1. Introduction
The digestion of foods in the gastrointestinal tract of humans and animals is determined by the activity of enzymes that break down macronutrients into smaller molecules to be absorbed in the gut and used by the body [
1]. Some of the most important digestive enzymes include α-amylase and α-glucosidase, which degrade carbohydrates to obtain energy, and lipases, which catalyze the cleavage of triglycerides to produce free fatty acids and monoacylglycerol that either meet metabolic needs or are re-esterified and stored as triglycerides in adipose tissue [
2]. Diets rich in carbohydrates can lead to hyperglycemia, which is associated with high insulin levels in the blood and increased uptake of nutrients, leading to the accumulation of adipose tissue and obesity [
3]. On the other hand, high-fat diets are associated with abnormally high levels of circulating fatty acids and subsequent ectopic deposition in non-adipose tissues, as well as lipid accumulation in the liver, heart, endothelium, nervous system, pancreas, and skeletal muscle, thereby causing an imbalance in homeostatic mechanisms regulating metabolism [
2,
4]. This imbalance may lead to health complications such as metabolic disorders (e.g., dyslipidemia, hypertension, type 2 diabetes), cancers, respiratory diseases, digestive problems, and osteoarthritis [
5].
Regulation of nutrient absorption (e.g., carbohydrates) through the inhibition of digestive enzymes is an effective manner to control metabolism. For example, acarbose can inhibit the activity of α-amylase and α-glucosidase enzymes by reducing glucose absorption and decreasing insulin secretion in postprandial glycemia, establishing a glycemic control mechanism associated with reduced glycosylated hemoglobin. This class of enzymatic inhibitors is indicated in patients with adequate fasting blood glucose and elevated postprandial blood glucose levels. In patients with impaired glucose tolerance, enzymatic inhibitors have been associated with a marked reduction in cardiovascular events and no risk of adverse side effects, such as weight gain or hypoglycemia [
6]. Therefore, the development of α-amylase and α-glucosidase inhibitors is increasingly recognized as a therapeutic strategy for patients with carbohydrate metabolic disorders, including postprandial hyperglycemia and type 2 diabetes mellitus [
7,
8].
Obesity is a complex disease that involves an abnormal or excessive accumulation of fat in the body and constitutes a public health problem worldwide [
9]. The control and treatment of this pathology are mainly aimed at avoiding health complications, as well as increasing life expectancy [
9]. Among the available drugs, lipase inhibitors (e.g., orlistat) act by reducing the absorption of monoacylglycerol and thus lead to weight loss [
7,
8,
10,
11]. However, new synthetic anti-obesity agents, which may bring better benefits to patients, have been investigated [
12].
In this context, the preparation of novel amino acid derivatives obtained from organic synthesis processes is a promising area, which has been subjected to numerous biological studies. In addition to the functionalization of carboxylic and amine groups attached to the stereogenic center, the coupling of carbon side chains may also result in functional amino acid derivative drugs synthesized by conventional chemical reactions (i.e., acylation, alkylation, and amidation) [
13]. These derivatives have attracted recent scientific interest due to their multiple biological properties [
14,
15]. For example, cationic antimicrobial peptides hold promise as new alternative antibiotics with the potential to inhibit multi-drug resistant bacteria [
16].
In the present study, the inhibitory effects of synthetic amino acid derivatives on digestive enzymes were assessed using in vitro assays. This exploratory study may have predictive value for developing new therapeutic agents against metabolic disorders such as type 2 diabetes mellitus and obesity.
2. Materials and Methods
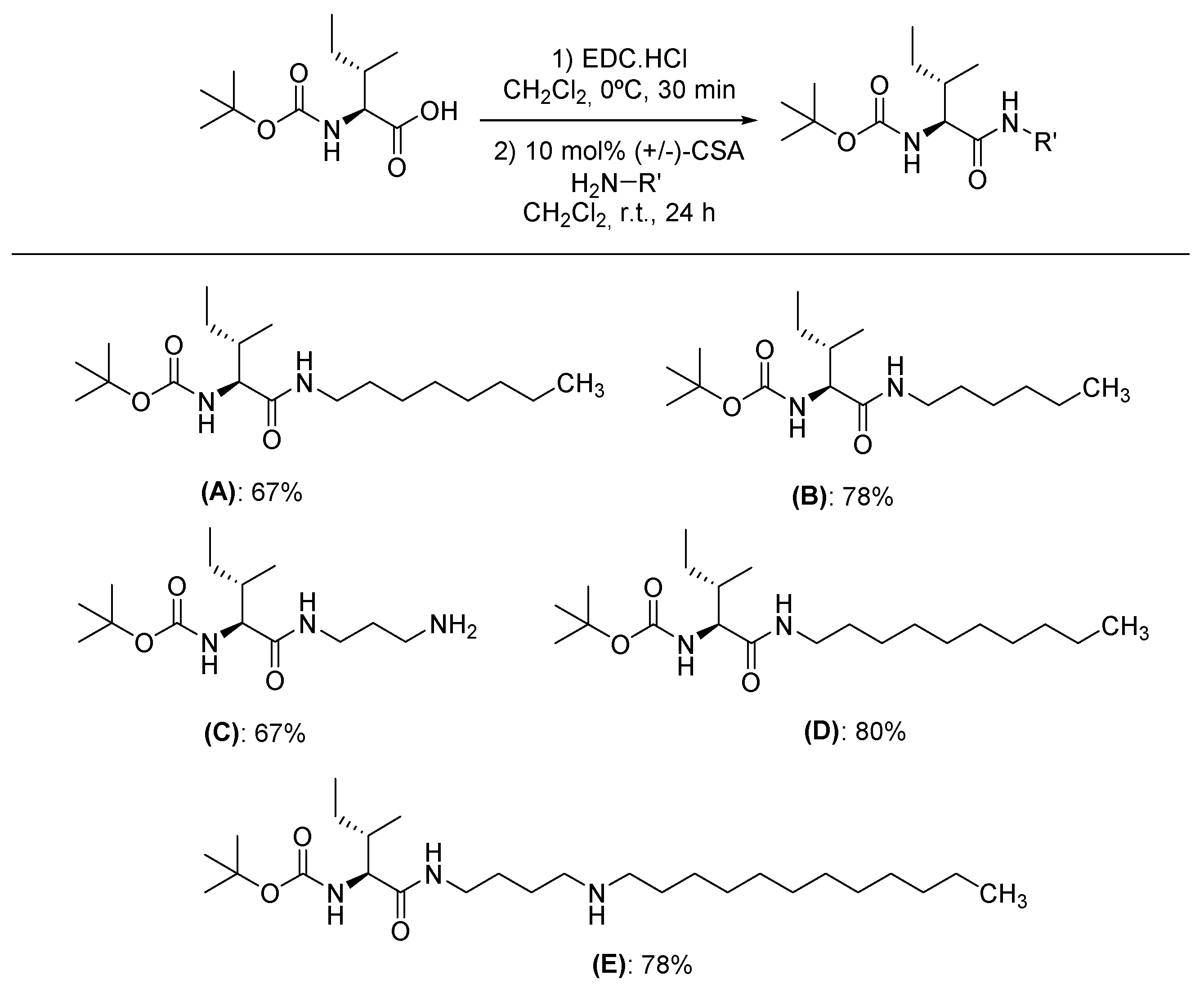
2.1. Synthesis of Amino Acid Derivatives
The evaluated as amino acid derivatives, compounds PPC80 (342.52 g/mol), PPC82 (314.47 g/mol), PPC84 (287.40 g/mol), PPC89 (370.58 g/mol), and PPC101 (469.76 g/mol), were synthesized according to our previous report in the literature [
17].
