1. Introduction
Coronary artery and aortic interventions procedures are used in the treatment of a wide spectrum of disease regarding the heart and systemic vessels.
Cardiac and noncardiac complications can occur at variable times after ptocedures, with the clinical presentation ranging from asymptomatic to devastating symptoms.
Some of the cardiac complications can be seen with any cardiac interventions and are related to a possible direct damage to the coronaries or cardiac structures, while others are specific to the interventional treatment. Some noncardiac complications must be taken in account and can be found on the vascular access site (pseudoaneurism formation or hematoma) or on a distant site because material embolization (stroke, or parenchymal injury). These complications occur at variable time frames after the procedure.
Invasive coronary angiography represents the gold standard technique for the diagnosis of coronary artery disease (CAD), but nowadays Coronary CT Angiography (CCTA) is considered the first-choice non-invasive alternative method, mainly in the chronic chest pain and in the acute phase for patients presenting low to intermediate pre-test risk [
1].
Thechnical development of cardiac CT scanners has moved in two different directions in recent years: some vendors implemented the effective volume coverage for single rotation, increasing detector rows width up to 16 centimeters, allowing the acquisition of the all cardiac volume in a single beat.
On the other hand other manufacturers introduced, on the same system, two radion tubes, allowed to work in a simoultaneous manner: this option guarantees a significant increase in effective temporal resolution but makes possible the so called “high pitch-flash” acquisition: a solution to acquire all the heart and coronaries in a single beat as well.
Both solutions has enabled volumetric imaging of the whole heart without artifacts, with a high temporal resolution acquisition and optimal diagnostic performance. Technological implementation significantly reduced the image acquisition time and consequently the radiation dose and the volume of contrast injected [
2].
Further advances have also been achieved by improving spatial and temporal resolution making CCTA increasingly used in the diagnostic work-up of patients with suspected CAD [
3,
4] .
Currently, ECG-gated CCTA is the gold-standard imaging technique for pre-procedural of structural heart surgery and for transcatheter aortic valve implantation (TAVI) planning [
5] .
ECG-gated CCTA assists interventional cardiologists, providing detailed anatomical information, for the adequate selction of catheters, guidewires, stent size selection, and for the correct timeline for multi-step revascularization [
6] .
CCTA also has prognostic value for procedural outcome, especially in cases of chronic total coronary artery occlusion [
7].
However, although ECG-gated CCTA is part of preoperative work-up [
8,
9], there are still few studies in the literature and no recommendation for its use in the follow-up of patients who underwent cardiac surgery.
Procedures such as percutaneous transluminal coronary angioplasty (PTCA), TAVI, or pacemaker (PMK) positioning, can still lead to potentially life-threatening complications.
Post-operative cardiac complications are rare but possible events with reported incidence ranging from 0.02% to 1% [
10,
11,
12,
13], however, other studies affirm that the true incidence of these complications is higher (4-5%) because often unrecognized and underestimated [
14,
15,
16].
The aim of our study was to report our experience of the role of the ECG-gated CCTA in the assessment of postoperative complications in patients undergoing cardiovascular procedures as a non-invasive imaging technique of choice for the diagnosis, management, and follow-up in post-procedural complications and to review the international literature
2. Materials and Methods
2.1. Patients
We have retrospectively evaluated a total of 294 CCTA with ECG-gating (retrospectively modulated acquisition) studies performed in our center from January 2020 to January 2023 after interventional/endovascular procedures or cardiac surgery. ECG-gating CCTA was performed in emergency settings if patients showed symptoms consistent with an acute complication or in standard settings as the regular follow-up (30 days to 6 months) after the procedure.
Two radiologists independently reviewed the CCTA exams on a dedicated workstation, blinded to the identity and the anamnestic data of all the patients.
Informed consent was obtained from all patients at the time of the exam.
2.2. CT Imaging
CCTA exams were performed using two multi-detector CT systems (SOMATOM Drive 256 - channels, Dual Source, Siemens, Germany and Aquilion Prime - 160 channnels, Canon Medical Systems, Japan) with modulated retrospective ECG gating and iterative reconstruction algorithm, for radiation and contrast dose reduction.
Oral nitrates were always used in order to widen coronary lumen and β-receptor blocker medication was employed, in case of heart rate >65 bpm.
CCTA protocol at our institution consists of a preliminary non-contrast, prospectively gated, low-radiation dose chest CT scan to define the correct anatomical volume for the following CCTA and for the evaluation of collateral findings (lungs, pleura, mediastinum, chest wall, etc.). After bolus tracking in the ascending aorta (100 UH) and breathing command (6 seconds), a bolus of 80-100 ml (according to scanner characteristics and acquisition length) of nonionic contrast agent (370-400 mgI/ml concentration) is injected at the rate of 4-5ml/s, followed by a 40-ml saline bolus at same injection rate used for contrast. CCTA is acquired at the thinnest collimation (0.5–0.625 mm in cranio-caudal direction with retrospective ECG gating in the arterial phase, using tube current modulation (maximum mAs between 40% and 80% of carcdiac cycle). Contrast volume and velocity injection are adapted to the IDR (iodine delivery rate) target.
For cardiac structures evaluation, in all cases a multiphase reconstruction 40-80% of cardiac cycle was made, with a 10% increment, including best sistolic and best diastolic phase.
All arterial ECG gated acquisition were followed by a late non gated scan, in order to asses or exclude medium contrast vessel extravasation.
After data acquisition, the image data set are sent to the picture communication system (PACS) and were reported with dedicated imaging reporting software (Syngovia workstation, Siemens Healthcare, Forchheim, Germany).
3. Results
Post-procedural complications were encountered in 27/294 patients (9.2%). The 27 patients enrolled in the study were 18 males and 9 females (male/female ratio: 2), with ages ranging between 47 to 86 years old (mean age 68.3 years old). Demographic data and complications correctly doagnosed at CTA, grouped according to clinical presentation are summarized in
Table 1.
Among 27 patients presenting post procedural complications 10 (37%) showed acute manifestations in an emergency setting and 17 (63%) were asymptomatic: diagnosis was made in course of a routnely performed follow up study.
