1. Introduction
Dye-sensitized solar cell (DSSC) was introduced for the first time by Gratzel and O’Regan in 1991 [
1,
2] as the promising third-generation solar cells owing to low-cost of raw materials [
3,
4,
5], simple fabrication process [
6,
7], and relatively good energy conversion efficiency [
8,
9,
10]. The configuration of DSSC consists of a dye adsorbed on the surface of a semiconductor oxide as photoanode, an electrolyte solution, and a counter electrode material [
11]. The working principle of DSSC is commonly utilizing electron transfer reactions. The working scheme of DSSC is shown in
Figure 1.
The series of chemical reactions that occur in DSSC are listed in Equations (1)–(5) [
12]:
The development research of DSSC has been focusing on increasing the performance of DSSC in order to get high efficiency. One of the materials that have been used in sandwich structure DSSC both as counter electrode and electrolyte is a conductive polymer. Conductive polymers are a group of organic polymers with high intrinsic conductivity and π-conjugated structure that possess the electrical and optical properties while maintain the mechanical properties [
13]. The unique properties of conductive polymer i.e. good optical properties, high conductivity, lightweight, low cost, flexibility, and excellent processability in industrial manufacturing are utilized in DSSC. One of the conductive polymers that have been used in DSSC as a counter electrode and an electrolyte is poly(3,4-ethylenedoxythiophene) (PEDOT).
PEDOT is one of the derivates of polythiophene that has been drawing attention due to its high conductivity, good stability, good transparency, low oxidation potential, and low band gap [
1,
11]. It is soluble in many solvents due to the presence of polystyrene sulfonate (PSS) as a dopant. The role of PSS is reliable for the stability of PEDOT:PSS dispersion owing to its being negatively charged as the counter ion while doping to the PEDOT. PSS has ester sulphate (-SO
3H) functional group as the hydrophilic part that assists the dispersion in PEDOT:PSS [
12]. In DSSC, PEDOT:PSS mostly is used as a counter electrode with the efficiency from 2-7%, reportedly [
3,
4,
8].
On the other hand, a similar thing has been found that PEDOT is dispersible in an aqueous solution in the presence of carrageenan. Carrageenan is an anionic heteropolysaccharide extracted from seaweed with an ester sulphate group, which is the same functional group as in PSS [
12]. Therefore, carrageenan has the potential as an alternative dopant for PEDOT to form PEDOT:Carrageenan. In recent research, PEDOT:Carrageenan is used as an electrolyte in DSSC with low efficiency.
Study of PEDOT and PEDOT:PSS related to their applications has been reviewed in several papers. Most of these review papers discuss the characteristics of PEDOT and PEDOT:PSS as a counter electrode of DSSC. L. Fagiolari
et al. have offered an overview of PEDOT-based materials and proposed the use of them in replacing Pt as counter electrodes in DSSCs [
14]. Wei Wei
et al. addressed the progress of PEDOT-based counter electrodes for DSSC with a specific discussion on the characteristics of PEDOT which was composited with other materials such as carbon, metal, metal oxide, etc [
15]. However, there are a few research articles that have been published for PEDOT:Carrageenan as an electrolyte for DSSC. Therefore, there is no comprehensive paper comparing PEDOT:PSS and PEDOT:Carrageenan in one paper related to their applications in DSSC. In this review article, we studied the different roles between PEDOT:PSS as a counter electrode and PEDOT:Carrageenan as an electrolyte in DSSC applications, respectively. This paper aims to compare the role of PEDOT:PSS as counter electrode and PEDOT:Carrageenan as electrolyte for DSSC applications.
2. Materials and Methods
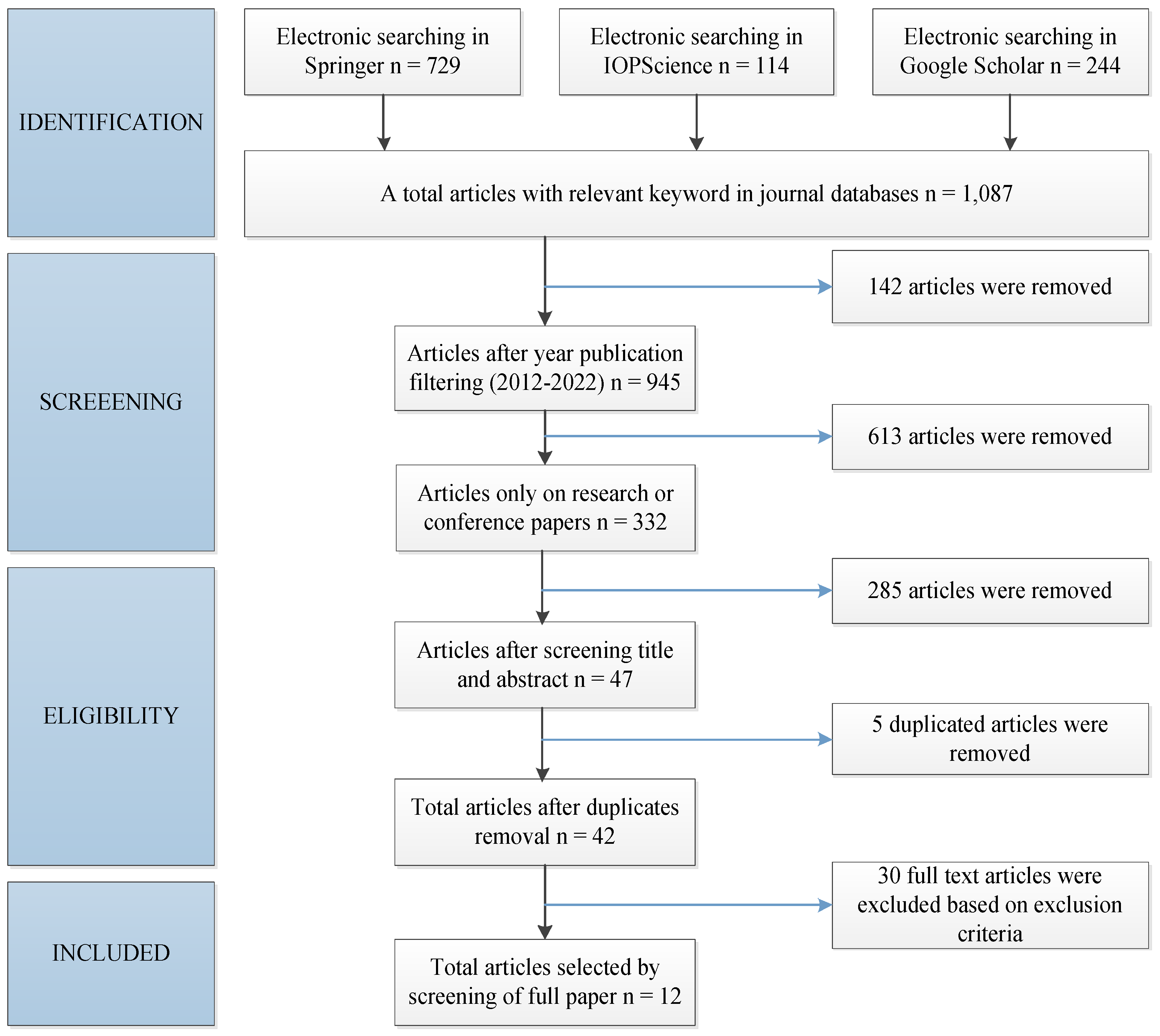
A systematic literature review (SLR) is applied to analyze the whole data that is related to some research at other’s previous works in the detail process. This review gives the overall description of the use of PEDOT:PSS and PEDOT:Carrageenan for DSSC application, respectively. This SLR has been done with four steps. They are the identification database based on the chosen keyword, the screening process, the eligibility test, and the included paper election. Furthermore, the selected papers in the full text were analyzed. The electronic search was conducted to find the papers in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Figure 2 shows selection procedures according to the PRISMA guidelines in this literature review.
