1. Introduction
Glaucoma comprises a group of eye diseases which can lead to irreversible blindness [
1]. Elevated intraocular pressure (IOP) is the primary risk factor for glaucoma and lowering IOP is the only therapy available to slow disease progression [
2]. However, the reduction of IOP, albeit important, is not sufficient to effectively and completely counteract the retinal and optic nerve progressive degeneration observed in these patients [
1]. Among others, ischemia due to vasospastic and/or occlusive ocular circulatory disturbances followed by tissue reperfusion are regarded as important events in the progression of the glaucomatous neuropathy [
3]. Ischemia/reperfusion leads to the production of reactive oxygen species (ROS) and several evidence suggest that ROS play a major role in the genesis of post-ischemic retinopathy as well as of glaucoma [
3,
4]. Indeed, increased levels of oxidative stress markers such as superoxide dismutase were recently shown to positively correlate with the onset and progression of glaucoma [
5].
The role of inflammation in glaucoma has been poorly investigated. Cytokines and chemokines are proteins secreted by immune cells and exert a central role in the progression of glaucoma and ischemic insult [
6,
7]. Among others, interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6) act as primary initiators of inflammation following infection or tissue damage in the eye as in other organs.
Prostaglandin F
2 alpha receptor (FP) agonists, including latanoprost, bimatoprost, travoprost, tafluprost and unoprostone isopropyl are potent and effective Food and Drug Administration (FDA)-approved IOP-lowering prodrugs for the treatment of ocular hypertension and glaucoma [
8]. These drugs mostly act
via an increase of aqueous humor (AH) drainage from the uveoscleral pathway upon the release of the respective free acids. However, as with other commonly used IOP-lowering agents, despite their satisfactory IOP control and safety profile, these compounds seem to only partially affect ocular blood flow with tafluprost being the most effective one [
9]. Furthermore, FP agonists seem to lack any direct meaningful activity on the inflammatory component sometime observed in glaucomatous eyes.
On the other hand, nitric oxide (NO) and its intracellular second messenger cyclic guanosine monophosphate (cGMP), lowers IOP mainly
via an increase of AH drainage from the Trabecular meshwork/Schlemm’s canal (TM/SC) pathway (conventional outflow facility) [
10]. Moreover, it has been shown that, independently from its effect on IOP, physiologic concentrations of NO may protect retinal cells from irreversible functional damage [
11]. In a rat model of retinal ischemia, endothelial NO synthase (eNOS) mRNA decreases and treatment with the NO precursor, L-arginine, increases the recovery of retinal function [
12] thereby demonstrating that NO plays an important role in protecting the retina from ischemic damage. Likewise, low nanomolar concentration of NO has also been shown to inhibit pro-inflammatory cytokines [
7] while NO depletion increases ROS generation and pro-inflammatory biomarkers [
13].
Recently, an entirely new class of drugs has been proposed for the treatment of ocular hypertension and glaucoma, namely: NO-donating FP agonists including, Vyzulta® (latanoprostene bunod 0.024% ophthalmic solution) that is marketed in many countries, including the USA, and NCX 470 (a novel dual acting NO-donating bimatoprost – 0.1% ophthalmic solution) that recently demonstrated ‘non-inferiority’ against latanoprost 0.005% ophthalmic solution [
https://clinicaltrials.gov/ct2/show/NCT04445519] in a pivotal phase 3 clinical trial at lowering IOP. These latter compounds act by concomitantly activating two independent mechanisms: a) FP receptor-mediated increase in uveoscleral outflow and, b) cGMP-dependent activation of TM/SC conventional outflow facility [
10]. Interestingly, these dual acting agents have also been shown to affect ocular blood flow in patients [
14,
15] as well as in a model of endothelin-1 (ET-1)-induced ischemia/reperfusion in New Zealand white (NZW) rabbits where repeated ocular dosing of NCX 470 reversed the ocular hemodynamic changes as well as the impairments in retinal function observed after ET-1 administration [
16].
Here we used the same ET-1-induced ischemia/reperfusion model to compare the effect of NCX 470 to that of bimatoprost administered as the FDA-approved drug (Lumigan®, 0.01% bimatoprost ophthalmic solution) or at a dose (0.072%) equimolar to that released by 0.1% NCX 470. Furthermore, in an attempt to understand the cellular and molecular mechanism/s involved, we investigated changes in various biochemical markers potentially affecting retinal cell physiology.
2. Results
2.1. Intraocular pressure (IOP) and Ophthalmic Artery Resistive Index (OA-RI) changes after repeated Vehicle (VEH), NCX 470, Lumigan® (LUM) or equiM bimatoprost (BIM) dosing in endothelin-1 (ET-1)-treated rabbits
Baseline IOP before ET-1 injection did not differ significantly between eyes later randomized for the different groups [20.5±0.8, 20.7±0.7, 21.5±0.5 and 19.5±1.1 mmHg for eyes later randomized for vehicle (VEH), NCX 470 (0.1%), Lumigan® (bimatoprost 0.01% ophthalmic solution, LUM) and bimatoprost administered at equimolar dose (0.072%, BIM) as released by 0.1% NCX 470, respectively] (
Table 1). Twice weekly treatments with ET-1 for 6 weeks increased average IOP to 24.8±0.3 mmHg. Conversely, in eyes treated with ET-1 and NCX 470 (30 µL/eye, bid, starting from week 3), IOP did not change significantly (18.1±0.6 mmHg at week 6) over the course of treatment remaining comparable to that registered prior to ET-1 treatment (non statistically significant vs respective baseline) (
Table 1). Treatment with LUM and BIM only provided partial reversal of ET-1 induced IOP changes (
Table 1).
Ophthalmic Artery Resistive Index (OA-RI) was calculated as previously reported [
16]. OA-RI values did not differ significantly under baseline conditions for eyes later randomized for VEH, NCX 470, LUM and BIM. Following 6 weeks of ET-1 (twice/week, 200 µL of 250 nM) injections, OA-RI increased significantly from 0.36±0.02 to 0.55±0.01 (
Table 2). Conversely, in eyes treated with NCX 470 OA-RI did not increase (0.34±0.02 and 0.33±0.01, at baseline and week 6, respectively) (
Table 2). LUM and BIM only partially reversed OA-RI changes elicited by ET-1 (
Table 2).
2.2. Electroretinogram (ERG) changes after repeated Vehicle (VEH), NCX 470, Lumigan® (LUM) or equiM bimatoprost (BIM) dosing in endothelin-1 (ET-1)-treated rabbits
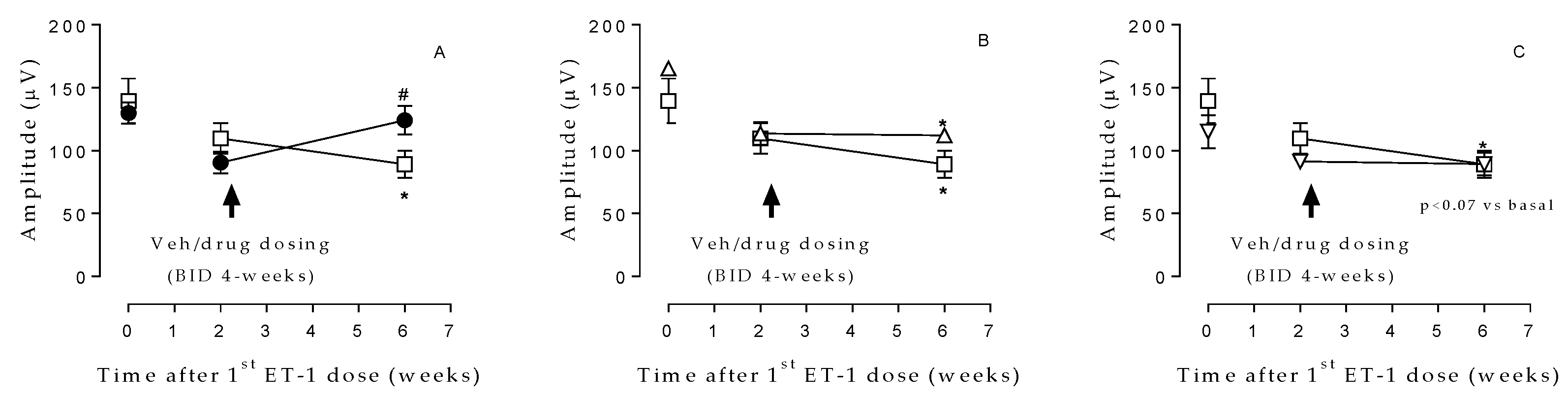
ET-1 treatment resulted in a marked decline in cones and rods function (as determined by ERG assessments) after 2 weeks of ET-1 injection and continued to decrease during the following weeks in the VEH group [139.5±17.8 and 89.1±10.7µV at time 0 (baseline) and week 6, respectively] (
Figure 1). Eyes treated with NCX 470 (0.1%, 30 µL/eye bid) exhibited significantly less impairment [129.8±8.7 and 124.1±11.3 µV, at time 0 (baseline) and week 6, respectively] in ERG wave amplitude at week 6 than those treated with vehicle (
Figure 1A). Interestingly, these effects were not evident in eyes treated (30 µL/eye bid) with LUM (
Figure 1B) or BIM (
Figure 1C).
