Submitted:
03 May 2023
Posted:
04 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fungal strains and growth conditions
2.2. Vaginal epithelial cells
2.3. Respecta® Balance Gel (RBG)
2.4. Establishment of A-431 epithelial cells monolayer infected with C. albicans in the presence or not of RBG
2.5. Assessment of vaginal epithelial cells damage
2.6. RBGs impact on C. albicans growth during vaginal epithelial cell infection
2.7. RBGs direct effect on Candida growth and metabolic activity
2.8. Effect of RBGs on C. albicans adhesion
2.9. Evaluation of RBGs effect on C. albicans hyphal formation
2.10. Quantification of IL-1β and IL-8 production after C. albicans infection and LPS stimulation of vaginal epithelial cells in the presence or not of RBGs
2.11. Statistical analysis
3. Results
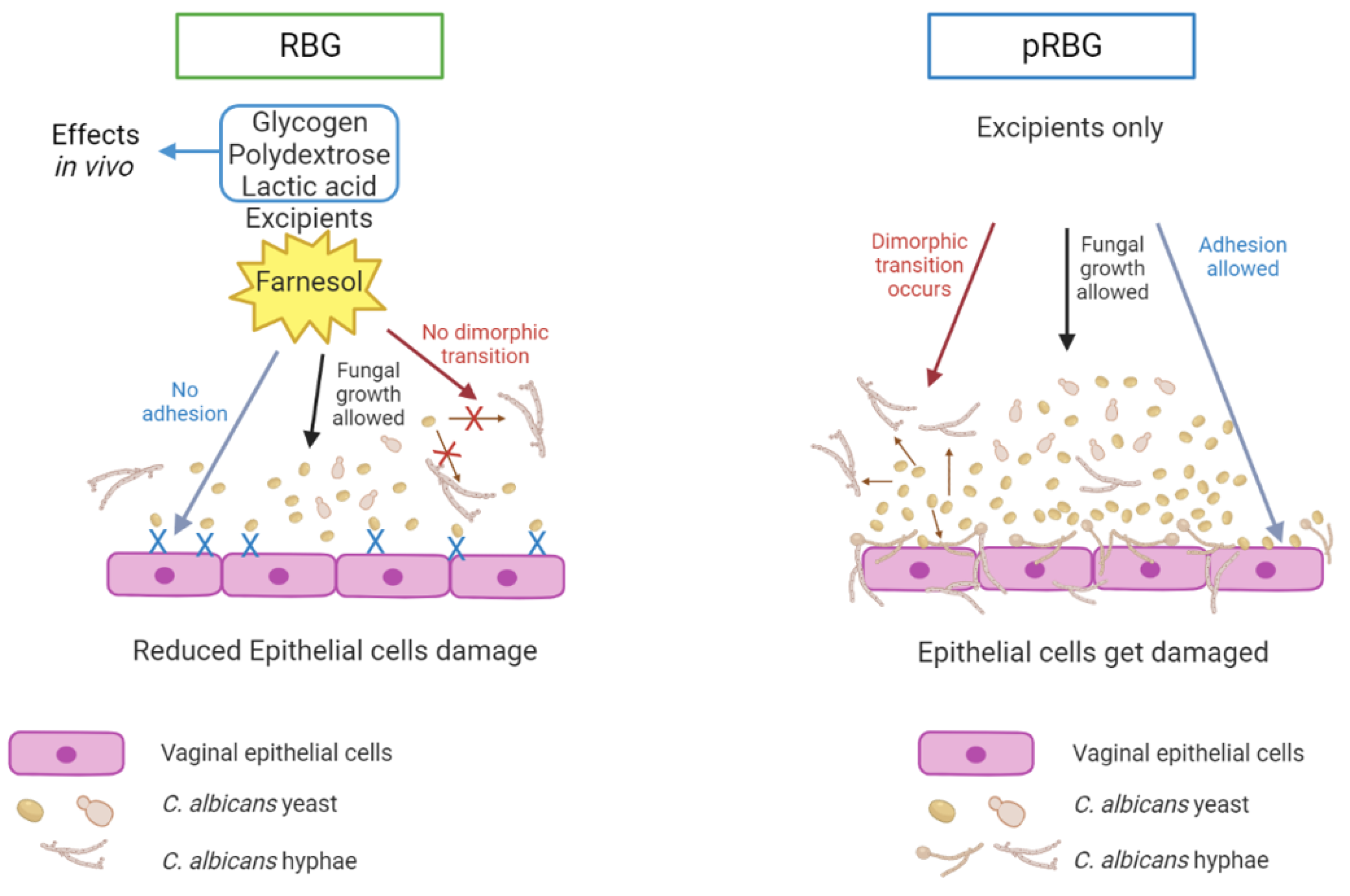
3.1. RBG reduced C. albicans-induced vaginal epithelial cell damage without affecting fungal growth.
3.2. RBG reduced C. albicans adhesion and hyphae formation.
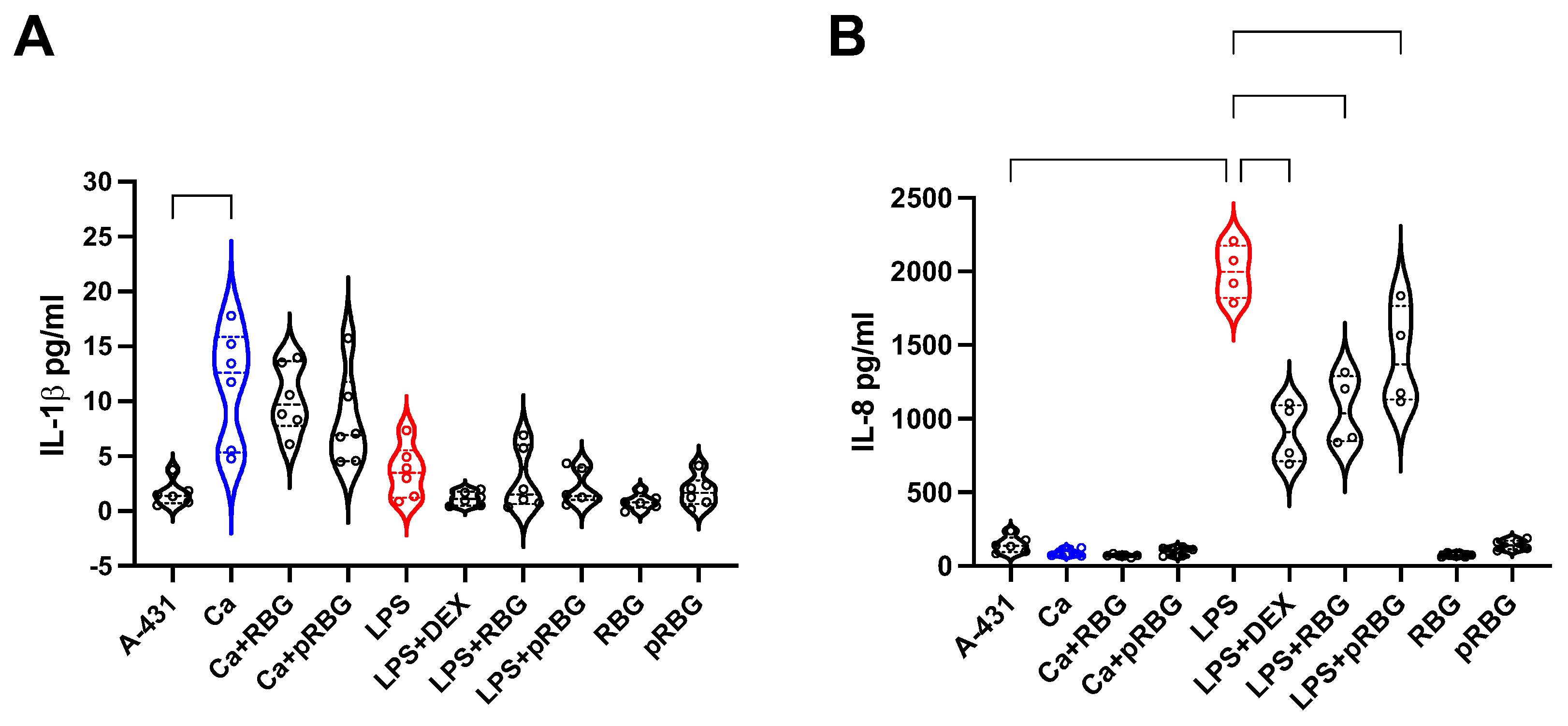
3.3. RBG effects on IL-1β and IL-8 production by vaginal epithelial cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blostein F, Levin-Sparenberg E, Wagner J, Foxman B. 2017. Recurrent vulvovaginal candidiasis. Ann Epidemiol 27:575-582.e3.
- Cassone, A. 2015. Vulvovaginal Candida albicans infections: pathogenesis, immunity and vaccine prospects. BJOG: Int J Obstet Gy 122:785–794.
- Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. 2018. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis 18:e339–e347.