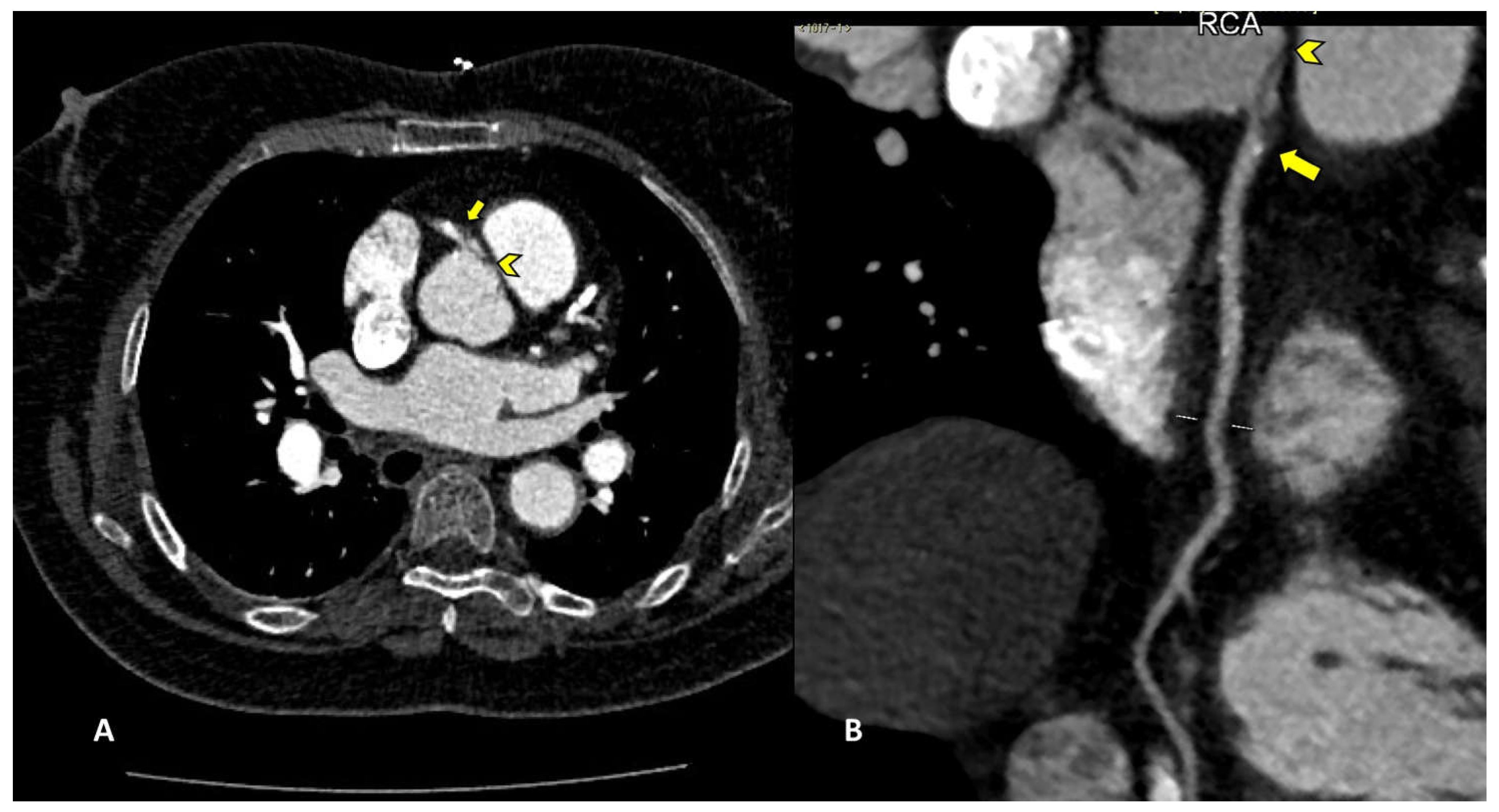
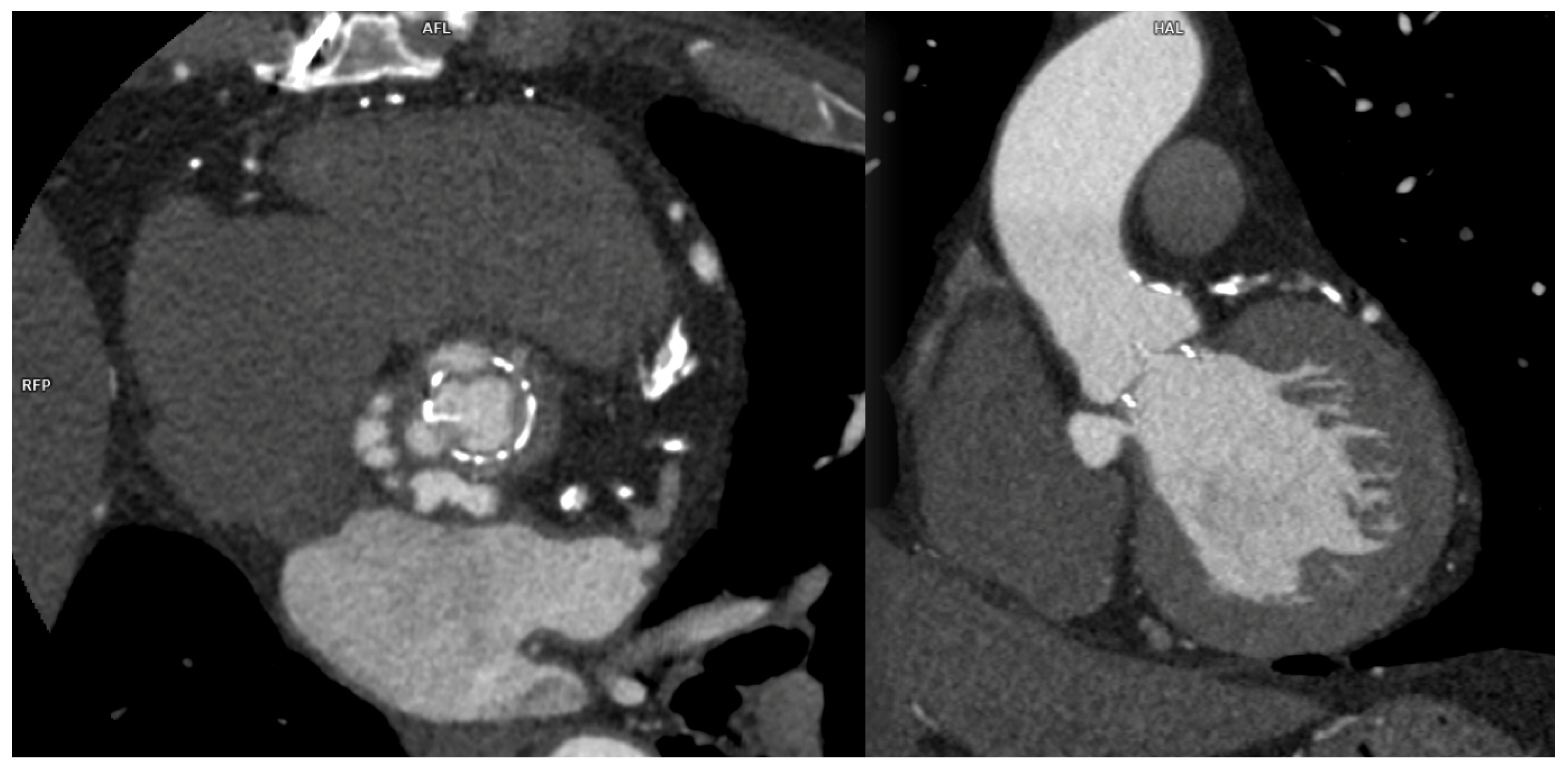
Among percutaneous coronary intervention (PCI) complications, coronary intimal dissection was found to be the most frequent event after PTCA (6/27 patients, 22.2%), associated with involvement of the ascending aorta (
Figure 1). All coronary dissections encountered were noted in an emergency setting and all of them involved the right coronary ostia, with a constant flap extension to the Valsalva sinus and ascendig aorta wall (less tanh 40 mm distance from the ostium).