2.1. Identification
The literature search employed the following databases at Springer, IOPscience, and Google Scholar with the keyword combination: (“PEDOT:PSS” AND “counter electrode”) OR (“PEDOT:Carrageenan” OR “PEDOT:Carr” AND “electrolyte”) OR (“polymer conductive” OR “polymer electrolyte”) AND (“DSSC” OR “Dye-Sensitized Solar Cell”). We restricted the search to articles published in international journals that were written in English and full-text available (open-access articles). The total documents were 1,087 papers as shown in
Table 1 in detail.
2.2. Screening
This section started with the year of publication screening from the total identified documents. The years of publication were limited from 2012 to 2022. As a result, 612 documents from Springer, 111 documents from IOPscience, and 222 documents from Google Scholar. Then, the type of paper screening was used to exclude all documents except for research and conference papers, and 332 articles were received. This screening process was done in September – October 2022.
2.3. Eligibility
The search in databases is selected to get more relevant articles. The search begins by screening the titles and analysing the abstracts of all articles and comparing them to predefined criteria. The inclusion criteria were studies on the performance of DSSC with PEDOT:PSS as a counter electrode and PEDOT:Carrageenan as an electrolyte, respectively. The exclusion criteria include articles that just describe PEDOT:PSS or carrageenan in other applications. After screening the title and abstract, there were 47 articles that meet the inclusion criteria and 285 excluded articles were removed. Out of 47 articles, five articles were excluded due to duplication and 30 articles were removed because the data was not complete to be analyzed. As result, there are 12 full-text articles to be analyzed.
2.4. Inclusion
Twelve full articles that have been studied in this review paper consist of nine articles for PEDOT:PSS as counter electrode and three articles for PEDOT:Carrageenan as electrolyte for DSSC.
3. Synthesis and Characteristics of PEDOT:PSS and PEDOT:Carrageenan
3.1. Synthesis and Characteristics of PEDOT:PSS
Poly(3,4-ethylenedioxythiphene) (PEDOT) was first synthesized and commercialized in 1988 by scientists in Germany [
13]. PEDOT has succeeded in becoming one of the electronically conducting polymers (ECPs) that have immense potential applications. However, PEDOT was difficult to be synthesized by the standard chemo/electro-polymerization due to its insolubility and infusibility in many solvents. Therefore, PEDOT needs some materials as a dopant which is a solvent-dispersible material with maintaining the properties of PEDOT.
Polystyrene sulfonate (PSS), water-dispersible polyelectrolyte, is a polymer surfactant that is able to be used as a dopant for PEDOT due to its -SO
3H functional group as the hydrophilic part. PEDOT and PSS formed a complex material as PEDOT:PSS.
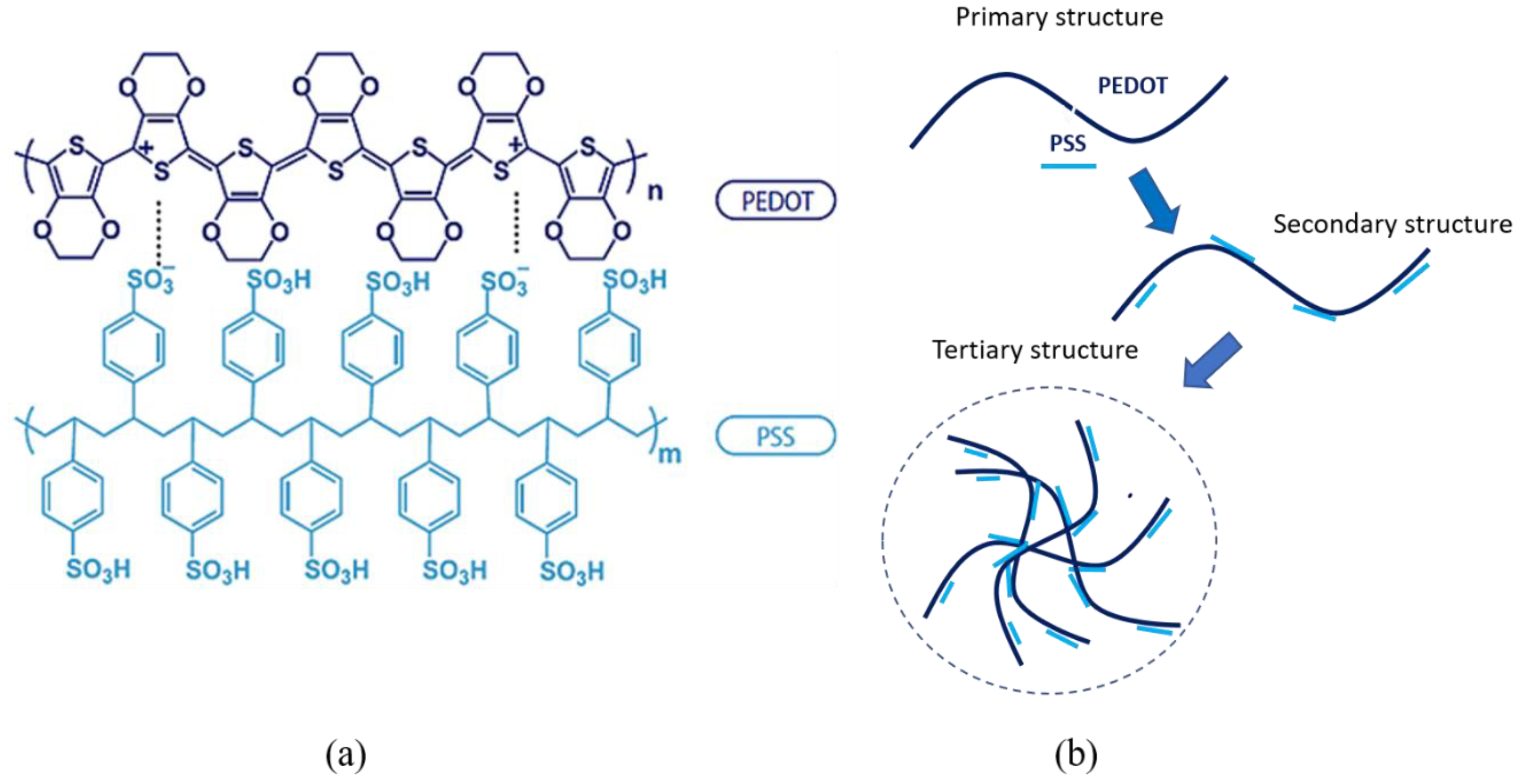
Figure 3 shows the structure of PEDOT:PSS and the identification of PEDOT:PSS structure was completed with FTIR analysis in
Table 2. In PEDOT:PSS, PSS has two roles: (i) it acts as a counterion to stabilize doped-PEDOT, and (ii) provides a matrix for PEDOT to form an aqueous dispersion [
16]. PEDOT:PSS consists of both positive charges conjugated PEDOT and negatively charge saturated PSS. A poly-ion complex between PEDOT cation and PSS anion was formed by monomer units of PEDOT and PSS through electrostatic interaction.
