Submitted:
04 May 2023
Posted:
05 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Cannabis and the Endocannabinoid System in the Human Hystory
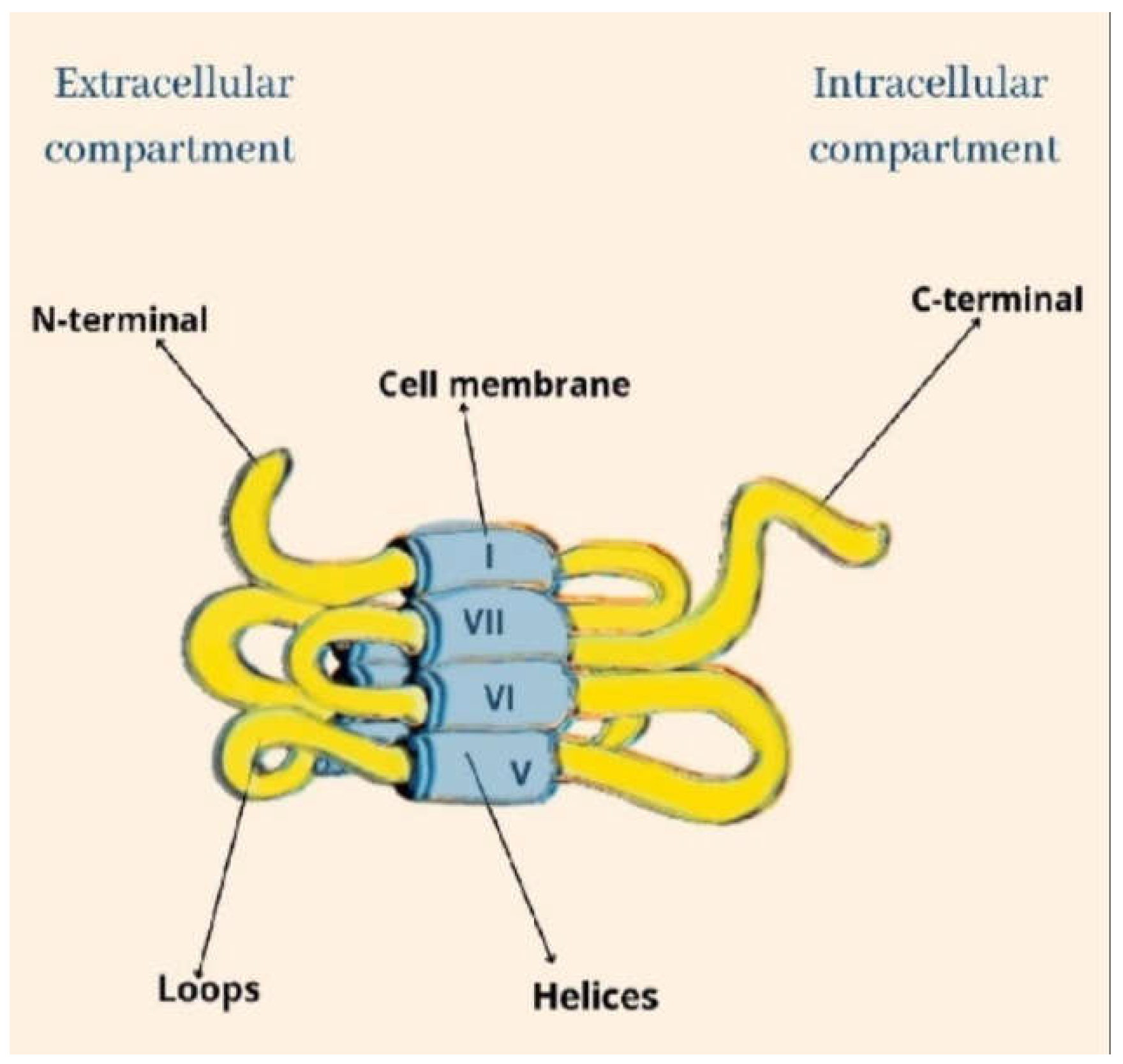
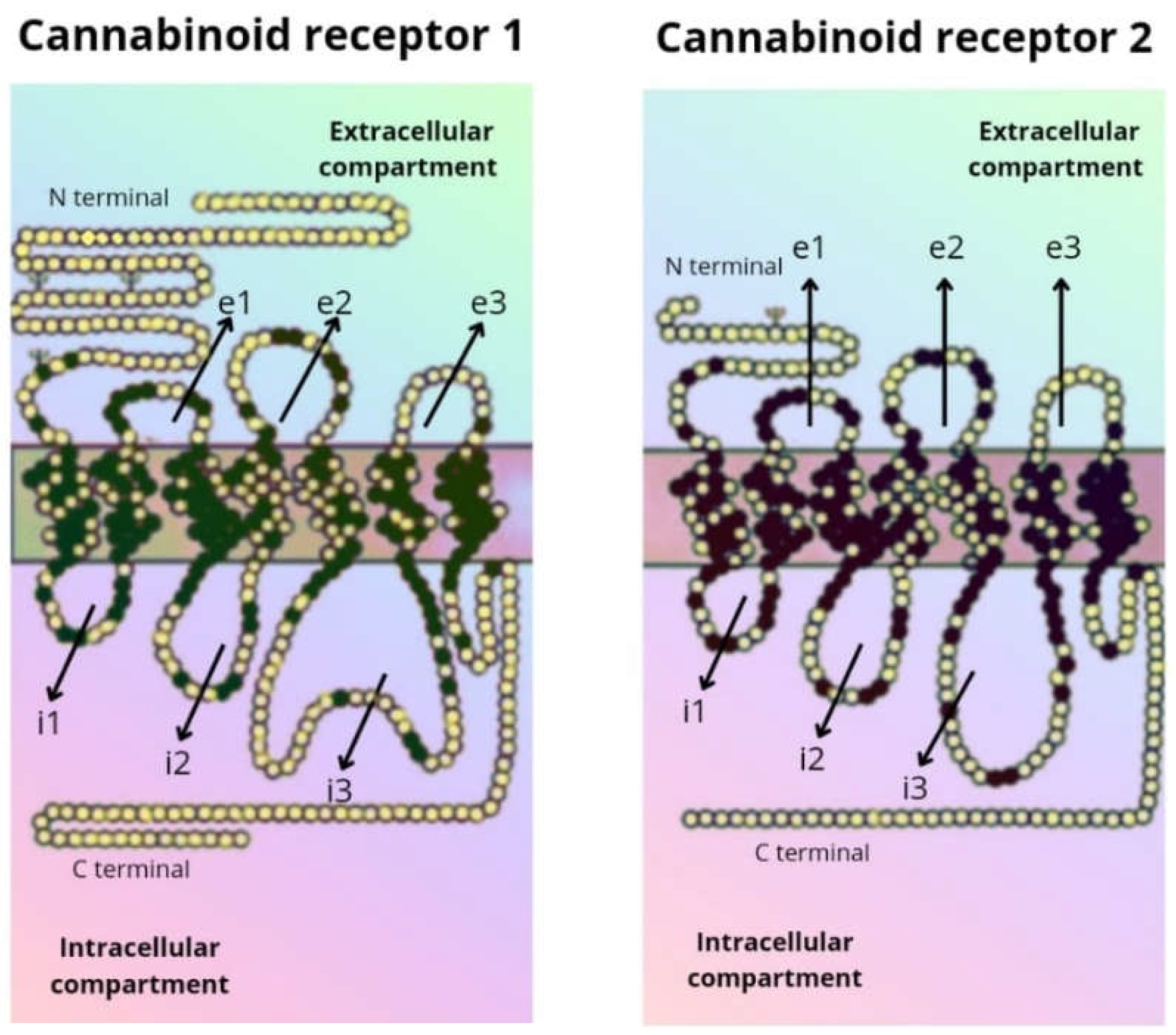
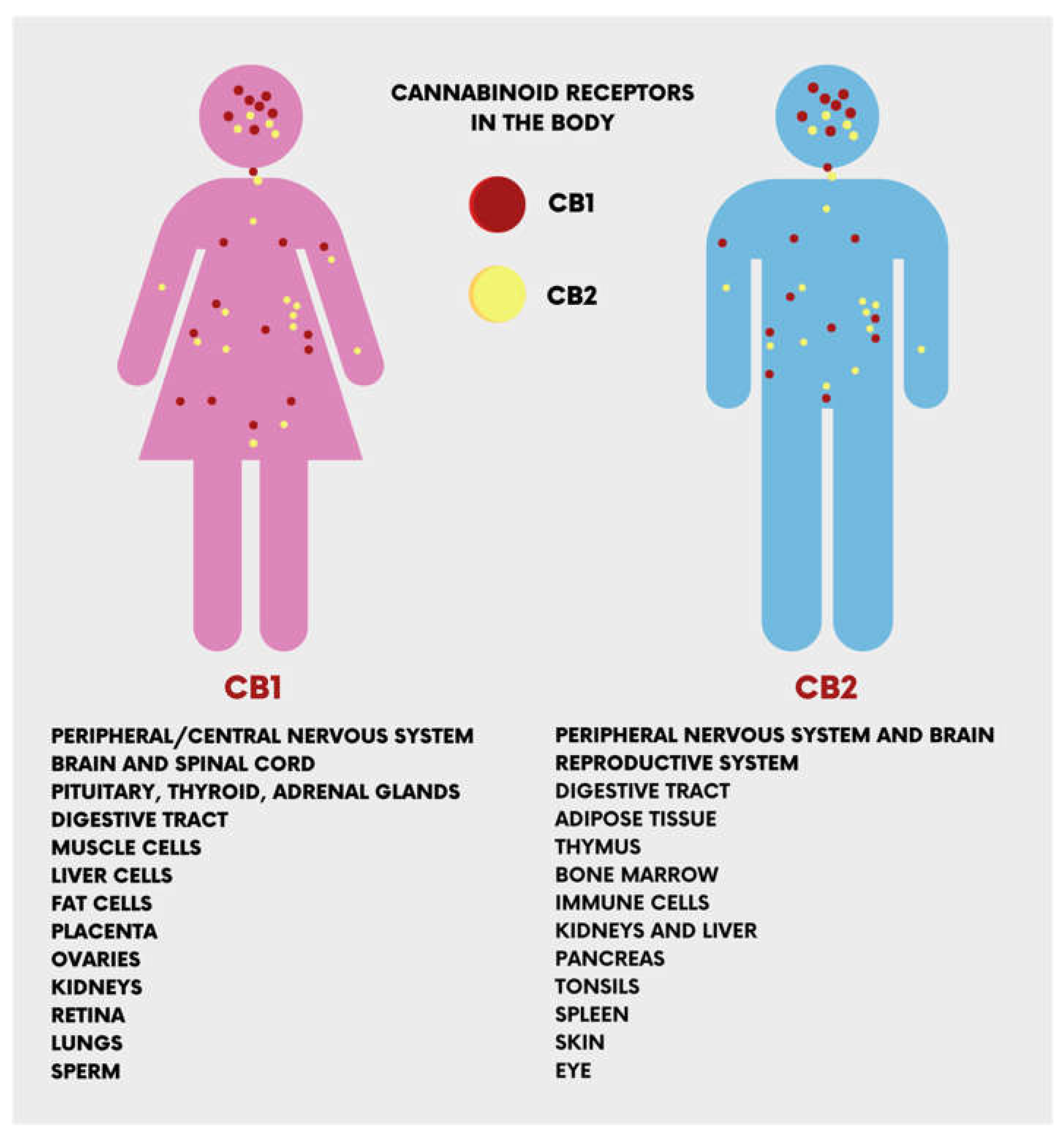
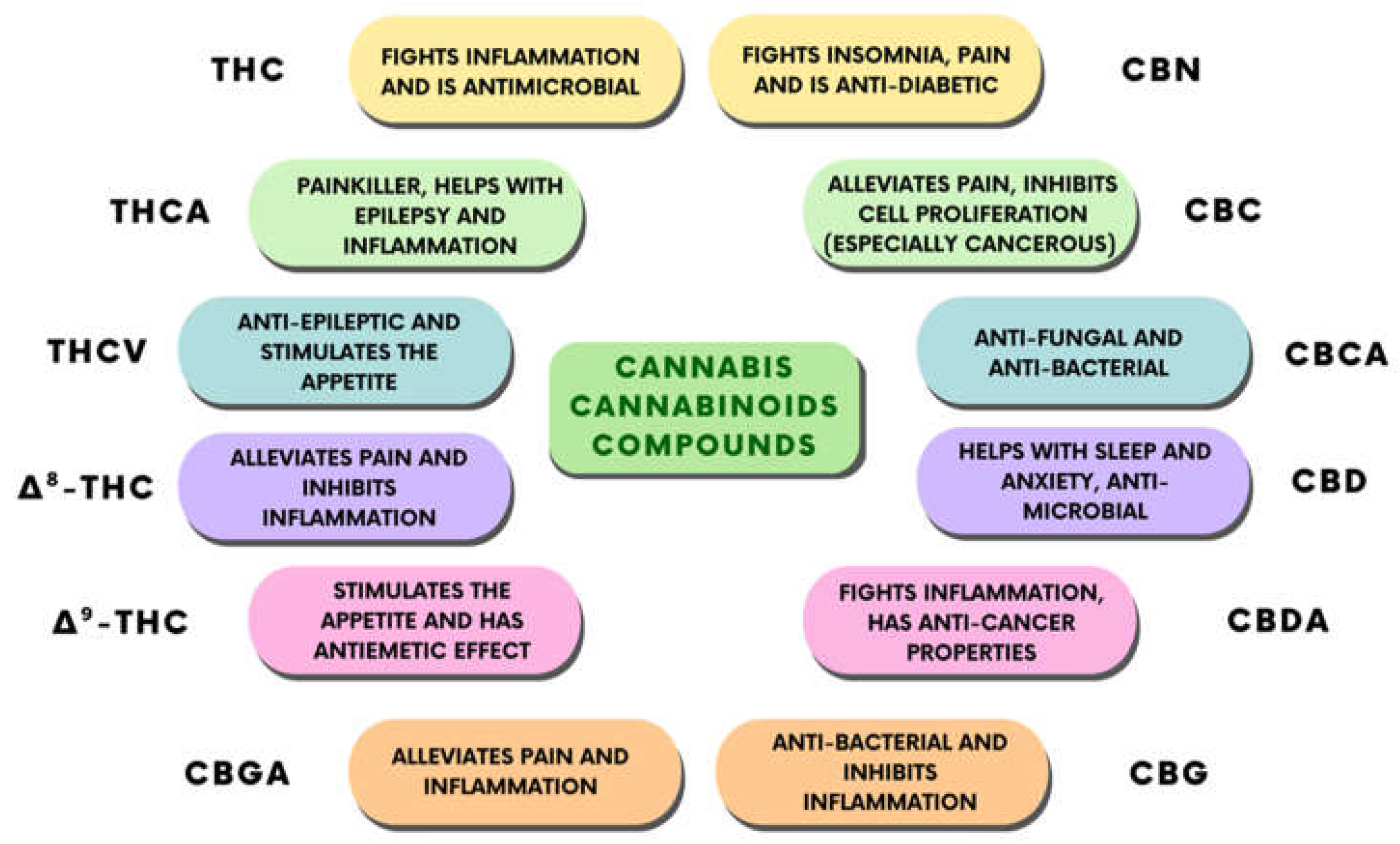
1.2. Biological Targets of CBD Action
1.3. CBD in Dentistry
1.3.1. Oral mucosa
1.3.2. Periodontal tissue
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Δ9-THC | Δ9tetrahydrocannabinol |
| CBD | cannabidiol |
| ECS | endocannabinoid system |
| GPCR | cannabinoid receptors |
| AEA | anandamide |
| 2AG | 2-arachidonoylglycerol |
| FAAH | fatty acid amide hydrolase |
| MAGL | monoacylglycerol lipase |
| 5HT1a | 5HT1a receptor |
| TRP | transient receptor potential |
| GlyRs | glycine |
| FABP | fatty acid binding proteins |
| ENT | equilibrative nucleoside transporter |
| ABCG2 | ATP-binding cassette super-family G member 2 |
| LOXs | Lipooxygenases |
| PLA2 | phospholipase A2 |
| IDO | indoleamine-pyrrole 2,3-dioxygenase |
| ACAT | acyl-CoA cholesterin acyltransferase |
| PPARγ, | peroxisome proliferator-activated receptor gamma |
| Nrf2 | nuclear factor erythroid-derived 2-like 2 |
| IFN- γ | Interferon-γ |
| IL-1 | Interleukin-1 |
| NLR, | nucleotide-binding oligomerization domain-like receptors |
| TGFβ | transforming growth factor beta |
References
- Russo, E.B. History of Cannabis and Its Preparations in Saga, Science, and Sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- Ladha, K.S.; Ajrawat, P.; Yang, Y.; Clarke, H. Understanding the Medical Chemistry of the Cannabis Plant Is Critical to Guiding Real World Clinical Evidence. Molecules 2020, 25, 4042. [Google Scholar] [CrossRef]
