Submitted:
04 May 2023
Posted:
05 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
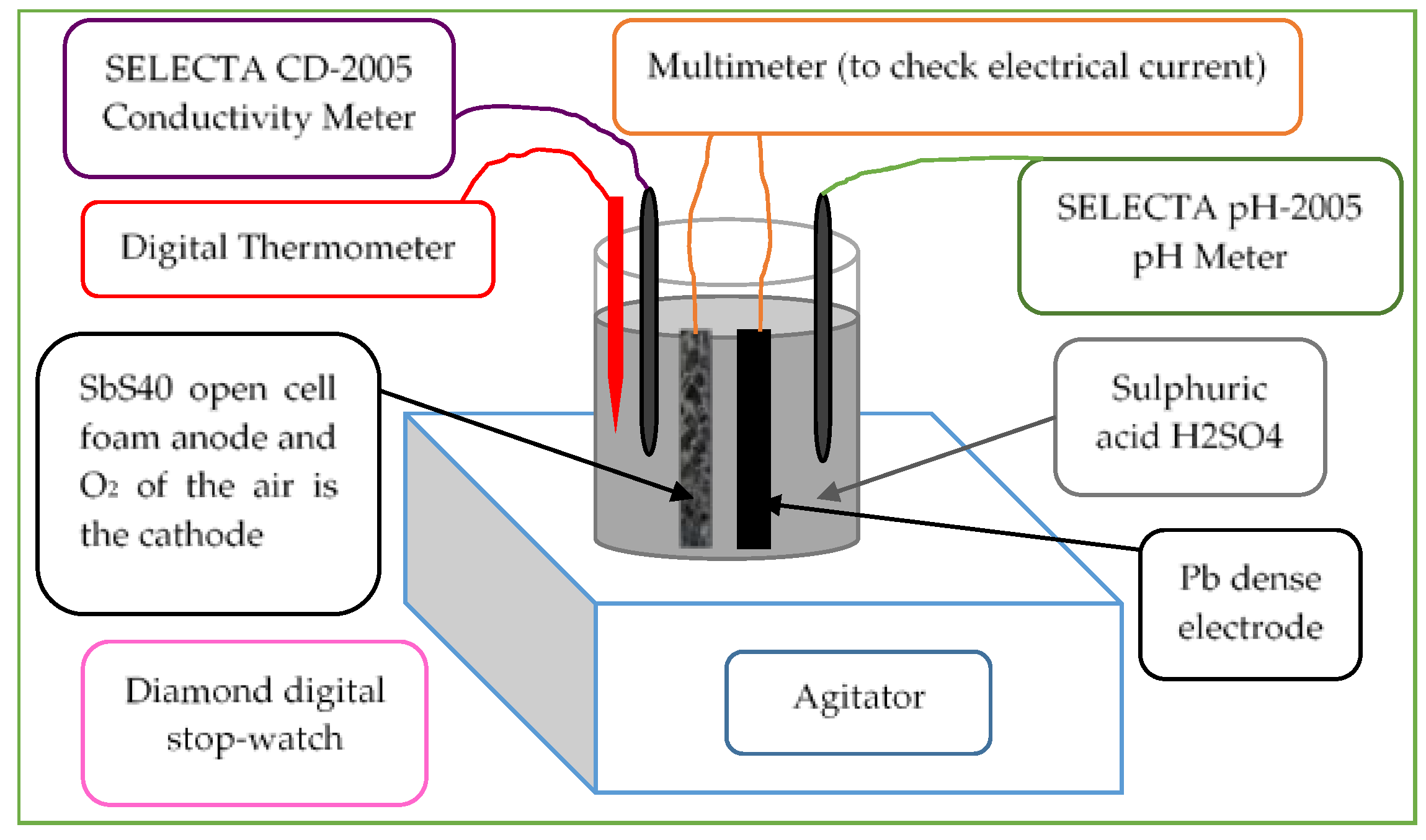
- The ESR foam electrodes were carefully cut into regular stick shapes before the salt removal (Figure 1).
- Density of the dried foams was measured by Archimedes’ principle, then porosity P was calculated. [42]
- Before and after each test, the ESR foam anode and the dense Pb were weighed.
- Stirring was carried out by the laboratory stirrer (Figure 1) for a good distribution of the species existing during the chemical reactions and for better dissolution of oxygen from the air in the electrolyte.
- The last test used the same 25% Sb-Pb dense alloy (non-porous material) as anode for comparison.
- A SELECTA CD-2005 Conductivity Meter (Figure 1) for electrolyte conductivity measurement and SELECTA pH-2005 pH Meter (Figure 1) electrochemical ex perimental apparatus were used simultaneously to meas ure the electrolyte electrical conductivity Km and pH every 1 minute for 30 minutes, timed by Diamond digital stopwatch (Figure 1).
- The electrical current was measured once the electrodes were immersed in an agitated sulphuric acid H2SO4, with an average pH between 1.11 and 1.28 (Figure 1).
- The entire sample had to be immersed in the sulphuric acid electrolyte to ensure that the properties studied were representative of the totality of the sample's interactions with the electrolyte.
- The tem perature of the 100 ml of sulphuric acid was also measured with a digital thermometer (Figure 1) during every manipulation (T0 before test and Tf at the end of the test) to explore the thermal stability of the cell.
- Note that the battery cells were named “SbSPb X”, where SbS stands for the anode foams obtained from the ESR process (see [42]); Pb was the non-porous dense lead electrode used for electrical circuit closure. A multimeter is used to check electrical current flow too and it will be shown later that this electrode does not contribute to the electrochemical reactions of the cell. X repre sents the value of the decimal salt grain diameter. Table 1 regroups the measured and calculated parameters of all tested samples.