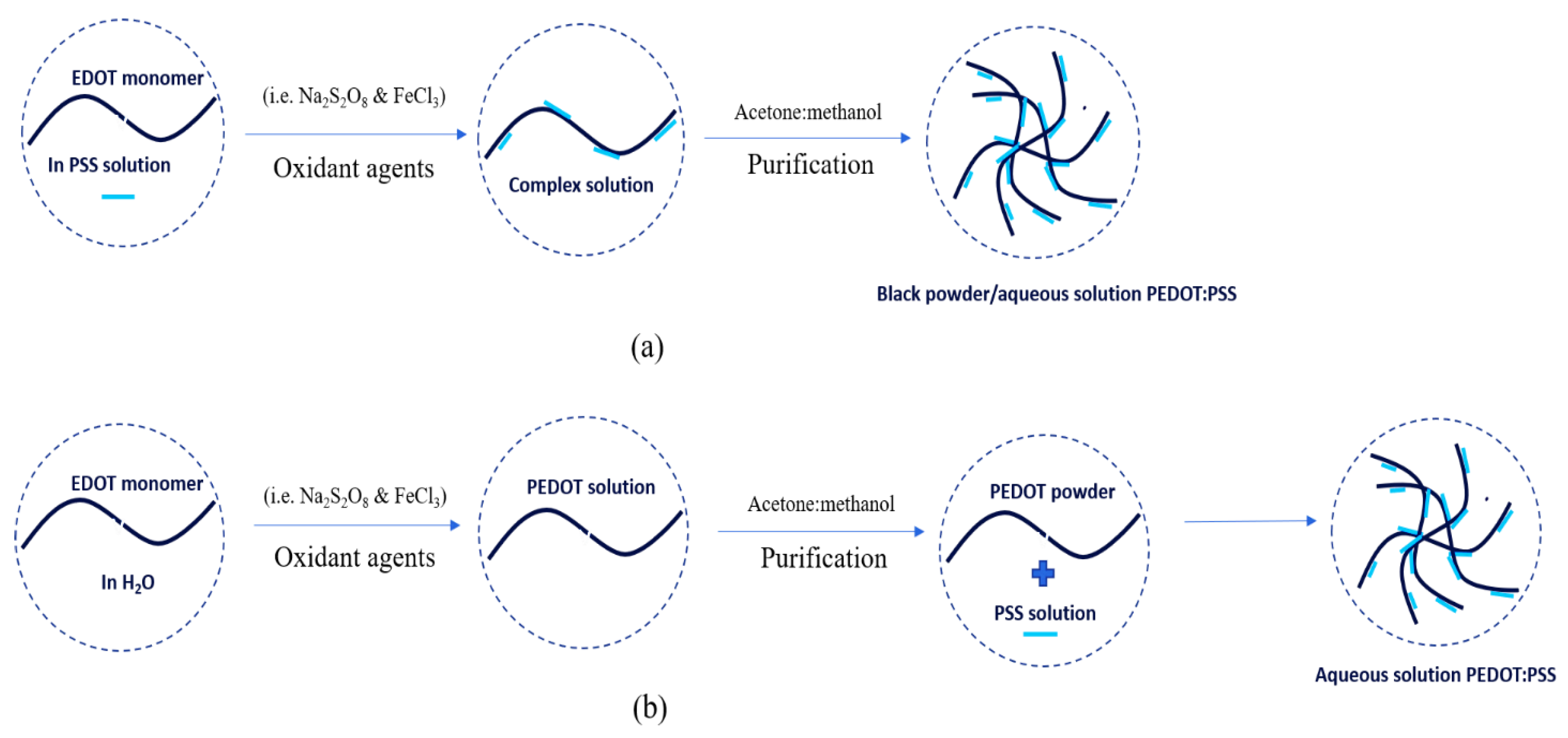
PEDOT:PSS was first synthesized by scientist research laboratories in German. PEDOT:PSS is the most successful commercially available in the form of an aqueous dispersion with good-water-dispersibility, superior miscibility, high transparency, high electrical conductivity, excellent flexibility, and satisfactory stretchability. PEDOT:PSS is typically synthesized via oxidative polymerization in two simple ways: in-situ and post-polymerization [
18,
19]. The synthesis process is shown in
Figure 4. In-situ polymerization has several steps to do as follows: first, monomer powder EDOT was added to an aqueous of PSS solution. Then, the mixtures were stirred vigorously in a water bath at room temperature under nitrogen. The oxidant agents (e.g. N
2S
2O
8 and FeCl
3 or Fe
2(SO
4)
3) were immediately added to the mixture solution to produce a complex and stirred again at room temperature for 24 hours. The precipitate was collected from the complex solution by centrifugation at a certain rpm and then rinsed with acetone:methanol at a certain ratio. The final result can be dispersed in water to be an aqueous in dark-blue solution or be dried in an oven at 60°C for 24 hours to get a black-powder. Post-polymerization includes the steps as follows: first, monomer powder EDOT were dispersed in water. The oxidant agents (e.g. N
2S
2O
8 and FeCl
3 or Fe
2(SO
4)
3) were immediately added to EDOT solution and stirred at room temperature for 24 hours. Then, the mixture was purified by mixing water and ethanol at a certain ratio. The PEDOT powder as a result was added to the PSS solution. The PEDOT:PSS solution was stirred at room temperature for 24 hours. The final result was the PEDOT:PSS aqueous in a dark-blue solution.
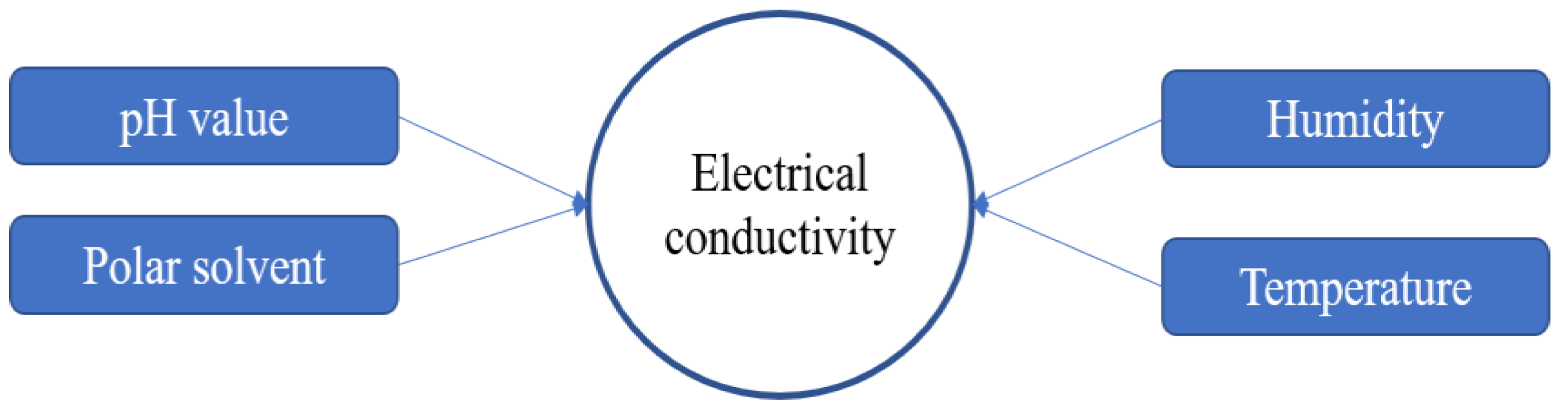
The properties of PEDOT:PSS are crucial things to consider in the synthesis process, especially for electrical conductivity. There are some factors that affect the electrical conductivity of PEDOT:PSS as shown in
Figure 5. In the synthesis process, some parameters should be controlled such as pH solution, temperature, humidity, and polar solvent as additives or blending components. PSS has hygroscopic and corrosive properties due to its strong acid (pH<2), which lowers lifetime and performance of the application devices. PSS can be neutralized with various alkalines, but it can change the structural and electrical conductivity of the resulting PEDOT:PSS [
20].
Several research showed that the higher the pH of the solution resulting from the synthesis process, for example, due to adding alkaline materials (e.g. NaOH), the electrical conductivity decreased and vice versa. If the resulting solution was in a lower pH, by adding acid materials (e.g. HCl) during the synthesis process, the electrical conductivity increased [
13,
20]. This is associated with (i) removal of the insulting PSS from the surface of the colloidal particles and (ii) crystallization of the PEDOT molecule which improve both intra- and inter-particle transfer to charge carrier [
20]. The high electrical conductivity affected the higher carrier mobility and the structure of PEDOT:PSS. PEDOT molecules with amorphous state partly change into the crystalline state by the lower pH and also adding polar solvents, i.e. ethylene glycol (EG), dimethyl sulfoxide (DMSO), dimethyl formamide (DMF), etc that were added during the synthesis process. PEDOT in the crystalline state has orthorhombic phase that represents
stacking of the PEDOT molecules with XRD peak at 2θ=26° (020) [
20,
21,
22].