- 2020. Vaginitis in Nonpregnant Patients: ACOG Practice Bulletin, Number 215. Obstetrics & Gynecology 135:e1–e17.
- Rosati D, Bruno M, Jaeger M, ten Oever J, Netea MG. 2020. Recurrent Vulvovaginal Candidiasis: An Immunological Perspective. Microorganisms 8:144.
- Miró MS, Caeiro JP, Rodriguez E, Vargas L, Vigezzi C, Icely PA, Castillo GDV, Azcurra AI, Abiega CD, Riera FO, Sotomayor CE. 2021. Candida albicans Modulates Murine and Human Beta Defensin-1 during Vaginitis. JoF 8:20.
- Ardizzoni A, Wheeler RT, Pericolini E. 2021. It Takes Two to Tango: How a Dysregulation of the Innate Immunity, Coupled With Candida Virulence, Triggers VVC Onset. Front Microbiol 12:692491.
- Farr A, Effendy I, Frey Tirri B, Hof H, Mayser P, Petricevic L, Ruhnke M, Schaller M, Schaefer APA, Sustr V, Willinger B, Mendling W. 2021. Guideline: Vulvovaginal candidosis (AWMF 015/072, level S2k). Mycoses 64:583–602.
- McClelland RS, Richardson BA, Hassan WM, Graham SM, Kiarie J, Baeten JM, Mandaliya K, Jaoko W, Ndinya-Achola JO, Holmes KK. 2009. Prospective study of vaginal bacterial flora and other risk factors for vulvovaginal candidiasis. J Infect Dis 199:1883–1890.
- Liu M-B, Xu S-R, He Y, Deng G-H, Sheng H-F, Huang X-M, Ouyang C-Y, Zhou H-W. 2013. Diverse vaginal microbiomes in reproductive-age women with vulvovaginal candidiasis. PLoS One 8:e79812.
- Roselletti E, Perito S, Sabbatini S, Monari C, Vecchiarelli A. 2019. Vaginal Epithelial Cells Discriminate Between Yeast and Hyphae of Candida albicans in Women Who Are Colonized or Have Vaginal Candidiasis. J Infect Dis 220:1645–1654.
- Pericolini E, Perito S, Castagnoli A, Gabrielli E, Mencacci A, Blasi E, Vecchiarelli A, Wheeler RT. 2018. Epitope unmasking in vulvovaginal candidiasis is associated with hyphal growth and neutrophilic infiltration. PLoS One 13:e0201436.
- Naglik JR, Moyes DL, Wächtler B, Hube B. 2011. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect 13:963–976.
- Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Höfs S, Gratacap RL, Robbins J, Runglall M, Murciano C, Blagojevic M, Thavaraj S, Förster TM, Hebecker B, Kasper L, Vizcay G, Iancu SI, Kichik N, Häder A, Kurzai O, Luo T, Krüger T, Kniemeyer O, Cota E, Bader O, Wheeler RT, Gutsmann T, Hube B, Naglik JR. 2016. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 532:64–68.
- Ho J, Camilli G, Griffiths JS, Richardson JP, Kichik N, Naglik JR. 2021. Candida albicans and candidalysin in inflammatory disorders and cancer. Immunology 162:11–16.
- Ho J, Yang X, Nikou S-A, Kichik N, Donkin A, Ponde NO, Richardson JP, Gratacap RL, Archambault LS, Zwirner CP, Murciano C, Henley-Smith R, Thavaraj S, Tynan CJ, Gaffen SL, Hube B, Wheeler RT, Moyes DL, Naglik JR. 2019. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat Commun 10:2297.
- Richardson JP, Willems HME, Moyes DL, Shoaie S, Barker KS, Tan SL, Palmer GE, Hube B, Naglik JR, Peters BM. 2018. Candidalysin Drives Epithelial Signaling, Neutrophil Recruitment, and Immunopathology at the Vaginal Mucosa. Infect Immun 86.
- Calderone RA, Fonzi WA. 2001. Virulence factors of Candida albicans. Trends in Microbiology 9:327–335.
- Ardizzoni A, Boaretto G, Pericolini E, Pinetti D, Capezzone de Joannon A, Durando L, Ragni L, Blasi E. 2022. Effects of benzydamine and mouthwashes containing benzydamine on Candida albicans adhesion, biofilm formation, regrowth, and persistence. Clin Oral Invest 26:3613–3625.
- Hornby JM, Kebaara BW, Nickerson KW. 2003. Farnesol Biosynthesis in Candida albicans : Cellular Response to Sterol Inhibition by Zaragozic Acid B. Antimicrob Agents Chemother 47:2366–2369.