3. Results and discussions
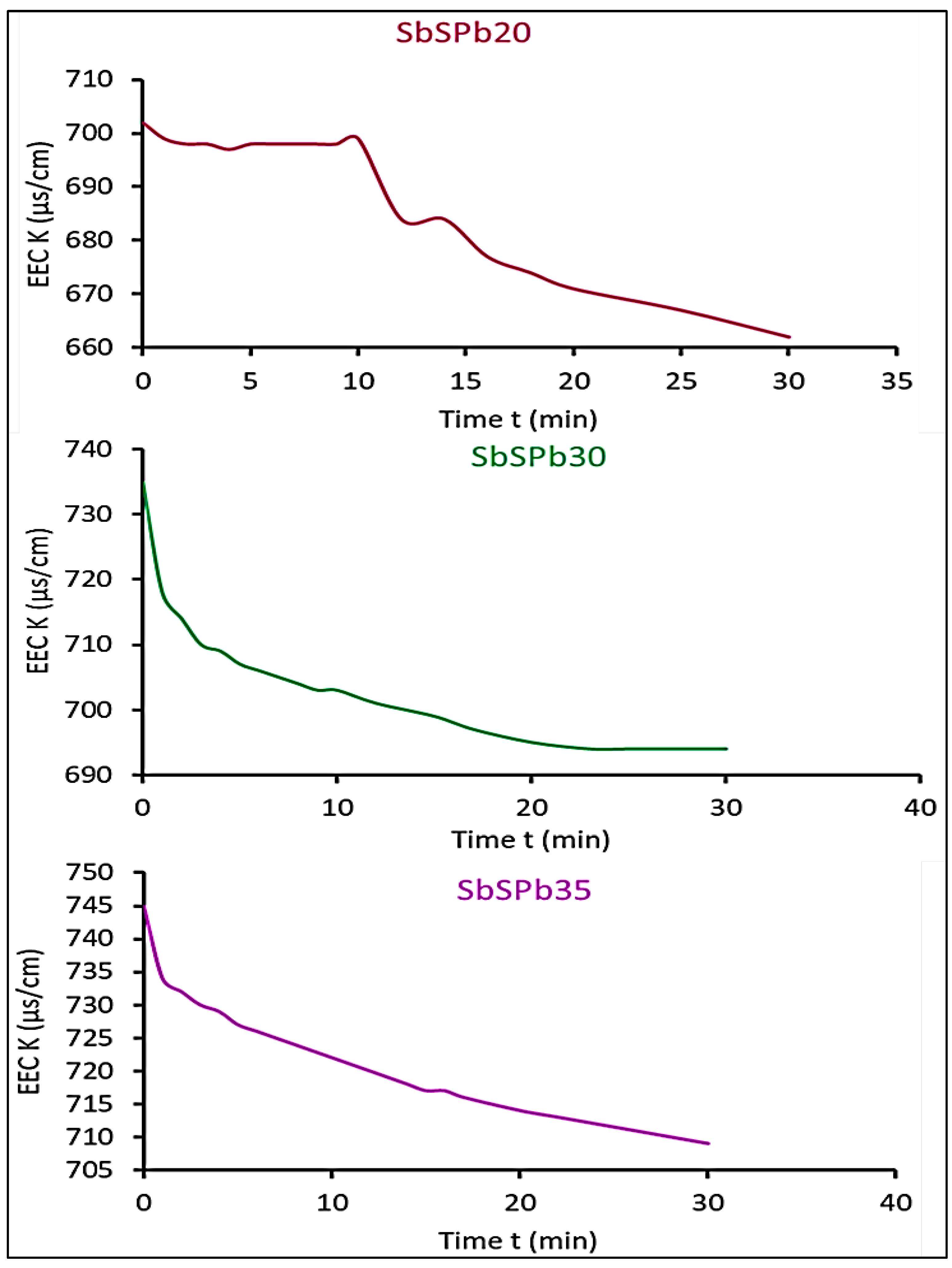
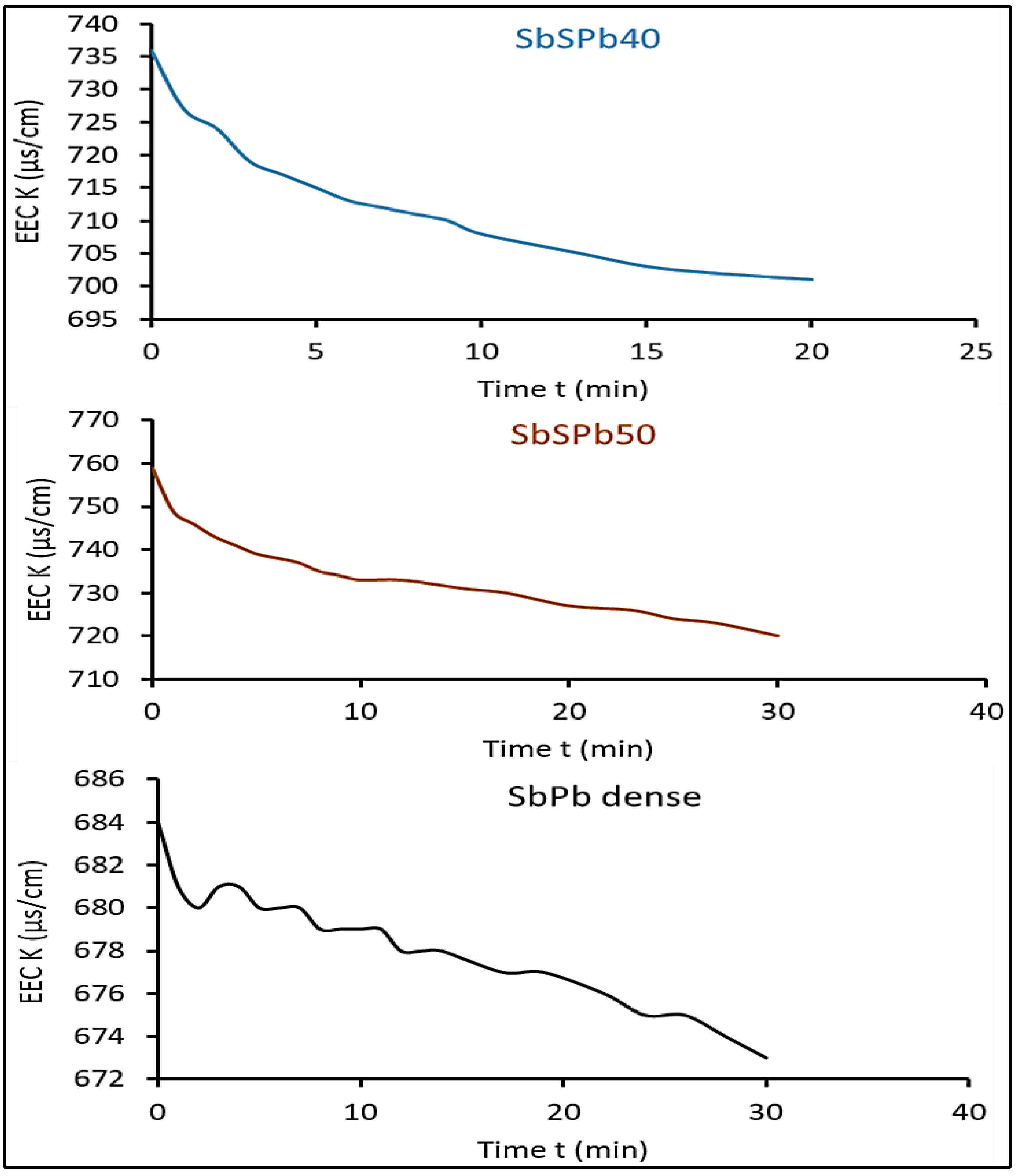
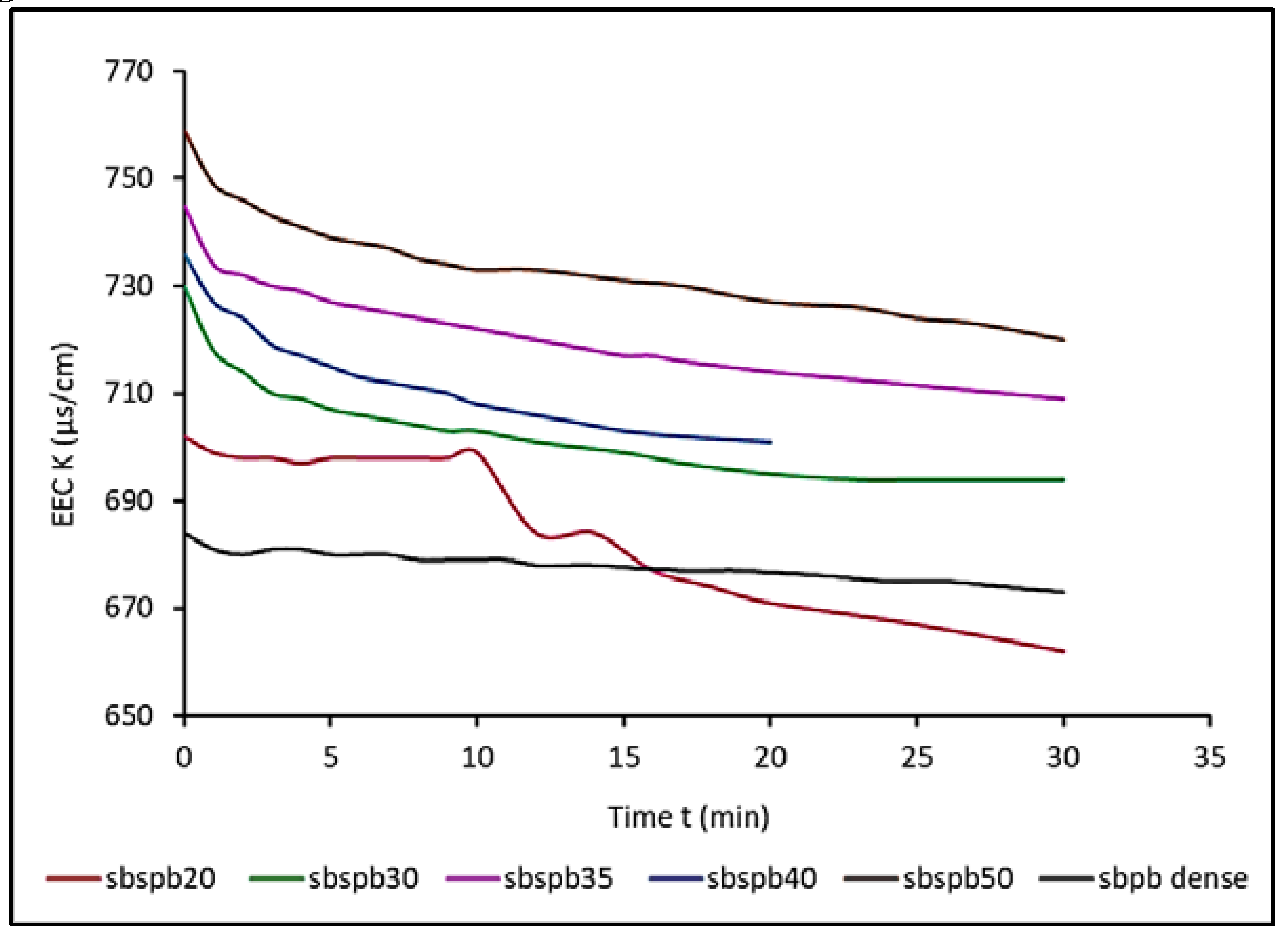
3.1. Interpretation of measured effective electrical conductivity results
3.3. Advantages of ESR foams and electrodes reactions
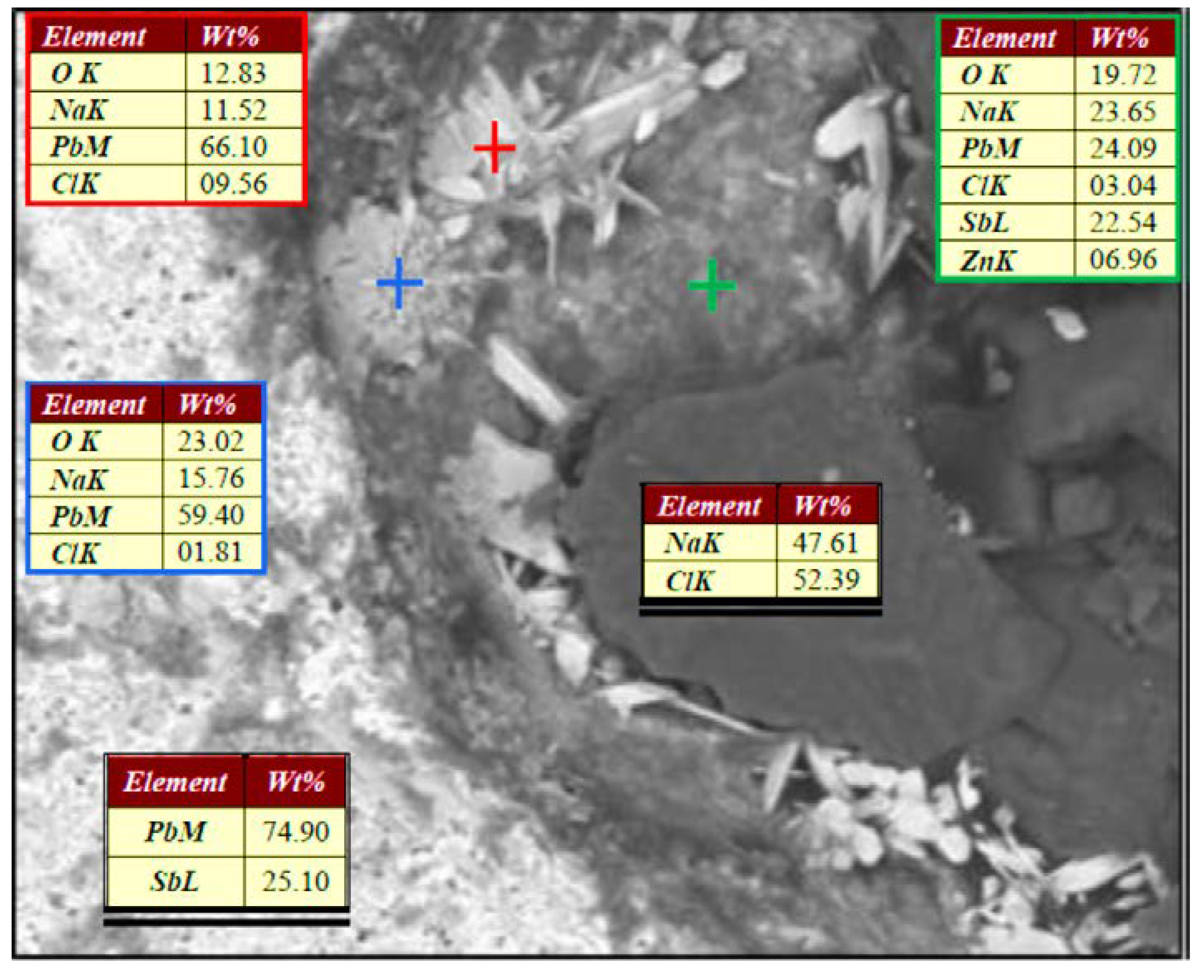
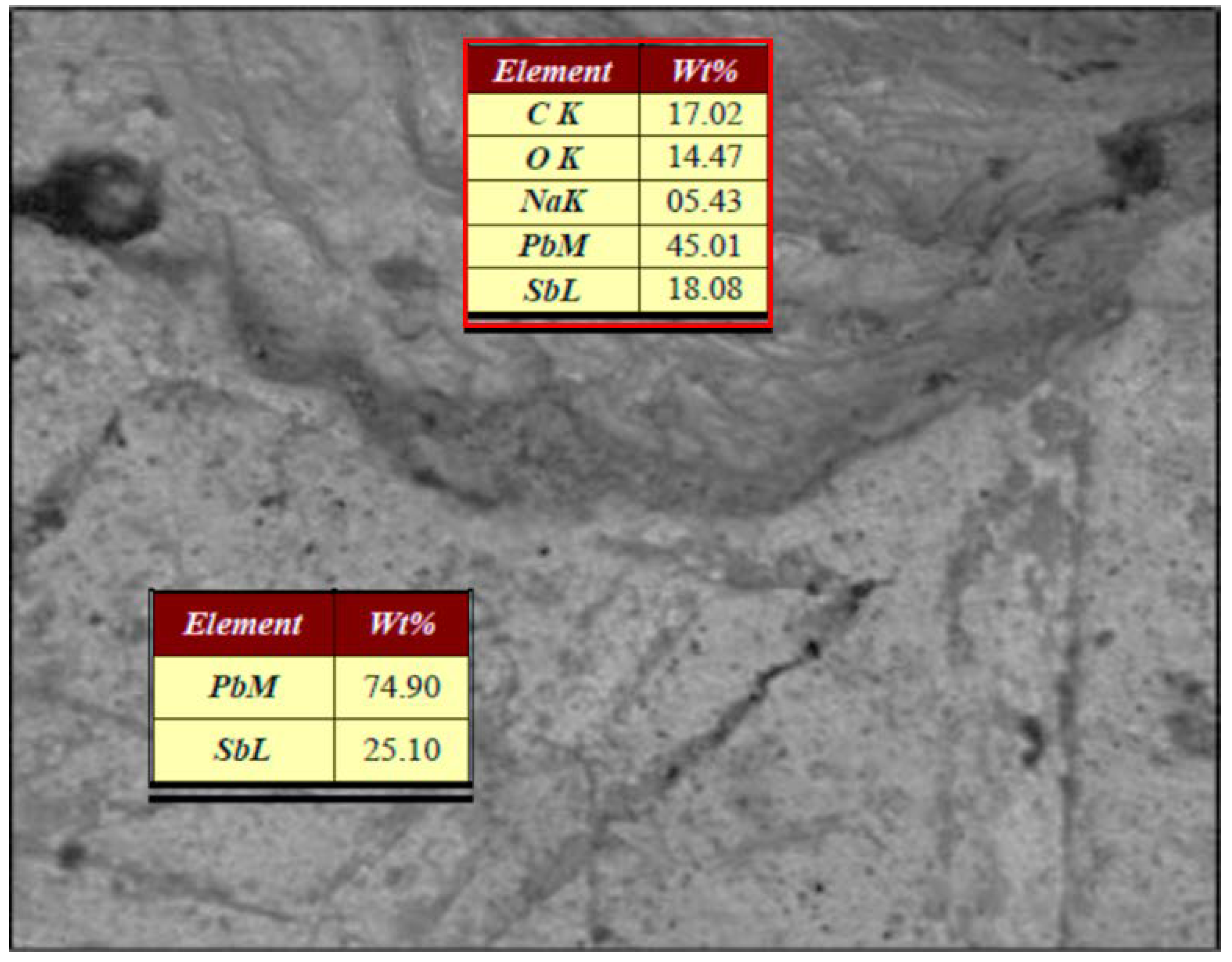
3.4. Identification of electrodes chemical reactivity
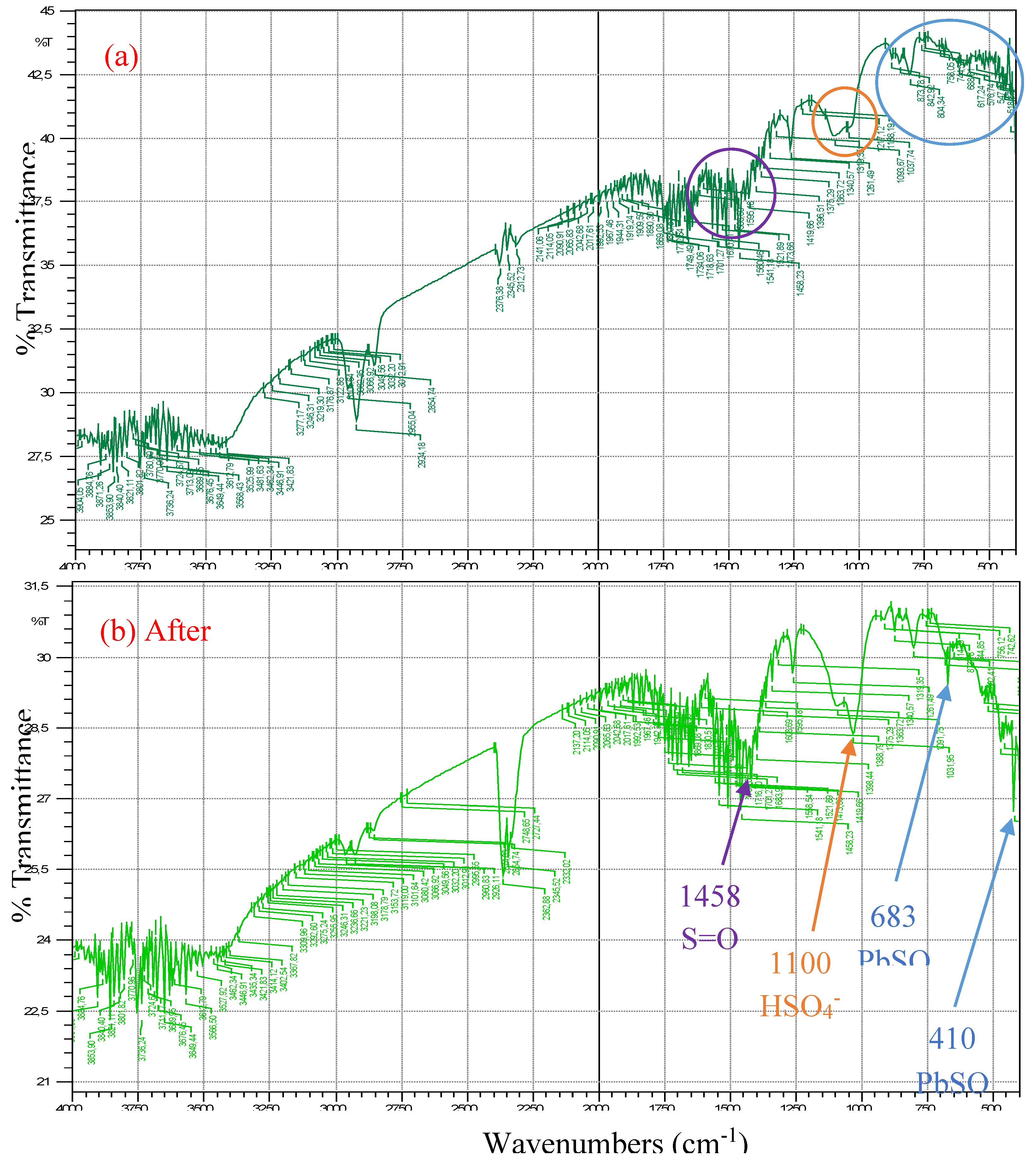
3.5. FTIR of SbPb dense electrode
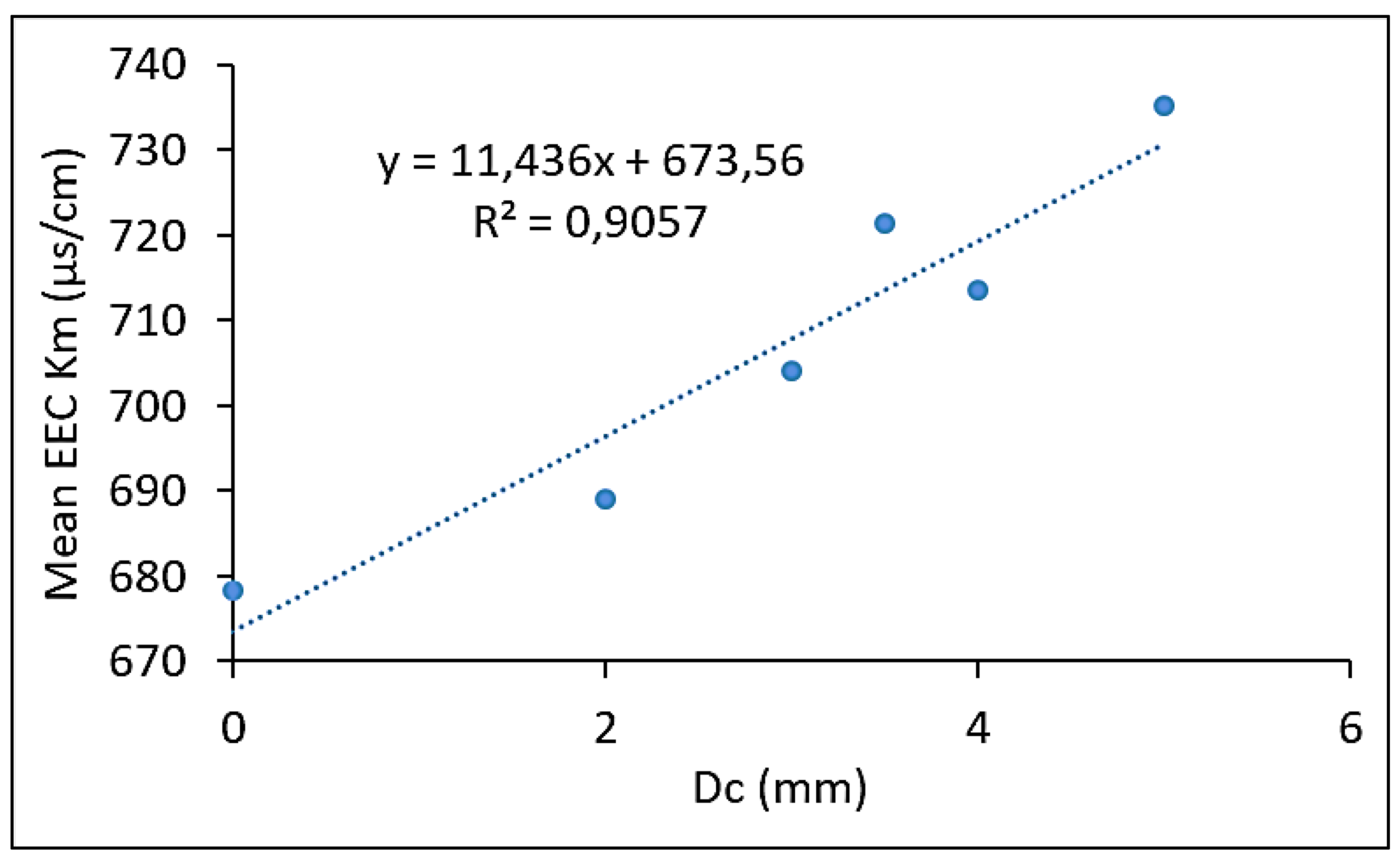
3.6. Effect of cell diameter of ESR foam anode on the measured effective electrical conductivity
4. Conclusion
References
- Hossain, M.D.; Islam, M.M.; Hossain, M.J.; Yasmin, S.; Shingho, S.R.; Ananna, N.A.; Mustafa, C.M. Effects of additives on the morphology and stability of PbO2 films electrodeposited on nickel substrate for light weight lead-acid battery application. Journal of Energy Storage 2020, 27, 101108. [Google Scholar] [CrossRef]
