Submitted:
03 May 2023
Posted:
04 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
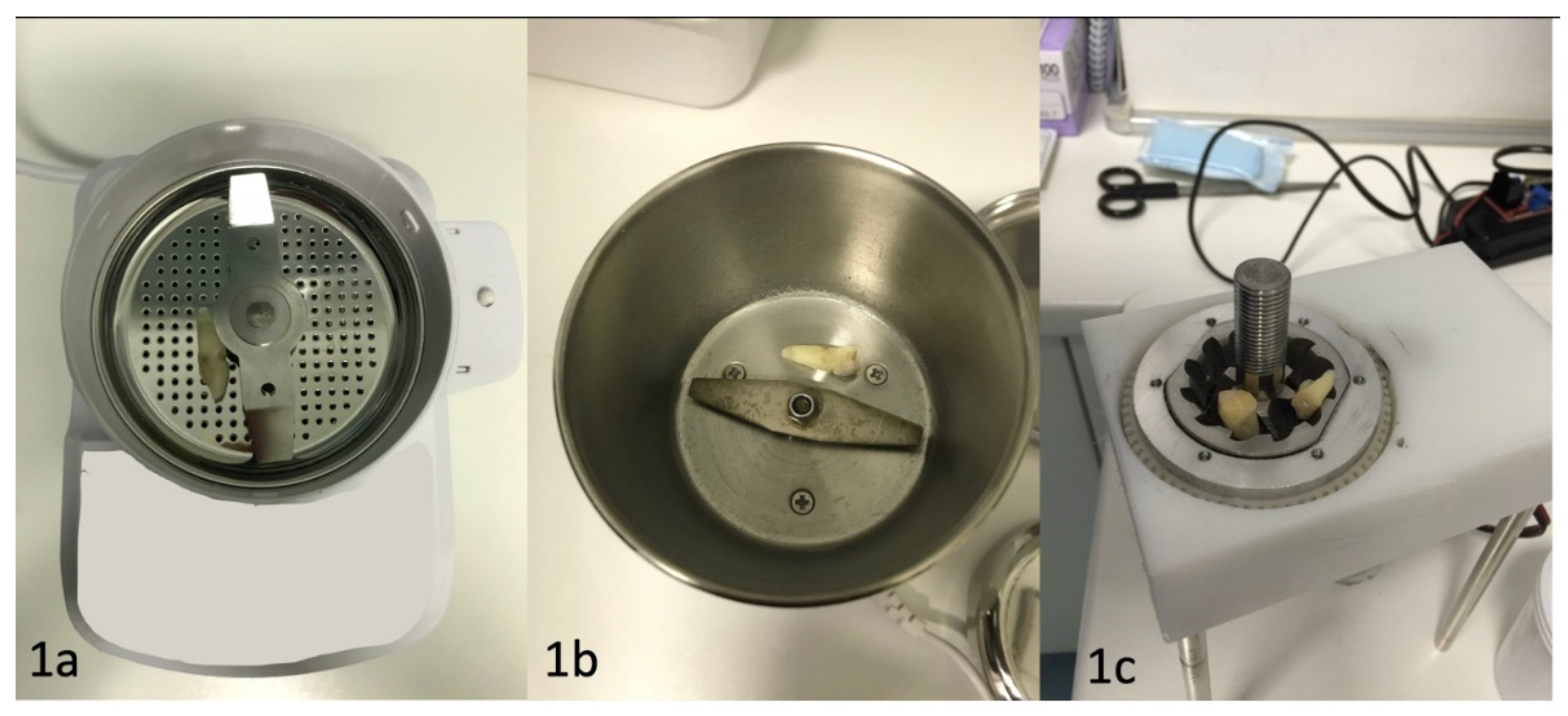
2. Materials and Methods
3. Results
4. Discussion
- average tooth weight before the test : each tooth was weighted to be able to compare the weight after the treatement
- average tooth weight after the test: each pulverized tooth was weighed after the treatment to be able to compare with the same tooth weight before the treatment
- lost tooth weight (%): the difference between before and after the treatment was measured in percentage of weight loss
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMPs | Bone morphogenetic proteins |
| GFs | Growth factors |
| DDM | Demineralized Dentin Matrix |
| TGF-B | Transforming growth factor-B |
| TT® | Tooth transformer ®
|
References
- Guided Bone Regeneration in Implant Dentistry. Available online: http://www.quintpub.com/display_detail.php3?psku=B2494#.Y7sD6uzMKdY (accessed on 8 January 2023).