- Lowe, H.; Steele, B.; Bryant, J.; Toyang, N.; Ngwa, W. Non-Cannabinoid Metabolites of Cannabis Sativa L. with Therapeutic Potential. Plants 2021, 10, 400. [Google Scholar] [CrossRef]
- Joshi, S.; Ashley, M. Cannabis: A Joint Problem for Patients and the Dental Profession. Br. Dent. J. 2016, 220, 597–601. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell 2016, 167, 750–762.e14. [Google Scholar] [CrossRef]
- Li, X.; Hua, T.; Vemuri, K.; Ho, J.-H.; Wu, Y.; Wu, L.; Popov, P.; Benchama, O.; Zvonok, N.; Locke, K.; et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176, 459–467.e13. [Google Scholar] [CrossRef]
- Moriconi, A.; Cerbara, I.; Maccarrone, M.; Topai, A. GPR55: Current Knowledge and Future Perspectives of a Purported “Type-3” Cannabinoid Receptor. Curr. Med. Chem. 2010, 17, 1411–1429. [Google Scholar] [CrossRef]
- Godlewski, G.; Offertáler, L.; Wagner, J.A.; Kunos, G. Receptors for Acylethanolamides—GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 2009, 89, 105–111. [Google Scholar] [CrossRef]
- Whyte, L.S.; Ryberg, E.; Sims, N.A.; Ridge, S.A.; Mackie, K.; Greasley, P.J.; Ross, R.A.; Rogers, M.J. The Putative Cannabinoid Receptor GPR55 Affects Osteoclast Function in Vitro and Bone Mass in Vivo. Proc. Natl. Acad. Sci. 2009, 106, 16511–16516. [Google Scholar] [CrossRef]
- Overton, H.A.; Fyfe, M.C.T.; Reynet, C. GPR119, a Novel G Protein-Coupled Receptor Target for the Treatment of Type 2 Diabetes and Obesity. Br. J. Pharmacol. 2008, 153, S76–S81. [Google Scholar] [CrossRef]