- Polke M, Leonhardt I, Kurzai O, Jacobsen ID. 2018. Farnesol signalling in Candida albicans – more than just communication. Critical Reviews in Microbiology 44:230–243.
- Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, Dussault P, Nickerson KW. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol 67:2982–2992.
- Deveau A, Piispanen AE, Jackson AA, Hogan DA. 2010. Farnesol Induces Hydrogen Peroxide Resistance in Candida albicans Yeast by Inhibiting the Ras-Cyclic AMP Signaling Pathway. Eukaryot Cell 9:569–577.
- Abe S, Tsunashima R, Iijima R, Yamada T, Maruyama N, Hisajima T, Abe Y, Oshima H, Yamazaki M. 2009. Suppression of anti- Candida activity of macrophages by a quorum-sensing molecule, farnesol, through induction of oxidative stress. Microbiology and Immunology 53:323–330.
- Jung Y, Hwang S, Sethi G, Fan L, Arfuso F, Ahn K. 2018. Potential Anti-Inflammatory and Anti-Cancer Properties of Farnesol. Molecules 23:2827.
- Casadevall A, Pirofski LA. 1999. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun 67:3703–3713.
- Yano J, Lilly E, Barousse M, Fidel PL. 2010. Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun 78:5126–5137.
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 108 Suppl 1:4680–4687.
- Wang S, Wang Q, Yang E, Yan L, Li T, Zhuang H. 2017. Antimicrobial Compounds Produced by Vaginal Lactobacillus crispatus Are Able to Strongly Inhibit Candida albicans Growth, Hyphal Formation and Regulate Virulence-related Gene Expressions. Front Microbiol 08.
- Matsuda Y, Cho O, Sugita T, Ogishima D, Takeda S. 2018. Culture Supernatants of Lactobacillus gasseri and L. crispatus Inhibit Candida albicans Biofilm Formation and Adhesion to HeLa Cells. Mycopathologia 183:691–700.
- Jang SJ, Lee K, Kwon B, You HJ, Ko G. 2019. Vaginal lactobacilli inhibit growth and hyphae formation of Candida albicans. Sci Rep 9:8121.
- De Seta F, Parazzini F, De Leo R, Banco R, Maso GP, De Santo D, Sartore A, Stabile G, Inglese S, Tonon M, Restaino S. 2014. Lactobacillus plantarum P17630 for preventing Candida vaginitis recurrence: a retrospective comparative study. European Journal of Obstetrics & Gynecology and Reproductive Biology 182:136–139.
- Spaggiari L, Sala A, Ardizzoni A, De Seta F, Singh DK, Gacser A, Blasi E, Pericolini E. 2022. Lactobacillus acidophilus, L. plantarum, L. rhamnosus, and L. reuteri Cell-Free Supernatants Inhibit Candida parapsilosis Pathogenic Potential upon Infection of Vaginal Epithelial Cells Monolayer and in a Transwell Coculture System In Vitro. Microbiol Spectr 10:e02696-21.
- Parolin C, Croatti V, Laghi L, Giordani B, Tondi MR, De Gregorio PR, Foschi C, Vitali B. 2021. Lactobacillus Biofilms Influence Anti-Candida Activity. Front Microbiol 12:750368.
- Chew SY, Cheah YK, Seow HF, Sandai D, Than LTL. 2015. Probiotic L actobacillus rhamnosus GR -1 and L actobacillus reuteri RC -14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing C andida glabrata isolates. J Appl Microbiol 118:1180–1190.
- Salari S, Ghasemi Nejad Almani P. 2020. Antifungal effects of Lactobacillus acidophilus and Lactobacillus plantarum against different oral Candida species isolated from HIV/ AIDS patients: an in vitro study. Journal of Oral Microbiology 12:1769386.
- Noverr MC, Huffnagle GB. 2004. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect Immun 72:6206–6210.
- Liang W, Guan G, Dai Y, Cao C, Tao L, Du H, Nobile CJ, Zhong J, Huang G. 2016. Lactic acid bacteria differentially regulate filamentation in two heritable cell types of the human fungal pathogen Candida albicans. Mol Microbiol 102:506–519.
- Goh YJ, Klaenhammer TR. 2014. Insights into glycogen metabolism in Lactobacillus acidophilus: impact on carbohydrate metabolism, stress tolerance and gut retention. Microb Cell Fact 13:94.
- do Carmo MMR, Walker JCL, Novello D, Caselato VM, Sgarbieri VC, Ouwehand AC, Andreollo NA, Hiane PA, Dos Santos EF. 2016. Polydextrose: Physiological Function, and Effects on Health. Nutrients 8:553.