- Romasco, T.; Tumedei, M.; Inchingolo, F.; Pignatelli, P.; Montesani, L.; Iezzi, G.; Petrini, M.; Piattelli, A.; Di Pietro, N. A Narrative Review on the Effectiveness of Bone Regeneration Procedures with OsteoBiol® Collagenated Porcine Grafts: The Translational Research Experience over 20 Years. J Funct Biomater 2022, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Hazballa, D.; Inchingolo, A.D.; Malcangi, G.; Marinelli, G.; Mancini, A.; Maggiore, M.E.; Bordea, I.R.; Scarano, A.; Farronato, M.; et al. Innovative Concepts and Recent Breakthrough for Engineered Graft and Constructs for Bone Regeneration: A Literature Systematic Review. Materials 2022, 15, 1120. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Xhajanka, E.; Romeo, D.M.; Romeo, M.; Zappone, C.M.F.; Malcangi, G.; Scarano, A.; Lorusso, F.; et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 2021, 14, 1147. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Inchingolo, A.D.; Dipalma, G.; Flace, P.; Girolamo, F.; Tarullo, A.; Laino, L.; et al. Regenerative Surgery Performed with Platelet-Rich Plasma Used in Sinus Lift Elevation before Dental Implant Surgery: An Useful Aid in Healing and Regeneration of Bone Tissue. Eur Rev Med Pharmacol Sci 2012, 16, 1222–1226. [Google Scholar] [PubMed]
- Inchingolo, A.D.; Malcangi, G.; Semjonova, A.; Inchingolo, A.M.; Patano, A.; Coloccia, G.; Ceci, S.; Marinelli, G.; Di Pede, C.; Ciocia, A.M.; et al. Oralbiotica/Oralbiotics: The Impact of Oral Microbiota on Dental Health and Demineralization: A Systematic Review of the Literature. Children 2022, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Liu, Z.; Liu, Y.; Yu, J.; Wang, X.; Tan, Z.; Ye, X. Recent Trends in the Development of Bone Regenerative Biomaterials. Frontiers in Cell and Developmental Biology 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Mummolo, S.; Mancini, L.; Quinzi, V.; D’Aquino, R.; Marzo, G.; Marchetti, E. Rigenera® Autologous Micrografts in Oral Regeneration: Clinical, Histological, and Radiographical Evaluations. Applied Sciences 2020, 10, 5084. [Google Scholar] [CrossRef]
- Linkhart, T.A.; Mohan, S.; Baylink, D.J. Growth Factors for Bone Growth and Repair: IGF, TGF Beta and BMP. Bone 1996, 19, 1S–12S. [Google Scholar] [CrossRef]
- Canalis, E.; Pash, J.; Varghese, S. Skeletal Growth Factors. Crit Rev Eukaryot Gene Expr 1993, 3, 155–166. [Google Scholar]
- Gargiulo Isacco, C.; Ballini, A.; Paduanelli, G.; Inchingolo, A.D.; Nguyen, K.C.D.; Inchingolo, A.M.; Pham, V.H.; Aityan, S.K.; Schiffman, M.; Tran, T.C.; et al. Bone Decay and beyond: How Can We Approach It Better. J Biol Regul Homeost Agents 2019, 33, 143–154 DENTAL SUPPLEMENT. [Google Scholar]
- Del Corso, M.; Vervelle, A.; Simonpieri, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current Knowledge and Perspectives for the Use of Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Oral and Maxillofacial Surgery Part 1: Periodontal and Dentoalveolar Surgery. Curr Pharm Biotechnol 2012, 13, 1207–1230. [Google Scholar] [CrossRef] [PubMed]
- Simonpieri, A.; Del Corso, M.; Vervelle, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current Knowledge and Perspectives for the Use of Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Oral and Maxillofacial Surgery Part 2: Bone Graft, Implant and Reconstructive Surgery. Curr Pharm Biotechnol 2012, 13, 1231–1256. [Google Scholar] [CrossRef] [PubMed]
- Sieverts, M.; Obata, Y.; Rosenberg, J.L.; Woolley, W.; Parkinson, D.Y.; Barnard, H.S.; Pelt, D.M.; Acevedo, C. Unraveling the Effect of Collagen Damage on Bone Fracture Using in Situ Synchrotron Microtomography with Deep Learning. Commun Mater 2022, 3, 1–13. [Google Scholar] [CrossRef]