- Chu, Z.-L.; Jones, R.M.; He, H.; Carroll, C.; Gutierrez, V.; Lucman, A.; Moloney, M.; Gao, H.; Mondala, H.; Bagnol, D.; et al. A Role for β-Cell-Expressed G Protein-Coupled Receptor 119 in Glycemic Control by Enhancing Glucose-Dependent Insulin Release. Endocrinology 2007, 148, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C. International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Garrett, E.R.; Hunt, C.A. Physicochemical Properties, Solubility, and Protein Binding of Δ9 -Tetrahydrocannabinol. J. Pharm. Sci. 1974, 63, 1056–1064. [Google Scholar] [CrossRef]
- Piomelli, D. The Molecular Logic of Endocannabinoid Signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Bellocchio, L.; Inchingolo, A.D.; Inchingolo, A.M.; Lorusso, F.; Malcangi, G.; Santacroce, L.; Scarano, A.; Bordea, I.R.; Hazballa, D.; D’Oria, M.T.; et al. Cannabinoids Drugs and Oral Health—From Recreational Side-Effects to Medicinal Purposes: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 8329. [Google Scholar] [CrossRef] [PubMed]
- Toczek, M.; Malinowska, B. Enhanced Endocannabinoid Tone as a Potential Target of Pharmacotherapy. Life Sci. 2018, 204, 20–45. [Google Scholar] [CrossRef] [PubMed]
- Felder, C.C.; Joyce, K.E.; Briley, E.M.; Mansouri, J.; Mackie, K.; Blond, O.; Lai, Y.; Ma, A.L.; Mitchell, R.L. Comparison of the Pharmacology and Signal Transduction of the Human Cannabinoid CB1 and CB2 Receptors. Mol. Pharmacol. 1995, 48, 443–450. [Google Scholar] [PubMed]
- Maccarrone, M.; Di Rienzo, M.; Battista, N.; Gasperi, V.; Guerrieri, P.; Rossi, A.; Finazzi-Agrò, A. The Endocannabinoid System in Human Keratinocytes. J. Biol. Chem. 2003, 278, 33896–33903. [Google Scholar] [CrossRef]
- Howlett, A.C.; Abood, M.E. CB 1 and CB 2 Receptor Pharmacology. In; 2017; pp. 169–206.