- De Seta F, Larsen B. 2021. Antimicrobial Activity of a Vaginal Gel Formulation: Considerations Related to Vaginal Infection and Dysbiosis. Pathogens 10:1576.
- Tsui C, Kong EF, Jabra-Rizk MA. 2016. Pathogenesis of Candida albicans biofilm. Pathogens and Disease 74:ftw018.
- Desai, J. 2018. Candida albicans Hyphae: From Growth Initiation to Invasion. JoF 4:10.
- Kovács R, Majoros L. 2020. Fungal Quorum-Sensing Molecules: A Review of Their Antifungal Effect against Candida Biofilms. J Fungi (Basel) 6.
- Amabebe E, Anumba DOC. 2018. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front Med 5:181.
- Petrova MI, Lievens E, Malik S, Imholz N, Lebeer S. 2015. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol 6.
- Sun Z, Ge X, Qiu B, Xiang Z, Jiang C, Wu J, Li Y. 2023. Vulvovaginal candidiasis and vaginal microflora interaction: Microflora changes and probiotic therapy. Front Cell Infect Microbiol 13:1123026.
- Hedges SR, Barrientes F, Desmond RA, Schwebke JR. 2006. Local and Systemic Cytokine Levels in Relation to Changes in Vaginal Flora. J INFECT DIS 193:556–562.
