1. Introduction
Amphetamine was first introduced as an over-the-counter medication and eventually restricted for specific conditions such as narcolepsy and attention deficit hyperactivity disorder [
1]. Globally, amphetamine and its analogs are widely misused psychostimulant drugs causing fatalities [
2]. A study from Saudi Arabia reported that amphetamine is the second in line for misuse after hashish [
3]. Around 4-70 % of Saudi patients undergoing addiction treatment were previously on amphetamine substance abuse [
4]. Attafi et al. have reported an exponential rise in fatalities from 18 % in 2018 to 80 % in 2020, with the use of amphetamine along with other drugs in Jazan, Saudi Arabia [
5]. Amphetamine abuse has found favor as it produces euphoria, increases performance, induces sexual arousal, and promotes weight loss [
6]. Amphetamine directly impacts the central nervous system, increases the availability of monoamines, interferes with monoamine reuptake via monoamine transporters, and inhibits monoamine oxidase [
7]. The primary site of action is the dopaminergic pathways in the mesolimbic system, particularly the reinforcement and reward circuitry [
8]. They enhance dopamine concentration causing sustained stimulation of D1 and D2 receptors [
9]. Amphetamine could also elicit its action on the corticostriatal system, affecting decision-making, emotion regulation, reinforcement, and reward regulation [10, 11].