2.2. Chemicals
The drugs and reagents used in this study were as follows: porcine pancreatic lipase, 50 mmol/L tris-HCl buffer (pH 8.0), p-nitrophenol palmitate, Triton-X 100, orlistat, porcine pancreatic α-amylase, 50 mmol/L Tris-HCl (pH 7.0), α-Glucosidase, 100 mmol/L citrate-phosphate buffer (pH 7.0), acarbose, and p-nitrophenyl-α-D-glycopyranoside (Sigma-Aldrich® Co., St. Louis, MO, USA), while dimethylsulfoxide and starch (Loja Synth®, Diadema, SP, Brazil). Unless noted, all chemicals utilized in the synthetic protocol were acquired from Sigma-Aldrich® Co., St. Louis, MO, USA and used as received.
2.3. Inhibitory Activity on Digestive Enzymes
2.3.1. Pancreatic Lipase Inhibition Assay
The pancreatic lipase inhibition assay was performed according to Santos et al. [
18] with some modifications. The porcine pancreatic lipase (10 g/L) was incubated in 50 mmol/L Tris-HCl buffer (pH 8.0) containing 10 mmol/L CaCl
2 and 25 mmol/L NaCl. The
p-nitrophenol palmitate substrate (8 mmol/L) was dissolved in 0.5%, w/v Triton-X 100. PPC80, while PPC82, PPC84, PPC89, and PPC101 amino acid derivatives and orlistat were solubilized in dimethylsulfoxide (DMSO) prepared at increasing concentrations ranging from 0.5 to 13.92 × 10
−1 mmol/L. A total of 100 µL of enzyme solution, 50 µL of
p-nitrophenol palmitate substrate, and 50 µL of amino acid derivative sample or orlistat were added to the microplate wells. Next, microplates were incubated at four different time intervals (10, 20, 30, and 40 min) in a water bath at 37 °C, and the reaction was stopped in an ice bath. All reactions were carried out in triplicate. The absorbance of the products was measured at 405 nm using a microplate reader (Thermoplate
®, TP-Reader, Wuxi City, Jiangsu, China).
2.3.2. Pancreatic α-Amylase Inhibition Assay
The pancreatic α-amylase inhibition assay was carried out according to Freitas et al. [
19] with some modifications. The porcine pancreatic α-amylase (1 mg/mL) was incubated in 50 mmol/L Tris-HCl buffer (pH 7.0) containing 10 mmol/L CaCl
2 and 1% starch. PPC80, PPC82, PPC84, PPC89, and PPC101 amino acid derivatives and acarbose were solubilized in DMSO prepared at increasing concentrations ranging from 0.15 to 15.90 × 10
−1 mmol/L. A total of 50 µL enzyme solution, 50 µL substrate, and 50 µL amino acid derivative sample or acarbose were added to the microplate wells. Afterward, microplates were pre-incubated for 10 min in a water bath at 37°C. A total of 100 µL substrate was added at each well, and microplates were incubated at four different time intervals (10, 20, 30, and 40 min) in a water bath at 37°C. The reaction was stopped using an ice bath. All reactions were carried out in triplicate. The absorbance of the products was measured at 405 nm using a microplate reader (Thermoplate
®, TP-Reader, Wuxi City, Jiangsu, China).
2.3.3.α-. Glucosidase Inhibition Assay
The inhibitory effect against α-glucosidase was carried out according to Chelladurai and Chinnachamy [
20] with some modifications. A total of 2 U/mL α-Glucosidase and 5 mmol/L ρ-nitrophenyl-α-D-glucopyranoside substrate were solubilized in 100 mmol/L citrate-phosphate buffer (pH 7.0). PPC80, PPC82, PPC84, PPC89, and PPC101 amino acid derivatives and acarbose were solubilized in DMSO prepared at increasing concentrations ranging from 0.24 to 17.40 × 10
−1 mmol/L. A total of 100 µL α-glucosidase solution, 50 µL amino acid derivative sample or acarbose, and 50 µL substrate were added to the microplate wells. Afterward, microplates were incubated at different intervals (10, 20, 30, and 40 min) in a water bath at 37°C. The reaction was stopped in an ice bath. All enzyme reactions were carried out in triplicate. The absorbance of the products was measured at 405 nm using a microplate reader (Thermoplate
®, TP-Reader, Wuxi City, Jiangsu, China).
2.3.4. Determination of the inhibitory effect and IC50
The percentage of inhibition (
I%) was determined using ‘absorbance versus time’ graphs. By means of linear regression, using the method of least-squares, the equations of the straight lines and the angular coefficients were obtained to determine the inhibition (I%) of the enzymatic activities by the equation:
where
A is the angular coefficient of the straight-line equation (enzyme + substrate),
a is the angular coefficient of the equation of the line (substrate),
B is the angular coefficient of the straight-line equation (enzyme + substrate + sample), and
b is the value of the angular coefficient of the straight-line equation (enzyme + sample).
The 50% inhibitory concentrations (IC50) were determined through ‘response versus concentration’ plots using the linear least-squares regression model.
2.3.5. Determination of Kinetic Parameters
Kinetic parameters were determined using the same experimental conditions described above for each enzyme [
21]. The reactions were prepared using increased substrate concentrations (16 to 0.06 mmol/L), both in the absence and presence of PPC80, PPC82, PPC84, PPC89, and PPC101 derivatives or positive control (orlistat or acarbose). The enzyme concentrations were maintained as described above. The absorbance of the products was measured at 405 nm using a microplate reader (Thermoplate
®, TP-Reader, Wuxi City, Jiangsu, China) as a function of time (60 sec). The absorbance values were converted into product concentration (µmol/L) using standard curves of glucose (α-amylase) and
p-nitrophenol (pancreatic lipase and α-glucosidase). The value of the initial velocity (
v0) of enzymatic reactions was estimated to create the ‘
v0 versus substrate concentration’ graph. Kinetic constants (
Km or
Ki and
Vmax) were calculated, and the inhibition model was verified using Lineweaver–Burk plots [
21].
2.4. Statistical Analysis
The data were subject to the analysis of variance (ANOVA) and Tukey’s test (p < 0.05) to determine the differences between mean groups using the GraphPad Prism 5 program. Data were presented as mean ± S.E.M.