- Li X, Wang X, Gao X. 2015. [Synergistic effects of lysozyme with EDTA-2Na on antibacterial activity]. Beijing Da Xue Xue Bao Yi Xue Ban 47:52–56.
- Schumacher GFB, Kim MH, Hosseinian AH, Dupon C. 1977. Immunoglobulins, proteinase inhibitors, albumin, and lysozyme in human cervical mucus. American Journal of Obstetrics and Gynecology 129:629–636.
- Mitsukawa K, Otsuki K, Yanaihara A, Sawada M, Iwasaki S, Okai T. 2006. Concentration of lactoferrin and interleukin-6 in cervical mucus from patients being treated for infertility: Lactoferrin and IL-6 in cervical mucus. Reproductive Medicine and Biology 5:105–109.
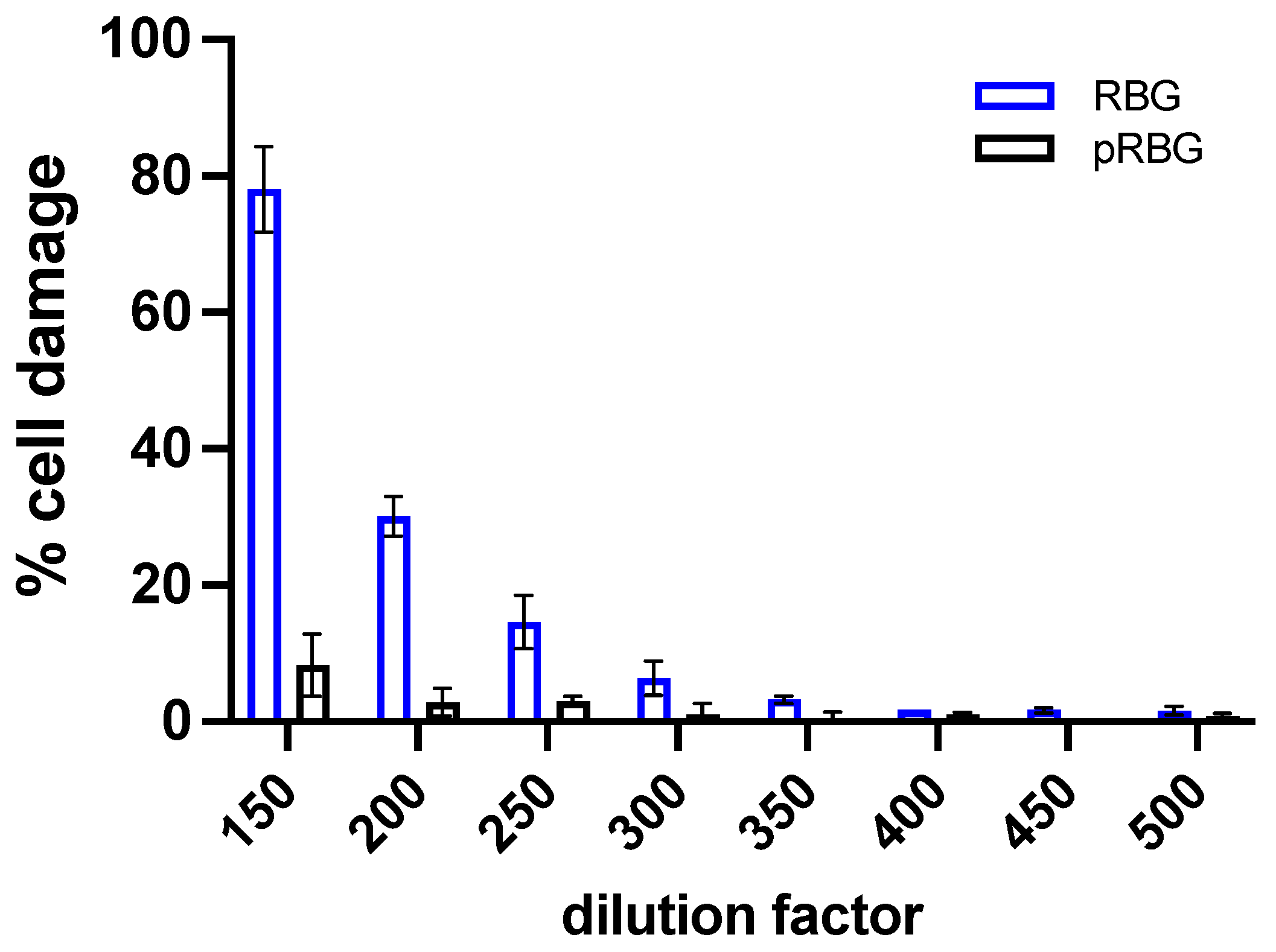