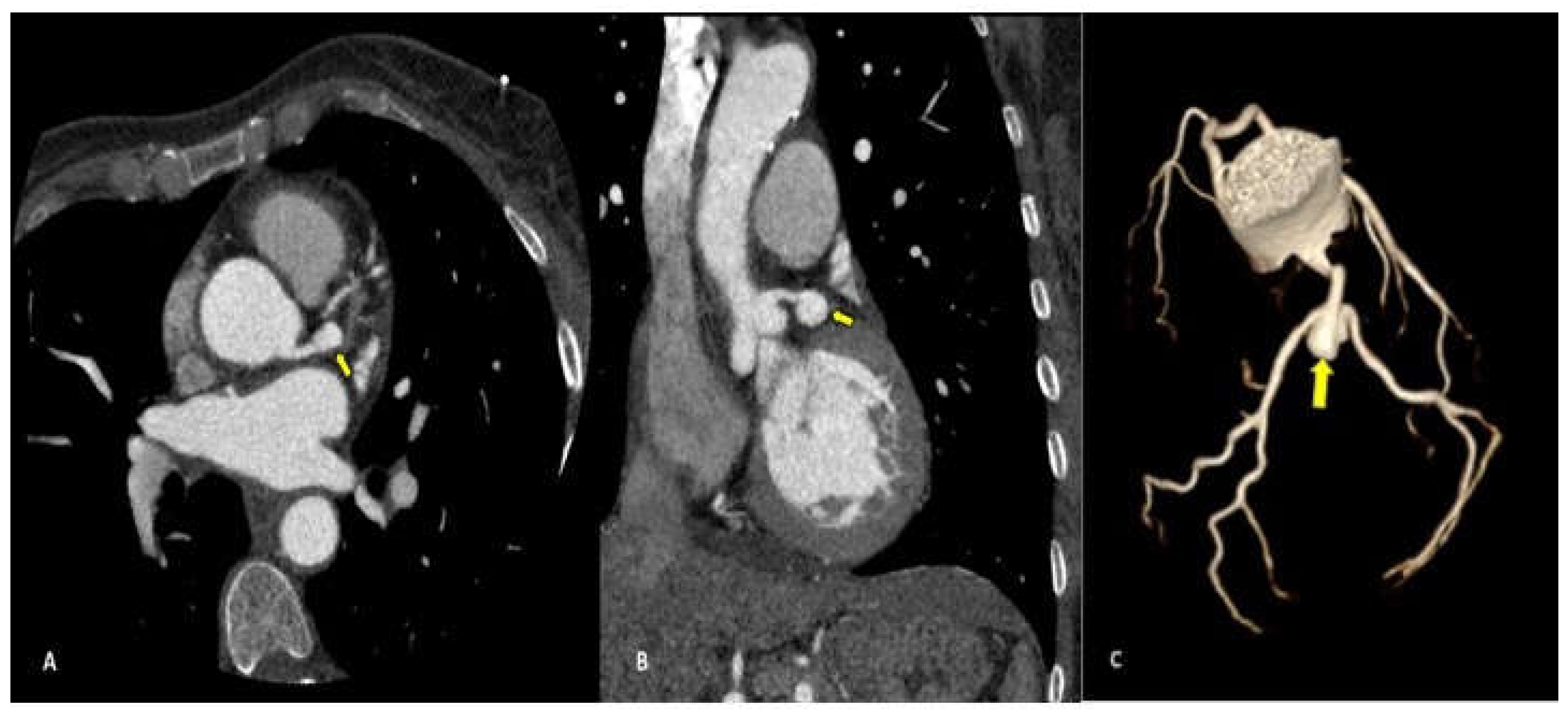
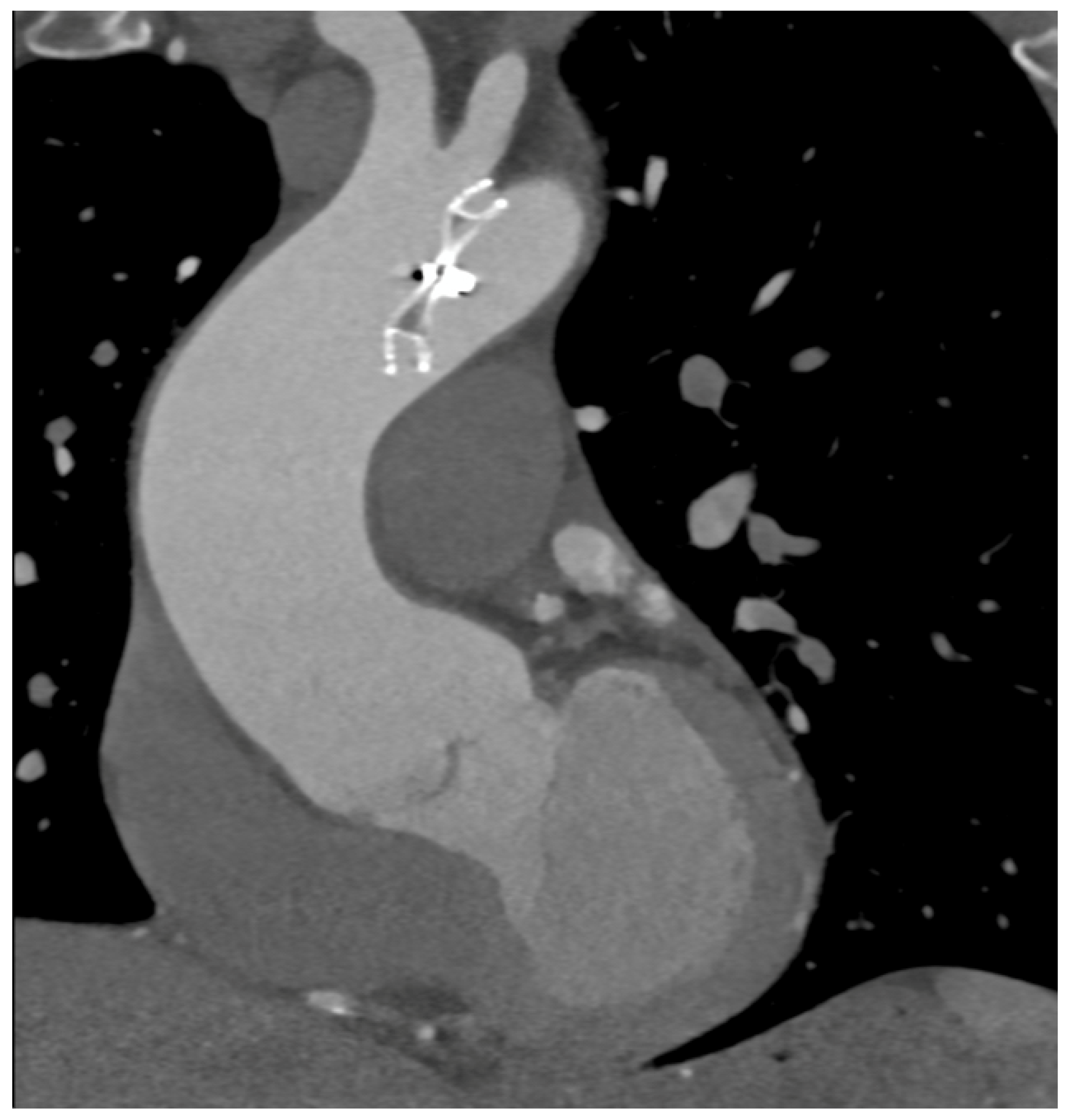
Complications less frequently presented after PTCA were vascular wall pseudoaneurysm formation (3/27 patients, 11.1%) (
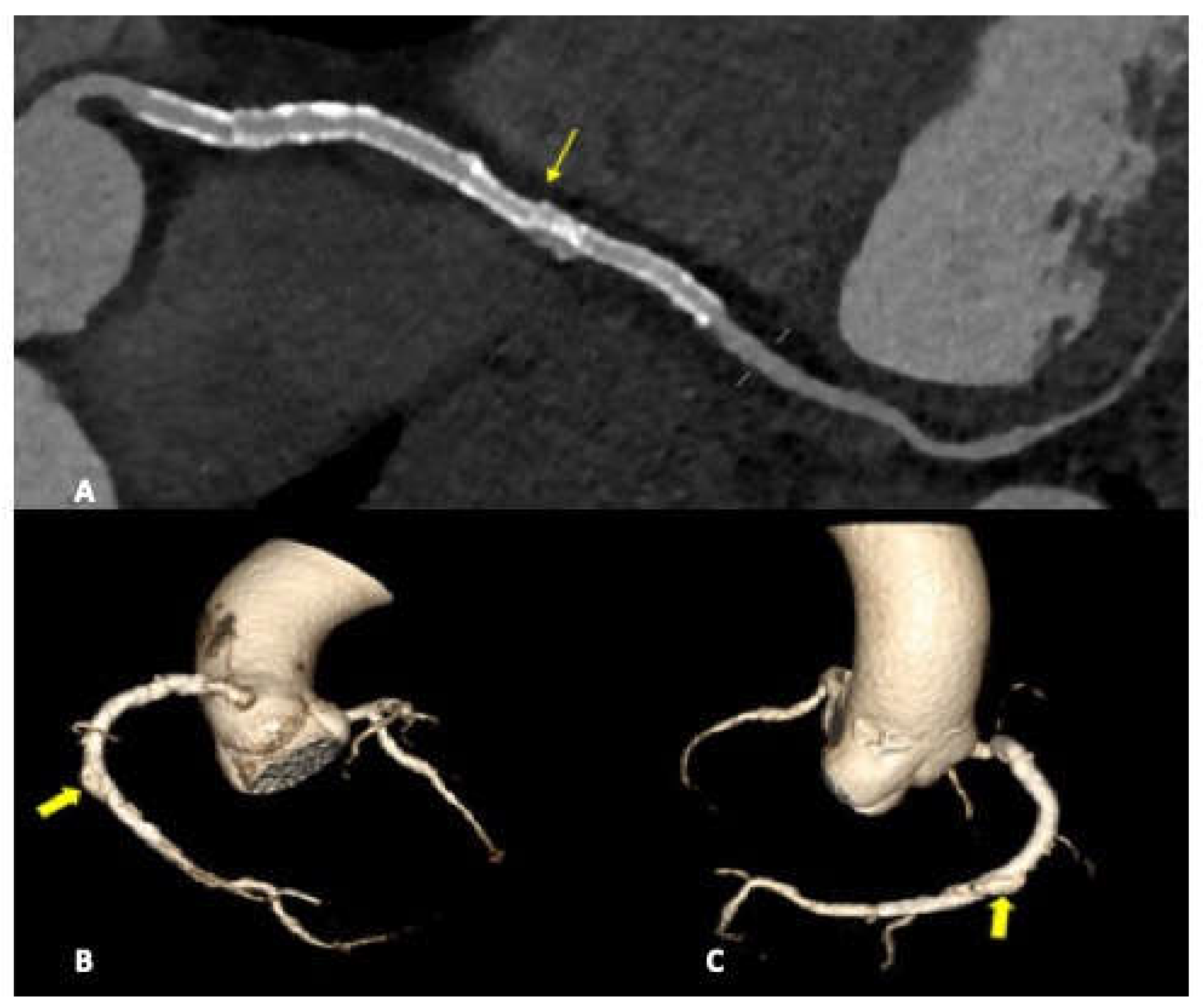
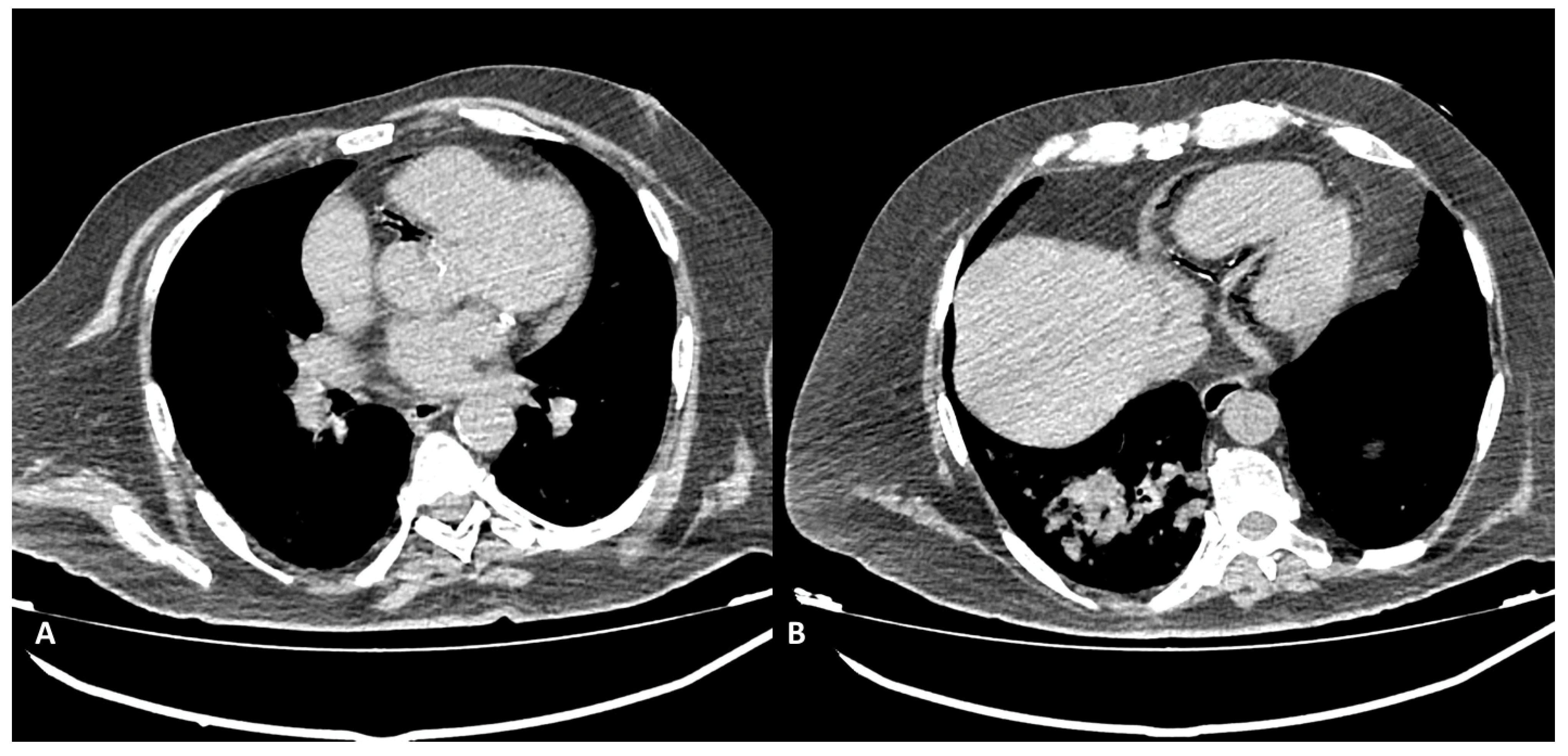
Figure 2) or coronary stent migration or displacement (4/27 