1. Introduction
Leaf color mutations are common and easily detectable morphological phenotype variations in higher plants [
1,
2]. They represent essential resources for insight into plant physiology and metabolism as they mostly affect plants’ photosynthetic efficiency, which results in poor growth and development performances and considerable economic losses [
1,
2]. Accordingly, LCMs have become ideal materials for investigating pigments’ metabolism, chloroplast formation and differentiation, photosynthesis, biotic and abiotic stress responses, etc. [
1,
2]. In rice, studies on LCMs have significantly contributed to the crop improvement [
1].
D. officinale (also called
D. catenatum) is an Orchid with high medicinal and ornamental values [
3,
4]. Therefore, understanding leaf color mutation mechanisms will contribute to developing diverse attractive
D. offficinale genotypes and deepen our knowledge of plant physiology and metabolism. In previous studies, we combined physiological and comparative transcriptomics analysis of LCMs of
D. officinale and unveiled that variation in leaf colors is associated with significantly reducing the number of chloroplasts and chlorophyll and carotenoid contents [
5]. We found that the photosynthetic efficiency of LCMs is greatly influenced by light intensity [
6]. Importantly, we have identified key DEGs (differentially regulated genes) related to variation in leaf color [
5]. Unfortunately, we could not validate these DEGs as potential candidate genes and perform functional investigations due to the unavailability of a reliable RG for qRT-PCR normalization.
Currently, qRT-PCR analysis is the widely used method to verify the reliability of transcriptome data due to its high sensitivity, accuracy, specificity, and reproducibility [
7,
8,
9,
10,
11]. But, its accuracy relies on various factors, including the integrity of initial samples, quality of RNA, primers specificity, reliability of RGs, and the efficiency of the reverse transcription and amplification [
12]. Of them, the suitability of RGs is very critical. The selection of inappropriate RG(s) will cause erroneous results by qRT-PCR analysis, resulting in wrong conclusions [
13]. Therefore, using one or more stable RGs as the calibration standard(s) is recommended [
11,
14]. In many plants, reliable RGs are often selected from housekeeping genes with stable expressions, such as actin (
ACT), polyubiquitin (
UBQ), glyceraldehyde 3-phosphate dehydrogenase (
GAPDH), elongation factor (
EF), 18S ribosomal RNA (
18S), Tubulin (
TUB), etc. [
14,
15,
16,
17]. For instance,
actin2 and
18SRNA are used to calibrate the expression levels of leaf color-related genes in
Lilium regale and wheat, respectively. Notably, a reliable RG should display stable expressions at different developmental stages or under diverse experimental conditions in all plant organs [
9,
18,
19]. But, other studies in many species have revealed that RGs do not always exhibit stable expression levels, specifically under changing environments [
19,
20,
21]. Therefore, it is essential to identify stable and reliable RG(s) for specific traits and experimental conditions in each plant species [
9,
22,
23].
In this study, based on the previously released RNA-seq data [
5], we selected ten candidate RGs and investigated their expression stability using common statistical algorithms (BestKeeper, GeNorm, and NormFinder). As a result, we identified and validated the most suitable RG for qRT-PCR normalization of leaf color-related genes in
D. officinale. Our findings will promote genomics studies on LCMs
D. officinale.
2. Materials and Methods
2.1. Plant Materials and Sampling
The wild-type (green leaf, CK) and a leaf color-mutant (yellow)
D. officinale tissue culture materials were used in this study (
Figure 1). When the tissue cultures were at the seedlings stage, leaves were sampled from ten plants of each genotype, mixed, and immediately frozen in liquid nitrogen. Subsequently, the collected samples were stored at −80 °C in a refrigerator until further experimentations. Three biological and experimental repeats were achieved for each analysis.
