Submitted:
28 April 2023
Posted:
04 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. In-house production of multi-enzyme preparation
2.3. Partial purification of the multi-enzyme preparation after extraction from solid state culture of Aspergillus niger P-19
2.4. Enzymatic hydrolysis of composite kitchen waste using inhouse produced multi-enzyme preparation from Aspergillus niger P-19
2.5. Fermentation of enzymatic hydrolysate of composite kitchen waste for transformation into biofertilizer formulations
2.6. Separation of Carrier and Liquid Biofertilizer
2.8. Seed germination test for the evaluation of biofertilizer formulations
Number of total seeds
Number of germinated seeds (control)
2.9. Plant growth experiment for evaluation of biofertilizer formulations
Yield of control plant
2.10. Determination of chlorophyll
2.11. Quantitative Analysis of Soil
3. Results and Discussion
3.1. In-house production of multi-enzyme preparation from Aspergillus niger P-19
3.2. Enzymatic hydrolysis of composite kitchen waste using inhouse produced multi-enzyme preparation from Aspergillus niger P-19
3.3. Fermentation of sugars released after enzymatic hydrolysis of composite kitchen waste into biofertilizer formulations
3.4. Separation of Carrier and Liquid Biofertilizer
3.5. Physico-chemical and biological characterization of developed biofertilizer formulations
3.6. Influence of biofertilizer formulations on seed germination
3.7. Influence of biofertilizer formulations plant development assay
3.8. Influence of biofertilizer formulations on chlorophyll content
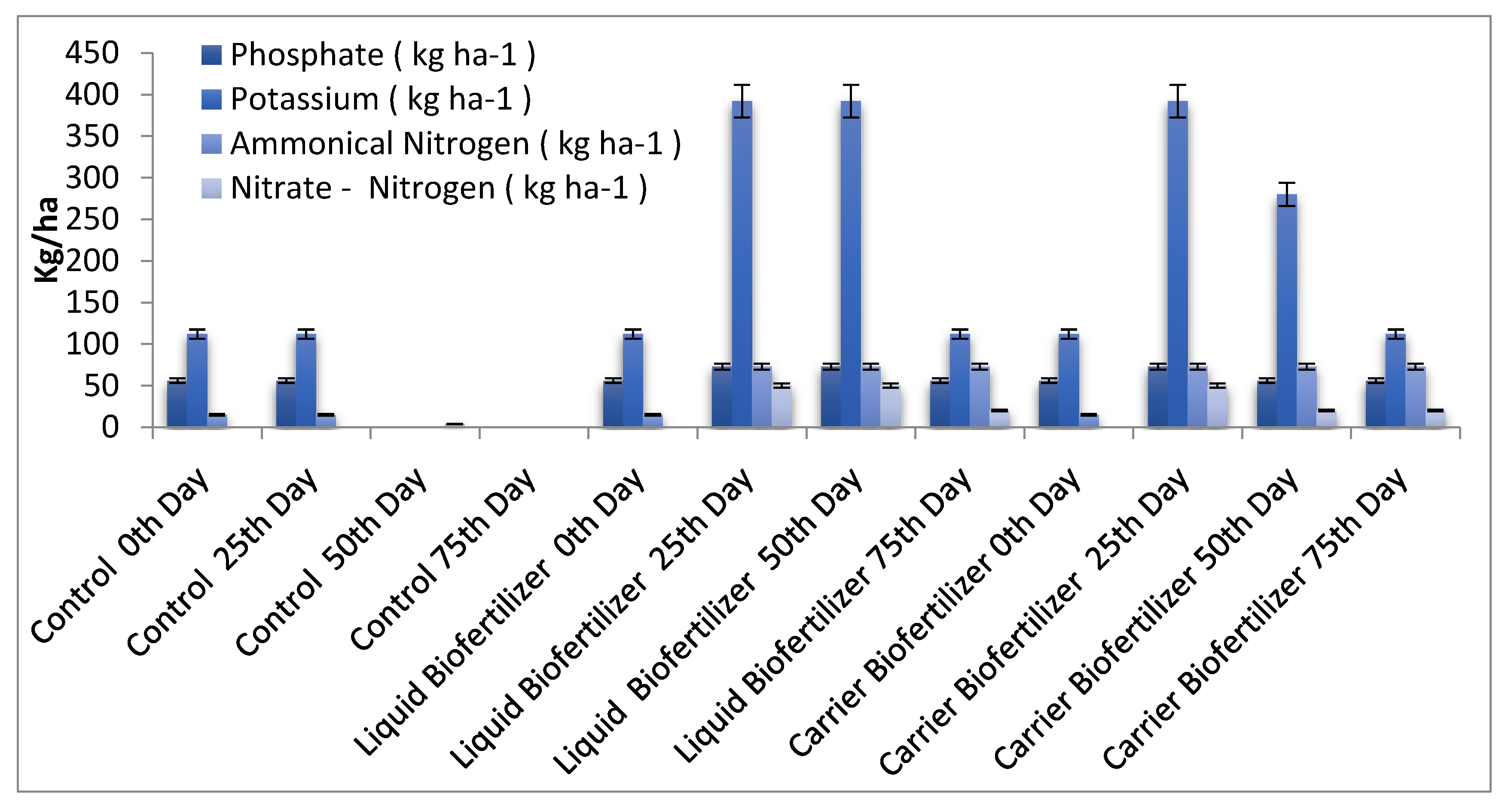
3.9. Quantitative Analysis of Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, F.; Hasan, S.; Rana, M.S.; Sharmin, N. A conceptual framework for zero waste management in Bangladesh. Int. J. Environ. Sci. Technol. 2023, 20, 1887–1904. [Google Scholar] [CrossRef]
- Helena de Sousa, M.; Ferreira da Silva, A.S.; Correia, R.C.; Leite, N.P.; Gonçalves Bueno, C.E.; Santos Pinheiro, R.L.D.; Santana, J.S.D.; Luna da Silva, J.; Sales, A.T.; Claudino de Souza C, Silva Aquino KAD, Souza RBD, Pinheiro IO, Henríquez JR, Pereira Schuler, A. R.; et al.; Valorizing municipal organic waste to produce biodiesel, biogas, organic fertilizer, and value-added chemicals: an integrated biorefinery approach. Biomass Conv. Bioref. 