patients, 14.8%) which were always diagnosed in non symtomatic patients (
Figure 3 and
Figure 4).
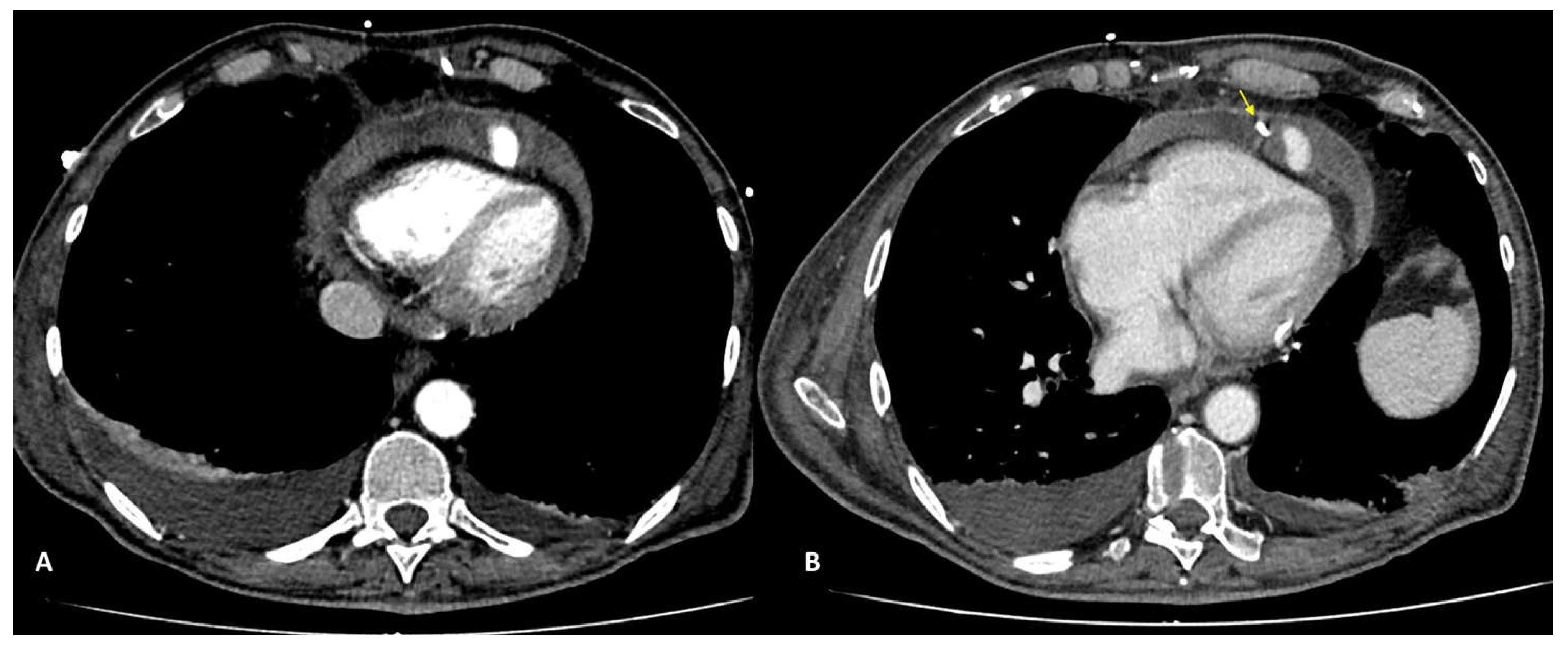
In case of PMK implantation, right atrium or ventricular perforation with associated hemopericardium (
Figure 5) were the most common complication (5/27 patients, 18.5%). All pacemaker included in the study had transvenous two or three leads. Damages caused by PMK leads were diagnosed mostly in a chronic phase: 4 by 5 patients, more than 30 days after implenation. Only one case presented as an acute complication (< 24h after PMK placement), showing a more worring and significamnt clinical picture, sustained by active bleeding in the pericardial sac.
