Kawasaki, E.; Yamaguchi, Y.; Eguchi, K.; Kanazawa, Y.; Nagataki, S. Antibodies to GAD in Japanese patients classified as Type 2 diabetes at diagnosis. High titre of GADAb is a predictive marker for early insulin treatment--report of west Japan (Kyushu, Yamaguchi, Osaka) study for GAD Ab(+) diabetes. Diabet Med 2002, 19, 730–734. [Google Scholar] [CrossRef]
- Kawasaki, E.; Nakamura, K.; Kuriya, G.; Satoh, T.; Kuwahara, H.; Kobayashi, M.; Abiru, N.; Yamasaki, H.; Eguchi, K. Autoantibodies to Insulin, Insulinoma-Associated Antigen-2, and Zinc Transporter 8 Improve the Prediction of Early Insulin Requirement in Adult-Onset Autoimmune Diabetes. J. Clin. Endocrinol. Metab. 2010, 95, 707–713. [Google Scholar] [CrossRef]
- Turner, R.; Stratton, I.; Horton, V.; Manley, S.; Zimmet, P.; Mackay, I.R.; Shattock, M.; Bottazzo, G.F.; Holman, R. UKPDS 25: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. Lancet 1997, 350, 1288–1293. [Google Scholar] [CrossRef]
- Yasui, J.; Kawasaki, E.; Tanaka, S.; Awata, T.; Ikegami, H.; Imagawa, A.; Uchigata, Y.; Osawa, H.; Kajio, H.; Kawabata, Y.; et al. Clinical and Genetic Characteristics of Non-Insulin-Requiring Glutamic Acid Decarboxylase (GAD) Autoantibody-Positive Diabetes: A Nationwide Survey in Japan. PLOS ONE 2016, 11, e0155643–e0155643. [Google Scholar] [CrossRef]
- Nederstigt, C.; Uitbeijerse, B.S.; Janssen, L.G.M.; Corssmit, E.P.M.; de Koning, E.J.P.; Dekkers, O.M. Associated auto-immune disease in type 1 diabetes patients: a systematic review and meta-analysis. Eur. J. Endocrinol. 2019, 180, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Kakleas, K.; Soldatou, A.; Karachaliou, F.; Karavanaki, K. Associated autoimmune diseases in children and adolescents with type 1 diabetes mellitus (T1DM). Autoimmun. Rev. 2015, 14, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E. Type 1 diabetes and autoimmunity. Clin Pediatr Endocrinol 2014, 23, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kordonouri, O.; Klinghammer, A.; Lang, E.B.; Grüters-Kieslich, A.; Grabert, M.; Holl, R.W. Thyroid autoimmunity in children and adolescents with type 1 diabetes: a multicenter survey. Diabetes Care 2002, 25, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Umpierrez, G.E.; Latif, K.A.; Murphy, M.B.; Lambeth, H.C.; Stentz, F.; Bush, A.; Kitabchi, A.E. Thyroid dysfunction in patients with type 1 diabetes: a longitudinal study. Diabetes Care 2003, 26, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Glastras, S.J.; Craig, M.E.; Verge, C.F.; Chan, A.K.; Cusumano, J.M.; Donaghue, K.C. The Role of Autoimmunity at Diagnosis of Type 1 Diabetes in the Development of Thyroid and Celiac Disease and Microvascular Complications. Diabetes Care 2005, 28, 2170–2175. [Google Scholar] [CrossRef]
- Kordonouri, O.; Klingensmith, G.; Knip, M.; Holl, R.W.; Aanstoot, H.-J.; Menon, P.S.; E Craig, M. Other complications and diabetes-associated conditions in children and adolescents. Pediatr. Diabetes 2014, 15, 270–278. [Google Scholar] [CrossRef]
- Horie, I.; Kawasaki, E.; Ando, T.; Kuwahara, H.; Abiru, N.; Usa, T.; Yamasaki, H.; Ejima, E.; Kawakami, A. Clinical and Genetic Characteristics of Autoimmune Polyglandular Syndrome Type 3 Variant in the Japanese Population. J. Clin. Endocrinol. Metab. 2012, 97, E1043–E1050. [Google Scholar] [CrossRef]
- Kawasaki, E.; Abiru, N.; Yano, M.; Uotani, S.; Matsumoto, K.; Matsuo, H.; Yamasaki, H.; Yamamoto, H.; Yamaguchi, Y.; Akazawa, S.; et al. Autoantibodies to Glutamic Acid Decarboxylase in Patients with Autoimmune Thyroid Disease: Relation to Competitive Insulin Autoantibodies. J. Autoimmun. 1995, 8, 633–643. [Google Scholar] [CrossRef]
- Balakhadze, M.; Giorgadze, E.; Lomidze, M. The frequency of Langerhans islets β-cells autoantibodies (Anti-GAD) in Georgian children and adolescents with chronic autoimmune thyroiditis. Int J Endocrinol. 2016, 2016, 6597091. [Google Scholar] [CrossRef] [PubMed]