2021, 12, 827–841. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Tabatabaei, M.; Soltanian, S.; Ghanavati, H. Biopower and biofertilizer producton from organic municipal solid waste: An exergo enviornmental analysis. Renew. Energ. 2019, 143, 64–76. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability. 2021, 13, 1140. [Google Scholar] [CrossRef]
- Soni, S.K.; Manhas, R.; Jakhar, Y.; Sharma, A.; Soni, R. Biofertilizers for Sustainable Agriculture. Genomic, Proteomics, and Biotechnology. 2022, 331–356. [Google Scholar]
- Sharma, A.; Saini, H.; Thakur, B.; Soni, R.; Soni, S.K. Consolidated bioprocessing of biodegradable municipal solid waste for transformation into biofertilizer formulations. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- Peng, L.; Chen, Y. Conversion of paper sludge to ethanol by separate hydrolysis and fermentation (SHF) using Saccharomyces cerevisiae. Biomass Bioenergy. 2011, 35, 1600–1606. [Google Scholar] [CrossRef]
- Méndez, J.; de França Passos, D.; Wischral, D.; Modesto, L.F.; Pereira Jr, N. Second-generation ethanol production by separate hydrolysis and fermentation from sugarcane bagasse with cellulose hydrolysis using a customized enzyme cocktail. Biofuels 2019, 12, 1225–1231. [Google Scholar] [CrossRef]
- Annamalai, N.; Al Battashi, H.; Anu, S.N.; Al Azkawi, A.; Al Bahry, S.; Sivakumar, N. Enhanced bioethanol production from waste paper through separate hydrolysis and fermentation. Waste Biomass Valorization. 2020, 11, 121–131. [Google Scholar] [CrossRef]
- Chugh, P.; Kaur, J.; Soni, R.; Sharma, A.; & Soni, S.K.; & Soni, S. K. A low-cost process for efficient hydrolysis of deoiled rice bran and ethanol production using an inhouse produced multi-enzyme preparation from Aspergillus niger P-19. J Mater Cycles Waste Manag. 2023, 25, 359–375. [Google Scholar] [CrossRef]
- Miller, G.L. Use of DNS reagent for determination of reducing sugars. Anal Chem. 1959, 31, 426–28. [Google Scholar] [CrossRef]
- Morin, L.G.; Prox, J. Single glucose oxidase- peroxidase reagent for two-minute determination of serum glucose. Clin. Chem. 1974, 19, 959–962. [Google Scholar] [CrossRef]
- James, G. Native Sherman Rockland Community College, State University of New York; Benjamin/Cummins Publishing Co, 1978; pp. 75–80. [Google Scholar]