Dislocation of aortic prostehtic valve after TAVI procedure in two cases was seen, with a symptomatich presentation referred by the patient as acute chest pain or thoracic discomfort.
In five patients previously underwent aortic valve surgery, a non emergency condition was diagnosed in course of routine follow up as: aortic root pseudoaneurysm (2/27 patients, 7.4%) or para-valvular leak (3/27 patients, 11.1%) (
Figure 6).
In one case (1/27 patient, 3.7%) of patent foramen ovale correction we found an Amplatzer septal occluder dislocation (
Figure 7).
We encounterded one case of intracoronary air embolism, in a patient presenting acute chest pain and dyspnea after a CT-guided lung biopsy procedure. At CT-gated non contrast scan a signifcant amount of air bubbles were found in the right coronary artery (mainly RCA) and its branches (Figure 8), due to transition through a pulmonary vein to the coronary circulation. CT acquisition was stopped after the non contrast gated preliminary acquisition and patient immedialtely deliverde to the cath lab for a coronary angiography.
4. Discussion
The primary endpoint of our study was to underline the importance of CCTA in the evaluation of cardiovascular post-procedural and post-surgical complications. CCTA gained over the time, thanks to scanner evolution and implemented post processing software capabilities a first row rule, in the evalutaion of postinterventional patients presenting with a clinical suspect. On the othe hand it must be underlined that CT is still not considered in the guidelines flowchart, although it can quickly and accurately recognize severe or life-threatening complications in acute care settings, allowing immediate treatment. Moreover, it can allow to define of chronic post-operative complications (30 days to 6 months after the procedure) and guide clinicians for optimal management.
In our study, all CCTA were performed using ECG-gating using two different CT systems and we found some indubitable advantages on the dual-source scanner which allowed a faster CT acquisition and a reduction in motion and breathing artifacts. The most important aspect in cardiac CT imaging is represented by the cardiac synchronization of the scan, which was adopted in arterial phase in all our studies, that allows a precise assessment especially on cardiac structures and ascending aorta, decreasing nondiagnostic examinations caused by structures movement [
2,
17].
Even if using a faster scanner we adopted drugs premedication (β-blocker and nitrates) to all patients except of those presenting specific contraindications.
In the 294 CCTAs examined, we found a total of 27 complications, among them coronary intimal dissection was found to be the most frequent (22.2%), in all cases associated with involvement of the ascending aorta.
Intimal dissection in the aortic or coronary wall in course of PCI in an unfrequent complication that is characterized by formation of a hematoma in the media, separation of the intimal layer, with compromise of the coronary blood flow. It can be explained by either direct trauma from the catheter tip or a vigorously expelled jet of contrast material from the catheter tip that abuts the wall of the coronary artery. Among risk factors must be taken in count female sex, mechanical trauma, technical factors such as inappropriate positioning of the catheter tip in the coronary artery ostium, overinflation of the angioplasty balloon. Ascending aortic dissection is more frequent in course of procedures involving RCA because of its smaller-caliber, and because of the hemodynamic force vector which is directed to the right convexity of the ascending aorta [
18]. In our study all cases of coronary artery dissection involved the RCA with flap extension to the right Valsalva sinus. CCTA can accurately define the aortic involvement guiding the correct management, because limited aortic involvment may be treated with stenting of coronary dissection entry point, whereas aortic dissection extending distant from the coronary os may require surgical intervention [
18]. The capability of CTA to precisely assess intimal flap extension and the presence or absence of intramural hematoma, is fundamental for the correct patient management. Moreover gated CTA scan is helpful for determining the coronary vessel partency impairment, when present and consequently to estabilishthe exact true and false lumen width.
Coronary pseudoaneurysms are relatively rare, they can be caused by iatrogenic procedural trauma after PTCA or more rarely after coronary angiography. An aneurysm is defined as >1.5 times the normal arterial diameter, pseudoanerysms do not involve all layers of the vessel wall. Coronary pseudoaneurysms are mostly detected incidentally and are asymptomatic, but they can result in fistulae, rupture, bleeding, tamponade and myocardial infarction, for these reasons a prompt diagnosis is crucial [
19] and CCTA allows a correct definition of the size, morphology and location of the lesion.
We found coronary pseudoaneurysm in two patients (7,4%) and both in the left coronary artery, one in the left main coronary artery (LMCA) and one in left anterior descending (LAD) respectively.
Stent displacement, fracture or migration is rare complication, risk factors include the use of drug eluting stents, manufacturing defects, or extensive vessel calcification. In case of multiple overlapping stents a possible inconvenience, hard to differentiate at CTA by struts fractures, is represented by stent misalignment or separation which can occure in the immediate post procedural period or later on. In both cases the consequences are essentially the same and consist of extravasation of contrast material through the rupture, with a small collection of it adjacent to the stent.
We detected coronary stent misalignement or dislocation in a total of three patients (14%) wich representes an high percentage compared to the literature [
20]. All cases were diagnosed in course of routine follow up scans, performed in order to asses stent patency and in absence of patient specific symptoms. Although in literature the stent dislodgements are reported to be more common in the LMCA and LAD artery , in our study in all three cases the RCA was involved. In one of the three cases, a patient underwent to multiple stenting of the middle and distal RCA, an unusual endoleak condition was diagnosed. A misalignement was found between two devices, with a consequential medium contrst extravasation, between stent meshes and the coronary vessel wall, configuriung a type III endoleak.
Pacemaker implantation has become a wide used practice all over the world in a growing population of patients, thanks to the increased indications.
Pacemaker complication may be related to pacemaker syndromes that represent the clinical manifestation of a suboptimal synchronization or to mechanical complication related to its positioning. They may be acute in the setting of pacemaker implantation or subacute, among them the more frequent are pneumothorax, cardiac perforation, pocket hematoma or bleeding, lead dislodgement, venous thrombosis, mechanical lead complications. One of the most insidious inconvenience is represented by perforation of the myocardial wall, both in acute and subacute phase. Symptoms of perforation include pleuritic chest pain from pericarditis, diaphragmatic or intercostal muscle stimulation and, in the presence of pericardial effusion, patients may develop shortness of breath and hypotension as tamponade develops [
21].
Complications encountered after PMK implantation represented 18,5% of all cases of the study. One patient only (0,03%) was scanned in the acute phase, less than 24 hours afterPMK positioning, presenting acute chest pain, dispnea and laboratory signs of anemization. In this case CTA was determining for the managemente beciasu of the clear demonstartion of acute bleeding and the presence of significatnt haemopericardium. Four patients presented at CT observation more than 30 days, after leads implantation (chronic complcations) and reporting aspecific symptopms (intermittent chest pain, dyspnea) [
22,
23,
24]. CT imaging confirmed the clinical or echocardiographic suspicion of cardiac wall perforation caused by leads edge. In chronic cases the amount of heaemopericardium was mild and no signs of active bleeding at CTA were shown. PMK leads in this cases always determined a buffering effect on the wall laceration.