Amphetamines are implicated in several adverse health outcomes such as intracerebral hemorrhage, cardiac arrest [
2], psychosis [
6] and suicide [
12]. Additionally, indirect fatalities include traffic accidents and participation in high-risk behaviors with poor judgment. Also, specific cases of amphetamine-induced hyperthermia result in rhabdomyolysis [
13] and multiple organ failure [
14]. Moreover, the use of amphetamine can also result in associated problems such as HIV and hepatitis, both of which inflict a considerable burden on the health system [
15]. An increase in amphetamine-dependent addicts among communities, hospitalization, rehabilitation, and costs contribute significantly to the global burden of disease.
More research is needed to determine how amphetamine and its metabolites affect the brain given the rise in mortality due to its use. Also, explicit judgments are difficult to make based on available data. Brain organic profiling is a reliable tool to illustrate alterations in organic components and the related mental illnesses. Moreover, in our previous study, we discovered changes in the organic profile of the brain in amphetamine users [
5]. Thus, we continued our investigation on the chemical-disease association based on the brain chemical profile of amphetamine in relation to the control and the occurrence of neuro disorders.
2. Results
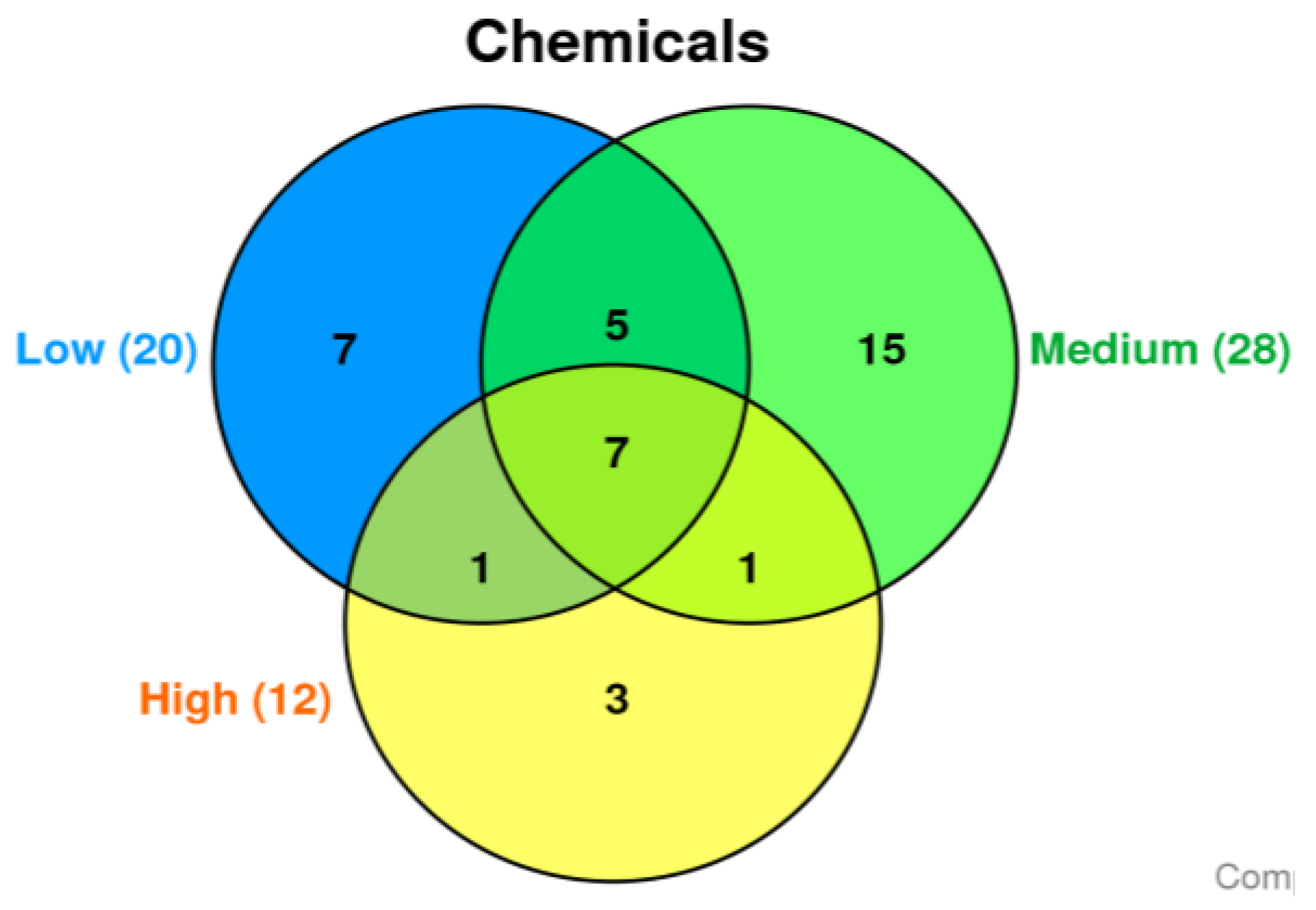
