2. Materials and Methods
2.1. General Considerations and Instrumentation
All reactions were performed in Schlenk or multi-neck flasks under nitrogen atmosphere and using the septum and syringe technique unless otherwise indicated. Dried solvents were taken from the MB-SPS 800 solvent drying system (M. Braun). Triethylamine was freshly distilled according to standard procedure under nitrogen atmosphere with potassium hydroxide and then with calcium hydride. The reaction temperature was adjusted using silicone oil baths preheated to the indicated temperatures or cooling baths (ice/water for 0 °C or dry ice/isopropanol for –78 °C). Column chromatography was performed on silica gel M60 (mesh 230-400, Macherey-Nagel, Düren, Germany). The column chromatographic separations were carried out using the flash technique (overpressure of approx. 2 bar compressed air). For the thin layer chromatography silica coated aluminum foils (60 F254 Merck) were used. The evaluation was performed under UV light (λ = 254 and 356 nm) and staining with iodine.
All commercially available chemicals were obtained from
ABCR,
ACROS,
Alfa Aesar,
Fluorochem,
Macherey-Nagel,
Merck,
Roth,
Sigma Aldrich, and
VWR and were used without further purification.
1H,
13C, and DEPT-135 NMR spectra were recorded at 293 K on
Bruker Avance III 600 (600 MHz),
Bruker Avance DRX 500 (500 MHz), and
Bruker Avance III 300 (300 MHz) instruments unless otherwise noted. Poorly soluble compounds were measured at elevated temperature to increase solubility. CDCl
3 and DMSO-
d6 served as solvents. As an internal standard, the residual proton signal of the corresponding solvents was locked when recording the
1H NMR spectra and
13C NMR spectra (CDCl
3,
δH 7.26,
δC 77.16; DMSO-
d6,
δH 2.50,
δC 39.52). Spin multiplicities are abbreviated as follows: s: singlet; d: doublet, dd: doublet of doublet; ddd: doublet of doublet of doublet; dt: doublet of a triplet; t: triplet; and m: multiplet. The quaternary carbon nuclei (C
quat) and the carbon nuclei of methine (CH), methylene (CH
2), and methyl (CH
3) groups were assigned based on DEPT-135 spectra. Melting points (uncorrected) were measured on the
Büchi B545 instrument according to the protocol of
Kofler [
15]. EI mass spectra were measured on the
TSQ 7000 triple quadrupole mass spectrometer (
Finnigan MAT). Indicated are all peaks with an intensity > 10% of the base peak, the mole peak, and any characteristic fragment peaks with an intensity < 10%. ESI mass spectra were measured on the
Finnigan LCQ Deca ion-trap
API mass spectrometer (
Thermo Quest), and HR-ESI mass spectra and HPLC chromatograms were measured on the
UHR-QTOF maXis 4G mass spectrometer (
Bruker Daltonics). IR spectra were measured on the
IRAffinity-1 instrument (
Shimadzu) (single reflection ATR unit with diamond ATR crystal, wavenumber range: 4000-600 cm-1). The intensities of the absorption bands are given as s (strong), m (medium), and w (weak). Elemental analyses were measured on the
Perkin Elmer Series II Analyzer 2400 at the Institute of Pharmaceutical Chemistry,
Heinrich Heine University. Rotational angle measurements were performed on the
Perkin Elmer 341 polarimeter.
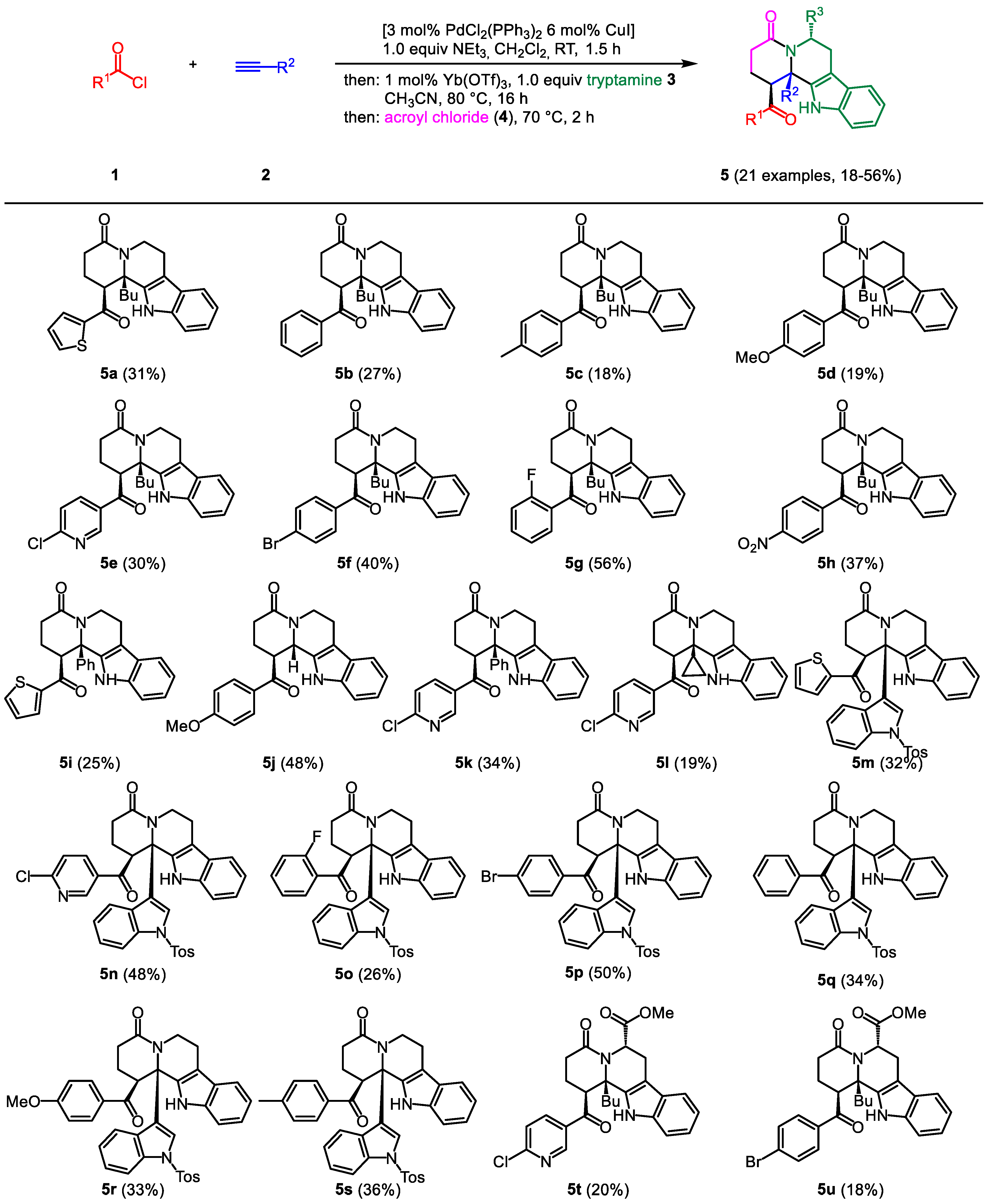
2.2. General procedure (GP) for the synthesis of THBC 5
In a sintered, dry screw-cap Schlenk tube with magnetic stir bar under nitrogen atmosphere PdCl
2(PPh
3)
2 (42 mg, 0.06 mmol), CuI (22 mg, 0.12 mmol), and acid chloride
1 (if a solid) were suspended in degassed dichloromethane (10 mL), and then stirred at rt for 5 min (for experimental details, see
Table 1). Acid chloride
1 (if a liquid), alkyne
2, and NEt
3 (0.28 mL, 2.00 mmol) were then added sequentially, and stirring was performed at rt for 1.5 h. Upon completion of the reaction (TLC control), Yb(OTf)
3 (12 mg, 0.02 mmol) was added, followed by tryptamine (
3a) (320 mg, 2.00 mmol) dissolved in CH
3CN (10 mL). After heating to 80 °C (oil bath) for 16 h, the reaction mixture was allowed to cool to rt, then acroyl chloride (
4) was added dropwise, and the mixture was heated at 70 °C (oil bath) for 2 h. After cooling to room temp the reaction mixture was diluted with MeOH (5 mL) and the crude product was adsorbed on Celite
© under reduced pressure and subsequently purified by chromatography on silica gel to give the analytically pure compound
5.
2.3. rac-12b-Butyl-1-(thiophene-2-carbonyl)-2,3,6,7,12,12b-hexahydroindolo-[2,3-a]quinolizin(1H)-4-one (5a)
According to the GP compound
5a (250 mg, 31%) was isolated as a colorless solid, Mp 245-248 °C (Lit.: 250-251 °C) [
13], R
f = 0.25 (diethyl ether).
1H NMR (600 MHz, CDCl
3): δ 0.84 (t,
3J
HH = 7.1 Hz, 3H), 1.08 (tdd,
2J
HH =
3J
HH = 12.2 Hz,
3J
HH = 9.0 Hz,
3J
HH = 5.4 Hz, 1H), 1.25 – 1.37 (m, 3H), 2.12 – 2.24 (m, 2H), 2.41 (ddt,
2J
HH = 17.3 Hz,
3J
HH = 13.8 Hz,
3J
HH = 8.3 Hz, 1H), 2.73 – 2.83 (m, 4H), 2.88 (ddd,
2J
HH = 15.2 Hz,
3J
HH = 3.8 Hz,
3J
HH = 1.6 Hz, 1H), 2.98 (dt,
2J
HH = 12.4 Hz,
3J
HH = 3.7 Hz, 1H), 3.74 (dd, 3J
HH = 13.6 Hz,
3J
HH = 5.1 Hz, 1H), 5.23 (ddd,
2J
HH = 13.0 Hz,
3J
HH = 5.0 Hz,
3J
HH = 1.6 Hz, 1H), 6.92 (dd,
3J
HH = 4.9 Hz,
3J
HH = 3.8 Hz, 1H), 7.04 – 7.11 (m, 2H), 7.14 – 7.18 (m, 1H), 7.39 (dd,
3J
HH = 3.9 Hz,
4J
HH = 1.1 Hz, 1H), 7.48 (dd,
3J
HH = 7.3 Hz,
4J
HH = 1.5 Hz, 1H), 7.55 (dd,
3J
HH = 4.9 Hz,
4J
HH = 0.9 Hz, 1H), 7.94 (s, 1H).