2.3. Glutathione (GSH) and 8-Hydroxy-2-deoxyguanosine (8-OH2dG) levels in retina after repeated Vehicle (VEH), NCX 470, Lumigan® (LUM) or equiM bimatoprost (BIM) dosing in endothelin-1 (ET-1)-treated rabbits
When compared to naïve conditions, ET-1 treatment over 6 weeks in eyes also receiving 4 weeks of VEH treatment led to a significant decrease in retina glutathione (GSH) content (1.30±0.04 and 0.37±0.04 μmoL/mg protein in naïve conditions and VEH at week 6, respectively). Interestingly, in the eyes treated with NCX 470 (0.1%, 30 µL/eye bid), the levels of GSH were similar to those of naïve animals and significantly increased compared to VEH-treated eyes (1.31±0.25 μmoL/mg protein) (
Table 3).
The levels of 8-Hydroxy-2-deoxyguanosine (8-OH2dG), a reliable marker of DNA oxidative stress, were significantly increased in retina of VEH-treated eyes vs naïve, from 18.9±0.7 pg/µg of DNA to 57.5±6.3 pg/µg of DNA. In contrast, 8-OH2dG content was significantly reduced in eyes treated with NCX 470 (34.1±5.0 pg/µg of DNA), (
Table 3). LUM and BIM were also effective on these parameters (
Table 3).
2.4. Interleukin-1ß (IL-1ß), Tumor Necrosis Factor alpha (TNF-α) and Interleukin-6 (IL-6) levels in retina after repeated Vehicle (VEH), NCX 470, Lumigan® (LUM) or equiM bimatoprost (BIM) dosing in endothelin-1 (ET-1)-treated rabbits
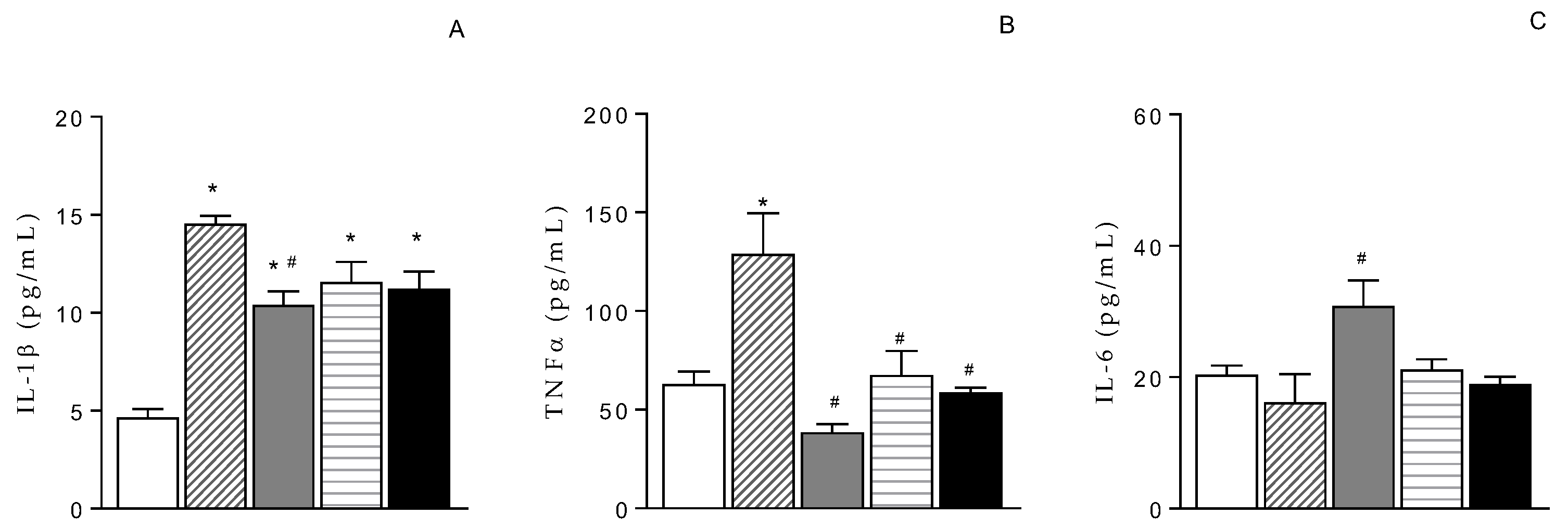
The treatment with ET-1 significantly increased IL-1β levels in retina from 4.7±0.4 pg/mL (naïve) to 14.5±0.4 pg/mL (ET-1 + VEH) (
Figure 2A). TNF-α content also increased in retina from 62.8±6.6 pg/mL (naïve) to 128.9±20.8 pg/mL (ET-1 + VEH) (
Figure 2B). The treatment with NCX 470 (0.1%, 30 µL/eye bid) counteracted these effects (10.4±0.7 pg/mL and 38.6±4.2 pg/mL for IL-1β and TNF-α, respectively) (
Figure 2A and B). Differently, IL-6 levels were unchanged in VEH-treated eyes (16.2±4.3 pg/mL) compared to naïve conditions (20.4±1.4 pg/mL), (
Figure 2C) while they were partially increased in NCX 470-treated eyes (30.8±3.9 pg/mL) (
Figure 2C). LUM and BIM also diminished the levels of IL-1β (11.2±0.9 and 11.6±1.0 pg/mL, for BIM and LUM, respectively) and TNF-α (58.7±2.5 and 67.6±12.2 pg/mL, for BIM and LUM, respectively) (
Figure 2A and B). Interestingly, however, neither LUM nor BIM affected IL-6 (19.0±1.1 and 21.2±1.5 pg/mL, for BIM and LUM, respectively) (
Figure 2C).