- Adams, R.; Hunt, M.; Clark, J.H. Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp. I. J. Am. Chem. Soc. 1940, 62, 196–200. [Google Scholar] [CrossRef]
- Mechoulam, R.; Shani, A.; Edery, H.; Grunfeld, Y. Chemical Basis of Hashish Activity. Science (80-. ). 1970, 169, 611–612. [Google Scholar] [CrossRef]
- Hall, W.; Degenhardt, L. Adverse Health Effects of Non-Medical Cannabis Use. Lancet 2009, 374, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Hanuš, L.O.; Pertwee, R.; Howlett, A.C. Early Phytocannabinoid Chemistry to Endocannabinoids and Beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the Art and New Challenges for Therapeutic Applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The Biosynthesis of the Cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef] [PubMed]
- Bazzaz, F.A.; Dusek, D.; Seigler, D.S.; Haney, A.W. Photosynthesis and Cannabinoid Content of Temperate and Tropical Populations of Cannabis Sativa. Biochem. Syst. Ecol. 1975, 3, 15–18. [Google Scholar] [CrossRef]
- Eichhorn Bilodeau, S.; Wu, B.-S.; Rufyikiri, A.-S.; MacPherson, S.; Lefsrud, M. An Update on Plant Photobiology and Implications for Cannabis Production. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- van Klingeren, B.; ten Ham, M. Antibacterial Activity of Δ9-Tetrahydrocannabinol and Cannabidiol. Antonie Van Leeuwenhoek 1976, 42, 9–12. [Google Scholar] [CrossRef]
- Schofs, L.; Sparo, M.D.; Sánchez Bruni, S.F. The Antimicrobial Effect behind Cannabis Sativa. Pharmacol. Res. Perspect. 2021, 9. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Tham, M.; Yilmaz, O.; Alaverdashvili, M.; Kelly, M.E.M.; Denovan-Wright, E.M.; Laprairie, R.B. Allosteric and Orthosteric Pharmacology of Cannabidiol and Cannabidiol-Dimethylheptyl at the Type 1 and Type 2 Cannabinoid Receptors. Br. J. Pharmacol. 2019, 176, 1455–1469. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and Its Analogs: A Review of Their Effects on Inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Mester, A.; Benea, H.; Oltean-Dan, D.; Onisor, F.; Baciut, M.; Bran, S. Cannabinoids and Bone Regeneration. Drug Metab. Rev. 2019, 51, 65–75. [Google Scholar] [CrossRef]
- Whyte, L.; Ford, L.; Ridge, S.; Cameron, G.; Rogers, M.; Ross, R. Cannabinoids and Bone: Endocannabinoids Modulate Human Osteoclast Function in Vitro. Br. J. Pharmacol. 2012, 165, 2584–2597. [Google Scholar] [CrossRef]
- Sholler, D.J.; Schoene, L.; Spindle, T.R. Therapeutic Efficacy of Cannabidiol (CBD): A Review of the Evidence From Clinical Trials and Human Laboratory Studies. Curr. Addict. Reports 2020, 7, 405–412. [Google Scholar] [CrossRef]
- Technavio CBD Oil Market by Product and Geography - Forecast and Analysis 2022-2026.
- Abidi, A.H.; Alghamdi, S.S.; Derefinko, K. A Critical Review of Cannabis in Medicine and Dentistry: A Look Back and the Path Forward. Clin. Exp. Dent. Res. 2022, 8, 613–631. [Google Scholar] [CrossRef]
- David, C.; Elizalde-Hernández, A.; Barboza, A.; Cardoso, G.; Santos, M.; Moraes, R. Cannabidiol in Dentistry: A Scoping Review. Dent. J. 2022, 10, 193. [Google Scholar] [CrossRef]
- Cuba, L. de F.; Salum, F.G.; Guimarães, F.S.; Cherubini, K.; Borghetti, R.L.; Figueiredo, M.A.Z. Cannabidiol on 5-FU-induced Oral Mucositis in Mice. Oral Dis. 2020, 26, 1483–1493. [Google Scholar] [CrossRef]
- Konermann, A.; Jäger, A.; Held, S.A.E.; Brossart, P.; Schmöle, A. In Vivo and In Vitro Identification of Endocannabinoid Signaling in Periodontal Tissues and Their Potential Role in Local Pathophysiology. Cell. Mol. Neurobiol. 2017, 37, 1511–1520. [Google Scholar] [CrossRef]
- Miyashita, K.; Oyama, T.; Sakuta, T.; Tokuda, M.; Torii, M. Anandamide Induces Matrix Metalloproteinase-2 Production through Cannabinoid-1 Receptor and Transient Receptor Potential Vanilloid-1 in Human Dental Pulp Cells in Culture. J. Endod. 2012, 38, 786–790. [Google Scholar] [CrossRef]
- Ataei, A.; Rahim Rezaee, S.A.; Moeintaghavi, A.; Ghanbari, H.; Azizi, M. Evaluation of Cannabinoid Receptors Type 1–2 in Periodontitis Patients. Clin. Exp. Dent. Res. 2022, 8, 1040–1044. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef]
- Crocq, M.-A. History of Cannabis and the Endocannabinoid System. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef]
- Miller, L.L.; Branconnier, R.J. Cannabis: Effects on Memory and the Cholinergic Limbic System. Psychol. Bull. 1983, 93, 441–456. [Google Scholar] [CrossRef]
- Cherney, J.; Small, E. Industrial Hemp in North America: Production, Politics and Potential. Agronomy 2016, 6, 58. [Google Scholar] [CrossRef]
- Monthony, A.S.; Page, S.R.; Hesami, M.; Jones, A.M.P. The Past, Present and Future of Cannabis Sativa Tissue Culture. Plants 2021, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Bifulco, M. Modern History of Medical Cannabis: From Widespread Use to Prohibitionism and Back. Trends Pharmacol. Sci. 2017, 38, 195–198. [Google Scholar] [CrossRef]
- Adams, R. Marihuana: Harvey Lecture, February 19, 1942. Bull. N. Y. Acad. Med. 1942, 18, 705–730. [Google Scholar] [PubMed]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Gul, W. Constituents of Cannabis Sativa. In Handbook of Cannabis; Oxford University Press, 2014; pp. 3–22.
- Mechoulam, R.; Hanuš, L. A Historical Overview of Chemical Research on Cannabinoids. Chem. Phys. Lipids 2000, 108, 1–13. [Google Scholar] [CrossRef]
- Alharbi, Y.N. Current Legal Status of Medical Marijuana and Cannabidiol in the United States. Epilepsy Behav. 2020, 112, 107452. [Google Scholar] [CrossRef]
- Howlett, A.C.; Fleming, R.M. Cannabinoid Inhibition of Adenylate Cyclase. Pharmacology of the Response in Neuroblastoma Cell Membranes. Mol. Pharmacol. 1984, 26, 532–538. [Google Scholar]
- Devane, W.A.; Dysarz, F.A.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and Characterization of a Cannabinoid Receptor in Rat Brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned CDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science (80-. ). 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an Endogenous 2-Monoglyceride, Present in Canine Gut, That Binds to Cannabinoid Receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Di Marzo, V.; Stella, N.; Zimmer, A. Endocannabinoid Signalling and the Deteriorating Brain. Nat. Rev. Neurosci. 2015, 16, 30–42. [Google Scholar] [CrossRef]