- Kaneko, K.; Hori, K.; Noda, S. Nanotubes make battery lighter and safer. Carbon 2020, 167, 596–600. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chemical Society Reviews 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Dunlop, J.; Beauchamp, R. Making space nickel/hydrogen batteries lighter and less expensive. NASA STI/Recon Technical Report N 1987, 88, 13530. [Google Scholar]
- Shaffer, B.; Auffhammer, M.; Samaras, C. Make electric vehicles lighter to maximize climate and safety benefits. 2021.
- Nyholm, L. Lighter and safer. Nature Energy 2020, 5, 739–740. [Google Scholar] [CrossRef]
- Landi, B.J.; Ganter, M.J.; Cress, C.D.; DiLeo, R.A.; Raffaelle, R.P. Carbon nanotubes for lithium ion batteries. Energy & Environmental Science 2009, 2, 638–654. [Google Scholar]
- Clemente, A.; Costa-Castelló, R. Redox flow batteries: A literature review oriented to automatic control. Energies 2020, 13, 4514. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T. Lead acid batteries. Handbook of batteries 2002, 1–88. [Google Scholar]
- Logeshkumar, S.; Manoharan, R. Influence of some nanostructured materials additives on the performance of lead acid battery negative electrodes. Electrochimica Acta 2014, 144, 147–153. [Google Scholar] [CrossRef]
- Hao, H.; Chen, K.; Liu, H.; Wang, H.; Liu, J.; Yang, K.; Yan, H. A review of the positive electrode additives in lead-acid batteries. Int. J. Electrochem. Sci 2018, 13, 2329–2340. [Google Scholar] [CrossRef]
- Pletcher, D.; Wills, R. A novel flow battery—A lead acid battery based on an electrolyte with soluble lead (II): III. The influence of conditions on battery performance. Journal of Power Sources 2005, 149, 96–102. [Google Scholar] [CrossRef]
- Jullian, E.; Albert, L.; Caillerie, J. New lead alloys for high-performance lead–acid batteries. Journal of power sources 2003, 116, 185–192. [Google Scholar] [CrossRef]