2.5. Caspase-3 activity and protein content in retina after repeated Vehicle (VEH), NCX 470, Lumigan® (LUM) or equiM bimatoprost (BIM) dosing in endothelin-1 (ET-1)-treated rabbits
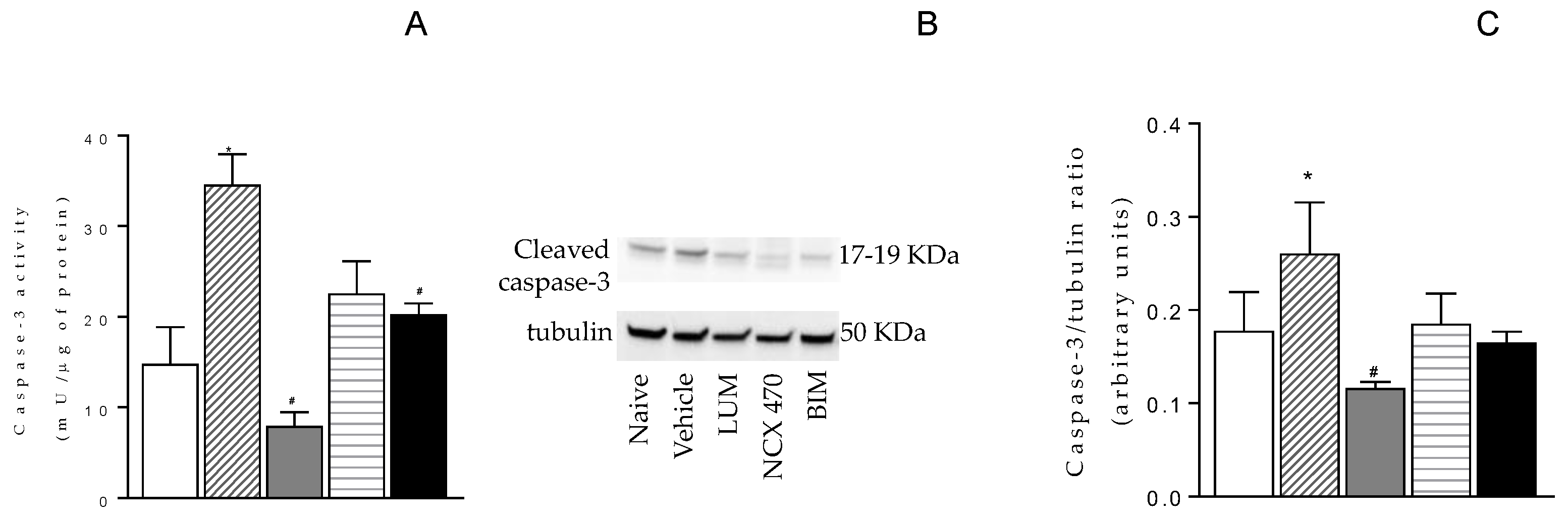
Caspase-3 activity was increased in retina 6 weeks post ET-1 treatment plus VEH for 4 weeks (35.9±4.4 mU/μg) compared with the activity observed in naïve retina (17.8±5.6 mU/μg) (
Figure 3A). NCX 470 treatment significantly reduced ET-1-induced changes (5.1±0.7 mU/μg) (
Figure 3A). LUM and BIM also diminished the levels of Caspase-3 although were numerically less effective than NCX 470 (20.5±1.7 and 23.7±3.6 mU/μg, for BIM and LUM, respectively) (
Figure 3A). Similar trend was observed in cleaved caspase-3 protein expression (
Figure 3 B and C).
2.6. Apoptotic cell determination in retinal sections after repeated NCX 470 dosing in endothelin-1 (ET-1)-treated rabbits
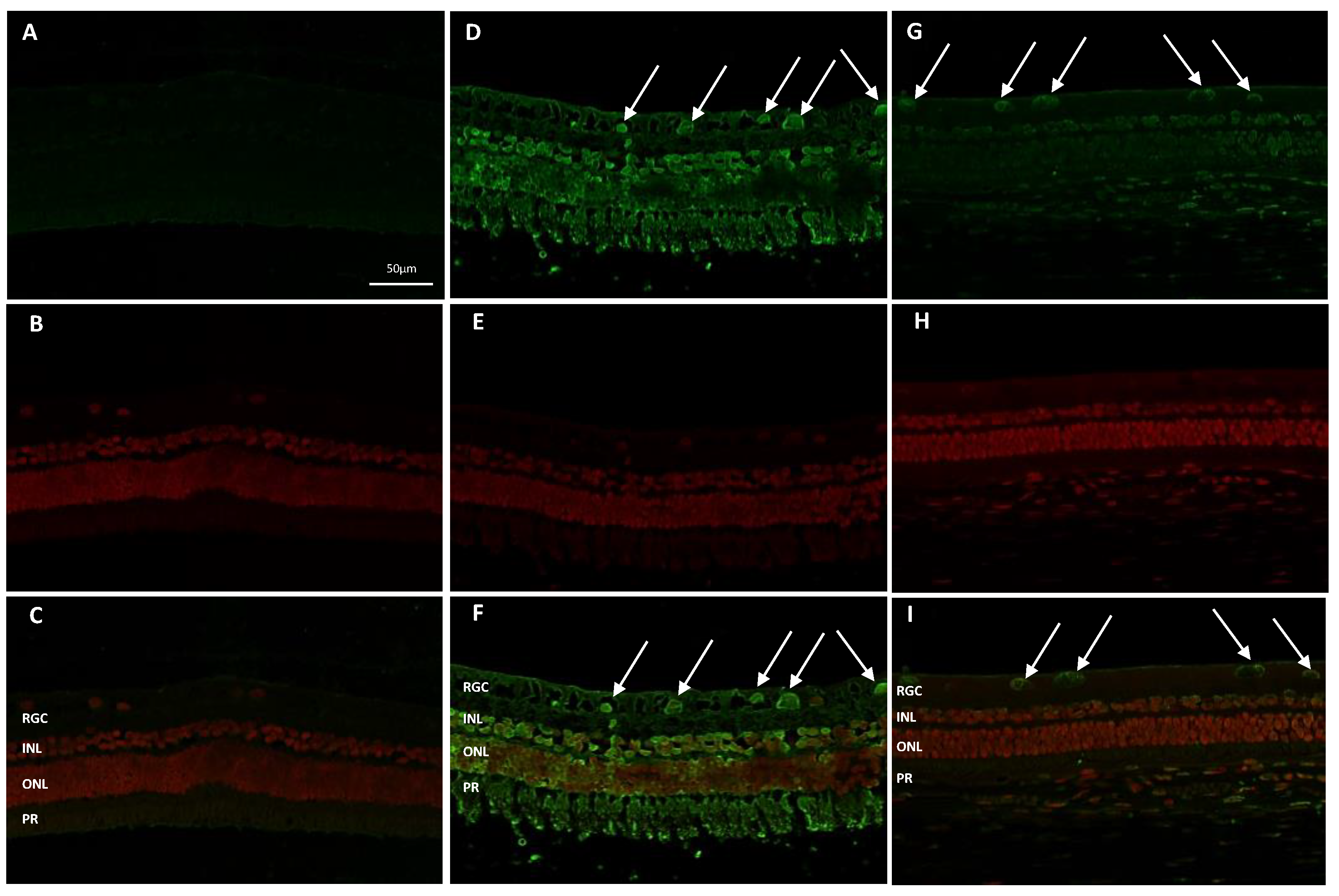
The degeneration of retinal glanglion cells (RGCs) in eyes undergoing ET-1 repeated injections was confirmed by TUNEL assay (
Figure 4) in retinal sessions counterstained with 7-amino actinomycin D (7-AAD)/RNase A to detect non-viable cells. As shown in
Figure 4, panel D-F, ET-1 treatment induced robust retinal cell death with a large proportion of apoptotic nuclei present within the RGCs layer as compared with naïve retina (
Figure 4, panel A-C). Four weeks repeated NCX 470 dosing significantly preserved retina tissue and reduced the apoptotic RGCs (
Figure 4, panel G-I).