Based on the amphetamine brain concentration, amphetamine related fatalities were subdivided into three groups (each group was contains six cases): The three subdivided groups produced three distinct chemical sets with some overlap. The degree to which chemicals are detected similarly between these groups is represented by Venn diagram (
Figure 1). In the low, medium, and high groups, 251, 238, and 134 chemicals did not match any annunciated CTD chemicals, respectively.
In the low, medium, and high groups, 20, 28, and 12 chemicals matched the CTD database, respectively. The Venn diagram shows the three divided groups' three distinct chemical sets with some overlap (
Figure 1). The common 7 chemicals, including 1-octadecene, 1-tridecene, 2,4-di-tert-butylphenol, arachidonic acid, docosahexaenoic acid (DHA), eicosane, and oleylamide, are differentially detected in all three groups (
Table 1).
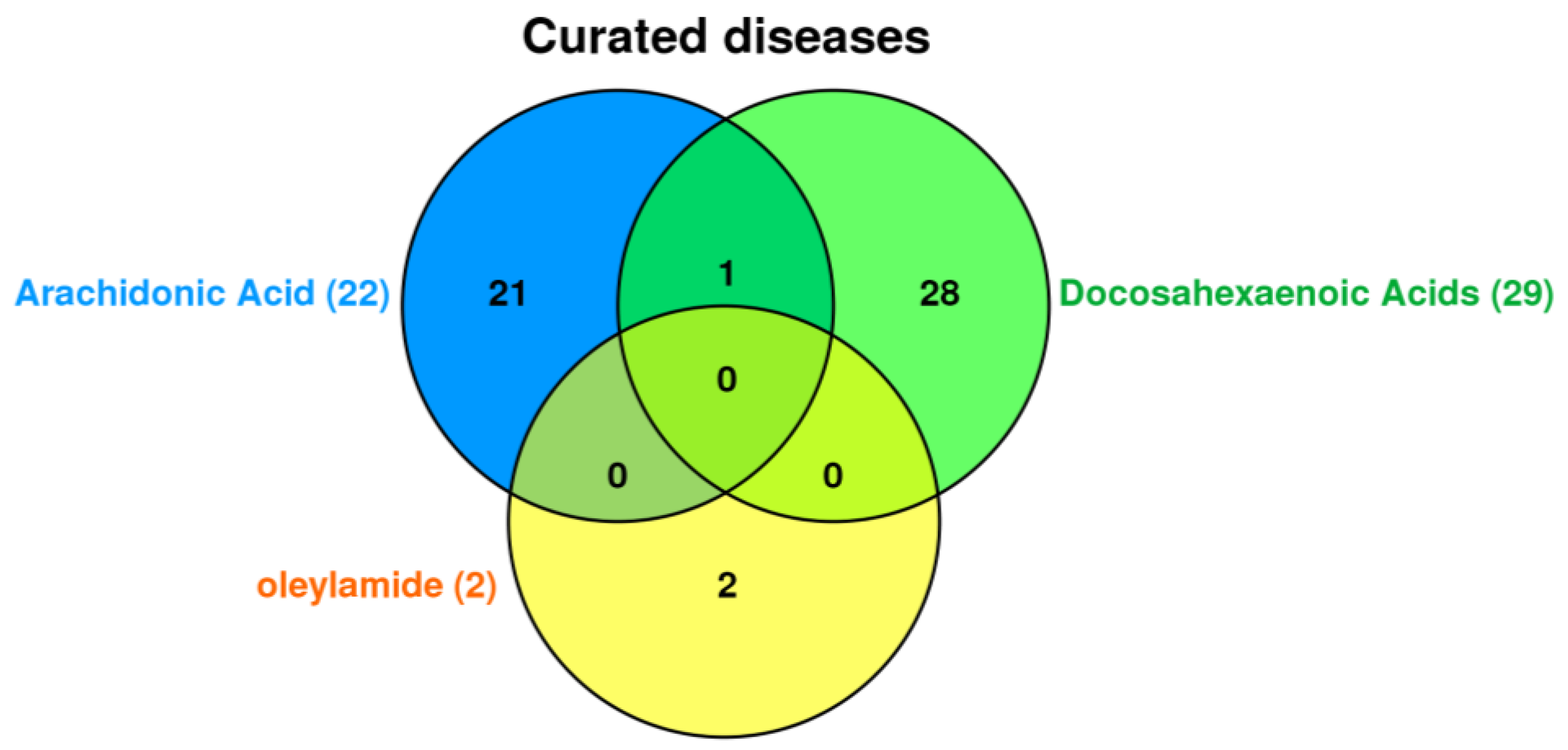
These chemicals have Gene Ontology (GO) annotations that describe the biological processes and molecular functions associated with them. The CTD database tools were used to investigate the association between the seven common chemicals in all groups and diseases such as cardiovascular disease, metabolic disorders, nervous system disease, mental disorder, and cancers. Finally, our focus was on nervous system disease and mental disorder, so we chose CTD tools for further investigation. Among chemical common to all, only docosahexaenoic acids (DHA), oleylamide, and arachidonic acid were associated with curated diseases in CTD database (