- Bhaskar, S.N. Orban’s Oral Histology and Embryology, 9th ed.; Orban, B.J., Ed.; Mosby: St. Louis, 1980; ISBN 978-0-8016-4609-6. [Google Scholar]
- Butler, W.T.; Ritchie, H. The Nature and Functional Significance of Dentin Extracellular Matrix Proteins. Int J Dev Biol 1995, 39, 169–179. [Google Scholar] [PubMed]
- Chen, J.; Shapiro, H.S.; Sodek, J. Development Expression of Bone Sialoprotein MRNA in Rat Mineralized Connective Tissues. J Bone Miner Res 1992, 7, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Ganss, B.; Kim, R.H.; Sodek, J. Bone Sialoprotein. Crit Rev Oral Biol Med 1999, 10, 79–98. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, Q.; Chen, J. Applications of Transgenics in Studies of Bone Sialoprotein. J Cell Physiol 2009, 220, 30–34. [Google Scholar] [CrossRef]
- Yeomans, J.D.; Urist, M.R. Bone Induction by Decalcified Dentine Implanted into Oral, Osseous and Muscle Tissues. Arch Oral Biol 1967, 12, 999–1008. [Google Scholar] [CrossRef]
- Bang, G.; Urist, M.R. Bone Induction in Excavation Chambers in Matrix of Decalcified Dentin. Arch Surg 1967, 94, 781–789. [Google Scholar] [CrossRef]
- Schmidt-Schultz, T.H.; Schultz, M. Intact Growth Factors Are Conserved in the Extracellular Matrix of Ancient Human Bone and Teeth: A Storehouse for the Study of Human Evolution in Health and Disease. Biol Chem 2005, 386, 767–776. [Google Scholar] [CrossRef]
- Bessho, K.; Tanaka, N.; Matsumoto, J.; Tagawa, T.; Murata, M. Human Dentin-Matrix-Derived Bone Morphogenetic Protein. J Dent Res 1991, 70, 171–175. [Google Scholar] [CrossRef] [PubMed]
- ten Dijke, P.; Miyazono, K.; Heldin, C.H. Signaling via Hetero-Oligomeric Complexes of Type I and Type II Serine/Threonine Kinase Receptors. Curr Opin Cell Biol 1996, 8, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Ramoshebi, L.N.; Matsaba, T.; Tasker, J.; Crooks, J.; Teare, J. Bone Induction by BMPs/OPs and Related Family Members in Primates. J Bone Joint Surg Am 2001, 83-A Suppl 1, S116-127. [Google Scholar] [CrossRef]
- Burchardt, H. The Biology of Bone Graft Repair. Clin Orthop Relat Res 1983, 28–42. [Google Scholar] [CrossRef]
- Lorusso, F.; Inchingolo, F.; Dipalma, G.; Postiglione, F.; Fulle, S.; Scarano, A. Synthetic Scaffold/Dental Pulp Stem Cell (DPSC) Tissue Engineering Constructs for Bone Defect Treatment: An Animal Studies Literature Review. International Journal of Molecular Sciences 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Vermesan, D.; Prejbeanu, R.; Haragus, H.; Poenaru, D.V.; Mioc, M.L.; Tatullo, M.; Abbinante, A.; Scacco, S.; Tarullo, A.; Inchingolo, F.; et al. Clinical Relevance of Altered Bone Immunopathology Pathways around the Elbow. Eur Rev Med Pharmacol Sci 2014, 18, 2846–2850. [Google Scholar]
- Arafat Kabir, Md.; Murata, M.; Kusano, K.; Mohammad Zakaria, S.; Hena Mohammad Noor, A.; Khuda, F.; Hossain, I.; Sultana, S.; Saito, T. Radiological Evaluation of Human Dentin Autografts in Bangladesh. J. Hard Tissue Biology. 2014, 23, 363–370. [Google Scholar] [CrossRef]
- Isacco, C.G.; Nguyen, K.C.D.; Pham, V.H.; Di Palma, G.; Aityan, S.K.; Tomassone, D.; Distratis, P.; Lazzaro, R.; Balzanelli, M.G.; Inchingolo, F. Searching for a Link between Bone Decay and Diabetes Type 2. Endocr Metab Immune Disord Drug Targets 2022, 22, 904–910. [Google Scholar] [CrossRef]