2.2. Selection of Candidate Internal RGs and Chlorophyll Pathway-Related Genes
We previously analyzed the transcriptome of wild-type and leaf color-mutant of
D. officinale [
5]. Based on the relative stability of the expression patterns of genes (FPKM values) in all groups based, ten commonly used internal RGs, including
Actin (Actin 7),
UBQ (polyubiquitin),
GAPDH (3-Glyceraldehyde phosphate dehydrogenase),
EF1α (elongation factor α),
β-TUB (β-Tubulin),
α-TUB,
RPL13AD (60S ribosomal protein l13a-4),
PIP1-2 (water protein channel pip1-2),
ALB3 (intimal protein ppf-1), and
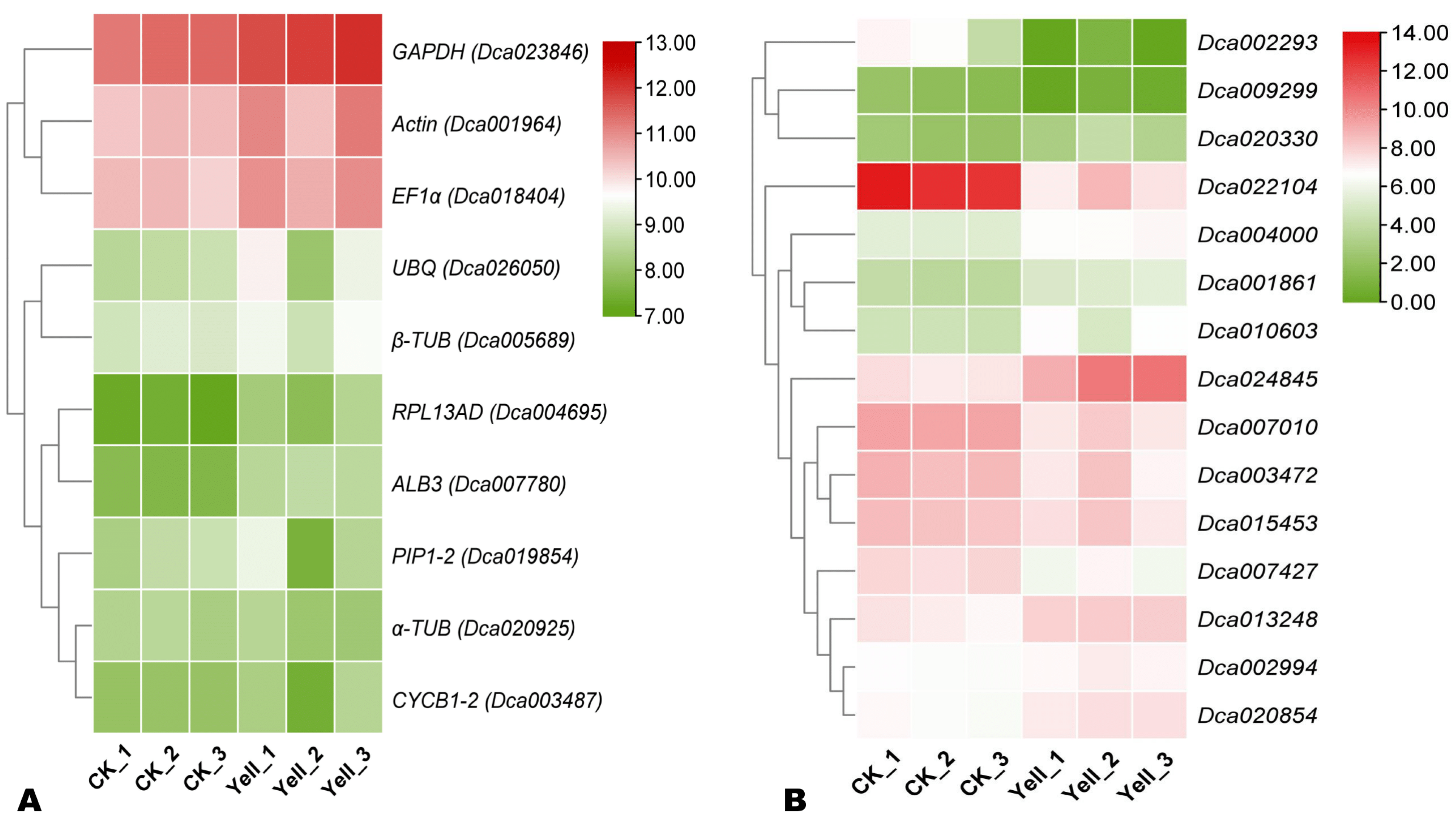
CYCB1-2 (Cyclin B1-2) were screened out as potential RGs for analyzing the relative expression of leaf color-related genes. The expression patterns and FPKM values of these genes are shown in
Figure 2A and
Table S1, respectively. In addition, based on the functional annotation of DEGs (differentially expressed genes) from the transcriptomics analysis [
5], we selected fifteen genes assigned to pigment synthesis (chlorophyll synthesis pathway) for validation of the most reliable RG (
Figure 2B,
Table S2). Primer Premier 5.0 software was used to design specific primers for each gene. Primers of the ten potential RG and fifteen genes are presented in
Table 1 and
Table S3, respectively.
2.3. Total RNA Extraction and cDNA Synthesis
Total RNA from all samples was extracted using the RNAprep pure polysaccharide polyphenol plant total RNA extraction kit according to the manufacturer’s instructions. The extracted RNA concentration and quality (OD260/OD280 value) were assessed with Nanodrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Finally, the integrity of total RNA was confirmed through electrophoresis (1% agarose gel).
The GeneAmp® PCR System 9700 (Applied Biosystems, Foster City, CA, USA) was used for cDNA construction. The reverse transcription was achieved using Hiscript II QRT super mix according to the manufacturer’s instructions for users. The reaction conditions were 15 min at 42 °C and 5 s at 85 °C. Each synthesized cDNA was ten-time diluted (with nuclease-free water)and stored in the refrigerator at −20 °C.
2.4. qRT-PCR Analysis and Validation of the Most Reliable RG
The qRT-PCR was conducted on Lightcycle
®480 type II fluorescent quantitative PCR (Roche, Switzerland) using the quantifast
®SYBR
®green PCR kit and following instructions by the manufacturer. The PCR reaction was a mixture of cDNA 1 μL; Upstream and downstream primers (10 μMol·L
−1) 0.2 μL for each; 2× QuantiFast
®SYBR
®Green PCR Master Mix 5 μL; and Nuclease-free H
2O 3.6 μL. The reaction procedure was as follows: pre-denaturation at 95 °C for 5 min; denaturation at 95 °C for 10 s (40 cycles); and annealing at 60 °C for 30 s. Three technical and biological repetitions were set for each gene. At the end of the cycle, the melting curve was used to detect the product specificity, and each gene’s Ct (cycle threshold) values were computed automatically [
15,
24].
The reliability of the most reliable RG was confirmed through qRT-PCR analysis of the relative expression of the fifteen selected chlorophyll pathway-related genes, followed by expression level calculation via the 2
−∆∆Ct method [
25]. The internal control was the most stable RG.
2.5. Stability and Statistical Analyses
The Ct values were considered as relative quantities to perform gene expression stability analysis [
15,
24]. Three commonly used software, including NormFinder [
7,
9], BestKeeper [
26], and geNorm [
22]. Finally, we applied the GM (geometric mean) method to fit the results from the three software and generate a comprehensive stability ranking for the candidate RG [
27]. Data Processing System, GraphPad Prism v9.0.0121 (GraphPad 159 Software Inc., La Jolla, CA, USA), and Microsoft Excel 2016 were used for data analysis and graphing [
27,
28]. Statistical differences were obtained via an independent t-test at
p < 0.05. Finally, we used TBtools software to construct the heatmap of genes [
29].