Complications of TAVI procedure can be immediate or periprocedural, the latter can appear shortly after the procedure [
13]. Prosthesis dislocation during TAVI is a rare but serious complication: Malpositioning, valve migration/embolization, conversion to open surgery, need for pacemaker implantation, stroke, and myocardial infarction are considered the major complications following TAVI [
25]. It can be managed effectively by implanting a second device and leaving the dislocated device safely in the aorta or complete retrieval of valve [
26].
ECG-gated CCTA plays an important role in the early diagnosis of local complications. CCTA examinations are carefully examined in all phases of the cardiac cycle and also dynamically, in order to assess the valve leaflets and their movements, the intactness of the aortic anulus and general complications such as hematomas. Stent dislocation after correct initial positioning is a very rare complication, valve can be dislocated too low determining significant hemodynamic paraaortic regurgitation, or too high with overlap of the coronary ostia [
13].
Surgical repair of atrial septal defects have been essentially replaced by percutaneous interventions. Even if these procedures are performed with excellent safety and efficacy in contemporary practice, some impairments have not been completely eliminated. Embolization of the device is still the most frequent complication and its clinical presentation varied from incidental finding to ventricular fibrillation and hemodynamic collapse. Amplatzer dislocation is rare but serious event [
27,
28]. In the literature, device dislocation and embolism are ranges from 4% to 21% and surgery being necessary in approximately 70% to 100% of those cases [
29,
30,
31] . Clinically, a deficient aortic rim is considered a potential cause of device migration [
32]. In one case (3.7%) of patent foramen ovale correction we found an Amplatzer septal occluder (AGA Medical Corporation, Golden Valley, MN) dislocated in the aortic arch.
In one case we noted air in RCA and its branches after a CT-guided lung biopsy procedure, without contrast agent usage. This was the only case in which we did not perform CCTA but only gated non contrast CT: the air in the coronary vessel and its branches was due to air transition in a pulmonary vein to the coronary circulation, the patient immediately underwent coronary angiography.
5. Conclusions
Although the role of ECG-gated CCTA as an integral part of pre-procedural and surgical work-up is well known, there are still few studies in the literature and no recommendation for its use as an imaging technique in the follow-up of patients undergoing coronary procedures and cardiac surgery.
The true incidence of cardiac post-procedural/surgical complications remains unrecognized and may be often underestimated.
Our experience demonstrate that ECG-gated CCTA represents a fundamental tool for the detection of post-procedural endovascular/surgical complications, in order to enable optimal patient management.
This experience promotes CCTA cardiac synchronization as the imaging technique of choice for the diagnosis, management, and follow-up in post-procedural complications.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, CL , GL, GF.; methodology, CL, GL, GF , ST, GT; software, CL, GL, GF, ST, GT ; validation, CL, GF; SM; MS.; formal analysis, CL.; investigation, CL, GL, GF, SGP, MS; resources, CL, GF, SGP, ST, GT, MS,; data curation, CL, GF, SGP, ST, GT, SM, MS ; writing—original draft preparation, CL, GL, SGP ; writing—review and editing, CL, GL, MS; visualization, CL, GL, MS; supervision, CL, GL; SM, MS; project administration, CL, GL, SM, MS. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Institutional Review Board Statement approval number 20222030 (28.12.2022)
Informed Consent Statement
Patient consent was waived due to the retrospective observational study; the IRB approved the study.
Data Availability Statement
available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CAD |
Coronary artery disease |
| CCTA |
Coronary Computed Tomography Angiography |
| TAVI |
Transcatheter aortic valve implantation |
| i.v. |
intra-venous |
| PMK |
Pacemaker |
| PCI |
Percutaneous coronary intervention |
| RCA |
Right coronary artery |
| LMCA |
Left main coronary artery |
| LAD |
Left anterior descending |
References
- Fyyaz, S.; Rasoul, H.; Miles, C.; Olabintan, O.; David, S.; Plein, S.; Alfakih, K. ESC 2019 guidelines on chronic coronary syndromes: Could calcium scoring improve detection of coronary artery disease in patients with low risk score. Findings from a retrospective cohort of patients in a district general hospital. JRSM Cardiovasc Dis 2021, 10, 20480040211032789. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.M.; Rybicki, F.J.; Steigner, M. CT coronary angiography: 256-slice and 320-detector row scanners. Curr Cardiol Rep 2010, 12, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.Y.; Zhou, S.C.; Wang, Y.J.; Li, Q.; Zhu, T.T.; Liu, C.X.; Guan, H.X. Application of Low Tube Voltage, Low-concentration Contrast Agent Using a 320-row CT in Coronary CT Angiography: Evaluation of Image Quality, Radiation Dose and Iodine Intake. Curr Med Sci 2020, 40, 178–183. [Google Scholar] [CrossRef]
- Liguori, C.; Tamburrini, S.; Ferrandino, G.; Leboffe, S.; Rosano, N.; Marano, I. Role of CT and MRI in Cardiac Emergencies. Tomography 2022, 8, 1386–1400. [Google Scholar] [CrossRef] [PubMed]
- Lehmkuhl, L.; Foldyna, B.; Haensig, M.; von Aspern, K.; Lucke, C.; Andres, C.; Grothoff, M.; Riese, F.; Nitzsche, S.; Holzhey, D.; et al. Role of preprocedural computed tomography in transcatheter aortic valve implantation. Rofo 2013, 184, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Opolski, M.P. Cardiac Computed Tomography for Planning Revascularization Procedures. J Thorac Imaging 2018, 33, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Roller, F.C.; Harth, S.; Rixe, J.; Krombach, G.A.; Schneider, C. Development and Suggestion of a Cardiac CTA Scoring System for the Prediction of Revascularization Success in Chronic Total Occlusions (CTO) of the Coronary Arteries. Rofo 2016, 188, 172–178. [Google Scholar] [CrossRef]