3. Results
3.1. Synthesis of Protected Amino Acid Derivatives
The synthesis started by reacting Boc-protected L-isoleucine amino acid with EDC.HCl as carboxylic acid coupling. After 30 min, the vessel was charged with the corresponding nucleophile in the presence of racemic camphorsulphonic acid (+/-)-CSA as organocatalyst [
17]. The corresponding synthetic amino acid derivatives PPC80, PPC82, PPC84, PPC89, and PPC101 were attaining in yields ranging from 67 to 80% (
Figure 1). It is worth to mention, no epimerization process was observed. Characterization data are in agreement with those previously described in the literature [
17]. The products were then used to carry out inhibitory activity assays against digestive enzymes.
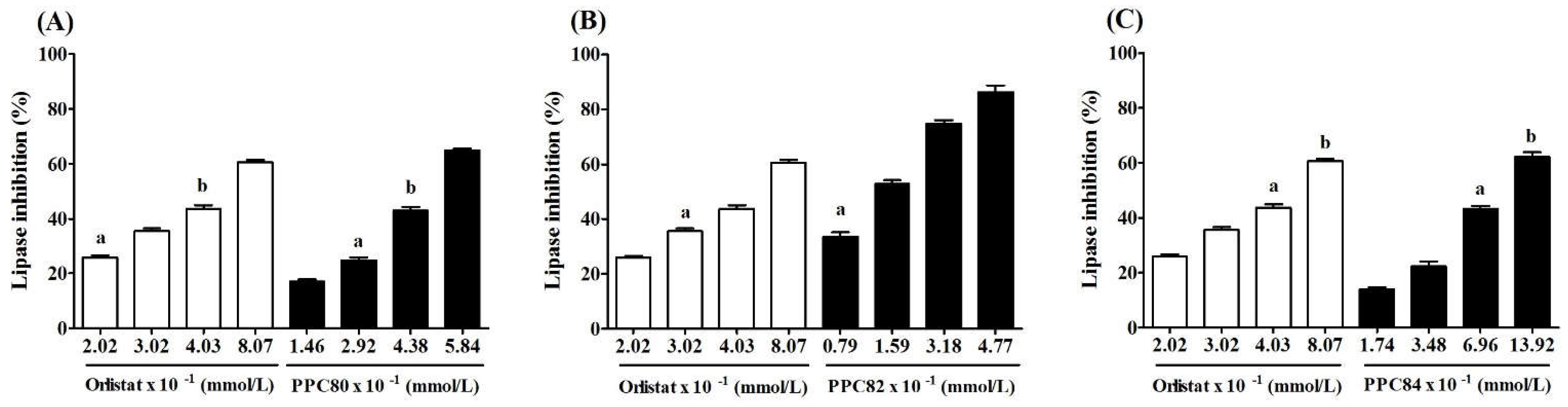
3.2. Inhibitory Effect of Amino Acid Derivatives on Pancreatic Lipase
The results showed that the inhibitory effect of PPC82, PPC80, and PPC84 amino acid derivatives on pancreatic lipase activity was concentration-dependent (
Figure 2). PPC80 (5.84 x 10
-1mmol/L) was more active (p < 0.05) than orlistat (8.07 x 10
-1 mmol/L, 60% inhibition) at a lower concentration, showing an inhibitory effect of about 65% on pancreatic lipase activity (
Figure 2A). Also, PPC82 (4.77 x 10
-1 mmol/L) was more effective in inhibiting pancreatic lipase at a lower concentration than orlistat (8.07 x 10
-1 mmol/L), with a response of about 86% (
Figure 2B). Moreover, PPC84 (13.92 x 10
-1 mmol/L) exerted a similar inhibitory effect on pancreatic lipase at a higher concentration than orlistat (8.07 x 10
-1 mmol/L), showing an inhibitory effect of about 62% (
Figure 2C). In this assay, PPC89 and PPC101 did not show inhibitory action on the reference enzyme.
As shown in
Table 1, the IC
50 values were also determined. PPC80 and PPC82 showed better IC
50 values than orlistat at the lower concentrations of 4.75 ± 0.08, 1.67 ± 0.06, and 5.88 ± 0.15 x 10
-1 mmol/L, respectively (p < 0.05). On the contrary, PPC84 (10.23 ± 0.20 x 10
-1 mmol/L) had a higher IC
50 value at a lower concentration than orlistat (p < 0.05), thereby showing a lower inhibitory effect on pancreatic lipase activity. As noted in
Figure 2C, PPC84 concentration was almost 2-fold higher than the reference compound, which may be associated with its high concentration (13.92 x 10
-1 mmol/L) observed in the inhibitory effect assay (
Figure 2C) and shows a low inhibitory affinity with the target enzyme.
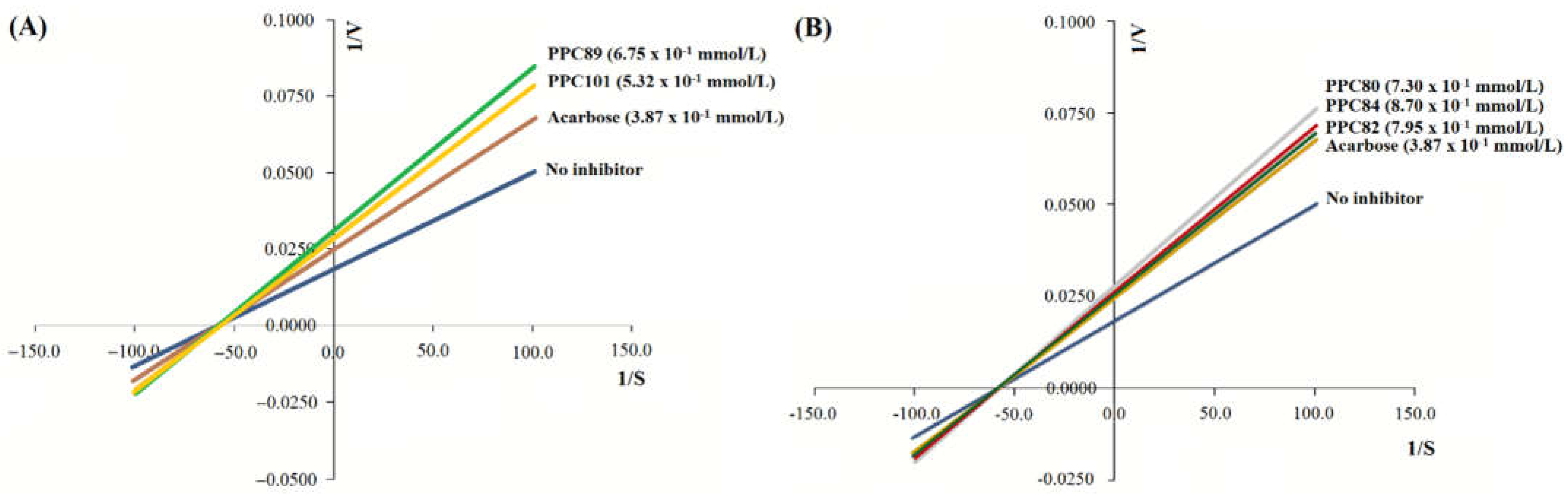
3.3. Kinetic Parameters on Pancreatic Lipase Activity
The inhibitory mechanism of PPC80, PPC82, and PPC84 compounds on the pancreatic lipase activity was assessed. The Lineweaver–Burk profiles showed that PPC80 and PPC82 follow a non-competitive inhibition mechanism (
Figure 3A-C). As observed in the Lineweaver–Burk plots, the straight lines did intersect the x-axis but did not the y-axis, thereby indicating a decrease in the maximum velocity (
Vmax) of the reaction [
21]. On the other hand, PPC84 followed a competitive inhibition mechanism since an intersection on the y-axis was observed (
Figure 3). These profiles were used to determine the values of kinetic parameters (
Table 2).