3. Results
3.1. Primers Specificity and Expression Profiles Analyses of Candidate RGs
To uncover a suitable RG for normalizing the relative expression levels of leaf color-related genes in
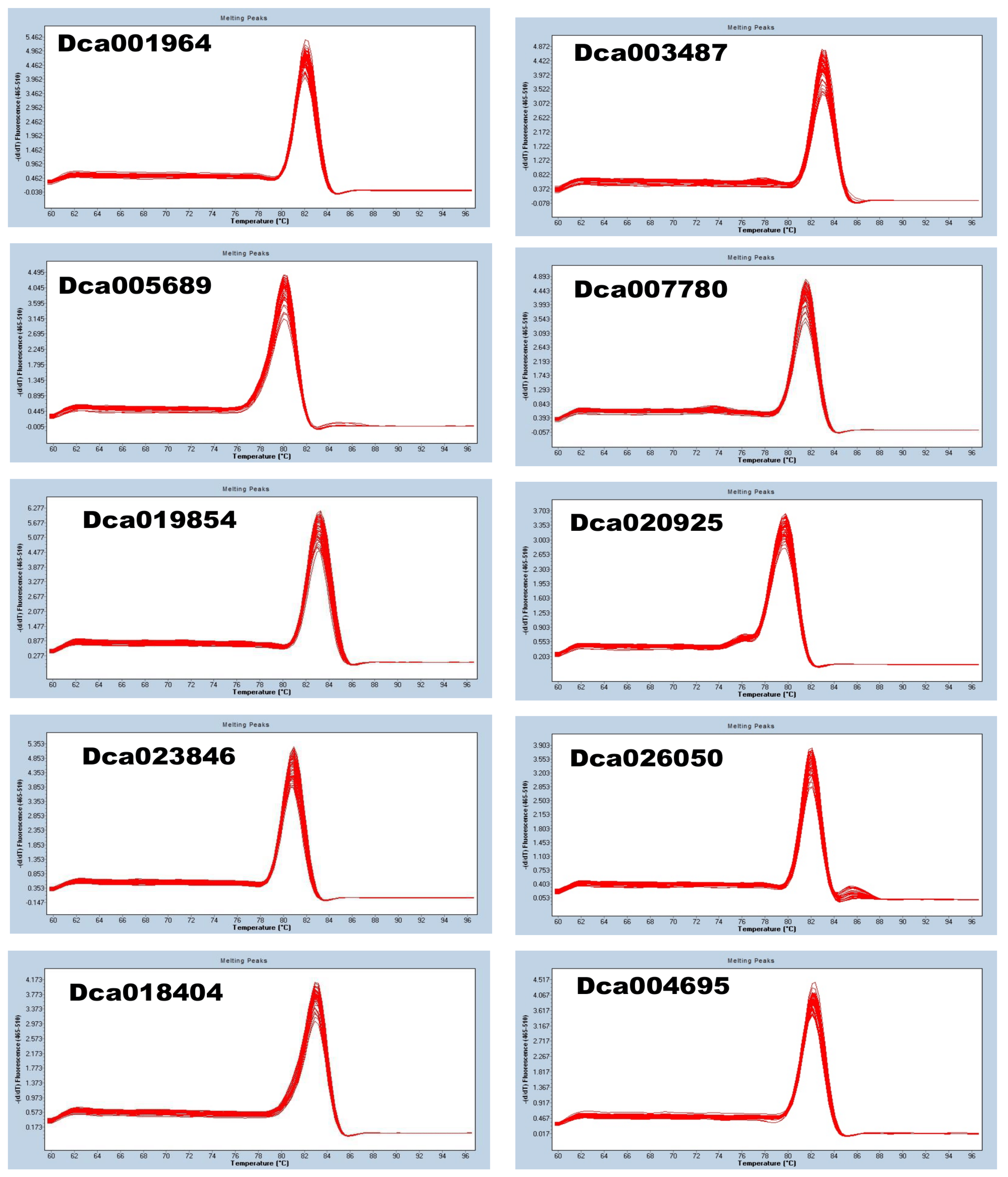
D. officinale via qRT-PCR, we selected ten candidate RGs from transcriptome data and subjected them to qRT-PCR analysis. The melting curves showed that the primers of each potential RG were highly specific (
Figure 3). All ten genes exhibited a single peak with no primer dimer, and all amplicons showed good repeatability, confirming the accuracy, reliability, and specificity of primers.