13C NMR (151 MHz, CDCl
3): δ 14.12 (CH
3), 21.11 (CH
2), 21.94 (CH
2), 23.47 (CH
2), 27.35 (CH
2), 29.76 (CH
2), 36.10 (CH
2), 40.18 (CH
2), 55.13 (CH), 62.09 (C
quat), 111.20 (CH), 111.26 (C
quat),118.33 (CH), 119.69 (CH), 122.31 (CH), 126.14 (C
quat), 128.64 (CH), 132.68 (CH), 134.05 (C
quat), 135.41 (CH), 135.95 (C
quat), 144.09 (C
quat), 169.75 (C
quat), 195.69 (C
quat). IR: ν
̃ [cm
-1] 3296 (w), 3271 (w), 3202 (w), 3177 (w), 3100 (w), 3057 (w), 3034 (w), 2953 (w), 2928 (w), 2893 (w), 2849 (w), 1655 (w), 1614 (s), 1584 (w), 1518 (w), 1489 (w),1433 (m), 1406 (m), 1352 (w), 1317 (w), 1304 (w), 1290 (w), 1263 (w), 1236 (m), 1219 (w), 1200 (w), 1190 (w), 1146 (w), 1126 (w), 1084 (w), 1059 (w), 1036 (w), 1005 (w), 845 (w), 804 (w), 745 (m), 727 (m), 696 (w), 644 (w). ESI MS: 407 ([M]
+). HR-ESI MS calcd. for C
24H
27N
2O
2S: 407.1788; Found: 407.1784. HPLC (254 nm): t
R = 4.9 min, 99%.
2.4. rac-1-Benzoyl-12b-butyl-2,3,6,7,12,12b-hexahydroindolo[2,3-a]quinolizin-4(1H)-on (5b)
According to the GP compound 5b (213 mg, 27%) was isolated as a colorless solid, Mp 241-244 °C, Rf = 0.4 (n-hexane/ethyl acetate 1:1). 1H NMR (600 MHz, CDCl3): δ 0.85 (t, 3JHH = 7.1 Hz, 3H), 1.08 – 1.14 (m, 1H), 1.27 – 1.40 (m, 3H), 2.05 – 2.11 (m, 1H), 2.19 – 2.34 (m, 2H), 2.75 – 2.91 (m, 5H), 3.01 (dt, 2JHH = 12.3 Hz, 3JHH = 3.8 Hz, 1H), 3.95 (dd, 3JHH = 13.6 Hz, 3JHH = 4.7 Hz, 1H), 5.25 (dd, 2JHH = 13.3 Hz, 3JHH = 4.4 Hz, 1H), 7.04 – 7.11 (m, 2H), 7.11 – 7.16 (m, 1H), 7.29 – 7.36 (m, 2H), 7.44 – 7.51 (m, 2H), 7.67 (d, 3JHH = 7.7 Hz, 2H), 7.97 (s, 1H). 13C NMR (151 MHz, CDCl3): δ 14.08 (CH3), 21.24 (CH2), 21.79 (CH2), 23.51 (CH2), 27.52 (CH2), 29.89 (CH2), 36.32 (CH2), 40.25 (CH2), 53.55 (CH), 62.41 (Cquat), 111.27 (CH), 118.39 (CH), 119.80 (CH), 122.43 (CH), 126.26 (Cquat), 128.17 (2 CH), 128.61 (Cquat), 128.94 (2 CH), 133.81 (CH), 134.39 (Cquat), 136.07 (Cquat), 136.94 (Cquat), 169.94 (Cquat), 203.71 (Cquat). IR: ν̃ [cm-1] 3252 (w), 3246 (w), 3217 (w), 3192 (w), 3159 (w), 3140 (w), 3105 (w), 3084 (w), 3057 (w), 3032 (w), 2953 (w), 2930 (w), 2891 (w), 2870 (w), 2845 (w), 1676 (m), 1614 (s), 1595 (w), 1578 (w), 1489 (w), 1466 (w), 1449 (m), 1433 (m), 1402 (m), 1352 (w), 1302 (w), 1288 (w), 1263 (w), 1223 (m), 1182 (w), 1152 (w), 1123 (w), 1059 (w), 1038 (w), 1026 (w), 1002 (w), 968 (w), 926 (w), 870 (w), 822 (w), 760 (w), 743 (s), 708 (s), 685 (m), 644 (w), 631 (w). ESI MS: 401 ([M]+). HR-ESI MS calcd. for C26H28N2O2: 401.2224; Found: 401.2229. HPLC (254 nm): tR = 5.1 min, 99%.
2.5. rac-12b-Butyl-1-(4-methylbenzoyl)-2,3,6,7,12,12b-hexahydroindolo-[2,3-a]quinolizin-4(1H)-one (5c)
According to the GP compound 5c (150 mg, 18%) was isolated as a colorless solid, Mp 214-216 °C, Rf = 0.36 (n-hexane/ethyl acetate 1:1). 1H NMR (600 MHz, CDCl3): δ 0.86 (t, 3JHH = 7.1 Hz, 3H), 1.09 – 1.16 (m, 1H), 1.29 – 1.40 (m, 3H), 1.96 – 2.02 (m, 1H), 2.22 (dddd, 2JHH = 17.9 Hz, 3JHH = 13.7 Hz, 3JHH = 10.8 Hz, 3JHH = 5.8 Hz, 2H), 2.35 (s, 3H), 2.68 (ddd, 2JHH = 18.3 Hz, 3JHH = 9.9 Hz, 3JHH = 7.7 Hz, 1H), 2.73 – 2.92 (m, 4H), 3.01 (td, 2JHH = 3JHH = 12.4 Hz, 3JHH = 3.8 Hz, 1H), 3.75 (dd, 3JHH = 13.6 Hz, 3JHH = 4.7 Hz, 1H), 5.23 (ddd, 2JHH = 12.8 Hz, 3JHH = 5.0, 3JHH = 1.5 Hz, 1H), 6.83 (d, 3JHH = 7.8 Hz, 1H), 6.98 (t, 3JHH = 7.6 Hz, 1H), 7.09 – 7.15 (m, 2H), 7.17 – 7.23 (m, 2H), 7.23 – 7.29 (m, 2H, superimposed by CDCl3), 7.51 – 7.57 (m, 1H), 8.06 (s, 1H).13C NMR (151 MHz, CDCl3): δ 14.17 (CH3), 20.53 (CH3), 21.01 (CH2), 21.18 (CH2), 23.56 (CH2), 27.56 (CH2), 29.88 (CH2), 36.36 (CH2), 40.01 (CH2), 56.88 (CH), 62.07 (Cquat), 111.21 (Cquat), 111.43 (CH), 118.45 (CH), 119.79 (CH), 122.44 (CH), 126.00 (CH), 126.22 (Cquat), 127.43 (CH), 131.63 (CH), 131.84 (CH), 134.62 (Cquat), 136.01(Cquat), 137.48(Cquat), 138.75(Cquat), 169.61(Cquat), 208.46(Cquat). IR: ν̃ [cm-1] 3231 (w), 3177 (w), 3069 (w), 2951 (w), 2928 (w), 2887 (w), 2870 (w), 2839 (w), 2818 (w), 2359 (w), 2342 (w), 2313 (w), 1967 (w), 1948 (w), 1701 (w), 1672 (m), 1672 (m), 1612 (s), 1599 (m), 1587 (w), 1570 (w), 1522 (w), 1487 (w), 1452 (w), 1429 (m), 1404 (m), 1366 (w), 1354 (w), 1317 (w), 1302 (w), 1281 (w), 1261 (w), 1236 (w), 1217 (w), 1198 (w), 1186 (w), 1165 (w), 1155 (w), 1136 (w), 1121 (w), 1078 (w), 1057 (w), 1036 (w), 1026 (w), 1009 (w), 964 (w), 895 (w), 826 (w), 777 (w), 743 (s), 725 (s), 692 (w), 669 (w), 648 (w). ESI MS: 415 ([M]+). HR-ESI MS calcd. for C26H28N2O2: 415.2380; Found: 415.2385. HPLC (254 nm): tR = 5.4 min, 99%.
2.6. rac-12b-Butyl-1-(4-methoxybenzoyl)-2,3,6,7,12,12b-hexahydroindolo-[2,3a]quinolizin-4(1H)-one (5d)
According to the GP compound
5d (163 mg, 19%) was isolated as a colorless solid, Mp 207-208 °C (Lit.: 201-202 °C) [
13], R
f = 0.24 (diethyl ether).
1H NMR (600 MHz, CDCl
3): δ 0.84 (t,
3J
HH = 7.1 Hz, 3H), 1.09 (dddd,
2J
HH = 12.5 Hz,
3J
HH = 10.9 Hz,
3J
HH = 9.3 Hz,
3J
HH = 5.5 Hz, 1H), 1.26 – 1.39 (m, 3H), 2.03 – 2.11 (m, 1H), 2.22 (ddd,
2J
HH = 14.4 Hz,
3J
HH = 12.2 Hz,
3J
HH = 4.3 Hz, 1H), 2.30 (ddd,
2J
HH = 13.8 Hz,
3J
HH = 11.3 Hz,
3J
HH = 6.8 Hz, 1H), 2.73 – 2.90 (m, 5H), 3.00 (td,
2J
HH =
3J
HH = 12.4 Hz,
3J
HH = 3.8 Hz, 1H), 3.78 (s, 3H), 3.89 (dd,
3J
HH = 13.6 Hz,
3J
HH = 4.9 Hz, 1H), 5.25 (ddd,
2J
HH = 13.0 Hz,
3J
HH = 5.1 Hz,
3J
HH = 1.7 Hz, 1H), 6.73 – 6.82 (m, 2H), 7.03 – 7.12 (m, 2H), 7.13 – 7.17 (m, 1H), 7.45 – 7.52 (m, 1H), 7.63 – 7.73 (m, 2H), 8.01 (s, 1H).
13C NMR (151 MHz, CDCl
3): δ 14.15 (CH
3), 21.13 (CH
2), 21.92 (CH
2), 23.53 (CH
2), 27.50 (CH
2), 29.92 (CH
2), 36.37 (CH
2), 40.08 (CH
2), 52.99 (CH), 55.65 (CH
3), 62.23 (C
quat), 111.01 (C
quat), 111.27 (CH), 114.09 (2CH), 118.30 (CH), 119.61 (CH), 122.23 (CH), 126.14 (C
quat), 129.61 (C
quat),, 130.64 (2CH), 134.51 (C
quat), 135.92 (C
quat), 164.13 (C
quat), 169.85 (C
quat), 201.95 (C
quat). IR: ν
̃ [cm
-1] 3366 (w), 3341 (w), 3319 (w), 3028 (w), 3015 (w), 2955 (w), 2928 (w), 2899 (w), 2866 (w), 2839 (w), 2357 (w), 1653 (w), 1634 (s), 1599 (m), 1576 (m), 1558 (w), 1514 (w), 1456 (w), 1423 (m), 1402 (m), 1377 (w), 1354 (w), 1339 (w), 1304 (m), 1281 (w), 1254 (m), 1231 (m), 1184 (m), 1152 (w), 1115 (w), 1084 (w), 1065 (w), 1040 (m), 1020 (m), 999 (w), 966 (w), 945 (w), 918 (w), 872 (w), 835 (m), 824 (w), 762 (m), 748 (s), 733 (m), 714 (m), 692 (m), 638 (w). ESI MS: 431 ([M]
+). Anal. calcd. for C
27H
30N
2O
3 (430.55): C 75.32, H 7.02, N 6.51; Found: C 75.02, H 6.88, N 6.42.