The kinetic parameters of the enzyme-substrate reaction in the absence of enzyme inhibitors were
Km = 0.204 ± 0.003 mmol/L and
Vmax = 55.78 ± 2.05 µmol/min per L (
Table 2). The addition of 1.01 mmol/L orlistat to the reaction caused a significant decrease in
Vmax (43.75 ± 0.76 µmol/min per L; p < 0.05) and an increase in
Ki (0.209 ± 0.001 mmol/L; p < 0.05), indicating that the reaction rate became slower in the presence of this compound. Moreover, the addition of 1.46 mmol/L PPC80 to the reaction reduced the reaction rate, causing an increase in
Ki (0.211 ± 0.004 mmol/L) and a decrease in
Vmax (41.36 ± 0.05 µmol/min per L), while 1.59 mmol/L PPC82 was more active (
Ki = 0.221 ± 0.001 mmol/L;
Vmax = 35.05 ± 0.13 µmol/min per L). Taken together, these results and those shown in Supplementary materials 1A and 1B suggest that PPC80 and PPC82 exert a non-competitive inhibitory effect on pancreatic lipase. Moreover, 1.74 mmol/L PPC84 reduced
Vmax (41.36 ± 0.52 µmol/min per L) and
Ki (0.181± 0.003 mmol/L), showing that this compound exerted competitive inhibition as observed in the Lineweaver–Burk plot (
Figure 3C).
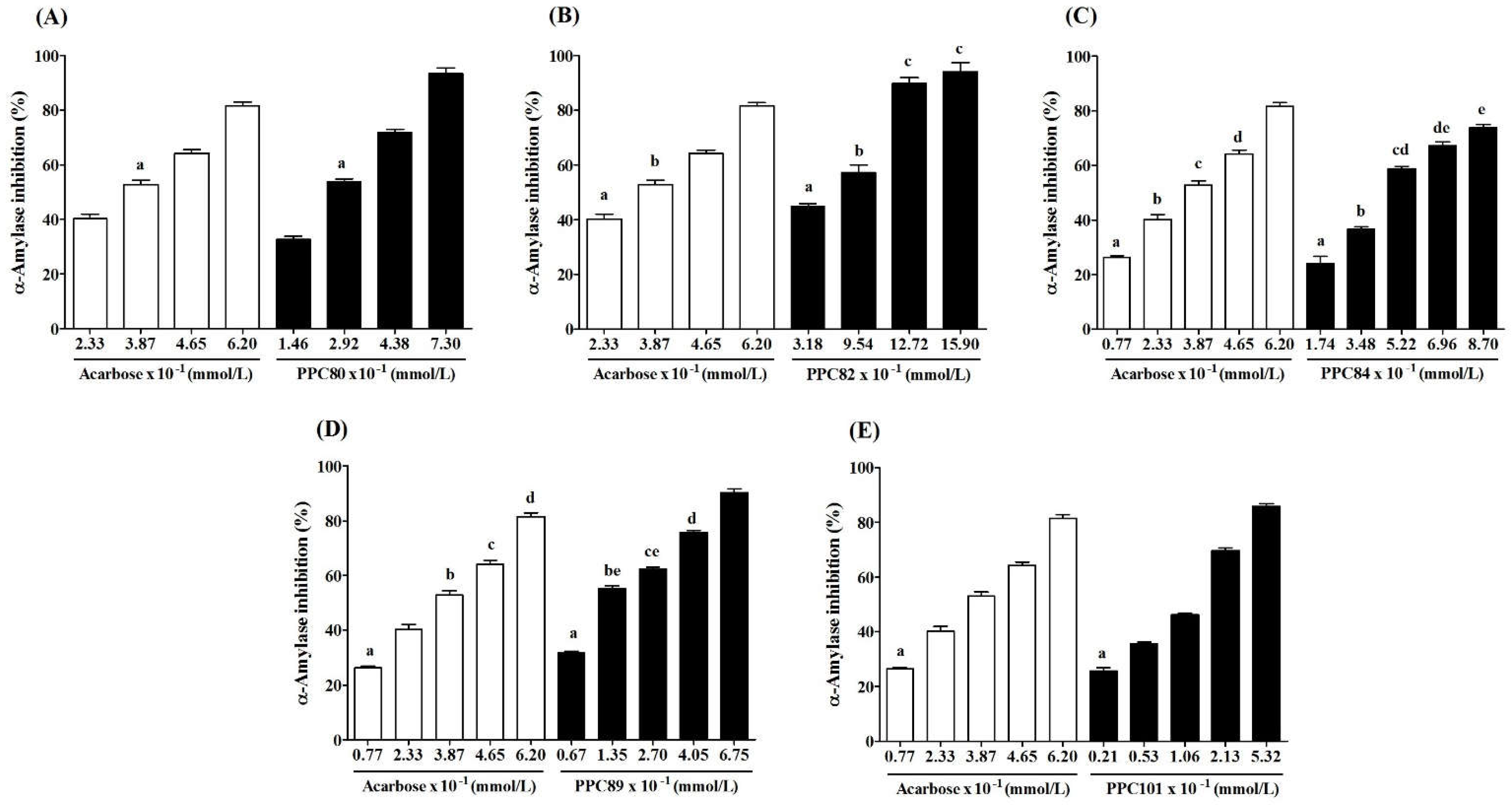
3.4. Inhibitory Effect of Amino Acid Derivatives on Pancreatic α-Amylase
The results showed that the inhibitory effect of PPC80, PPC82, PPC84, PPC89, and PPC101 amino acid derivatives on pancreatic α-amylase activity was concentration-dependent (
Figure 4). PPC80 (7.30 x 10
-1 mmol/L), PPC82 (15.90 x 10
-1 mmol/L), PPC84 (8.70 x 10
-1 mmol/L), PPC89 (6.75 x 10
-1 mmol/L), and PPC101 (5.32 x 10
-1 mmol/L) inhibited pancreatic α-amylase by nearly 93%, 94%, 74%, 90%, and 86% (p < 0.05), respectively, while acarbose (6.20 x 10
-1 mmol/L) reduced the specific enzymatic activity by about 86% (
Figure 4A–4E). PPC101 was more active than acarbose at a lower concentration, while PPC80 and PPC82 showed better inhibitory effects than acarbose at higher concentrations. Moreover, PPC89 (4.05 x 10
-1 mmol/L) produced the same inhibitory effect as acarbose (6.20 x 10
-1 mmol/L).