Besides pH and polar solvent, the humidity and temperature affected the electrical conductivity of PEDOT:PSS in thin film form. The PEDOT:PSS can be applied in many application devices if it was made in thin film form. Several methods such as spin-coating, screen-printing, electrospinning, etc. are used as a technique for thin film deposition. The electrical conductivity of
PEDOT:PSS film was changed in water. At first, the electrical conductivity initially increased significantly, but then it slowly decreased when its film was gradually damaged depending on the longer immersed time of PEDOT:PSS film in water. Otherwise, the electrical conductivity of PEDOT:PSS film decreased slowly as the temperature decreased [
13,
16]
.
3.2. Synthesis and Characteristics of PEDOT:Carrageenan
Carrageenan is a general name for a polysaccharides family, extracted from many species of red algae (
Rhodophyta). Chemically, this polymer is linear, sulphated gulactans, which is composed of alternating
disaccharide repeating units of 3-linked β-D-
galactopyranose (G-units) and 4-linked α-D-
galactopyranose (D-Units) or 4-linked 3,6-
anhydrogalactose (DA-Units) [
23,
24]. The first called
phycocolloid carrageenin was found by the British pharmacist Stanford in 1862 who extracted it from Irish moss (
Chandrus crispus) [
24]. The name was later changed to
carrageenan so as to fulfill with the ‘-an’ suffix for the names of polysaccharides that was accepted in the industry dates from the 1940s [
25].
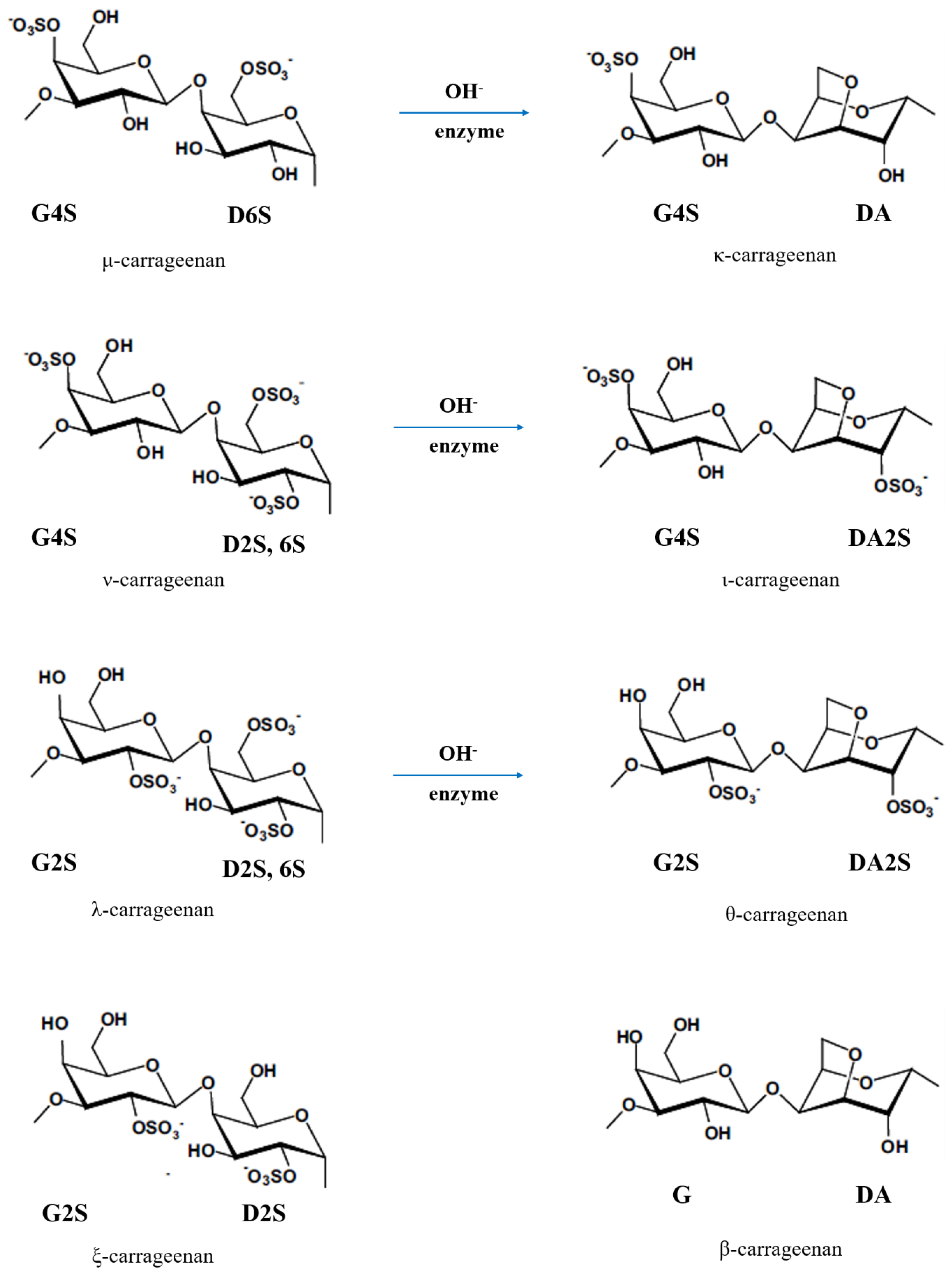
Depending on the number and position of sulphate groups,
carrageenan has three commercially main types:
kappa (κ)-, iota (ι)-, and
lambda (λ)-carrageenans. The
κ-carrageenan is extracted from the seaweed
Kappaphycus alvarezii, known as
Euchema cottonii (or simply
cottonii). The
ι-carrageenan is mainly produced from
Euchema denticulum, known as
Euchemaspinosum (or simply
spinosum species). The
λ-carrageenan is extracted from the species of
Gigantana and
Chondrus ginera. There are also several other
carrageenan repeating units, e.g.
mu (µ)-, nu (υ)-, xi(ξ)-, theta (θ)-, and beta (β)-carrageenans [
25,
26,
27].
Figure 6 shows all types of
carrageenan. Based on IUPAC nomenclature and on the letter codes (
Table 3), their corresponding names of
κ-, ι-, and
λ-carrageenan are
carrageenase 2,40-disulphate (G4S-DA2S),
carrageenase 40-sulphate (G4S-DA), and
carrageenan 2,6,20-trisulphate (G2S-D2S,6S), respectively [
25,
26].
Carrageenan is one of natural polymers with ester sulphate (-SO
3H) groups that has the capability to produce thermo-reversible gels or high viscous solution, and it is commonly used as gelification, stabilizing, and emulsifying agents in several foods and pharmaceutical and cosmetic products [
28]. Carrageenan has the similarity of ester sulpahte (-SO
3H) groups to PSS. PSS has been used as a dopant in complex material PEDOT:PSS, then carrageenan also has the potential to be used as dopant to form a complex material PEDOT:Carrageenan.