Figure 2).
Arachidonic acid is commonly associated 22 diseases including cardiovascular diseases, thrombosis, and inflammation. Docosahexaenoic acid (DHA) is commonly associated 29 diseases including amyotrophic lateral sclerosis, parkinsonian disorders, nerve degeneration, and dyskinesia related only to docosahexaenoic acid (DHA). While oleylamide associated with amnesia and seizures. Autistic disorder is related to both docosahexaenoic acid (DHA) and arachidonic acid.
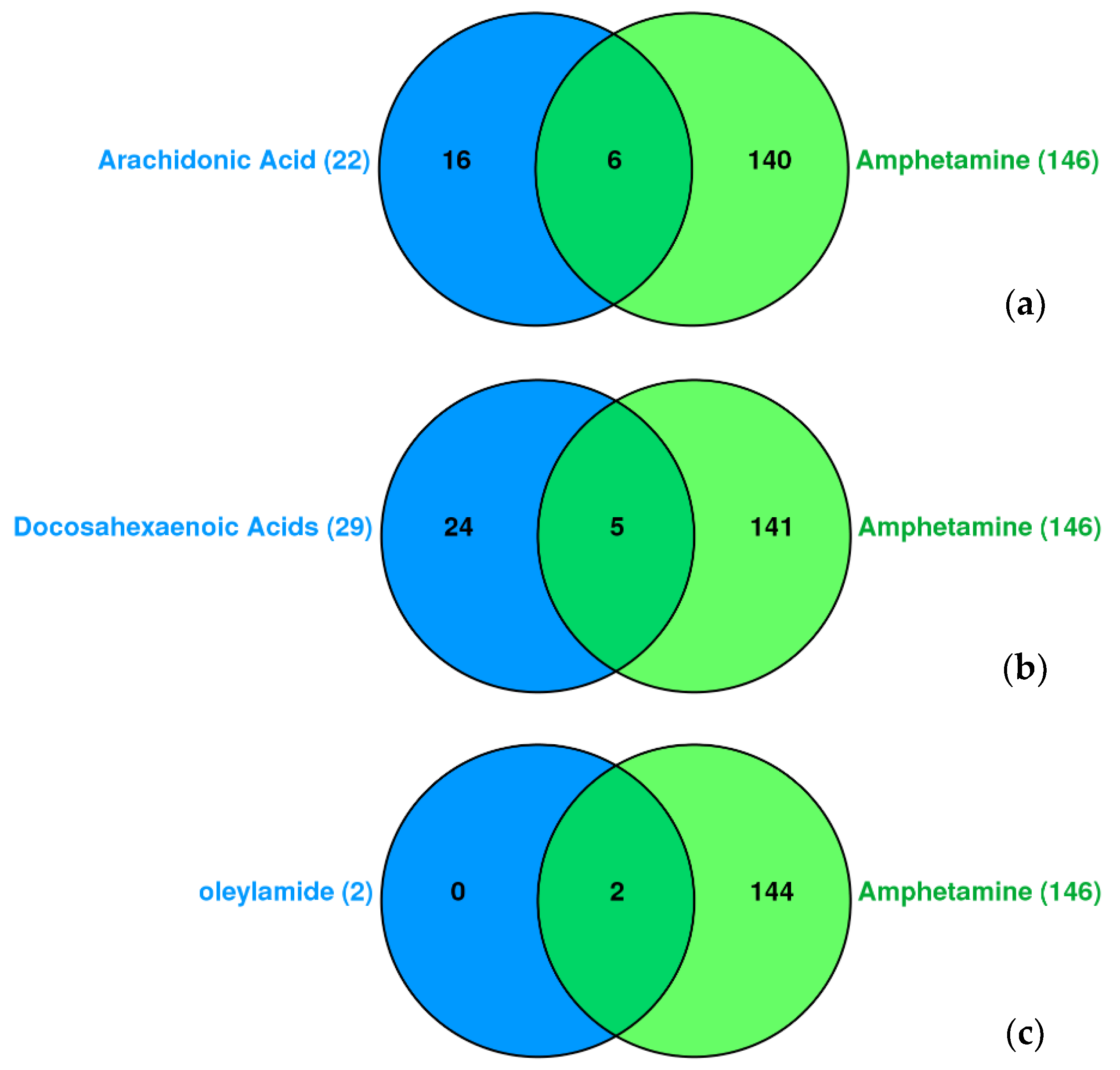
In related to amphetamine, we analyzed the chemical-disease interaction association of docosahexaenoic acid (DHA), arachidonic acid, and oleylamide in compare with amphetamine (
Figure 3). Congenital abnormalities, bradycardia, substance-related disorder, fever, hyperalgesia, and hypotension are common to arachidonic acid and amphetamine. Dyskinesia, nerve degeneration, pain, liver diseases, and arrhythmias are common to docosahexaenoic acid (DHA) and amphetamine. Amnesia and seizures are common to oleylamide and amphetamine.
Arachidonic acid is commonly associated with cardiovascular diseases, thrombosis, and inflammation. While amyotrophic lateral sclerosis, parkinsonian disorders, and dyskinesia related only to docosahexaenoic acid (DHA).