2.7. rac-12b-Butyl-1-(6-chloronicotinoyl)-2,3,6,7,12,12b-hexahydroindolo-[2,3a]quinolizin-4(1H)-one (5e)
According to the GP compound 5e (260 mg, 30%) was isolated as a colorless solid, Mp 237-242 °C, Rf = 0.17 (diethyl ether). 1H NMR (600 MHz, CDCl3): δ 0.85 (t, 3JHH = 7.04 Hz, 3H), 1.05 – 1.14 (m, 1H), 1.26 – 1.39 (m, 3H), 1.98 – 2.09 (m, 1H), 2.22 (dt, 2JHH = 13.7 Hz, 3JHH = 6.4 Hz, 1H), 2.35 (tt, 2JHH = 3JHH = 14.5 Hz, 3JHH = 8.2 Hz, 1H), 2.63 – 2.71 (m, 1H), 2.72 – 2.87 (m, 3H), 2.88 – 2.94 (m, 1H), 2.96 – 3.04 (m, 1H), 3.83 (dd, 3JHH = 13.7 Hz, 3JHH = 5.0 Hz, 1H), 5.24 (dd, 2JHH = 13.1 Hz, 3JHH = 4.7 Hz, 1H), 7.05 – 7.15 (m, 3H), 7.22 (d, 3JHH = 8.2 Hz, 1H), 7.50 (d, 3JHH = 7.1 Hz, 1H), 7.69 – 7.82 (m, 2H), 8.62 (s, 1H). 13C NMR (151 MHz, CDCl3): δ 14.13 (CH3), 21.25 (2CH2), 23.45 (CH2), 27.20 (CH2), 29.51 (CH2), 35.84 (CH2), 40.28 (CH2), 54.20 (CH), 62.02 (Cquat), 111.19 (CH), 111.99 (Cquat), 118.59 (CH), 120.16 (CH), 122.79 (CH), 124.52 (CH), 126.21 (Cquat), 131.04 (Cquat), 133.60 (Cquat), 135.89 (Cquat), 137.75 (CH), 149.69 (CH), 156.26 (Cquat), 169.48 (Cquat), 201.35 (Cquat). IR: ν̃ [cm-1] 3227 (w), 3219 (w), 3167 (w), 3154 (w), 3107 (w), 3059 (w), 2953 (w), 2930 (w), 2847 (w), 1684 (m), 1620 (s), 1578 (m), 1555 (w), 1452 (m), 1433 (m), 1406 (m), 1366 (m), 1352 (m), 1319 (w), 1288 (m), 1263 (m), 1227 (m), 1196 (w), 1148 (w), 1136 (w), 1103 (m), 1034 (w), 1007 (w), 968 (w), 870 (w), 822 (w), 743 (s), 712 (w), 702 (w), 662 (w). ESI MS: 438 ([M(37Cl)+], 436 ([M(35Cl)]+). HR-ESI MS calcd. for C25H27ClN3O2: 436.1786; Found: 436.1786. HPLC (254 nm): tR = 4.8 min, 99%.
2.8. rac-1-(4-Bromobenzoyl)-12b-butyl-2,3,6,7,12,12b-hexahydroindolo-[2,3-a]quinolizin-4(1H)one (5f)
According to the GP compound 5f (385 mg, 40%) was isolated as a colorless solid, Mp 228-232 °C, Rf = 0.35 (n-hexane/ethyl acetate 1:1). 1H NMR (600 MHz, CDCl3): δ 0.85 (t, 3JHH = 7.1 Hz, 3H), 1.10 (dddd, 2JHH = 15.7 Hz, 3JHH = 12.4 Hz, 3JHH = 8.7 Hz, 3JHH = 5.4 Hz, 1H), 1.27 – 1.39 (m, 3H), 2.03 (ddt, 2JHH = 13.7 Hz, 3JHH = 9.1 Hz, 3JHH = 4.6 Hz, 1H), 2.18 – 2.26 (m, 1H), 2.29 (ddd, 2JHH = 12.6 Hz, 3JHH = 9.0, 3JHH = 5.5 Hz, 1H), 2.72 – 2.82 (m, 4H), 2.89 (ddd, 2JHH = 15.2 Hz, 3JHH = 3.8 Hz, 3JHH = 1.6 Hz, 1H), 3.00 (td, 2JHH = 3JHH = 12.4 Hz, 3JHH = 3.7 Hz, 1H), 3.87 (dd, 3JHH = 13.6 Hz, 3JHH = 5.0 Hz, 1H), 5.24 (ddd, 2JHH = 13.0 Hz, 3JHH = 5.1 Hz, 3JHH = 1.6 Hz, 1H), 7.06 – 7.15 (m, 3H), 7.44 (d, 3JHH = 8.7 Hz, 2H), 7.46 – 7.53 (m, 3H), 7.86 (s, 1H). 13C NMR (151 MHz, CDCl3): δ 14.14 (CH3), 21.18 (CH2), 21.59 (CH2), 23.50 (CH2), 27.37 (CH2), 29.68 (CH2), 36.13 (CH2), 40.18 (CH2), 53.54 (CH), 62.17 (Cquat), 111.25 (CH), 111.44 (Cquat), 118.40 (CH), 119.87 (CH), 122.51 (CH), 126.15 (Cquat), 129.20 (Cquat), 129.56 (2CH), 132.21 (2CH), 134.17 (Cquat), 135.55 (Cquat), 135.92 (Cquat), 169.75 (Cquat), 202.74 (Cquat). IR: ν̃ [cm-1] 3225 (w), 3156 (w), 3146 (w), 3105 (w), 3057 (w), 2951 (w), 2927 (w), 2893 (w), 2868 (w), 2361 (w), 1680 (m), 1616 (s), 1585 (m), 1566 (w), 1485 (w), 1449 (w), 1431 (m), 1406 (m), 1352 (w), 1319 (w), 1300 (w), 1283 (m), 1263 (m), 1221 (m), 1179 (w), 1153 (w), 1146 (w), 1121 (w), 1072 (m), 1036 (w), 1009 (m), 966 (w), 926 (w), 912 (w), 870 (w), 837 (w), 820 (w), 760 (w), 741 (s), 683 (w). ESI MS: 481 ([M(81Br)]+), 479 ([M(79Br)]+). HR-ESI MS calcd. for C26H28BrN2O2: 479.1329; Found: 479.1325. HPLC (254 nm): tR = 5.6 min, 99%.
2.9. rac-12b-Butyl-1-(2-fluorobenzoyl)-2,3,6,7,12,12b-hexahydroindolo[2,3-a]-quinolizin-4(1H)one (5g)
According to the GP compound 5g (472 mg, 56%) was isolated as a colorless solid, Mp 233-236 °C, Rf = 0.21 (n-hexane/ethyl acetate 3:2). 1H NMR (600 MHz, CDCl3): δ 0.85 (t, 3JHH = 7.1 Hz, 3H), 1.12 (tdd, 2JHH = 3JHH = 12.0 Hz, 3JHH = 8.7 Hz, 3JHH = 5.3 Hz, 1H), 1.27 – 1.39 (m, 3H), 2.13 – 2.20 (m, 1H), 2.23 (ddt, 2JHH = 16.7 Hz, 3JHH = 12.2 Hz, 3JHH = 2.1 Hz, 2H), 2.72 – 2.87 (m, 5H), 3.00 (td, 2JHH = 3JHH = 12.4 Hz, 3JHH = 3.8 Hz, 1H), 3.86 (dd, 3JHH = 13.1 Hz, 3JHH = 4.8 Hz, 1H), 5.23 (ddd, 2JHH = 12.9 Hz, 3JHH = 5.0 Hz, 3JHH = 1.6 Hz, 1H), 7.02 (ddd, 3JHF = 11.5 Hz, 3JHH = 8.3 Hz, 4JHH = 1.0 Hz, 1H), 7.06 – 7.11 (m, 1H), 7.13 (tdd, 3JHH = 7.0 Hz, 5JHF = 2.9 Hz, 4JHH = 1.2 Hz, 2H), 7.24 (d, 3JHH = 8.0 Hz, 1H), 7.44 (dddd, 3JHH = 8.6 Hz, 3JHH = 7.0 Hz, 4JHF = 4.9 Hz, 4JHH = 1.8 Hz, 1H), 7.49 (d, 3JHH = 7.8 Hz, 1H), 7.62 (td, 3JHH = 7.7 Hz, 4JHH = 1.9 Hz, 1H), 8.11 (s, 1H). 13C NMR (151 MHz, CDCl3): δ 14.13 (CH3), 21.08 (CH2), 21.14 (CH2), 23.53 (CH2), 27.56 (CH2), 30.14 (CH2), 36.75 (CH2), 40.06 (CH2), 57.41(d, 4JCF = 6.18 Hz, CH), 62.21 (Cquat), 111.25 (CH), 111.29 (Cquat), 117.13(d, 2JCF = 23.61 Hz, CH), 118.39 (CH), 119.70 (CH), 122.33 (CH), 124.76(d, 4JCF = 3.27 Hz, CH), 125.93(d, 2JCF = 11.39 Hz, Cquat), 126.19 (Cquat), 130.52(d, 3JCF = 1.57 Hz, CH), 134.39 (Cquat), 135.27(d, 3JCF = 9.27 Hz, CH), 135.98 (Cquat), 161.08(d, 1JCF = 255.70 Hz, Cquat), 169.77 (Cquat), 202.04(d, 3JCF = 4.05 Hz, Cquat). IR: ν̃ [cm-1] 3250 (w), 3196 (w), 3181 (w), 3109 (w), 3059 (w), 3040 (w), 2955 (w), 2932 (w), 2891 (w), 2872 (w), 2859 (w), 2847 (w), 1682 (w), 1611 (s), 1574 (w), 1557 (w), 1528 (w), 1479 (w), 1450 (m), 1433 (m), 1404 (m), 1362 (w), 1352 (m), 1317 (w), 1269 (m), 1261 (m), 1234 (m), 1213 (m), 1190 (w), 1152 (w), 1123 (w), 1101 (w), 1076 (w), 1061 (w), 1036 (w), 1007 (w), 968 (w), 926 (w), 912 (w), 897 (w), 872 (w), 827 (w), 808 (w), 779 (w), 743 (s), 733 (s), 696 (m), 665 ( ), 640 (m), 621 (w). ESI MS: 419 ([M]+). HR-ESI MS calcd. for C26H28FN2O2: 419.2129; Found: 419.2134. HPLC (254 nm): tR = 5.1 min, 99%.