The IC
50 values showed the inhibitory potential of PPC80, PPC82, PPC84, PPC89 and PPC1010 derivatives (
Table 1). PPC89 (1.71 ± 0.14 x 10
-1 mmol/L) and PPC101 (1.62 ± 0.02 x 10
-1 mmol/L) had lower IC
50 values than acarbose (3.26 ± 0.03 x 10
-1 mmol/L), thereby showing a more potent inhibitory activity against pancreatic amylase, while PPC82 and PPC84 were less effective in inhibiting the activity of the enzyme (p < 0.05). Moreover, PPC80 showed a similar suppressive potential as the reference compound (acarbose). The inhibitory effects were corroborated in
Figure 4, where the inhibition of the pancreatic α-amylase enzyme occurred at higher concentrations of PPC82 and PPC84.
3.5. Kinetic Parameters against Pancreatic α-Amylase
The kinetic parameters of pancreatic α-amylase activity for PPC89, PPC101, PPC80, PPC84, and PPPC82 amino acid derivatives were determined. The Lineweaver–Burk plots showed that the inhibition mechanism of PPC80, PPC82, PPC84, PPC89, and PPC101 is non-competitive (
Figure 5A-B), as a single point of intersection on the x-axis was verified [
21]. These profiles were then used to determine the values of kinetic parameters (
Table 3).
In the absence of enzyme inhibitors, the reaction had a lower
Km (0.153 ± 0.006 mmol/L) and a higher
Vmax (102.06 ± 0.77 µmol/min per L) than in the presence of acarbose or amino acid derivatives (p < 0.05), showing that these compounds can inhibit pancreatic amylase and reduce the reaction rate (
Table 3). PPC80, PPC82, PPC84, PPC89, and PPC101 derivatives decreased
Vmax in a range from 57.35 ± 1.09 µmol/min per L to 71.78 ± 0.78 µmol/min per L, and inhibition constant (
Ki) values ranged from 0.219 ± 0.007 mmol/L to 0.312 ± 0.005 mmol/L. The kinetic data and Lineweaver–Burk plots (
Figure 5A-B) suggested that amino acid derivatives inhibit enzymatic function through a non-competitive inhibitory mechanism. In line with these results, a previous study also reported non-competitive inhibition for synthetic peptides that showed
Vmax values ranging from 0.313 and 0.558 [
22].
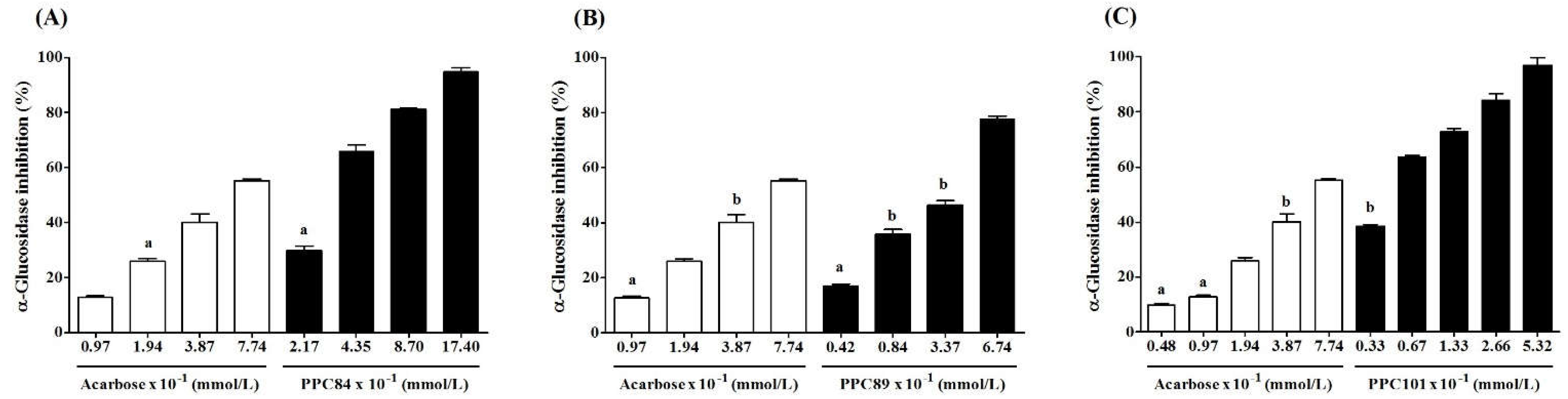
3.6. Inhibitory Effect of Amino Acid Derivatives on α-Glucosidase
As shown in
Figure 6, the inhibitory effect of PPC84, PPC89, and PPC101 amino acid derivatives on α-glucosidase activity was concentration-dependent. PPC84 (4.35 x 10
-1 mmol/L), PPC89 (6.74 x 10
-1 mmol/L), and PPC101 (0.67 x 10
-1 mmol/L) inhibited α-glucosidase by nearly 66, 78 and 64% (p < 0.05), respectively, inhibiting enzyme activity at lower concentrations than acarbose (positive control). These derivatives were also more effective at higher concentrations (
Figure 6A-C). Moreover, PPC80 and PPC82 did not show inhibitory action against the tested enzyme.
The IC
50 values showed the inhibitory potential of PPC84, PPC89, and PPC101 amino acid derivatives on α-glucosidase activity (
Table 1). PC89, PPC84, and PPC101 derivatives had lower IC
50 and therefore were more effective for inhibiting α-glucosidase than acarbose (IC
50 = 6.39 ± 0.05 x 10
-1 mmol/L). It is worth noting that PPC101 was 12-fold more potent than acarbose, exhibiting an outstanding potential to inhibit the target enzyme.