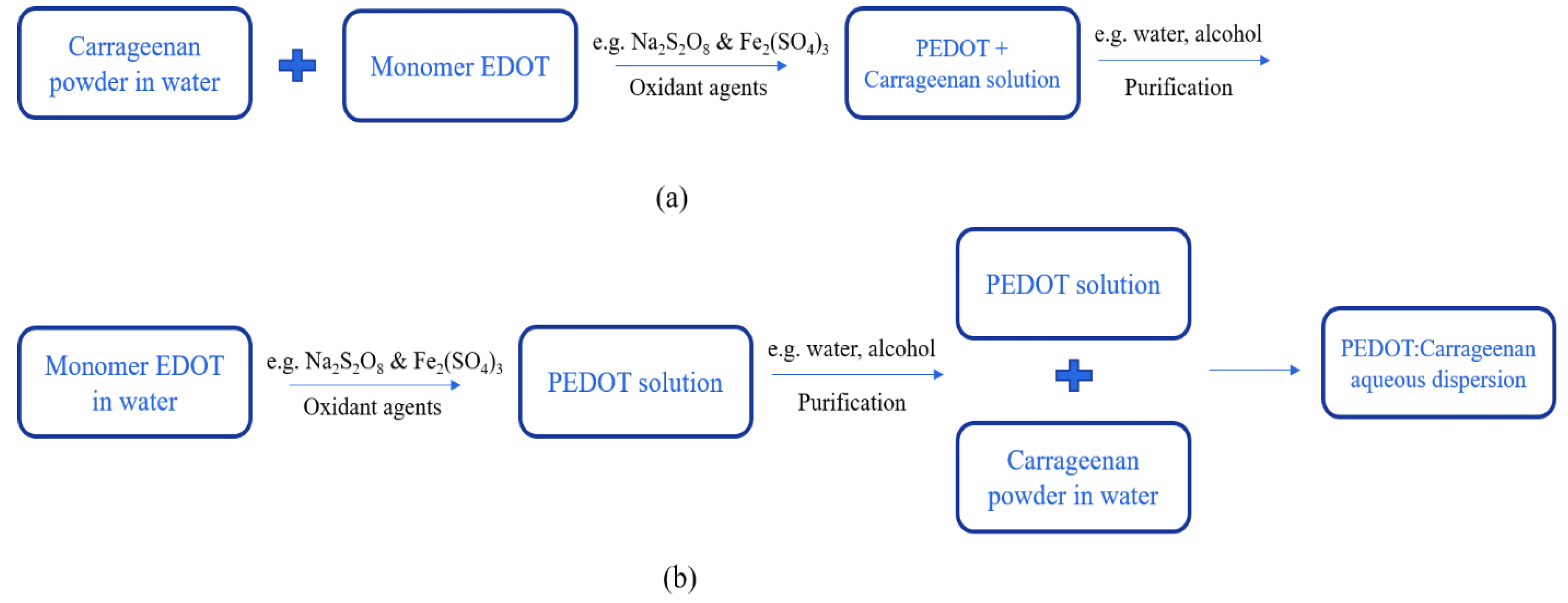
PEDOT:Carrageenan was synthesized via oxidative polymerization [
12,
30] as synthesis of PEDOT:PSS. The oxidative polymerization was done in two simple ways: in-situ and post-polymerization. The procedures of both two ways as similar as PEDOT:PSS, but the carrageenan powder were first diluted in water under stirring at 70°C. Then, monomer EDOT, oxidant agents, and surfactant were mixed together until the PEDOT:Carrageenan solution was obtained in homogenous solution. The process synthesis of PEDOT:Carrageenan is shown in
Figure 7. To make sure obtaining the PEDOT:Carrageenan aqueous dispersion, the FTIR analysis (
Table 4) could be used to identify the bonding atoms in PEDOT:Carrageenan.
4. Results and Discussions
4.1. Conductive Polymer in Dye-Sensitized Solar Cells
A dye-sensitized solar cell (DSSC) has a traditional structure that consists of a transparent photoanode with a dye-sensitized mesoporous thin film, / redox electrolyte, and a counter electrode with a catalytic layer deposited on a conductive substrate. Recently, there are many papers that researched how to increase the performance of DSSC, starting from the material selection for photoanode materials like some metal-oxide semiconductors, e.g. TiO2, ZnO, ZrO2, etc., even up in composite form between two or more metal-oxide semiconductors such as a composite of TiO2:ZnO, TiO2:ZrO2, etc. Also, many researchers are starting to use natural dyes as dye-sensitized like extracted from leaves, vegetables, etc., which can be absorbed well by photoanode materials. As well as in the selected materials for electrolyte and counter electrode, where these two materials work together in electron transfer from the external circuit to the internal circuit for obtaining the current.
In DSSC, conductive polymers have been mostly used as a counter electrode and an electrolyte system. These two components have a crucial role to create charge transport electrons. The counter electrode is defined as the electrode at which electrons enter the cell and reduction occurs. Meanwhile, the electrolyte is sandwiched between the photoanode and the counter electrode to stable the operation of DSSC as it must carry the charge between the photoanode and the counter electrode for the regeneration of the dye and itself.
In DSSC, the counter electrode has been responsible for three roles: (i) as a catalyst to accelerate the reduction reaction of redox couple within the electrolyte, (ii) as a positive electrode to collect the electrons from the external circuit and transmit them into the cell, and (iii) as mirror or reflector to reflect the unabsorbed light from the cell back into the active area, hence increasing the absorption efficiency of sunlight into DSSC [
30,
31]. An ideal counter electrode material is effectively expected to have a large surface area and porosity and good catalytic activity for the reduction of redox couple within the electrolyte that has matched energy levels for redox couple potential, high electrical conductivity, good reflectively, low-cost, easy synthesis process, optimum thickness and strong adhesivity on TCO substrate, chemically, mechanically, and electrochemically stable. Several papers had written a review about various types of conductive polymers as counter electrodes for DSSC. They said that the potency of conductive polymers to be a counter electrode material for DSSC had given the promise to replace Pt-based counter electrodes with high efficiency [
30,
31,
32,
33]. PEDOT:PSS is one of the counter electrode polymers that has been used in DSSC with a high performance of ~10% [
30].
Electrolytes have roles in DSSC as an electron transfer with the function of regenerating the dye-sensitized from the oxidized state. The electrolyte must have long-term stability and high ionic conductivity. In general, electrolytes for DSSC are formed in liquid, quasi-solid, and solid. Liquid electrolytes have been mostly used for DSSC due to easy fabrication. The utilize of a liquid electrolyte leads to relevant technological problems, such as limited long-term stability, difficulty in tough and airtight sealing, evaporation and seepage of electrolyte in case of breaking of the glass substrates, corrosion of platinized cathode, shift of the adsorbed dye, water, and oxygen permeability [
34]. To address this problem, the polymer could be used as an electrolyte due to its properties such as high ionic conductivity, transparency, thin-film forming ability, flexibility, and easy processability. Some papers have reviewed the polymer electrolytes for DSSC with high ionic conductivity of 4.75 x 10
-2 S/cm [
35,
36]. Polymer electrolytes is classified into three different types based on their physical state and composition: (i) gel polymer electrolytes, (ii) solid polymer electrolytes, and (iii) composite polymer electrolytes [
37].
The conductive polymer as a counter electrode and electrolyte becomes interesting research since the properties of the polymer are matched to the criteria of ideal material both the counter electrode and electrolyte. One of the conductive polymers that have been mostly used as a counter electrode and electrolyte is poly(3,4-ethylenedioxythiophene) (PEDOT) and their composites like PEDOT:PSS and PEDOT:Carrageenan.
4.2. PEDOT:PSS as Counter Electrode
The recent development research of PEDOT:PSS as a counter electrode for DSSC application is summarized in
Table 5. There are factors that have affected the quality of PEDOT:PSS-based counter electrode in DSSC performance, either intrinsically or extrinsically. Intrinsically, the characteristics results should obtain the match properties of PEDOT:PSS as a counter electrode, such as electrical conductivity value and electrocatalytic activity capability. It was related to the chemical synthesis process that has done in each experiment. Extrinsically, the quality of the thin film can be seen from thickness, morphology, and roughness, which it was related to the treatment that has given during the thin film fabricated process.