- Osório, W.R.; Rosa, D.M.; Garcia, A. The roles of cellular and dendritic microstructural morphologies on the corrosion resistance of Pb–Sb alloys for lead acid battery grids. Journal of power sources 2008, 175, 595–603. [Google Scholar] [CrossRef]
- Li, A.; Cheni, Y.; Chen, H.; Shu, D.; Li, W.; Wang, H.; Dou, C.; Zhang, W.; Chen, S. Electrochemical behavior and application of lead–lanthanum alloys for positive grids of lead-acid batteries. Journal of power sources 2009, 189, 1204–1211. [Google Scholar] [CrossRef]
- Abdelghani-Idrissi, S. La charge rapide d'une batterie métal-air par la maîtrise de la fluidique diphasique. Université Paris sciences et lettres, 2020.
- Hariprakash, B.; Mane, A.; Martha, S.; Gaffoor, S.; Shivashankar, S.; Shukla, A. A low-cost, high energy-density lead/acid battery. Electrochemical and Solid-State Letters 2004, 7, A66. [Google Scholar] [CrossRef]
- Tabaatabaai, S.; Rahmanifar, M.; Mousavi, S.; Shekofteh, S.; Khonsari, J.; Oweisi, A.; Hejabi, M.; Tabrizi, H.; Shirzadi, S.; Cheraghi, B. Lead-acid batteries with foam grids. Journal of power sources 2006, 158, 879–884. [Google Scholar] [CrossRef]
- Bullock, K.R. Lead/acid batteries. Journal of power sources 1994, 51, 1–17. [Google Scholar] [CrossRef]
- Martha, S.; Hariprakash, B.; Gaffoor, S.; Trivedi, D.; Shukla, A. A low-cost lead-acid battery with high specific-energy. Journal of Chemical Sciences 2006, 118, 93–98. [Google Scholar] [CrossRef]
- Albert, L.; Chabrol, A.; Torcheux, L.; Steyer, P.; Hilger, J. Improved lead alloys for lead/acid positive grids in electric-vehicle applications. Journal of power sources 1997, 67, 257–265. [Google Scholar] [CrossRef]
- Hariprakash, B.; Gaffoor, S. Lead-acid cells with lightweight, corrosion-protected, flexible-graphite grids. Journal of power sources 2007, 173, 565–569. [Google Scholar] [CrossRef]
- LaFollette, R.M. Design and performance of high specific power, pulsed discharge, bipolar lead acid batteries. In Proceedings of the Proceedings of the Tenth Annual Battery Conference on Applications and Advances, 1995; pp. 43–47.