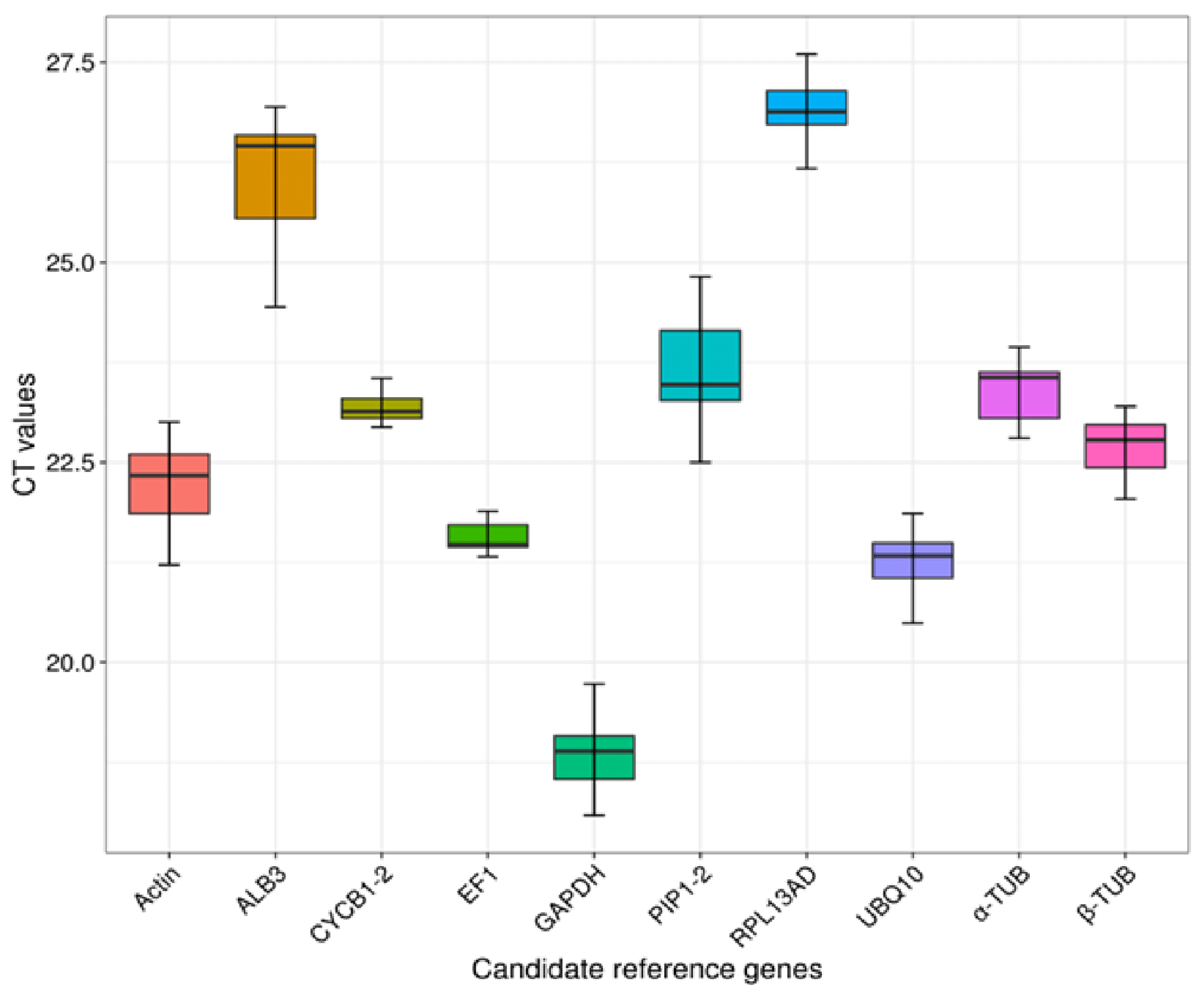
In order to assess the expression levels of each potential RGs, we automatically computed their respective Ct values. The results showed that the Ct values of the ten genes ranged from 18.858 (
GAPDH) to 26.915 (
RPL13AD) on average (
Figure 4). The average Ct value of
actin-7,
UBQ10,
EF1α,
β-TUB,
α-TUB,
PIP1-2,
ALB3, and
CYCB1-2 was 22.241, 21.288, 21.493, 22.678, 23.386, 23.571, 26.069, and 23.230, respectively.
CYCB1-2 exhibited the least expression variation from 22.94 to 23.93, followed by
EF1α from 20.84 to 22.20 (
Figure 4). The summary of the variation in expression levels (Ct values) of all candidate RGs is presented in
Table S4.
3.2. Stability Analysis of Candidate Internal RGs
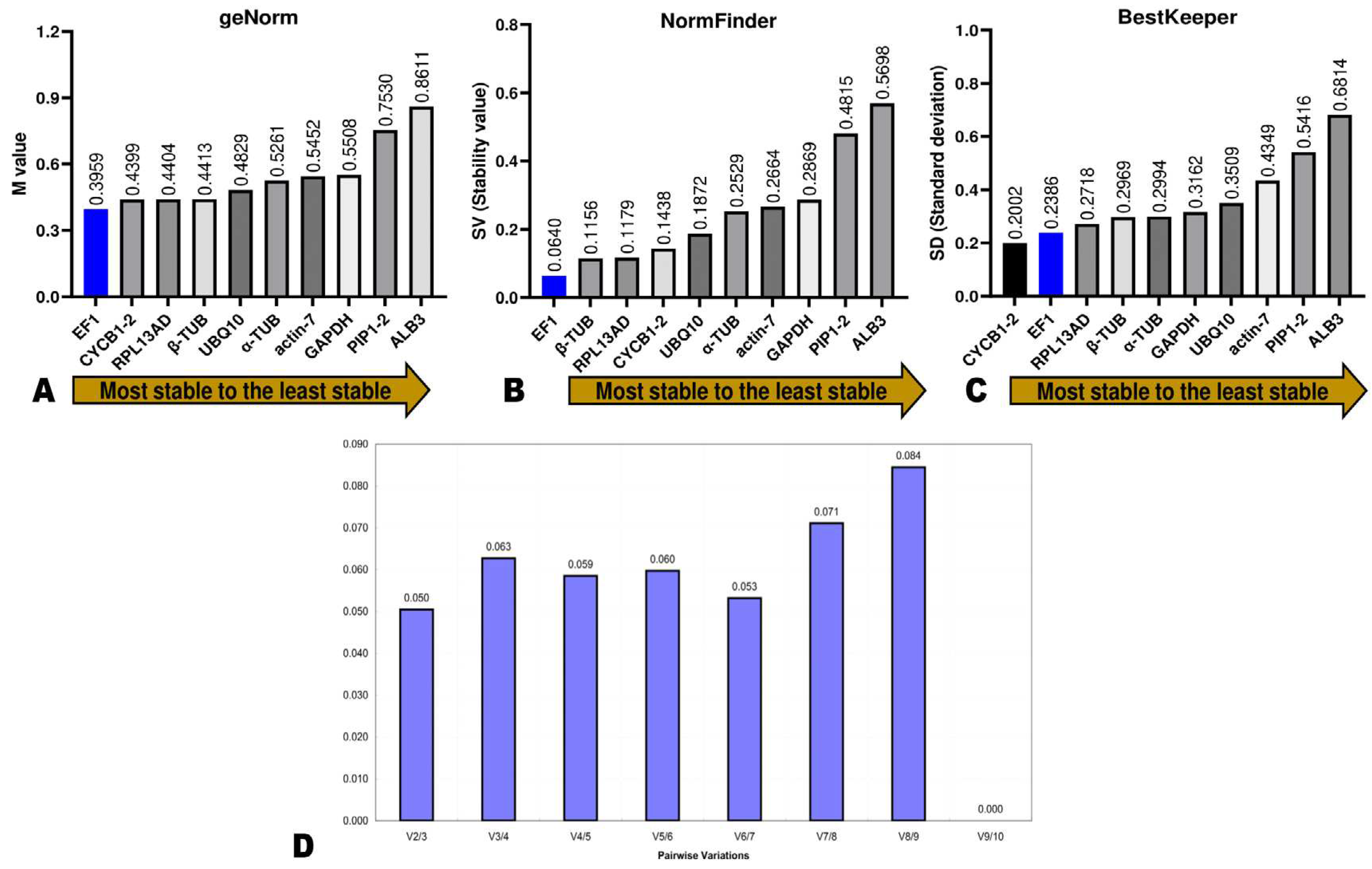
To determine the stability rankings of the ten potential RGs, three independent software (BestKeeper, GeNorm, and NormFinde) were used, and the results were integrated via the GM method to obtain the comprehensive stability ranking. Firstly, we used geNorm to compute the expression stability measured (M) of each potential RG based on average pairwise expression ratios. A gene can be selected as an internal RG if its M value is below 1.5, and the lower the M value, the more the gene is stable [
15]. The analysis by geNorm showed that the M values of all ten genes were > 0.9, indicating they both met the basic requirements for an internal RG (
Figure 5a).
EF1α was the most stable gene, with an M value of 0.395, followed by
CYCB1-2 (M value of 0.439) (
Figure 5a). Usually, a single RG is insufficient to achieve high accuracy. Accordingly, sometimes using two or more RGs for precise and reliable normalization is recommended. The optimal number of RGs is determined by pairwise variation value (Vn/n + 1, V value). If Vn/n + 1 ≥ 0.15, the adequate number of RGs equals n + 1. In contrast, if Vn/n + 1 < 0.15, the number is n [
14,
22]. The V value of all pairwise comparisons was less than 0.15 (
Figure 5D), indicating two RGs might be required. Thus, the M values indicate