The thin film of PEDOT:PSS with different number layers and temperature annealing can be affected to the performance DSSC. PEDOT:PSS thin film with three layers of coating at 120 °C resulted the highest efficiency of 1.77% [
38]. Increasing the number of layers and the annealing temperature of PEDOT:PSS film has enhanced the electrical conductivity value. However, the higher annealing temperature produced a thinner layer of PEDOT:PSS film and caused the decreasing PEDOT:PSS roughness. It hinder the electrocatalytic activity of PEDOT:PSS that cause low the electron mobility. Therefore, increasing the number of layers and temperature of PEDOT:PSS film can cause low the short-circuit current density (J
sc) that the DSSC efficiency decrease.
PEDOT:PSS-based DSSC has still low efficiency with low J
sc and fill factor (FF) due to the high series resistance of the cell that was caused by the poor contact between PEDOT:PSS and substrate. Polyethylene glycol (PEG), a water-soluble polymer with excellent film-forming, emulsifying, and adhesive properties, can assist the binding strength between PEDOT:PSS and substrate. PEG also serves as a binder to improve the mechanical properties of the counter electrode. The highest efficiency of 4.39% was obtained by mixing the PEDOT:PSS and 5wt% of PEG in 0.2wt% of acetylene black [
39]. It was proven that the best binding strength between PEDOT:PSS and substrate caused a low sheet resistance value that indicated high electrical conductivity and improved the J
sc and FF values. These results enhanced the performance of DSSC using PEDOT:PSS as a counter electrode.
The stability of PEDOT:PSS during the preparation process is most important to keep the good properties and quality of the counter electrode. PEDOT:PSS can be easily combined with many other materials and compounds, such as metal-oxide semiconductors, metal materials, sulphide compounds, etc. Titanium dioxide (TiO
2) is one of the metal-oxide semiconductors which can be mixed with PEDOT:PSS as a counter electrode. DSSC with a mixture of PEDOT:PSS and TiO
2 nanoparticles have the highest efficiency of 8.49% [
40]. TiO
2 nanoparticles can be used to increase polymer surface for enhancing film redox reaction and solar performance. Other materials, e.g. Ni, NiSO
4, and NiS, can be also combined with PEDOT:PSS as a counter electrode. Composite of PEDOT:PSS with those materials, as well as composite PEDOT:PSS with TiO
2 nanoparticles, can enhance the electrical conductivity and electrocatalytic activity by increasing the large surface area and decreasing the sheet resistance. The highest efficiency of 2.25% and 3.05% could be obtained from composite of Ni-PEDOT:PSS and NiSO
4-PEDOT:PSS, respectively [
41]. Meanwhile, composite of NiS-PEDOT:PSS obtained the highest efficiency of 8.18% [
42].
Combination of PEDOT:PSS and carbon materials can also be used as counter electrodes to enhance the performance of DSSC. Carbon materials with polymers enhanced mechanical and thermal stability, electrical conductivity, and electrocatalytic activity due to increased surface area. PEDOT:PSS/Carbon composite was formed by mixing the PEDOT:PSS solution and a small amount of graphite powder. The composite film was coated on FTO using a scratch method at vacuum at 80°C with a thickness of 3-4μm. The highest efficiency of 7.60% was obtained due to the high electrical conductivity and high electrocatalytic activity in the
redox reduction [
3]. In the same way, PEDOT:PSS/Graphene composite obtained the highest efficiency of 4.66% [
43]. Other carbon materials such as single-wall carbon nano-horn (SWCNH) and single-wall carbon nanotube (SWCNT) can be composited with PEDOT:PSS. PEDOT:PSS/SWCNH bilayer films obtained the highest efficiency of 5.10%. The proposed bilayer is more valuable since it couple of merits of SWCNH like high specific surface area, excellent electrocatalytic ability, and merits of PEDOT:PSS like high conductivity [
44]. The performance of DSSC increased due to highly textured surface, large surface area, increased surface roughness, evidencing more number of catalytic active sites for reduction of
to
ions. Increasing surface area in case of bilayer counter electrode could be attributed to the compositional/stacked effect of PEDOT:PSS and SWCNH. Meanwhile, combination of PEDOT:PSS and SWCNT with addition of molybdenum disulphide (MoS
2) obtained the highest efficiency of 8.14%. MoS
2 is transition metal sulphide, as a typical lamellar compound that has the similar structure to graphene. MoS
2 can be prospective used as a lubricant hydrogen evolution reaction, electrode materials for lithium battery and DSSC [
45]. The DSSC performance increased due to the high catalytic activity of counter electrode. The enhanced electrocatalytic activity corresponds to its synergetic catalytic effect, such as distinctive structure with large surface area of PEDOT:PSS, the improvement conductivity of SWCNT, and excellent catalytic activity of MoS
2, which could make electron transmit across the counter electrode/FTO interface easily [
45].
4.3. PEDOT:Carrageenan as Electrolyte
Research related to PEDOT:Carrageenan for DSSC was found in some papers as presented in
Table 6. In general, characteristics of carrageenan is similar to cellulose which is always used as electrolyte in electronic devices. However, the performance of DSSC is still low using PEDOT:Carrageenan as electrolyte.
The first study of PEDOT:Carrageenan as electrolyte for DSSC obtained the highest efficiency of 0.42% using κ-carrageenan [
46]. In this study, the synthesis process has been done by in situ polymerization with mixing EDOT monomer and κ-carrageenan solution. The different concentration of κ-carrageenan was used to investigate the effect of κ-carrageenan on PEDOT. The degraded-carrageenan was also prepared to observe the different molecular weight of carrageenan in PEDOT and its effects to conductivity value. Increasing the concentration of κ-carrageenan along with increasing the molecular weight enhanced the conductivity value and vice versa for degraded-carrageenan, decreasing the molecular weight of the carrageenan caused low the conductivity value. It caused low the performance of DSSC.
In other studies, PEDOT:Carrageenan was synthesized using λ-carrageenan with ratio of 1:1 [
47] and κ-carrageenan with ratio of 1:2 [
48] in weight percent (wt%). Both showed low the performance of DSSC. It was caused by the rigidity of PEDOT chain, electron transport was not as efficient as compared to liquid system [
47,
48]. Both studies have synthesized the PEDOT:Carrageenan with similar process via in-situ polymerization by mixing EDOT monomer and carrageenan solution. However, the conductivity of each samples was not measured which was related to the DSSC performance.
5. Author’s Perspective
Polystyrene sulfonate (PSS) and carrageenan can be as a dopant for PEDOT due to their ester sulphate group (-SO3) through similar synthesis process which is oxidative polymerization. PSS and carrageenan are a water-soluble polymer that assist PEDOT to be dispersed in many solvents. PEDOT:PSS as counter electrode has been successfully combined with other materials in composite form to optimize the electrical conductivity and catalytic activity that can enhance the DSSC performance. PEDOT:Carrageenan as electrolyte on DSSC still obtained low performance. PEDOT:Carrageenan can be probably combined with other materials as well as PEDOT:PSS to enhance ionic conductivity and viscosity as electrolyte for DSSC to enhance the DSSC performance. PEDOT:Carrageenan as electrolyte polymer can be formed in gel polymer electrolyte, solid polymer electrolyte, or composite polymer electrolyte.
PSS and carrageenan have differences. PSS has pH condition in acidic environmental, while carrageenan has pH condition in alkaline environmental. pH value could affect to the degree of crystallinity of materials that could affect to the electrical conductivity. Increasing pH value decreased the electrical conductivity due to low the degree of crystallinity. It can be the one of the reasons that PEDOT:PSS can be used as counter electrode due to acidic pH that show the high electrical conductivity. Meanwhile, PEDOT:Carrageenan has alkaline pH that show the low electrical conductivity which means PEDOT:Carrageenan can be used as electrolyte.