2.10. rac-12b-Butyl-1-(4-nitrobenzoyl)-2,3,6,7,12,12b-hexahydroindolo[2,3-a]-quinolizin-4(1H)-one (5h)
According to the GP compound 5h (334 mg, 37%) was isolated as a colorless solid, Mp 222-224 °C, Rf = 0.30 (n-hexane/ethyl acetate 1:1). 1H NMR (600 MHz, CDCl3): δ 0.86 (t, 3JHH = 7.1 Hz, 3H), 1.11 (dtt, 2JHH = 12.9 Hz, 3JHH = 9.7 Hz, 3JHH = 5.3 Hz, 1H), 1.28 – 1.41 (m, 3H), 1.98 - 2.06 (m, 1H), 2.23 (ddd, 2JHH = 14.2 Hz, 3JHH = 13.3 Hz, 3JHH = 4.1 Hz, 1H), 2.30 – 2.40 (m, 1H), 2.67 (td, 2JHH = 3JHH = 13.4 Hz, 3JHH = 3.8 Hz, 1H), 2.82 (ddt, 2JHH = 17.2 Hz, 3JHH = 10.5 Hz, 3JHH = 6.0 Hz, 3H), 2.91 (dd, 2JHH = 15.3 Hz, 3JHH = 3.7 Hz, 1H), 3.00 (td, 2JHH = 3JHH = 12.5 Hz, 3JHH = 3.7 Hz, 1H), 3.93 (dd, 3JHH = 13.5 Hz, 3JHH = 4.9 Hz, 1H), 5.26 (dd, 2JHH = 13.0 Hz, 3JHH = 4.6 Hz, 1H), 7.03 – 7.12 (m, 3H), 7.46 – 7.54 (m, 1H), 7.70 (d, 3JHH = 8.5 Hz, 2H), 7.74 (s, 1H), 8.09 (d, 3JHH = 8.4 Hz, 2H). 13C NMR (151 MHz, CDCl3): δ 14.13 (CH3), 21.25 (2CH2), 23.46 (CH2), 27.19 (CH2), 29.52 (CH2), 35.71 (CH2), 40.30 (CH), 54.28 (CH), 62.12 (Cquat), 111.16 (CH), 112.00 (Cquat), 118.53 (CH), 120.18 (CH), 122.78 (CH), 123.96 (2CH), 126.22 (Cquat), 128.97 (2CH), 133.68 (Cquat), 135.88 (Cquat), 141.44 (Cquat), 150.43 (Cquat), 169.49 (Cquat), 202.23 (Cquat). IR: ν̃ [cm-1] 3273 (w), 3221 (w), 3113 (w), 3053 (w), 2978 (w), 2947 (w), 2909 (w), 2868 (w), 2845 (w), 2156 (w), 1971 (w), 1690 (m), 1616 (s), 1582 (w), 1526 (s), 1495 (w), 1452 (m), 1431 (w), 1406 (m), 1383 (w), 1344 (s), 1319 (w), 1302 (w), 1277 (w), 1254 (w), 1233 (m), 1204 (w), 1173 (m), 1150 (w), 1101 (w), 1043 (w), 1032 (w), 1007 (w), 984 (m), 962 (w), 943 (w), 930 (w), 860 (m), 853 (m), 824 (w), 745 (s), 723 (m), 716 (m), 706 (w), 677 (w), 652 (w). ESI MS: 446 ([M]+). HR-ESI MS calcd. for C26H28N3O4: 446.2074; Found: 446.2075. HPLC (254 nm): tR = 5.1 min, 99%.
2.11. rac-12b-Phenyl-1-(thiophene-2-carbonyl)-2,3,6,7,12,12b-hexahydro-indolo-[2,3-a]quinolizin-4(1H)-one (5i)
According to the GP compound
5i (210 mg, 55%) was isolated as a colorless solid, Mp 302-303 °C (Lit.: 315-316 °C) [
13], R
f = 0.24 (diethyl ether).
1H NMR (600 MHz, DMSO-d
6): δ 1.85 (tt,
2J
HH =
3J
HH = 13.7 Hz,
3J
HH = 5.3 Hz, 1H), 1.92 – 2.01 (m, 1H), 2.27 (dd,
2J
HH = 17.7 Hz,
3J
HH = 5.7 Hz, 1H), 2.43 (dd,
2J
HH = 15.2 Hz,
3J
HH = 4.5 Hz, 1H), 2.79 (ddd,
2J
HH = 17.7 Hz,
3J
HH = 13.0 Hz,
3J
HH = 6.8 Hz, 1H), 2.92 (ddd,
2J
HH = 15.2 Hz,
3J
HH = 12.0 Hz,
3J
HH = 5.9 Hz, 1H), 3.00 (td,
2J
HH =
3J
HH = 12.4 Hz,
3J
HH = 4.7 Hz, 1H), 4.67 (dd,
2J
HH = 12.9 Hz,
3J
HH = 5.8 Hz, 1H), 4.77 – 4.86 (m, 1H), 7.02 (td,
3J
HH = 7.4 Hz,
4J
HH = 2.3 Hz, 2H), 7.09 – 7.21 (m, 4H), 7.27 (d,
3J
HH = 7.8 Hz, 2H), 7.40 (d,
3J
HH = 7.8 Hz, 1H), 7.52 (d,
3J
HH = 8.1 Hz, 1H), 7.89 (d,
3J
HH = 4.9 Hz, 1H), 8.07 (d,
3J
HH = 3.8 Hz, 1H), 11.76 (s, 1H) .
13C NMR (151 MHz, DMSO-d
6): δ 19.79 (CH
2), 21.77 (CH
2), 28.88 (CH
2), 39.00 (CH
2), 47.17 (CH), 66.70 (C
quat), 109.47 (C
quat), 111.39 (CH), 118.08 (CH), 118.98 (CH), 121.79 (CH), 126.62 (C
quat), 126.91 (2 CH), 126.95 (CH), 127.80 (2CH), 128.30 (CH), 134.01 (CH), 135.81 (C
quat), 136.05 (CH), 136.16 (C
quat), 141.31 (C
quat), 144.71 (C
quat), 171.69 (C
quat), 192.09 (C
quat). IR: ν
̃ [cm
-1] 3267 (w), 1738 (w), 1651 (m), 1607 (s), 1585 (w), 1574 (w), 1516 (w), 1495 (w), 1454 (w), 1416 (m), 1393 (m), 1377 (w), 1342 (m), 1298 (w), 1283 (w), 1263 (w), 1250 (m), 1231 (m), 1217 (w), 1186 (w), 1153 (w), 1140 (w), 1080 (w), 1065 (w), 1094 (w), 961 (w), 947 (w), 914 (w), 880 (w), 854 (w), 826 (w), 814 (w), 729 (s), 702 (s), 681 (w), 658 (w), 627 (w). MS (ESI): 427 (M
+). ESI MS: 427 ([M]
+). HR-ESI MS calcd. for C
26H
23N
2O
2S: 427.1475; Found: 427.1479. HPLC (254 nm): t
R = 4.8 min, 99%.
2.12. rac-1-(4-Methoxybenzoyl)-2,3,6,7,12,12b-hexahydroindolo[2,3-a]quinolizin-4(1H)-one (5j)
According to the GP compound 5j (360 mg, 48%) was isolated as a colorless solid, Mp 160 °C, Rf = 0.20 (n-hexane/ethyl acetate 1:2). 1H NMR (600 MHz, CDCl3): δ 1.94 – 2.02 (m, 1H), 2.17 – 2.23 (m, 1H), 2.64 (ddd, 2JHH = 17.9 Hz, 3JHH = 11.9 Hz, 3JHH = 6.0 Hz, 1H), 2.71 (ddd, 2JHH = 17.6 Hz, 3JHH = 5.8 Hz, 3JHH = 2.5 Hz, 1H), 2.76 – 2.82 (m, 1H), 2.84 – 2.93 (m, 2H), 3.73 (ddd, 2JHH = 12.8 Hz, 3JHH = 10.1 Hz, 3JHH = 3.1 Hz, 1H), 3.90 (s, 3H), 5.17 – 5.24 (m, 1H), 5.46 – 5.51 (m, 1H), 6.99 (d, 3JHH = 8.5 Hz, 2H), 7.06 – 7.12 (m, 2H), 7.17 (d, 3JHH = 7.9 Hz, 1H), 7.48 (d, 3JHH = 7.6 Hz, 1H), 7.71 (s, 1H), 7.99 (d, 3JHH = 8.5 Hz, 2H). 13C NMR (151 MHz, CDCl3): δ 21.37 (CH2), 25.97 (CH2), 32.10 (CH2), 40.96 (CH2), 48.92 (CH), 55.42 (CH), 55.81 (CH3), 111.17 (Cquat), 111.36 (CH), 114.51 (2CH), 118.36 (CH), 119.94 (CH), 122.41 (CH), 126.61 (Cquat), 128.04 (Cquat), 131.20 (2CH), 132.81 (Cquat), 136.26 (Cquat), 164.71 (Cquat), 168.54 (Cquat), 201.08 (Cquat). IR: ν̃ [cm-1] 3900 (m), 3647 (m), 3005 (w), 2924 (w), 2845 (w), 2438 (w), 2365 (w), 1622 (s), 1616 (m), 1597 (s), 1570 (m), 1506 (m), 1437 (m), 1420 (m), 1373 (w), 1350 (w), 1317 (m), 1304 (m), 1292 (w), 1260 (s), 1234 (m), 1215 (m), 1169 (s), 1155 (m), 1117 (w), 1099 (w), 1053 (w), 1028 (m), 1009 (m), 980 (w), 841 (m), 741 (s), 685 (w), 673 (w), 606 (s). EI MS (70 eV, m/z (%)): 374 (43), 318 ([C20H18N2O2]2+, 41), 317 (83), 240 (17), 239 ([C15H15N2O]+, 100), 170 ([C11H10N2]2+, 26), 169 (56), 168 (12), 167 (12), 142 (10), 135 ([C8H7O2]+, 50), 115 (14), 107 ([C7H7O]+, 12), 92 (11), 77 (18), 49 (12). HR-ESI MS calcd. for C23H23N2O3: 375.1703; Found: 375.1705. HPLC (254 nm): tR = 4.3 min, 97%.