- Di Marzo, V.; Melck, D.; Bisogno, T.; De Petrocellis, L. Endocannabinoids: Endogenous Cannabinoid Receptor Ligands with Neuromodulatory Action. Trends Neurosci. 1998, 21, 521–528. [Google Scholar] [CrossRef]
- Elphick, M.R. The Evolution and Comparative Neurobiology of Endocannabinoid Signalling. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3201–3215. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pinilla, E.; Varani, K.; Reyes-Resina, I.; Angelats, E.; Vincenzi, F.; Ferreiro-Vera, C.; Oyarzabal, J.; Canela, E.I.; Lanciego, J.L.; Nadal, X.; et al. Binding and Signaling Studies Disclose a Potential Allosteric Site for Cannabidiol in Cannabinoid CB2 Receptors. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Thomas, A.; Baillie, G.L.; Phillips, A.M.; Razdan, R.K.; Ross, R.A.; Pertwee, R.G. Cannabidiol Displays Unexpectedly High Potency as an Antagonist of CB 1 and CB 2 Receptor Agonists in Vitro. Br. J. Pharmacol. 2007, 150, 613–623. [Google Scholar] [CrossRef]
- Navarro, G.; Reyes-Resina, I.; Rivas-Santisteban, R.; Sánchez de Medina, V.; Morales, P.; Casano, S.; Ferreiro-Vera, C.; Lillo, A.; Aguinaga, D.; Jagerovic, N.; et al. Cannabidiol Skews Biased Agonism at Cannabinoid CB1 and CB2 Receptors with Smaller Effect in CB1-CB2 Heteroreceptor Complexes. Biochem. Pharmacol. 2018, 157, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.; Bolognini, D.; Limebeer, C.; Cascio, M.; Anavi-Goffer, S.; Fletcher, P.; Mechoulam, R.; Pertwee, R.; Parker, L. Cannabidiol, a Non-Psychotropic Component of Cannabis, Attenuates Vomiting and Nausea-like Behaviour via Indirect Agonism of 5-HT1A Somatodendritic Autoreceptors in the Dorsal Raphe Nucleus. Br. J. Pharmacol. 2012, 165, 2620–2634. [Google Scholar] [CrossRef]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- Ryberg, E.; Larsson, N.; Sjögren, S.; Hjorth, S.; Hermansson, N.-O.; Leonova, J.; Elebring, T.; Nilsson, K.; Drmota, T.; Greasley, P.J. The Orphan Receptor GPR55 Is a Novel Cannabinoid Receptor. Br. J. Pharmacol. 2007, 152, 1092–1101. [Google Scholar] [CrossRef]
- Lauckner, J.E.; Jensen, J.B.; Chen, H.-Y.; Lu, H.-C.; Hille, B.; Mackie, K. GPR55 Is a Cannabinoid Receptor That Increases Intracellular Calcium and Inhibits M Current. Proc. Natl. Acad. Sci. 2008, 105, 2699–2704. [Google Scholar] [CrossRef]
- Laun, A.S.; Shrader, S.H.; Brown, K.J.; Song, Z.-H. GPR3, GPR6, and GPR12 as Novel Molecular Targets: Their Biological Functions and Interaction with Cannabidiol. Acta Pharmacol. Sin. 2019, 40, 300–308. [Google Scholar] [CrossRef]
- Petrescu, N.B.; Jurj, A.; Sorițău, O.; Lucaciu, O.P.; Dirzu, N.; Raduly, L.; Berindan-Neagoe, I.; Cenariu, M.; Boșca, B.A.; Campian, R.S.; et al. Cannabidiol and Vitamin D3 Impact on Osteogenic Differentiation of Human Dental Mesenchymal Stem Cells. Medicina (B. Aires). 2020, 56, 607. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of Cannabinoids and Cannabinoid-Enriched Cannabis Extracts on TRP Channels and Endocannabinoid Metabolic Enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular Targets for Cannabidiol and Its Synthetic Analogues: Effect on Vanilloid VR1 Receptors and on the Cellular Uptake and Enzymatic Hydrolysis of Anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Neeper, M.P.; Liu, Y.; Hutchinson, T.L.; Lubin, M.L.; Flores, C.M. TRPV2 Is Activated by Cannabidiol and Mediates CGRP Release in Cultured Rat Dorsal Root Ganglion Neurons. J. Neurosci. 2008, 28, 6231–6238. [Google Scholar] [CrossRef] [PubMed]
- Nabissi, M.; Morelli, M.B.; Santoni, M.; Santoni, G. Triggering of the TRPV2 Channel by Cannabidiol Sensitizes Glioblastoma Cells to Cytotoxic Chemotherapeutic Agents. Carcinogenesis 2013, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Vellani, V.; Schiano-Moriello, A.; Marini, P.; Magherini, P.C.; Orlando, P.; Di Marzo, V. Plant-Derived Cannabinoids Modulate the Activity of Transient Receptor Potential Channels of Ankyrin Type-1 and Melastatin Type-8. J. Pharmacol. Exp. Ther. 2008, 325, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, M.; Keun-Hang, S.Y.; Sydorenko, V.; Ashoor, A.; Kabbani, N.; Al Kury, L.; Sadek, B.; Howarth, C.F.; Isaev, D.; Galadari, S.; et al. Effects of Cannabidiol on the Function of A7-Nicotinic Acetylcholine Receptors. Eur. J. Pharmacol. 2013, 720, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-H.; Galadari, S.; Isaev, D.; Petroianu, G.; Shippenberg, T.S.; Oz, M. The Nonpsychoactive Cannabinoid Cannabidiol Inhibits 5-Hydroxytryptamine 3A Receptor-Mediated Currents in Xenopus Laevis Oocytes. J. Pharmacol. Exp. Ther. 2010, 333, 547–554. [Google Scholar] [CrossRef]
- Ahrens, J.; Demir, R.; Leuwer, M.; de la Roche, J.; Krampfl, K.; Foadi, N.; Karst, M.; Haeseler, G. The Nonpsychotropic Cannabinoid Cannabidiol Modulates and Directly Activates Alpha-1 and Alpha-1-Beta Glycine Receptor Function. Pharmacology 2009, 83, 217–222. [Google Scholar] [CrossRef]
- Bakas, T.; van Nieuwenhuijzen, P.S.; Devenish, S.O.; McGregor, I.S.; Arnold, J.C.; Chebib, M. The Direct Actions of Cannabidiol and 2-Arachidonoyl Glycerol at GABA A Receptors. Pharmacol. Res. 2017, 119, 358–370. [Google Scholar] [CrossRef]
- Ross, H.R.; Napier, I.; Connor, M. Inhibition of Recombinant Human T-Type Calcium Channels by Δ9-Tetrahydrocannabinol and Cannabidiol. J. Biol. Chem. 2008, 283, 16124–16134. [Google Scholar] [CrossRef]
- Ghovanloo, M.-R.; Shuart, N.G.; Mezeyova, J.; Dean, R.A.; Ruben, P.C.; Goodchild, S.J. Inhibitory Effects of Cannabidiol on Voltage-Dependent Sodium Currents. J. Biol. Chem. 2018, 293, 16546–16558. [Google Scholar] [CrossRef] [PubMed]
- Rimmerman, N.; Ben-Hail, D.; Porat, Z.; Juknat, A.; Kozela, E.; Daniels, M.P.; Connelly, P.S.; Leishman, E.; Bradshaw, H.B.; Shoshan-Barmatz, V.; et al. Direct Modulation of the Outer Mitochondrial Membrane Channel, Voltage-Dependent Anion Channel 1 (VDAC1) by Cannabidiol: A Novel Mechanism for Cannabinoid-Induced Cell Death. Cell Death Dis. 2013, 4, e949–e949. [Google Scholar] [CrossRef] [PubMed]
- Elmes, M.W.; Kaczocha, M.; Berger, W.T.; Leung, K.; Ralph, B.P.; Wang, L.; Sweeney, J.M.; Miyauchi, J.T.; Tsirka, S.E.; Ojima, I.; et al. Fatty Acid-Binding Proteins (FABPs) Are Intracellular Carriers for Δ9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD). J. Biol. Chem. 2015, 290, 8711–8721. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol Enhances Anandamide Signaling and Alleviates Psychotic Symptoms of Schizophrenia. Transl. Psychiatry 2012, 2, e94–e94. [Google Scholar] [CrossRef] [PubMed]