- Luo. Y.; Liang, J.; Zeng, G.; Chen. M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manage. 2018, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, Y. ; Meenakshisundaram. S.; Raja, K.; Balaiah, A. Sustainable and efficient-recycling approach of chicken feather waste into liquid protein hydrolysate with biostimulant efficacy on plant, soil fertility and soil microbial consortium: A perspective to promote the circular economy. Process Saf Environ Prot. 2023, 170, 573–583. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant physiol. 1949, 24, 1. [Google Scholar]
- Esteban-Lustres, R.; Torres, M.D.; Piñeiro, B.; Enjamio, C.; Domínguez, H. Intensification and biorefinery approaches for the valorization of kitchen wastes–A review. Bioresour Technol. 2022, 127652. [Google Scholar] [CrossRef]
- Blanco-Vargas, A.; Chacón-Buitrago, M.A.; Quintero-Duque, M.C.; Poutou-Piñales, R.A.; Díaz-Ariza, L.A.; Devia-Castillo, C.A.; Pedroza-Rodríguez, A.M. Production of pine sawdust biochar supporting phosphate-solubilizing bacteria as an alternative bioinoculant in Allium cepa L., culture. Sci Rep. 2022, 12, 12815. [Google Scholar] [CrossRef]
- Soni, S.K.; Sharma. A.; Soni, R. Microbial Enzyme Systems in the Production of Second Generation Bioethanol. Sustainability. 2023, 15, 3590. [Google Scholar] [CrossRef]
- Soni, S.K.; Sharma, A.; Soni, R. (2022b) Method for preparation of stable microbial inoculants.Indian patent 202211050475 https://ipindiaservices.gov.in/PublicSearch/PublicationSearch/ApplicationDetails.
- Roslan, M.A.M.; Sobri, Z.M.; Zuan, A.T.K.; Cheak, S.C.; Rahman, N.A.A. Bioprospecting microwave-alkaline hydrolysate cocktail of defatted soybean meal and jackfruit peel biomass as carrier additive of molasses-alginate-bead biofertilizer. Sci Rep. 2022, 12, 254. [Google Scholar] [CrossRef]
- Xu, S.; Bai, Z.; Jin, B.; Xiao, R.; & Zhuang, G.; & Zhuang, G. Bioconversion of wastewater from sweet potato starch production to Paenibacillus polymyxa biofertilizer for tea plants. Sci Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Khurana A, Kumar V 2020. State of Organic and Natural Farming: Challenges and Possibilities. New Delhi: Centre for Science and Environment. https://www. cseindia. org/state-of-organic-and-natural-farming-in-india-10346.