- Nakashima, M. Bone Morphogenetic Proteins in Dentin Regeneration for Potential Use in Endodontic Therapy. Cytokine Growth Factor Rev 2005, 16, 369–376. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Scacco, S.; Perillo, L.; Scarano, A.; Aityan, S.K.; Contaldo, M.; Cd Nguyen, K.; Santacroce, L.; Syed, J.; et al. A Comparative Study on Different Stemness Gene Expression between Dental Pulp Stem Cells vs. Dental Bud Stem Cells. Eur Rev Med Pharmacol Sci 2019, 23, 1626–1633. [Google Scholar] [CrossRef]
- Scarano, A.; Inchingolo, F.; Murmura, G.; Traini, T.; Piattelli, A.; Lorusso, F. Three-Dimensional Architecture and Mechanical Properties of Bovine Bone Mixed with Autologous Platelet Liquid, Blood, or Physiological Water: An In Vitro Study. Int J Mol Sci 2018, 19, 1230. [Google Scholar] [CrossRef] [PubMed]
- Rijal, G.; Shin, H.-I. Human Tooth-Derived Biomaterial as a Graft Substitute for Hard Tissue Regeneration. Regen Med 2017, 12, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Hazballa, D.; Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Santacroce, L.; Minetti, E.; Di Venere, D.; Limongelli, L.; Bordea, I.R.; Scarano, A.; et al. The Effectiveness of Autologous Demineralized Tooth Graft for the Bone Ridge Preservation: A Systematic Review of the Literature. J Biol Regul Homeost Agents 2021, 35, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Inchingolo, A.D.; Trasarti, S.; Ferrara, E.; Qorri, E.; Mancini, A.; Montemurro, N.; Scarano, A.; Inchingolo, A.M.; Dipalma, G.; et al. Long-Term Outcomes of Implants Placed in Maxillary Sinus Floor Augmentation with Porous Fluorohydroxyapatite (Algipore® FRIOS®) in Comparison with Anorganic Bovine Bone (Bio-Oss®) and Platelet Rich Plasma (PRP): A Retrospective Study. Journal of Clinical Medicine 2022, 11, 2491. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.J. Experimental Evaluation of the Osteogenic Potential of Bone Graft Materials. Annu Meet Am Inst Oral Biol 1969, 13–21. [Google Scholar]
- Mellonig, J.T.; Bowers, G.M.; Cotton, W.R. Comparison of Bone Graft Materials. Part II. New Bone Formation with Autografts and Allografts: A Histological Evaluation. J Periodontol 1981, 52, 297–302. [Google Scholar] [CrossRef]
- Colnot, C.; Romero, D.M.; Huang, S.; Helms, J.A. Mechanisms of Action of Demineralized Bone Matrix in the Repair of Cortical Bone Defects. Clin Orthop Relat Res 2005, 69–78. [Google Scholar] [CrossRef]
- Araújo, M.G.; Sonohara, M.; Hayacibara, R.; Cardaropoli, G.; Lindhe, J. Lateral Ridge Augmentation by the Use of Grafts Comprised of Autologous Bone or a Biomaterial. An Experiment in the Dog. J Clin Periodontol 2002, 29, 1122–1131. [Google Scholar] [CrossRef]
- Minamizato, T.; Koga, T.; I, T.; Nakatani, Y.; Umebayashi, M.; Sumita, Y.; Ikeda, T.; Asahina, I. Clinical Application of Autogenous Partially Demineralized Dentin Matrix Prepared Immediately after Extraction for Alveolar Bone Regeneration in Implant Dentistry: A Pilot Study. Int J Oral Maxillofac Surg 2018, 47, 125–132. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, Y.-K.; Park, Y.-H.; Park, J.-C.; Ku, J.-K.; Um, I.-W.; Kim, J.-Y. Evaluation of the Healing Potential of Demineralized Dentin Matrix Fixed with Recombinant Human Bone Morphogenetic Protein-2 in Bone Grafts. Materials (Basel) 2017, 10, 1049. [Google Scholar] [CrossRef]
- Libonati, A.; Marzo, G.; Klinger, F.G.; Farini, D.; Gallusi, G.; Tecco, S.; Mummolo, S.; De Felici, M.; Campanella, V. Embryotoxicity Assays for Leached Components from Dental Restorative Materials. Reprod Biol Endocrinol 2011, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Clinical Application of Autogenous Tooth as Bone Graft Material in Extraction Socket- A Prospective Study - Clinical Epidemiology and Global Health. Available online: https://cegh.net/article/S2213-3984(22)00105-1/fulltext (accessed on 3 April 2023)..