- Maugendre, D.; Verite, F.; Guilhem, I.; Genetet, B.; Allannic, H.; Delamaire, M. Anti-pancreatic autoimmunity and Graves' disease: study of a cohort of 600 Caucasian patients. Eur. J. Endocrinol. 1997, 137, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Lethagen, Å.L.; Ericsson, U.B.; Hallengren, B.; Groop, L.; Tuomi, T. Glutamic acid decarboxylase antibody positivity is associated with an impaired insulin response to glucose and arginine in nondiabetic patients with autoimmune thyroiditis. J Clin Endocrinol Metab. 2002, 87, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir, B.; Jönsson, I.; Lantz, M. Prevalence of diabetes and presence of autoantibodies against zinc transporter 8 and glutamic decarboxylase at diagnosis and at follow up of Graves’ disease. Endocrine 2019, 64, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Erdö, S.L.; Wolff, J.R. γ-aminobutyric acid outside the mammalian brain. J Neurochem. 1990, 54, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Christie, M.R.; Brown, T.J.; Cassidy, D. Binding of antibodies in sera from Type 1 (insulin- dependent) diabetic patients to glutamate decarboxylase from rat tissues. Evidence for antigenic and non-antigenic forms of the enzyme. Diabetologia 1992, 35, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Bottazzo, G.F.; Gleichmann, H. Immunology and Diabetes Workshops: report of the first international workshop on the standardisation of cytoplasmic islet cell antibodies. Diabetologia 1986, 29, 125–126. [Google Scholar] [CrossRef]
- Bonifacio, E.; Dawkins, R.L.; Lernmark, Å. Immunology and Diabetes Workshops: Report of the Second International Workshop on the Standardisation of Cytoplasmic Islet Cell Antibodies. Diabetologia 1987, 30, 273–273. [Google Scholar] [CrossRef]
- Martino, G.V.; Tappaz, M.L.; Braghi, S.; Dozio, N.; Canal, N.; Pozza, G.; Bottazzo, G.F.; Grimaldi, L.M.; Bosi, E. Autoantibodies to glutamic acid decarboxylase (GAD) detected by an immuno-trapping enzyme activity assay: Relation to insulin-dependent diabetes mellitus and islet cell antibodies. J. Autoimmun. 1991, 4, 915–923. [Google Scholar] [CrossRef]
- Rowley, M.J.; Mackay, I.R.; Chen, Q.Y.; Knowles, W.J.; Zimmet, P.Z. Antibodies to glutamic acid decarboxylase discriminate major types of diabetes mellitus. Diabetes. 1992, 41, 548–551. [Google Scholar] [CrossRef]
- Kawasaki, E.; Takino, H.; Yano, M.; Uotani, S.; Matsumoto, K.; Takao, Y.; Yamaguchi, Y.; Akazawa, S.; Nagataki, S. Autoantibodies to glutamic acid decarboxylase in patients with IDDM and autoimmune thyroid disease. Diabetes. 1994, 43, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Schmidli, R.S.; Colman, P.G.; Bonifacio, E.; Bottazzo, G.F.; Harrison, L.C. High level of concordance between assays for glutamic acid decarboxylase antibodies. The first international glutamic acid decarboxylase antibody workshop. Diabetes 1994, 43, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Schmidli, R.S.; Colman, P.G.; Bonifacio, E. Disease sensitivity and specificity of 52 assays for glutamic acid decarboxylase antibodies. The second international GADAb Workshop. Diabetes 1995, 44, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, E.; Tanaka, M.; Miwa, M.; Abiru, N.; Kawakami, A. Novel enzyme-linked immunosorbent assay for bivalent ZnT8 autoantibodies. Acta Diabetol. 2013, 51, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Raab, J.; Haupt, F.; Scholz, M.; Matzke, C.; Warncke, K.; Lange, K.; Assfalg, R.; Weininger, K.; Wittich, S.; Löbner, S.; et al. Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results of the Fr1da study. BMJ Open 2016, 6, e011144. [Google Scholar] [CrossRef] [PubMed]
- Lind, A.; Cortez, F.d.J.; Ramelius, A.; Bennet, R.; Robinson, P.V.; Seftel, D.; Gebhart, D.; Tandel, D.; Maziarz, M.; Agardh, D.; et al. Multiplex agglutination-PCR (ADAP) autoantibody assays compared to radiobinding autoantibodies in type 1 diabetes and celiac disease. J. Immunol. Methods 2022, 506, 113265. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Yu, L. Effective assay technologies fit for large-scale population screening of type 1 diabetes. Front. Clin. Diabetes Heal. 2023, 3, 1034698. [Google Scholar] [CrossRef]