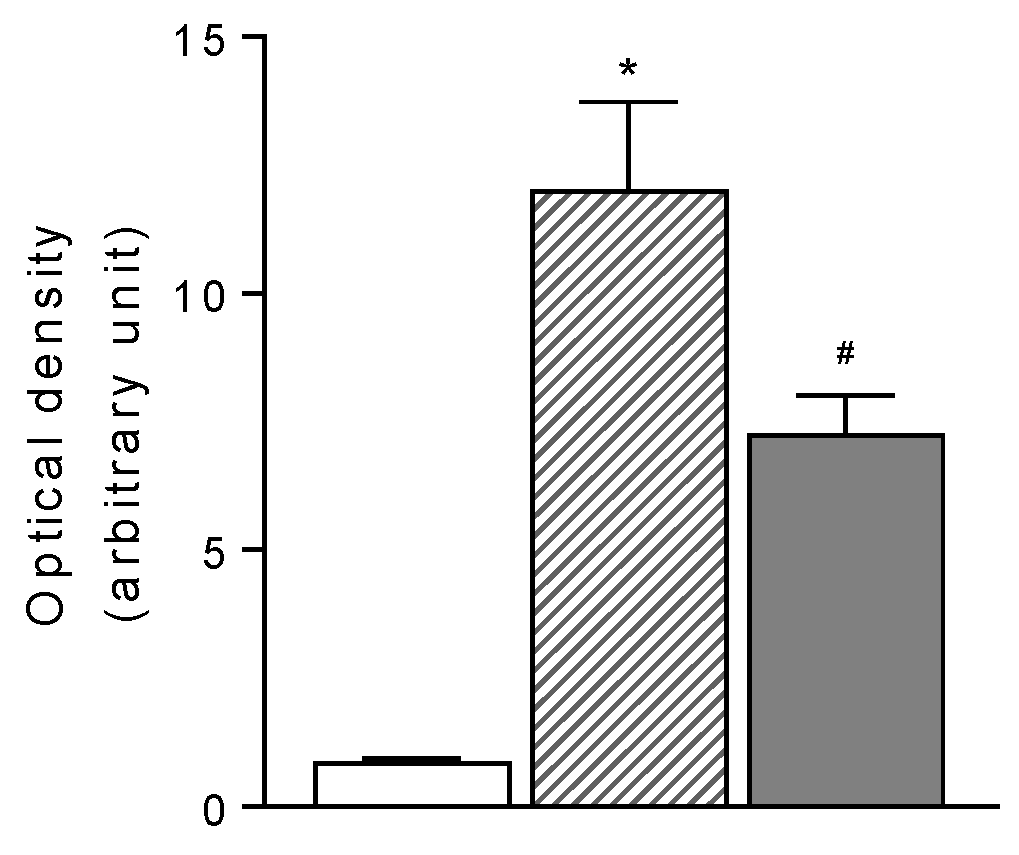
Findings were confirmed by the fluorometric quantification of RGC layer shown in
Figure 5. Previous exploratory work done using in situ identification of the apoptotic cell by DNA fragmentation also documented the presence of apoptotic nuclei within the RGCs layer (data not shown)
3. Discussion
NCX 470 is a novel nitric oxide (NO)-donating derivative where the prostamide bimatoprost is esterified at the hydroxyl group in position 15 with the 6-(nitrooxy) hexanoic acid residue which serves as the NO-donating moiety [
17]. The compound has demonstrated robust and dose-dependent IOP reduction in patients with ocular hypertension and glaucoma [
18], an effect recently confirmed within the first pivotal phase 3 clinical trial where NCX 470 0.1% was tested against latanoprost 0.005% ophthalmic solution in a similar patients population [
https://clinicaltrials.gov/ct2/show/NCT04445519].
Furthermore, NCX 470 resulted effective in reversing hemodynamic changes as well as retinal dysfunction in a rabbit model of ischemia/reperfusion injury of the optic nerve head and retina induced by the repeated injection of ET-1 [
16].
In the present study we confirmed and extended previous results [
16] by comparing the activity of NCX 470 (0.1% ophthalmic solution) to that of bimatoprost administered at the commercial dose (Lumigan®, bimatoprost 0.01% ophthalmic solution – LUM) or at a dose equimolar to 0.1% NCX 470 (0.072% - approximately 7 times the commercial dose – BIM). Both LUM and BIM were less effective that NCX 470 on reversing ET-1-mediated IOP changes as well as on hemodynamic endpoints suggesting that both active metabolites of NCX 470, bimatoprost acid and NO, contribute to the overall effects of this drug on ocular hemodynamics. NO donors have been shown to diminish OA-RI in this model (unpublished results) and Vyzulta® (Latanoprostene bunod ophthalmic solution, 0.024%), a FDA-approved drug for the lowering of IOP in patients with ocular hypertension or glaucoma sharing similar NO-donating technology anchored to an FP receptor agonist as NCX 470, has been shown to enhance ocular perfusion pressure in glaucoma patients [
14] and retinal vessel density in a similar patients population [
15]. Further confirming the NO contribution on these parameters is the finding suggesting an increase of second NO-stimulated messenger, cGMP in retina and ciliary body found after NCX 470 dosing [
17]. A bit more controversial is, however, the effect of bimatoprost on ocular blood flow, with some authors reporting efficacy [
19] and others suggesting no effect [
20]. Additional testing is needed to fully understand the contribution of bimatoprost in the overall effect of NCX 470.
In addition, we further investigated the findings of NCX 470-mediated activity on retinal function. Confirming previous work [
16], NCX 470 enhanced rod and cone responses to scotopic stimulation as determined by an increased b-wave ERG amplitude in these eyes. Again, these effects were not as evident in eyes treated with LUM or BIM thus pointing to NO as the differentiating factor for NCX 470 activity.
NO has been associated with both neuroprotective and neurodegenerative effects, with the circulating amount of NO being the discriminating factor. Low nanomolar concentrations are essential for maintaining aqueous humor homeostasis [
10], ocular blood flow [
21], and promote rods and cones activity [
22], whereas excessive NO concentrations (µM – mM), as that produced by inducible nitric oxide synthase (NOS2), might result cytotoxic [
23]. In our study, NCX 470 diminished ET-1-induced changes in ophthalmic artery resistive index thereby suggesting that an increase in ocular blood flow likely contributed to the beneficial effect of NCX 470 on retina function. However, a bimatoprost-mediated contribution on this phenomenon seems also plausible as indicated in previous work by Emre and coworkers [
24] where topical dosing of bimatoprost was found to protect rabbit retina from ischemia/reperfusion damage.
Little is known on the cellular and molecular mechanism/s involved in the beneficial effects of NCX 470 in retina. We found that NCX 470 treatment prevented cellular apoptosis of retinal ganglion cells as highlighted by a decrease in caspase-3 activity, a marker of the early phase of the apoptotic process [
25]. Likewise, caspase-3 protein content in dissected retina from NCX 470 eyes also decreased compared to those receiving VEH suggesting that NCX 470 lowers the overall expression of caspase-3 rather than its activity. Accordingly, in retinal slices from NCX 470-treated eyes we found less positive cells showing DNA fragmentation compared to VEH-treated eyes, thus indicating that NCX 470 exerts protective effects on RGCs survival when exposed to ischemia/reperfusion insult by inhibiting the apoptotic cascades. As to whether these latter effects are equally shared by bimatoprost was not investigated in this study; however, a bimatoprost contribution could likely be a possibility. Indeed, BIM and LUM, albeit to lesser extent as compared to NCX 470, inhibited caspase-3 activity and protein expression in treated eyes. These latter findings are in agreement with previous reports suggesting an anti-apoptotic activity of bimatoprost in cultured RGCs [
26] and
in vivo following topical dosing in a rabbit model where various prostaglandins including bimatoprost were found to reduce apoptotic cell nuclei in retinal layers following ischemia/reperfusion injury [
24].
Reduced glutathione (GSH), an anti-oxidant defensive marker [
27] decreased after ET-1 plus VEH treatment. Likewise, pro-inflammatory cytokines such as IL-1β, and TNF-α increased after ET-1 plus VEH treatment, while IL-6 remained unchanged. Interestingly, IL-1β and TNF-α were brought back to physiologic levels by NCX 470, on the contrary IL-6 increased following NCX 470 treatment. An effect observed also in eyes treated with BIM or LUM, albeit to numerically lesser extent. These findings once again suggest that the concomitant contribution of NO- and prostaglandin-mediated mechanism/s on oxidative stress and inflammatory signaling pathways could have contributed to the overall effect of NCX 470. The increase in IL-6 is controversial; this cytokine is typically considered pro-inflammatory. However, some papers report that this cytokine shows regenerative and/or anti-inflammatory activities [
28], so it is possible that the protective effects on retinal cells shown by NCX 470 compared with BIM or LUM are related to IL-6 production. Additional investigations are needed to provide more meaningful interpretation of this finding.