EF-1α +
CYCB1-2 is the best combination for accurate normalization of leaf color-related genes’ expression levels in
D. officinale.
NormFinder serves to compute the SV (stability value) of RGs and unveil the optimal number of RGs for precise normalization through analysis of intra- and inter-group variations [
15]. The most stable gene is one that exhibits a lower expression level than the average SVs. The stability ranking by NormFinder and GeNorm analyses were somewhat similar, with a little variation in the classification of the top four genes (
Figure 5B). Interestingly,
EF1α (SV = 0.064) occupied the first rank, confirming it was the most stable RG.
β-TUB (0.115),
RPL13AD (0.117), and
CYCB1-2 (0.143) ranked second, third, and fourth in terms of stability by NormFinder, respectively (
Figure 5B).
Figure 5.
Expression stability of candidate RGs (reference genes) by geNorm analysis (A), NormFinder (B), and BestKeeper (C). (D) The pairwise variation values (Vn/Vn + 1, V) of candidate RGs.
Figure 5.
Expression stability of candidate RGs (reference genes) by geNorm analysis (A), NormFinder (B), and BestKeeper (C). (D) The pairwise variation values (Vn/Vn + 1, V) of candidate RGs.
BestKeeper helps to evaluate the stability index of RGs by calculating mainly the SD (standard deviation) [
26]. A gene is stable if its SD is lower than 1.0 [
15,
26]. The results by BestKeeper indicated that the SD of all ten genes was lower than 0.7, supporting that they are suitable for use as internal RGs
Figure 5C).
CYCB1-2 (SV = 0.2002) and
EF1α (SV = 0.2386) ranked first and second in terms of stability, respectively (
Figure 5C). Both three software revealed that
PIP1-2 and
ALB3 were the least stable genes.
We applied the GM method to integrate the results from the three software and determine the comprehensive stability ranking of the ten RGs. As presented in
Table 2, GM values confirmed that
EF1α was the most suitable and reliable RG, followed by
CYCB1-2,
RPL13AD,
β-TUB,
UBQ10,
α-TUB,
GAPDH,
Actin,
PIP1-2, and
ALB3.