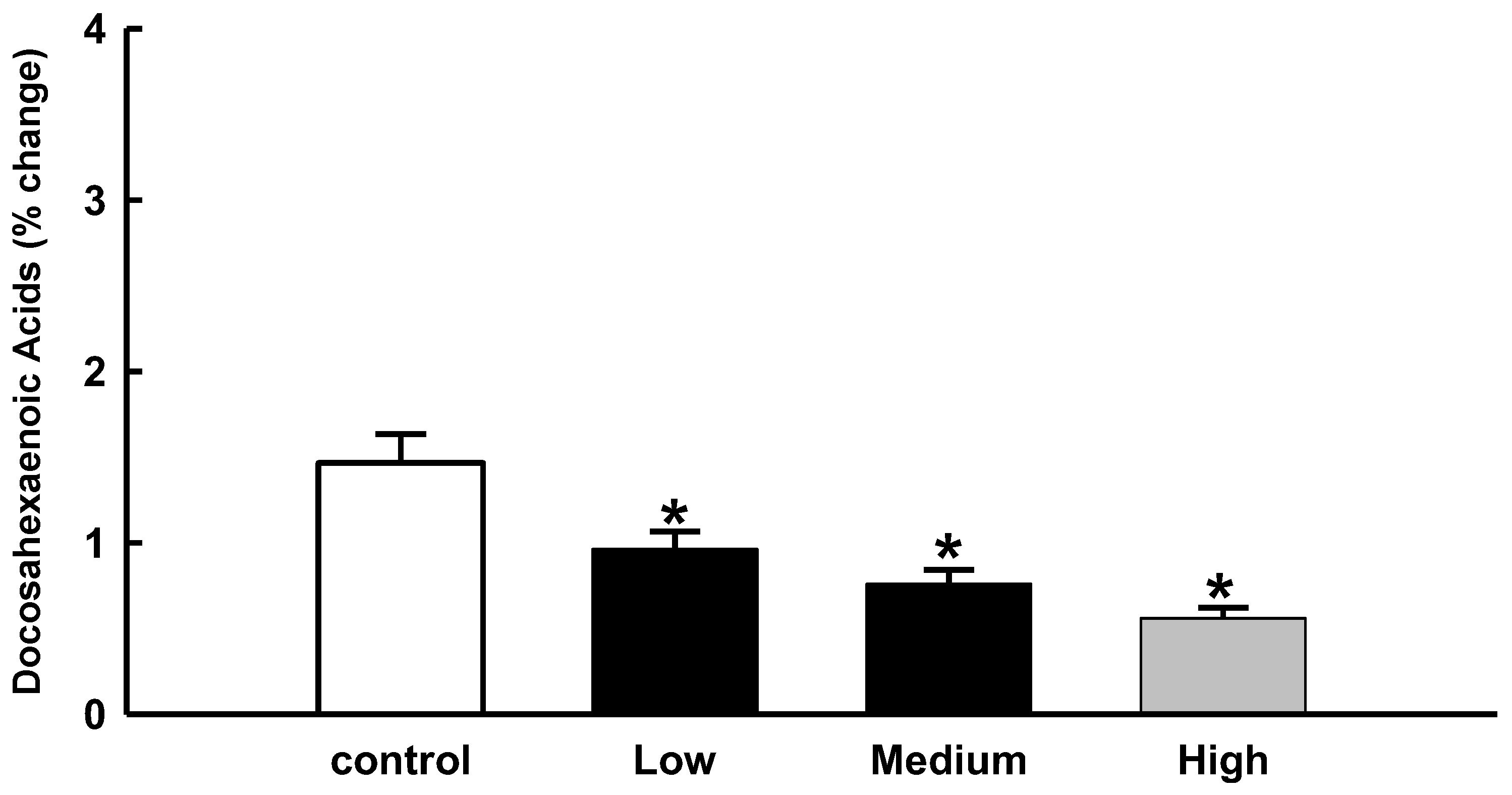
In the present study, we were interested in docosahexaenoic acid (DHA), so we analyzed the relative percentage change in docosahexaenoic acid (DHA) levels in brain samples from amphetamine-related fatalities.
In the current study, our focus was on docosahexaenoic acid (DHA), so we analyzed the relative percentage change in the level docosahexaenoic acid from the brain samples of amphetamine-related fatalities. The level of decoshexaenoic acid (DHA) in the brain differed significantly from that of the control group. The results also indicated that as amphetamine levels rose, docosahexaenoic acid (DHA) levels decreased. (
Figure 4).
3. Discussion
Amphetamines disrupt biochemical processes in the body and allow several chemicals to accumulate in the brain. The severity of amphetamine toxicity is determined by the number of affected organs, the dose consumed, the presence of potent decongestants, and the patient's comorbidities.
Hundreds of chemical entities have been identified in brain tissue of fatal amphetamine cases. Many of these chemicals have not yet been explicitly studied. However, we found seven common chemicals in the three categories created based on the amphetamine concentration in the brain. These compounds have been linked to various diseases in CTD, including neurocognitive disorders, dementia, neurodevelopmental disorders, alzheimer's disease, major depression, and mood disorders. CTD, a well-known database, provides manually curated information about chemical–gene/protein interactions, chemical–disease, and gene-disease relationships and is used to better understand how chemical exposure affects human health. The generated data is combined with functional and pathway data to help develop hypotheses about the mechanisms underlying environmentally influenced diseases.