In conclusion, NCX 470 by virtue of its dual mechanism of action involving NO-mediated as well as bimatoprost-mediated effects improves ocular hemodynamics and retinal cell physiology in ET-1-induced ischemia/reperfusion injury model.
4. Materials and Methods
4.1. Animals
Twentyfour (24) adult New Zealand White (NZW) male rabbits weighting 2.0–2.5 kg were used. The experimental procedures were conducted in accordance with the Italian regulation on protection of animals used for experimental and other scientific purpose (Italian Legislative Decree 26, March 13, 2014) and with the EU Regulations (Council Directive of the European Community 2010/63/EU). The study was approved by the local animal care committee of the University of Florence (Italy) and followed Ministry of Health recommendations (authorization n. 110/2021-PR). Every effort was made to minimize animal suffering and to reduce the number of animals used.
The animals were kept in individual cages, food and water were provided ad libitum. The animals were identified with a tattoo on the ear, numbered consecutively, and housed on a 12–12 h light/dark cycle in a temperature-controlled room (22–23 °C).
4.2. Experimental animal model and test item dosing
The endothelin-1 (ET-1)-induced ischemia/reperfusion model previously described [
16] was used. Briefly, the ischemia/reperfusion injury was induced by the subtenon injection (twice/week for 6 weeks) of 200 µL of 250 nM ET-1 (Fluka, Israel) dissolved in water using a lacrimal cannula under anesthesia produced by ketamine (20 mg/kg) and xylazine (5 mg/kg) injected intramuscularly.
Vehicle (Kolliphor® HS15, benzalkonium chloride, boric acid, EDTA, sorbitol, dibasic sodium phosphate, pH=6.0, 30 µL/eye, bid), NCX 470 (0.1% dissolved in vehicle, 30 µL/eye, bid), Lumigan® (bimatoprost 0.01% ophthalmic solution, 30 µL/eye, bid) or bimatoprost (0.072% dissolved in vehicle, 30 µL/eye, bid) were administered as eye drops (at approximately 10:00 AM and 4:00 PM) by a treatment-masked investigator starting on week 3 until the end of the experiment.
4.3. Functional measurements
Out of the 24 animals used in this study, 12 animals (left and right eyes were randomly assigned to vehicle (VEH, n=7 eyes), NCX 470 (0.1%, n=7 eyes), Lumigan® (bimatoprost 0.01% ophthalmic solution, LUM, n=4 eyes) or bimatoprost (0.072%, equimolar to 0.1% NCX 470, BIM, n=6 eyes) were treated and tested for intraocular pressure (IOP) and Ophthamic Artery Resistive Index (OA-RI). The remaining 12 animals, although receiving similar treatment, were used for Electroretinogram (ERG) determinations.
4.3.1. IOP determinations
IOP was measured at the indicated time points using a pneumatonometer (Model 30 Classic; Reichert, Depew, NY) on Monday (after 36 h free of treatment) before AM dosing with test items. One drop of oxybuprocaine hydrochloride (4 mg/mL) was instilled immediately before each set of pressure measurements. Values reported for each time point are the mean of 2 consecutive measurements taken 1 min apart. IOP changes from baseline were calculated as follows:
IOP
Tx-IOP
T0 where IOP
Tx and IOP
T0 are the IOPs at the time of interest and at baseline [
16].
4.3.2. OA-RI determinations
Measurements were taken using an Echo Color Doppler (Philips Ultrasound HD7XE; Philips, Milan, Italy) with the S12–4 ultrasound transducer and a frequency of 6.0 MHz, before ET-1 treatment (basal, time 0), and weekly thereafter on Mondays until the end of the study. Pourcelot resistive index for ophthalmic artery (OA-RI) was calculated using the following formula:
OA-PSV–OA-EDV/OA-PSV where OA-PSV and OA-EDV are the ophthalmic artery peak systolic velocity and ophthalmic artery end diastolic velocity, respectively [
29].
4.3.3. ERG analysis
Topical anesthesia was achieved using 1 drop of oxybuprocaine hydrochloride (4 mg/mL). The eyes were then dilated by topical application of tropicamide 1% and, when needed, adapted to darkness for at least 2 h before standard ERGs recording of both eyes using contact lens corneal electrodes so to have sufficiently stable and amplified recordings. The ERG signals were recorded using Retimax (CSO, Florence, Italy) and according to the current International Society for Clinical Electrophysiology of Vision (ISCEV) indications, as previously reported [
16]. Measurements were taken before ET-1 first dose (baseline, time 0), then at the end of week 2 (before vehicle-, NCX 470-, Lumigan®- or bimatoprost-first day-first dose) and at the end of week 6 (36 h after vehicle, NCX 470, LUM or BIM last day-last dose).
4.4. Biochemical measurements
Retina was collected from all animals at the end of the study as well as from naïve animals for comparative assessments. Animals were euthanized with an overdose of anesthetic (pentothal sodium 0.15 g/kg, iv bolus) and tissues dissected and frozen until further processing. Reduced glutathione (GSH) and 8-Hydroxy-2-deoxyguanosine (8-OH2dG) were used as representative markers of oxidative stress. Caspase-3 was used as a representative marker of cell apoptosis.
4.4.1. GSH levels
Retina was homogenized in 2 mL of 10 mM phosphate buffer, pH 7.4 and then centrifuged at 10,000g for 30 min at 4°C, then a fixed volume of supernatant was diluted with phosphate buffer added with ethylene diamine tetra-acetic acid (EDTA) to 2 mL. The resulting solution was then processed to determine the levels of GSH, as previously reported [
16].
4.4.2. 8-OH2dG levels
The isolation of DNA was performed as described previously by our group [
30] with minor modifications. Briefly, samples were homogenized in 1 mL of 10 mM PBS, pH 7.4, sonicated on ice for 1 min, added with 1 ml of 10 mmol/L Tris-HCl buffer, pH 8, containing 10 mmol/L EDTA, 10 mmol/L NaCl, and 0.5% SDS, incubated for 1 h at 37°C with 20 µg/mL RNase 1 (Sigma-Aldrich, Saint Louis, MO, USA) and overnight at 37°C in the presence of 100 µg/mL proteinase K (Sigma-Aldrich). The mixture was extracted with chloroform/isoamyl alcohol (10/2 v/v). DNA was precipitated from the aqueous phase with 0.2 volumes of 10 mmol/L ammonium acetate, solubilized in 200 µL of 20 mmol/L acetate buffer, pH 5.3, and denatured at 90°C for 3 min. The extract was then supplemented with 10 IU of P1 nuclease (Sigma-Aldrich) in 10 µL and incubated for 1 h at 37°C with 5 IU of alkaline phosphatase (Sigma-Aldrich) in 0.4 mol/L phosphate buffer, pH 8.8. The mixture was filtered by an Amicon Micropure-EZ filter (Merck-Millipore), and 50 µL of each sample was used for 8-OH2dG determination using an ELISA kit (JalCA, Shizuoka, Japan), following the instructions provided by the manufacturer. The absorbance of the chromogenic product was measured at 450 nm and expressed as ng/mg of DNA. The results were calculated from a standard curve based on 8-OH2dG solution. The values are expressed as pg 8-OH2dG/µg total DNA.