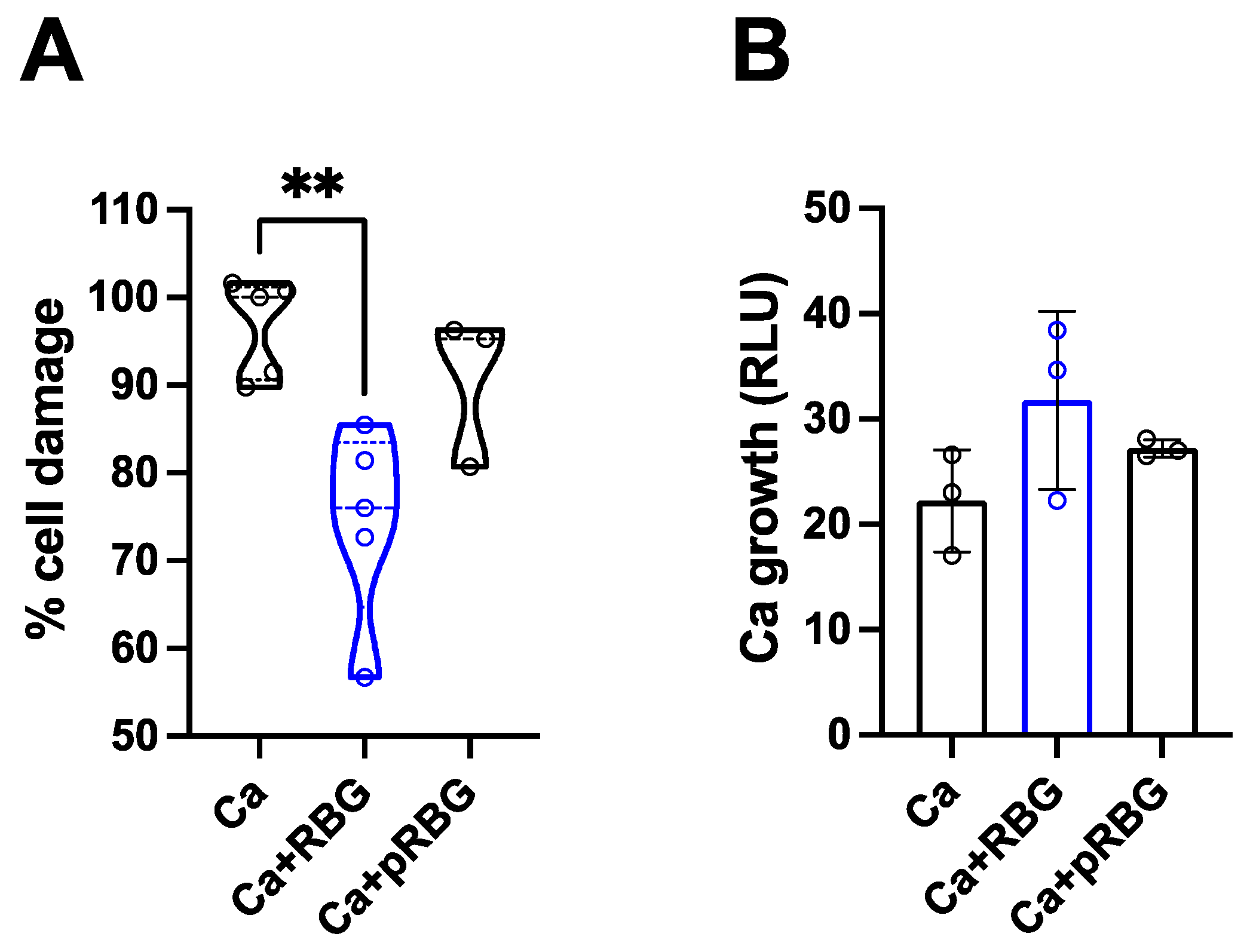
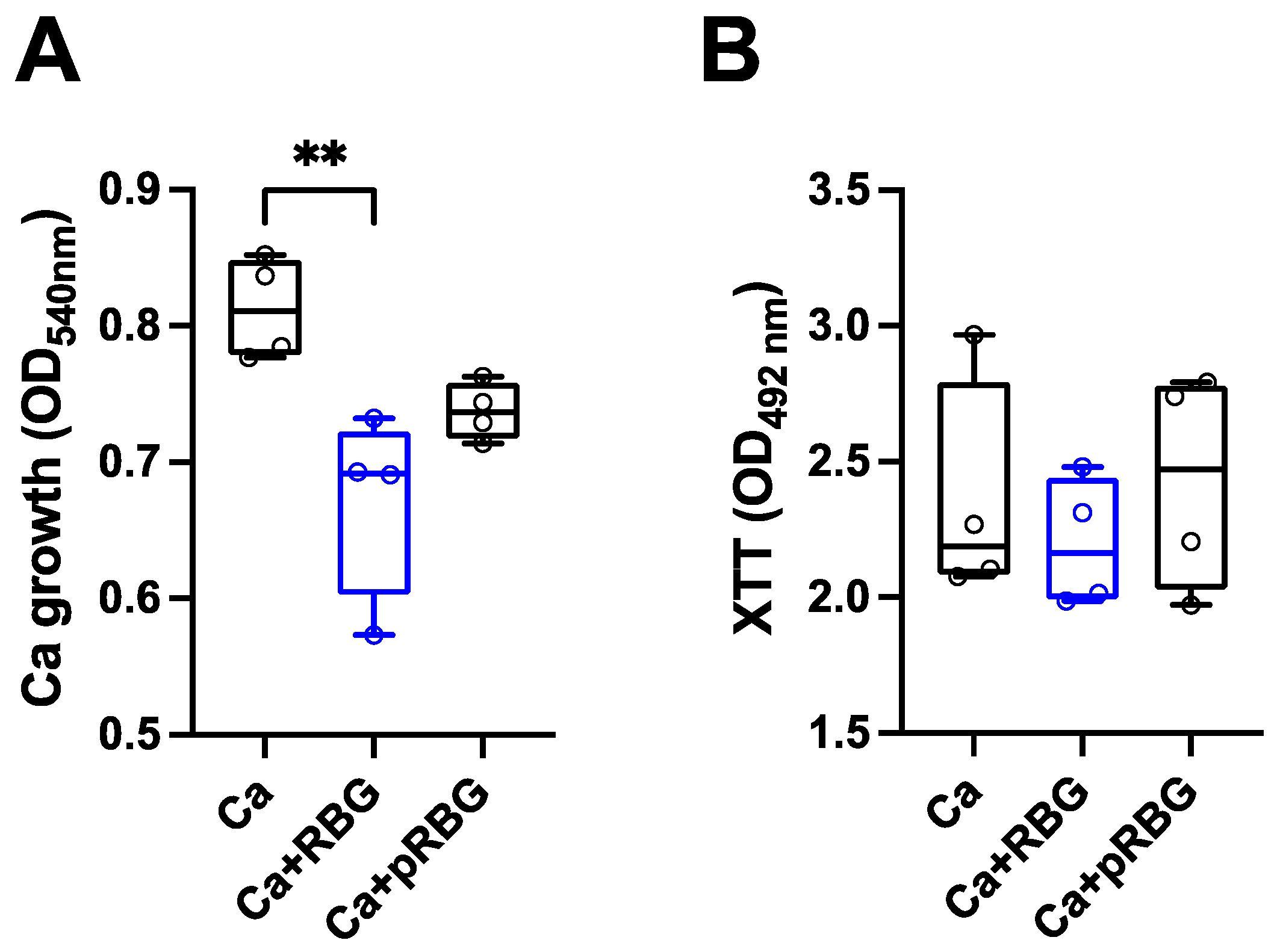
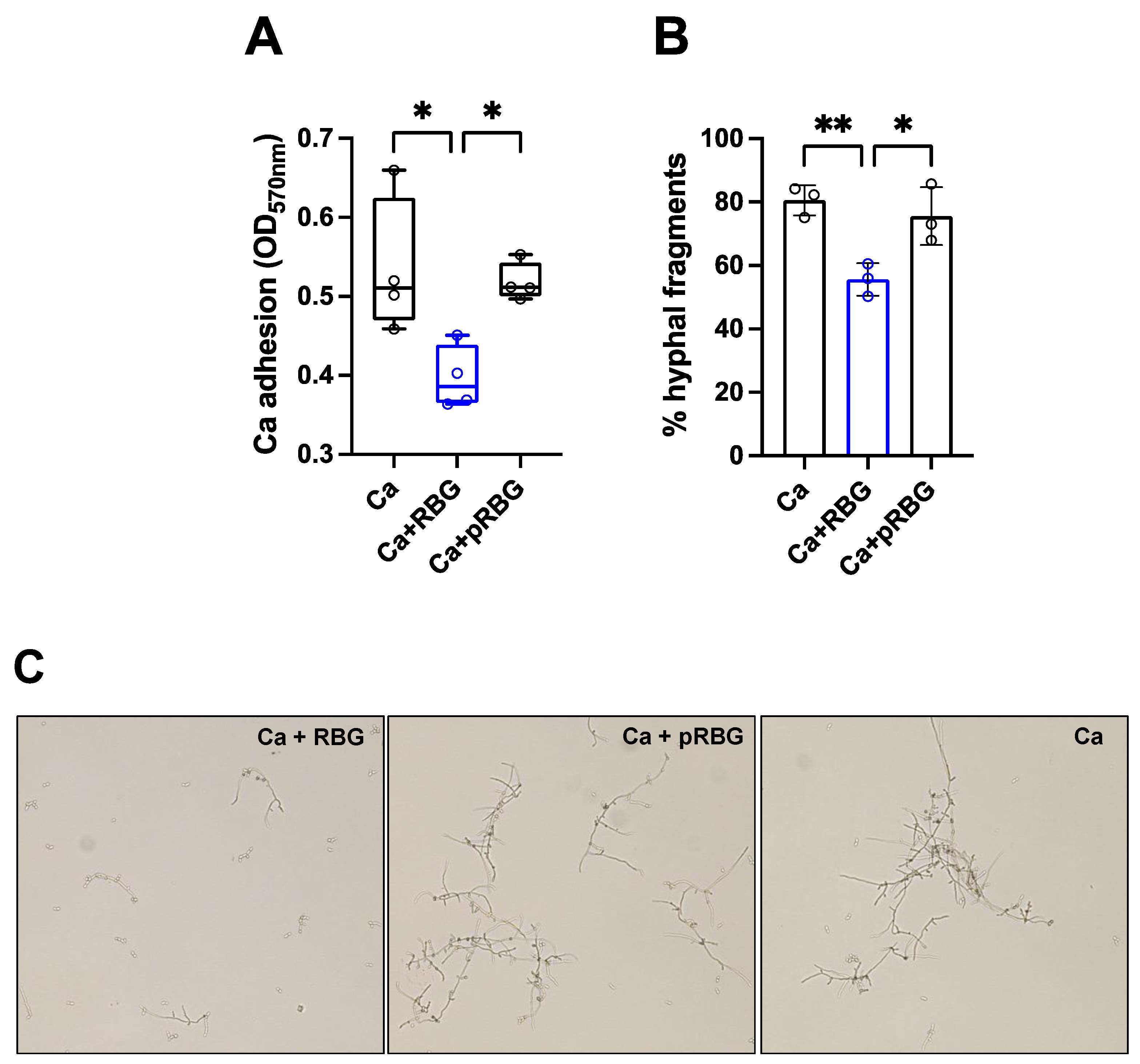
| RBG | pRBG |
| Water | Water |
| Disodium EDTA 0.2% Xanthan gum Sodium hyaluronate Propylene glycol Decylene glycol Hydroxyacetophenone Hydrogenated castor oil Tocopherol acetate PEG-40 Polydextrose Lactic acid Farnesol Glycogen |
Disodium EDTA 0.2% Xanthan gum Sodium hyaluronate Propylene glycol Decylene glycol Hydroxyacetophenone Hydrogenated castor oil Tocopherol acetate PEG-40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