Only docosahexaenoic acid (DHA), arachidonic acids, and oleylamide were linked to curated diseases in the CTD database. In related to amphetamine, amnesia is common to both oleylamide and amphetamine. They have a potential therapeutic role in amnesia [1, 2]. The findings also revealed that they are linked to seizures. In this context, Amphetamine abuse causes seizures, whereas oleylamide has an anti-seizure effect [3, 4]. The link between amphetamine, oleylamide, amnesia, and seizure needs to be explored. In addition, arachidonic acid was identified as one of the brain chemicals in all the cases. We hypothesize that the disordered activation of lipoxygenase (LO) and COX during amphetamine abuse could result in various inflammatory diseases. According to Bhattacharjee et al. (2008), acute amphetamine administration to rats results in the release of arachidonic acid as a second messenger following indirect agonism at dopamine D2-like receptors in the brain. This is likely due to neuroplastic changes in brain arachidonic acid signaling, which correspond to depressive behaviors observed in humans and rats after chronic amphetamine withdrawal [
5]. Methamphetamine's neurotoxicity may be due to its prostaglandin H synthase (PHS)-dependent bioactivation, which releases free radical intermediates that eventually generate reactive oxygen species and damage cellular macromolecules [
6]. In response to the amphetamine challenge, docosahexaenoic acid-deficient mice had a significantly larger ventral striatum. The arachidonic acid composition in the ventral striatum, as well as the arachidonic acid: docosahexaenoic acid (DHA) ratio, were found to be positively correlated with DA concentrations. During the preceding amphetamine challenge, the arachidonic acid composition in the ventral striatum and DA concentrations were positively associated with locomotor activity [
7].
Our research revealed a lack of docosahexaenoic acid (DHA) and a change in arachidonic acid levels, suggesting a link to the development of depression and its detrimental effects. According to our research results, there may be a connection between the rise in amphetamine levels and the decline in docosahexaenoic acid (DHA) levels. It has been demonstrated that docosahexaenoic acids deficiency may play a role in the etiology of neurological disorders. Docosahexaenoic acid (DHA) and arachidonic acid were frequently linked to Autistic disorders, cocaine-related disorders, Alzheimer's disease, and cognitive dysfunction. These chemicals matched with the standard curated disease list in the CTD and were considered as neuro disroders. Docosahexaenoic acid (DHA), a polyunsaturated fatty acid (FA), is selectively esterified to amino phospholipids and is abundant at the cytofacial site of the plasma membrane, where it plays a specific role in intracellular events. docosahexaenoic acid (DHA) is involved in cellular oxidative processes and intracellular signaling to gene expression and growth regulation modulation [
8]. It helps circulate blood in the brain during mental tasks [9, 10], which is especially beneficial for patients with dementia and Alzheimer's disease [11, 12]. Also, as there is limited clinical literature, empirical evidence from preclinical studies can also be supportive. According to one study, dietary docosahexaenoic acid (DHA) deficits significantly alter mesocorticolimbic dopamine (DA) neurotransmission in rat brains (13). Levant et al. (2004) demonstrated that rats fed a diet with a relatively minor reduction in brain docosahexaenoic acid (DHA) content exhibit adult behavioral changes consistent with altered dopaminergic function (14). Also, serotonin, a neurotransmitter, may require enough docosahexaenoic acid (DHA) to function normally in the brain and is essential to stabilize mood and reduce nerve cell inflammation (7, 15). Thus, our preliminary findings suggest that amphetamine abusers may experience a deficiency of docosahexaenoic acid (DHA) which can impact cell membrane fluidity, neurotransmission, and cause neuroinflammation.
Other chemicals found among the groups in our study are eicosane and 2, 4 di-tert-butylphenol (DTBP). They are naturally occurring substances derived from polyunsaturated fatty acids with 20 carbons. Eicosane is effective at suppressing pro-inflammatory cytokines (TNF- and IL-12), while the latter is cytoprotective against various oxidants. The correlation between 2,4-di-tert-butylphenol and vitamin C equivalent antioxidant capacity was strong. In a dose-dependent manner, 2,4-di-tert-butylphenol scavenged the ABTS radical anions. Furthermore, in the mouse model, the 2,4-di-tert-butylphenol diet had a significant anti-amnesic effect [
16]. Similarly, trace amounts of other chemicals such as 1-Octadecene, 1-tridecene, and oleylamide have also been observed in amphetamine fatal cases but did match annunciated CTD chemicals database. However, future studies would be needed to justify the down- or up-regulation of these chemicals in various tissues.
Further, the amphetamine challenge of the human brain produced several chemicals that require further investigation. In forensic investigations of drug-related fatalities, chemical profiling along with autopsy findings and toxicological analysis can provide conclusive evidence. Hence, more research is necessary to understand the relationship between the concentrations of tested substances and their toxic effects to confirm and expand on these findings.