4.4.3. Caspase-3 activity and protein expression
The activity of caspase-3 was determined using a fluorescent substrate following the methods described by Stennicke and Salvesen [
31]. The Ac-Asp-Glu-Val-Asp-AMC (AC-DEVD-AMC; Bachen) was used as a fluorescent substrate for caspase-3. Briefly, frozen tissue samples were homogenized with 10 mM N-2-hydroxymethylpiperazine-N-2-ethansulfone acid (HEPES, pH 7.4) containing 0.5% [(3-cholamidopropyl) dimethylammino]-1-propane-sulfonate (CHAPS), 42 mM KCl, 5 mM NaCl, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 μg/mL leupeptin and 1 μg/mL pepstatin A. The homogenate was then centrifuged at 10,000g for 10 min. The resulting supernatant (containing about 70–80 μg total protein) was incubated with 40 μM of the caspase-3 substrate AC-DEVD-AMC for 60 min at 37 °C. At the end of incubation, substrate cleavage was monitored fluorometrically with a λ excitation at 380 nm and λ emission at 460 nm. Data are expressed as arbitrary mU/μg proteins where one unit of enzyme activity is defined as the amount of enzyme required to liberate 40 μmol of AC-DEVD-AMC for 60 min at 37 °C.
Retina tissues were homogenized with radioimmunoprecipitation assay (RIPA) buffer enriched in protease inhibitors and PMSF (1 mM). Homogenates were then centrifuged at 15,000 g for 10 min and total protein levels were determined in the collected supernatants using BCA Protein Assay (ThermoFisher Scientific, Waltham, MA, USA). Forty (40) μg of proteins were separated by SDS-PAGE, electro-transferred on PVDF membranes and incubated with Caspase-3 Monoclonal Antibody 2 μg/ml (74T2, Invitrogen, Massachusetts, USA) followed by incubation with appropriated HRP-conjugated secondary antibodies. The loading transfer of equal amounts of proteins was checked by reblotting the membrane with a tubulin antibody (1:5000; Sigma-Aldrich, St. Louis, MO, USA). Bands were visualized by enhanced chemiluminescence (ECL; ThermoFisher Scientific, Waltham, MA, USA) and quantified by densitometric analysis with the ImageJ 1.33 software.
4.4.4. IL-1β, IL-6 and TNFα levels
The levels of inflammatory cytokines, interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumour necrosis factor-α (TNFα), were quantitatively determined on aliquots (100 μL) of retina homogenate supernatants, using the RayBio® Rabbit ELISA kit for IL-1β , IL-6 and TNFα (RayBiotech, Norcross, GA, USA), following the protocol provided by the manufacturer. Briefly, the antibody specific for rabbit cytokine is coated on a 96-well plate, where the cytokine present in the sample is bound to the well by the immobilized antibody. The biotinylated anti-rabbit cytokine antibody was added to the well and successively, the horseradish peroxidase (HRP) conjugated streptavidin. The addition of 3,3’,5,5’-tetramethylbenzidine (TMB) substrate solution permitted the developing of colour in proportion to the amount of the cytokine bound. Finally, the stop solution changed the colour from blue to yellow and the intensity of the colour was measured at 450nm. Cytokine quantification was performed using standard curves obtained by using cytokine standards that underwent the same protocol as the samples. Values are indicated as mean ± S.E.M. and expressed as pg/mL.
4.5. In situ staining
Some eyes from each treatment group were dissected, paraffin-embedded and later processed for the presence of apoptotic nuclei.
4.5.1. TUNEL DNA fragmentation staining
To determine DNA fragmentation the paraffin-embedded eyes were cut into 5μm thick sections, deparaffinized, and rehydrated. Tissues were then incubated with proteinase K solution at room temperature and refixed with formaldehyde 4%. Sections were incubated with DNA labeling solution, containing Br-dUTP, for 60 min at 37ºC and then with the antibody solution for 30 min at room temperature. Finally, sections were counterstained with 7-amino-actinomycin D (7-AAD)/RNase A, a fluorescent intercalant that binds selectively GC regions of DNA and can be excited at 488 nm. Sections were analyzed with a confocal Leica TCS SP5 microscope. All sections were stained in a single session to minimize artefactual inconsistencies during the staining process. Optical density (OD) of RGC layer was measured using the free-share ImageJ 1.33 image analysis program.
4.6. Statistical Analysis
The values were expressed as mean ± standard error of the mean (S.E.M.). Statistical analysis was done using GraphPad Prism Statistic (release 5.0; GraphPad Software Inc. CA, USA) using ANOVA analysis. P-values were calculated with the use of either one- or two-sided statistical tests, when not otherwise specified the one-way test was used; p < 0.05 was considered statistically significant.
Author Contributions
Conceptualization, L.L., E.M., E.B. and F.I.; methodology, L.L., S.S., E.B., F.I., C.G., S.B. S.M. and S.V.; data curation, S.S., L.L., G.P., E.B., C.G., S.B., F.I.,S.M. and S.V.; writing—original draft preparation, E.M.; writing—review and editing, S.S., S.M., L.L., E.B., C.G., S.B. and F.I.; performed the histological evaluations, S.S, S.M. and S.V.; synthesized and developed the compound NCX 470, E.B., C.G., S.B. and F.I.; funding acquisition, E.M. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially sponsored by Nicox.
Institutional Review Board Statement
The experimental procedures were conducted in accordance with the Italian regulation on protection of animals used for experimental and other scientific purpose (Italian Legislative Decree 26, March 13, 2014) and with the EU Regulations (Council Directive of the European Community 2010/63/EU). The study was approved by the local animal care committee of the University of Florence (Italy) and followed Ministry of Health recommendations (authorization n. 110/2021-PR).
Data Availability Statement
Data are available upon request.
Conflicts of Interest
E. Bastia, C. Galli, S. Brambilla and F. Impagnatiello were employed at Nicox Research Institute at the time this work was performed. The remaining authors have no conflict of interest.
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. J. Am. Med. Assoc. 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Caprioli, J. Intraocular Pressure Fluctuation: Is It Important? J Ophthalmic Vis Res. 2018, 13, 170–174. [Google Scholar] [PubMed]
- Hardy, P.; Dumont, I.; Bhattacharya, M.; Hou, X.; Lachapelle, P.; Varma, D.R.; Chemtob, S. Oxidants, nitric oxide and prostanoids in the developing ocular vasculature: a basis for ischemic retinopathy. Cardiovasc Res. 2000, 47, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Fan Gaskin, J.C.; Shah, M.H.; Chan, E.C. Oxidative Stress and the Role of NADPH Oxidase in Glaucoma. Antioxidants. 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shao, M.; Li, Y.; Li, X.; Wan, Y.; Sun, X.; Cao, W. Relationship between Oxidative Stress Biomarkers and Visual Field Progression in Patients with Primary Angle Closure Glaucoma. Oxid Med Cell Longev. 2020, 2701539. [Google Scholar] [CrossRef]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E.M. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog Retin Eye Res. 2021, 83, 10091016. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, V.; Foulds, W.S.; Luu, C.D.; Ling, E.A.; Kaur, C. Retinal ganglion cell death is induced by microglia derived pro-inflammatory cytokines in the hypoxic neonatal retina. J Pathol. 2011, 224, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Impagnatiello, F.; Bastia, E.; Almirante, N.; Brambilla, S.; Duquesroix, B.; Kothe, A.C.; Bergamini, M.V.W. Prostaglandin analogues and nitric oxide contribution in the treatment of ocular hypertension and glaucoma. Br J Pharmacol., 2019, 8, 1079–1089. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Zhao, Y.; Yang, X.; Zhou, D.; Chen, B.; Duan, X. Effects of Tafluprost on Ocular Blood Flow. Ophthalmol Ther. 2022, 11, 1991–2003. [Google Scholar] [CrossRef]