- Minetti, E.; Berardini, M.; Trisi, P. A New Tooth Processing Apparatus Allowing to Obtain Dentin Grafts for Bone Augmentation: The Tooth Transformer. TODENTJ 2019, 13, 6–14. [Google Scholar] [CrossRef]
- Bono, N.; Tarsini, P.; Candiani, G. BMP-2 and Type I Collagen Preservation in Human Deciduous Teeth after Demineralization. Journal of Applied Biomaterials & Functional Materials 2019, 17, 228080001878423. [Google Scholar] [CrossRef]
- Bianchi, S.; Mancini, L.; Torge, D.; Cristiano, L.; Mattei, A.; Varvara, G.; Macchiarelli, G.; Marchetti, E.; Bernardi, S. Bio-Morphological Reaction of Human Periodontal Ligament Fibroblasts to Different Types of Dentinal Derivates: In Vitro Study. Int J Mol Sci 2021, 22, 8681. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Palermo, A. Comparison between the Bone Regeneration Using Tooth Graft with or without Tooth Transformer in Sheep. BAOJ Dentistry 5.
- Minetti, E.; Corbella, S.; Taschieri, S.; Canullo, L. Tooth as Graft Material: Histologic Study. Clin Implant Dent Relat Res 2022, 24, 488–496. [Google Scholar] [CrossRef] [PubMed]
- DENTAL SUPPLEMENT; Minetti, E. ; Palermo, A.; Savadori, P.; Barlattani, A.; Franco, R.; Michele, M.; Gianfreda, F.; Bollero, P. Autologous Tooth Graft: A Histological Comparison between Dentin Mixed with Xenograft and Dentin Alone Grafts in Socket Preservation. J Biol Regul Homeost Agents 2019, 33, 189–197. [Google Scholar]
- Minetti, E.; Celko, M.; Contessi, M.; Carini, F.; Gambardella, U.; Giacometti, E.; Santillana, J.; Beca Campoy, T.; Schmitz, J.H.; Libertucci, M.; et al. Implants Survival Rate in Regenerated Sites with Innovative Graft Biomaterials: 1 Year Follow-Up. Materials 2021, 14, 5292. [Google Scholar] [CrossRef]
- Minetti, E.; Giacometti, E.; Gambardella, U.; Contessi, M.; Ballini, A.; Marenzi, G.; Celko, M.; Mastrangelo, F. Alveolar Socket Preservation with Different Autologous Graft Materials: Preliminary Results of a Multicenter Pilot Study in Human. Materials 2020, 13, 1153. [Google Scholar] [CrossRef]
- Santos, A.; Botelho, J.; Machado, V.; Borrecho, G.; Proença, L.; Mendes, J.J.; Mascarenhas, P.; Alcoforado, G. Autogenous Mineralized Dentin versus Xenograft Granules in Ridge Preservation for Delayed Implantation in Post-Extraction Sites: A Randomized Controlled Clinical Trial with an 18 Months Follow-Up. Clin Oral Implants Res 2021, 32, 905–915. [Google Scholar] [CrossRef]