- Allouzi, M.M.A.; Allouzi, S.M.A.; Keng, Z.X.; Supramaniam, C.V.; Singh, A.; & Chong, S.; & Chong, S. Liquid biofertilizers as a sustainable solution for agriculture. Heliyon. 2022, 2609. [Google Scholar] [CrossRef] [PubMed]
- Raimi, A.; Roopnarain, A.; Adeleke, R. Biofertilizer production in Africa: current status, factors impeding adoption and strategies for success. Sci Afr. 2021, 11, e00694. [Google Scholar] [CrossRef]
- El-Ghamry, A.; Mosa, A.A.; Alshaal, T.; El-Ramady, H. Nanofertilizers vs. biofertilizers: new insights. Env. Biodivers. Soil Secur. 2018, 2, 51–72. [Google Scholar]
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. Adv. Agron. 2020, 162, 31–87. [Google Scholar]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharyya, P.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: a potential approach for sustainable agriculture development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, G.; Shah, R.; Joshi, B.; Patel, P. Klebsiella pneumoniae VRE36 as a PGPR isolated from Saccharum officinarum cultivar Co99004. J. Appl. Biol. Biotechnol, 2017, 5, 47–52. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, V.; Bruno, L.B.; Rajkumar, M. Synergism of Industrial and Agricultural Waste as a Suitable Carrier Material for Developing Potential Biofertilizer for Sustainable Agricultural Production of Eggplant. Horticulturae. 2022, 8, 444. [Google Scholar]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol Lett. 2010, 32, 32,1559–1570. [Google Scholar] [CrossRef]
- Jimtha John, C.; Jishma, P.; Karthika, N.R.; Nidheesh, K.S.; Ray, J.G.; Mathew, J.; Radhakrishnan, E.K. Pseudomonas fluorescens R68 assisted enhancement in growth and fertilizer utilization of Amaranthus tricolor (L.). 3 Biotech. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Wickramasinghe, W.R.K.D.W.K.V.; Girija, D.; Gopal, K.S.; & Kesevan, S.; & Kesevan, S. Multi-phasic nitrogen fixing plant growth promoting rhizobacteria as biofertilizer for rice cultivation. Res. J. Agric. Sci. 2021, 12, 399–404. [Google Scholar]
- El_Komy, M.H.; Hassouna, M.G.; Abou-Taleb, E.M.; Al-Sarar, A.S.; Abobakr, Y. A mixture of Azotobacter, Azospirillum, and Klebsiella strains improves root-rot disease complex management and promotes growth in sunflowers in calcareous soil. Eur J Plant Pathol. 2020, 156, 713–726. [Google Scholar] [CrossRef]
- Badawy, I.H.; Hmed, A.A.; Sofy, M.R.; Al-Mokadem, A.Z. Alleviation of cadmium and nickel toxicity and phyto-stimulation of tomato plant l. by endophytic Micrococcus luteus and Enterobacter cloacae. Plants. 2022, 11, 2018. [Google Scholar]
- Muniswami, D.M.; Chinnadurai, S.; Sachin, M.; Jithin, H.; Ajithkumar, K.; Narayanan, G.S.; Dineshkumar, R. Comparative study of biofertilizer/biostimulant from seaweeds and seagrass in Abelmoschus esculentus crop. Biomass Convers Biorefin. 2021, 1–18. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Saeid, A.; Mironiuk, M.; Witek-Krowiak, A.; Kozioł, K.; Grzesik, R.; Chojnacka, K. Sustainable method of phosphorus biowaste management to innovative biofertilizers: A solution for circular economy of the future. Sustain Chem Pharm. 2022, 27, 100634. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Naeem, M.; Danish, S.; Khan, M.J.; Fahad, S.; Datta, R.; Brtnicky, M.; Kintl, A.; Hussain, G.S.; El-Esawi, M.A. Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants. 2020, 9, 1386. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Kwon, S.W.; & Weon, H.Y.; Weon, H.Y. Y. Isolation, molecular characterization and growth-promoting activities of endophytic sugarcane diazotroph Klebsiella sp. GR9. World J Microbiol Biotechnol. 2017, 23, 997–1006. [Google Scholar] [CrossRef]