2.13. rac-1-(6-Chloronicotinoyl)-12b-phenyl-2,3,6,7,12,12b-hexahydroindolo-[2,3-a]quinolizin-4(1H)-one (5k)
According to the GP compound 5k (313 mg, 34%) was isolated as a colorless solid, Mp 233 °C (dec.), Rf = 0.27 (n-hexane/ethyl acetate 1:1). 1H NMR (600 MHz, DMSO-d6): δ 1.77 – 1.86 (m, 1H), 2.05 (dd, 2JHH = 14.2 Hz, 3JHH = 6.6 Hz, 1H), 2.29 (dd, 2JHH = 17.8 Hz, 3JHH = 5.9 Hz, 1H), 2.44 (dd, 2JHH = 15.2 Hz, 3JHH = 4.3 Hz, 1H), 2.82 (ddd, 2JHH = 18.7 Hz, 3JHH = 12.8 Hz, 3JHH = 6.9 Hz, 1H), 2.92 (ddd, 2JHH = 13.6 Hz, 3JHH = 12.1 Hz, 3JHH = 5.6 Hz, 1H), 2.99 (td, 2JHH = 3JHH = 12.4 Hz, 3JHH = 4.4 Hz, 1H), 4.68 (dd, 2JHH = 12.8 Hz, 3JHH = 5.6 Hz, 1H), 4.88 – 4.95 (m, 1H), 6.98 – 7.04 (m, 2H), 7.13 (t, 3JHH = 7.8 Hz, 2H), 7.17 (t, 3JHH = 7.6 Hz, 1H), 7.24 (d, 3JHH = 7.9 Hz, 2H), 7.40 (d, 3JHH = 7.8 Hz, 1H), 7.51 (d, 3JHH = 8.2 Hz, 1H), 7.55 (d, 3JHH = 8.4 Hz, 1H), 8.09 (dd, 3JHH = 8.5 Hz, 4JHH = 2.4 Hz, 1H), 8.85 (d, 4JHH = 2.4 Hz, 1H), 11.76 (s, 1H). 13C NMR (151 MHz, DMSO-d6): δ 19.84 (CH2), 21.06 (CH2), 28.78 (CH2), 39.00 (CH2), 46.35 (CH), 66.65 (Cquat), 109.63 (Cquat), 111.44 (CH), 118.15 (CH), 119.06 (CH), 121.89 (CH), 124.11 (CH), 126.58 (Cquat), 126.88 (2CH), 127.15 (CH.), 128.10 (2CH), 131.58 (Cquat), 135.84 (2Cquat), 138.90 (CH), 141.06 (Cquat), 150.01 (CH), 153.94 (Cquat), 171.71 (Cquat), 198.28 (Cquat). IR: ν̃ [cm-1] 3271 (w), 1686 (m), 1630 (m), 1605 (s), 1574 (w), 1555 (w), 1491 (w), 1449 (m), 1423 (w), 1398 (w), 1387 (w), 1364 (w), 1341 (w), 1325 (w), 1290 (w), 1277 (w), 1263 (w), 1221 (w), 1200 (w), 1182 (w), 1138 (w), 1101 (m), 1080 (w), 1047 (w), 986 (w), 947 (w), 901 (w), 835 (w), 779 (w), 758 (s), 743 (m), 704 (s), 685 (m), 656 (w), 625 (m), 607 (s). ESI MS: 458 ([M(37Cl)]+), 456 ([M(35Cl)]+). HR-ESI MS calcd. for C27H23ClN3O2: 456.1473; Found: 427.1475. HPLC (254 nm): tR = 4.8 min, 99%.
2.14. rac-1-(6-Chloronicotinoyl)-12b-cyclopropyl-2,3,6,7,12,12b-hexahydroindolo[2,3-a]quinolizin-4(1H)-on (5l)
According to the GP compound 5l (160 mg, 19%) was isolated as a colorless solid, Mp 234-236 °C, Rf = 0.21 (n-hexane/ethyl acetate 1:2). 1H NMR (600 MHz, CDCl3): δ 1.85 – 1.92 (m, 1H), 2.05 – 2.11 (m, 1H), 2.30 – 2.39 (m, 2H), 2.77 – 2.85 (m, 3H), 2.88 – 2.98 (m, 3H), 3.47 – 3.56 (m, 2H), 3.85 (dd, 2JHH = 13.5 Hz, 3JHH = 5.1 Hz, 1H), 5.26 (dd, 2JHH = 12.5 Hz, 3JHH = 4.7 Hz, 1H), 7.07 – 7.15 (m, 3H), 7.25 (d, 3JHH = 8.8 Hz, 1H), 7.50 (d, 3JHH = 7.5 Hz, 1H), 7.81 (dd, 3JHH = 8.4 Hz, 4JHH = 2.5 Hz, 1H), 7.86 (s, 1H), 8.64 (d, 4JHH = 2.5 Hz, 1H). 13C NMR (151 MHz, CDCl3): δ 21.17 (CH2), 21.27 (CH2), 28.12 (CH2), 29.52 (CH2), 33.49 (CH2), 40.25 (CH2), 45.07 (CH2), 54.14 (CH), 61.57 (Cquat), 111.33 (CH), 112.35 (Cquat), 118.64 (2CH), 120.29 (CH), 123.03 (CH), 124.61 (CH), 126.10 (Cquat), 130.86 (Cquat), 132.76 (Cquat), 136.03 (Cquat), 137.83 (CH), 149.73 (CH), 156.42 (Cquat), 169.45 (Cquat), 201.17 (Cquat). IR: ν̃ [cm-1] 3582 (w), 3271 (w), 3248 (w), 3171 (w), 3113 (w), 3084 (w), 3055 (w), 2965 (w), 2922 (w), 2891 (w), 2843 (w), 2360 (w), 2008 (w), 1686 (m), 1618 (s), 1578 (m), 1555 (w), 1497 (w), 1433 (m), 1412 (m), 1369 (m) 1350 (w), 1314 (m), 1296 (m), 1283 (m), 1260 (m), 1234 (m), 1223 (m), 1200 (w), 1173 (w), 1157 (w), 1148 (w), 1103 (m), 1078 (w), 1063 (w), 1030 (w), 1003 (w), 964 (w), 920 (w), 907 (w), 891 (w), 878 (w), 845 (w), 833 (w), 818 (w), 772 (w), 745 (s), 731 (m), 708 (m), 679 (w), 656 (w). ESI MS: 422 ([M(37Cl)]+), 420 ([M(35Cl)]+). HR-ESI MS calcd. for C24H22ClN3O2: 420.1473; Found: 420.1480. HPLC (254 nm): tR = 4.5 min, 97%.
2.15. rac-1-(Thiophene-2-carbonyl)-12b-(1-tosyl-1H-indol-3-yl)-2,3,6,7,12,12b-hexahydroindolo[2,3-a]quinolizin-4(1H)-one (5m)
According to the GP compound 5m (390 mg, 32%) was isolated as a colorless solid, Mp 314-316 °C, Rf = 0.24 (n-hexane/ethyl acetate 1:1). 1H NMR (600 MHz, CDCl3): δ 2.14 – 2.22 (m, 1H), 2.34 (s, 3H), 2.55 – 2.71 (m, 3H), 2.91 (td, 2JHH = 3JHH = 12.5 Hz, 3JHH = 4.2 Hz, 1H), 2.99 (d, 2JHH = 17.5 Hz, 1H), 3.07 (ddd, 2JHH = 16.0 Hz, 3JHH = 12.1 Hz, 3JHH = 5.4 Hz, 1H), 4.08 (d, 2JHH = 11.0 Hz, 1H), 4.94 (dd, 2JHH = 13.0 Hz, 3JHH = 5.3 Hz, 1H), 6.87 (t, 3JHH = 4.1 Hz, 1H), 7.12 – 7.18 (m, 3H), 7.18 – 7.28 (m, 4H, ücalclagert von CDCl3), 7.32 – 7.36 (m, 1H), 7.39 (d, 3JHH = 8.1 Hz, 1H), 7.48 – 7.56 (m, 4H), 7.66 (d, 3JHH = 8.0 Hz, 2H), 7.81 (d, 3JHH = 8.3 Hz, 1H), 9.18 (s, 1H). 13C NMR (151 MHz, CDCl3): δ 20.87 (CH2), 21.77 (CH3), 23.45 (CH2), 32.36 (CH2), 38.81 (CH2), 55.55 (CH), 62.90 (Cquat), 110.30 (Cquat), 111.93 (CH), 113.78 (CH), 118.83 (CH), 120.17 (CH), 120.88 (CH), 122.54 (Cquat), 122.93 (CH), 124.03 (CH), 124.90 (CH), 126.55 (Cquat), 127.16 (2CH), 127.64 (CH), 128.34 (CH), 128.94 (Cquat), 130.00 (2CH), 132.46 (CH), 134.94 (Cquat), 134.96 (Cquat), 135.31 (CH), 135.92 (Cquat), 136.03 (Cquat), 143.48 (Cquat), 145.15 (Cquat), 169.45 (Cquat), 193.93 (Cquat). IR: ν̃ [cm-1] 3152 (s), 3134 50 (s), 3103 (s), 3063 (s), 2959 (s), 2932 (s), 2916 (s), 2841 (s), 2720 (s), 1672 (m), 1609 (m), 1601 (m), 1445 (m), 1420 (m), 1406 (m), 1391 (m), 1369 (m), 1342 (m), 1329 (s), 1294 (s), 1277 (m), 1263 (s), 1240 (m), 1233 (m), 1217 (s), 1175 (w), 1159 (m), 1140 (m), 1123 (m), 1088 (m), 1040 (m), 1015 (s), 988 (m), 978 (m), 957 (s), 903 (s), 876 (s), 853 (m), 822 (m), 810 (m), 745 (w), 721 (w), 696 (w), 669 (m), 654 (w), 604 (m). EI MS (70 eV, m/z (%)): 619 ([M]+, 26), 465 ([C28H22N3O2S]+, 17), 464 ([C28H22N3O2S]+, 52), 439 (15), 438 (28), 327 (21), 326 (87), 323 (13), 298 (24), 285 (16), 284 (42), 283 (31), 282 (26), 281 (11), 269 (22), 257 (24), 256 (61), 255 (29), 155 ([C7H7O2S]+, 11), 143 (10), 111 ([C5H3OS]+, 100), 91 ([C7H7]+, 53), 65 (10). HR-ESI MS calcd. for C35H29N3O4S2: 620.1672; Found: 620.1675. HPLC (254 nm): tR = 5.8 min, 99%.