The main difference properties between counter electrode and electrolyte materials is electrical and ionic conductivity. As counter electrode, a material should have high electrical conductivity, while as electrolyte, a material should have high ionic conductivity. When a material has high electrical conductivity, it means that material has low ionic conductivity and vice versa. Since PEDOT:PSS has been successfully used as counter electrode for DSSC with high performance and the DSSC performance is still low with PEDOT:Carrageenan electrolyte, PEDOT:Carrageenan has a chance to be used as counter electrode also as well as PEDOT:PSS through some synthesis treatments, such as combined with other materials that can enhance the electrical conductivity and catalytic activity or decrease the pH value with added the acidic solution e.g. HCl, H2SO4, etc. during synthesis process.
6. Conclusions
The study of PEDOT:PSS as counter electrode and PEDOT:Carrageenan as electrolyte for DSSC application has been reviewed in this paper. PEDOT:PSS and PEDOT:carrageenan can be synthesized through similar synthesis process due to their similar ester sulphate (-SO3H) functional groups that assist PEDOT to be well dispersed in many solvents. The combination materials between PEDOT:PSS and metal oxide materials have enhanced the electrical conductivity and catalytic activity through increasing the roughness of film morphology and large surface area. This combination material has been successfully used as a counter electrode with highest DSSC performance of ~8%. Meanwhile, the DSSC performance was still low by using PEDOT:Carrageenan as electrolyte. However, there is a chance to use PEDOT:Carrageenan as a counter electrode as well as PEDOT:PSS through some synthesis treatments with increasing the electrical conductivity than ionic conductivity of PEDOT:Carrageenan.
Author Contributions
Conceptualization, E.S.N., A.A., R.R., and L.S.; methodology, E.S.N. and R.R.; review investigation, E.S.N.; data curation, E.S.N.; writing—original draft preparation, E.S.N.; writing—review and editing, E.S.N., L.S., A.A., and R.R.; visualization, E.S.N.; supervision, L.S., A.A., and R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Academic Leadership Grant (ALG) of Universitas Padjadjaran 2022, No. 2203/UN6.3.1/PT.00/2022.
Institutional Review Board Statement
Not applicable
Data Availability Statement
Not applicable
Acknowledgments
The authors are grateful for the financial support from Hibah Riset Internal Universitas Padjadjaran under project no. 1549/UN6.3.1/PT.00/2023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- P. Balraju, M. Kumar, M. S. Roy, and G. D. Sharma, “Dye sensitized solar cells (DSSCs) based on modified iron phthalocyanine nanostructured TiO2 electrode and PEDOT:PSS counter electrode,” Synth. Met., vol. 159, pp. 1325–1331, Apr. 2009.
- C.-H. Chiang and C.-G. Wu, “High-efficient dye-sensitized solar cell based on highly conducting and thermally stable PEDOT:PSS/glass counter electrode,” Org. Electr Onics, vol. 14, pp. 1769–1776, 2013. [CrossRef]
- G. Yue et al., “A dye-sensitized solar cell based on PEDOT:PSS counter electrode,” Chin. Sci. Bull., vol. 58, no. 4–5, pp. 559–566, Feb. 2013. [CrossRef]
- Anil, S. Sambhudevan, C. O. Sreekala, and B. Shankar, “Effect of silver nanoparticle in the PEDOT: PSS counter electrode of dye sensitized solar cell,” presented at the PROCEEDINGS OF THE INTERNATIONAL CONFERENCE ON ADVANCED MATERIALS: ICAM 2019, Kerala, India, 2019, p. 020123. [CrossRef]
- H. Pujiarti, W. S. Arsyad, Shobih, L. Muliani, and R. Hidayat, “Efficient and Stable Photovoltaic Characteristics of Quasi-Solid State DSSC using Polymer Gel Electrolyte Based on Ionic Liquid in Organosiloxane Polymer Gels,” J. Phys. Conf. Ser., vol. 1011, p. 012020, Apr. 2018. [CrossRef]
- B. I. Ito, J. N. de Freitas, M.-A. D. Paoli, and A. F. Nogueira, “Application of a composite polymer electrolyte based on montmorillonite in dye-sensitized solar cells,” J. Braz. Chem. Soc., vol. 19, no. 4, pp. 688–696, 2008. [CrossRef]
- S. Sarwar et al., “Transformation of a liquid electrolyte to a gel inside dye sensitized solar cells for better stability and performance,” Thin Solid Films, vol. 704, p. 138024, Jun. 2020. [CrossRef]
- P. Gemeiner et al., “Pt–free counter electrodes based on modified screen–printed PEDOT:PSS catalytic layers for dye–sensitized solar cells,” Mater. Sci. Semicond. Process., vol. 66, pp. 162–169, Aug. 2017. [CrossRef]
- K. Moolsarn et al., “A Dye-Sensitized Solar Cell Using a Composite of PEDOT:PSS and Carbon Derived from Human Hair for a Counter Electrode,” Int. J. Photoenergy, vol. 2017, pp. 1–11, 2017. [CrossRef]
- C. Bu, Q. Tai, Y. Liu, S. Guo, and X. Zhao, “A transparent and stable polypyrrole counter electrode for dye-sensitized solar cells,” J. Power Sources, vol. 221, pp. 78–83, 2013.
- W. M. Diah, S. Saehana, and C. I. Holdsworth, “Potency of Carrageenan as the doping agent for poly(3,4-ethylenedioxythiophene) conductive polymer,” J. Phys. Conf. Ser., vol. 1242, no. 1, p. 012007, Jun. 2019. [CrossRef]
- W. M. Diah et al., “The Effect of Synthetic Conditions on the Characteristics of Carrageenan-Doped Poly(3,4-ethylenedioxythiophene),” Macromol. Symp., vol. 391, no. 1, p. 1900162, Jun. 2020. [CrossRef]
- Y. Wen and J. Xu, “Scientific Importance of Water-Processable PEDOT-PSS and Preparation, Challenge and New Application in Sensors of Its Film Electrode: A Review,” J. Polym. Sci. Part Polym. Chem., vol. 55, no. 7, pp. 1121–1150, Apr. 2017. [CrossRef]
- L. Fagiolari, E. Varaia, N. Mariotti, M. Bonomo, C. Barolo, and F. Bella, “Poly(3,4-ethylenedioxythiophene) in Dye-Sensitized Solar Cells: Toward Solid-State and Platinum-Free Photovoltaics,” Adv. Sustain. Syst., vol. 5, 2021. [CrossRef]
- W. Wei, H. Wang, and Y. H. Hu, “A review on PEDOT-based counter electrodes for dye-sensitized solar cells: PEDOT-Based Counter Electrodes for DSSCs,” Int. J. Energy Res., vol. 38, no. 9, pp. 1099–1111, Jul. 2014. [CrossRef]
- L. V. Kayser and D. J. Lipomi, “Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS,” Adv. Mater., vol. 31, no. 10, p. 1806133, Mar. 2019. [CrossRef]
- P. Sakunpongpitiporn, K. Phasuksom, N. Paradee, and A. Sirivat, “Facile synthesis of highly conductive PEDOT:PSS via surfactant templates,” RSC Adv., vol. 9, no. 11, pp. 6363–6378, 2019. [CrossRef]
- F. Louwet et al., “PEDOT/PSS: synthesis, characterization, properties and applications,” Synth. Met., vol. 135–136, pp. 115–117, Apr. 2003. [CrossRef]
- S. Panigrahy and B. Kandasubramanian, “Polymeric thermoelectric PEDOT: PSS & composites: Synthesis, progress, and applications,” Eur. Polym. J., vol. 132, p. 109726, Jun. 2020. [CrossRef]