- Lach, J.; Wróbel, K.; Wróbel, J.; Podsadni, P.; Czerwiński, A. Applications of carbon in lead-acid batteries: a review. Journal of Solid State Electrochemistry 2019, 23, 693–705. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, Z.; Wang, E.; An, L.; Sun, G. Aqueous metal-air batteries: Fundamentals and applications. Energy Storage Materials 2020, 27, 478–505. [Google Scholar] [CrossRef]
- Olabi, A.G.; Sayed, E.T.; Wilberforce, T.; Jamal, A.; Alami, A.H.; Elsaid, K.; Rahman, S.M.A.; Shah, S.K.; Abdelkareem, M.A. Metal-air batteries—a review. Energies 2021, 14, 7373. [Google Scholar] [CrossRef]
- Das, S.K.; Lau, S.; Archer, L.A. Sodium–oxygen batteries: a new class of metal–air batteries. Journal of Materials Chemistry A 2014, 2, 12623–12629. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C. High energy density metal-air batteries: a review. Journal of The Electrochemical Society 2013, 160, A1759. [Google Scholar] [CrossRef]
- Han, X.; Li, X.; White, J.; Zhong, C.; Deng, Y.; Hu, W.; Ma, T. Metal–air batteries: from static to flow system. Advanced Energy Materials 2018, 8, 1801396. [Google Scholar] [CrossRef]
- Milusheva, Y.; Popov, I.; Shirov, B.; Banov, K.; Boukoureshtlieva, R.; Obretenov, W.; Naidenov, V. Lead-Air Electrochemical System with Acid Electrolyte. Journal of The Electrochemical Society 2021, 168, 060524. [Google Scholar] [CrossRef]
- Olabi, A.G.; Sayed, E.T.; Wilberforce, T.; Jamal, A.; Alami, A.H.; Elsaid, K.; Rahman, S.M.A.; Shah, S.K.; Abdelkareem, M.A.J.E. Metal-air batteries—a review. 2021, 14, 7373.
- Pei, P.; Wang, K.; Ma, Z. Technologies for extending zinc–air battery’s cyclelife: A review. Applied Energy 2014, 128, 315–324. [Google Scholar] [CrossRef]
- Akhtar, N.; Akhtar, W.J.I.J.o.E.R. Prospects, challenges, and latest developments in lithium–air batteries. 2015, 39, 303–316.
- Chang, S.; Hou, M.; Xu, B.; Liang, F.; Qiu, X.; Yao, Y.; Qu, T.; Ma, W.; Yang, B.; Dai, Y.J.A.F.M. High-performance quasi-solid-state Na-air battery via gel cathode by confining moisture. 2021, 31, 2011151.
- Farsak, M.; Kardaş, G. 2.12 Electrolytic Materials. 2018.
- Zhao, Y.; Huang, G.; Zhang, C.; Peng, C.; Pan, F. Effect of phosphate and vanadate as electrolyte additives on the performance of Mg-air batteries. Materials Chemistry and Physics 2018, 218, 256–261. [Google Scholar] [CrossRef]
- Sumathi, S.; Sethuprakash, V.; Basirun, W.; Zainol, I.; Sookhakian, M.J.J.o.s.-g.s. ; technology. Polyacrylamide-methanesulfonic acid gel polymer electrolytes for tin-air battery. 2014, 69, 480–487. [Google Scholar]
- Ashby, M.F.; Evans, T.; Fleck, N.; Hutchinson, J.W.; Wadley, H.N.G.; Gibson, L.J. Metal Foams: A Design Guide; Elsevier Science: 2000.
- Song, M.J.; Kim, I.T.; Kim, Y.B.; Shin, M.W. Self-standing, binder-free electrospun Co3O4/carbon nanofiber composites for non-aqueous Li-air batteries. Electrochimica Acta 2015, 182, 289–296. [Google Scholar] [CrossRef]
- Zhu, G.; Li, X.; Liu, Y.; Mao, Y.; Liang, Z.; Ji, Z.; Shen, X.; Sun, J.; Cheng, X.; Mao, J. Scalable surface engineering of commercial metal foams for defect-rich hydroxides towards improved oxygen evolution. Journal of Materials Chemistry A 2020, 8, 12603–12612. [Google Scholar] [CrossRef]
- Wang, H.-F.; Xu, Q. Materials Design for Rechargeable Metal-Air Batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Hassein-Bey, A.H.; Belhadj, A.-E.; Gavrus, A.; Abudura, S. Elaboration and Mechanical-Electrochemical Characterisation of Open Cell Antimonial-lead Foams Made by the “Excess Salt Replication Method” for Eventual Applications in Lead-acid Batteries Manufacturing. Kemija u industriji: Časopis kemičara i kemijskih inženjera Hrvatske 2020, 69, 387–398. [Google Scholar] [CrossRef]
- Prengaman, R.D. SECONDARY BATTERIES – LEAD– ACID SYSTEMS | Lead Alloys. In Encyclopedia of Electrochemical Power Sources, Garche, J., Ed.; Elsevier: Amsterdam, 2009; pp. 648–654. [Google Scholar]
- Hill, R.J. Structure of PbSb2O6 and its relationship to the crystal chemistry of PbO2 in antimonial lead-acid batteries. Journal of Solid State Chemistry 1987, 71, 12–18. [Google Scholar] [CrossRef]
- Dharmasena, K.; Wadley, H. Electrical conductivity of open-cell metal foams. Journal of materials research 2002, 17, 625–631. [Google Scholar] [CrossRef]
- Ashby, M.F.; Evans, A.G.; Fleck, N.A.; Gibson, L.J.; Hutchinson, J.W.; Wadley, H.N.G. Metal Foams: A Design Guide; Butterworth-Heinemann: 2000; p. 263.
- Liu, P.; Li, T.; Fu, C. Relationship between electrical resistivity and porosity for porous metals. Materials Science and Engineering: A 1999, 268, 208–215. [Google Scholar] [CrossRef]
- Majima, H.; Peters, E.; Awakura, Y.; Park, S.K. Electrical conductivity of acidic sulfate solution. Metallurgical Transactions B 1987, 18, 41–47. [Google Scholar] [CrossRef]
- Biagetti, R. Fabrication of lead-acid batteries. 1973.