- Shimahara, Y.; Kobayashi, J.; Kanzaki, S.; Higashi, M. [Role of cardiac computed tomography in cardiac surgery]. Kyobu Geka 2014, 67, 612–617. [Google Scholar]
- Marwan, M.; Achenbach, S. Role of Cardiac CT Before Transcatheter Aortic Valve Implantation (TAVI). Curr Cardiol Rep 2016, 18, 21. [Google Scholar] [CrossRef]
- Refaat, M.M.; Hashash, J.G.; Shalaby, A.A. Late perforation by cardiac implantable electronic device leads: Clinical presentation, diagnostic clues, and management. Clin Cardiol 2010, 33, 466–475. [Google Scholar] [CrossRef]
- Baumann, S.; Behnes, M.; Sartorius, B.; Becher, T.; El-Battrawy, I.; Fastner, C.; Ansari, U.; Lossnitzer, D.; Mashayekhi, K.; Henzler, T. Follow-up of iatrogenic aorto-coronary "Dunning" dissections by cardiac computed tomography imaging. BMC Med Imaging 2017, 17, 64. [Google Scholar] [CrossRef]
- Boxma, R.P.J.; Kolff-Kamphuis, M.G.M.; Gevers, R.M.M.; Boulaksil, M. Subacute right ventricular pacemaker lead perforation: Evaluation by echocardiography and cardiac CT. J Echocardiogr 2017, 15, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Soschynski, M.; Capilli, F.; Ruile, P.; Neumann, F.J.; Langer, M.; Krauss, T. Post-TAVI Follow-Up with MDCT of the Valve Prosthesis: Technical Application, Regular Findings and Typical Local Post-Interventional Complications. Rofo 2018, 190, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Tanasie, C.; Chandonnet, M.; Chin, A.; Kokis, A.; Ly, H.; Perrault, L.P.; Chartrand-Lefebvre, C. Catheter-induced aortic dissection after invasive coronary angiography: Evaluation with MDCT. AJR Am J Roentgenol 2011, 197, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Kakefuda, Y.; Hayashida, K.; Yamada, Y.; Yashima, F.; Inohara, T.; Yanagisawa, R.; Tanaka, M.; Arai, T.; Kawakami, T.; Maekawa, Y.; et al. Impact of Subclinical Vascular Complications Detected by Systematic Postprocedural Multidetector Computed Tomography After Transcatheter Aortic Valve Implantation Using Balloon-Expandable Edwards SAPIEN XT Heart Valve. Am J Cardiol 2017, 119, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Joseph, G.; Khaykin, Y.; Ziada, K.M.; Wilkoff, B.L. Delayed lead perforation: A disturbing trend. Pacing Clin Electrophysiol 2005, 28, 251–253. [Google Scholar] [CrossRef]
- Kim, J.H.; Baek, S.E.; Kim, Y.J.; Suh, Y.J. Coronary CTA for Acute Chest Pain in the Emergency Department: Comparison of 64-Detector Row Single-Source and Third-Generation Dual-Source Scanners. AJR Am J Roentgenol 2023. [CrossRef]
- Dunning, D.W.; Kahn, J.K.; Hawkins, E.T.; O'Neill, W.W. Iatrogenic coronary artery dissections extending into and involving the aortic root. Catheter Cardiovasc Interv 2000, 51, 387–393. [Google Scholar] [CrossRef]
- Biswas, S.; Hristov, B.; Kinthala, S.; Abrol, S. Post Traumatic Pseudoaneurysm of Left Anterior Descending Artery Presenting as Acute Coronary Syndrome. Cardiol Res 2019, 10, 114–119. [Google Scholar] [CrossRef]
- Eggebrecht, H.; Haude, M.; von Birgelen, C.; Oldenburg, O.; Baumgart, D.; Herrmann, J.; Welge, D.; Bartel, T.; Dagres, N.; Erbel, R. Nonsurgical retrieval of embolized coronary stents. Catheter Cardiovasc Interv 2000, 51, 432–440. [Google Scholar] [CrossRef]
- Lak, H.M.; Goyal, A. Pacemaker Types and Selection. In: StatPearls. edn. Treasure Island (FL); 2022.
- Ellenbogen, K.A.; Wood, M.A.; Shepard, R.K. Delayed complications following pacemaker implantation. Pacing Clin Electrophysiol 2002, 25, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Cano, O.; Andres, A.; Alonso, P.; Osca, J.; Sancho-Tello, M.J.; Olague, J.; Martinez-Dolz, L. Incidence and predictors of clinically relevant cardiac perforation associated with systematic implantation of active-fixation pacing and defibrillation leads: A single-centre experience with over 3800 implanted leads. Europace 2017, 19, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Sterlinski, M.; Przybylski, A.; Maciag, A.; Syska, P.; Pytkowski, M.; Lewandowski, M.; Kowalik, I.; Firek, B.; Kolsut, P.; Religa, G.; et al. Subacute cardiac perforations associated with active fixation leads. Europace 2009, 11, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Wendler, O.; Thielmann, M.; Schroefel, H.; Rastan, A.; Treede, H.; Wahlers, T.; Eichinger, W.; Walther, T. Worldwide experience with the 29-mm Edwards SAPIEN XT transcatheter heart valve in patients with large aortic annulus. Eur J Cardiothorac Surg 2013, 43, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Ussia, G.P.; Barbanti, M.; Sarkar, K.; Aruta, P.; Scarabelli, M.; Cammalleri, V.; Imme, S.; Pistritto, A.M.; Gulino, S.; Mule, M.; et al. Transcatheter aortic bioprosthesis dislocation: Technical aspects and midterm follow-up. EuroIntervention 2012, 7, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Berdat, P.A.; Chatterjee, T.; Pfammatter, J.P.; Windecker, S.; Meier, B.; Carrel, T. Surgical management of complications after transcatheter closure of an atrial septal defect or patent foramen ovale. J Thorac Cardiovasc Surg 2000, 120, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Chessa, M.; Carminati, M.; Butera, G.; Bini, R.M.; Drago, M.; Rosti, L.; Giamberti, A.; Pome, G.; Bossone, E.; Frigiola, A. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol 2002, 39, 1061–1065. [Google Scholar] [CrossRef]
- Latson, L.A. Transcatheter closure of paraprosthetic valve leaks after surgical mitral and aortic valve replacements. Expert Rev Cardiovasc Ther 2009, 7, 507–514. [Google Scholar] [CrossRef]
- Swan, L.; Varma, C.; Yip, J.; Warr, M.; Webb, G.; Benson, L.; McLaughlin, P. Transcatheter device closure of atrial septal defects in the elderly: Technical considerations and short-term outcomes. Int J Cardiol 2006, 107, 207–210. [Google Scholar] [CrossRef]
- Piatkowski, R.; Kochanowski, J.; Scislo, P.; Kochman, J.; Opolski, G. Dislocation of Amplatzer septal occluder device after closure of secundum atrial septal defect. J Am Soc Echocardiogr 2010, 23, 1007 e1001–1002. [Google Scholar] [CrossRef]
- Scott, N.S.; King, M.E.; McQuillan, B.; Shariff, S.; Hung, J.W.; Januzzi, J.L.; Palacios, I.F.; Picard, M.H. Effect of atrial septal mobility on transcatheter closure of interatrial communications. Echocardiography 2003, 20, 711–714. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).