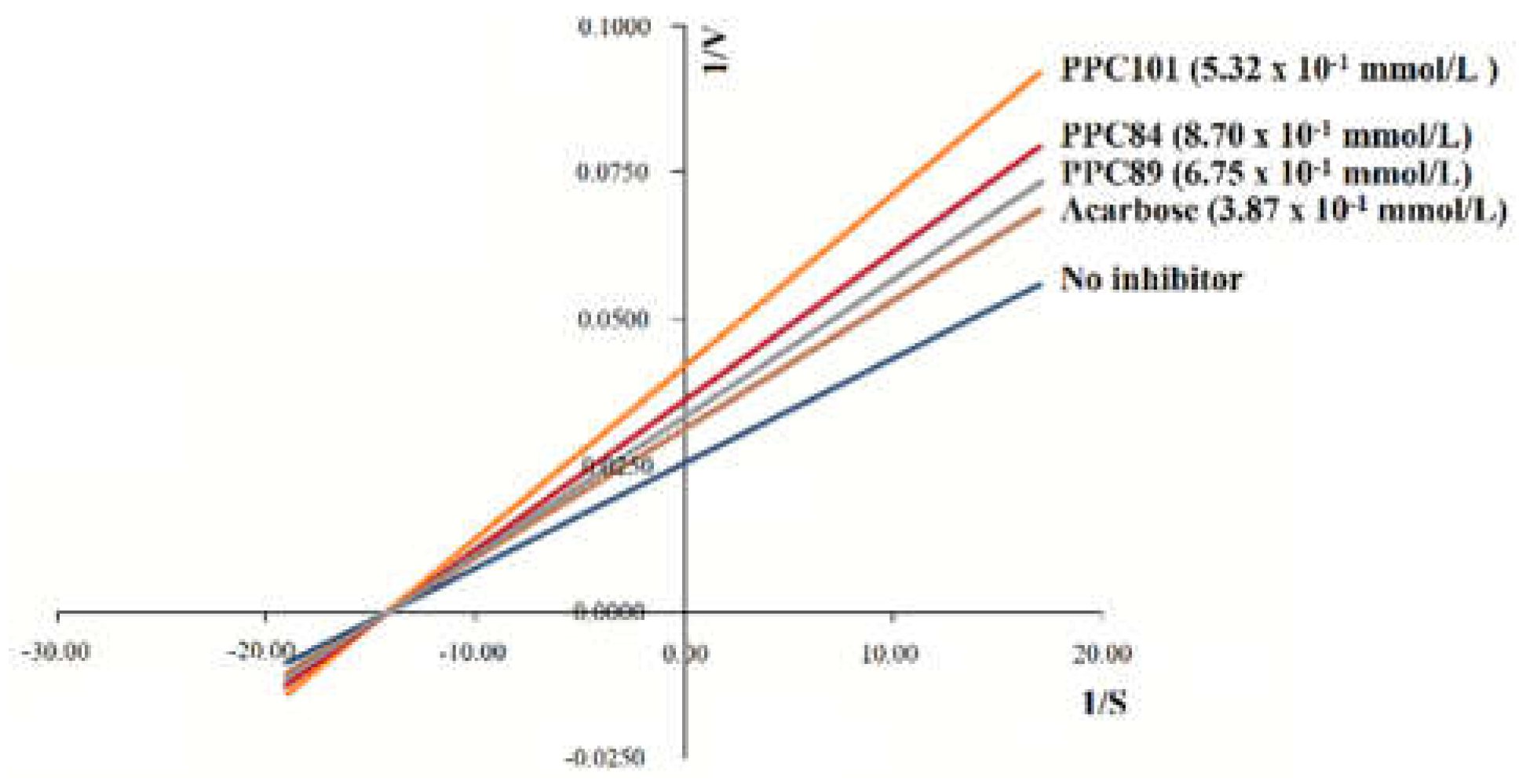
3.7. Kinetic Parameters of α-Glucosidase
The inhibition assay and the Lineweaver–Burk plot (
Figure 7) revealed that the inhibition mechanism of PPC101, PPC89, and PPC84 against α-glucosidase is non-competitive. This mechanism is related to the crossing of inhibition lines at an intersection point on the x-axis [
21]. These data were then used to determine the values of kinetic parameters (
Table 4).
In the absence of enzyme inhibitors, the reaction was faster than acarbose and had a
Km of 0.184 ± 0.006 mmol/L and a
Vmax of 78.26 ± 0.09 µmol/min per L (
Table 4). The presence of acarbose reduced the reaction rate, showing
Ki and
Vmax values of 0.209 ± 0.006 mmol/L and 56.64 ± 0.15 µmol/min per L, respectively. PPC84, PPC89, and PPC101 showed significantly lower
Vmax and higher
Ki values (p < 0.05) than the reaction without the inhibitor. Therefore, PPC84, PPC89, and PPC101 derivatives reduced the reaction rate and confirmed the results observed in the Lineweaver–Burk plots (
Figure 7).
4. Discussion
The structure of amino acid derivatives suggested that the hydrocarbon chain is involved in their inhibitory effects since compounds with a side chain with more than eight carbon atoms did not inhibit pancreatic lipase. The inclusion of a amino group at the carbon side chain of PPC84 (
Figure 1C) may have led to additional hydrogen bonding interactions (non-covalent interactions) at the catalytic site, resulting in an impaired ability to competitive inhibit the enzymatic activity. Besides, the possibility of the amine to act as a nucleophile (covalent bonding) cannot be ruled out [
23].
These findings are consistent with previous studies assessing the effects of amino acid and peptide derivatives on pancreatic lipase activity. Ngoh and Gan [
24] identified different peptides from common bean (
Phaseolus vulgaris) that inhibited pancreatic lipase in the range of 23–87%. Polylysine is a synthetic peptide that also acts as a lipase inhibitor, showing a remarkable inhibition (80%) on the activity of porcine pancreatic lipase at a concentration of 100 mg/mL [
25]. Furthermore, synthetic peptides [
26] and hydrolyzed peptides [
23] inhibited pancreatic lipases with IC
50 values below 50 µM.
The results of the present study showed that PPC80 and PPC82 have inhibitory potential on the activity of pancreatic lipase, which may promote a reduction of intestinal fat absorption and potentially affect body weight [
27]. Therefore, PPC80 and PPC82 derivatives constitute promising therapeutic agents for the treatment of obesity and related lipid disorders.
Unlike competitive inhibitors, non-competitive inhibitors are unaffected by the substrate concentration, and therefore high concentrations of the inhibitor are not required to compete with the substrate [
28]. Non-competitive inhibitors do not bind to the active site of the enzyme, but at another region called the allosteric site. Thus, non-competitive inhibitors can interact with the free enzyme to form the enzyme-inhibitor complex or with potential binding sites of the ES complex to form the enzyme-substrate-inhibitor complex, both of which are catalytically inactive. The formation of these complexes induces structural changes in the enzyme, modifying the conformation of the active site to prevent its interaction with the substrate [
29].
The function of amino acid derivatives may be associated with the size of the hydrocarbon chain since compounds such as PPC89 and PPC101, which exhibit a high number of carbon atoms after nitrogen in their aliphatic chains, were more effective in inhibiting the activity of α-amylase at low IC50 values. PPC80 (eight carbons) showed a similar inhibitory effect as acarbose, thus being the third most effective compound. Moreover, PPC82 (six carbons) and PPC84 (three carbons and one amino group) showed the lowest inhibitory activities.
The catalytic mechanism of the α-amylase family is stable and specific because of the α-retaining double displacement reaction. This two-step mechanism is a distinctive feature of the α-amylase family and may contribute to its broad specificity due to the attachment of different domains to the catalytic site or to extra sugar-binding subsites around the catalytic site [
30]. However, the carboxylic groups of aspartate and glutamate residues can act as acid/base catalysts and nucleophilic reagents during the formation of covalent intermediates in the catalytic cycle. The presence of Cl ions may lead to the activation and facilitates the protonation of a carboxyl group [
31].
An increasing number of studies have shown that synthetic compounds derived from amino acids and peptides exhibit an inhibitory action on α-amylase [
22,
32,
33]. Admassu et al. [
22] identified two α-amylase inhibitor peptides (GGSK and ELS) released by hydrolysis of proteolytic enzymes obtained from the red seaweed (
Porphyra species). The IC
50 values for GGSK and ELS were 2.58 mM ± 0.08 and 2.62 mM ± 0.05, respectively. Another study reported peptides extracted from basil (
Ocimum basilicum) seeds that showed 36% inhibition on α-amylase [
33]. Similarly, González-Montoya et al. [
32] also identified peptides from soy (
Glycine max) protein capable of inhibiting pancreatic α-amylase activity at IC
50 values ranging from 0.16 to 8.30 mg/mL.