- Newman, J.; Tiedemann, W. Porous-electrode theory with battery applications. AIChE Journal 1975, 21, 25–41. [Google Scholar] [CrossRef]
- Mai, T.T.T.; Phan, T.B.; Pham, T.T.; Vu, H.H. Nanostructured PbO2-PANi composite materials for electrocatalytic oxidation of methanol in acidic sulfuric medium. Advances in Natural Sciences: Nanoscience and Nanotechnology 2014, 5, 025004. [Google Scholar]
- Ma, X.; Peyton, A.; Zhao, Y. Measurement of the electrical conductivity of open-celled aluminium foam using non-contact eddy current techniques. NDT & E International 2005, 38, 359–367. [Google Scholar]
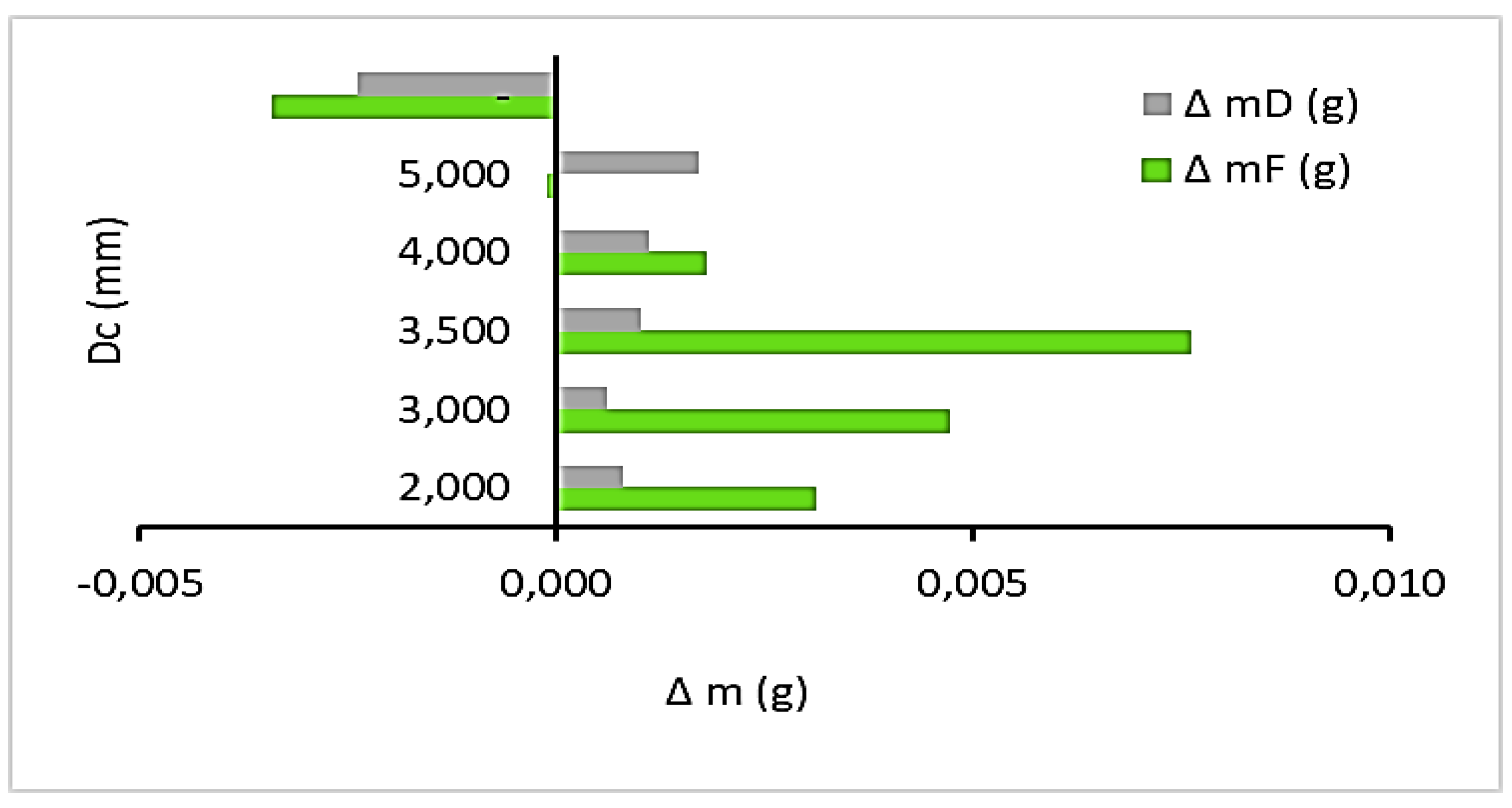
| mF0 (g) | mD0 (g) | mFf (g) | mDf (g) | Δ mF (g) | ΔmD (g) | Dc (mm) | P (%) | T0-Tf (°C) | Km (µS/cm) | pH0-pHf | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sbspb20 | 5,780 | 3,696 | 5,777 | 3,695 | 0,003 | 0,001 | 2,000 | 46 | 19,6-19,7 | 689,00 | 1,16-1,18 | ||
| sbspb30 | 2,350 | 3,664 | 2,345 | 3,663 | 0,005 | 0,001 | 3,000 | 66 | 17,9-17,5 | 704,05 | 1,25-1,29 | ||
| sbspb35 | 6,311 | 3,374 | 6,303 | 3,373 | 0,008 | 0,001 | 3,500 | 48 | 19,7-19,9 | 721,42 | 1,11-1,13 | ||
| sbspb40 | 8,068 | 3,752 | 8,066 | 3,750 | 0,002 | 0,001 | 4,000 | 56 | 17,8-17,7 | 713,53 | 1,13-1,11 | ||
| sbspb50 | 3,458 | 3,734 | 3,458 | 3,732 | 0,000 | 0,002 | 5,000 | 60 | 17-16,7 | 735,16 | 1,25-1,26 | ||
| sbpb dense | 3,695 | 3,869 | 3,698 | 3,871 | -0,003 | -0,002 | - | 0 | 17,3 | 678,36 | 1,15-1,17 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