4. Materials and Methods
4.1. Sample population
The age groups of the amphetamine-related fatal cases were between 21-44 years, and the cause of death was accidental (40 %), gunshot (10 %), traffic accidents (10 %), and suicidal (40 %). The age group of the control samples (not amphetamine fatality cases) was between 20-56 years, and the cause of mortality reported was gunshot (50 %) and undetermined (50 %).
4.2. Study Design
A retrospective design was used to retrieve information on amphetamine fatalities from the Online Toxicology Analysis Requests and Results (OTARR) system between January 2019 and December 2021. OTARR is a computerized toxicological database developed to harmonize labor policies in all Kingdom of Saudi Arabia toxicological institutes. The data is confidential and not available to the public. However, access is through the Ministry of Health electronic portal for health services in Saudi Arabia. All data pertaining to toxicological study results, including the manner of death in fatalities involving amphetamine were obtained from the OTARR system. Using a data collection form we retrieved, analyzed, and quantified fatalities associated with the use of amphetamine.
4.3. Data collection
Relevant information such as the age, substance of abuse (amphetamine) that led to fatality, information about non-amphetamine fatal cases (control), and brain toxicological data was extracted from the database. Amphetamine-related fatalities were classified into three groups depending on amphetamine brain concentration based on a previous investigation [
17], with each group having six cases and the fourth group for non-amphetamine-related fatalities (control) containing six cases. The low concentration category includes instances with less than 0-0.5 g/ml, more than 0.5 g/ml but less than 1.5 g/ml, and more than 1.5 g/ml cases (high group). We quantified the outcomes and chemical profiles in amphetamine-related fatalities. We obtained information on the brain chemical profile of amphetamine-related fatalities while excluding fatalities associated with other drug abuse substances.
4.4. Bioinformatics tools and analysis
The chemical profile was uploaded to the Comparative Toxicogenomics Database (CTD) analysis application and analyzer tools on the CTD website (
http://ctdbase.org/), which identified the chemical disease association. CTD captures curated associations, which are real associations between chemicals and a disease. CTD biocurators mine published literature to extract chemical-disease associations. It reads the peer-reviewed scientific literature and manually curates three core molecular interactions between chemicals, genes, and diseases using official nomenclature, integrating third party-controlled vocabularies for chemicals, genes, diseases and organisms, and a novel controlled vocabulary for molecular interactions. Manual curation yields a strong, densely annotated dataset with extremely accurate and detailed data. Chemical A, for example, is linked to disease B because of a curated interaction with gene C, and gene C is linked to disease B. We identified the chemicals found in all the tested samples using a Venn diagram. Only chemicals that matched and were linked to curated neurodisorders were chosen.
4.5. Statistical analysis
To summarize the data, descriptive statistics were used. The results were presented as means, standard error of the mean (SEM), and median. All the data was explored and calculated using SigmaPlot 11 for Windows. One-way ANOVA and Dunnett's post hoc test were done to compare the groups. Chemical-Diseases Interaction Query (
http://ctdbase.org/tools/) was used to retrieve chemical-disease relationships by selecting 'Chemicals' as input type, inputting a list of chemical terms that are common in all three groups, and selecting 'Enriched Diseases' as output. The hypergeometric distribution is used to calculate the significance of enrichment and adjusted for multiple testing using the Bonferroni method. The default values were utilized for adjusted p-values (threshold 0.01).
5. Conclusions
Amphetamine abuse can produce several chemicals which can have a derogatory impact on brain function. The altered levels of DHA, formation of arachidonic acid and oxidative products suggest that amphetamine abuse can cause neuroinflammation and neurotoxicity. Also, the formation of these products is linked to the development of neurodisorders as evidenced by using the CTD tools. However, although this study provides evidence, we propose to conduct further studies to substantiate the correlation between the chemicals formed and neurodisorders.
Author Contributions
Conceptualization, I.A., D.B. and M.T.; methodology, I.A., M.T. and I.K.; software, I.A. and M.T.; validation, I.A., I.K., and M.T.; formal analysis, M.T. and I.A. ; investiga-tion, S.M., A.A., M.O., I.A. and W.S.; resources, I.K. and S.-A-S.; data curation, I.A., D.B. and M.T.; writing—original draft preparation, D.B., O.B. and I.A.; writing—review and editing, D.B., O.B., I.K., S.A-S. and I.A.; visualization, M.T. and I.A.; supervision, D.B. and I.A.; project administration, D.B. and I.A.; funding acquisition, S.A-Q. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, for funding this research work through the project number – RUP20-01.