- Leishman, E.; Manchanda, M.; Thelen, R.; Miller, S.; Mackie, K.; Bradshaw, H.B. Cannabidiol’s Upregulation of N -Acyl Ethanolamines in the Central Nervous System Requires N -Acyl Phosphatidyl Ethanolamine-Specific Phospholipase D. Cannabis Cannabinoid Res. 2018, 3, 228–241. [Google Scholar] [CrossRef]
- Carrier, E.J.; Auchampach, J.A.; Hillard, C.J. Inhibition of an Equilibrative Nucleoside Transporter by Cannabidiol: A Mechanism of Cannabinoid Immunosuppression. Proc. Natl. Acad. Sci. 2006, 103, 7895–7900. [Google Scholar] [CrossRef] [PubMed]
- Mijangos-Moreno, S.; Poot-Aké, A.; Arankowsky-Sandoval, G.; Murillo-Rodríguez, E. Intrahypothalamic Injection of Cannabidiol Increases the Extracellular Levels of Adenosine in Nucleus Accumbens in Rats. Neurosci. Res. 2014, 84, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.L.; Allen, J.D.; Arnold, J.C. Interaction of Plant Cannabinoids with the Multidrug Transporter ABCC1 (MRP1). Eur. J. Pharmacol. 2008, 591, 128–131. [Google Scholar] [CrossRef]
- GILBERT, J.C.; PERTWEE, R.G.; WYLLIE, M.G. EFFECTS OF Δ9-TETRAHYDROCANNABINOL AND CANNABIDIOL ON A Mg2+-ATPase OF SYNAPTIC VESICLES PREPARED FROM RAT CEREBRAL CORTEX. Br. J. Pharmacol. 1977, 59, 599–601. [Google Scholar] [CrossRef]
- Doohan, P.T.; Oldfield, L.D.; Arnold, J.C.; Anderson, L.L. Cannabinoid Interactions with Cytochrome P450 Drug Metabolism: A Full-Spectrum Characterization. AAPS J. 2021, 23, 91. [Google Scholar] [CrossRef]
- Takeda, S.; Usami, N.; Yamamoto, I.; Watanabe, K. Cannabidiol-2′,6′-Dimethyl Ether, a Cannabidiol Derivative, Is a Highly Potent and Selective 15-Lipoxygenase Inhibitor. Drug Metab. Dispos. 2009, 37, 1733–1737. [Google Scholar] [CrossRef] [PubMed]
- Wheal, A.J.; Cipriano, M.; Fowler, C.J.; Randall, M.D.; O’Sullivan, S.E. Cannabidiol Improves Vasorelaxation in Zucker Diabetic Fatty Rats through Cyclooxygenase Activation. J. Pharmacol. Exp. Ther. 2014, 351, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.T.; Formukong, E.; Evans, F.J. Activation of Phospholipase A 2 by Cannabinoids. FEBS Lett. 1987, 211, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Fišar, Z.; Singh, N.; Hroudová, J. Cannabinoid-Induced Changes in Respiration of Brain Mitochondria. Toxicol. Lett. 2014, 231, 62–71. [Google Scholar] [CrossRef]
- Koch, M.; Dehghani, F.; Habazettl, I.; Schomerus, C.; Korf, H.-W. Cannabinoids Attenuate Norepinephrine-Induced Melatonin Biosynthesis in the Rat Pineal Gland by Reducing Arylalkylamine N-Acetyltransferase Activity without Involvement of Cannabinoid Receptors. J. Neurochem. 2006, 98, 267–278. [Google Scholar] [CrossRef]
- Jenny, M.; Santer, E.; Pirich, E.; Schennach, H.; Fuchs, D. Δ9-Tetrahydrocannabinol and Cannabidiol Modulate Mitogen-Induced Tryptophan Degradation and Neopterin Formation in Peripheral Blood Mononuclear Cells in Vitro. J. Neuroimmunol. 2009, 207, 75–82. [Google Scholar] [CrossRef]
- Cornicelli, J.A.; Gilman, S.R.; Krom, B.A.; Kottke, B.A. Cannabinoids Impair the Formation of Cholesteryl Ester in Cultured Human Cells. Arterioscler. An Off. J. Am. Hear. Assoc. Inc. 1981, 1, 449–454. [Google Scholar] [CrossRef]
- Watanabe, K.; Motoya, E.; Matsuzawa, N.; Funahashi, T.; Kimura, T.; Matsunaga, T.; Arizono, K.; Yamamoto, I. Marijuana Extracts Possess the Effects like the Endocrine Disrupting Chemicals. Toxicology 2005, 206, 471–478. [Google Scholar] [CrossRef]
- Hegde, V.L.; Singh, U.P.; Nagarkatti, P.S.; Nagarkatti, M. Critical Role of Mast Cells and Peroxisome Proliferator–Activated Receptor γ in the Induction of Myeloid-Derived Suppressor Cells by Marijuana Cannabidiol In Vivo. J. Immunol. 2015, 194, 5211–5222. [Google Scholar] [CrossRef]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Gao, F.; Coppola, G.; Geschwind, D.; Vogel, Z. Microarray and Pathway Analysis Reveal Distinct Mechanisms Underlying Cannabinoid-Mediated Modulation of LPS-Induced Activation of BV-2 Microglial Cells. PLoS ONE 2013, 8, e61462. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. Cannabidiol as a Novel Therapeutic for Immune Modulation. ImmunoTargets Ther. 2020, Volume 9, 131–140. [Google Scholar] [CrossRef]
- Costa, B.; Giagnoni, G.; Franke, C.; Trovato, A.E.; Colleoni, M. Vanilloid TRPV1 Receptor Mediates the Antihyperalgesic Effect of the Nonpsychoactive Cannabinoid, Cannabidiol, in a Rat Model of Acute Inflammation. Br. J. Pharmacol. 2004, 143, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. Cannabinoid-Induced Cell Death in Endometrial Cancer Cells: Involvement of TRPV1 Receptors in Apoptosis. J. Physiol. Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Δ9-Tetrahydrocannabinol and Cannabidiol Differentially Inhibit the Lipopolysaccharide-Activated NF-ΚB and Interferon-β/STAT Proinflammatory Pathways in BV-2 Microglial Cells. J. Biol. Chem. 2010, 285, 1616–1626. [Google Scholar] [CrossRef]
- Liu, C.; Ma, H.; Slitt, A.L.; Seeram, N.P. Inhibitory Effect of Cannabidiol on the Activation of NLRP3 Inflammasome Is Associated with Its Modulation of the P2X7 Receptor in Human Monocytes. J. Nat. Prod. 2020, 83, 2025–2029. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Iñigo, P.M.; Mestre, L.; Carrillo-Salinas, F.J.; Guaza, C. Cannabidiol Provides Long-Lasting Protection against the Deleterious Effects of Inflammation in a Viral Model of Multiple Sclerosis: A Role for A2A Receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef]
- Borsani, E.; Majorana, A.; Cocchi, M.A.; Conti, G.; Bonadeo, S.; Padovani, A.; Lauria, G.; Bardellini, E.; Rezzani, R.; Rodella, L.F. Epithelial Expression of Vanilloid and Cannabinoid Receptors: A Potential Role in Burning Mouth Syndrome Pathogenesis. Histol. Histopathol. 2014, 29, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, S.; Giaginis, C.; Alexandrou, P.; Rodriguez, J.; Tasoulas, J.; Danas, E.; Patsouris, E.; Klijanienko, J. Evaluation of Cannabinoid CB1 and CB2 Receptors Expression in Mobile Tongue Squamous Cell Carcinoma: Associations with Clinicopathological Parameters and Patients’ Survival. Tumor Biol. 2016, 37, 3647–3656. [Google Scholar] [CrossRef]