3.3. Validation of EF1α as the Most Reliable and Stable Internal RG
To confirm the reliability and stability of
EF1α for accurate normalization of relative expression levels of leaf color-related genes, we investigated the expression of fifteen selected genes from the chlorophyll biosynthesis pathway (
Figure 2B,
Table S2) via qRT-PCR. As shown in
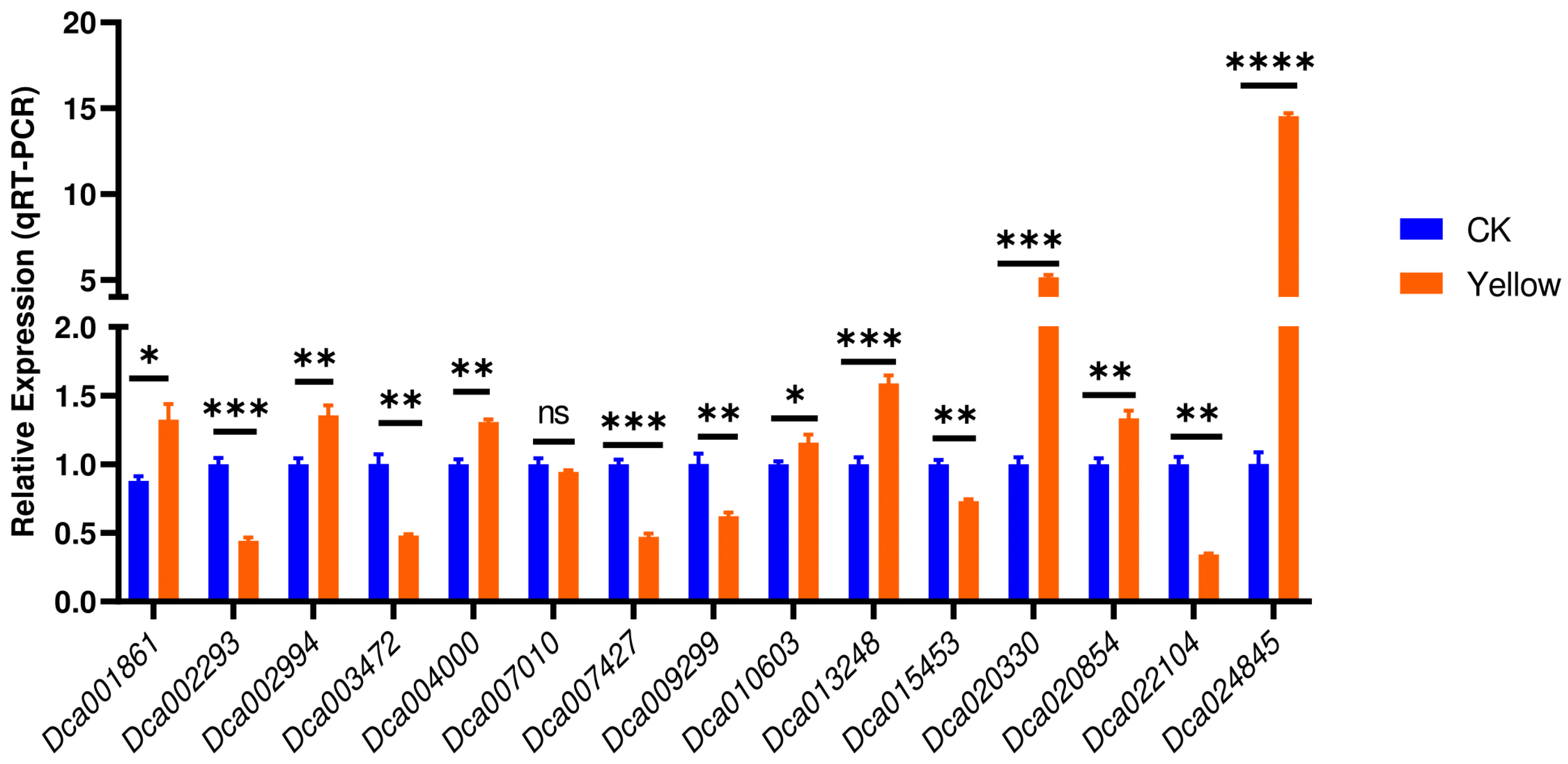
Figure 6, the expression patterns of the targeted genes via qRT-PCR and RNA-Seq were consistent with identical regulation patterns.
4. Discussion
Leaf color is a critical agronomic trait, and its variation significantly affects global plant metabolism. Principally, leaf color mutations induce less photosynthetic efficiency, causing poor growth, reduced yield, and important economic losses [
1,
2]. Accordingly, leaf color mutants have become materials of considerable research interest. They are ideal for studying variations in plant metabolism and physiology, especially the molecular mechanisms governing chloroplast biogenesis and differentiation, pigment synthesis and accumulation, photosynthesis, diverse stress response, etc. [
1,
2]. In
D. officinale, previous studies have shown that leaf color mutation is associated with a reduction in plant height, stem diameter, and the number of chloroplasts; low chlorophyll content; and altered chloroplast structure [
5,
6]. In addition, they have provided insight into the underlying molecular mechanisms through comparative transcriptome analysis and unveiled DEGs [
5]. However, the identified potential candidate genes have not been validated, and functional characterizations are yet to be conducted due to the unavailability of a reliable RG for qRT-PCR analysis. Thus, the present study took advantage of the RNA-seq data to comprehensively select candidate RGs, examine their stability, and validate the most stable and reliable to promote genomics studies on leaf color mutants in
D. officinale. This approach has been widely used in several plants to identify suitable RGs for specific traits and experimental conditions [
11,
15,
30,
31,
32].