- Orban, T.; Bundy, B.; Becker, D.J.; DiMeglio, L.A.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Greenbaum, C.J.; Marks, J.B.; Monzavi, R.; et al. Costimulation Modulation With Abatacept in Patients With Recent-Onset Type 1 Diabetes: Follow-up 1 Year After Cessation of Treatment. Diabetes Care 2014, 37, 1069–1075. [Google Scholar] [CrossRef]
- Rigby, M.R.; Harris, K.M.; Pinckney, A.; DiMeglio, L.A.; Rendell, M.S.; Felner, E.I.; Dostou, J.M.; Gitelman, S.E.; Griffin, K.J.; Tsalikian, E.; et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J. Clin. Investig. 2015, 125, 3285–3296. [Google Scholar] [CrossRef]
- Pescovitz, M.D.; Greenbaum, C.J.; Krause-Steinrauf, H.; Becker, D.J.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Marks, J.B.; McGee, P.F.; Moran, A.M.; Raskin, P.; Rodriguez, H.; Schatz, D.A.; Wherrett, D.; Wilson, D.M.; Lachin, J.M.; Skyler, J.S. Rituximab, B-lymphocyte depletion, and preservation of β-cell function. N Engl J Med 2009, 361, 2143–52. [Google Scholar] [CrossRef]
- Haller, M.J.; Long, S.A.; Blanchfield, J.L.; Schatz, D.A.; Skyler, J.S.; Krischer, J.P.; Bundy, B.N.; Geyer, S.M.; Warnock, M.V.; Miller, J.L.; et al. Low-Dose Anti-Thymocyte Globulin Preserves C-Peptide, Reduces HbA1c, and Increases Regulatory to Conventional T-Cell Ratios in New-Onset Type 1 Diabetes: Two-Year Clinical Trial Data. Diabetes 2019, 68, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Herold, K.C.; Bundy, B.N.; Long, S.A.; Bluestone, J.A.; DiMeglio, L.A.; Dufort, M.J.; Gitelman, S.E.; Gottlieb, P.A.; Krischer, J.P.; Linsley, P.S.; Marks, J.B.; Moore, W.; Moran, A.; Rodriguez, H.; Russell, W.E.; Schatz, D.; Skyler, J.S.; Tsalikian, E.; Wherrett, D.K.; Ziegler, A.G.; Greenbaum, C.J.; et al. An anti-CD3 antibody, Teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019, 381, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Sims, E.K.; Bundy, B.N.; Stier, K.; Serti, E.; Lim, N.; Long, S.A.; Geyer, S.M.; Moran, A.; Greenbaum, C.J.; Evans-Molina, C.; et al. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
| Subject | Prevalence |
| Healthy control | <1% |
| Acute-onset type 1 diabetes (at onset) | 60~80% |
| Fulminant type 1 diabetes | 5~9% |
| LADA (SPIDDM) | 100% |
| Type 2 diabetes (diet/OHA) | 4~5% |
| Polyglandular autoimmune syndrome, type 1 | 30~40% |
| Polyglandular autoimmune syndrome, type 2 | 30~50% |
| Autoimmune thyroid disease | 6~8% |
| Stiff-person syndrome | 60~70% |
| Required item: |
| 1) the presence of anti-islet autoantibodies at some time point during the diseasecourse a) |
| 2) the absence of ketosis or ketoacidosis at the diagnosis of diabetes and the unnecessity for insulin treatment to correct hyperglycemia immediately afterdiagnosis in principle |
| 3) gradual decrease of insulin secretion overtime, requirement of insulin treatment more than 3 months b) after diagnosis of diabetes, and exhausted endogenous insulin secretion (fasting serum C-peptide immunoreactivity < 0.6 ng/ml) at last observedtime point |
| Judgement: |
| when the case fulfills the criteria all of the three described above (1), 2), and 3)), the case is diagnosed with “slowly progressive type 1 diabetes (definite)”when the case fulfills the criteria only 1) and 2), but not 3), the case is diagnosed with“slowly progressive type 1 diabetes (probable)” |
| a) Anti-islet autoantibodies include glutamic acid decarboxylase (GAD) autoantibody, insulinoma-associated antigen-2 (IA-2) autoantibody, islet cell antibody (ICA), zinc transporter 8 (ZnT8) autoantibody or insulin autoantibody (IAA). The measurementof IAA should be performed before starting insulin treatment. |
| b) more than 6 months in typical case |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