- Cavet, M.E.; Vittitow, J.L.; Impagnatiello, F.; Ongini, E.; Bastia, E. Nitric oxide (NO): an emerging target for the treatment of glaucoma. Invest Ophthalmol Vis Sci. 2014, 55, 5005–5015. [Google Scholar] [CrossRef]
- Maynard, K.I.; Chen, D.; Arango, P.M.; Ogilvy, C.S. Nitric oxide produced during ischemia improves functional recovery in the rabbit retina. NeuroReport, 1996, 8, 81–85. [Google Scholar] [CrossRef]
- Hangai, M.; Yoshimura, N.; Hiroi, K.; Mandai, M.; Honda, Y. Role of nitric oxide during the initial phase of reperfusion after retinal ischemia in the rat. Ophthalmic Res. 1999, 31, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Santana-Garrido, Á.; Reyes-Goya, C.; Arroyo-Barrios, A.; André, H.; Vázquez, C.M.; Mate, A. Hypertension secondary to nitric oxide depletion produces oxidative imbalance and inflammatory/fibrotic outcomes in the cornea of C57BL/6 mice. J Physiol Biochem. 2022, 78, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.K.; Slight, J.R.; Vittitow, J.L.; Scassellati Sforzolini, B.; Weinreb, R.N. Efficacy of Latanoprostene Bunod 0. 024% Compared With Timolol 0.5% in Lowering Intraocular Pressure Over 24 Hours. Am J Ophthalmol. 2016, 169, 249–257. [Google Scholar] [PubMed]
- El-Nimri, N.W.; Moghimi, S.; Penteado, R.C.; Ghahari, E.; Yang, D.; Brye, N.; Proudfoot, J.; Do, J.L.; Camp, A.; Salcedo, M.; Rubio, V.; Weinreb, R.N. Comparison of the Effects of Latanoprostene Bunod and Timolol on Retinal Blood Vessel Density: A Randomized Clinical Trial. Am J Ophthalmol. 2022, 241, 120–129. [Google Scholar] [CrossRef]
- Bastia, E.; Sgambellone, S.; Lucarini, L.; Provensi, G.; Brambilla, S.; Galli, C.; Almirante, N.; Impagnatiello, F. NCX 470 Restores Ocular Hemodynamics and Retinal Cell Physiology After ET-1-Induced Ischemia/Reperfusion Injury of Optic Nerve and Retina in Rabbits. J Ocul Pharmacol Ther. 2022, 38, 496–504. [Google Scholar] [CrossRef]
- Impagnatiello, F.; Toris, C.B.; Batugo, M.; Prasanna, G.; Borghi, V.; Bastia, E.; Ongini, E.; Krauss, A.H. Intraocular Pressure-Lowering Activity of NCX 470, a Novel Nitric Oxide-Donating Bimatoprost in Preclinical Models. Invest Ophthalmol Vis Sci. 2015, 56, 6558–6564. [Google Scholar] [CrossRef]
- Walters, T.R.; Kothe, A.C.; Boyer, J.L.; Usner, D.W.; Lopez, K.; Duquesroix, B.; Fechtner, R.D.; Navratil, T. A Randomized, Controlled Comparison of NCX 470 (0.021%, 0.042%, and 0.065%) and Latanoprost 0.005% in Patients With Open-angle Glaucoma or Ocular Hypertension: The Dolomites Study. J Glaucoma. 2022, 31, 382–391. [Google Scholar] [CrossRef]
- Koz, O.G.; Ozsoy, A.; Yarangumeli, A.; Kose, S.K.; Kural, G. Comparison of the effects of travoprost, latanoprost and bimatoprost on ocular circulation: a 6-month clinical trial. Acta Ophthalmol Scand. 2007, 85, 838–843. [Google Scholar] [CrossRef]
- Alagoz, G.; Gürel, K.; Bayer, A.; Serin, D.; Celebi, S.; Kukner, S. A comparative study of bimatoprost and travoprost: effect on intraocular pressure and ocular circulation in newly diagnosed glaucoma patients. Ophthalmologica. 2008, 222, 88–95. [Google Scholar] [CrossRef]
- Toda, N.; Nakanishi-Toda, M. Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res. 2007, 26, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.S.; Xuan, B.; Chiou, G.C. Effects of nitric oxide donors on the retinal function measured with electroretinography. J Ocul Pharmacol Ther. 2000, 16, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Opatrilova, R.; Kubatka, P.; Caprnda, M.; Büsselberg, D.; Krasnik, V.; Vesely, P.; Saxena, S.; Ruia, S.; Mozos, I.; Rodrigo, L.; Kruzliak, P.; Dos Santos, K.G. Nitric oxide in the pathophysiology of retinopathy: evidences from preclinical and clinical researches. Acta Ophthalmol. 2018, 96, 222–231. [Google Scholar] [CrossRef]
- Emre, S.; Gul, M.; Ates, B.; Esrefoglu, M.; Koc, B.; Erdogan, A.; Yesilada, E. Comparison of the protective effects of prostaglandin analogues in the ischemia and reperfusion model of rabbit eyes. Exp Anim. 2009, 58, 505–513. [Google Scholar] [CrossRef]
- Ola, M.S.; Alhomida, A.S.; LaNoue, K.F. Gabapentin Attenuates Oxidative Stress and Apoptosis in the Diabetic Rat Retina. Neurotox Res 2019, 36, 81–90. [Google Scholar] [CrossRef]
- Yamagishi, R.; Aihara, M.; Araie, M. Neuroprotective effects of prostaglandin analogues on retinal ganglion cell death independent of intraocular pressure reduction. Exp Eye Res. 2011, 93, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gong, J.; Yoshida, T.; Eberhart, C.G.; Xu, Z.; Kombairaju, P.; Sporn, M.B.; Handa, J.T.; Duh, E.J. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free Radic Biol Med. 2011, 51, 216–224. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Galassi, F.; Sodi, A.; Ucci, F.; Renieri, G.; Pieri, B.; Masini, E. Ocular haemodynamics and nitric oxide in normal pressure glaucoma. Acta Ophthalmol Scand Suppl. 2000, 232, 37–38. [Google Scholar] [CrossRef]
- Masini, E.; Bani, D.; Vannacci, A.; Pierpaoli, S.; Mannaioni, P.F.; Comhair, S.A.; Xu, W.; Muscoli, C.; Erzurum, S.C.; Salvemini, D. Reduction of antigen-induced respiratory abnormalities and airway inflammation in sensitized guinea pigs by a superoxide dismutase mimetic. Free Radic Biol Med. 2005, 39, 520–531. [Google Scholar] [CrossRef]
- Stennicke, H.R.; Salvesen, G.S. Caspases: preparation and characterization. Methods. 1999, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Rods and cones response prior to (baseline) and 2 and 6 weeks after ET-1-induced ischemia/reperfusion injury in eyes receiving A) NCX 470 (0.1%, closed circle, n=7) and VEH (open square, n=7); B) LUM (0.01%, open triangle, n=4) and VEH (open square, n=7); C) BIM (0.072%, open triangle, n=6) and VEH (open square, n=7). Reported amplitude reflects the difference between b-wave and a-wave maximal intensities; data are reported as mean ± S.E.M., Student’s t-test. *p<0.05 vs time 0 (baseline); #p<0.05 vs vehicle at the same time point.
Figure 1.
Rods and cones response prior to (baseline) and 2 and 6 weeks after ET-1-induced ischemia/reperfusion injury in eyes receiving A) NCX 470 (0.1%, closed circle, n=7) and VEH (open square, n=7); B) LUM (0.01%, open triangle, n=4) and VEH (open square, n=7); C) BIM (0.072%, open triangle, n=6) and VEH (open square, n=7). Reported amplitude reflects the difference between b-wave and a-wave maximal intensities; data are reported as mean ± S.E.M., Student’s t-test. *p<0.05 vs time 0 (baseline); #p<0.05 vs vehicle at the same time point.
Figure 2.
A) Interleukin-1ß (IL-1ß), B) Tumor Necrosis Factor alpha (TNF-α) and C) Interleukin-6 (IL-6) levels under naïve condition (open bar, n=3-4) and 6 weeks after ET-1-induced ischemia/reperfusion injury in eyes treated with VEH (dashed bar, n=4), NCX 470 (grey bar, n=4), LUM (squared bar, n=4) or BIM (closed bar, n=4). Data are mean ± S.E.M., One-way ANOVA followed by Bonferroni post hoc test. *p<0.05 vs naïve, #p<0.05 vs vehicle.