The efficiency of qRT PCR results depends on the reliability of internal RGs. Only a stable expression of internal RG can guarantee the accuracy of the results. Previous studies have demonstrated that internal RGs are species-specific, and their stability varies according to the traits and experimental conditions [
11,
15,
30,
31,
32]. For instance, different RGs have been identified for studying traits-related pigment synthesis in different plant species. In
Catalpa fargesii,
CfMADH and
CfEF-1 have been identified as the most reliable RGs to be used individually or in combination for normalizing the expression levels of leaf color-related genes [
31]. In
Lagerstroemia indica,
LiEF1α-2 and
LiEF1α-1 were identified as the most suitable for analyzing the expression levels of leaf color-related genes [
33]. In wheat, 18S rRNA is used as the internal RG to calibrate the expression of leaf color-related candidate genes [
17]. In
Lilium regale,
LrActin2 was identified as the best RG for normalizing photosynthesis-related candidate genes’ expression levels [
16]. Herein, we investigated ten genes, including
EF1α,
CYCB1-2,
RPL13AD,
β-TUB,
UBQ10,
α-TUB,
GAPDH,
Actin,
PIP1-2, and
ALB3. Expression stability analysis via GeNorm, NormFinder, and BestKeeper revealed that these ten genes could be used as internal RGs to calibrate leaf color-related genes’ expression levels in
D. officinale.
EF1α exhibited the highest stability among them, indicating it is the most suitable RG. Using this RG may promote the molecular dissection of the regulatory network of leaf color mutations in
D. officinale.
To verify the suitability and reliability of EF1α, we analyzed fifteen chlorophyll pathway-related DEGs’ expression levels via qRT qPCR. The expression patterns of the targeted genes via EF1α normalization were consistent with the transcriptome sequencing results. This result further confirms the accuracy of using EF1α as the primary RG for qRT-PCR calibration of leaf color-related genes’ expression levels in D. officinale. Furthermore, of the fifteen analyzed genes, Dca024845 and Dca020330 were the most significantly up-regulated in the yellow leaf mutant genotype. Accordingly, these genes may represent key candidate genes for deciphering chlorophyll synthesis-related molecular mechanisms in D. offinale. Functional characterization of these genes is required to reveal their roles.
5. Conclusions
In summary, this study investigates ten RGs for qRT-PCR normalization of expression levels of leaf color-related candidate genes in D. officinale. Of them, EF1α was the most reliable RG, based on stability rankings analysis and confirmatory through the accurate calibration of the expression levels of fifteen chlorophyll pathway-related DEGs. Dca024845 and Dca020330 were the most significantly up-regulated genes in the yellow leaf mutant genotype. Therefore, they should be subjected to functional genomics studies to deepen our understanding of the molecular mechanisms involved in leaf color mutations. Our results will enable the identification of candidate genes and functional genomics studies related to leaf color in D. officinale. Furthermore, they may provide comprehensive guidelines for uncovering suitable RGs in other plant species.
Supplementary Materials
The following supporting information can be downloaded at:
Preprints.org, Table S1: List of the ten candidate reference genes with their FPKM expression values; Table S2: List of the fifteen chlorophyll pathway-related genes with their FPKM expression values; Table S3: Primers of the fifteen chlorophyll pathway-related genes for qRT-PCR analysis; Table S4: Summary of the qRT-PCR Ct value of ten candidate genes.
Author Contributions
Resources, H.C.; writing—original draft preparation, H.C.; writing—review and editing, H.L., L.M. and H.C.; visualization, L.L. and Y.J.; supervision, S.L.; project administration, H.C.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from Yunnan Province Science and Technology Department Technology Talent and Platform Program (202005AD160014), the Green Food Brand-Build a Special Project (Floriculture) supported by Science and Technology (530000210000000013742), Major science and technology project in Yunnan Province (202002AA100007), and Yunnan Province Seed Industry Joint Laboratory Project (202205AR070001-05).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets analyzed or generated during the study are included in this manuscript and its
Supplementary File.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Li, W.; Zhang, Y.; Mazumder, M.A.R.; Pan, R.; Akhter, D. Research progress on rice leaf color mutants. Crop Des. 2022, 1, 100015. [Google Scholar] [CrossRef]
- Zhao, M.H.; Li, X.; Zhang, X.X.; Zhang, H.; Zhao, X.Y. Mutation mechanism of leaf color in plants: A review. Forests 2020, 11, 851. [Google Scholar] [CrossRef]
- Qi, L.; Shi, Y.; Li, C.; Liu, J.; Chong, S.-L.; Lim, K.-J.; Si, J.; Han, Z.; Chen, D. Glucomannan in Dendrobium catenatum: Bioactivities, Biosynthesis and Perspective. Genes 2022, 13, 1957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-Q.; Xu, Q.; Bian, C.; Tsai, W.-C.; Yeh, C.-M.; Liu, K.-W.; Yoshida, K.; Zhang, L.-S.; Chang, S.-B.; Chen, F.; et al. The Dendrobium catenatum Lindl. genome sequence provides insights into polysaccharide synthase, floral development and adaptive evolution. Sci. Rep. 2016, 6, 19029. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Li, H.; Miao, Z.; Fu, G.; Yang, C.; Wu, L.; Zhao, P.; Shan, Q.; Ruan, J.; Wang, G.; et al. The preliminary study of leaf-color mutant in Dendrobium officinale. J. Nucl. Agric. Sci. 2017, 31, 0461–0471. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, W.; Li, H.; Cao, H.; Lu, L.; Tian, M.; Sun, D.; Li, D. Study on chloroplast ultrastructure, photosynthetic pigments, and chlorophyll fluorescence characteristics of leaf color mutants in Dendrobium officinale Kimura et Migo. Plant Sci. J. 2020, 38, 260–268. [Google Scholar] [CrossRef]