- Rosenblatt, K.A.; Daling, J.R.; Chen, C.; Sherman, K.J.; Schwartz, S.M. Marijuana Use and Risk of Oral Squamous Cell Carcinoma. Cancer Res. 2004, 64, 4049–4054. [Google Scholar] [CrossRef]
- Beneng, K.; Renton, T.; Yilmaz, Z.; Yiangou, Y.; Anand, P. Cannabinoid Receptor CB1-Immunoreactive Nerve Fibres in Painful and Non-Painful Human Tooth Pulp. J. Clin. Neurosci. 2010, 17, 1476–1479. [Google Scholar] [CrossRef]
- Que, K.; He, D.; Jin, Y.; Wu, L.; Wang, F.; Zhao, Z.; Yang, J.; Deng, J. Expression of Cannabinoid Type 1 Receptors in Human Odontoblast Cells. J. Endod. 2017, 43, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Saiz, L.; Bernal-Cepeda, L.; Castellanos, J. Immune Challenges Upregulate the Expression of Cannabinoid Receptors in Cultured Human Odontoblasts and Gingival Fibroblasts. Acta Odontológica Latinoam. 2022, 35, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, M.; Sobhan, U.; Muramatsu, T.; Sato, M.; Ichikawa, H.; Sahara, Y.; Tazaki, M.; Shibukawa, Y. TRPV1-Mediated Calcium Signal Couples with Cannabinoid Receptors and Sodium–Calcium Exchangers in Rat Odontoblasts. Cell Calcium 2012, 52, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Thoungseabyoun, W.; Tachow, A.; Pakkarato, S.; Rawangwong, A.; Krongyut, S.; Sakaew, W.; Kondo, H.; Hipkaeo, W. Immunohistochemical Localization of Cannabinoid Receptor 1 (CB1) in the Submandibular Gland of Mice under Normal Conditions and When Stimulated by Isoproterenol or Carbachol. Arch. Oral Biol. 2017, 81, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Pirino, C.; Cappai, M.G.; Maranesi, M.; Tomassoni, D.; Giontella, A.; Pinna, W.; Boiti, C.; Kamphues, J.; Dall’Aglio, C. The Presence and Distribution of Cannabinoid Type 1 and 2 Receptors in the Mandibular Gland: The Influence of Different Physical Forms of Diets on Their Expression in Piglets. J. Anim. Physiol. Anim. Nutr. (Berl). 2018, 102, e870–e876. [Google Scholar] [CrossRef]
- Yoshida, R.; Ohkuri, T.; Jyotaki, M.; Yasuo, T.; Horio, N.; Yasumatsu, K.; Sanematsu, K.; Shigemura, N.; Yamamoto, T.; Margolskee, R.F.; et al. Endocannabinoids Selectively Enhance Sweet Taste. Proc. Natl. Acad. Sci. 2010, 107, 935–939. [Google Scholar] [CrossRef]
- Prestifilippo, J.P.; Fernandez-Solari, J.; Medina, V.; Rettori, V.; Elverdin, J.C. Role of the Endocannabinoid System in Ethanol-Induced Inhibition of Salivary Secretion. Alcohol Alcohol. 2009, 44, 443–448. [Google Scholar] [CrossRef]
- Prestifilippo, J.P.; Fernández-Solari, J.; Cal, C. de la; Iribarne, M.; Suburo, A.M.; Rettori, V.; McCann, S.M.; Elverdin, J.C. Inhibition of Salivary Secretion by Activation of Cannabinoid Receptors. Exp. Biol. Med. 2006, 231, 1421–1429. [Google Scholar] [CrossRef]
- Andreis, K.; Billingsley, J.; Naimi Shirazi, K.; Wager-Miller, J.; Johnson, C.; Bradshaw, H.; Straiker, A. Cannabinoid CB1 Receptors Regulate Salivation. Sci. Rep. 2022, 12, 14182. [Google Scholar] [CrossRef]
- Jirasek, P.; Jusku, A.; Simanek, V.; Frankova, J.; Storch, J.; Vacek, J. Cannabidiol and Periodontal Inflammatory Disease: A Critical Assessment. Biomed. Pap. 2022, 166, 155–160. [Google Scholar] [CrossRef]
- Liu, C.; Qi, X.; Alhabeil, J.; Lu, H.; Zhou, Z. Activation of Cannabinoid Receptors Promote Periodontal Cell Adhesion and Migration. J. Clin. Periodontol. 2019, 46, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Raphael-Mizrahi, B.; Gabet, Y. The Cannabinoids Effect on Bone Formation and Bone Healing. Curr. Osteoporos. Rep. 2020, 18, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Kozono, S.; Matsuyama, T.; Biwasa, K.K.; Kawahara, K.; Nakajima, Y.; Yoshimoto, T.; Yonamine, Y.; Kadomatsu, H.; Tancharoen, S.; Hashiguchi, T.; et al. Involvement of the Endocannabinoid System in Periodontal Healing. Biochem. Biophys. Res. Commun. 2010, 394, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Miyazu, M.; Xiang, P.; Li, S.N.; Sokabe, M.; Naruse, K. Stretch-Induced Cell Proliferation Is Mediated by FAK-MAPK Pathway. Life Sci. 2005, 76, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Lanza Cariccio, V.; Scionti, D.; Raffa, A.; Iori, R.; Pollastro, F.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Treatment of Periodontal Ligament Stem Cells with MOR and CBD Promotes Cell Survival and Neuronal Differentiation via the PI3K/Akt/MTOR Pathway. Int. J. Mol. Sci. 2018, 19, 2341. [Google Scholar] [CrossRef] [PubMed]
- Ossola, C.A.; Surkin, P.N.; Mohn, C.E.; Elverdin, J.C.; Fernández-Solari, J. Anti-Inflammatory and Osteoprotective Effects of Cannabinoid-2 Receptor Agonist HU-308 in a Rat Model of Lipopolysaccharide-Induced Periodontitis. J. Periodontol. 2016, 87, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.Y.; Dabbous, M.K.; Tipton, D.A. Effect of Cannabidiol on Human Gingival Fibroblast Extracellular Matrix Metabolism: MMP Production and Activity, and Production of Fibronectin and Transforming Growth Factor β. J. Periodontal Res. 2012, 47, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Napimoga, M.H.; Benatti, B.B.; Lima, F.O.; Alves, P.M.; Campos, A.C.; Pena-dos-Santos, D.R.; Severino, F.P.; Cunha, F.Q.; Guimarães, F.S. Cannabidiol Decreases Bone Resorption by Inhibiting RANK/RANKL Expression and pro-Inflammatory Cytokines during Experimental Periodontitis in Rats. Int. Immunopharmacol. 2009, 9, 216–222. [Google Scholar] [CrossRef]
- Gu, Z.; Singh, S.; Niyogi, R.G.; Lamont, G.J.; Wang, H.; Lamont, R.J.; Scott, D.A. Marijuana-Derived Cannabinoids Trigger a CB2/PI3K Axis of Suppression of the Innate Response to Oral Pathogens. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Stahl, V.; Vasudevan, K. Comparison of Efficacy of Cannabinoids versus Commercial Oral Care Products in Reducing Bacterial Content from Dental Plaque: A Preliminary Observation. Cureus 2020. [Google Scholar] [CrossRef]
- Klein, M.; de Quadros De Bortolli, J.; Guimarães, F.S.; Salum, F.G.; Cherubini, K.; de Figueiredo, M.A.Z. Effects of Cannabidiol, a Cannabis Sativa Constituent, on Oral Wound Healing Process in Rats: Clinical and Histological Evaluation. Phyther. Res. 2018, 32, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Furuichi, Y.; Biswas, K.K.; Hashiguchi, T.; Kawahara, K.; Yamaji, K.; Uchimura, T.; Izumi, Y.; Maruyama, I. Endocannabinoid, Anandamide in Gingival Tissue Regulates the Periodontal Inflammation through NF-ΚB Pathway Inhibition. FEBS Lett. 2006, 580, 613–619. [Google Scholar] [CrossRef]