Figure 2.
A) Interleukin-1ß (IL-1ß), B) Tumor Necrosis Factor alpha (TNF-α) and C) Interleukin-6 (IL-6) levels under naïve condition (open bar, n=3-4) and 6 weeks after ET-1-induced ischemia/reperfusion injury in eyes treated with VEH (dashed bar, n=4), NCX 470 (grey bar, n=4), LUM (squared bar, n=4) or BIM (closed bar, n=4). Data are mean ± S.E.M., One-way ANOVA followed by Bonferroni post hoc test. *p<0.05 vs naïve, #p<0.05 vs vehicle.
Figure 3.
Caspase-3 activity (A) and cleaved Caspase-3 protein expression (B) and respective densitometries (C) under naïve condition (open bar, n=6) and 6 weeks after ET-1-induced ischemia/reperfusion injury in eyes treated with VEH (dashed bar, n=8), NCX 470 (grey bar, n=8), LUM (squared bar, n=7) or BIM (closed bar, n=7). Data are mean ± S.E.M., One-way ANOVA followed by Bonferroni post hoc test. *p<0.05 vs naïve, #p<0.05 vs vehicle.
Figure 3.
Caspase-3 activity (A) and cleaved Caspase-3 protein expression (B) and respective densitometries (C) under naïve condition (open bar, n=6) and 6 weeks after ET-1-induced ischemia/reperfusion injury in eyes treated with VEH (dashed bar, n=8), NCX 470 (grey bar, n=8), LUM (squared bar, n=7) or BIM (closed bar, n=7). Data are mean ± S.E.M., One-way ANOVA followed by Bonferroni post hoc test. *p<0.05 vs naïve, #p<0.05 vs vehicle.
Figure 4.
Representative retina sections immunostained for BrdU (green, panels A, D, and G for Naïve, ET-1+VEH and ET-1+NCX 470, respectively) and 7-AAD/Rnase A (red, panels B, E, and H for Naïve, ET-1+VEH and ET-1+NCX 470, respectively) to counterstain the nuclei (magnification 20x). Panels C, F and I show respective merging pictures. RGC, Retinal Ganglion Cells layer; INL, Inner Nuclear Layer; ONL, Outer Nuclear Layer; PR, Photoreceptors. White arrows indicate apoptotic bodies within retinal ganglion cells.
Figure 4.
Representative retina sections immunostained for BrdU (green, panels A, D, and G for Naïve, ET-1+VEH and ET-1+NCX 470, respectively) and 7-AAD/Rnase A (red, panels B, E, and H for Naïve, ET-1+VEH and ET-1+NCX 470, respectively) to counterstain the nuclei (magnification 20x). Panels C, F and I show respective merging pictures. RGC, Retinal Ganglion Cells layer; INL, Inner Nuclear Layer; ONL, Outer Nuclear Layer; PR, Photoreceptors. White arrows indicate apoptotic bodies within retinal ganglion cells.
Figure 5.
Fluorometric quantification of apoptotic RGCs under naïve condition (open bar, n=5) and 6 weeks after ET-1-induced ischemia/reperfusion injury in eyes treated with VEH (dashed bar, n=5) and NCX 470 (grey bar, n=5), Densitometric data are reported as relative optical density in each experimental group. Data are mean ± S.E.M., One-way ANOVA followed by Bonferroni post hoc test. *p<0.05 vs naïve, #p<0.05 vs vehicle.
Figure 5.
Fluorometric quantification of apoptotic RGCs under naïve condition (open bar, n=5) and 6 weeks after ET-1-induced ischemia/reperfusion injury in eyes treated with VEH (dashed bar, n=5) and NCX 470 (grey bar, n=5), Densitometric data are reported as relative optical density in each experimental group. Data are mean ± S.E.M., One-way ANOVA followed by Bonferroni post hoc test. *p<0.05 vs naïve, #p<0.05 vs vehicle.
Table 1.
Intraocular pressure (IOP) after repeated (30 µL/eye, bid) VEH, NCX 470, LUM or BIM treatments in ET-1-treated eyes.
Table 1.
Intraocular pressure (IOP) after repeated (30 µL/eye, bid) VEH, NCX 470, LUM or BIM treatments in ET-1-treated eyes.
| |
Intraocular pressure (IOP, mmHg) |
Time after
ET-1
1st dose
|
Week 0 Baseline
|
Week 2
ET-1
|
Week 3 ET-1
+
Test items or vehicle |
Week 4 ET-1
+
Test items or vehile |
Week 5 ET-1
+
Test items or vehicle |
Week 6 ET-1
+
Test items or vehicle |
| VEH |
20.5±0.8 |
23.9±0.4 |
24.0±0.6 |
24.7±0.4 |
24.8±0.3 |
24.8±0.3 |
| NCX 470 |
20.7±0.7 |
22.4±0.9#
|
18.7±0.9*#
|
18.4±0.5*#
|
18.1±0.5*#
|
18.1±0.6*#
|
| LUM |
21.5±0.5 |
22.1±0.9 |
20.5±1.3*
|
20.6±0.9*
|
21.1±0.4*
|
20.7±0.7*
|
| BIM |
19.5±1.1 |
25.4±0.3 |
23.1±0.8 |
22.8±1.1 |
22.3±0.7 |
21.6±0.5*
|
Table 2.
Ophthalmic Artery Resistive Index (OA-RI) changes after repeated (30µL/eye, bid) VEH, NCX 470, LUM or BIM treatments in ET-1-treated eyes.
Table 2.
Ophthalmic Artery Resistive Index (OA-RI) changes after repeated (30µL/eye, bid) VEH, NCX 470, LUM or BIM treatments in ET-1-treated eyes.
| |
Ophthalmic Artery Resistive Index (OA-RI) |
Time after
ET-1
1st dose
|
Week 0 Baseline
|
Week 2
ET-1
|
Week 3 ET-1
+
Test items or vehicle |
Week 4 ET-1
+
Test items or vehile |
Week 5 ET-1
+
Test items or vehicle |
Week 6 ET-1
+
Test items or vehicle |
| VEH |
0.36±0.02 |
0.43±0.03 |
0.49±0.01 |
0.50±0.02 |
0.52±0.02 |
0.55±0.01 |
| NCX 470 |
0.34±0.02 |
0.43±0.02 |
0.38±0.01*
|
0.34±0.02*#
|
0.33±0.01*#
|
0.33±0.01*#
|
| LUM |
0.38±0.03 |
0.43±0.03 |
0.41±0.04 |
0.45±0.02 |
0.44±0.01 |
0.43±0.02*
|
| BIM |
0.31±0.02 |
0.39±0.03 |
0.43±0.02 |
0.41±0.03*
|
0.41±0.02*
|
0.44±0.01*
|
Table 3.
Glutathione (GSH) and 8-Hydroxy-2-deoxyguanosine (8-OH2dG) levels in retina after repeated VEH, NCX 470, LUM or BIM in ET-1-treated eyes.
Table 3.
Glutathione (GSH) and 8-Hydroxy-2-deoxyguanosine (8-OH2dG) levels in retina after repeated VEH, NCX 470, LUM or BIM in ET-1-treated eyes.
| Group ID |
Dose
(% w/w) |
GSH
(µmol/mg protein) |
8-OH2dG
(pg/µg DNA) |
| Naïve |
-- |
1.30±0.04 (n=4) |
18.9±0.7 (n=3) |
| VEH |
-- |
0.37±0.04* (n=6) |
57.5±6.3* (n=6) |
| NCX 470 |
0.1 |
1.31±0.25# (n=6) |
34.1±5.0# (n=6) |
| LUM |
0.01 |
1.09±0.07# (n=4) |
51.8±3.5* (n=5) |
| BIM |
0.072 |
1.39±0.23# (n=4) |
41.5±1.9* (n=6) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).