2.16. rac-1-(6-Chloronicotinoyl)-12b-(1-tosyl-1H-indol-3-yl)-2,3,6,7,12,12b-hexa-hydroindolo[2,3-a]quinolizin-4(1H)-one (5n)
According to the GP compound 5n (624 mg, 48%) was isolated as a colorless solid, Mp 294-296 °C, Rf = 0.18 (n-hexane/ethyl acetate 1:1). 1H NMR (600 MHz, DMSO-d6): δ 1.83 (tt, 2JHH = 3JHH = 13.9 Hz, 3JHH = 5.5 Hz, 1H), 2.01 (dd, 2JHH = 15.1 Hz, 3JHH = 6.7 Hz, 1H), 2.27 (s, 3H), 2.30 – 2.37 (m, 1H), 2.44 – 2.49 (m, 1H), 2.70 (ddd, 2JHH = 18.6 Hz, 3JHH = 12.8 Hz, 3JHH = 6.6 Hz, 1H), 2.81 – 2.95 (m, 2H), 4.68 – 4.74 (m, 1H), 4.98 – 5.03 (m, 1H), 7.05 – 7.12 (m, 3H), 7.21 – 7.30 (m, 3H), 7.45 (d, 3JHH = 7.8 Hz, 1H), 7.50 (d, 3JHH = 10.3 Hz, 2H), 7.55 (d, 3JHH = 8.0 Hz, 2H), 7.59 (d, 3JHH = 8.1 Hz, 1H), 7.62 – 7.68 (m, 2H), 7.98 (dd, 3JHH = 8.4 Hz, 4JHH = 2.6 Hz, 1H), 8.82 (d, 4J = 2.5 Hz, 1H), 11.76 (s, 1H). 13C NMR (151 MHz, DMSO-d6): δ 19.77 (CH2), 20.99 (CH3), 21.30 (CH2), 28.64 (CH2), 40.06 (CH2), 47.49 (CH), 63.04 (Cquat), 109.25 (Cquat), 111.61 (CH), 112.54 (CH), 118.29 (CH), 119.19 (CH), 122.07 (CH), 122.45 (CH), 123.50 (CH), 124.13 (CH), 124.87 (CH), 125.10 (CH), 125.11 (Cquat), 126.32 (2CH), 126.60 (Cquat), 127.80 (Cquat), 129.92 (2CH), 130.85 (Cquat), 133.54 (Cquat), 133.55 (Cquat), 135.67 (Cquat), 136.31 (Cquat), 138.74 (CH), 145.30 (Cquat), 149.86 (CH), 154.24 (Cquat), 170.72 (Cquat), 197.1 (Cquat). IR: ν̃ [cm-1] 3838 (s), 3233 (s), 3194 (s), 3157 (s), 2911 (s), 2847 (s), 1688 (m), 1605 (m), 1582 (m), 1557 (s), 1449 (m), 1420 (s), 1404 (m), 1368 (m), 1348 (m), 1331 (s), 1310 (s), 1298 (s), 1279 (m), 1263 (s), 1231 (m), 1192 (m), 1175 (w), 1142 (m), 1125 (m), 1107 (m), 1086 (m), 1072 (s), 1059 (s), 1028 (s), 988 (m), 959 (s), 893 (s), 874 (s), 827 (s), 804 (m), 777 (m), 750 (w), 702 (m), 677 (m), 656 (m), 633 (m). EI MS (70 eV, m/z (%)): 650 ([M (37Cl)]+, 4), 648 ([M (35Cl)]+, 11), 495 ([C29H2237ClN4O2]+, 16), 494 (15), 493 ([C29H2235ClN4O2]+, 40), 439 (12), 438 (33), 327 (23), 326 (100), 298 (22), 285 (13), 284 (35), 283 (26), 282 (21), 269 (19), 257 (20), 256 (50), 255 (24), 144 (13), 143 (59), 142 ([C6H337ClNO]+, 11), 140 ([C6H335ClNO]+, 25), 130 (37), 91 ([C7H7]+, 28). Anal. calcd. for C36H29ClN4O4S (649.16): C 66.61, H 4.50, N 8.63, S 4.94; Found: C 66.34, H 4.55, N 8.47, S 5.19.
2.17. rac-1-(2-Fluorobenzoyl)-12b-(1-tosyl-1H-indol-3-yl)-2,3,6,7,12,12b-hexa-hydroindolo[2,3-a]quinolizin-4(1H)-one (5o)
According to the GP compound 5o (332 mg, 26%) was isolated as a colorless solid, Mp 268-270 °C, Rf = 0.26 (n-hexane/ethyl acetate 1:1). 1H NMR (600 MHz, CDCl3,): δ 2.02 – 2.09 (m, 1H), 2.24 – 2.33 (m, 4H), 2.60 (dd, 2JHH = 15.5 Hz, 3JHH = 4.2 Hz, 1H), 2.70 (ddd, 2JHH = 18.1 Hz, 3JHH = 10.0 Hz, 3JHH = 6.4 Hz, 1H), 2.78 (td, 2JHH = 3JHH = 12.6 Hz, 3JHH = 4.3 Hz, 1H), 2.98 – 3.08 (m, 2H), 4.09 – 4.13 (m, 1H), 4.89 (dd, 2JHH = 13.0 Hz, 3JHH = 5.4 Hz, 1H), 6.90 (td, 3JHH = 7.5 Hz, 4JHH = 1.9 Hz, 1H), 6.94 – 6.99 (m, 1H), 7.06 (dd, 3JHF = 11.1 Hz, 3JHH = 8.3 Hz, 1H), 7.12 – 7.22 (m, 4H), 7.25 – 7.30 (m, 3H, superimposed by CDCl3), 7.38 – 7.48 (m, 3H), 7.53 (d, 3JHH = 7.8 Hz, 1H), 7.59 (s, 1H), 7.69 (d, 3jHH = 8.3 Hz, 2H), 7.89 (d, 3JHH = 8.3 Hz, 1H), 8.99 (s, 1H). 13C NMR (151 MHz, CDCl3): δ 20.90 (CH2), 21.73 (CH3), 22.46 (CH2), 32.26 (CH2), 38.85 (CH2), 56.17(d, 4JCF = 5.93 Hz, CH), 63.39 (Cquat), 110.77 (Cquat), 111.89 (CH), 113.80 (CH), 116.57(d, 2JCF = 23.1 Hz, CH), 118.85 (CH), 120.19 (CH), 121.09 (CH), 122.60 (Cquat), 122.99 (CH), 124.06 (CH), 124.81(d, 4JCF = 3.17 Hz, CH), 124.85 (CH), 126.45(d, 2JCF = 13.7 Hz, Cquat), 126.73 (Cquat), 127.16 (2CH), 128.29 (CH), 128.99 (Cquat), 129.69(d, 3JCF = 2.24 Hz, CH), 129.95 (2CH), 134.51(d, 3JCF = 9.10 Hz, CH), 134.86 (Cquat), 134.92 (Cquat), 135.70 (Cquat), 135.74 (Cquat), 145.15 (Cquat), 160.00(d, 1JCF = 251 Hz, Cquat), 169.37 (Cquat), 200.83(d, 3JCF = 3.56 Hz, Cquat). IR: ν̃ [cm-1] 3981 (w), 3854 (w), 3802 (w), 3736 (w), 3723 (w), 3588 (w), 3524 (w), 3424 (w), 3404 (w), 3271 (w), 3175 (w), 3159 (w), 3136 (w), 3105 (w), 3084 (w), 3067 (w), 3040 (w), 3019 (w), 2974 (w), 2953 (w), 2934 (w), 2913 (w), 2886 (w), 2841 (w), 2810 (w), 2752 (w), 2714 (w), 2695 (w), 2621 (w), 2488 (w), 2359 (w), 2342 (w), 1690 (w), 1609 (s), 1576 (w), 1452 (m), 1410 (m), 1396 (w), 1368 (m), 1350 (m), 1333 (w), 1294 (w), 1277 (m), 1261 (w), 1223 (w), 1213 (w), 1175 (s), 1142 (m), 1123 (m) 1086 (m), 1042 (w), 986 (m), 961 (w), 876 (w), 841 (w), 808 (w), 787 (w), 746 (s), 702 (m), 669 (s), 656 (s), 629 (m). EI MS (70 eV, m/z (%)): 631 ([M]+, 21), 476 ([C30H23FN3O2]+, 57), 438 ([C26H20N3O2S]+, 31), 326 ([C21H16N3O]2+, 98), 298 ([C19H12N3O]3+, 23), 284 ([C19H14N3]+, 51), 256 ([C17H10N3]4+, 68), 123 ([C7H4FO]+, 100), 91 ([C7H7]+, 51). Anal. calcd. for C37H30FN3O4S (631.72): C 70.35, H 4.79, N 6.65, S 5.08; Found: C 70.24, H 4.87, N 6.38, S 4.97.
2.18. rac-1-(4-Bromobenzoyl)-12b-(1-tosyl-1H-indol-3-yl)-2,3,6,7,12,12b-hexahydroindolo[2,3-a]quinolizin-4(1H)-one (5p)
According to the GP compound 5p (687 mg, 50%) was isolated as a colorless solid, Mp 290-292 °C, Rf = 0.21 (n-hexane/ethyl acetate 1:1). 1H NMR (600 MHz, CDCl3): δ 1.95 – 2.03 (m, 1H), 2.28 – 2.43 (m, 4H), 2.65 (d, 2JHH = 15.2 Hz, 2H), 2.83 – 2.97 (m, 2H), 3.01 – 3.10 (m, 1H), 4.07 – 4.16 (m, 1H), 4.90 – 4.98 (m, 1H), 7.07 – 7.12 (m, 2H), 7.13 – 7.18 (m, 1H), 7.20 – 7.27 (m, 5H, superimposed by CDCl3), 7.28 – 7.32 (m, 1H), 7.33 – 7.41 (m, 3H), 7.44 – 7.48 (m, 1H), 7.50 – 7.54 (m, 1H), 7.56 (s, 1H), 7.65 (d, 3JHH = 7.4 Hz, 2H), 7.85 – 7.92 (m, 1H), 9.11 (s, 1H).13C NMR (151 MHz, CDCl3): δ 20.90 (CH2), 21.79 (CH3), 23.22 (CH2), 32.06 (CH2), 38.98 (CH2), 53.55 (CH), 63.10 (Cquat), 110.51 (Cquat), 111.86 (CH), 113.87 (CH), 118.88 (CH), 120.22 (CH), 120.98 (CH), 122.68 (Cquat), 123.01 (CH), 124.18 (CH), 125.04 (CH), 126.60 (Cquat), 127.05 (2CH), 127.85 (CH), 128.49 (Cquat), 128.90 (Cquat), 129.33 (2CH), 129.90 (2CH), 131.98 (2CH), 134.79 (Cquat), 134.87 (Cquat), 135.83 (Cquat), 135.89 (Cquat), 135.93 (Cquat), 145.26 (Cquat), 169.51 (Cquat), 201.69 (Cquat). IR: ν̃ [cm-1] 3244 (w), 3082 (w), 2913 (w), 1676 (w), 1618 (s), 1582 (w), 1489 (w), 1447 (m), 1420 (w), 1398 (m), 1377 (m), 1366 (w), 1342 (w), 1298 (w), 1279 (w), 1265 (w), 1233 (m), 1209 (w), 1175 (s), 1144 (m), 1125 (m), 1092 (m), 1072 (w), 1053 (w), 1009 (w), 988 (m), 955 (w), 890 (w), 878 (w), 810 (m), 746 (s), 719 (w), 691 (w), 675 (s), 656 (m). EI MS (70 eV, m/z (%)): 693 ([81Br-M]+, 3), 692 ([M]+, 3), 691 ([79Br-M]+, 6), 538 ([C30H2381BrN3O2]+, 21), 536 ([C30H2379BrN3O2]+, 22), 439 ([C26H21N3O2S]+, 13), 438 ([C26H20N3O2S]+, 30), 327 (25), 326 ([C21H16N3O]2+, 100), 298 ([C19H12N3O]4+, 21), 285 (20), 284 ([C19H14N3]+, 55), 283 (33), 282 (34), 257 (24), 256 ([C17H10N3]4+, 58), 255 (27), 185 ([C7H481BrO]+, 38), 183 (35, [C7H479BrO]+, 35), 155 ([C7H7O2S]+, 22), 143 (15), 92 (16), 91 ([C7H7]+, 51), 65 (22). Anal. calcd. for C37H30BrN3O4S (692.63): C 64.16, H 4.37, N 6.07, S 4.63; Found: C 64.22, H 4.46, N 5.90, S 4.47.