Institutional Review Board Statement
The study was approved by the Jazan Health Ethics Committee (No. 2055).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant details are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heo H-J, Park Y-J, Suh Y-M, Choi S-J, Kim M-J, Cho H-Y, et al. Effects of oleamide on choline acetyltransferase and cognitive activities. Bioscience, biotechnology, and biochemistry 2003, 67, 1284–91. [Google Scholar] [CrossRef] [PubMed]
- Gibbs ME, Ng KT. Counteractive effects of norepinephrine and amphetamine on ouabain-induced amnesia. Pharmacology Biochemistry and Behavior 1977, 6, 533–7. [Google Scholar] [CrossRef] [PubMed]
- Alldredge BK, Lowenstein DH, Simon RP. Seizures associated with recreational drug abuse. Neurology 1989, 39, 1037. [Google Scholar] [CrossRef] [PubMed]
- Wu C-F, Li C-L, Song H-R, Zhang H-F, Yang J-Y, Wang Y-L. Selective effect of oleamide, an endogenous sleepinducing lipid amide, on pentylenetetrazole-induced seizures in mice. Journal of pharmacy and pharmacology 2003, 55, 1159–62. [Google Scholar]
- Bhattacharjee AK, Chang L, Chen M, White L, Bell JM, Bazinet RP, et al. Chronic d-amphetamine depresses an imaging marker of arachidonic acid metabolism in rat brain. International Journal of Neuropsychopharmacology 2008, 11, 957–69. [Google Scholar] [CrossRef] [PubMed]
- Ramkissoon A, Wells PG. Human prostaglandin H synthase (hPHS)-1 and hPHS-2 in amphetamine analog bioactivation, DNA oxidation, and cytotoxicity. Toxicological Sciences 2011, 120, 154–62. [Google Scholar] [CrossRef] [PubMed]
- McNamara, RK. Role of omega-3 fatty acids in the etiology, treatment, and prevention of depression: current status and future directions. Journal of nutrition & intermediary metabolism 2016, 5, 96–106. [Google Scholar]
- Yavin, E. Versatile roles of docosahexaenoic acid in the prenatal brain: from pro-and anti-oxidant features to regulation of gene expression. Prostaglandins, leukotrienes and essential fatty acids 2006, 75, 203–11. [Google Scholar] [CrossRef] [PubMed]
- Jackson PA, Reay JL, Scholey AB, Kennedy DO. DHA-rich oil modulates the cerebral haemodynamic response to cognitive tasks in healthy young adults: a near IR spectroscopy pilot study. British journal of nutrition 2012, 107, 1093–8. [Google Scholar] [CrossRef] [PubMed]
- Jackson PA, Forster JS, Bell JG, Dick JR, Younger I, Kennedy DO. DHA supplementation alone or in combination with other nutrients does not modulate cerebral hemodynamics or cognitive function in healthy older adults. Nutrients 2016, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Yassine HN, Braskie MN, Mack WJ, Castor KJ, Fonteh AN, Schneider LS, et al. Association of docosahexaenoic acid supplementation with Alzheimer disease stage in apolipoprotein E ε4 carriers: a review. JAMA neurology 2017, 74, 339–47. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H. Effects of N-3 polyunsaturated fatty acids on dementia. Journal of clinical medicine research 2017, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- McNamara RK, Hahn C-G, Jandacek R, Rider T, Tso P, Stanford KE, et al. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biological psychiatry 2007, 62, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Levant B, Radel JD, Carlson SE. Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behavioural brain research 2004, 152, 49–57. [Google Scholar]
- Bozzatello P, Brignolo E, De Grandi E, Bellino S. Supplementation with omega-3 fatty acids in psychiatric disorders: a review of literature data. Journal of clinical medicine 2016, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Choi SJ, Kim JK, Kim HK, Harris K, Kim C-J, Park GG, et al. 2, 4-Di-tert-butylphenol from sweet potato protects against oxidative stress in PC12 cells and in mice. Journal of medicinal food 2013, 16, 977–83. [Google Scholar] [CrossRef] [PubMed]
- Attafi IM, Tumayhi MM, Banji D, Albeishy MY, Khardali IA, Korashy HM. Analysis of fatalities involving amphetamine in Jazan, Saudi Arabia. Forensic Science International: Reports 2021, 4, 100237. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).