- Oguić, M.; Čandrlić, M.; Tomas, M.; Vidaković, B.; Blašković, M.; Jerbić Radetić, A.T.; Zoričić Cvek, S.; Kuiš, D.; Cvijanović Peloza, O. Osteogenic Potential of Autologous Dentin Graft Compared with Bovine Xenograft Mixed with Autologous Bone in the Esthetic Zone: Radiographic, Histologic and Immunohistochemical Evaluation. Int J Mol Sci 2023, 24, 6440. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.; Patano, A.; Di Pede, C.; Inchingolo, A.D.; Palmieri, G.; de Ruvo, E.; Campanelli, M.; Buongiorno, S.; Carpentiere, V.; Piras, F.; et al. Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. Journal of Functional Biomaterials 2023, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Malcangi, G.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; De Leonardis, N.; et al. Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. International Journal of Molecular Sciences 2022, 23, 4027. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, M. a. I.; Briels, W.J.; Rinzema, A.; Boom, R.M. Numerical Simulation and PEPT Measurements of a 3D Conical Helical-Blade Mixer: A High Potential Solids Mixer for Solid-State Fermentation. Biotechnology and Bioengineering 2003, 84, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.H.; Holmfred, E.; Cornett, C.; Maldonado, C.; Rønsted, N. An Efficient, Robust, and Inexpensive Grinding Device for Herbal Samples like Cinchona Bark. Sci Pharm 2015, 83, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, T.; Antikainen, O.; Yliruusi, J. Simultaneous Comparison of Two Roller Compaction Techniques and Two Particle Size Analysis Methods. AAPS PharmSciTech 2017, 18, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, M.; Terranova, L.; Mallet, R.; Mabilleau, G.; Chappard, D. Three-Dimensional Arrangement of β-Tricalcium Phosphate Granules Evaluated by Microcomputed Tomography and Fractal Analysis. Acta Biomaterialia 2015, 11, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Kim, S.-G.; Yun, P.-Y.; Yeo, I.-S.; Jin, S.-C.; Oh, J.-S.; Kim, H.-J.; Yu, S.-K.; Lee, S.-Y.; Kim, J.-S.; et al. Autogenous Teeth Used for Bone Grafting: A Comparison with Traditional Grafting Materials. Oral Surg Oral Med Oral Pathol Oral Radiol 2014, 117, e39–45. [Google Scholar] [CrossRef]
- Jun, S.-H.; Ahn, J.-S.; Lee, J.-I.; Ahn, K.-J.; Yun, P.-Y.; Kim, Y.-K. A Prospective Study on the Effectiveness of Newly Developed Autogenous Tooth Bone Graft Material for Sinus Bone Graft Procedure. J Adv Prosthodont 2014, 6, 528–538. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Lee, J.; Um, I.-W.; Kim, K.-W.; Murata, M.; Akazawa, T.; Mitsugi, M. Tooth-Derived Bone Graft Material. J Korean Assoc Oral Maxillofac Surg 2013, 39, 103–111. [Google Scholar] [CrossRef]
- Murata, M. Bone Engineering Using Human Demineralized Dentin Matrix and Recombinant Human BMP-2. J. Hard Tissue Biology. 2005, 14, 80–81. [Google Scholar] [CrossRef]