- Cao, A.; Shao, D.; Cui, B.; Tong, X.; Zheng, Y.; Sun, J.; Li, H. Screening the reference genes for quantitative gene expression by RT-qPCR during SE initial dedifferentiation in four Gossypium hirsutum cultivars that have different SE capability. Genes 2019, 10, 497. [Google Scholar] [CrossRef]
- Huggett, J.; Dheda, K.; Bustin, S.; Zumla, A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005, 6, 279–284. [Google Scholar] [CrossRef]
- Liu, Q.; Qi, X.; Yan, H.; Huang, L.; Nie, G.; Zhang, X. Reference gene selection for quantitative real-time reverse-transcriptase PCR in annual ryegrass (Lolium multiflorum) subjected to various abiotic stresses. Molecules 2018, 23, 172. [Google Scholar] [CrossRef]
- Wang, L.; Dossou, S.S.K.; Wei, X.; Zhang, Y.; Li, D.; Yu, J.; Zhang, X. Transcriptome dynamics during black and white sesame (Sesamum indicum L.) seed development and identification of candidate genes associated with black pigmentation. Genes 2020, 11, 1399. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.; Liu, Y.; Gu, Y.; Zhang, H.; Ahmad, F.; Wang, G.; Ren, L. Selection and Validation of Reliable Reference Genes for qRT-PCR Normalization of Bursaphelenchus xylophilus from Different Temperature Conditions and Developmental Stages. Appl. Sci. 2022, 12, 2880. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, L.; Yang, S.; Li, G.; Ye, J. Selection and validation of reference genes for quantitative RT-PCR analysis in peach fruit under different experimental conditions. Sci. Hortic. 2017, 225, 195–203. [Google Scholar] [CrossRef]
- Dheda, K.; Huggett, J.F.; Chang, J.S.; Kim, L.U.; Bustin, S.A.; Johnson, M.A.; Rook, G.A.W.; Zumla, A. The implications of using an inappropriate reference gene for real-time reverse transcription PCR data normalization. Anal. Biochem. 2005, 344, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, H.; Cao, Y.; Yang, P.; Feng, Y.; Tang, Y.; Yuan, S.; Ming, J. Validation of reference genes for quantitative real-time pcr during bicolor tepal development in asiatic hybrid lilies (Lilium spp.). Front. Plant Sci. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, J.; Hua, Q.; Tel-Zur, N.; Xie, F.; Zhang, Z.; Chen, J.; Zhang, R.; Hu, G.; Zhao, J.; et al. Identification of reliable reference genes for quantitative real-time PCR normalization in pitaya. Plant Methods 2019, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Hu, F.; Yuan, S.; Liu, C. Selection of reference genes for quantitative real-time PCR analysis of photosynthesis-related genes expression in Lilium regale. Physiol. Mol. Biol. Plants 2019, 25, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shi, N.; An, X.; Liu, C.; Fu, H.; Cao, L.; Feng, Y.; Sun, D.; Zhang, L. Candidate Genes for Yellow Leaf Color in Common Wheat (Triticum aestivum L.) and Major Related Metabolic Pathways according to Transcriptome Profiling. Int. J. Mol. Sci. 2018, 19, 1594. [Google Scholar] [CrossRef]
- Wan, H.; Zhao, Z.; Qian, C.; Sui, Y.; Malik, A.A.; Chen, J. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal. Biochem. 2010, 399, 257–261. [Google Scholar] [CrossRef]
- Liang, L.; He, Z.; Yu, H.; Wang, E.; Zhang, X.; Zhang, B.; Zhang, C.; Liang, Z. Selection and Validation of Reference Genes for Gene Expression Studies in Codonopsis pilosula Based on Transcriptome Sequence Data. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Shan, T.; Pang, S. Selection of reference genes for real-time RT-PCR normalization in brown alga Undaria pinnatifida. J. Appl. Phycol. 2019, 31, 787–793. [Google Scholar] [CrossRef]
- Yi, S.; Qian, Y.; Han, L.; Sun, Z.; Fan, C.; Liu, J.; Ju, G. Selection of reliable reference genes for gene expression studies in Rhododendron micranthum Turcz. Sci. Hortic. 2012, 138, 128–133. [Google Scholar] [CrossRef]
- Derveaux, S.; Vandesompele, J.; Hellemans, J. How to do successful gene expression analysis using real-time PCR. Methods 2010, 50, 227–230. [Google Scholar] [CrossRef]
- Ren, R.; Dai, P.-H.; Li, M.; Liu, Z.; Cao, F. Selection and stability evaluation of reference genes for real-time quantitative PCR in dove tree (Davidia involucrata). 2016, 52, 1565–1575. [Google Scholar] [CrossRef]
- Ma, L.; Cui, G.; Wang, X.; Jia, W.; Duan, Q.; Du, W.; Wang, J. Cloning and expression analysis of catalase (Ls-Cat1) gene in Lilium sargentiae Wilson. J. Nucl. Agric. Sci. 2017, 31, 1700–10707. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cheng, Y.; Ma, L.; Li, S.; Wang, J. Identification of reference genes provides functional insights into meiotic recombination suppressors in Gerbera hybrida. Hortic. Plant J. 2022, 8, 123–132. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Ma, K.S.; Li, F.; Liang, P.Z.; Chen, X.W.; Liu, Y.; Gao, X.W. Identification and validation of reference genes for the normalization of gene expression data in qRT-PCR Analysis in aphis gossypii (Hemiptera: Aphididae). J. Insect Sci. 2016, 16. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Zhao, L.J.; Yang, G.J.; Zhang, Y.; Fu, P.Y.; Hu, J.W.; Liu, Y.; Wang, N. Selection and Validation of Reference Genes for Leaf Color Phenotype in “Maiyuanjinqiu”, a Catalpa fargesii Variety, by qRT-PCR. For. Res. 2022, 35, 123–131. [Google Scholar] [CrossRef]
- Zhang, J.R.; Feng, Y.Y.; Yang, M.J.; Xiao, Y.; Liu, Y.S.; Yuan, Y.; Li, Z.; Zhang, Y.; Zhuo, M.; Zhang, J.; et al. Systematic screening and validation of reliable reference genes for qRT-PCR analysis in Okra (Abelmoschus esculentus L.). Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Q.; Li, Y.; Gao, L.; Lv, F.; Yang, R.; Wang, P. Candidate reference genes for quantitative gene expression analysis in Lagerstroemia indica. Mol. Biol. Rep. 2021, 48, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).