2.19. rac-1-Benzoyl-12b-(1-tosyl-1H-indol-3-yl)-2,3,6,7,12,12b-hexahydro-indolo[2,3-a]quinolizin-4(1H)-one (5q)
According to the GP compound 5q (418 mg, 34%) was isolated as a colorless solid, Mp 294-297 °C, Rf = 0.22 (n-hexane/ethyl acetate 1:1). 1H NMR (600 MHz, CDCl3): δ 2.01 – 2.09 (m, 1H), 2.31 (s, 3H), 2.33 – 2.42 (m, 1H), 2.61 – 2.75 (m, 2H), 2.85 (td, 2JHH = 12.5 Hz, 3JHH = 4.2 Hz, 1H), 2.95 (ddd, 2JHH = 17.8 Hz, 3JHH = 5.6 Hz, 3JHH = 3.8 Hz, 1H), 3.05 (ddd, 2JHH = 15.4 Hz, 3JHH = 12.1 Hz, 3JHH = 5.5 Hz, 1H), 4.23 (dd, 2JHH = 11.6 Hz, 3JHH = 3.2 Hz, 1H), 4.96 (dd, 2JHH = 12.9 Hz, 3JHH = 5.3 Hz, 1H), 7.10 (d, 3JHH = 8.1 Hz, 2H), 7.15 (t, 3JHH = 7.4 Hz, 1H), 7.19 – 7.24 (m, 2H), 7.25 – 7.36 (m, 4H), 7.43 (d, 3JHH = 8.1 Hz, 1H), 7.47 – 7.55 (m, 4H), 7.60 (s, 1H), 7.67 (d, 3JHH = 8.2 Hz, 2H), 7.89 (d, 3JHH = 8.3 Hz, 1H), 8.98 (s, 1H).13C NMR (151 MHz, CDCl3): δ 20.99 (CH2), 21.74 (CH3), 23.25 (CH2), 32.35 (CH2), 38.69 (CH2), 53.47 (CH), 63.16 (Cquat), 110.44 (Cquat), 111.85 (CH), 113.92 (CH), 118.83 (CH), 120.20 (CH), 120.85 (CH), 122.56 (Cquat), 122.96 (CH), 124.10 (CH), 124.83 (CH), 126.52 (Cquat), 127.10 (2CH), 127.92 (2CH), 128.24 (CH), 128.89 (2CH), 129.20 (Cquat), 129.91 (2CH), 133.43 (CH), 134.90 (Cquat), 134.94 (Cquat), 135.81 (Cquat), 135.87 (Cquat), 137.19 (Cquat), 145.09 (Cquat), 169.22 (Cquat), 202.45 (Cquat). IR: ν̃ [cm-1] 3136 (w), 3132 (w), 3107 (w), 3086 (w), 3067 (w), 3030 (w), 2959 (w), 2934 (w), 2843 (w), 1734 (w), 1686 (w), 1609 (m), 1582 (w), 1545 (w), 1493 (w), 1447 (m), 1408 (m), 1389 (w), 1369 (m), 1350 (w), 1331 (w), 1294 (w), 1275 (w), 1261 (w), 1227 (w), 1211 (w), 1175 (s), 1159 (m), 1140 (m), 1123 (m), 1070 (w), 1047 (w), 986 (m), 959 (w), 903 (w), 876 (w), 829 (w), 810 (w), 797 (w), 746 (s), 712 (m), 692 (w), 671 (m), 656 (s), 627 (w). EI MS (70 eV, m/z (%)): 613 ([M]+, 16), 459 (18), 458 ([C30H24N3O2]+, 54), 438 ([C26H20N3O2S]+, 26), 327 (23), 326 ([C21H16N3O]2+, 100), 298 (22), 285 (18), 284 ([C19H14N3]+, 46), 283 (31), 282 (26), 269 (18), 257 (25), 256 ([C17H10N3]4+, 66), 255 (29), 105 ([C7H5O]+, 76), 91 ([C7H7]+, 32), 77 (31). HR-ESI MS calcd. for C37H32N3O4S: 614.2108; Found: 614.2108. HPLC (254 nm): tR = 5.8 min, 99 %. Anal. calcd. for C37H31N3O4S (613.73): C 72.41, H 5.09, N 6.85, S 5.22; Found: C 71.56, H 5.04, N 6.52, S 4.97.
2.20. rac-1-(4-Methoxybenzoyl)-12b-(1-tosyl-1H-indol-3-yl)-2,3,6,7,12,12b-hexahydroindolo[2,3-a]quinolizin-4(1H)-one (5r)
According to the GP compound 5r (423 mg, 33%) was isolated as a colorless solid, Mp 273-274 °C, Rf = 0.16 (n-hexane/ethyl acetate 1:1). 1H NMR (600 MHz, CDCl3): δ 2.01 – 2.07 (m, 1H), 2.32 (s, 3H), 2.36 – 2.46 (m, 1H), 2.63 – 2.69 (m, 1H), 2.74 (ddd, 2JHH = 18.0 Hz, 3JHH = 10.8 Hz, 3JHH = 6.8 Hz, 1H), 2.84 (td, 2JHH = 3JHH = 12.5 Hz, 3JHH = 4.2 Hz, 1H), 2.97 (ddd, 2JHH = 18.3 Hz, 3JHH = 5.7 Hz, 3JHH = 3.4 Hz, 1H), 3.03 (ddd, 2JHH = 15.4 Hz, 3JHH = 12.1 Hz, 3JHH = 5.5 Hz, 1H), 3.82 (s, 3H), 4.17 (dd, 2JHH = 11.8 Hz, 3JHH = 3.1 Hz, 1H), 4.92 – 4.98 (m, 1H), 6.78 – 6.81 (m, 2H), 7.12 – 7.16 (m, 3H), 7.18 – 7.23 (m, 2H), 7.24 – 7.28 (m, 2H, superimposed by CDCl3), 7.30 – 7.33 (m, 1H), 7.39 (d, 3JHH = 8.1 Hz, 1H), 7.52 (d, 3JHH = 7.8 Hz, 1H), 7.59 (s, 1H), 7.59 – 7.63 (m, 2H), 7.66 – 7.70 (m, 2H), 7.86 – 7.89 (m, 1H), 8.81 (s, 1H).13C NMR (151 MHz, CDCl3): δ 21.02 (CH2), 21.72 (CH3), 23.31 (CH2), 32.32 (CH2), 38.74 (CH2), 52.90 (CH), 55.70 (CH3), 63.30 (Cquat), 110.29 (Cquat), 111.85 (CH), 113.88 (CH), 114.10 (2CH), 118.80 (CH), 120.17 (CH), 120.82 (CH), 122.62 (Cquat), 122.92 (CH), 124.06 (CH), 124.75 (CH), 126.48 (Cquat), 127.13 (2CH), 128.20 (CH), 129.30 (Cquat), 129.59 (Cquat), 129.91 (2CH), 130.62 (2CH), 134.92 (Cquat), 134.95 (Cquat), 135.81 (Cquat), 135.88 (Cquat), 145.08 (Cquat), 163.99 (Cquat), 169.51 (Cquat), 200.04 (Cquat). IR: ν̃ [cm-1] 3298 (w), 3258 (w), 3063 (w), 2947 (w), 2851 (w), 1668 (w), 1618 (m), 1599 (m), 1574 (w), 1508 (w), 1445 (w), 1422 (w), 1395 (m), 1375 (m), 1323 (w), 1300 (w), 1279 (w), 1233 (m), 1209 (w), 1175 (s), 1144 (m), 1124 (m), 1090 (w), 1082 (w), 1051 (w), 1032 (w), 1013 (w), 988 (m), 835 (w), 810 (w), 760 (m), 750 (m), 737 (s), 706 (w), 675 (s), 656 (w), 621 (w). EI MS (70 eV, m/z (%)): 643 ([M]+, 14), 488 ([C31H26N3O3]+, 39), 439 ([C26H21N3O2S]+, 17), 438 ([C26H20N3O2S]+, 21), 326 ([C21H16N3O]2+, 62), 284 ([C19H14N3]+, 36), 283 (19), 282 (16), 269 (15), 257 (19), 256 ([C17H10N3]4+, 51), 255 (20), 135 (100), 91 ([C7H7]+, 20). Anal. calcd. for C38H33N3O5S (643.76): C 70.90, H 5.17, N 6.53, S 4.98; Found: C 70.86, H 5.12, N 6.39, S 4.82.