Pancreatic α-amylase and α-glucosidase are critical enzymes involved in the digestion of dietary starch, catalyzing the release of oligosaccharides that are further degraded into glucose. Therapeutic approaches for the treatment of type 2 diabetes include the inhibition of these enzymes to decrease the absorption of glucose in the digestive tract and reduce postprandial hyperglycemia [
34,
35]. Acarbose, miglitol, and voglibose are major inhibitors that reduce the rate of glucose absorption, attenuating the postprandial increase in plasma glucose level and thus helping in the treatment of obesity [
36,
37]. Our results indicate that amino acid derivatives are potent inhibitors of pancreatic α-amylase and can be promising agents for the treatment of diabetes and metabolic disorders.
Several studies have reported the promising potential of amino acid and peptide derivatives as α-glucosidase inhibitors [37-39]. For example, KLPGF and NVLQPS peptides obtained from albumin showed inhibitory activity on α-glucosidase at IC
50 values of 59.5 ± 5.7 µM and 100.0 ± 5.7 μM, respectively [
39]. In this study, the inhibitory activity of the KLPGF peptide motif was similar to acarbose (IC
50 = 60.8 μM). Furthermore, three peptides isolated from quinoa (
Chenopodium quinoa) showed similar inhibitory activity against α-glucosidase [
37]. Singh and Kaur [
38] reported serine-threonine-tyrosine-valine-containing peptides isolated from the endophytic fungi
Acacia nilotica that exhibited potent inhibitory effects against α-glucosidase at low IC
50 values (3.75 µg/mL).
The human α-glucosidase is an enzyme found in the epithelium of the small intestine that catalyzes starch breakdown and the consequent release of glucose. Therefore, inhibition of this enzyme constitutes a promising strategy to reduce serum glucose levels in metabolic diseases, including type 2 diabetes [
20]. Our results showed that PPC89, PPC84, and PPC101 amino acid derivatives inhibit α-glucosidase, exhibiting potential as agents for lowering blood glucose levels in carbohydrate-related metabolic diseases.
Our results are in line with the reported inhibition mechanism for F5-SP, a peptide derivative with a
Ki value of 86.63 ± 0.014 ng/mL that could bind to multiple sites of the α-glucosidase enzyme to modify its conformation [
40].
5. Conclusions
In summary the results showed that PPC80, PPC82, PPC84, PPC89, and PPC101 amino acid derivatives are potential inhibitors of lipase, α-amylase, and α-glucosidase enzymes. For instance, PPC80, PPC82, and PPC84 inhibited pancreatic lipase, with IC50 values as low as 1.67 mmol/L. The activity of pancreatic α-amylase was suppressed by PPC80, PPC82, PPC84, PPC89, and PPC101, with IC50 values in a range of 1.62–5.19 mmol/L. In addition, PPC84, PPC89, and PPC101 also presented an inhibitory effect on α-glucosidase, with IC50 values as low as 0.51 mmol/L. PPC80 and PPC82 followed a non-competitive inhibition mechanism against pancreatic lipase, while PPC84 acted through competitive inhibition. Moreover, PPC80 and PPC82 followed a non-competitive inhibition mechanism against pancreatic lipase, while PPC94 acted through competitive inhibition. PPC80, PPC82, PPC84, PPC89, and PPC101 displayed non-competitive inhibition against α-amylase, while PPC84, PPC89, and PPC101 against α-glucosidase. The present study supports that amino acid derivatives are promising therapeutic agents for metabolic disorders, including type II diabetes and obesity.
Author Contributions
Conceptualization, O.V.S. and G.W.A.; methodology, F.C.S.; software, B.C.S.S.; validation, P.P.C., F.C.S. and B.C.S.S.; formal analysis, O.V.S. and G.W.A.; investigation, P.P.C., F.C.S. and B.C.S.S.; resources, O.V.S. and G.W.A.; data curation, P.P.C., O.V.S. and G.W.A.; writing—original draft preparation, P.P.C., O.V.S. and G.W.A.; writing—review and editing, O.V.S. and G.W.A.; visualization, F.C.S., B.C.S.S, P.P.C., O.V.S. and G.W.A.; supervision, O.V.S.; project administration, O.V.S. and G.W.A.; funding acquisition, O.V.S. and G.W.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (Grant n° CDS-APQ-03302-18) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (Finance Code 001).
Acknowledgments
The Authors are grateful to Éder Luis Tostes and Jésus de Paula Sarmento for the technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.K.; Trivedi, H.H.; Kumar, S.; Prakash, A.; Roy, S.; Qamra, A.; Mukherjee, S. A review of digestive enzyme and probiotic supplementation for functional gastrointestinal disorders. Indian Pract. 2020, 7, 35–39. [Google Scholar]
- Hosseini, F.; Jayedi, A.; Khan, T.A.; Shab-Bidar, S. Dietary carbohydrate and the risk of type 2 diabetes: an updated systematic review and dose–response meta-analysis of prospective cohort studies. Sci. Rep. 2022, 12, 1–9. [Google Scholar] [CrossRef]
- Cohen, B-C. ; Shamay, A.; Argov-Argaman, N. Lipid metabolism in mammary epithelial cells-A comparison of common in vitro models. Adv. Diary Res. 2018, 6, 1–9. [Google Scholar] [CrossRef]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 12, 1–8. [Google Scholar] [CrossRef]
- Dinicolantonio, J.J.; Bhutani, J.; O’keefe, J.H. Acarbose: Safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart 2015, 2, 1–13. [Google Scholar] [CrossRef]
- Santoso, M.; Ong, L.L.; Aijijiyah, N.P.; Wati, F.A.; Azminah, A.; Annuur, R.M.; Fadlan, A.; Judeh, Z.M.A. Synthesis, α-glucosidase inhibition, α-amylase inhibition, and molecular docking studies of 3,3-di(indolyl)indolin-2-ones. Heliyon 2021, 8, 1–8. [Google Scholar] [CrossRef]
- Singh, A.; Singh, K.; Sharma, A.; Kaur, K.; Kaur, K.; Chadha, R.; Bedi, P.M.S. Recent developments in synthetic α-glucosidase inhibitors: A comprehensive review with structural and molecular insight. J. Mol. Struct. 2023, 1281, 1–33. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Liu, T-T. ; Liu, X-T.; Chen, Q-X.; Shi, Y. Lipase inhibitors for obesity: A review. Biomed. Pharmacother. 2020, 128, 1–9. [Google Scholar] [CrossRef]
- Kushner, R.F. Weight loss strategies for treatment of obesity. Prog. Cardiovasc. Dis. 2014, 56, 465–472. [Google Scholar] [CrossRef]