- Nampo, T.; Watahiki, J.; Enomoto, A.; Taguchi, T.; Ono, M.; Nakano, H.; Yamamoto, G.; Irie, T.; Tachikawa, T.; Maki, K. A New Method for Alveolar Bone Repair Using Extracted Teeth for the Graft Material. J Periodontol 2010, 81, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Kim, S.-G.; Byeon, J.-H.; Lee, H.-J.; Um, I.-U.; Lim, S.-C.; Kim, S.-Y. Development of a Novel Bone Grafting Material Using Autogenous Teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010, 109, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Dozza, B.; Lesci, I.G.; Duchi, S.; Della Bella, E.; Martini, L.; Salamanna, F.; Falconi, M.; Cinotti, S.; Fini, M.; Lucarelli, E.; et al. When Size Matters: Differences in Demineralized Bone Matrix Particles Affect Collagen Structure, Mesenchymal Stem Cell Behavior, and Osteogenic Potential. J Biomed Mater Res A 2017, 105, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone Substitutes in Orthopaedic Surgery: From Basic Science to Clinical Practice. J Mater Sci Mater Med 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Testori, T.; Wallace, S.S.; Trisi, P.; Capelli, M.; Zuffetti, F.; Del Fabbro, M. Effect of Xenograft (ABBM) Particle Size on Vital Bone Formation Following Maxillary Sinus Augmentation: A Multicenter, Randomized, Controlled, Clinical Histomorphometric Trial. Int J Periodontics Restorative Dent 2013, 33, 467–475. [Google Scholar] [CrossRef]
- Carano, R.A.D.; Filvaroff, E.H. Angiogenesis and Bone Repair. Drug Discov Today 2003, 8, 980–989. [Google Scholar] [CrossRef]
- Shapoff, C.A.; Bowers, G.M.; Levy, B.; Mellonig, J.T.; Yukna, R.A. The Effect of Particle Size on the Osteogenic Activity of Composite Grafts of Allogeneic Freeze-Dried Bone and Autogenous Marrow. J Periodontol 1980, 51, 625–630. [Google Scholar] [CrossRef]
- Pallesen, L.; Schou, S.; Aaboe, M.; Hjørting-Hansen, E.; Nattestad, A.; Melsen, F. Influence of Particle Size of Autogenous Bone Grafts on the Early Stages of Bone Regeneration: A Histologic and Stereologic Study in Rabbit Calvarium. Int J Oral Maxillofac Implants 2002, 17, 498–506. [Google Scholar]
- Gideon Hallel, I.B. A Novel Procedure to Process Extracted Teeth for Immediate Grafting of Autogenous Dentin. J Interdiscipl Med Dent Sci 2014, 02. [Google Scholar] [CrossRef]
- Klüppel, L.E.; Antonini, F.; Olate, S.; Nascimento, F.F.; Albergaria-Barbosa, J.R.; Mazzonetto, R. Bone Repair Is Influenced by Different Particle Sizes of Anorganic Bovine Bone Matrix: A Histologic and Radiographic Study in Vivo. J Craniofac Surg 2013, 24, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Kon, K.; Shiota, M.; Ozeki, M.; Yamashita, Y.; Kasugai, S. Bone Augmentation Ability of Autogenous Bone Graft Particles with Different Sizes: A Histological and Micro-Computed Tomography Study. Clin Oral Implants Res 2009, 20, 1240–1246. [Google Scholar] [CrossRef]