- Semerci, N.; Kunt, B.; Calli, B. Phosphorus recovery from sewage sludge ash with bioleaching and electrodialysis. Int Biodeterior Biodegradation. 2019, 144, 104739. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate solubilizing microorganisms: promising approach as biofertilizers. Int. J. Agron. 2019, 1–7. [Google Scholar] [CrossRef]
- Tiquia, S.M. Evaluation of organic matter and nutrient composition of partially decomposed and composed and composted spent pig litter. Environ Technol 2002, 24, 97–107. [Google Scholar] [CrossRef]
- Nagarajan, S.; Eswaran, P.; Masilamani, R.P.; Natarajan, H. Chicken feather compost to promote the plant growth activity by using keratinolytic bacteria. Waste Biomass Valorization. 2018, 9, 531–538. [Google Scholar] [CrossRef]
- Muhammad, I.; Lv, J.Z.; Yang, L.; Ahmad, S.; Farooq, S.; Zeeshan, M.; Zhou, X.B. Low irrigation water minimizes the nitrate nitrogen losses without compromising the soil fertility, enzymatic activities and maize growth. BMC Plant Biol. 2022, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Ding, J.; Li, H.; Wang, X.; Li, W.; Li, K.; Sun, S. Mitigating nitrate leaching in cropland by enhancing microbial nitrate transformation through the addition of liquid biogas slurry. Agric. Ecosyst. Environ. 2023, 345, 108324. [Google Scholar] [CrossRef]
- Tsai, S.H.; Liu, C.P.; Yang, S.S. Microbial conversion of food wastes for biofertilizer production with thermophilic lipolytic microbes. Renew. Energ. 2007, 32, 32–904. [Google Scholar] [CrossRef]
- Wang, H.Y.; Shen, L.I.U.; Zhai, L.M.; Zhang, J.Z.; Ren, T.Z.; Fan, B.Q.; LIU, H.B. Preparation and utilization of phosphate biofertilizers using agricultural waste. J. Integr. Agric. 2015, 14, 158–167. [Google Scholar] [CrossRef]
- Devi, V.; Sumathy, V.J.H. Production of biofertilizer from fruit waste. Eur. J. Pharm. Med. Res. 2017, 4, 436–443. [Google Scholar]
- Asadu, C.O.; Aneke, N.G.; Egbuna, S.O.; Agulanna, A.C. Comparative studies on the impact of bio-fertilizer produced from agro-wastes using thermo-tolerant actinomycetes on the growth performance of Maize (Zea-mays) and Okro (Abelmoschus esculentus). Environ Technol. Innov. 2018, 12, 55–71. [Google Scholar] [CrossRef]
- Blanco-Vargas, A.; Chacón-Buitrago, M.A.; Quintero-Duque, M.C.; Poutou-Piñales, R.A.; Díaz-Ariza, L.A.; Devia-Castillo, C.A.; Pedroza-Rodríguez, A.M. Production of pine sawdust biochar supporting phosphate-solubilizing bacteria as an alternative bioinoculant in Allium cepa L., culture. Sci. Rep. 2022, 12, 12815. [Google Scholar] [CrossRef]

| Time (h) | Total reducing sugars (%) |
Glucose (%) |
Klebsiella pneumoniae AP-407 (CFU/ml) |
|---|---|---|---|
| 0 | 3.10 | 1.5 | 1.00 ×106 |
| 24 | 1.80 | 0 | 2.45 ×108 |
| 48 | 0.75 | 0 | 1.10 ×1010 |
| 72 | 0.08 | 0 | 3.00 ×1012 |
| Parameter (s) | Kitchen Waste Hydrolysate | Carrier Biofertilizer | Liquid Biofertilizer |
|---|---|---|---|
| pH | 4.0±0.5 | 6.5±0.5 | 6.5±0.5 |
| Viable Count | - | 1.00 ×108 CFU/g | 3.00 ×1012 CFU/ml |
| IAA | - | 31.75±1.75 µg/ml | 34.40±1.60 µg/ml |
| HCN | - | + | + |
| Siderophore | - | Hydroxymate(+) | Hydroxymate(+) |
| Treatment | Number of Seed germinated (out of a total of 20) |
Seed germination (SG %) |
Relative seed germination (RSG %) |
Vigor Index |
|---|---|---|---|---|
| Control | 12.0±0.45 | 60.0±2.25 | - | 185.25 |
| Liquid biofertilizer | 18.0±0.75 | 90.0±3.75 | 150.0 | 620.00 |
| Carrier biofertilizer | 14.0±0.50 | 70.0±2.50 | 116.6 | 275.90 |
| Parameter | Day 25 | Day 50 | Day 75 | Relative yield increase (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Carrier | Liquid | Control | Carrier | Liquid | Control | Carrier | Liquid | Carrier | Liquid | |
| Plant height cm) | 11.7± 0.280 |
13.0± 0.500 |
15.5± .650 |