- Prieto-Rodríguez, J.A.; Lévuok-Mena, K.P.; Cardozo-Muñoz, J.C.; Parra-Amin, J.E.; Lopez-Vallejo, F.; Cuca-Suárez, L.E.; Patiño-Ladino, O.J. In vitro and in silico study of the α-glucosidase and lipase inhibitory activities of chemical constituents from Piper cumanense (Piperaceae) and synthetic analogs. Plants 2022, 11, 1–15. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T. Unusual amino acids in medicinal chemistry. J. Med. Chem. 2016, 59, 10807–10836. [Google Scholar] [CrossRef]
- De Castro, P.P.; Campos, D.L.; Pavan, F.R.; Amarante, G.W. Dual-protected amino acid derivatives as new antitubercular agents. Chem. Biol. Drug. Des. 2018, 92, 1576–1580. [Google Scholar] [CrossRef]
- De Castro, P.P.; Siqueira, R.; Conforte, L.; Franco, C.; Bressan, G.; Amarante, G.W. Cytotoxic Activity of Synthetic Chiral Amino Acid Derivatives. J. Braz. Chem. Soc., 2020, 31, 193–200. [Google Scholar] [CrossRef]
- Oliva, R.; Chino, M.; Pane, K.; Pistorio, V.; Santis, A.; Pizzo, E.; D’errico, G.; Pavone, V.; Lombardi, A.; Vecchio, P.; Notomista, E.; Nastri, F.; Petraccone, L. Exploring the role of unnatural amino acids in antimicrobial peptides. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Castro, P.P.; Rimulo, I.M.R.; Almeida, A.M.; Diniz, R.; Amarante, G.W. Brønsted acid-catalyzed epimerization-free preparation of dual-protected amino acid derivatives. ACS Omega 2017, 2, 2967–2976. [Google Scholar] [CrossRef]
- Santos, B.C.S.; Pires, A.S.; Yamamoto, C.H.; Couri, M.R.C.; Taranto, A.G.; Alves, M.S.; Araújo, A.L.S.M.; Sousa, O.V. Methyl chavicol and its synthetic analogue as possible antioxidant and antilipase agents based on the in vitro and in silico assays. Oxid. Med. Cell. Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Freitas, T.C.; Oliveira, R.J.; Mendonça, R.J.; Candido, P.A.; Pereira, L.S.S.; Devienne, K.F.; Silva, A.C.; Pereira, C.A. Identification of bioactive compounds and analysis of inhibitory potential of the digestive enzymes from Syzygium sp. extracts. J. Chem. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Chelladurai, G.R.M; Chinnachamy, C. Alpha amylase and alpha glucosidase inhibitory effects of aqueous stem extract of Salacia oblonga and its GC-MS analysis. Braz. J. Pharm. Sci. 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Robin, T.; Reuveni, S.; Urbakh, M. Single-molecule theory of enzymatic inhibition. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, A.A.M.; Yang, R.; Zhao, W. Identification of bioactive peptides with α-amylase inhibitory potential from enzymatic protein hydrolysates of red seaweed (Porphyra spp). J. Agric. Food Chem. 2018, 66, 4872–4882. [Google Scholar] [CrossRef]
- Awosika, T.O.; Aluko, R.E. Inhibition of the in vitro activities of α-amylase, α-glucosidase and pancreatic lipase by yellow field pea (Pisum sativum L.) protein hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 2021–2034. [Google Scholar] [CrossRef]
- Ngoh, Y.; Gan, C. Enzyme-assisted extraction and identification of antioxidativeanda-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2015, 190, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Kido, Y.; Hiramoto, S.; Murao, M.; Horio, Y.; Toshiyuki, M.; Kodama, T.; Nokabou, Y. ε-Polylysine inhibits pancreatic lipase activity and suppresses postprandial hypertriacylglyceridemia in rats. J. Nutr. 2003, 133, 1887–1891. [Google Scholar] [CrossRef] [PubMed]
- Lunder, M.; Bratkovic, T.; Kreft, S.; Strukelj, B. Peptide inhibitor of pancreatic lipase selected by phage display using different elution strategies. J. Lip. Res. 2005, 46, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Lunagariya, N.A.; Patel, N.K.; Jagtap, S.C.; Bhutani, K.K. Inhibitors of pancreatic lipase: state of the art and clinical perspectives. EXCLI J. 2014, 13, 897–921. [Google Scholar] [PubMed]
- Ghadyale, V.; Takalikar, S.; Haldavnekar, V.; Arvindekar, A. Effective control of postprandial glucose level through inhibition of intestinal alpha glucosidase by Cymbopogon martinii (Roxb.). Evid.-Based Complement. Alternat. Med. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Johnson, K.A. A century of enzyme kinetic analysis, 1913 to 2013. FEBS Lett. 2013, 587, 2753–2766. [Google Scholar] [CrossRef]
- Rani, K.; Rana, R.; Datt, S. Review on characteristics and application of amylases. Int. J. Microbiol. Bioinform. 2015, 5, 1–5. [Google Scholar]
- Butterworth, P.J.; Warren, F.J.; Ellis, P.R. Humana-amylase and starch digestion: An interesting marriage. Starch/Stärke 2011, 63, 395–405. [Google Scholar] [CrossRef]
- González-Montoya, M.; Hernández-Ledesma, B.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Bioactive peptides from germinated soybean with anti-diabetic potential by inhibition of dipeptidyl peptidase-IV, α-amylase, and α-Glucosidase enzymes. Int. J. Mol. Sci. 2019, 19, 2883–2897. [Google Scholar] [CrossRef] [PubMed]
- Afifah, N.H.; Gan, C-Y. Antioxidative and amylase inhibitor peptides from Basil Seeds. Int. J. Pept. Res. Ther. 2015, 22, 3–10. [Google Scholar] [CrossRef]
- Mohamed, E.A.H.; Siddiqui, M.J.A.; Ang, L.F.; Sadikun, A.; Chan, S.H.; Tan, S.C.; Asmawi, M.Z.; Yam, M.F. Potent α-glucosidase and α-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Complement. Altern. Med. 2012, 12, 1–7. [Google Scholar] [CrossRef]
- Jayaraj, S.; Suresh, S. ; Kadeppagari, R-K. Amylase inhibitors and their biomedical applications. Starch/Stärke. [CrossRef]
- Bays, H.E.; Fitch, A.; Christensen, S.; Burridge, K.; Tondt, J. Anti-obesity medications and investigational agents: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars 2022, 2, 1–33. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Martínez-Villalueng, C.; Hernández-Ledesma, B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, A. Antidiabetic potential of a peptide isolated from an endophytic Aspergillus awamori. J. Appl. Microbiol. 2016, 120, 301–311. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, Y.; Zhao, W.; Liu, J.; Chen, F. Anti-diabetic activity peptides from albumin against α-glucosidase and α-amylase. Food Chem. 2012, 135, 2078–2085. [Google Scholar] [CrossRef]
- Yuwen, F.; Shaoyun, W.; Jinhong, W.; Li, Z.; Zhengwu, W.; Li, G.; Jiajun, H.; Haiming, S.; Jingli, H. The kinetics and mechanism of α-glucosidase inhibition by F5-SP, a novel compound derived from sericin peptides. Food Funct. 2017, 8, 323–332. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).