- Fucini, S.E.; Quintero, G.; Gher, M.E.; Black, B.S.; Richardson, A.C. Small versus Large Particles of Demineralized Freeze-Dried Bone Allografts in Human Intrabony Periodontal Defects. J Periodontol 1993, 64, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shimizu, Y.; Asai, S.; Ooya, K. Experimental Sinus Grafting with the Use of Deproteinized Bone Particles of Different Sizes. Clin Oral Implants Res 2003, 14, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Coathup, M.J.; Cai, Q.; Campion, C.; Buckland, T.; Blunn, G.W. The Effect of Particle Size on the Osteointegration of Injectable Silicate-Substituted Calcium Phosphate Bone Substitute Materials. J Biomed Mater Res B Appl Biomater 2013, 101, 902–910. [Google Scholar] [CrossRef]
- Prieto, E.M.; Talley, A.D.; Gould, N.R.; Zienkiewicz, K.J.; Drapeau, S.J.; Kalpakci, K.N.; Guelcher, S.A. Effects of Particle Size and Porosity on in Vivo Remodeling of Settable Allograft Bone/Polymer Composites. J Biomed Mater Res B Appl Biomater 2015, 103, 1641–1651. [Google Scholar] [CrossRef]
- Gultekin, B.A.; Bedeloglu, E.; Kose, T.E.; Mijiritsky, E. Comparison of Bone Resorption Rates after Intraoral Block Bone and Guided Bone Regeneration Augmentation for the Reconstruction of Horizontally Deficient Maxillary Alveolar Ridges. Biomed Res Int 2016, 2016, 4987437. [Google Scholar] [CrossRef]
- Koga, T.; Minamizato, T.; Kawai, Y.; Miura, K.; I, T.; Nakatani, Y.; Sumita, Y.; Asahina, I. Bone Regeneration Using Dentin Matrix Depends on the Degree of Demineralization and Particle Size. PLoS One 2016, 11, e0147235. [Google Scholar] [CrossRef]
- Chackartchi, T.; Iezzi, G.; Goldstein, M.; Klinger, A.; Soskolne, A.; Piattelli, A.; Shapira, L. Sinus Floor Augmentation Using Large (1-2 Mm) or Small (0.25-1 Mm) Bovine Bone Mineral Particles: A Prospective, Intra-Individual Controlled Clinical, Micro-Computerized Tomography and Histomorphometric Study. Clin Oral Implants Res 2011, 22, 473–480. [Google Scholar] [CrossRef]
- Kon, K.; Shiota, M.; Ozeki, M.; Kasugai, S. The Effect of Graft Bone Particle Size on Bone Augmentation in a Rabbit Cranial Vertical Augmentation Model: A Microcomputed Tomography Study. Int J Oral Maxillofac Implants 2014, 29, 402–406. [Google Scholar] [CrossRef]
- Minetti, E.; Corbella, S.; Taschieri, S. The Weight of Permanent Teeth: An Exploratory Study on a Total of 205 Teeth. Quintessenza Internazionale & Jomi 2019, 34, 85–89. [Google Scholar]
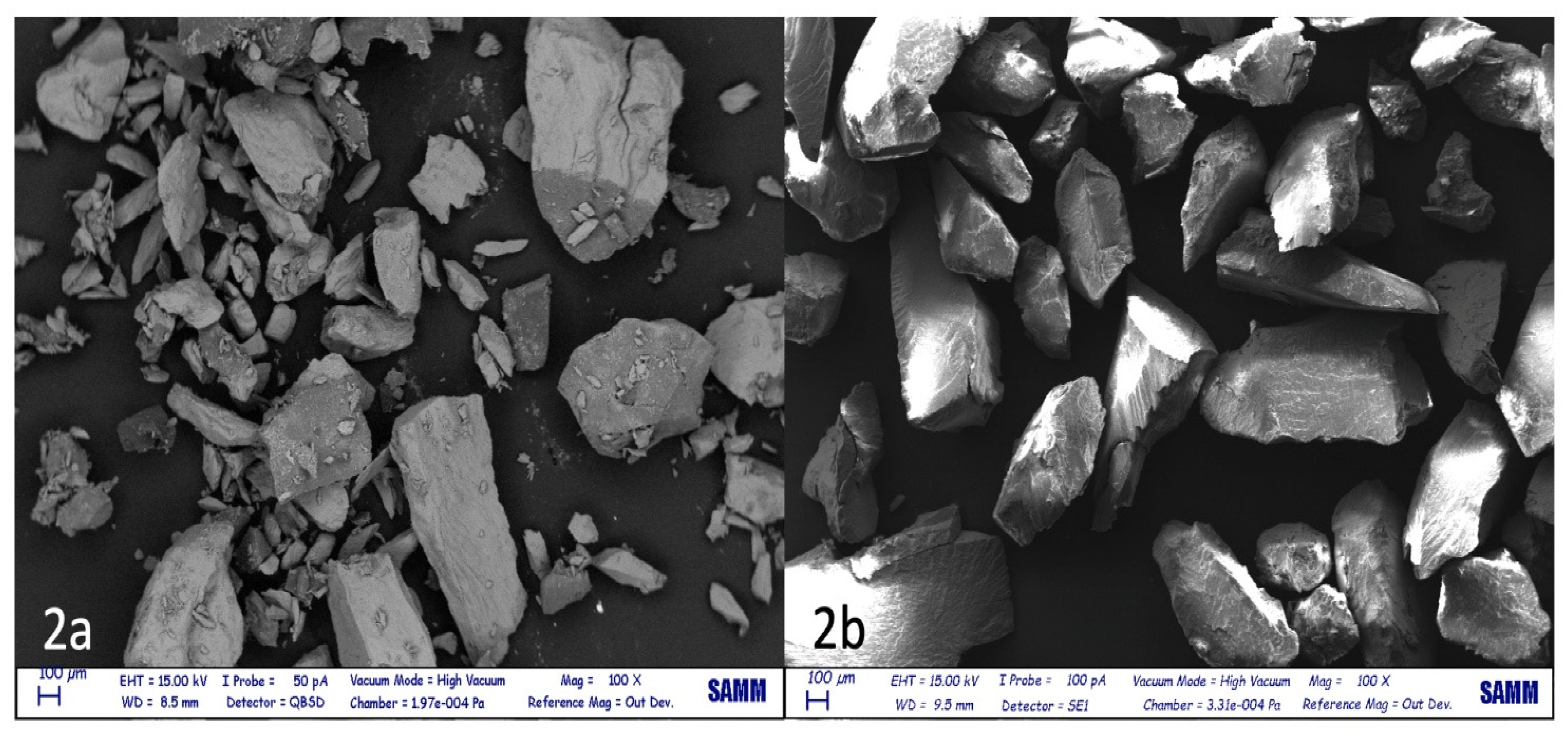


| Type of test | Average tooth weight before test | Average tooth weight after test | Lost tooth weight (%) |
|---|---|---|---|
| High speed | 1.2+/-0.53 | 0.56 +/-0.29 | 53.5+/-9.89 |
| Low speed | 1.44 +/-0.62 | 1.32+/-0.58 | 9.16+/-2.34 |
| Authors | Granules’ dimensions (mm) |
|---|---|
| Kim, Y., 2013 [61] |
0.5–1.0 |
| Kim, Y., 2013 [63] |
0.4–0.8 |
| Murata M., 2005 [64] |
0.4–0.8 |
| Nampo T., 2010 [65] |
0.5 |
| Kim Y., 2010 [66] |
0.2–1.2 |
| Jun S.H.,2014 [62] |
0.5–1 |
| Binderman I., 2014 [73] |
0.3–1.2 |
| Dozza B., 2017 [67] |
0.5–1.0 |
| Testori T., 2013 [69] |
1–2.0 |
| Kluppel L.E., 2013 [74] |
0.2–0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