- Y. Mochizuki, T. Horii, and H. Okuzaki, “Effect of pH on Structure and Conductivity of PEDOT/PSS,” Trans. Mater. Res. Soc. Jpn., vol. 37, no. 2, pp. 307–310, 2012. 2012. [CrossRef]
- J. Dong and G. Portale, “Role of the Processing Solvent on the Electrical Conductivity of PEDOT:PSS,” Adv. Mater. Interfaces, vol. 7, no. 18, p. 2000641, Sep. 2020. [CrossRef]
- T. Horii, H. Hikawa, M. Katsunuma, and H. Okuzaki, “Synthesis of highly conductive PEDOT:PSS and correlation with hierarchical structure,” Polymer, vol. 140, pp. 33–38, Mar. 2018. [CrossRef]
- F. van de Velde et al., “The structure of κ/ι-hybrid carrageenans II. Coil–helix transition as a function of chain composition,” Carbohydr. Res., vol. 340, no. 6, pp. 1113–1129, May 2005. [CrossRef]
- T. R. Thrimawithana, S. Young, D. E. Dunstan, and R. G. Alany, “Texture and rheological characterization of kappa and iota carrageenan in the presence of counter ions,” Carbohydr. Polym., vol. 82, no. 1, pp. 69–77, Aug. 2010. [CrossRef]
- L. Pereira, A. M. Amado, A. T. Critchley, F. van de Velde, and P. J. A. Ribeiro-Claro, “Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman),” Food Hydrocoll., vol. 23, no. 7, pp. 1903–1909, Oct. 2009. [CrossRef]
- V. L. Campo, D. F. Kawano, D. B. da Silva, and I. Carvalho, “Carrageenans: Biological properties, chemical modifications and structural analysis – A review,” Carbohydr. Polym., vol. 77, no. 2, pp. 167–180, Jun. 2009. [CrossRef]
- P. Volery, R. Besson, and C. Schaffer-Lequart, “Characterization of Commercial Carrageenans by Fourier Transform Infrared Spectroscopy Using Single-Reflection Attenuated Total Reflection,” J. Agric. Food Chem., vol. 52, no. 25, pp. 7457–7463, Dec. 2004. [CrossRef]
- V. Webber, S. M. de Carvalho, P. J. Ogliari, L. Hayashi, and P. L. M. Barreto, “Optimization of the extraction of carrageenan from Kappaphycus alvarezii using response surface methodology,” Food Sci. Technol., vol. 32, no. 4, pp. 812–818, Oct. 2012. [CrossRef]
- E. Gómez-Ordóñez and P. Rupérez, “FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds,” Food Hydrocoll., vol. 25, no. 6, pp. 1514–1520, Aug. 2011. [CrossRef]
- J. Wu et al., “Counter electrodes in dye-sensitized solar cells,” Chem. Soc. Rev., vol. 46, no. 19, pp. 5975–6023, 2017. [CrossRef]
- E. Oktaviani, N. M. Nursam, N. Prastomo, and Shobih, “Effect of counter electrode materials on the performance of dye-sensitized solar cell,” presented at the PROCEEDINGS OF THE 3RD INTERNATIONAL SEMINAR ON METALLURGY AND MATERIALS (ISMM2019): Exploring New Innovation in Metallurgy and Materials, Tangerang Selatan, Indonesia, 2020, p. 050001. [CrossRef]
- J. Theerthagiri, A. R. Senthil, J. Madhavan, and T. Maiyalagan, “Recent Progress in Non-Platinum Counter Electrode Materials for Dye-Sensitized Solar Cells,” ChemElectroChem, vol. 2, no. 7, pp. 928–945, Jul. 2015. [CrossRef]
- K. Saranya, Md. Rameez, and A. Subramania, “Developments in conducting polymer based counter electrodes for dye-sensitized solar cells – An overview,” Eur. Polym. J., vol. 66, pp. 207–227, May 2015. 20 May. [CrossRef]
- F. Bella and R. Bongiovanni, “Photoinduced polymerization: An innovative, powerful and environmentally friendly technique for the preparation of polymer electrolytes for dye-sensitized solar cells,” J. Photochem. Photobiol. C Photochem. Rev., vol. 16, pp. 1–21, Sep. 2013. [CrossRef]
- M. S. Su’ait, M. Y. A. Rahman, and A. Ahmad, “Review on polymer electrolyte in dye-sensitized solar cells (DSSCs),” Sol. Energy, vol. 115, pp. 452–470, May 2015. 20 May. [CrossRef]
- L. P. Teo, M. H. Buraidah, and A. K. Arof, “Polyacrylonitrile-based gel polymer electrolytes for dye-sensitized solar cells: a review,” Ionics, vol. 26, no. 9, pp. 4215–4238, Sep. 2020. [CrossRef]
- K. S. Ngai, S. Ramesh, K. Ramesh, and J. C. Juan, “A review of polymer electrolytes: fundamental, approaches and applications,” Ionics, vol. 22, no. 8, pp. 1259–1279, Aug. 2016. [CrossRef]
- K. K. Putra, E. S. Rosa, Shobih, and N. Prastomo, “Development of monolithic dye sensitized solar cell fabrication with polymer-based counter electrode,” J. Phys. Conf. Ser., vol. 1191, p. 012029, Mar. 2019. [CrossRef]
- X. Yan and L. Zhang, “Polyethylene glycol-modified poly(3,4-ethylene dioxythiophene):poly(styrene sulfonate) counter electrodes for dye-sensitized solar cell,” J. Appl. Electrochem., vol. 43, no. 6, pp. 605–610, Jun. 2013. [CrossRef]
- W. Maiaugree, S. Pimanpang, M. Towannang, S. Saekow, W. Jarernboon, and V. Amornkitbamrung, “Optimization of TiO2 nanoparticle mixed PEDOT–PSS counter electrodes for high efficiency dye sensitized solar cell,” J. Non-Cryst. Solids, vol. 358, no. 17, pp. 2489–2495, Sep. 2012. [CrossRef]
- W. Maiaugree et al., “Composited NiSO4 and PEDOT:PSS counter electrode for efficient dye-sensitized solar cell based on organic T2/T− electrolyte,” Mater. Lett., vol. 111, pp. 197–200, Nov. 2013. [CrossRef]
- W. Maiaugree, P. Pimparue, W. Jarernboon, S. Pimanpang, V. Amornkitbamrung, and E. Swatsitang, “NiS(NPs)-PEDOT-PSS composite counter electrode for a high efficiency dye sensitized solar cell,” Mater. Sci. Eng. B, vol. 220, pp. 66–72, Jun. 2017. [CrossRef]
- L. Wan et al., “Well-dispersed PEDOT:PSS/graphene nanocomposites synthesized by in situ polymerization as counter electrodes for dye-sensitized solar cells,” J. Mater. Sci., vol. 50, no. 5, pp. 2148–2157, Mar. 2015. [CrossRef]
- C. K. Reddy, M. Gurulakshmi, K. Susmitha, M. Raghavender, N. Thota, and Y. P. V. Subbaiah, “A novel PEDOT:PSS/SWCNH bilayer thin film counter electrode for efficient dye-sensitized solar cells,” J. Mater. Sci. Mater. Electron., vol. 31, no. 6, pp. 4752–4760, Mar. 2020. [CrossRef]
- G. Yue et al., “PEDOT:PSS and glucose assisted preparation of molybdenum disulfide/single-wall carbon nanotubes counter electrode and served in dye-sensitized solar cells,” Electrochimica Acta, vol. 142, pp. 68–75, Oct. 2014. [CrossRef]
- Ng and D. H. Camacho, “Polymer electrolyte system based on carrageenan-poly(3,4- ethylenedioxythiophene) (PEDOT) composite for dye sensitized solar cell,” IOP Conf. Ser. Mater. Sci. Eng., vol. 79, p. 012020, Jun. 2015. [CrossRef]
- J. Palamba, N. Sari, A. W. M. Diah, and S. Saehana, “A preliminary study of DSSC with PEDOT carrageenan as electrolyte system,” J. Phys. Conf. Ser., vol. 1763, no. 1, p. 012090, Jan. 2021. [CrossRef]
- N. Sari, A. J. Palamba, S. Saehana, and A. W. M. Diah, “DSSC with PEDOT-Carrageenan Electrolyte as Learning Media for Photovoltaic Concept Physics,” J. Phys. Conf. Ser., vol. 2126, no. 1, p. 012004, Nov. 2021. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).