28.5±0 .425 |
32.5± 0.625 |
47.5± 0.875 |
37.0± 1.350 |
49.5± 1.475 |
66.0± 1.800 |
133.7 | 178.3 |
| Shoot height (cm) | 9.7±0 .230 |
10.0± 0.350 |
11.5± 0.380 |
21.5± 0.250 |
24.0± 0.400 |
38.0± 0.625 |
27.1± 0.905 |
37.2± 1.010 |
50.2± 1.310 |
137.2 | 185.2 |
| Root height (cm) | 2.0± 0.050 |
3.0± 0.150 |
4.0± 0.410 |
6.5± 0.115 |
8.5± 0.220 |
9.5± 0.325 |
10.0± 0.445 |
12.3± 0.465 |
15.8± 0.490 |
123.0 | 158.0 |
| Parameter | Control | Carrier | Liquid | Relative yield increase (%) | |
|---|---|---|---|---|---|
| Carrier | Liquid | ||||
| Number of flowers | 40±2 | 56±3 | 65±3 | 140.0 | 162.5 |
| Flower diameter (cm) | 5.85±0.092 | 6.4±0.150 | 7.0±0.105 | 109.4 | 119.6 |
| Average Flower weight (g) | 6.15±0.236 | 7.4±0.220 | 8.5±0.210 | 120.3 | 138.2 |
| Treatment | Chlorophyll a (µg/ml) | Chlorophyll b (µg/ml) | Total Chlorophyll (µg/ml) |
|---|---|---|---|
| Control | 24.4±1.2 | 22.4±0.98 | 46.8±1.09 |
| Carrier Biofertilizer | 29.5±1.3 | 35.75±1.11 | 65.25±1.20 |
| Liquid Biofertilizer | 42.2±1.2 | 41.3±1.32 | 83.5±1.26 |
| Agro-industrial waste | Process involved | Microorganism involved | Agro-industrial commodity generated | Impact | Reference |
|---|---|---|---|---|---|
| Food waste | Food waste inoculated with microbes in a composter at 50 ◦C for 28 days | Brevibacillus borstelensis SH168 | Biofertilizer 1.82×109 CFU/g | Food waste in addition to biofertilizer production | [46] |
| Wastewater from sweet potato starch | Inoculation in 100 ml of sterilized (121°C, 20 min) SPSW and incubated at 24–32 h incubation at 30°C | Paenibacillus polymyxa | Biofertilizer having 9.7×109 CFU/ml | Biofertilizer which improves the growth of tea plant | [22] |
| Peat, corn cobs with 20% (w/w) perlite (CCP), wheat husks with 20% (w/w) perlite (WHP), and composted cattle manure with 20% (w/w) perlite |
Adsorption of Aspergillus niger 1107 on carrier material developed from waste | Aspergillus niger 1107 | Phosphate Biofertilizer | Higher growth and high content of phosphate in soil | [47] |
| Fruit waste | 30-40 days of the composting process | Bacillus spp. and Aspergillus spp | Carrier-based biofertilizer | Better seed germination, shoot, and root height, and the ability to prevent root diseases | [48] |
| Sawdust and agricultural waste | Biofertilizer was produced from Agro wastes by composting | Actinomyces spp., Streptomyces spp., and Rothia spp. | Biofertilzier (compost) | Better plant height and higher leaf width indicate a higher rate of photosynthesis | [49] |
| Chicken feather waste | 30 days of degradation process by 20–25% inoculum w/w | Bacillus subtilis | Compost | Management of chicken feather Increase in N, P, K content of the soil | [43] |
| Caribbean pine sawdust | 2.0 g Biochar adsorbed with inoculum and shaken at 150 RPM, 24 h at 30 ± 2 °C | Pseudomonas sp., Serratia sp., and Kosakonia sp | Biofertilizer having 1.0 × 107 CFU/ml | Increases seedling growth nutrient in soil and growth of Allium cepa L. | [50] |
| Chicken feather waste | White chicken feathers inoculated with B. pumilus AR57 in 1 % v/v; 1.25 ×108 CFU/ml) and incubated at 150 rpm, 37 ◦C for 28 h | Bacillus pumilus AR57 | Biofertilizer | Enhances total phosphate and potassium solubilizers and nitrifying bacteria in the soil of Zea mays L. | [15] |
| Kitchen waste | Separate hydrolysis and fermentation for 5 days | Aspergillus niger P-19 and Klebsiella pneumoniae AP-407 | Carrier and liquid biofertilizer formulations having 3.00 × 1012 CFU/g and 3.00 ×1012 CFU/ml, respectively | Kitchen waste management in addition to biofertilizer production improves both plant growth of Tagetes erecta (Marigold) and soil quality | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