- Qi, X.; Liu, C.; Li, G.; Luan, H.; Li, S.; Yang, D.; Zhou, Z. Investigation of in Vitro Odonto/Osteogenic Capacity of Cannabidiol on Human Dental Pulp Cell. J. Dent. 2021, 109, 103673. [Google Scholar] [CrossRef] [PubMed]
- Luiz de Oliveira da Rosa, W.; Machado da Silva, T.; Fernando Demarco, F.; Piva, E.; Fernandes da Silva, A. Could the Application of Bioactive Molecules Improve Vital Pulp Therapy Success? A Systematic Review. J. Biomed. Mater. Res. Part A 2017, 105, 941–956. [Google Scholar] [CrossRef]
- Qi, X.; Liu, C.; Li, G.; Al-Alfe, D.; Paurazas, S.; Askar, M.; Yang, D.; Zhou, Z. Evaluation of Cannabinoids on the Odonto/Osteogenesis in Human Dental Pulp Cells In Vitro. J. Endod. 2021, 47, 444–450. [Google Scholar] [CrossRef]
- Yu, L.; Zeng, L.; Zhang, Z.; Zhu, G.; Xu, Z.; Xia, J.; Weng, J.; Li, J.; Pathak, J.L. Cannabidiol Rescues TNF-α-Inhibited Proliferation, Migration, and Osteogenic/Odontogenic Differentiation of Dental Pulp Stem Cells. Biomolecules 2023, 13, 118. [Google Scholar] [CrossRef]
- Duncan, H.F.; Galler, K.M.; Tomson, P.L.; Simon, S.; El-Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H.; Markvart, M.; et al. European Society of Endodontology Position Statement: Management of Deep Caries and the Exposed Pulp. Int. Endod. J. 2019, 52, 923–934. [Google Scholar] [CrossRef]
- Sáez, M.D.M.; López, G.L.; Atlas, D.; de la Casa, M.L. Evaluation of PH and Calcium Ion Diffusion from Calcium Hydroxide Pastes and MTA. Acta Odontol. Latinoam. 2017, 30, 26–32. [Google Scholar]
- Schmuhl, E.; Ramer, R.; Salamon, A.; Peters, K.; Hinz, B. Increase of Mesenchymal Stem Cell Migration by Cannabidiol via Activation of P42/44 MAPK. Biochem. Pharmacol. 2014, 87, 489–501. [Google Scholar] [CrossRef]
- Smith, A.J.; Smith, J.G.; Shelton, R.M.; Cooper, P.R. Harnessing the Natural Regenerative Potential of the Dental Pulp. Dent. Clin. North Am. 2012, 56, 589–601. [Google Scholar] [CrossRef]
- Libro, R.; Scionti, D.; Diomede, F.; Marchisio, M.; Grassi, G.; Pollastro, F.; Piattelli, A.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol Modulates the Immunophenotype and Inhibits the Activation of the Inflammasome in Human Gingival Mesenchymal Stem Cells. Front. Physiol. 2016, 7. [Google Scholar] [CrossRef]
- Cuba, L.F.; Salum, F.G.; Cherubini, K.; Figueiredo, M.A.Z. Cannabidiol: An Alternative Therapeutic Agent for Oral Mucositis? J. Clin. Pharm. Ther. 2017, 42, 245–250. [Google Scholar] [CrossRef]
- Kamali, A.; Oryan, A.; Hosseini, S.; Ghanian, M.H.; Alizadeh, M.; Baghaban Eslaminejad, M.; Baharvand, H. Cannabidiol-Loaded Microspheres Incorporated into Osteoconductive Scaffold Enhance Mesenchymal Stem Cell Recruitment and Regeneration of Critical-Sized Bone Defects. Mater. Sci. Eng. C 2019, 101, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Rock, E.M.; Limebeer, C.L.; Pertwee, R.G.; Mechoulam, R.; Parker, L.A. Therapeutic Potential of Cannabidiol, Cannabidiolic Acid, and Cannabidiolic Acid Methyl Ester as Treatments for Nausea and Vomiting. Cannabis Cannabinoid Res. 2021, 6, 266–274. [Google Scholar] [CrossRef]
- Good, P.; Haywood, A.; Gogna, G.; Martin, J.; Yates, P.; Greer, R.; Hardy, J. Oral Medicinal Cannabinoids to Relieve Symptom Burden in the Palliative Care of Patients with Advanced Cancer: A Double-Blind, Placebo Controlled, Randomised Clinical Trial of Efficacy and Safety of Cannabidiol (CBD). BMC Palliat. Care 2019, 18, 110. [Google Scholar] [CrossRef]
- Frane, N.; Stapleton, E.; Iturriaga, C.; Ganz, M.; Rasquinha, V.; Duarte, R. Cannabidiol as a Treatment for Arthritis and Joint Pain: An Exploratory Cross-Sectional Study. J. Cannabis Res. 2022, 4, 47. [Google Scholar] [CrossRef]
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The Nonpsychoactive Cannabis Constituent Cannabidiol Is an Oral Anti-Arthritic Therapeutic in Murine Collagen-Induced Arthritis. Proc. Natl. Acad. Sci. 2000, 97, 9561–9566. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. The Endocannabinoid System in Obesity and Type 2 Diabetes. Diabetologia 2008, 51, 1356–1367. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.; Zeira, M.; Reich, S.; Har-Noy, M.; Mechoulam, R.; Slavin, S.; Gallily, R. Cannabidiol Lowers Incidence of Diabetes in Non-Obese Diabetic Mice. Autoimmunity 2006, 39, 143–151. [Google Scholar] [CrossRef]
- Kudiyirickal, M.G.; Pappachan, J.M. Diabetes Mellitus and Oral Health. Endocrine 2015, 49, 27–34. [Google Scholar] [CrossRef]
- Lattanzi, S.; Trinka, E.; Striano, P.; Rocchi, C.; Salvemini, S.; Silvestrini, M.; Brigo, F. Highly Purified Cannabidiol for Epilepsy Treatment: A Systematic Review of Epileptic Conditions Beyond Dravet Syndrome and Lennox–Gastaut Syndrome. CNS Drugs 2021, 35, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D. Cannabidiol: A Review of Clinical Efficacy and Safety in Epilepsy. Pediatr. Neurol. 2019, 96, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Nonato, E.R.; Borges, M.A. Oral and Maxillofacial Trauma in Patients with Epilepsy: Prospective Study Based on an Outpatient Population. Arq. Neuropsiquiatr. 2011, 69, 491–495. [Google Scholar] [CrossRef]
- Stahl, V. Cannabis and Derivatives Thereof for the Treatment of Pain and Inflammation Related with Dental Pulp and Bone Regeneration Related to Dental Jaw Bone Defects.
- Dick, T.N.A.; Marques, L.C.; Lopes, A. de A.L.; Candreva, M.S.; Santos, L.R.; Picciani, B.L.S. Phytotherapy in Dentistry: A Literature Review Based on Clinical Data. European J. Med. Plants 2020, 1–13. [Google Scholar] [CrossRef]
- Nelson, K.M.; Bisson, J.; Singh, G.; Graham, J.G.; Chen, S.-N.; Friesen, J.B.; Dahlin, J.L.; Niemitz, M.; Walters, M.A.; Pauli, G.F. The Essential Medicinal Chemistry of Cannabidiol (CBD). J. Med. Chem. 2020, 63, 12137–12155. [Google Scholar] [CrossRef]
- Koturbash, I.; MacKay, D. Cannabidiol and Other Cannabinoids: From Toxicology and Pharmacology to the Development of a Regulatory Pathway. J. Diet. Suppl. 2020, 17, 487–492. [Google Scholar] [CrossRef]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural Products for Human Health: An Historical Overview of the Drug Discovery Approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef]
- Abubakar, A.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