2.21. rac-1-(4-Methylbenzoyl)-12b-(1-tosyl-1H-indol-3-yl)-2,3,6,7,12,12b-hexahydroindolo[2,3-a]quinolizin-4(1H)-one (5s)
According to the GP compound 5s (458 mg, 36%) was isolated as a colorless solid, Mp 293-296 °C, Rf = 0.29 (n-hexane/ethyl acetate 1:1). 1H NMR (600 MHz, CDCl3): δ 2.01 – 2.07 (m, 1H), 2.32 (s, 3H), 2.36 – 2.44 (m, 4H), 2.66 (dd, 2JHH = 15.5 Hz, 3JHH = 4.0 Hz, 1H), 2.72 (ddd, 2JHH = 18.1 Hz, 3JHH = 11.2 Hz, 3JHH = 6.8 Hz, 1H), 2.82 (td, 2JHH = 3JHH = 12.5 Hz, 3JHH = 4.2 Hz, 1H), 2.93 (ddd, 2JHH = 18.2 Hz, 3JHH = 5.7 Hz, 3JHH = 3.1 Hz, 1H), 3.03 (ddd, 2JHH = 15.6 Hz, 3JHH = 12.0 Hz, 3JHH = 5.4 Hz, 1H), 4.15 (dd, 2JHH = 12.1 Hz, 3JHH = 3.0 Hz, 1H), 4.93 – 4.97 (m, 1H), 7.11 – 7.16 (m, 5H), 7.17 – 7.24 (m, 2H), 7.25 – 7.29 (m, 2H, superimposed by CDCl3), 7.32 (dd, 3JHH = 8.1 Hz, 4JHH = 1.1 Hz, 1H), 7.38 (d, 3JHH = 8.1 Hz, 1H), 7.47 (d, 3JHH = 8.0 Hz, 2H), 7.52 (d, 3JHH = 7.8 Hz, 1H), 7.59 (d, 4JHH = 1.1 Hz, 1H), 7.67 – 7.71 (m, 2H), 7.89 (d, 3JHH = 8.3 Hz, 1H), 8.77 (s, 1H). 13C NMR (151 MHz, CDCl3): δ 21.02 (CH2), 21.75 (CH3), 21.77 (CH3), 23.32 (CH2), 32.46 (CH2), 38.58 (CH2), 53.35 (CH), 63.16 (Cquat), 110.37 (Cquat), 111.85 (CH), 113.93 (CH), 118.82 (CH), 120.18 (CH), 120.79 (CH), 122.55 (Cquat), 122.93 (CH), 124.07 (CH), 124.76 (CH), 126.49 (Cquat), 127.14 (2CH), 128.19 (2CH), 128.34 (CH), 129.31 (Cquat), 129.62 (2CH), 129.90 (2CH), 134.48 (Cquat), 134.96 (Cquat), 134.98 (Cquat), 135.79 (Cquat), 135.91 (Cquat), 144.66 (Cquat), 145.05 (Cquat), 169.15 (Cquat), 201.80 (Cquat). IR: ν̃ [cm-1] 3323 (w), 3285 (w), 3238 (w), 1668 (w), 1618 (s), 1580 (w), 1491 (w), 1447 (m), 1420 (w), 1396 (w), 1377 (m), 1344 (w), 1323 (w), 1300 (w), 1279 (w), 1263 (w), 1236 (w), 1209 (w), 1186 (m), 1175 (m), 1144 (m), 1125 (m), 1086 (w), 1053 (w), 1022 (w), 988 (m), 957 (w), 837 (w), 810 (w), 748 (s), 704 (w), 675 (s), 656 (w). EI MS (70 eV, m/z (%)): 627 ([M]+, 14), 473 (15), 472 ([C31H26N3O2]+, 47), 438 ([C26H20N3O2S]+, 25), 327 (22), 326 ([C21H16N3O]2+, 88), 323 (16), 298 ([C19H12N3O]4+, 20), 285 (20), 284 ([C19H14N3]+, 55), 283 (31), 282 (25), 269 (19), 257 (28), 256 ([C17H10N3]4+, 75), 255 (30), 119 ([C8H7O]+, 100), 91 ([C7H7]+, 72). Anal. calcd. for C38H33N3O4S (627.76): C 72.71, H 5.30, N 6.69, S 5.11; Found: C 72.91, H 5.34, N 6.55, S 5.03.
2.22. Methyl (6S)-12b-butyl-1-(6-chloronicotinoyl)-4-oxo-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-6-carboxylate (5t)
According to the GP compound 5t (198 mg, 20%) was isolated as a colorless solid, Mp 220-230 °C, Rf = 0.33 (n-hexane/ethyl acetate 5:7). [α]D25: +92 ° (c = 1 mg/mL, CH2Cl2), 1H NMR (500 MHz, CDCl3) δ 0.79 (t, 3JHH = 7.0 Hz, 3H), 1.19 – 1.25 (m, 4H), 2.57 – 2.65 (m, 1H), 2.81 – 2.89 (m, 2H), 3.13 (dd, 2JHH = 16.0 Hz, 3JHH = 6.9 Hz, 1H), 3.47 (dd, 2JHH = 16.0 Hz, 3JHH = 2.7 Hz, 1H), 3.69 (s, 3H), 5.05 – 5.11 (m, 1H), 5.58 (dd, 2JHH = 6.9 Hz, 3JHH = 2.6 Hz, 1H), 7.08 – 7.19 (m, 3H), 7.42 (d, 3JHH = 8.4 Hz, 1H), 7.48 (d, 3JHH = 7.7 Hz, 1H), 7.95 (s, 1H), 8.17 (dd, 3JHH = 8.4 Hz, 4JHH = 2.5 Hz, 1H), 8.99 (d, 4JHH = 2.5 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 14.08 (CH3), 22.62 (CH2), 22.67 (CH2), 22.84 (CH2), 25.68 (CH2), 30.08 (CH2), 37.40 (CH2), 51.45 (CH), 52.80 (CH3), 55.10 (CH), 63.05 (Cquat), 108.39 (Cquat), 111.51 (CH), 118.54 (CH), 120.18 (CH), 122.92 (CH), 124.83 (CH), 125.48 (Cquat), 131.34 (Cquat), 133.81 (Cquat), 136.43 (Cquat), 138.54 (CH), 150.61 (CH), 156.80 (Cquat), 172.93 (Cquat), 173.14 (Cquat), 204.15 (Cquat). IR: ν̃ [cm-1] 3377 (w), 3055 (w), 2955 (w), 2926 (w), 2860 (w), 2359 (w), 1726 (m), 1688 (m), 1630 (s), 1574 (w), 1555 (w), 1472 (w), 1445 (w), 1406 (m), 1381 (w), 1354 (w), 1337 (m), 1304 (m), 1288 (w), 1275 (w), 1261 (w), 1224 (m), 1206 (w), 1177 (w), 1148 (w), 1126 (m), 1099 (m), 1067 (w), 1013 (w), 974 (w), 962 (w), 943 (w), 912 (w), 839 (w), 787 (w), 766 (w), 743 (s), 712 (w), 677 (w), 633 (w). EI MS (70 eV, m/z (%)): 495 ([M(37Cl)]+), 493 ([M(35Cl)]+, 4), 438 ([C23H1937ClN3O4]+, 16), (16), 437 (12), 436 ([C23H1935ClN3O4]+, 49), 283 (10), 237 ([C15H13N2O]3+, 15), 225 ([C15H17N2]2+, 21), 195 (16), 183 (17), 182 (26), 181 ([C12H9N2]5+, 10), 142, ([C6H337ClNO]+, 33), 140 ([C6H325ClNO]+, 100), 112 (14). Anal. calcd. for C27H28ClN3O4 (493.99): C 65.65, H 5.71, N 8.51; Found: C 65.43, H 5.51, N 8.23.
2.23. Methyl (6S)-1-(4-bromobenzoyl)-12b-butyl-4-oxo-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-6-carboxylate (5u)
According to the GP compound 5u (195 mg, 18%) was isolated as a yellow solid, Mp 245-248 °C, Rf = 0.19 (n-hexane/ethyl acetate 7:3). [α]D25: +58 ° (c = 1 mg/mL, CH2Cl2), 1H NMR (600 MHz, CDCl3) δ 0.86 (t, 3JHH = 7.3 Hz, 3H), 1.07 – 1.15 (m, 1H), 1.17 – 1.23 (m, 1H), 1.27 1.36 (m, 2H), 2.00 – 2.08 (m, 1H), 2.30 – 2.44 (m, 2H), 2.59 (ddd, 2JHH = 14.7 Hz, 3JHH = 12.7 Hz, 3JHH = 4.2 Hz, 1H), 2.85 (ddd, 2JHH = 18.5 Hz, 3JHH = 10.3 Hz, 3JHH = 5.8 Hz, 1H), 2.91 - 3.00 (m, 2H), 3.61 (s, 4H), 3.83 (dd, 2JHH = 13.4 Hz, 3JHH = 5.6 Hz, 1H), 6.08 – 6.18 (m, 1H), 7.04 – 7.10 (m, 3H), 7.34 – 7.46 (m, 4H), 7.50 – 7.58 (m, 1H), 7.74 (s, 1H). 13C NMR (151 MHz, CDCl3) δ 14.24 (CH3), 21.36 (CH2), 21.39 (CH2), 23.64 (CH2), 26.72 (CH2), 29.89 (CH2), 35.62 (CH2), 50.73 (CH), 52.36 (CH3), 53.94 (CH), 62.73 (Cquat), 108.69 (Cquat), 111.09 (CH), 118.66 (CH), 119.97 (CH), 122.76 (CH), 126.04 (Cquat), 129.12 (Cquat), 129.33 (2CH), 132.09 (2CH), 132.58 (Cquat), 135.54 (Cquat), 136.10 (Cquat), 171.19 (Cquat), 171.39 (Cquat), 202.11 (Cquat). IR: ν̃ [cm-1] 3275 (w), 3057 (w), 2953 (w), 2928 (w), 2901 (w), 2870 (w), 2857 (w), 1736 (m), 1672 (m), 1630 (s), 1584 (m), 1566 (w), 1483 (w), 1454 (m), 1435 (m), 1387 (s), 1356 (m), 1327 (m), 1292 (m), 1279 (m), 1254 (m), 1202 (s), 1179 (m), 1167 (m), 1153 (m), 1109 (m), 1070 (m), 1028 (m), 1007 (s), 970 (m), 912 (w), 889 (w), 839 (m), 812 (m), 741 (s), 679 (m). EI MS (70 eV, m/z (%)): 538 ([M(81Br)]+, 3), 536 ([M(79Br)]+, 3), 481 ([C24H20BrN2O4(81Br)]+, 33), 479 ([C24H20BrN2O4(79Br)]+, 27), 283 ([C16H15N2O3]3+, 33), 242 (17), 225 ([C15H17N2]2+, 33), 201 (13), 195 (13), 185 ([C7H4BrO(81Br)]+, 66), 184 (10), 183 ([C7H4BrO(79Br)]+, 100), 182 (26), 155 (11), 130 (31). HR-ESI MS calcd. for C28H30BrN2O4: 537.1383. Found. 537.1375. HPLC (245 nm): tR = 5.4 min, 99 %. Anal. calcd. for C28H29BrN2O4 (537.45): C 62.57, H 5.44, N 5.21; Found: C 61.68, H 5.67, N 4.93.